Abstract
Background
Brodalumab is a fully human anti–interleukin-17 receptor A monoclonal antibody efficacious for the treatment of adults with moderate-to-severe plaque psoriasis.
Objective
This study summarizes malignancy rates in psoriasis clinical studies of brodalumab.
Methods
Data were pooled from one phase II study and three large, multicenter, phase III randomized studies of brodalumab for the treatment of psoriasis, including two studies with randomization to brodalumab, ustekinumab, or placebo. Data from the 52-week (brodalumab and ustekinumab) and long-term (brodalumab) pools were summarized as exposure-adjusted or follow-up time-adjusted event rates per 100 patient-years (PY).
Results
Exposure-adjusted event rates per 100 PY at 52 weeks were lower with brodalumab (n = 4019; 3446 total PY of exposure) than with ustekinumab (n = 613; 495 total PY of exposure), including adjudicated malignancies (0.9 vs 2.6) and Surveillance, Epidemiology, and End Results (SEER)-adjudicated malignancies (0.3 vs 0.4). The exposure-adjusted event rate of adjudicated malignancies in the brodalumab group remained stable in the long-term analysis (0.9 [82 events]).
Conclusions
Rates of malignancy among brodalumab-treated patients with psoriasis were generally low.
Trial registry
ClinicalTrials.gov identifier NCT00975637; NCT01101100; NCT01708590 (AMAGINE-1); NCT01708603 (AMAGINE-2); NCT01708629 (AMAGINE-3).
Electronic supplementary material
The online version of this article (10.1007/s40257-020-00512-4) contains supplementary material, which is available to authorized users.
Key Points
| Because psoriasis is associated with an increased incidence of some malignancies such as skin cancer, we assessed the malignancy rates in multiple clinical studies of brodalumab, a fully human anti–interleukin-17 receptor A monoclonal antibody efficacious for the treatment of moderate-to-severe plaque psoriasis. |
| We found that brodalumab appears to have a low carcinogenic risk of malignancy on the basis of an analysis of > 8000 patient-years of exposure. |
Introduction
Psoriasis is a chronic inflammatory disease associated with increased incidence of some cancers [1], a risk that may be related to impaired immune function or predisposing therapies in patients with this disorder [2]. In a population-based study of 67,761 individuals that examined the association between the risk of incident cancers and psoriasis, patients with psoriasis were found to have an increased risk of developing malignancies such as lymphohematopoietic and pancreatic cancers, and the risk of certain cancers was elevated in patients with long-term disease history [1]. Accordingly, patients with psoriasis should be monitored and routinely screened for potential malignancies, particularly skin cancer [3].
There may be an increased risk of malignancy among patients with psoriasis compared with the general population. A systematic review showed increase risk of solid cancers in psoriasis, including respiratory tract cancer (standardized incidence ratio [SIR], 1.52), upper aerodigestive tract cancer (SIR, 3.05), urinary tract cancer (SIR, 1.31), and liver cancer (SIR, 1.90) [2]. Increased incidence of cancer in patients with psoriasis has also been linked to the use of certain immunosuppressive and potentially carcinogenic treatments, such as cyclosporine, methotrexate, and psoralen and ultraviolet A light therapy [3]. For example, in a 5-year cohort study of patients with psoriasis, use of the immunosuppressive therapy cyclosporine was associated with a 2-fold greater incidence of malignancy and a 6-fold greater incidence of nonmelanoma skin cancer (NMSC) compared with incidence in the general population [4]. In a meta-analysis of seven studies, there was a higher risk of squamous cell carcinoma in psoriasis (SIR, 5.3), which was strongly related to the use of 8-methoxypsoralen-ultraviolet-A and, potentially, cyclosporine and methotrexate [2]. This meta-analysis also showed an increased incidence of basal cell carcinoma in patients with psoriasis (SIR, 2.00) [2].
An association between use of anti-tumor necrosis factor α (anti-TNFα) agents and NMSC, especially squamous cell carcinoma, has also been reported in several immune-mediated conditions. A comparison of patients receiving anti-TNFα therapy showed a significantly elevated risk of NMSC in those with psoriasis relative to the risk in those with rheumatoid arthritis (hazard ratio, 6.0 [95% confidence interval 1.6–22.4]) [5]. Expert opinion recommends avoiding the use of TNFα inhibitors in patients who currently have malignancy or those with history of malignancy [6].
Recently developed biologic therapies for psoriasis target immune system messengers such as interleukin-17 (IL-17), and related pathways have also been identified as novel cancer targets. For example, IL-12 plays a role in antitumor immunity through activation of antitumor effectors and blocking the expansion of intratumoral regulatory T cells [7]. IL-12 also contributes to maintaining cancer cells in equilibrium, allowing them to persist in a dormant rather than metastatic state [8]. Consistent with this role of IL-12 in cancer surveillance, inhibition of IL-12/23p40 in a preclinical model enhanced tumor outgrowth [8]. There may be a theoretical risk of compromising cancer surveillance through mechanisms of blocking IL-12, such as with the anti–IL-12/23 p40 antibody ustekinumab. Potential malignancies are considered an event of interest for immunomodulatory biologics, and treatments for psoriasis should be assessed for any possible contribution to risk of malignancy in these patients.
Brodalumab is a fully human anti–IL-17 receptor A monoclonal antibody [9]. Across three large phase III studies of brodalumab for the treatment of moderate-to-severe plaque psoriasis, 75% improvement in psoriasis area severity index (PASI 75) response rates ranged from 83% to 86% in patients receiving brodalumab 210 mg every 2 weeks (Q2W) compared with 3% to 8% in those receiving placebo [10, 11]. Brodalumab is a targeted immunomodulatory agent and is not expected to be broadly immunosuppressive [12, 13]. A network meta-analysis of 109 studies of treatments for chronic plaque psoriasis (which included three studies of brodalumab versus placebo and two studies of brodalumab versus the active comparator ustekinumab and placebo) did not find evidence of increased risk of serious adverse events (AEs) with brodalumab [14]. However, the long-term effect of brodalumab on risk of malignancies remains unknown. The objective of this analysis was to summarize malignancy rates in clinical studies of brodalumab for the treatment of moderate-to-severe plaque psoriasis.
Methods
Study Design and Treatments
Data from a 12-week phase II study (NCT00975637) [15], its open-label extension (NCT01101100; cut-off date: June 16, 2014) [16], and three randomized, multicenter, 52-week, phase III studies (AMAGINE-1, NCT01708590 [11]; AMAGINE-2, NCT01708603; and AMAGINE-3, NCT01708629 [10]) were pooled. The methodologies of these studies have previously been described, and the AMAGINE-1 and AMAGINE-2/-3 study designs are included as Supplementary Material (Electronic Supplementary Material 1) [10, 11, 15, 16]. Briefly, each study enrolled adults ≥18 years of age with plaque psoriasis and PASI ≥12. Use of most systemic psoriasis treatments including corticosteroids, phototherapy, methotrexate, and cyclosporine within 14–28 days before the first dose of study drug was an exclusion criteria in the phase III psoriasis trials (details in Electronic Supplementary Material 2).
Each study was placebo controlled for the first 12 weeks, and AMAGINE-2 and -3 also included ustekinumab as an active comparator up to week 52. Treatment changes were incorporated into the design of each study; therefore, patients had exposure to different study therapies (described briefly below) and experienced different durations of treatment with those therapies. Patients with active malignancy or history of malignancy within 5 years (except for treated or cured cutaneous squamous or basal cell carcinoma, in situ cervical cancer, or in situ breast ductal carcinoma) were excluded from the phase III clinical program.
In the initial 12-week period of the phase II study, brodalumab was administered at dosages of 70, 140, or 210 mg Q2W, with an additional dose at week 1, or at a dosage of 280 mg Q4W. During the open-label extension (up to data cutoff at week 192), patients received brodalumab 210 mg Q2W, except some patients with body weight ≤100 kg had doses decreased to 140 mg Q2W per a protocol amendment. In AMAGINE-1, patients were assigned to brodalumab 140 or 210 mg Q2W (with an additional dose at week 1), which persisted through the termination of the open-label extension at week 266, except for certain patients who received three weekly doses of brodalumab 140 or 210 mg during retreatment after withdrawal and then returned to the Q2W treatment interval. In AMAGINE-2 and -3, patients were assigned to brodalumab 140 or 210 mg Q2W (with an additional dose at week 1), which persisted through week 12. From week 12 to week 266, patients assigned to brodalumab received brodalumab 140 mg Q2W, Q4W, or Q8W or brodalumab 210 mg Q2W. In AMAGINE-2 and -3, ustekinumab was administered according to the label at 45 mg in patients with body weight ≤ 100 kg and 90 mg in patients with body weight > 100 kg and was given on day 1, week 4, and every 12 weeks thereafter through week 52.
Assessments
All AEs reported in the neoplasms benign, malignant, and unspecified (including cysts and polyps) System Organ Class were medically reviewed in a blinded manner by study staff, and confirmed malignancies were categorized as adjudicated malignancies. Malignancies were further categorized as Surveillance, Epidemiology, and End Results (SEER)-adjudicated malignancies [17] if they corresponded to malignancies included in SEER reporting. The category of SEER-adjudicated malignancies excluded basal cell carcinoma and squamous cell carcinoma of the skin, in situ cancers (except for urinary bladder), benign neoplasms, and recurrent tumors. Adjudicated malignancies were also separately classified as NMSCs.
Statistical Analyses
Baseline demographics and disease characteristics by treatment group in the initial 12-week studies (Table 1) were summarized as mean (range) for continuous variables and number of patients (%) for categorial variables. Data from the 12-week induction period (placebo, ustekinumab, and brodalumab), 52-week extensions (brodalumab and ustekinumab), and long-term pool (brodalumab) were summarized. The long-term pool included data collected from the phase II and III studies until their open-label extensions were terminated at 192 or 266 weeks. The all-brodalumab group included all patients who received one or more doses of brodalumab. At week 12 and week 52, data are also shown for patients who received brodalumab 210 mg Q2W. An exploratory analysis of malignancy events occurring among patients with a history of malignancy in the phase III studies was also conducted.
Table 1.
Baseline demographics and disease characteristics by treatment group in the initial 12-week studies
| Placebo (n = 879) |
Ustekinumab (n = 613) |
Brodalumab 210 mg Q2W (n = 1496) |
All brodalumab (n = 3066)a |
|
|---|---|---|---|---|
| Female, n (%) | 272 (30.9) | 196 (32.0) | 459 (30.7) | 942 (30.7) |
| Age, mean (range), years | 44.6 (18–86) | 45.1 (18–75) | 45.0 (18–75) | 44.8 (18–75) |
| Race, n (%) | ||||
| White | 799 (90.9) | 551 (89.9) | 1351 (90.3) | 2775 (90.5) |
| Asian | 29 (3.3) | 24 (3.9) | 51 (3.4) | 116 (3.8) |
| Black | 29 (3.3) | 20 (3.3) | 40 (2.7) | 85 (2.8) |
| Native Hawaiian/Pacific Islander | 3 (0.3) | 1 (0.2) | 10 (0.7) | 18 (0.6) |
| American Indian/Alaska Native | 2 (0.2) | 2 (0.3) | 8 (0.5) | 16 (0.5) |
| Other/unknown | 17 (1.9) | 15 (2.4) | 36 (2.4) | 56 (1.8) |
| Psoriasis duration, mean (range), years | 18.5 (1–67) | 18.5 (1–57) | 18.6 (1–65) | 18.4 (1–66) |
| Psoriasis area and severity index, mean (range) | 20.0 (12–66) | 20.0 (12–60) | 20.2 (12–72) | 20.2 (12–72) |
| Static physician’s global assessment score, n (%) | ||||
| 0 or 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 2 (0.1) | 8 (0.3) |
| 3 | 500 (56.9) | 345 (56.3) | 827 (55.3) | 1789 (58.3) |
| 4 | 330 (37.5) | 235 (38.3) | 583 (39.0) | 1112 (36.3) |
| 5 | 49 (5.6) | 33 (5.4) | 84 (5.6) | 157 (5.1) |
| Psoriatic arthritis, n (%) | 180 (20.5) | 114 (18.6) | 310 (20.7) | 654 (21.3) |
| Prior malignancy, n (%) | 18 (2.0) | 17 (2.8) | 34 (2.3) | 69 (2.3) |
Q2W every 2 weeks
aThe all-brodalumab group includes all patients who received ≥ 1 dose of brodalumab
Rates of malignancy events were calculated as exposure-adjusted or follow-up time-adjusted event rates per 100 patient-years (PY). Exposure-adjusted event rates, which exclude gaps or interruptions in treatment, were calculated as the number of events/total PY of exposure × 100. Follow-up observation time included gaps or interruptions in treatment and post-treatment follow-up beyond the exposure period. Follow-up observation time-adjusted event rates were calculated as the number of events/total PY of follow-up × 100.
Results
Patient Treatment Exposure and Baseline Characteristics
During the initial 12-week studies, 3066 patients in the all-brodalumab group had a total of 688 PY of brodalumab exposure; of these, 1496 patients received brodalumab 210 mg Q2W. A total of 613 patients in the ustekinumab group had 140 total PY of ustekinumab exposure. At the end of 52 weeks, 4019 patients had received brodalumab for a total of 3446 PY, and total ustekinumab exposure increased to 495 PY of exposure. In the long-term pool, 4464 patients were treated with brodalumab, of whom 1304 received brodalumab 210 mg Q2W and had no ustekinumab exposure. In the long-term pool, there were a total of 8655 PY of exposure and a total of 9174 PY of follow-up in the all-brodalumab group, and mean duration of exposure to brodalumab was 23.3 months. In the overall brodalumab 210 mg Q2W group, there were a total of 2543 PY of exposure and a total of 2686 PY of follow-up, and mean duration of exposure was 23.4 months.
Baseline characteristics among patients enrolled in the initial 12-week period, including sex, age, and duration of psoriasis, were similar across all groups (Table 1). Overall, ~ 70% of patients were men and ~ 90% were White, and most patients (57%) were ≥ 40 to < 65 years of age. The mean (standard deviation [SD]) duration of psoriasis was ~ 18.5 (12.1) years, 21% of patients had psoriatic arthritis, the mean (SD) PASI score was 20.1 (8.1), and almost all patients (> 99%) had a static physician’s global assessment of psoriasis score of ≥ 3. At study baseline, 2–3% of patients across treatment groups reported a history of malignancy (Table 1).
Event Rates Through Week 12
Few malignancy events were reported during the 12-week induction period (Table 2). Within this period, no adjudicated malignancies were reported over a total of 195 PY of exposure in those receiving placebo, one was reported over a total of 140 PY in those receiving ustekinumab, and four were reported over a total of 688 PY among all patients receiving brodalumab. Exposure-adjusted event rates for adjudicated malignancies were similar in the ustekinumab, brodalumab 210 mg Q2W, and all-brodalumab treatment groups, ranging from 0.6 to 0.7 events per 100 PY of exposure. There were three cases of NMSC among all patients receiving brodalumab and no cases in the placebo or ustekinumab groups. Through week 12, one SEER-adjudicated malignancy (prostate cancer) occurred in a patient receiving ustekinumab, and one (penile squamous cell cancer) occurred among all patients receiving brodalumab. One patient in the brodalumab 140 mg Q2W group had pancreatic carcinoma, a grade 4 serious AE that was reported on study day 39 (after the exposure period); however, this patient received only one dose of brodalumab before being discontinued from the study.
Table 2.
Malignancy exposure-adjusted event rates (12-week results)
| Placebo (n = 879; 195 PY) |
Ustekinumab (n = 613; 140 PY) |
Brodalumab 210 mg Q2W (n = 1496; 336 PY) |
All brodalumab (n = 3066; 688 PY)a |
|
|---|---|---|---|---|
| Adjudicated malignancies | 0 | 1 (0.7) | 2 (0.6) | 4 (0.6) |
| NMSC | 0 | 0 | 1 (0.3) | 3 (0.4) |
| Basal cell carcinoma | 0 | 0 | 0 | 2 (0.3) |
| Squamous cell carcinoma | 0 | 0 | 1 (0.3) | 1 (0.1) |
| SEER-adjudicated malignancies | 0 | 1 (0.7) | 1 (0.3) | 1 (0.1) |
| Penile squamous cell carcinoma | 0 | 0 | 1 (0.3) | 1 (0.1) |
| Prostate cancer | 0 | 1 (0.7) | 0 | 0 |
Values are the number of events (exposure-adjusted event rate per 100 patient-years [n/PY × 100])
NMSC nonmelanoma skin cancer, PY total patient-years of exposure through week 12, Q2W every 2 weeks, SEER Surveillance, Epidemiology, and End Results
aThe all-brodalumab group includes all patients who received ≥ 1 dose of brodalumab
Event Rates Through Week 52
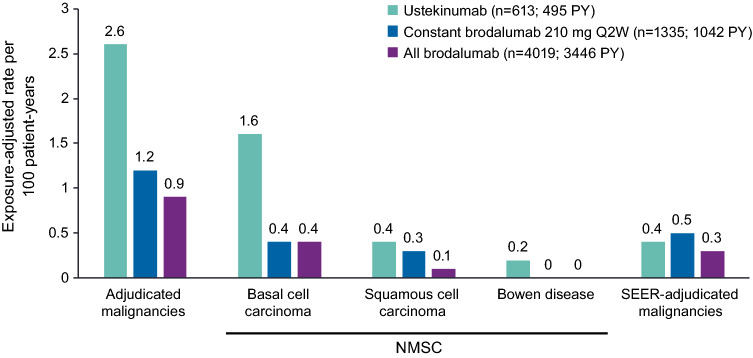
Exposure-adjusted adjudicated malignancy event rates through 52 weeks were lower in the all-brodalumab group (0.9 events per 100 PY) than in the ustekinumab group (2.6 events per 100 PY; Fig. 1). Exposure-adjusted rates of NMSC through 52 weeks were 0.6 events per 100 PY in the all-brodalumab group (basal cell carcinoma [n = 15], 0.4 events per 100 PY and squamous cell carcinoma [n = 4], 0.1 events per PY) and were 2.2 events per 100 PY in the ustekinumab group (basal cell carcinoma [n = 8], 1.6 events per PY; squamous cell carcinoma [n = 2], 0.4 events per PY; and Bowen disease [n = 1], 0.2 events per PY; Fig. 1). The ratio of basal cell carcinoma to squamous cell carcinoma events was 4:1 in the ustekinumab group and 3.8:1 in the all-brodalumab group. Most malignancy AEs through week 52 were grade ≤ 2 in severity.
Fig. 1.
Malignancy event rates (52-week results). The all-brodalumab group includes all patients who received ≥ 1 dose of brodalumab. NMSC nonmelanoma skin cancer, PY total patient-years of exposure through week 52, Q2W every 2 weeks, SEER Surveillance, Epidemiology, and End Results
Exposure-emergent serious AEs occurring in more than one patient included adenocarcinoma of the pancreas in two brodalumab-treated patients, basal cell carcinoma in two ustekinumab-treated patients, one case of prostate cancer each for brodalumab and ustekinumab, and two cases of uterine leiomyoma with brodalumab. Two brodalumab-treated patients had grade 4 treatment-emergent serious malignancy AEs, which were bile duct adenocarcinoma and follicle center lymphoma. One fatal malignancy AE, pancreatic carcinoma, occurred in the ustekinumab group.
Event Rates Through End of Study
Rates of malignancy events in brodalumab-treated patients in the long-term analysis (Table 3) were consistent with the 52-week results. NMSC was experienced by 56 patients in the all-brodalumab group (basal cell carcinoma [n = 39] and squamous cell carcinoma [n = 9] follow-up time-adjusted NMSC event rate: 0.6 per 100 PY) and in 11 patients in the brodalumab 210-mg Q2W group (basal cell carcinoma [n = 7] and squamous cell carcinoma [n = 2] follow-up time-adjusted NMSC event rate: 0.4 per 100 PY). A total of 37 SEER-adjudicated malignancies were reported over a total of 9174 PY of exposure; the most common of these was prostate cancer (n = 8; 0.1 events per 100 PY in the all-brodalumab group; Table 3). There was one case of B-cell lymphoma and one case of follicle center lymphoma in the all-brodalumab group.
Table 3.
Malignancy exposure-adjusted and follow-up observation time–adjusted event rates with brodalumab (long-term pool)
| Overall brodalumab 210 mg Q2W (n = 1304)a |
All brodalumab (n = 4464)b |
|||
|---|---|---|---|---|
| Exposure-adjusted event rate per 100 patient-years (2543 PY) | Follow-up time-adjusted event rate per 100 patient-years (2686 PY) | Exposure-adjusted event rate per 100 patient-years (8655 PY) | Follow-up time-adjusted event rate per 100 patient-years (9174 PY) | |
| Adjudicated malignancies | 19 (0.7) | 21 (0.8) | 82 (0.9) | 93 (1.0) |
| NMSC | 11 (0.4) | 11 (0.4) | 56 (0.6) | 56 (0.6) |
| Basal cell carcinoma | 7 (0.3) | 7 (0.3) | 39 (0.5) | 39 (0.4) |
| Squamous cell carcinoma | 2 (0.1) | 2 (0.1) | 9 (0.1) | 9 (0.1) |
| Squamous cell carcinoma of the skin | 1 (< 0.1) | 1 (< 0.1) | 6 (0.1) | 6 (0.1) |
| Basosquamous carcinoma of the skin | 0 | 0 | 1 (< 0.1) | 1 (<0.1) |
| Squamous cell carcinoma of the oral cavity | 1 (< 0.1) | 1 (< 0.1) | 1 (< 0.1) | 1 (<0.1) |
| SEER-adjudicated malignancies | 8 (0.3) | 10 (0.4) | 26 (0.3) | 37 (0.4) |
| Prostate cancer | 0 | 0 | 7 (0.1) | 8 (0.1) |
| Breast cancer | 1 (< 0.1) | 1 (< 0.1) | 3 (< 0.1) | 3 (<0.1) |
| Adenocarcinoma of the pancreas | 1 (< 0.1) | 1 (< 0.1) | 2 (< 0.1) | 2 (<0.1) |
| Adenocarcinoma of the colon | 0 | 0 | 1 (< 0.1) | 1 (<0.1) |
| B-cell lymphoma | 0 | 0 | 0 | 1 (<0.1) |
| Bile duct adenocarcinoma | 0 | 0 | 1 (< 0.1) | 1 (<0.1) |
| Bladder cancer | 0 | 1 (< 0.1) | 0 | 1 (<0.1) |
| Bladder transitional cell carcinoma | 0 | 0 | 1 (< 0.1) | 1 (<0.1) |
| Carcinoid tumor | 0 | 0 | 1 (< 0.1) | 2 (<0.1) |
| Colon cancer | 1 (< 0.1) | 1 (< 0.1) | 1 (< 0.1) | 1 (<0.1) |
| Endometrial cancer | 0 | 0 | 0 | 1 (<0.1) |
| Esophageal adenocarcinoma | 0 | 0 | 0 | 1 (<0.1) |
| Esophageal carcinoma | 1 (< 0.1) | 1 (< 0.1) | 1 (< 0.1) | 1 (<0.1) |
| Follicle center lymphoma, follicular grade 1–3 | 0 | 0 | 1 (< 0.1) | 1 (<0.1) |
| Invasive ductal breast carcinoma | 1 (< 0.1) | 1 (< 0.1) | 1 (< 0.1) | 1 (<0.1) |
| Malignant anorectal neoplasm | 0 | 0 | 0 | 1 (<0.1) |
| Metastases to lymph nodes | 0 | 1 (< 0.1) | 0 | 1 (<0.1) |
| Pancreatic carcinoma | 0 | 0 | 1 (< 0.1) | 2 (<0.1) |
| Papillary thyroid cancer | 1 (< 0.1) | 1 (< 0.1) | 1 (< 0.1) | 1 (<0.1) |
| Penile squamous cell carcinoma | 0 | 0 | 1 (< 0.1) | 1 (<0.1) |
| Rectal cancer | 1 (< 0.1) | 1 (< 0.1) | 1 (< 0.1) | 1 (<0.1) |
| Renal cancer | 0 | 0 | 1 (< 0.1) | 1 (<0.1) |
| Renal cell carcinoma | 0 | 0 | 0 | 1 (<0.1) |
| Small cell lung cancer | 0 | 0 | 0 | 1 (<0.1) |
| Small intestine carcinoma metastatic | 1 (< 0.1) | 1 (< 0.1) | 1 (< 0.1) | 1 (<0.1) |
Values are the number of events (exposure- or follow-up observation time-adjusted event rate per 100 patient-years [n/PY × 100]). The mean (SD) duration of cumulative exposure in the overall brodalumab 210-mg Q2W group was 23.4 (7.9) months and was 23.3 (10.9) months in the all-brodalumab group
NMSC nonmelanoma skin cancer, PY total patient-years of exposure or follow-up through end of study, Q2W every 2 weeks, SD standard deviation, SEER Surveillance, Epidemiology, and End Results
aIn the overall brodalumab 210-mg Q2W group, ≥75% of doses were 210 mg only
bThe all-brodalumab group includes all patients who received ≥1 dose of brodalumab
History of Malignancy
In an exploratory analysis, 2–3% of patients across treatment groups reported history (typically > 5 years before screening, with exceptions listed in Methods) of malignancy (Table 1). Among the most common types of prior malignancy were basal cell carcinoma, squamous cell carcinoma, breast cancer, malignant melanoma, squamous cell carcinoma of the skin, thyroid neoplasm, prostate cancer, and uterine cancer (Supplementary Table 1, see Electronic Supplementary Material). Of patients with prior malignancy (n = 138), 41–67% of patients across treatment groups reported previous treatment with any phototherapy (placebo [n = 18], 66.7%; ustekinumab [n = 17], 41.2%; brodalumab 210 mg Q2W [n = 34], 55.9%; all-brodalumab group [n = 69], 52.2%). One patient in the brodalumab 210 mg Q2W group with a history of resolved basal cell carcinoma experienced an adjudicated malignancy event of squamous cell carcinoma during the 12-week induction phase. Overall, three adjudicated malignancies, also classified as NMSC AEs (one basal cell carcinoma, two squamous cell carcinoma), were reported in patients with malignancy history who received constant brodalumab 210 mg Q2W through 52 weeks. The three patients who experienced these NMSC AEs entered the clinical program with a history of resolved breast cancer (n = 1), basal cell carcinoma (n = 3), and squamous cell carcinoma (n = 1).
Discussion
In clinical studies of brodalumab, rates of malignancy were generally low. In the long-term analysis of 4464 patients receiving brodalumab over 8655 total PY of exposure, the event rate for all malignancies was 0.9 per 100 PY, and the event rate for NMSC was 0.6 per 100 PY. Through 52 weeks, the event rate for all malignancies was 2.6 per 100 PY for the active comparator ustekinumab. Three NMSCs were reported in patients with malignancy history receiving constant brodalumab 210 mg Q2W through 52 weeks.
The risk of malignancy among patients receiving treatment for psoriasis has been previously assessed in numerous studies of commonly used treatments, including the anti-TNFα antibodies infliximab and adalimumab, the anti–IL-12/23 p40 antibody ustekinumab, and the anti–IL-17A antibodies secukinumab and ixekizumab. Comparison of event rates between studies must be made with caution because there are differences in study setting, inclusion and exclusion criteria (including definition of psoriasis and psoriatic arthritis), design, treatment type and regimen, and methods of defining, determining, and reporting AEs. However, malignancy findings from studies of other psoriasis agents provide context for the current analysis of brodalumab safety.
Malignancy risk with use of anti–IL-12/23 or anti–IL-17 treatment for psoriasis has been investigated in long-term analyses of clinical study data. After 5 years of follow-up of ustekinumab treatment for psoriasis, an analysis of 8998 total PY of follow-up found event rates of 0.64 per 100 PY (45 mg) and 0.44 per 100 PY (90 mg) for NMSC and 0.59 per 100 PY (45 mg) and 0.61 per 100 PY (90 mg) for other malignancies [18]. In an analysis of 2725 total PY of secukinumab exposure over 52 weeks, the event rate was 0.48 per 100 PY for NMSC and also 0.48 per 100 PY for other malignant or unspecified tumors [19]. Over 6480 total PY of ixekizumab exposure over 60 weeks, the incidence rate was 0.4 per 100 PY for NMSC and 0.5 per 100 PY for malignancies excluding NMSC [20]. Finally, over 1373 total PY of ixekizumab exposure in psoriatic arthritis, the incidence rate of malignancy was 0.7 per 100 PY [21]. As stated previously, comparisons between different studies must be made with caution, but rates of malignancy in the current study of brodalumab (0.9 events per 100 PY for all malignancies and 0.6 events per 100 PY for NMSC) appear consistent with rates reported for other psoriasis treatments. Three NMSCs were reported over 52 weeks in patients with malignancy history who were treated with brodalumab 210 mg Q2W. Furthermore, 1-year post-marketing pharmacovigilance monitoring showed three cases of malignancy (hepatic, lung, and ovarian), all considered unrelated to brodalumab [22]. Overall, there is limited clinical evidence that use of biologic treatments for psoriasis increases malignancy risk. The complex role of inflammatory cytokines such as IL-17 in mediating tumor growth, development, and immunity is an active area of clinical and preclinical investigation [23]. Real-world studies are needed to understand any potential long-term malignancy risk with anti–IL-17 biologic treatments for psoriasis.
Several studies have assessed malignancy risk with anti-TNFα therapy. In one registry study among patients with psoriasis, an analysis including 23,660 total PY of adalimumab use found a rate of 1.0 events of malignancy per 100 PY of exposure [24]. In a retrospective cohort study, incidence rates per 100 PY of observation for malignancies excluding NMSC were higher for infliximab (2.30) and lower for adalimumab (0.78) compared with the rate in the overall psoriasis population (1.42). However, for NMSC, incidence rates per 100 PY were lower for infliximab (1.68) and higher for adalimumab (2.41) compared with the psoriasis population (1.80) [25]. A systematic review of adalimumab safety in psoriasis registries found event rates of 0.9 per 100 PY for malignancy, < 0.6 per 100 PY for malignancies excluding NMSC, and a range of < 0.5–0.6 per 100 PY for NMSC [26]. Another systematic review found an increased risk of NMSC, but not other malignancies, among patients with psoriasis treated with anti-TNFα therapy, compared with the general population and with patients with rheumatoid arthritis treated with anti-TNF therapy [27].
Multiple studies in disease states other than psoriasis have investigated risk of malignancy, particularly lymphoma, associated with use of anti-TNFα agents [28–31]. These studies found no increase in risk of malignancy compared with patients with the same disease treated with other classes of agents [28–31]. One study of self-reported data among patients with rheumatoid arthritis found a small (hazard ratio, 1.24) but not statistically significant increase in the rate of NMSC with use of anti-TNFα therapy [32]. A systematic review found no evidence of increased risk of overall cancer, lymphoma, or melanoma with use of anti-TNFα therapy across disease states, but findings for NMSC were inconsistent [33]. A prospective cohort study in Sweden showed that patients with rheumatoid arthritis treated with TNF inhibitors had an increased risk of invasive melanoma relative to patients not treated with biologics (hazard ratio, 1.5) [34]. An examination of electronic medical records from an academic medical center identified 35 cases of melanoma among 6045 patients exposed to a TNFα inhibitor [35]. There were also 972 reports of melanoma associated with TNFα inhibitors in the US Food and Drug Administration Adverse Events Reporting System database (from the date of approval for each drug through August 2012) [35]. However, a systematic review found no sufficient evidence to conclude that anti-TNFα agents increase malignancy risk [36].
Among studies that reported findings across multiple drug classes in patients with psoriasis, a network meta-analysis including clinical trials of anti-TNFα, anti–IL-12/23, and anti–IL-17 therapies found that malignancies were reported in both the intervention and placebo groups across all of these classes; however, event rates were not reported [14]. In the Psoriasis Longitudinal Assessment Registry, treatment with ustekinumab was not associated with increased malignancy risk compared with no ustekinumab treatment, but treatment for >12 months with a TNFα inhibitor (infliximab, adalimumab, or etanercept) was associated with a 1.5-fold increase in malignancy risk compared with no TNFα inhibitor treatment [37]. In a Psoriasis Longitudinal Assessment and Registry (PSOLAR) analysis of 12,095 patients treated with biologics who were followed up for 31,818 PY, the rate of malignancies excluding NMSC was 0.68 per 100 PY in the overall group; this rate was lower for patients in the infliximab (0.58 per 100 PY) or ustekinumab (0.53 per 100 PY) groups [38]. In a later PSOLAR analysis over a total of 40,388 PY of exposure to biologics, the rate of malignancies excluding NMSC remained 0.68 per 100 PY in the overall group; this rate was 0.79 per 100 PY in the infliximab group and 0.48 per 100 PY in the ustekinumab group, possibly indicating increasing incidence of malignancy over time for the anti-TNFα agent infliximab [39]. Further investigations are needed to characterize long-term malignancy risk across these biologic classes.
This study had some limitations. Fewer patients received ustekinumab than those receiving brodalumab, resulting in less exposure to ustekinumab. Patients in the clinical studies received varying doses of brodalumab on varying schedules, which limits the ability to generalize to patients receiving the recommended dose of 210 mg Q2W [9]. Longer follow-up time is needed to understand the potential clinical implications of the ratio of basal cell carcinoma to squamous cell carcinoma (3.8:1 through 52 weeks) in patients who received any dose of brodalumab, and observations such as the occurrence of eight cases of prostate cancer over a total of 9174 PY of follow-up. Follow-up in this analysis continued for only 266 weeks at most, and the mean exposure was <2 years in the long-term pool. Increased rates of malignancy beyond this time period could not be assessed.
Conclusions
In conclusion, on the basis of an analysis of >8000 PY of exposure, brodalumab appears to have a low carcinogenic risk. Rates of malignancy among brodalumab-treated patients with psoriasis were low in clinical studies. Few events of malignancy occurred in patients with a history of malignancy through 52 weeks. Longer follow-up and real-world evidence are needed to characterize the long-term risk of malignancy with brodalumab.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Medical writing assistance was provided under the direction of the authors by Lisa Baker, PhD, Rebecca Slager, PhD, and David Boffa, ELS, of MedThink SciCom, with support from Ortho Dermatologics. Ortho Dermatologics is a division of Bausch Health US, LLC. Some results of this study were previously presented at the Fall Clinical Dermatology Conference, October 18–21, 2018, Las Vegas, NV; the Orlando Dermatology Aesthetic & Clinical Conference, January 18–21, 2019, Orlando, FL; the 43rd Annual Hawaii Dermatology Seminar, February 17–22, 2019, The Big Island, HI; and the 2020 Winter Clinical Dermatology Conference; January 17–22, 2020; Kohala Coast, HI.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Compliance with Ethical Standards
Funding/support
This analysis was sponsored by Ortho Dermatologics (a division of Bausch Health US, LLC). The AMAGINE-1-2/-3 studies were sponsored by Amgen Inc. Open Access of this article was funded by Ortho Dermatologics (a division of Bausch Health US, LLC).
Ethical approval
Study protocols were in compliance with federal regulations and the International Conference on Harmonisation Tripartite Guideline on Good Clinical Practice and were approved by individual institutional review boards at each participating study center. All participants signed and personally dated the informed consent form approved by the independent ethics committees and institutional review boards before any study-specific procedures were performed.
Conflicts of interest and financial disclosures
Alice Gottlieb has served as a consultant or as an advisory board member for Janssen, Celgene, Bausch Health, Bristol-Myers Squibb, Beiersdorf, AbbVie, UCB, Novartis, Incyte, Eli Lilly, Dr. Reddy’s Laboratories, Dermira, Allergan, Sun Pharma, XBiotech, LEO Pharmaceuticals, Avotres Therapeutics, and Boehringer Ingelheim and has received research or educational grants from Janssen, Incyte, Novartis, XBiotech, UCB, and Boehringer Ingelheim. Mark Lebwohl is an employee of Mount Sinai, which receives research funds from AbbVie, Amgen, Arcutis, AstraZeneca, Boehringer Ingelheim, Celgene, Clinuvel, Eli Lilly, Incyte, Janssen Research & Development, LLC, Kadmon Corp., LLC, LEO Pharmaceuticals, Medimmune, Novartis, Ortho Dermatologics, Pfizer, Sciderm, UCB, Inc., and ViDac and has been a consultant for Allergan, Almirall, Arcutis, Inc., Avotres Therapeutics, BirchBioMed Inc., Boehringer Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Evelo, Foundation for Research and Education in Dermatology, Inozyme Pharma, LEO Pharmaceuticals, Meiji Seika Pharma, Menlo, Mitsubishi, Neuroderm, Pfizer, Promius/Dr. Reddy’s Laboratories, Theravance, and Verrica. Clive Liu has served on speaker bureaus and participated in research and advisory boards for AbbVie, Celgene, Novartis, Lilly, Regeneron, Sanofi, Sun Pharma, Ortho Dermatologics, and Janssen. Robert J. Israel is an employee of Bausch Health US, LLC (an affiliate of Bausch Health Companies Inc.) and holds stock and/or stock options in the company. Abby Jacobson is an employee of Ortho Dermatologics (a division of Bausch Health US, LLC) and holds stocks and/or stock options in Bausch Health.
References
- 1.Brauchli YB, Jick SS, Miret M, Meier CR. Psoriasis and risk of incident cancer: an inception cohort study with a nested case-control analysis. J Invest Dermatol. 2009;129(11):2604–2612. doi: 10.1038/jid.2009.113. [DOI] [PubMed] [Google Scholar]
- 2.Pouplard C, Brenaut E, Horreau C, Barnetche T, Misery L, Richard MA, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36–46. doi: 10.1111/jdv.12165. [DOI] [PubMed] [Google Scholar]
- 3.Kimball AB, Gladman D, Gelfand JM, Gordon K, Horn EJ, Korman NJ, et al. National Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screening. J Am Acad Dermatol. 2008;58(6):1031–1042. doi: 10.1016/j.jaad.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paul CF, Ho VC, McGeown C, Christophers E, Schmidtmann B, Guillaume JC, et al. Risk of malignancies in psoriasis patients treated with cyclosporine: a 5 y cohort study. J Invest Dermatol. 2003;120(2):211–216. doi: 10.1046/j.1523-1747.2003.12040.x. [DOI] [PubMed] [Google Scholar]
- 5.Geller S, Xu H, Lebwohl M, Nardone B, Lacouture ME, Kheterpal M. Malignancy risk and recurrence with psoriasis and its treatments: a concise update. Am J Clin Dermatol. 2018;19(3):363–375. doi: 10.1007/s40257-017-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80(1):27–40. doi: 10.1016/j.jaad.2018.06.057. [DOI] [PubMed] [Google Scholar]
- 7.Ngiow SF, Teng MW, Smyth MJ. A balance of interleukin-12 and -23 in cancer. Trends Immunol. 2013;34(11):548–555. doi: 10.1016/j.it.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Teng MW, Vesely MD, Duret H, McLaughlin N, Towne JE, Schreiber RD, et al. Opposing roles for IL-23 and IL-12 in maintaining occult cancer in an equilibrium state. Cancer Res. 2012;72(16):3987–3996. doi: 10.1158/0008-5472.CAN-12-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siliq [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2017.
- 10.Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328. doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
- 11.Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286. doi: 10.1111/bjd.14493. [DOI] [PubMed] [Google Scholar]
- 12.Roman M, Chiu MW. Spotlight on brodalumab in the treatment of moderate-to-severe plaque psoriasis: design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:2065–2075. doi: 10.2147/DDDT.S113683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong Y, Peranteau AJ, Nawas Z, Tyring SK. A review of brodalumab, an IL-17 receptor antagonist, for moderate-to-severe plaque psoriasis. Skin Therapy Lett. 2017;22(1):1–6. [PubMed] [Google Scholar]
- 14.Sbidian E, Chaimani A, Garcia-Doval I, Do G, Hua C, Mazaud C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017;12:CD011535. [DOI] [PMC free article] [PubMed]
- 15.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 16.Papp K, Leonardi C, Menter A, Thompson EH, Milmont CE, Kricorian G, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71(6):1183–90 e3. [DOI] [PubMed]
- 17.National Cancer Institute. Surveillance, Epidemiology, and End Results Program 2019. https://seer.cancer.gov/. Accessed 29 October 2019.
- 18.Papp KA, Griffiths CE, Gordon K, Lebwohl M, Szapary PO, Wasfi Y, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168(4):844–854. doi: 10.1111/bjd.12214. [DOI] [PubMed] [Google Scholar]
- 19.van de Kerkhof PC, Griffiths CE, Reich K, Leonardi CL, Blauvelt A, Tsai TF, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75(1):83–98 e4. [DOI] [PubMed]
- 20.Strober B, Leonardi C, Papp KA, Mrowietz U, Ohtsuki M, Bissonnette R, et al. Short- and long-term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: etanercept comparisons and integrated data. J Am Acad Dermatol. 2017;76(3):432–40 e17. [DOI] [PubMed]
- 21.Mease P, Roussou E, Burmester GR, Goupille P, Gottlieb A, Moriarty SR, et al. Safety of ixekizumab in patients with psoriatic arthritis: results from a pooled analysis of three clinical trials. Arthritis Care Res (Hoboken). 2019;71(3):367–378. doi: 10.1002/acr.23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebwohl M, Leonardi C, Wu JJ, et al. One-year pharmacovigilance update of brodalumab. Poster presented at: 20th Annual Las Vegas Dermatology Seminar; November 7–9, 2019; Las Vegas, NV.
- 23.Blake SJ, Teng MW. Role of IL-17 and IL-22 in autoimmunity and cancer. Actas Dermosifiliogr. 2014;105(suppl 1):41–50. doi: 10.1016/S0001-7310(14)70017-1. [DOI] [PubMed] [Google Scholar]
- 24.Menter A, Thaci D, Wu JJ, Abramovits W, Kerdel F, Arikan D, et al. Long-term safety and effectiveness of adalimumab for moderate to severe psoriasis: results from 7-year interim analysis of the ESPRIT registry. Dermatol Ther (Heidelb). 2017;7(3):365–381. doi: 10.1007/s13555-017-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimball AB, Schenfeld J, Accortt NA, Anthony MS, Rothman KJ, Pariser D. Cohort study of malignancies and hospitalized infectious events in treated and untreated patients with psoriasis and a general population in the United States. Br J Dermatol. 2015;173(5):1183–1190. doi: 10.1111/bjd.14068. [DOI] [PubMed] [Google Scholar]
- 26.Strober B, Crowley J, Langley RG, Gordon K, Menter A, Leonardi C, et al. Systematic review of the real-world evidence of adalimumab safety in psoriasis registries. J Eur Acad Dermatol Venereol. 2018;32(12):2126–2133. doi: 10.1111/jdv.15203. [DOI] [PubMed] [Google Scholar]
- 27.Peleva E, Exton LS, Kelley K, Kleyn CE, Mason KJ, Smith CH. Risk of cancer in patients with psoriasis on biological therapies: a systematic review. Br J Dermatol. 2018;178(1):103–113. doi: 10.1111/bjd.15830. [DOI] [PubMed] [Google Scholar]
- 28.D’Haens G, Reinisch W, Colombel JF, Panes J, Ghosh S, Prantera C, et al. Five-year safety data from ENCORE, a European observational safety registry for adults with Crohn’s disease Treated with infliximab [Remicade(R)] or conventional therapy. J Crohns Colitis. 2017;11(6):680–689. doi: 10.1093/ecco-jcc/jjw221. [DOI] [PubMed] [Google Scholar]
- 29.D’Haens G, Reinisch W, Panaccione R, Satsangi J, Petersson J, Bereswill M, et al. Lymphoma risk and overall safety profile of adalimumab in patients with Crohn’s disease with up to 6 years of follow-up in the pyramid registry. Am J Gastroenterol. 2018;113(6):872–882. doi: 10.1038/s41395-018-0098-4. [DOI] [PubMed] [Google Scholar]
- 30.Hellgren K, Dreyer L, Arkema EV, Glintborg B, Jacobsson LT, Kristensen LE, et al. Cancer risk in patients with spondyloarthritis treated with TNF inhibitors: a collaborative study from the ARTIS and DANBIO registers. Ann Rheum Dis. 2017;76(1):105–111. doi: 10.1136/annrheumdis-2016-209270. [DOI] [PubMed] [Google Scholar]
- 31.Mercer LK, Galloway JB, Lunt M, Davies R, Low AL, Dixon WG, et al. Risk of lymphoma in patients exposed to antitumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis. 2017;76(3):497–503. doi: 10.1136/annrheumdis-2016-209389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakravarty EF, Michaud K, Wolfe F. Skin cancer, rheumatoid arthritis, and tumor necrosis factor inhibitors. J Rheumatol. 2005;32(11):2130–2135. [PubMed] [Google Scholar]
- 33.Chen Y, Friedman M, Liu G, Deodhar A, Chu CQ. Do tumor necrosis factor inhibitors increase cancer risk in patients with chronic immune-mediated inflammatory disorders? Cytokine. 2018;101:78–88. doi: 10.1016/j.cyto.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Raaschou P, Simard JF, Holmqvist M, Askling J, Group AS. Rheumatoid arthritis, anti-tumour necrosis factor therapy, and risk of malignant melanoma: nationwide population based prospective cohort study from Sweden. BMJ. 2013;346:f1939. [DOI] [PubMed]
- 35.Nardone B, Hammel JA, Raisch DW, Weaver LL, Schneider D, West DP. Melanoma associated with tumour necrosis factor-alpha inhibitors: a Research on Adverse Drug events And Reports (RADAR) project. Br J Dermatol. 2014;170(5):1170–1172. doi: 10.1111/bjd.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Sun J, Yang Y, Huang Y, Liu G. Malignancy risk of anti-tumor necrosis factor alpha blockers: an overview of systematic reviews and meta-analyses. Clin Rheumatol. 2016;35(1):1–18. doi: 10.1007/s10067-015-3115-7. [DOI] [PubMed] [Google Scholar]
- 37.Fiorentino D, Ho V, Lebwohl MG, Leite L, Hopkins L, Galindo C, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77(5):845–854. doi: 10.1016/j.jaad.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Gottlieb AB, Kalb RE, Langley RG, Krueger GG, de Jong EM, Guenther L, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13(12):1441–1448. [PubMed] [Google Scholar]
- 39.Papp K, Gottlieb AB, Naldi L, Pariser D, Ho V, Goyal K, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) J Drugs Dermatol. 2015;14(7):706–714. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.