Abstract
Eph (erythropoietin-producing hepatoma) receptors and Ephrin ligands constitute the largest subfamily of receptor tyrosine kinase (RTK), which were first discovered in tumors. Heretofore, Eph protein has been shown to be involved in various tumor biological behaviors including proliferation and progression. The occurrence of specific types of tumor is closely related to the virus infection. Virus entry is a complex process characterized by a series of events. The entry into target cells is an essential step for virus to cause diseases, which requires the fusion of the viral envelope and host cellular membrane mediated by viral glycoproteins and cellular receptors. Integrin molecules are well known as entry receptors for most herpes viruses. However, in recent years, Eph receptors and their Ephrin ligands have been reported to be involved in virus infections. The main mechanism may be the interaction between Eph receptors and conserved viral surface glycoprotein, such as the gH/gL or gB protein of the herpesviridae. This review focuses on the relationship between Eph receptor family and virus infection that summarize the processes of viruses such as EBV, KSHV, HCV, RRV, etc., infecting target cells through Eph receptors and activating its downstream signaling pathways resulting in malignancies. Finally, we discussed the perspectives to block virus infection, prevention, and treatment of viral-related tumors via Eph receptor family.
Keywords: Eph receptor, Virus infection, Virus-associated cancer, EBV, KSHV
Introduction
Eph (erythropoietin-producing hepatoma) is a big family of receptor tyrosine kinases and plays key roles in physiological and pathological processes in development and disease [1–3]. A total of 14 Eph receptors have been found in humans, which can be subdivided into two subfamilies including EphA and EphB (Fig. 1) based on amino acid sequence homology and relative binding affinities to glycosylphosphatidylinositol (GPI) linked Ephrin-A or transmembrane Ephrin-B ligands [4, 5]. There are nine EphA receptors, which promiscuously bind five Ephrin-A ligands, and five EphB receptors, which promiscuously bind three Ephrin-B ligands [6]. Given Eph receptors and their ligands are often overexpressed in human malignancies and associated with poor prognosis, Eph receptors and Ephrins are considered as very promising drug targets [7, 8].
Fig. 1.
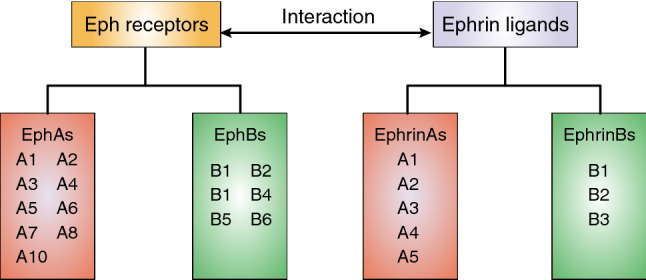
Members of Eph family. In the human genome, there are totally nine EphA and five EphB receptors. The EphA receptors promiscuously bind five glycosylphosphatidylinositol (GPI) linked Ephrin-A ligands, and the EphB receptors promiscuously bind three transmembrane Ephrin-B ligands
Virus infection is closely related to the occurrence and development of many diseases. In recent years, many studies have identified the relationship between virus infection and tumors. Well-known virus-related tumors include: (1) EBV-positive lymphoma, nasopharyngeal carcinoma, and gastric cancer [9–11], (2) Kaposi’s sarcoma-associated herpesvirus (KSHV) in Kaposi’s sarcoma (KS) [12], primary effusion lymphoma (PEL) [13], and multicentric castleman’s disease (MCD) [14], (3) HBV and HCV in liver cancer, etc. [15].
Virus infection of the host involves a complex multi-step process. The first step is viral attachment and entry through interaction between viral glycoprotein and receptors on the surface of the host. For example, EBV-infecting epithelial cells mainly rely on the interaction of gH/gL glycoproteins with host surface integrin receptors (αvβ5, αvβ6, αvβ8) [16–18]. KSHV interacts with integrin receptors (α3β1, αvβ3, α5β5, α9β1) on the surface of epithelial cells and fibroblasts through the encoded gB glycoprotein to facilitate its entry [19]. In addition, HCV entry into the target cells is mediated through binding of HCV envelope glycoproteins to glycosaminoglycans involving viral envelope glycoproteins as well as several cellular attachments and entry factors [20, 21] including CD81 [22], scavenger receptor class B type I (SRBI) [23], claudin1(CLDN1) [24], and occludin (OCLN) [25].
Integrin family is well known as an entry receptor for most herpes viruses. However, in recent years, some studies have reported that the Eph receptors family can also act as an entry receptor-mediating infection of pathogenic microorganisms. Given the tyrosinase properties of Eph receptors, there have been a large number of small-molecule inhibitors targeting Eph receptors, which provide a valuable opportunity for the treatment and prevention of Eph receptor-associated virus infection. In this review, we focus on the relationship between Eph receptor and virus infection and discuss the possibility of targeting Eph receptor signaling pathways as alternative antivirus therapeutic strategies.
Structure and function of Eph family
Structure of Eph and Ephrin families
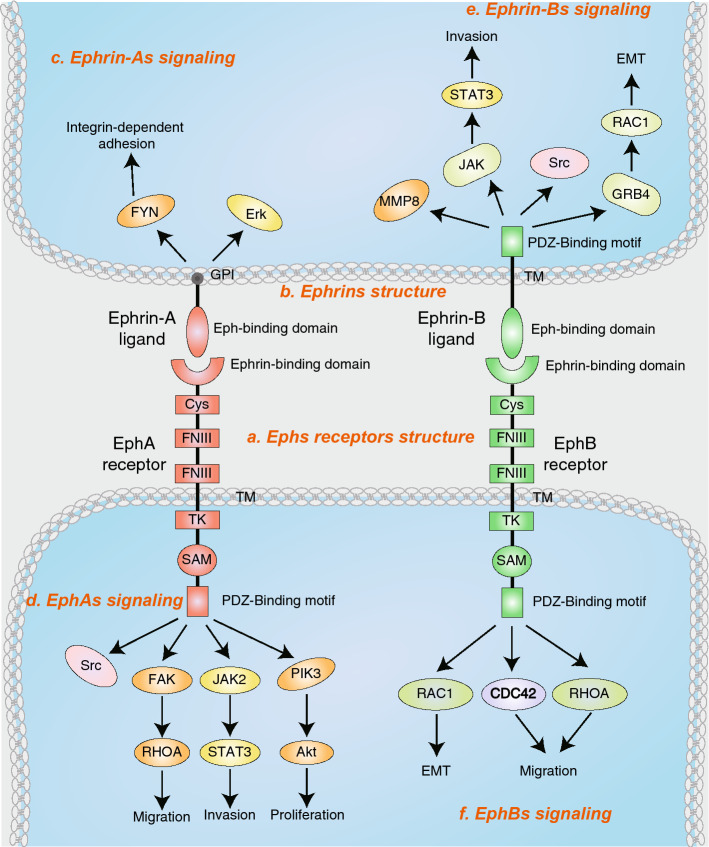
The Eph receptor consists of three parts [6]: (1) extracellular domain, including a ligand-binding domain, a cysteine-rich domain, and two fibronectin type III repeats, (2) transmembrane domain, (3) intracellular domain, consisting of a juxtamembrane region, a tyrosine kinase domain, a sterile alpha motif (SAM), and a C-terminal PSD95/discs large/zona occludens 1 protein (PDZ)-binding motif. The Ephrin-A ligands, unlike the Eph receptor, have no intracellular domain and are anchored on the membrane by the glycosyl lipoinositol (GPI) group. Ephrin-B ligands have a hydrophobic transmembrane region and a short intracellular region (Fig. 2).
Fig. 2.
Domain structure and signaling concepts of Ephs and Ephrins. a Eph receptors (Ephs) consist of a ligand-binding domain (LBD), cysteine-rich region (Cys), two fibronectin III repeat (FNIII), a transmembrane region (TM), a juxtamembrane region (JM), a tyrosine kinase domain (TK), a sterile alpha motif (SAM) a PSD-95/Dlg/ZO-1. GPI and glycosylphosphatidylinositol binding motif (PDZ). b Ephrin-As are linked to the membrane via a glycosylphosphatidylinositol (GPI) moiety, Ephrin-Bs are anchored by a transmembrane domain and contain a cytoplasmic tail. c Ephrin-A signaling promotes activation of FYN, and Erk. d EphA receptors directly activate Src and RHOA through focal adhesion kinase (FAK). EphA receptors activate JAK2 by STAT3 (signal transducer and activator of transcription 3). EphA2 activates Akt in pancreatic cancer cells. e Ephrin-Bs promote EMT and invasion by activating Src, STAT3, MMP8 (matrix metalloproteinase 8), and RAC1. f EphBs activate RHOA, RAC1, and CDC42 which promote cancer cell migration and invasion
Signaling modes of Ephs and Ephrins
Binding of Eph receptor to Ephrin proteins in adjacent cells produces cell-dependent bi-directional signaling that regulates cell shape, movement, survival, and proliferation [3, 7, 8]. The Eph forward signaling is dependent on binding to the ephrin proteins, which can undergo clustering, auto-phosphorylation, and activation of kinase activity. Many studies have reported that Eph receptor’s forward signaling could activate Src, RHOA, RAC1, CDC42, STAT3 (signal transducer and activator of transcription 3), and PIK3/Akt in a variety of tumors which promote cancer-cell migration and invasion [26–31]. In addition, binding of Eph receptors to Ephrin proteins can also lead to endocytosis and proteolysis [8, 32–35]. The Ephrin reverse signaling is activated by interacting with the Eph receptor. Ephrin-As transduct signaling via glycosylphosphatidylinositol groups interacting with transmembrane partners. However, signaling transduction of Ephrin-Bs is involved in tyrosine and serine phosphorylation by associating transmembrane structure of Ephrin-Bs with various effector proteins [8, 36]. Some studies have reported that Ephrin reverse signaling could promote EMT and invasion in a variety of tumor cells by activating Src, STAT3, MMP8 (matrix metalloproteinase 8), and RAC1 [30]. Given the involvement of Eph in multiple life processes and their roles in cancer progression, researchers have conducted intensive research on the function of the Eph family over the past few decades. Moreover, several studies have shown that Eph family is closely related to virus infection in recent years (Table 1).
Table 1.
Ephs involved in virus entry
| Virus | Entry receptors | Binding virus glycoprotein | Target cells | Refs. |
|---|---|---|---|---|
| EBV | EphA2 | gH/gL and gB | Human gastric adenocarcinoma cell (AGS), human embryonic kidney epithelial cells (HEK293) | [66, 67] |
| KSHV | EphA2 | gH/gL | Human embryonic kidney epithelial cells (HEK293T) | [81, 85] |
| HCV | EphA2 | E2 (HCV envelope glycoprotein)a | Human hepatocarcinoma cell (Huh7.5.1) | [93] |
| RRV | EphA4, EphA5, EphA7, EphB2, EphB3 | gH/gL | B cells and endothelial cells | [102, 104] |
aNot confirmed
Function of Ephs and Ephrins in malignancies
Many studies have verified Ephs and Ephrins, aberrantly expressed in tumors which can drastically affect malignancy including progression, metastatic spread, and patient survival [30, 37]. Ephs and Ephrins expression can increase or decrease during cancer progression caused due to transcriptional regulation by oncogenic signaling pathways, promoter methylation, and microRNAs [38, 39]. EphA2, EphB2, and EphB4 are the Eph receptors that most widely deregulated expression in tumors [40–42]. EphA2 is frequently overexpressed in melanoma, glioma, breast cancer, prostate cancer, lung cancer, cervical cancer, colon cancer, esophageal cancer, gastric cancer, ovarian cancer, bladder cancer, and renal-cell carcinomas [42, 43]. Many studies have shown that overexpression of EphA2 is closely related to the activation of some tumor-associated signaling pathways, such as Wnt/β-catenin pathway, Ras/MAPK pathway, and Akt/mTOR pathway [44–46]. Some mutations of Eph receptors are predicted to play a vital role in cancer progression. For example, EphB2 mutations have been identified in human gastric, colorectal, and prostate tumors, some of which can impair kinase function [47–50]. Furthermore, EphB2 has been shown to be upregulated in glioblastoma as a consequence of decreased miR-204, which led promote invasiveness [51, 52]. Another Eph receptor, EphB4, the interaction partners of Ephrin-B2, is a prominent marker of normal and tumor vasculature [53]. In addition, EphB4 can trigger RAS–MAPK-dependent proliferation of MCF7 cancer cells which are considered to be prognostic for poor survival of patients with breast cancer [54, 55].
Role of Eph family in virus infection
EphA2 is an EBV entry receptor
EBV is a ubiquitous human gamma-herpes virus that is present in over 90% of adults worldwide. EBV is closely related to nasopharyngeal carcinoma, 10% of gastric cancer, and various B-cell lymphomas. EBV is associated with B cells and epithelial malignancies, indicating that its main tropism is the infection of B cells and epithelial cells [56]. Human B cells are the primary target of EBV infection. The mechanisms of EBV entering B cells have been well documented. EBV infects B cells requiring interaction between its glycoproteins gp350 or splicing mutant gp220 and the cell surface receptor CD21, and interaction of viral glycoproteins gp42 with MHC II [57, 58]. It is generally believed that activated-gp42 is able to transduce signals to gH/gL and gB to prevent it from interacting with other cellular receptors, including integrin receptors. Therefore, gp42 is considered to be indispensable for EBV infecting B cells specifically [59–61].
Unlike B cells, EBV entry into epithelial cells is independent of gp350/gp220 and gp42, while glycoproteins gH/gL and gB are required for EBV infection of epithelial cells [62–65]. Studies from Chesnokova et al. have shown that the interaction of glycoprotein gH/gL with three epithelial integrin receptors (αvβ5, αvβ6, αvβ8) is associated with EBV entry into epithelial cells [16, 18]. Recently, Zhang et al. found that Ephrin receptor A2 (EphA2) was associated with EBV entry into epithelial cells through microarray and RNAi library screening. Knockdown of EphA2 by siRNA or CRISPR-Cas9 can significantly reduce EBV infection of epithelial cells, while overexpression of EphA2 can restore the EBV infection of epithelial cells [66]. Further studies found that the interaction of EphA2 and EBV-encoded proteins gH/gL and gB can promote the fusion and internalization of EBV, and the Ephrin ligand binding domain and fibronectin domain of EphA2 arerequired for EphA2-mediated EBV infection. These results indicate that EphA2 is critical for EBV entry into epithelial cells [66]. Chen et al. also found that EphA2, but not the extracellular region of EphA4, interacts with EBV-encoded gH/gL to promote EBV fusion and endocytosis [67]. Moreover, they show that the integrin receptors αvβ5, αvβ6, and αvβ8 have no effect on the entry of EBV into epithelial cells, which is distinct from the findings of Chesnokova et al. [67]. The discovery of EphA2 as a novel EBV-infected epithelial cell receptor is of great significance and may uncover new attractive targets, which could be used to develop new intervention strategies for blocking EBV infection.
Although EphA2 is required for EBV infection of epithelial cells, EBV-encoded proteins also regulate the expression of Eph family. Huang et al. found that EphA4 expression was down-regulated in EBV-positive diffuse large B-cell lymphoma and correlated with patient prognosis. Mechanism studies found that down-regulation of EphA4 expression is mainly caused by the regulation of ERK-SP1 signaling pathway by EBV-encoded LMP1 protein. These findings suggest that EphA4 may be a potential therapeutic target for diffuse large B-cell lymphoma [68]. In addition, Zhao et al. reported that there were six hypermethylated genes in EBV-positive gastric cancer cells (AGS-EBV), including EphB6 [69]. In contrast, Xiang et al. found that the expression level of EphA2 was significantly higher in EBV-positive nasopharyngeal carcinoma cells than in EBV-negative nasopharyngeal carcinoma cells (CNE2-EBV vs. CNE2) using RNA-seq analysis. In NPC samples, the upregulation of EphA2 expression in EBV-positive NPC samples was further confirmed. Mechanistically, PI3K/Akt signaling pathway is significantly activated in both xenografts and clinical samples of NPC and EBVaGC [70]. Miao et al. reported that a reciprocal regulatory loop between EphA2 and Akt, which was characterized by unligated EphA2 was a substrate for Akt and negatively regulated by the ligand-activated EphA2 in turn [71]. These findings suggest that EBV may regulate the expression of EphA2 through the AKT signaling pathway. Furthermore, Kim et al. also reported EphA2 and EBV-associated gastric tumor cells were involved in the formation of vasculogenic mimicry (VM) channels [72]. Interestingly, EphA2 expression was not detectable in B-cell lymphoma cell lines (Akata, Akata-EBV and Raji) susceptible to EBV infection. These findings suggest that EphA2 is essential for EBV infection of epithelial cells, but not required for B cell infection [66].
EphA2 is a KSHV entry receptor
Kaposi’s sarcoma-associated herpesvirus (KSHV) or human herpesvirus-8 (HHV-8) was first isolated from patients with AIDS-related Kaposi’s sarcoma (AIDS-KS) in 1994 by Chang et al. [73]. KSHV is a tumor-associated virus that is the causative agent of Kaposi’s sarcoma (KS), primary effusion lymphoma (PEL), and multicentric castleman’s disease (MCD) [73–75]. The KSHV genome is highly consistent with γ-1 Epstein-Barr virus (EBV), γ-2 herpesvirus saimiri (HVS), and rhesus r virus (RRV). Similar to all members of the herpesvirus family, KSHV has a double-stranded DNA genome (~ 160 kb) packaged in the capsid, and the capsid is surrounded by a lipid envelope containing five conserved glycoproteins [76, 77]. The KSHV genome encodes more than 100 open reading frames (ORFs), of which 4–75 are classified according to their homology to the HVS ORF [78]. KSHV has a wide range of cellular tropism, and it can infect various target cells in vitro and in vivo. KSHV entry and signal transduction are complex events that vary greatly depending on the host cell [79]. Moreover, KSHV can form a variety of different internalization pathways into the host through different combinations of host cell surface receptors [79]. Studies have shown that multiple KSHV glycoproteins are involved in binding to host cell membranes and are involved in interacting with surface receptor of host cells, thereby inducing a cascade of signaling pathways to promote endocytosis. Subsequent steps include fusion of the viral envelope with the endosomal membrane, release of the viral capsid in the cytoplasm, and transport of KSHV DNA into the nucleus. These processes are essential for the virus infection, which relies on intricate intermolecular interactions.
Ephrins have been reported to regulate macropinocytosis and clathrin-dependent endocytosis in a variety of cells [3, 80]. A recent study reported that the interaction of EphA2 and KSHV glycoprotein gH/gL could promote virus entry [81]. Pretreatment of target cells with soluble EphA2 ligand or incubation of KSHV virions with soluble EphA2 protein inhibited KSHV infection [81]. Knockdown of EphA2 significantly reduced the entry of KSHV, while overexpression of EphA2 increased the entry of KSHV [81]. Significantly, examined EphA2 expression in tissue sections from individuals with Kaposi’s sarcoma using quantitative RT-PCR and in situ histochemistry showed a strong correlation between EphA2 expression and KSHV infection both in cultured Kaposi’s sarcoma-derived cells and in Kaposi’s sarcoma tissues [47]. Previous studies have shown that KSHV-induced ERK and NF-κB signaling pathways are essential for the initiation of viral or host gene expression [82–84]. Interestingly, the MAPK pathway can control the expression of the EphA2 receptor [45]. These results explain, at least in part, the reason why the expression of EphA2 is strongly correlated with KSHV infection. Moreover, binding of gH/gL to EphA2 would induce phosphorylation of EphA2 and promote the internalization of KSHV. These findings indicate that EphA2 is a specific entry receptor for KSHV infection [81].
Chakraborty et al. also reported that EphA2 bind to FAK, Src, and other signaling molecules in a lipid-raft to form a signal complex, which could promote KSHV entry into macropinosomes [85]. Another group reported that EphA2 played a crucial role in the coordination and amplification of KSHV-induced signaling in fibroblasts and the virus is endocytosed by the clathrin-mediated endocytic pathway [86]. Interestingly, a recent study found that androgen receptors could also promote KSHV-infected host cells by interacting with EphA2, which revealed why KSHV had a higher infection ratio in male than female [87].
EphA2 is a co-factor for hepatitis C virus entry
HCV was originally isolated from serum in 1989, and its genome is a single-stranded RNA of approximately 9 kb [88]. HCV is the leading cause of cirrhosis and hepatocellular carcinoma. Although newly developed antiviral drugs targeting HCV proteins have been shown to increase virological response, there are still some toxicity and resistance [89]. HCV entry is a multi-step process, which is mainly mediated by viral envelope glycoproteins, adhesion proteins, and entry factors of cell surface [90]. The attachment of the virus to the target cells is mediated by the binding of the HCV envelope glycoprotein to the glycosaminoglycan [91]. The involvement of CD81 is essential for HCV in the process of clathrin-dependent endocytosis [92]. Joachim Lupberger et al. screened EphA2 and epidermal growth factor receptor (EGFR) as co-receptor factors to facilitate HCV entry using RNAi kinase libraries [93]. Small molecule inhibitors targeting tyrosine kinase can significantly attenuate HCV infection (55). Mechanistic studies indicate that EphA2 and EGFR mediate HCV entry by modulating the interaction of the CD81-claudin-1 (CLDN1) co-receptor with viral glycoproteins [93]. EphA2 has been reported highly expressed in human liver. Cui et al. found that EphA2 expression was prominent in highly invasive hepatoma cells, and its overexpression was significantly correlated with decreased differentiation and poor survival for HCC patients [94]. In addition, Lee et al. identified that high expression of EphA2 was related to lymph node metastasis in 32 human hepatocellular carcinoma patients, all of these patients were infected with HCV or HBV [95]. However, direct evidence of whether the malignant phenotype of liver cancer through control the expression of EphA2 caused by HCV was not reported. These results demonstrate that EphA2 can act as new co-receptors for HCV entry and indicate that tyrosine kinase inhibitors have significant antiviral effects. Therefore, inhibition of EphA2 may be a new strategy for preventing and counteracting HCV infection.
EphAs and EphBs are rhesus monkey rhadinovirus (RRV) entry receptors
Rhesus monkey is a primate species that is genetically and physiologically similar to humans. Scientists have decoded the genome of the rhesus monkey and compared it with humans. Nucleotide sequences that aligned between the humans and rhesus monkey averaged at 93.54% identity [96]. The γ-2 herpesvirus is a unique subfamily of the lymphotropic herpesviruses. Rhesus monkey rhadinovirus (RRV) is a natural infectious agent with a high frequency of infection in both feeding and wild rhesus monkey populations [97]. RRV is a rhesus monkey ortholog of the human Kaposi’s sarcoma-associated herpesvirus (KSHV, human herpesvirus-8, HHV-8) [98, 99]. RRV has been reported to be associated with B-cell malignancies and is similar to B-cell malignancies caused by KSHV [100–103]. Hahn et al. reported the gH/gL glycoprotein complex of rhesus monkey rhadinovirus could bind to the virus and mediate its entry into target cells via cellular Ephrin receptor tyrosine kinase proteins [104]. Unlike KSHV, which enters into host cells through EphA2 receptor mainly, RRV can utilize more type A and B Eph receptors as entry receptors (10 of 14 Eph receptors can interact with gH/gL glycoprotein complexes) [104]. Furthermore, RRV entry into B cells and endothelial cells is almost entirely dependent on the Eph receptor pathway, whereas RRV entry into fibroblasts and epithelial cells is via the Eph receptor-independent pathway [100]. Therefore, it suggests that KSHV may also infect host cells through Eph receptor-independent pathways in some cases.
Eph family as an entry receptor for other viruses
Although the Eph family has been widely involved in virus infections such as EBV, KSHV, and HCV, the role of Eph receptors and Ephrins in other virus infections remain to be discovered. For example, Karlas et al. found that EphB6 played an important role in H1N1 influenza virus entry and replication using siRNA library screening in A549 lung cancer cell [105]. Xu et al. reported that the expression patterns of Ephrin-B2 and Ephrin-B3 might be the reason of acute lymphatic necrosis caused by henipavirus infection [106]. Moreover, Ephrin-B2 and Ephrin-B3 promote the entry mechanism of henipavirus mainly by interacting with the viral G protein to activate the viral F protein, thereby inducing fusion of the viral capsid and the host cell membrane [107]. In the study by Dewannieux et al. the mouse IAPE (Intracisternal A-type Particles elements with an Envelope) family was able to utilize five Ephrin-A family members, such as Ephrin-A4 as entry receptor to infect host cell. Interestingly, Ephrin-B family with higher homology to Ephrin-A family do not mediate IAPE entry [108]. In recent years, Eph family has also been reported as entry receptor for other pathogenic microorganisms. Swidergall et al. found that EphA2 could act as a pattern recognition receptor to promote fungal infection of host cells by interacting with fungal surface glycoproteins [109].
Role of Eph family in antiviral therapies
The Eph family has been increasingly recognized as an attractive therapeutic target for many diseases, ranging from anticancer therapeutics to modulators of synaptic plasticity, bone homeostasis, and remodeling and stem cell biology [110–112]. Some therapeutic approaches have been developed to modulate Eph–Ephrin function, including small-molecule kinase inhibitors, Ephrin-mimetic peptides, short hairpin RNAs, and monoclonal antibodies [113–116]. The involvement of Eph family in virus infection provides new strategies for antiviral therapies. Zhang et al. reported that soluble EphA2 protein, antibodies against EphA2, soluble EphA2 ligand Ephrin-A1, or the EphA2 inhibitor 2,5-dimethylpyrrolyl benzoic acid could efficiently block EBV epithelial cell infection [66]. Xu et al. found that Ephrin-B2’s extracellular domain (ECD) and an antibody to the henipavirus, glycoprotein that bind Ephrin-Bs had antiviral activity [106]. In addition, EphA2/Ephrin-A ECDs and EphA2-targeting antibodies have also been successfully used to inhibit KSHV and HCV of cultured cells [81, 85, 93]. These studies demonstrated that suitable targeting of Eph–Ephrin function holds considerable promise for antiviral therapies.
Conclusions
Virus infection is closely related to the occurrence and development of many diseases, posing a great threat to human life and health. Antiviral therapies are currently limited by drug resistance, toxicity, and high cost. Therefore, new antiviral prevention and treatment strategies are imminent. The mechanism by which viral entry involves a range of events, including interactions with target cell surface receptors, endocytosis, and nuclear release (Fig. 3). Host cell surface receptors are key molecules for viral recognition and are involved in linking viruses to host cells. Therefore, there is a significant potential for the preparation of antiviral drugs for specific target cell receptors.
Fig. 3.
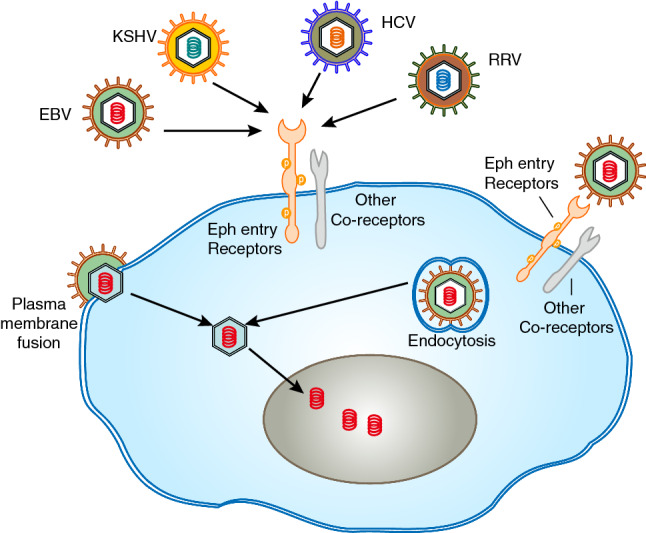
Interaction of virus and Ephs is crucial for viral entry. A generic virus is shown binding to Ephs entry receptors and co-receptors (integrins). The virion interaction with entry receptors triggers specific entry pathways, two examples are shown. Fusion at the plasma membrane mediated by entry receptors (e.g., Epstein–Barr virus infection of epithelial cells). Endocytosis mediated by entry receptors (e.g., Kaposi’s sarcoma-associated herpesvirus infection of multiple cell types)
Current research has found that Eph family is involved in the entry of multiple viruses, and because of the involvement of Eph–Ephrin in many biological processes, there are already a large number of approved targeted inhibitors against the Eph family. These provide a rich opportunity for intervention and treatment for virus infection.
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (81702919, 81802478), Guangxi Natural Science Foundation (2017GXNSFBA198071, 2018GXNSFBA050042), Guangxi Science and Technology Program Project (No. 2018AD19360) and the Applied Basic Research Programs of Science and Technology Commission Foundation of Shanxi Province (Grant No. 201901D211472).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jia Wang and Xiang Zheng have contributed equally to this study.
Contributor Information
Xuemei Zhang, Email: 474044435@qq.com.
Zailong Qin, Email: qinzailong@hotmail.com.
References
- 1.Batlle E, Wilkinson DG. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. J Cold Spring Harb Perspect Biol. 2012;4(1):a008227. doi: 10.1101/cshperspect.a008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. J Cell. 2008;133(1):38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. J Nat Rev Mol Cell Biol. 2005;6(6):462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 4.Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17(1):9–19. doi: 10.1016/S0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 5.Eph Nomenclature Committee Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell. 1997;90(3):403–404. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- 6.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6(6):462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 7.Arvanitis DN, Davy A. Regulation and misregulation of Eph/ephrin expression. J Cell Adh Migr. 2012;6(2):131–137. doi: 10.4161/cam.19690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. J Nat Rev Cancer. 2010;10(3):165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu AD, Lu LX, Xie YB, Chen LZ, Feng QS, Kang T, Jia WH, Zeng YX. Clinical values of multiple Epstein-Barr virus (EBV) serological biomarkers detected by xMAP technology. J J Transl Med. 2009;7:73. doi: 10.1186/1479-5876-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh ST, Kim M, Lee SK. Maintenance of the viral episome is essential for the cell survival of an Epstein-Barr virus positive gastric carcinoma cell line. J Arch Pharm Res. 2009;32(5):729–736. doi: 10.1007/s12272-009-1512-7. [DOI] [PubMed] [Google Scholar]
- 11.Ozyar E, Ayhan A, Korcum AF, Atahan IL. Prognostic role of Ebstein-Barr virus latent membrane protein-1 and interleukin-10 expression in patients with nasopharyngeal carcinoma. J Cancer Invest. 2004;22(4):483–491. doi: 10.1081/CNV-200026386. [DOI] [PubMed] [Google Scholar]
- 12.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266(5192):1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 13.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332(18):1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 14.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay MF, Clauvel JP, Raphael M, Degos L, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86(4):1276–1280. doi: 10.1182/blood.V86.4.1276.bloodjournal8641276. [DOI] [PubMed] [Google Scholar]
- 15.Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. J Nat Rev Cancer. 2013;13(2):123–135. doi: 10.1038/nrc3449. [DOI] [PubMed] [Google Scholar]
- 16.Chesnokova LS, Hutt-Fletcher LM. Fusion of Epstein-Barr virus with epithelial cells can be triggered by alphavbeta5 in addition to alphavbeta6 and alphavbeta8, and integrin binding triggers a conformational change in glycoproteins gHgL. J J Virol. 2011;85(24):13214–13223. doi: 10.1128/jvi.05580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chesnokova LS, Jiang R, Hutt-Fletcher LM. Viral entry. J Curr Top Microbiol Immunol. 2015;391:221–235. doi: 10.1007/978-3-319-22834-1_7. [DOI] [PubMed] [Google Scholar]
- 18.Chesnokova LS, Nishimura SL, Hutt-Fletcher LM. Fusion of epithelial cells by Epstein–Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. J Proc Natl Acad Sci USA. 2009;106(48):20464–20469. doi: 10.1073/pnas.0907508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veettil MV, Bandyopadhyay C, Dutta D, Chandran B. Interaction of KSHV with host cell surface receptors and cell entry. J Viruses. 2014;6(10):4024–4046. doi: 10.3390/v6104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Hahn T, Rice CM. Hepatitis C virus entry. J Biol Chem. 2008;283(7):3689–3693. doi: 10.1074/jbc.R700024200. [DOI] [PubMed] [Google Scholar]
- 21.Barth H, Schnober EK, Zhang F, Linhardt RJ, Depla E, Boson B, Cosset FL, Patel AH, Blum HE, Baumert TF. Viral and cellular determinants of the hepatitis C virus envelope-heparan sulfate interaction. J Virol. 2006;80(21):10579–10590. doi: 10.1128/JVI.00941-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282(5390):938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 23.Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G, Traboni C, Nicosia A, Cortese R, Vitelli A. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21(19):5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446(7137):801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 25.Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, Rice CM. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457(7231):882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12(1):15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- 27.Fang WB, Ireton RC, Zhuang G, Takahashi T, Reynolds A, Chen J. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci. 2008;121(Pt 3):358–368. doi: 10.1242/jcs.017145. [DOI] [PubMed] [Google Scholar]
- 28.Lai KO, Chen Y, Po HM, Lok KC, Gong K, Ip NY. Identification of the Jak/Stat proteins as novel downstream targets of EphA4 signaling in muscle: implications in the regulation of acetylcholinesterase expression. J Biol Chem. 2004;279(14):13383–13392. doi: 10.1074/jbc.M313356200. [DOI] [PubMed] [Google Scholar]
- 29.Chang Q, Jorgensen C, Pawson T, Hedley DW. Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer. Br J Cancer. 2008;99(7):1074–1082. doi: 10.1038/sj.bjc.6604676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10(3):165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakada M, Niska JA, Tran NL, McDonough WS, Berens ME. EphB2/R-Ras signaling regulates glioma cell adhesion, growth, and invasion. Am J Pathol. 2005;167(2):565–576. doi: 10.1016/S0002-9440(10)62998-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binda E, Visioli A, Giani F, Lamorte G, Copetti M, Pitter KL, Huse JT, Cajola L, Zanetti N, DiMeco F, De Filippis L, Mangiola A, Maira G, Anile C, De Bonis P, Reynolds BA, Pasquale EB, Vescovi AL. The EphA2 receptor drives self-renewal and tumorigenicity in stem-like tumor-propagating cells from human glioblastomas. J Cancer Cell. 2012;22(6):765–780. doi: 10.1016/j.ccr.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi KM, Park GL, Hwang KY, Lee JW, Ahn HJ. Efficient siRNA delivery into tumor cells by p19-YSA fusion protein. J Mol Pharm. 2013;10(2):763–773. doi: 10.1021/mp300344p. [DOI] [PubMed] [Google Scholar]
- 34.Hwang YS, Lee HS, Kamata T, Mood K, Cho HJ, Winterbottom E, Ji YJ, Singh A, Daar IO. The Smurf ubiquitin ligases regulate tissue separation via antagonistic interactions with ephrinB1. J Genes Dev. 2013;27(5):491–503. doi: 10.1101/gad.208355.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lisabeth EM, Falivelli G, Pasquale EB. Eph receptor signaling and ephrins. J Cold Spring Harb Perspect Biol. 2013;5(9):a009159. doi: 10.1101/cshperspect.a009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daar IO. Non-SH2/PDZ reverse signaling by ephrins. J Semin Cell Dev Biol. 2012;23(1):65–74. doi: 10.1016/j.semcdb.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miao H, Wang B. EphA receptor signaling–complexity and emerging themes. Semin Cell Dev Biol. 2012;23(1):16–25. doi: 10.1016/j.semcdb.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arvanitis DN, Davy A. Regulation and misregulation of Eph/ephrin expression. Cell Adh Migr. 2012;6(2):131–137. doi: 10.4161/cam.19690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishimura M, Jung EJ, Shah MY, Lu C, Spizzo R, Shimizu M, Han HD, Ivan C, Rossi S, Zhang X, Nicoloso MS, Wu SY, Almeida MI, Bottsford-Miller J, Pecot CV, Zand B, Matsuo K, Shahzad MM, Jennings NB, Rodriguez-Aguayo C, Lopez-Berestein G, Sood AK, Calin GA. Therapeutic synergy between microRNA and siRNA in ovarian cancer treatment. Cancer Discov. 2013;3(11):1302–1315. doi: 10.1158/2159-8290.CD-13-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merlos-Suarez A, Batlle E. Eph-ephrin signalling in adult tissues and cancer. Curr Opin Cell Biol. 2008;20(2):194–200. doi: 10.1016/j.ceb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Noren NK, Pasquale EB. Paradoxes of the EphB4 receptor in cancer. Cancer Res. 2007;67(9):3994–3997. doi: 10.1158/0008-5472.CAN-07-0525. [DOI] [PubMed] [Google Scholar]
- 42.Wykosky J, Debinski W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res. 2008;6(12):1795–1806. doi: 10.1158/1541-7786.MCR-08-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ireton RC, Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr Cancer Drug Targets. 2005;5(3):149–157. doi: 10.2174/1568009053765780. [DOI] [PubMed] [Google Scholar]
- 44.Peng Q, Chen L, Wu W, Wang J, Zheng X, Chen Z, Jiang Q, Han J, Wei L, Wang L, Huang J, Ma J. EPH receptor A2 governs a feedback loop that activates Wnt/beta-catenin signaling in gastric cancer. Cell Death Dis. 2018;9(12):1146. doi: 10.1038/s41419-018-1164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macrae M, Neve RM, Rodriguez-Viciana P, Haqq C, Yeh J, Chen C, Gray JW, McCormick F. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell. 2005;8(2):111–118. doi: 10.1016/j.ccr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Yang NY, Fernandez C, Richter M, Xiao Z, Valencia F, Tice DA, Pasquale EB. Crosstalk of the EphA2 receptor with a serine/threonine phosphatase suppresses the Akt-mTORC1 pathway in cancer cells. Cell Signal. 2011;23(1):201–212. doi: 10.1016/j.cellsig.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alazzouzi H, Davalos V, Kokko A, Domingo E, Woerner SM, Wilson AJ, Konrad L, Laiho P, Espin E, Armengol M, Imai K, Yamamoto H, Mariadason JM, Gebert JF, Aaltonen LA, Schwartz S, Jr, Arango D. Mechanisms of inactivation of the receptor tyrosine kinase EPHB2 in colorectal tumors. Cancer Res. 2005;65(22):10170–10173. doi: 10.1158/0008-5472.CAN-05-2580. [DOI] [PubMed] [Google Scholar]
- 48.Huusko P, Ponciano-Jackson D, Wolf M, Kiefer JA, Azorsa DO, Tuzmen S, Weaver D, Robbins C, Moses T, Allinen M, Hautaniemi S, Chen Y, Elkahloun A, Basik M, Bova GS, Bubendorf L, Lugli A, Sauter G, Schleutker J, Ozcelik H, Elowe S, Pawson T, Trent JM, Carpten JD, Kallioniemi OP, Mousses S. Nonsense-mediated decay microarray analysis identifies mutations of EPHB2 in human prostate cancer. Nat Genet. 2004;36(9):979–983. doi: 10.1038/ng1408. [DOI] [PubMed] [Google Scholar]
- 49.Davalos V, Dopeso H, Velho S, Ferreira AM, Cirnes L, Diaz-Chico N, Bilbao C, Ramirez R, Rodriguez G, Falcon O, Leon L, Niessen RC, Keller G, Dallenbach-Hellweg G, Espin E, Armengol M, Plaja A, Perucho M, Imai K, Yamamoto H, Gebert JF, Diaz-Chico JC, Hofstra RM, Woerner SM, Seruca R, Schwartz S, Jr, Arango D. High EPHB2 mutation rate in gastric but not endometrial tumors with microsatellite instability. Oncogene. 2007;26(2):308–311. doi: 10.1038/sj.onc.1209780. [DOI] [PubMed] [Google Scholar]
- 50.Zogopoulos G, Jorgensen C, Bacani J, Montpetit A, Lepage P, Ferretti V, Chad L, Selvarajah S, Zanke B, Hudson TJ, Pawson T, Gallinger S. Germline EPHB2 receptor variants in familial colorectal cancer. PLoS ONE. 2008;3(8):e2885. doi: 10.1371/journal.pone.0002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ying Z, Li Y, Wu J, Zhu X, Yang Y, Tian H, Li W, Hu B, Cheng SY, Li M. Loss of miR-204 expression enhances glioma migration and stem cell-like phenotype. Cancer Res. 2013;73(2):990–999. doi: 10.1158/0008-5472.CAN-12-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang SD, Rath P, Lal B, Richard JP, Li Y, Goodwin CR, Laterra J, Xia S. EphB2 receptor controls proliferation/migration dichotomy of glioblastoma by interacting with focal adhesion kinase. Oncogene. 2012;31(50):5132–5143. doi: 10.1038/onc.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salvucci O, Tosato G. Essential roles of EphB receptors and EphrinB ligands in endothelial cell function and angiogenesis. Adv Cancer Res. 2012;114:21–57. doi: 10.1016/B978-0-12-386503-8.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brantley-Sieders DM, Jiang A, Sarma K, Badu-Nkansah A, Walter DL, Shyr Y, Chen J. Eph/ephrin profiling in human breast cancer reveals significant associations between expression level and clinical outcome. PLoS ONE. 2011;6(9):e24426. doi: 10.1371/journal.pone.0024426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao Z, Carrasco R, Kinneer K, Sabol D, Jallal B, Coats S, Tice DA. EphB4 promotes or suppresses Ras/MEK/ERK pathway in a context-dependent manner: Implications for EphB4 as a cancer target. Cancer Biol Ther. 2012;13(8):630–637. doi: 10.4161/cbt.20080. [DOI] [PubMed] [Google Scholar]
- 56.Fingeroth JD, Weis JJ, Tedder TF, Strominger JL, Biro PA, Fearon DT. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. J Proc Natl Acad Sci USA. 1984;81(14):4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mullen MM, Haan KM, Longnecker R, Jardetzky TS. Structure of the Epstein-Barr virus gp42 protein bound to the MHC class II receptor HLA-DR1. J Mol Cell. 2002;9(2):375–385. doi: 10.1016/S1097-2765(02)00465-3. [DOI] [PubMed] [Google Scholar]
- 58.Nemerow GR, Mold C, Schwend VK, Tollefson V, Cooper NR. Identification of gp350 as the viral glycoprotein mediating attachment of Epstein-Barr virus (EBV) to the EBV/C3d receptor of B cells: sequence homology of gp350 and C3 complement fragment C3d. J J Virol. 1987;61(5):1416–1420. doi: 10.1128/JVI.61.5.1416-1420.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. Fusing structure and function: a structural view of the herpesvirus entry machinery. J Nat Rev Microbiol. 2011;9(5):369–381. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ressing ME, van Leeuwen D, Verreck FA, Gomez R, Heemskerk B, Toebes M, Mullen MM, Jardetzky TS, Longnecker R, Schilham MW, Ottenhoff TH, Neefjes J, Schumacher TN, Hutt-Fletcher LM, Wiertz EJ. Interference with T cell receptor-HLA-DR interactions by Epstein-Barr virus gp42 results in reduced T helper cell recognition. J Proc Natl Acad Sci USA. 2003;100(20):11583–11588. doi: 10.1073/pnas.2034960100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sathiyamoorthy K, Jiang J, Hu YX, Rowe CL, Mohl BS, Chen J, Jiang W, Mellins ED, Longnecker R, Zhou ZH, Jardetzky TS. Assembly and architecture of the EBV B cell entry triggering complex. J PLoS Pathog. 2014;10(8):e1004309. doi: 10.1371/journal.ppat.1004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. J Nat Med. 2002;8(6):594–599. doi: 10.1038/nm0602-594. [DOI] [PubMed] [Google Scholar]
- 63.Kirschner AN, Omerovic J, Popov B, Longnecker R, Jardetzky TS. Soluble Epstein-Barr virus glycoproteins gH, gL, and gp42 form a 1:1:1 stable complex that acts like soluble gp42 in B-cell fusion but not in epithelial cell fusion. J J Virol. 2006;80(19):9444–9454. doi: 10.1128/jvi.00572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turk SM, Jiang R, Chesnokova LS, Hutt-Fletcher LM. Antibodies to gp350/220 enhance the ability of Epstein-Barr virus to infect epithelial cells. J J Virol. 2006;80(19):9628–9633. doi: 10.1128/jvi.00622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, Kenyon WJ, Li Q, Mullberg J, Hutt-Fletcher LM. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J J Virol. 1998;72(7):5552–5558. doi: 10.1128/JVI.72.7.5552-5558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H, Li Y, Wang HB, Zhang A, Chen ML, Fang ZX, Dong XD, Li SB, Du Y, Xiong D, He JY, Li MZ, Liu YM, Zhou AJ, Zhong Q, Zeng YX, Kieff E, Zhang Z, Gewurz BE, Zhao B, Zeng MS. Ephrin receptor A2 is an epithelial cell receptor for Epstein-Barr virus entry. J Nat Microbiol. 2018;3(2):164–171. doi: 10.1038/s41564-017-0080-8. [DOI] [PubMed] [Google Scholar]
- 67.Chen J, Sathiyamoorthy K, Zhang X, Schaller S, Perez WB, Jardetzky TS, Longnecker R. Ephrin receptor A2 is a functional entry receptor for Epstein-Barr virus. J Nat Microbiol. 2018;3(2):172–180. doi: 10.1038/s41564-017-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang YC, Lin SJ, Lin KM, Chou YC, Lin CW, Yu SC, Chen CL, Shen TL, Chen CK, Lu J, Chen MR, Tsai CH. Regulation of EBV LMP1-triggered EphA4 downregulation in EBV-associated B lymphoma and its impact on patients’ survival. J Blood. 2016;128(12):1578–1589. doi: 10.1182/blood-2016-02-702530. [DOI] [PubMed] [Google Scholar]
- 69.Zhao J, Liang Q, Cheung KF, Kang W, Lung RW, Tong JH, To KF, Sung JJ, Yu J. Genome-wide identification of Epstein-Barr virus-driven promoter methylation profiles of human genes in gastric cancer cells. J Cancer. 2013;119(2):304–312. doi: 10.1002/cncr.27724. [DOI] [PubMed] [Google Scholar]
- 70.Xiang T, Lin YX, Ma W, Zhang HJ, Chen KM, He GP, Zhang X, Xu M, Feng QS, Chen MY, Zeng MS, Zeng YX, Feng L. Vasculogenic mimicry formation in EBV-associated epithelial malignancies. Nat Commun. 2018;9(1):5009. doi: 10.1038/s41467-018-07308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, Sloan AE, Cohen ML, Wang B. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell. 2009;16(1):9–20. doi: 10.1016/j.ccr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim HS, Won YJ, Shim JH, Kim HJ, Kim J, Hong HN, Kim BS. Morphological characteristics of vasculogenic mimicry and its correlation with EphA2 expression in gastric adenocarcinoma. Sci Rep. 2019;9(1):3414. doi: 10.1038/s41598-019-40265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. J Sci. 1994;266(5192):1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 74.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. J N Engl J Med. 1995;332(18):1186–1191. doi: 10.1056/nejm199505043321802. [DOI] [PubMed] [Google Scholar]
- 75.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, D'Agay MF, Clauvel JP, Raphael M, Degos L, Et A. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. J Blood. 1995;86(4):1276–1280. doi: 10.1182/blood.V86.4.1276.bloodjournal8641276. [DOI] [PubMed] [Google Scholar]
- 76.Neipel F, Albrecht JC, Fleckenstein B. Human herpesvirus 8–the first human Rhadinovirus. J J Natl Cancer Inst Monogr. 1998;23:73–77. doi: 10.1093/oxfordjournals.jncimonographs.a024178. [DOI] [PubMed] [Google Scholar]
- 77.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. J Nat Med. 1996;2(3):342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 78.Cai Q, Verma SC, Lu J, Robertson ES. Molecular biology of Kaposi's sarcoma-associated herpesvirus and related oncogenesis. J Adv Virus Res. 2010;78:87–142. doi: 10.1016/B978-0-12-385032-4.00003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chandran B. Early events in Kaposi’s sarcoma-associated herpesvirus infection of target cells. J J Virol. 2010;84(5):2188–2199. doi: 10.1128/jvi.01334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Himanen JP, Saha N, Nikolov DB. Cell-cell signaling via Eph receptors and ephrins. J Curr Opin Cell Biol. 2007;19(5):534–542. doi: 10.1016/j.ceb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hahn AS, Kaufmann JK, Wies E, Naschberger E, Panteleev-Ivlev J, Schmidt K, Holzer A, Schmidt M, Chen J, Konig S, Ensser A, Myoung J, Brockmeyer NH, Sturzl M, Fleckenstein B, Neipel F. The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus. J Nat Med. 2012;18(6):961–966. doi: 10.1038/nm.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naranatt PP, Akula SM, Zien CA, Krishnan HH, Chandran B. Kaposi’s sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-zeta-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J Virol. 2003;77(2):1524–1539. doi: 10.1128/jvi.77.2.1524-1539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi’s sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner. J Virol. 2006;80(23):11432–11446. doi: 10.1128/JVI.01342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sadagopan S, Sharma-Walia N, Veettil MV, Raghu H, Sivakumar R, Bottero V, Chandran B. Kaposi’s sarcoma-associated herpesvirus induces sustained NF-kappaB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression. J Virol. 2007;81(8):3949–3968. doi: 10.1128/JVI.02333-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chakraborty S, Veettil MV, Bottero V, Chandran B. Kaposi’s sarcoma-associated herpesvirus interacts with EphrinA2 receptor to amplify signaling essential for productive infection. J Proc Natl Acad Sci USA. 2012;109(19):E1163–1172. doi: 10.1073/pnas.1119592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dutta D, Chakraborty S, Bandyopadhyay C, Valiya VM, Ansari MA, Singh VV, Chandran B. EphrinA2 regulates clathrin mediated KSHV endocytosis in fibroblast cells by coordinating integrin-associated signaling and c-Cbl directed polyubiquitination. J PLoS Pathog. 2013;9(7):e1003510. doi: 10.1371/journal.ppat.1003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Zou Z, Deng Z, Liang D, Zhou X, Sun R, Lan K. Male hormones activate EphA2 to facilitate Kaposi’s sarcoma-associated herpesvirus infection: implications for gender disparity in Kaposi’s sarcoma. J PLoS Pathog. 2017;13(9):e1006580. doi: 10.1371/journal.ppat.1006580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. J Sci. 1989;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 89.Kao JH. Telaprevir for chronic HCV infection. J N Engl J Med. 2009;361(5):534. [PubMed] [Google Scholar]
- 90.von Hahn T, Rice CM. Hepatitis C virus entry. J J Biol Chem. 2008;283(7):3689–3693. doi: 10.1074/jbc.R700024200. [DOI] [PubMed] [Google Scholar]
- 91.Barth H, Schnober EK, Zhang F, Linhardt RJ, Depla E, Boson B, Cosset FL, Patel AH, Blum HE, Baumert TF. Viral and cellular determinants of the hepatitis C virus envelope-heparan sulfate interaction. J J Virol. 2006;80(21):10579–10590. doi: 10.1128/jvi.00941-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. J Sci. 1998;282(5390):938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 93.Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S, Royer C, Fischer B, Zahid MN, Lavillette D, Fresquet J, Cosset FL, Rothenberg SM, Pietschmann T, Patel AH, Pessaux P, Doffoel M, Raffelsberger W, Poch O, McKeating JA, Brino L, Baumert TF. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. J Nat Med. 2011;17(5):589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cui XD, Lee MJ, Yu GR, Kim IH, Yu HC, Song EY, Kim DG. EFNA1 ligand and its receptor EphA2: potential biomarkers for hepatocellular carcinoma. Int J Cancer. 2010;126(4):940–949. doi: 10.1002/ijc.24798. [DOI] [PubMed] [Google Scholar]
- 95.Lee CF, Ling ZQ, Zhao T, Fang SH, Chang WC, Lee SC, Lee KR. Genomic-wide analysis of lymphatic metastasis-associated genes in human hepatocellular carcinoma. World J Gastroenterol. 2009;15(3):356–365. doi: 10.3748/wjg.15.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rhesus Macaque Genome S, Analysis C, Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu YS, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers YH, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang SP, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csuros M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit AF, Ullmer B, Wang H, Xing J, Burhans R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas LJ, Han SG, Hopkins J, Karimpour-Fard A, Kim YH, Pollack JR, Vinar T, Addo-Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani K, Kehrer-Sawatzki H, Kolb J, Patil S, Pu LL, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O'Brien WE, Prufer K, Stenson PD, Wallace JC, Ke H, Liu XM, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE, Zwieg AS. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316(5822):222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 97.Desrosiers RC, Sasseville VG, Czajak SC, Zhang X, Mansfield KG, Kaur A, Johnson RP, Lackner AA, Jung JU. A herpesvirus of rhesus monkeys related to the human Kaposi’s sarcoma-associated herpesvirus. J J Virol. 1997;71(12):9764–9769. doi: 10.1128/JVI.71.12.9764-9769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alexander L, Denekamp L, Knapp A, Auerbach MR, Damania B, Desrosiers RC. The primary sequence of rhesus monkey rhadinovirus isolate 26–95: sequence similarities to Kaposi’s sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J J Virol. 2000;74(7):3388–3398. doi: 10.1128/JVI.74.7.3388-3398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Searles RP, Bergquam EP, Axthelm MK, Wong SW. Sequence and genomic analysis of a Rhesus macaque rhadinovirus with similarity to Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8. J J Virol. 1999;73(4):3040–3053. doi: 10.1128/JVI.73.4.3040-3053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bruce AG, Bielefeldt-Ohmann H, Barcy S, Bakke AM, Lewis P, Tsai CC, Murnane RD, Rose TM. Macaque homologs of EBV and KSHV show uniquely different associations with simian AIDS-related lymphomas. J PLoS Pathog. 2012;8(10):e1002962. doi: 10.1371/journal.ppat.1002962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Orzechowska BU, Powers MF, Sprague J, Li H, Yen B, Searles RP, Axthelm MK, Wong SW. Rhesus macaque rhadinovirus-associated non-Hodgkin lymphoma: animal model for KSHV-associated malignancies. J Blood. 2008;112(10):4227–4234. doi: 10.1182/blood-2008-04-151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wong SW, Bergquam EP, Swanson RM, Lee FW, Shiigi SM, Avery NA, Fanton JW, Axthelm MK. Induction of B cell hyperplasia in simian immunodeficiency virus-infected rhesus macaques with the simian homologue of Kaposi’s sarcoma-associated herpesvirus. J J Exp Med. 1999;190(6):827–840. doi: 10.1084/jem.190.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Großkopf AK, Schlagowski S, Hörnich BF, Fricke T, Desrosiers RC, Hahn AS (2019) EphA7 functions as receptor on BJAB cells for cell-to-cell transmission of the kaposi’s sarcoma-associated herpesvirus and for cell-free infection by the related rhesus monkey rhadinovirus. J Virol 93(15). 10.1128/JVI.00064-19 [DOI] [PMC free article] [PubMed]
- 104.Hahn AS, Desrosiers RC. Rhesus monkey rhadinovirus uses eph family receptors for entry into B cells and endothelial cells but not fibroblasts. PLoS Pathog. 2013;9(5):e1003360. doi: 10.1371/journal.ppat.1003360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karlas A, Machuy N, Shin Y, Pleissner KP, Artarini A, Heuer D, Becker D, Khalil H, Ogilvie LA, Hess S, Maurer AP, Muller E, Wolff T, Rudel T, Meyer TF. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature. 2010;463(7282):818–822. doi: 10.1038/nature08760. [DOI] [PubMed] [Google Scholar]
- 106.Xu K, Broder CC, Nikolov DB. Ephrin-B2 and ephrin-B3 as functional henipavirus receptors. J Semin Cell Dev Biol. 2012;23(1):116–123. doi: 10.1016/j.semcdb.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steffen DL, Xu K, Nikolov DB, Broder CC. Henipavirus mediated membrane fusion, virus entry and targeted therapeutics. Viruses. 2012;4(2):280–308. doi: 10.3390/v4020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dewannieux M, Vernochet C, Ribet D, Bartosch B, Cosset FL, Heidmann T. The mouse IAPE endogenous retrovirus can infect cells through any of the five GPI-anchored ephrin A proteins. PLoS Pathog. 2011;7(10):e1002309. doi: 10.1371/journal.ppat.1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Swidergall M, Solis NV, Lionakis MS, Filler SG. EphA2 is an epithelial cell pattern recognition receptor for fungal beta-glucans. J Nat Microbiol. 2018;3(1):53–61. doi: 10.1038/s41564-017-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Genander M, Frisen J. Ephrins and Eph receptors in stem cells and cancer. J Curr Opin Cell Biol. 2010;22(5):611–616. doi: 10.1016/j.ceb.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 111.Gallarda BW, Bonanomi D, Muller D, Brown A, Alaynick WA, Andrews SE, Lemke G, Pfaff SL, Marquardt T. Segregation of axial motor and sensory pathways via heterotypic trans-axonal signaling. J Sci. 2008;320(5873):233–236. doi: 10.1126/science.1153758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. J Dev Cell. 2004;7(4):465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 113.Jing X, Miwa H, Sawada T, Nakanishi I, Kondo T, Miyajima M, Sakaguchi K. Ephrin-A1-mediated dopaminergic neurogenesis and angiogenesis in a rat model of Parkinson’s disease. J PLoS One. 2012;7(2):e32019. doi: 10.1371/journal.pone.0032019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhuang G, Brantley-Sieders DM, Vaught D, Yu J, Xie L, Wells S, Jackson D, Muraoka-Cook R, Arteaga C, Chen J. Elevation of receptor tyrosine kinase EphA2 mediates resistance to trastuzumab therapy. J Cancer Res. 2010;70(1):299–308. doi: 10.1158/0008-5472.Can-09-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jackson D, Gooya J, Mao S, Kinneer K, Xu L, Camara M, Fazenbaker C, Fleming R, Swamynathan S, Meyer D, Senter PD, Gao C, Wu H, Kinch M, Coats S, Kiener PA, Tice DA. A human antibody-drug conjugate targeting EphA2 inhibits tumor growth in vivo. J Cancer Res. 2008;68(22):9367–9374. doi: 10.1158/0008-5472.Can-08-1933. [DOI] [PubMed] [Google Scholar]
- 116.Landen CJ, Lu C, Han LY, Coffman KT, Bruckheimer E, Halder J, Mangala LS, Merritt WM, Lin YG, Gao C, Schmandt R, Kamat AA, Li Y, Thaker P, Gershenson DM, Parikh NU, Gallick GE, Kinch MS, Sood AK. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J J Natl Cancer Inst. 2006;98(21):1558–1570. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]