Abstract
Since the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) has emerged from China, the infection (novel corona virus disease-2019, COVID-19) has affected many countries and led to many deaths worldwide. Like SARS-CoV, angiotencin converting enzyme (ACE)2 as a functional receptor for SARS-CoV2 is essential for the virus to make an entry into the cell. ACE2 is a part of Renin-Angiotensin-Aldosterone System, which is expressed in several organs that opposes the angiotensin (Ang) II functions by converting Ang II to Ang (1-7), the one with vasodilation effects. The death rate of COVID-19 is estimated to be approximately 3.4%; however, some comorbid conditions like underlying cardiovascular disease, hypertension, and diabetes increase the risk of mortality. In addition, cardiovascular involvement as a complication of SARS-CoV2 could be direct through either ACE2 receptors that are expressed tremendously in the heart, or by the surge of different cytokines or by acute respiratory distress syndrome-induced hypoxia. Traditional risk factors could aggravate the process of COVID-19 infection that urges the triage of these high-risk patients for SARS-CoV2. Currently, there is no effective, proven treatment or vaccination for COVID-19, but many investigators are struggling to find a treatment strategy as soon as possible. Some potential medications like chloroquine by itself or in combination with azithromycin and some protease inhibitors used for the treatment of COVID-19 have cardiovascular adverse effects, which should be kept in mind while the patients taking these medications are being closely monitored.
Keywords: COVID-19, SARS-CoV2, Cardiovascular disease, ACE2, RAAS
Graphical abstract
1. Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) was first detected in Wuhan, China, in December 2019. The novel corona virus disease-2019 (COVID-19) spread rapidly, reaching epidemic proportions in China and many other countries. Now, by 11 Mar 2020, there are over 118,000 cases of COVID-19 and 4628 deaths in 114 countries around the world.1
The coronavirus sweeping across the world, has been announced a pandemic on 11 Mar 2020 by WHO.1
The new corona virus belongs to the Betacorona virus genus, which is a positive-stranded RNA virus with a crown-like appearance as seen under an electron microscope (coronam is the Latin term for crown) because of the presence of spike glycoproteins on the envelope. It has a round or elliptic shape and often pleomorphic form with a diameter of approximately 60–140 nm.2
The Chinese CDC report has divided the clinical manifestations of COVID-19 according to the severity of symptoms. In all, 81% of cases have mild symptoms including mild pneumonia; 14% of cases showed severe manifestations like dyspnea, respiratory frequency ≥ 30 breaths/min, blood oxygen saturation ≤ 93%, PaO2/FiO2 ratio [the ratio between the blood pressure of the oxygen (partial pressure of oxygen, PaO2) and the percentage of oxygen supplied (fraction of inspired oxygen, FiO2)] < 300, and/or lung infiltrates > 50% within 24 - 48 h. In addition, 5% of cases showed critical expressions like respiratory failure, septic shock, and/or multiple organ dysfunction or failure.4
The death rate of Covid-19 is estimated to be approximately 3.4% globally according to WHO.1 However, the fatality rate of Covid-19 will be higher in special populations with comorbid diseases like cancer (5.6%), hypertension (6.0%), chronic respiratory disease (6.3%), diabetes (7.3%), and cardiovascular disease (CVD) (10.5%).3
Previous studies have shown a relationship between cardiovascular metabolic diseases and SARS and Middle East Respiratory coronavirus (MERS), the two other types of corona viruses that reached epidemic proportions a few years ago.5, 6, 7
In a systematic analysis of 637 MERS-CoV cases by Badawi, et al, it was reported that diabetes and hypertension were prevalent in about 50% of the patients and cardiac diseases were present in 30% of the cases.7
Currently, there is not any proven therapeutic medication for COVID-19, and conservative strategies including cardiorespiratory ventilation support are the main approach. Knowing the pathogenesis of COVID-19 infection will be helpful in developing effective medication.
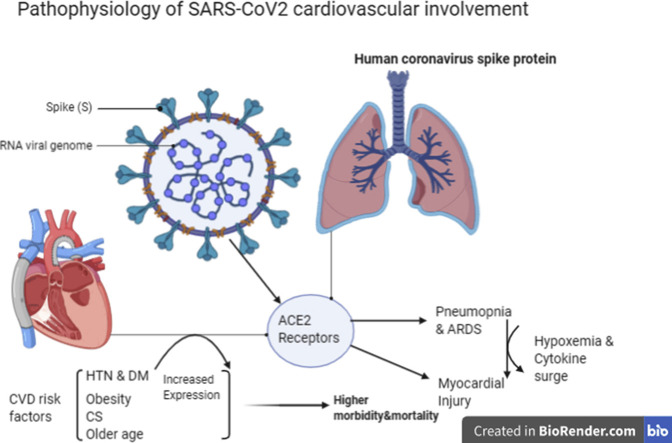
It has been identified that angiotensin-converting enzyme 2 (ACE2) is the essential receptor for SARS-CoV virus to enter into the cell. 8, 9, 10, 11Lung and cardiovascular involvement as complications of SARS-CoV2 are the two main causes of death among these patients. In this review, the pathophysiology of SARS-CoV2 infection along with a special focus on cardiovascular involvement has been explained.
2. SARS-CoV2 origin
The family Coronaviridae includes a large number of viruses that are found in birds and mammals . 12 , 13At first, human coronaviruses were characterized in the 1960s, and were linked with a large percentage of respiratory infections both in children and adults.12 During the SARS-CoV epidemic in the late 2002, globally, more than 8000 human cases and 774 deaths occurred.12 After the SARS epidemic, bats have been considered as a potential reservoir species that could be concerned with future coronavirus-related human pandemics.14
During 2012, MERS-CoV emerged in Saudi Arabia 15 , 16 and 919 out of 2521 (35%) deaths occurred.17 A main role in the transmission of the virus to humans has been attributed to dromedary camels,18 and its origin has been again suggested to bats.19
A full-genome sequence analysis of the new coronavirus (SARS-CoV2) revealed that it belongs to the Betacoronavirus genus, but it is divergent from SARS-CoV and MERS-CoV that caused epidemics in the past.20 The analysis of the genome of the SARS-CoV2 by Paraskevis and his colleagues showed that although it is closely related to BatCoV RaTG13 sequence throughout the genome (sequence similarity is 96.3%), there is a discordant clustering with the Bat_SARS-like coronavirus sequences showing that BatCoV RaTG13 does not provide the exact variant that caused the outbreak in humans, but the hypothesis that SARS-CoV2 has been derived from bats is very prospective.21
3. Renin-Angiotensin-Aldosterone System (RAAS)
Renin-angiotensin-aldosterone system (RAAS) is a vital complex system in the human body for survival by maintaining vascular tone, regulating extracellular fluid, and arterial pressure.22 , 23
Prorenin, the precursor of renin, which is a 406 amino-acid-long protein could be proteolytically activated in the kidney by neuroendocrine convertase 1 (proprotein convertase 1) or cathepsin B and nonproteolytically in many tissues by the renin/prorenin receptor.24 The secreted renin hydrolyzes liver-secreted protein angiotensinogen to angiotensin (Ang) I.25
Ang I undertakes cleavage in the lung capillaries, endothelial cells, and kidney epithelial cells by the endothelial-bound angiotensin-converting enzyme (ACE). ACE is a carboxypeptidase enzyme, also known as kininase II, converts Ang I into the peptide Ang II, which has a vasoconstrictive role on all blood vessels, and degrades bradykinin as well.26 , 27 It increases blood pressure, heart rate, and stimulates plasminogen activator inhibitor (PAI)-1 and PAI-2 that results in increasing prothrombotic activities.28
Furthermore, it stimulates the adrenal gland cortex to secrete aldosterone, which maintains sodium-potassium homeostasis by stimulating kidney proximal tubules to increase sodium transportation, resulting in sodium retention and potassium loss.29 Ang II can also impact the release of prostaglandins, which can influence renal vasoconstriction. Cyclooxygenase 1-derived prostaglandin E 2 and its receptor have been identified as crucial for the Ang II-dependent hypertension.31 Therefore, Ang II is a critical regulator of blood volume, pressure, and pH. After binding to prorenin/renin receptors, circulating renin and prorenin trigger the local generation of Ang II and facilitate Ang II-independent signaling cascades.30
Ang II can be degraded into Ang III by aminopeptidase-A enzymes on red blood cells.31 There are other degraded forms of Ang II as well, with a variable degree of affinity for Ang receptors. In fact, the degradation of Ang II with ACE2 enzyme is the basis for treating diabetic nephropathy.32 , 33
The cardiac RAAS is upregulated in all types of cardiac injuries such as volume overload, myocardial infarction, and heart failure 34, 35, 36, 37 as a homeostatic response to restore cardiac function. Compensatory hypertrophy of cardiac myocytes could be induced by Ang II, which is an inotropic and growth factor.38 Ang II is also an essential factor in left ventricular remodeling after myocardial infarction or afterload-induced cardiac hypertrophy.39
The zinc metallopeptidase, ACE2, is the only known human homologue of ACE. Since its discovery in 2000, ACE2 has been implicated in heart function, hypertension, and diabetes, with its effects being mediated, in part, through its ability to convert from Ang II to Ang-(1–7) that the latter opposes the pressor, proliferative, and profibrotic functions of Ag II.40 , 41 ACE and ACE2, both are central enzymes in the RAS but ACE2 is not inhibited by ACE inhibitors, it could not convert Ang I to Ang II, as well as, could not inactivate bradykinin.8 , 42 In contrast, ACE2 cleaves a single residue from Ang I to generate Ang 1–9, and a residue from Ang II to generate Ang 1–7. Hence, ACE2 negatively regulates the RAAS by inactivating Ang II.8
ACE2 is a type 1 integral membrane glycoprotein, being expressed and active in most tissues 43 , 44 that act as a carboxypeptidase rather than ACE, which acts as a dipeptidase. The main locations of ACE2 receptors are cells in contact with the external environment like lung alveolar epithelial cells and enterocytes of the small intestine.11 ACE2 is also present in arterial and venous endothelial cells, arterial smooth muscle cells, in renal, and cardiovascular tissue.43, 44, 45 The main substrate of ACE2 appears to be Ang II, but other peptides may also be degraded by ACE2 at lower affinity.44, 45, 46, 47
In the kidney, ACE2 and ACE also have antagonistic functions; while, ACE2 is critical for renal homeostasis and its deficit has been associated with albuminuria and glomerular injury (glomerulosclerosis), ACE is bad for the kidney.32 , 33 , 42
In the heart, ACE2 functions as the principal pathway for the metabolism of Ang II,39 , 48 likely through the Nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase system with the p 47 (phox) subunit, and consequently diminishes the adverse effects of Ang II in the heart.49 Interestingly, the triggered disruption of ACE2 in mice results in severe cardiac contractility defect, increased Ang II levels, and the upregulation of hypoxia-induced genes in the heart,50 possibly by acting as a natural counterpart of ACE1,51 suggesting that the balance of ACE and ACE2 in the heart is an important driving factor for progressive cardiac disease.52, 53, 54, 55
Lung is a major source of Ang II synthesis due to the high level of RAAS activity in this organ. In addition, ACE2 is also highly expressed in the lung that appears to have a role in regulating the balance of circulating Ang II/Ang 1–7 levels. Ang II induces pulmonary vasoconstriction in response to hypoxia, which is important in preventing shunting in patients with pneumonia or lung injury.54 Locally increased Ang II production also triggers increasing vascular permeability facilitating pulmonary edema.55
An interesting study by Crackower et al revealed that there is a Yin-Yang nature of ACE and ACE2 expression.55 They observed that Ace2 knockout mice show major cardiac contractility defects and increased levels of Ang II. However, double knockout mice, in which both Ace and Ace2 genes have been deleted, do not show the cardiac defects of the Ace2 knockout alone.
Donoghue et al examined the effect of the transgenic overexpression of ACE2 in the heart of a mouse, and demonstrated that there is a high rate of lethal ventricular arrhythmia, which is associated with the disruption of gap junction formation.56
4. ACE2 as the receptor for SARS-CoV2
ACE2 has been identified as the functional receptor for SARS-CoV by Li et al.8 They showed that ACE2 can be immunoprecipitated by the S1 domain of the SARS-CoV virus and that ACE2 can promote viral replication. The demonstration of ACE2 expression in human organs can potentially identify the possible routes of infection for SARS-CoV, and possible ways of spread and replication throughout the body.11 Hamming et al demonstrated that type I and type II pneumocytes are markedly positive for ACE2, while bronchial epithelial cells show only weak staining.11
Many similarities have been shown between SARS-CoV-2 with the original SARS-CoV using computer modeling.57 SARS-CoV spike protein has strong binding affinity to human ACE2, based on biochemical interaction studies and crystal structure analysis. SARS-CoV-2 and SARS-CoV spike proteins share 76.5% identity in amino acid sequences and, imperatively, the SARS-CoV-2 and SARS-CoV spike proteins have a high degree of homology.58 , 59
It was stated that residue 394 (glutamine) in the SARS-CoV-2 receptor-binding domain, corresponding to residue 479 in SARS-CoV, can be recognized by the critical lysine 31 on the human ACE2 receptor.60 , 61 Further analysis even suggested that SARS-CoV-2 distinguishes human ACE2 more competently than SARS-CoV increasing the ability of SARS-CoV-2 to transmit from person to person.60 Thus, the SARS-CoV-2 spike protein was also predicted to have a strong binding affinity to human ACE2.
While, upper respiratory tract does not appear to be a primary site of entrance for SARS-CoV due to the modest ACE2 expression 11 , 62 that results in low transmissibility of the virus; this is not the case for SARS-CoV2. The potentially higher level of transmissibility of SARS-CoV-2 when compared with SARS-CoV might be speculated that the new virus might exploit cellular attachment-promoting factors with higher efficiency than SARS-CoV, which leads to robust infection of ACE2+ cells in the upper respiratory tract.63 In addition to the lung, extrapulmonary ACE2 positive tissues are vulnerable to SARS-CoV and SARS Cov-2 spread.11 , 64 , 65
Both SARS-CoV and SARS-CoV2 spike glycoprotein (S) use ACE2 as the receptor for entrance into the cytoplasm. The spike (S) protein of coronaviruses facilitates viral entry into target cells. The S1 surface subunit of S protein binds to ACE2 receptor, which simplifies viral attachment to the surface of target cells. In addition, the entry requires S protein priming by the type 2 transmembrane cellular proteases (TMPRSS2) that entails S protein cleavage at the S1/S2 and the S2’ site and allows the fusion of viral and cellular membranes, a process driven by the S2 subunit.63 The efficiency of ACE2 usage in the process of the SARS-S/ACE2 interface determines the transmissibility of SARS-CoV.59 , 66
SARS-CoV uses the endosomal cysteine proteases cathepsin B and L (CatB/L),67 and the serine protease TMPRSS2 68, 69, 70 for S protein priming in cell lines, so the inhibition of both proteases is essential for the vigorous blockade of viral entry.71 However, only TMPRSS2 activity is indispensable for viral spread and pathogenesis in the infected host, while CatB/L activity is unnecessary.70 , 72, 73, 74
As endosomal low PH is essential for CatB/L activity; Hoffmann and his colleagues employed ammonium chloride to elevate endosomal PH and consequently block the CatB/L activity to evaluate the role of CatB/L in the process of SARS-CoV2 entrance into the cells. Ammonium chloride treatment strongly inhibited both SARS-CoV2 and SARS-CoV driven entry into TMPRSS2− 293T cells, suggesting CatB/L necessity.63
In addition, the employment of the serine protease inhibitor camostat mesylate, which is active against TMPRSS2 71 partially, and adding ammonium chloride to camostat mesylate completely blocked the entry of SARS-CoV 2 virus to the TMPRSS2− 293T cells.63
Furthermore, SARS-CoV 2 can use TMPRSS2 for S protein priming and camostat mesylate, an inhibitor of TMPRSS2, which blocks the SARS-CoV 2 infection of lung cells.63 They established that antibody responses against SARS-CoV could at least partially protect against SARS-CoV 2 infection.63
5. Cardiovascular involvement by SARS-CoV2
SARS-CoV2 particularly involves alveolar epithelial cells, resulting in respiratory symptoms that are more severe in patients with CVD, which might be linked with increased secretion of ACE2 in these patients compared with healthy individuals. In addition, ACE2 expression could be increased by taking ACE inhibitor medications in hypertensive patients.75 ACE inhibitors and angiotensin receptor blockers (ARBs) are widely used in various patients with kidney disease, heart failure, coronary artery disease (CAD), HTN, and diabetes.
The circulating and organ levels of ACE2 in the heart and atherosclerotic vessels are increased in patients with CAD and heart failure. In addition, a large proportion of the variation in plasma ACE2 levels has been attributed to hereditary factors.76
The cardiovascular system is at the risk of COVID-19 involvement by the direct infection as well as due to the indirect involvement of hypoxemia induced by pneumonia and acute respiratory distress syndrome (ARDS).
In a recent case report on 138 hospitalized COVID-19 patients, 16.7% of patients developed arrhythmia and 7.2% experienced acute cardiac injury as well as other COVID-19 associated complications. Some cases of acute onset heart failure, myocardial infarction, myocarditis, and cardiac arrest have also been reported. However, the reporting does not yet define the prevalence of cardiac complications in CVD-naive versus cardiac comorbid patients.77
In a retrospective, multicenter cohort Chinese study, 191 patients were included, of whom 137 were discharged and 54 died in hospital. A total of 48% of patients had a comorbidity: hypertension being the most common comorbidity in 30% of patients, followed by diabetes in 19% of patients, and coronary heart disease in 8% of patients.78
5.1. Endothelial dysfunction
Vascular endothelium is an active organ with paracrine, autocrine, and endocrine functions, which is vital for the regulation of vascular tone and the maintenance of vascular homoeostasis.79 Endothelial dysfunction is the primary factor of microvascular dysfunction characterized by vasoconstriction and subsequent organ ischemia, inflammation associated with tissue edema, and a procoagulant state.80
In a postmortem analysis, Varga, et al observed the direct viral infection of endothelial cells in several patients, all of whom had underlying conditions including HTN, kidney disease, CAD, and diabetes.81 The presence of viral elements within endothelial cells and an accumulation of inflammatory cells, with the evidence of endothelial and inflammatory cell death have been described by authors suggesting that SARS CoV-2 infection facilitates endotheliitis as a direct consequence of viral involvement and of the host inflammatory response.81 Moreover, it has been suggested that the induction of apoptosis and pyroptosis might have an important role in endothelial cell damage in patients with COVID-19. COVID-19-endotheliitis could explain the systemic impaired microcirculatory function in different vascular beds and their clinical sequelae in patients with COVID-19.81
The above-mentioned finding is much more important in patients with preexisting endothelial dysfunction including male sex, smoking, hypertension, diabetes, obesity, and established cardiovascular disease, all associated with adverse outcome.81
5.2. Myocardial injury
Myocardial injury could complicate the course of COVID-19. According to Chen's report, the first death was a 61-year-old man with no previous chronic underlying disease. The patient was admitted in ICU and developed severe respiratory failure, heart failure, and sepsis, and then experienced a sudden cardiac arrest on the 11th day of admission and was declared dead.82
In a report released by the National Health Commission of China (NHC), among the people who died from COVID-19, 11.8% of patients without underlying CVD had substantial heart damage, with elevated levels of cardiac troponin I (cTnI) or cardiac arrest during hospitalization.83
Li, et al conducted a meta-analysis of six studies including 1527 patients with COVID-19 infection to evaluate the prevalence of cardiovascular metabolic diseases in COVID-19 in ICU/severe and non-ICU/severe patients.84
They reported 8.0% patients with COVID-19 who suffered acute cardiac injury and this incidence was much higher in ICU/severe patients, about thirteenfold more than non-ICU/cardiac patients.
In another meta-analysis of four studies, it was suggested that in patients with severe infection, high sensitivity cTn I was significantly higher at admission compared to those with a nonsevere course.85
Sometimes, myocardial involvement could progress rapidly and aggressively, and leads to fulminant myocarditis. One case of fulminant myocarditis has been stated in a 37-year-old patient in a case report by Hu et al.6
It has been described that troponin levels remained high in nonsurvivors throughout the clinical course and increased with illness deterioration.86 Although, myocardial injury could occur in patients without a history of heart failure, it is more common in patients with preexisting heart failure (14.6% vs. 1.5%).87
The most common cause of myocarditis in developed countries is viral infections.169 Acute Myocardial injury could be the direct result of SARS-CoV2 through ACE2 receptors, which is highly expressed in the cardiovascular system. 43, 44, 45 SARS-CoV viral RNA was detected in 35% of autopsied human heart samples from SARS-CoV infected patients during the Toronto SARS outbreak in Oudit's report.89 They also endorsed that pulmonary infection with the human SARS-CoV in mice led to an ACE2-dependent myocardial infection.89
Another mechanism for cardiac involvement could be cytokines triggered by an imbalanced response by type 1 and type 2 T helper cells.51 , 88 Huang and his colleagues noted that the patients infected with SARS-CoV2 had high amounts of various cytokines like interleukin(IL)1β, Interferon (IFN) γ, IFN γ-induced protein 10 kDa (IP10), and monocyte chemoattractant protein 1 (MCP1), probably leading to activated T-helper-1 cell responses. Moreover, patients requiring ICU admission had higher concentrations of granulocyte colony-stimulating factor, IP10, MCP1, macrophage inflammatory protein 1A (MIP1A), and tumor necrosis factor α than did those not requiring ICU admission, suggesting that the cytokine storm was associated with disease severity.90 , 91 However, SARS-CoV2 infection also initiated increased secretion of T-helper-2 cytokines (e.g., IL4 and IL10) that suppresses inflammation, which differs from SARS-CoV infection.90 , 91
The third mechanism for cardiac injury is hypoxemia due to respiratory dysfunction caused by COVID-19.83 Huang et al reported that 32% COVID-19 patients had various degrees of hypoxemia and needed high-flow nasal cannula or higher-level oxygen support.90 Chen et al reported that up to 76% of patients required oxygen therapy.82 In fact, COVID-19 infection-induced severe pneumonia causes significant gas exchange alteration leading to hypoxemia, which ominously lessens the energy supply by cell metabolism, therefore increases anerobic fermentation, causing intracellular acidosis and oxygen-free radicals, which lead to the phospholipid layer of cell membrane detriment. Meanwhile, hypoxia-induced influx of calcium ions also leads to injury and apoptosis of cardio myocytes.84
5.3. Acute Heart Failure
Acute heart failure could evolve in patients with COVID-19 with several potential mechanisms. Myocardial injury as mentioned in the previous section could result in acute heart failure.92 Other mechanisms include ARDS-induced hypoxemia, acute kidney injury, and subsequent volume overload, exacerbation of chronic heart failure,92 and sustained/repetitive cardiac arrhythmia.93
In a report by Arentz et al, among 21 patients admitted to an ICU for severe COVID-19, 7 (33.3%) patients developed dilated cardiomyopathy, characterized by globally decreased LV systolic function, clinical signs of cardiogenic shock, elevated creatine kinase, or troponin I levels, or hypoxemia, without a past history of systolic dysfunction.94
5.4. Acute coronary events
Although, to our knowledge, there is no report indicating the incidence of ST elevation myocardial infarction in patients with COVID-19, it could be proposed that the rate of myocardial infarction, acute coronary syndrome (ACS), and non-ST-elevation myocardial infarction will increase among COVID-19 patients particularly among those with underlying CVD or risk factors. This conjecture comes from the previous studies reporting the occurrence of ACS and myocardial infarction (MI) among patients with SARS infection.95 , 96
In a study by Clyton, et al, there was an association between recent respiratory infection and MI.97 In this line, a meta-analysis of case–control studies found a significant association between recent respiratory infection and acute MI.98
Some pathophysiological mechanisms can be implicated in this matter. For instance, acute inflammation could result in endothelial dysfunction, consequent vasoconstriction, and ischemia in sensitive organs like the heart. Furthermore, inflammation-induced endothelial dysfunction could increase the risk of thrombosis and thrombotic events.80 In addition, fever and the surge of inflammation could increase the cardiac demand by increasing metabolism and heat rate, resulting in cardiac ischemia and cardiac events particularly in patients with underlying CVD.
5.5. Arrhythmia
As yet, there is extremely limited literature available on the occurrence of arrhythmia in the context of COVID-19. In a study of 138 hospitalized patients with COVID-19 in Wuhan, arrhythmia was reported in 16.7% of total patients and in 16 of 36 patients admitted to the ICU (44%), although the authors did not further specify its type.93 Thereafter, in a subsequent publication from the same institution, ventricular tachycardia/ventricular fibrillation was reported as a complication of the COVID-19 disease in 11 of 187 patients (5.9%), with a significantly higher incidence in patients with elevated troponin T.99 However, the largest observational study from China, with 1099 patients from 552 hospitals, did not report any arrhythmia.100
To our knowledge, there are no specific reports on the occurrence of bradycardia in COVID-19 infection. However, an experimental study has shown that coronavirus-infected rabbits have ECG abnormalities including 2nd degree AV block secondary to myocarditis and heart failure.101 In severely ill patients admitted in the ICU due to COVID-19 transient bradycardia and asystole may occur due to patient turning for prone position, intubation, or trachea suction that likely occurs due to the temporary increased vagal tone.102
Moreover, in patients with underlying CVD on CVD medications, increased QT interval-corrected (QTc) could be the reason of ventricular arrhythmia due to drug-drug interaction.
Also, a heart rate/temperature discordance as what was observed in typhoid fever was reported in patients with COVID-19.93 , 103
6. Underlying cardiovascular disease as a prompting factor for COVID-19 infection
There are many reports showing the increased rate of infection with SARS-CoV2 in patients with previous CVD. It appears that the underlying CVD could aggravate the severity of symptoms and pneumonia due to SARS-CoV2.83 In one study, among the patients with severe symptoms of COVID-19, 25% had heart disease and 44% had arrhythmia.77 Among the comorbid conditions, cardiac disease and diabetes increased the risk of death by twice as much as other risk factors.104
According to mortality data released by NHC, 17% of patients had a history of coronary heart disease. Furthermore, data showed that patients aged >60 years who were infected with SARS-CoV2 had more systemic symptoms and more severe pneumonia than patients aged ≤60 years.83
As stated by Li et al in a meta-analysis respecting the prevalence and impact of cardio-metabolic comorbidities on COVID-19, the proportion of cardio-cerebrovascular disease was statistically significantly higher in ICU/severe patients compared to the non-ICU/severe cases [RR = 3.30, 95% CI (2.03, 5.36), Z = 4.81, P < 0.00001].84
Patients with ACS and acute MI are expected to be at higher risk of mortality due to COVID-19 infection. This is truly supported by the fact that the patients with severe infection are susceptible to increased ischemia and cardiac demand, and consequently result in poorer prognosis.83 Correspondingly, in patients with an underlying cardiac insufficiency, infection with SARS-CoV2 could deteriorate the condition and lead to death.83
Cardiac troponins are known to have a crucial role in the evaluation of patients with acute chest pain.76 In a recent meta-analysis by Lippi et al, it was shown that cTnI values are significantly increased in patients with severe SARS-CoV2 infection compared to those with milder forms of disease.85 Therefore, it seems judicious to measure cardiac damage biomarkers instantly after hospitalization for SARS-CoV2 infection, and subsequent monitoring during hospital stay, which might help in identifying a subset of patients with possible cardiac injury and predicting the progression of COVID-19 toward a worse clinical picture.
7. Traditional risk factors in patients with COVID-19 infection
HTN is a comorbid condition, which has been reported to be associated with increased mortality in patients with COVID-19 infection. Chen and his colleagues reported that 113 (14.4%) patients who died from COVID-19 more likely had HTN and diabetes when compared with 161 patients who recovered.85 Furthermore, according to the China Center for Disease Control and Prevention's report of 44000 people with laboratory confirmed covid-19, HTN and diabetes were among comorbid conditions with higher risk of mortality.4 In addition, a meta-analysis of eight studies including 46248 patients with laboratory confirmed COVID-19 showed that the subjects with more severe disease more likely had HTN [odds ratio 2.36 (95% confidence interval 1.46 to 3.83)].105
In this track, Li et al described that HTN was the most prevalent cardiovascular metabolic comorbidity (17.1%, 95% CI 9.9–24.4%) followed by cardiocerebrovascular disease (16.4%, 95% CI 6.6–26.1%), and diabetes (9.7%, 95% CI 6.9–12.5%). Although, the overall proportion of HTN, cardiocerebrovascular diseases and diabetes were about twofolds, threefolds, and twofolds in ICU/severe cases compared with their non-ICU/severe counterparts, respectively; they did not find that people with HTN and diabetes were more susceptible to COVID-19 infection.84
Possible mechanisms of the adverse effect of HTN on the process of COVID-19 infection might be in part due to the increased expression of ACE2 receptors in hypertensive patients taking RAAS inhibitors based on some animal and human studies.106 , 107 Also, hypertensive patients might have underlying cardiovascular atherosclerotic disease, diastolic dysfunction, and chronic kidney disease, which all expose them to higher risk of morbidity and mortality.
Older age, which is a cardiovascular risk and the main cause of death among elderly population, 168 was associated with higher risk of severe COVID-19 in national surveys database in the US, China, and Italy.108 The case fatality rate in those three databases exceeded 1% around the age of 50-55 years and 10% above 80-85 years (above 70 years in Italy). Those studies reporting the results both based on unadjusted and multivariable models described the unchanged pattern of the effect size of age after adjustment for comorbidities.86 , 109
In the US, among individuals older than 60 years ranges from 17% to 27%, the percentage of hospitalized patients requiring care in an ICU is 27%–71% with an infection fatality rate ranging from 2.2% to 9.3%.110
According to the extensive evidence, Smoking has been accepted as a negative risk factor on lung health and its causal association with overabundance of respiratory diseases may be likely due to the detrimental effect on the immune system.111 , 112
Previous studies showed that smoking status was associated with higher mortality in the previous MERS-CoV outbreak.113 , 114 Among the observational studies concerning the clinical characteristics of patients with COVID-19, a large study consisting of 1099 patients conducted by Guan et al from multiple regions of mainland China.101 Descriptive results on the smoking status of patients revealed that 173 out of 1099 patients with COVID-19 had severe symptoms, and 926 had nonsevere symptoms. Among the patients with severe symptoms, 16.9% were current smokers and 5.2% were former smokers, in contrast to patients with nonsevere symptoms where 11.8% were current smokers and 1.3% were former smokers. Moreover, in the group of patients who either needed mechanical ventilation, admission to ICU or died, 25.5% were current smokers and 7.6% were former smokers. In contrast, in the group of patients who did not have these adverse outcomes, only 11.8% were current smokers and 1.6% were former smokers.
A systematic review and meta-analysis including 5 studies identified the association between smoking and severity of symptoms as smokers were 1.4 times more likely (RR = 1.4, 95% CI: 0.98–2.00) to have severe symptoms of COVID-19 and approximately 2.4 times more likely to be admitted to ICU, need mechanical ventilation or die compared to nonsmokers (RR = 2.4, 95% CI: 1.43–4.04). However, the results of this meta-analysis have not been adjusted for other factors, which might assist in the progression of disease.115
Diabetes is a well-known risk factor for CVD accompanied by obesity, HTN, and CVD in many cases. Diabates is associated with increased rate of mortality in patients with COVID-19. Evidence of the higher risk of mortality among persons with diabetes and the two previous SARS corona viruses (SARS and MERS) has been reported.116, 117, 118
Bearing in mind the high prevalence of CVD, obesity, and hypertension in patients with diabetes, it is still unknown whether diabetes independently contributes to this increased risk. However, according to one study, plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS.5
In diabetes, there has been an enhanced ACE2 expression in different organs like the lung, kidney, heart, and pancreas in rodents and in the lung of human models,119, 120, 121 which might result in facilitating the virus entry to the cell. In addition, circulating levels of furin, a cellular protease involved in facilitating viral entry by cleaving the S1 and S2 domain of the spike protein of the virus, are elevated in patients with diabetes.122 Interestingly, Chen et al revealed that in patients with diabetes and HTN, the clearance of SARS-CoV2 was delayed, a finding that needs to be confirmed in larger studies.123
Potential mechanisms that may increase the susceptibility for COVID-19 in patients with diabetes include higher affinity for cellular binding and efficient virus entry, decreased viral clearance, diminished T cell function, increased susceptibility to hyper inflammation, and cytokine storm syndrome, and comorbid conditions like CVD.124
Obesity and severe obesity are well established as risk factors for hospitalization and mechanical ventilation. The increased risk of hospitalization and mechanical ventilation in these patients is not surprising because obesity is connected with decreased respiratory reserve volume, functional capacity, and respiratory system compliance. In addition, abdominal obesity compromises pulmonary function further in supine position due to the decreased diaphragmatic excursion, making ventilation more difficult. Furthermore, increased inflammatory cytokines associated with obesity may contribute to the increased morbidity associated with obesity in COVID-19 infections.125
Zheng et al reported that the presence of obesity in metabolic-associated fatty liver disease patients was associated with a significant increased risk of severe COVID-19 illness even after adjusting for other risk factors.126
A French retrospective cohort study in patients with COVID-19 illness admitted in ICU, identified the significant increased need for invasive mechanical ventilation (IMV) with higher grade of obesity. Overall, 68.6% required IMV of whom the greatest proportion of patients needed IMV was the patients with body mass index (BMI) >35 kg/m2 (85.7%). BMI had an independent risk factor for the severity of the disease and need for IMV.127
According to the report of National Center for Health Statistics, the prevalence of adult obesity and severe obesity from 2017 to 2018 has increased in the US since 2009 - 2010 and is now 42% and 9%, respectively.128 Concerning this report, as well as based on the above-mentioned studies and the compatible results of the previous studies about other respiratory infection like H1N1 and their increased mortality and morbidity with increased BMI, it must serve as a caution for the care of patients with obesity and particularly patients with severe obesity. These observations also emphasize the need for increased observance, precedence on detection and testing, and forceful therapy for patients with obesity and COVID-19 infections.125
In addition to above-mentioned mechanisms, other various mechanisms concerning the increased risk of mortality and morbidity in COVID-19 patients with traditional risk factors are mentioned in the Table 1 .
Table 1.
Mechanisms of different CVD risk factors in the aggravation of COVID-19
| CVD risk factors | Potential mechanisms for aggravation of COVID-19 infection |
|---|---|
| DM | Increased expression of ACE2 129,131,132 |
| Underlying kidney disease 163 | |
| Underlying coronary artery disease 161 | |
| Impaired immunity 162 | |
| HTN | Increased expression of ACE2 106,107,160 |
| Underlying diastolic dysfunction 164 | |
| Underlying coronary artery disease 161 | |
| Underlying kidney disease 163 | |
| Smoking | Underlying coronary artery disease 161 |
| Decreased respiratory compliance 167 | |
| Decreased functional capacity 167 | |
| Underlying HTN 167 | |
| Impaired immunity 111,112 | |
| Old age | Underlying cardiovascular disease 161 |
| Impaired immunity 166 | |
| Obesity | Decreased respiratory reserve 125 |
| Decreased functional capacity 125 | |
| Underlying diabetes 165 | |
| Underlying coronary artery disease 161 | |
| Underlying HTN 165 | |
| Increased inflammatory cytokines125 |
8. Long-term CVD risk in recovered patients from COVID-19
COVID-19 infection may also be connected with an elevated long-term CV risk. Previous studies in patients with pneumonia has established the long period of hypercoagulability state and systemic inflammatory activity.130 Besides, follow-up studies of the SARS epidemic demonstrated that patients with a history of SARS-coronavirus infection often had hyperlipidemia, CV system abnormalities, or glucose metabolism disorder. However, the explanation for metabolic disorder in survivors of SARS could be due to the use of pulses of methylprednisolone and not because of the infection itself.130 Although, there is no long-term effects of SARS-CoV2 infection, more inspection is required for recovered patients in the future based on the information available from SARS.
9. Cardiovascular precautions of therapeutic strategies
Although, currently there is no proven therapeutic medication or vaccine for SARS-CoV2, and the present best strategy to treat patients with SARS-CoV2 infection is a supportive approach, there are some potential medications under investigation. Some of these medications might have cardiovascular adverse effects that must be closely monitored.
9.1. Renin-angiotensin-aldosterone (RAAS) inhibitors
Whether, ARBs like losartan and olmesartan, which are therapeutic medications widely used in hypertensive, diabetes, and kidney disease patients, aggravate the SARS-CoV2 infection by increasing the expression of ACE2 as the functional receptor of the virus or prevent infected patients to progress toward ARDS remain to be established.
Circulating levels of ACE2 are increased not only in patients with HTN 160 or diabetes,131 , 132 but also further elevated in patients using these medications.131 , 132 Utilizing these medications could increase the cardiac ACE2 expression about threefold following chronic treatment (28 days) after MI induced by coronary artery ligation of rats.75 Losartan was also shown to upregulate renal ACE2 expression in chronically treated rats.133
A recent hypothesis suggested that ARBs might be beneficial for patients infected by COVID-19 who experience pneumonia.134 At the first glance, it appears that the high expression of ACE2 by ARBs aggravates SARS-CoV2 infection; conversely, it might keep the patients against acute lung injury, which is one of the main causes of death due to the virus infection. The latter hypothesis comes from the fact that infection with SARS-CoV2 downregulates the ACE2 receptors and consequently increases the level of Ang II, which has vasoconstrictive functions and could worsen the lung involvement. Currently, taking ARBs diminishes the production of Ang caused by viral COVID-19-mediated downregulation of ACE2. In addition, upregulating ACE2 might be another potential mechanism that helps reduce Ang production by ACE and increasing the production of the vasodilator Ang 1–7.135
In this matter, multiple cardiac societies, including ACC, AHA, and ESC currently recommend not to start with ACE inhibitors or ARBs in patients with COVID-19 but to continue with prior, either class of ACE inhibitors or ARBs.136
9.2. Chloroquine plus azithromycin
A widely used antimalarial and autoimmune disease drug named chloroquine has been reported as a potential broad-spectrum antiviral drug through blocking virus infection by increasing endosomal PH required for virus/cell fusion, as well as by the glycosylation of cellular receptors of SARS-CoV.137, 138, 139 Chloroquine has been reported to be possibly effective in blocking SARS-CoV-2 infection at both entry and postentry stages in vitro.140 In addition, chloroquine acts as an immunomodulatory drug, which could synergistically enhance its antiviral effect in vivo.105
However, chloroquine has been shown to cause atrioventricular (AV) blocks and prolonged QTc. Also, hydroxychloroquine, which is the more potent synthetic form of chloroquine, is being used for empirical management of COVID-19 based on a small French trial along with azithromycin, a macrolide antibiotic.141 , 142 Hydroxychloroquine can similarly produce conduction defects in the heart. Additive effects of concomitant use of beta-blockers or calcium channel blockers can induce severe bradycardia leading to cerebral hypoperfusion with syncope and/or fall.143
The combination of azithromycin and chloroquine products has been utilized with improved recovery of symptoms.141 , 142 As azithromycin is known to increase QTc, it could theoretically further increase QTc in combination with chloroquine and increase the risk of torsade de point and ventricular arrhythmia. Monitoring of QTc along with electrolytes (particularly potassium) is necessary when these medications are going to be started by physicians.144
9.3. Protease inhibitors
Lopinavir/ritonavir are FDA-approved drugs for HIV-1 infection.145 A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19 by Cao B et al showed no survival benefit following treatment with lopinavir/ritonavir in hospitalized adult patients with severe COVID-19. At this time, this is not currently a recommended treatment option for COVID patients. However, as there is not a definite treatment for COVID-19 infection, these drugs might be used in severe ill patients in some centers. Therefore, it must be noticed that these medications could prolong PR and QT intervals leading to high-grade AV blocks and rarely torsade de Pointes. Moreover, they could increase serum lipid levels and decrease the serum concentration of clopidogrel and prasugrel, while they could increase the serum level of ticagrelor and statins.145
In addition, lopinavir/ritonavir enhances the effects of factor Xa inhibitors such as apixaban and rivaroxaban through the inhibition of CYP3A4, thereby increasing bleeding risk. Thus, extreme attention is warranted in patients on multiple cardiovascular medications.145
Ribavirin, which is sometimes combined with lopinavir/ritonavir, has not been established to directly affect the heart but can indirectly influence the heart by increasing the levels of lopinavir/ritonavir. It has also been reported to reduce the effect of warfarin and to have noncardiac side effects.146
9.4. Remdesivir
Remdesivir is an adenosine analog, which incorporates into nascent viral RNA chains and results in premature termination,147 which has been recognized as a promising antiviral drug against a wide range of RNA viruses including SARS CoV infection in cultured cells, mice, and nonhuman primate models.147
Wang et al demonstrated that remdesivir functioned at the stage of postvirus entry, and revealed its high efficacy in the control of SARS-CoV2 infection in vitro.147
Although its side effects have not yet been well known, there was a patient who developed hypotension and bradycardia when this medication was used to treat Ebola.148
9.5. Colchicine
Colchicine, which is an alkaloid extracted from plants of the genus Colchicum (autumn crocus) has a well-established therapeutic usage in gout and familial Mediterranean fever.149 However, it has also been used in other diseases including Behcet's disease, pericarditis, CAD, and other inflammatory and fibrotic conditions. Current results suggest that colchicine downregulates multiple inflammatory pathways and modulates innate immunity.149
Colchicine has been utilized safely in a variety of cardiovascular clinical conditions including acute pericarditis,155 prevention of postpericardiotomy syndrome,150 prevention of atrial fibrillation recurrence after cardiac surgery, or ablation procedures.151
Colchicine is a nonselective inhibitor of NLRP3 inflammasome.152 In the experimental models, it has been found that inflammasome NLRP3 is a major pathophysiological component in the development of ARDS.153 , 154 On the other hand, SARS-CoV2 proteins, such as viroporins E, 3a, and 8A, play a considerable role in viral replication and pathogenetic sequelae.155 There are evidence supporting that these 3 SARS-CoV2 proteins provoke the activation of inflammasome NLP3.156 , 157 Based on the said information, it is assumed that colchicine might be an impending useful and safe drug to limit myocardial necrosis and pneumonia in the context of COVID-19. A clinical trial based on this hypothesis is undergoing.
10. Conclusion
COVID-19 tends to be more severe in patients with CVD, HTN, and diabetes. The cTnI measurement and its monitoring as an easy and available laboratory test could be considered in high-risk hospitalized patients with COVID-19. On the other hand, in patients with underlying CVD and traditional risk factors, it is sensible to screen these patients for SARS-CoV2 to plan and integrate aggressive therapeutic strategies.
To note, there are currently more than 80 clinical trials to test a variety of potential SARS-CoV-2 treatments.158 As both SARS-CoV and SARS-CoV2 share ACE2 receptors to make an entry into the cells, it is wise to utilize the results of the previous researches of SARS-CoV for SARS-CoV2. Among the potential drugs, remdesivir and chloroquine are the two most focused drugs. Now, six clinical trials regarding the use of remdesivir are undergoing performance tests.159
Some potential used drugs have adverse cardiovascular side effects that must be noted by the physicians. Until a proper, approved medication is found, the conservative approach and focus on the adverse effects of potential and empirically used drugs must be the priority.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
There is no funding support to declare.
Acknowledgment
There is no acknowledgment to declare.
Footnotes
Peer review under responsibility of Hellenic Society of Cardiology.
References
- 1.World Health Organization Coronavirus disease 2019 (COVID-19) situation report–51. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available at:
- 2.Cascella M., Rajnik M., Cuomo A. StatPearls. StatPearls Publishing; Treasure Island (FL): 2020 Jan. Features, Evaluation and Treatment Coronavirus (COVID-19)https://www.ncbi.nlm.nih.gov/books/NBK554776/ [Internet]. [Updated 2020 Mar 8]. Available from: [PubMed] [Google Scholar]
- 3.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) — China, 2020. China CDC Week. 2020;2(8):113–122. doi: 10.46234/ccdcw2020.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Yang J.K., Feng Y., Yuan M.Y. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 6.Yu C.M., Wong R.S., Wu E.B. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med. 2006;82:140–144. doi: 10.1136/2Fpgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W., Moore M.J., Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/2Fnature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/2Fnm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X.H., Deng W., Tong Z. Mice transgenic from human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comput Med. 2007;57(5):450–459. [PubMed] [Google Scholar]
- 11.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., Van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/2Fpath.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn J.S., McIntosh K. History and recent advances in coronavirus discovery Pediatr. Inf Disp J. 2005;24(11):S223–S227. doi: 10.1097/01.inf.0000188166.17324.60. [DOI] [PubMed] [Google Scholar]
- 13.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis Methods. Mol Biol. 2015;1282:1–23. doi: 10.1007/2F978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajjar S.A., Memish Z.A., McIntosh K. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): a perpetual challenge. Ann Saudi Med. 2013;33(5):427–436. doi: 10.5144/0256-4947.2013.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Centre for Disease Prevention and Control ECDC Risk assessment guidelines for infectious diseases transmitted on aircraft (RAGIDA) Middle East Respiratory Syndrome Coronavirus (MERS-CoV) ECDC Tech Rep. 2020 (January 2020) [Google Scholar]
- 17.Alagaili A.N., Briese T., Mishra N. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. mBio. 2014;5(2) doi: 10.1128/mBio.00884-14. e00884–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ithete N.L., Stoffberg S., Corman V.M. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg Infect Dis. 2013;19(10):1697–1699. doi: 10.3201/eid1910.130946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paraskevis D., Kostaki E.G., Magiorkinis G. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect Genet Evol. 2020;79:104212. doi: 10.1016/j.meegid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atlas S.A. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13:9–20. doi: 10.18553/jmcp.2007.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navar L.G. Physiology, hemodynamics, endothelial function, renin-angiotensin-aldosterone system, sympathetic nervous system. J Am Soc Hypertens. 2014;8:519–524. doi: 10.1016/j.jash.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z., Cappiello M.G., Scott B.B., Bukhtiyarov Y., McGeehan G.M. Purification and characterization of recombinant human renin for X-ray crystallization studies. BMC Biochem. 2008;9:19. doi: 10.1186/1471-2091-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verdecchia P., Angeli F., Mazzotta G., Gentile G., Reboldi G. The renin angiotensin system in the development of cardiovascular disease: role of aliskiren in risk reduction. Vasc Health Risk Manag. 2008;4:971–981. doi: 10.2147/vhrm.s3215. http://www.ncbi.nlm.nih.gov/pubmed/19183745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crisan D., Carr J. Angiotensin I-converting enzyme: genotype and disease associations. J Mol Diagn. 2000;2:105–115. doi: 10.1016/S1525-1578(10)60624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernstein K.E., Gonzalez-Villalobos R.A., Giani J.F. Angiotensin-converting enzyme overexpression in myelocytes enhances the immune response. Biol Chem. 2014;395:1173–1178. doi: 10.1515/hsz-2013-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Studdy P.R., Lapworth R., Bird R. Angiotensin-converting enzyme and its clinical significance-a review. J Clin Pathol. 1983;36(8):938–947. doi: 10.1136/jcp.36.8.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feener E.P., Northrup J.M., Aiello L.P., King G.L. Angiotensin II induces plasminogen activator inhibitor-1 and −2 expression in vascular endothelial and smooth muscle cells. J Clin Invest. 1995;95:1353–1362. doi: 10.1172/JCI117786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiolero A., Maillard M., Nussberger J., Brunner H.R., Burnier M. Proximal sodium reabsorption: an independent determinant of blood pressure response to salt Hypertens. Hypertension. 2000;36:631–636. doi: 10.1161/01.hyp.36.4.631. http://www.ncbi.nlm.nih.gov/pubmed/11040249 Dallas, Tex. 1979. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Zhao S. Morgan & Claypool Life Sciences; San Rafael (CA): 2010. Vascular Biology of the Placenta.https://www.ncbi.nlm.nih.gov/books/NBK53257/ Chapter 8, Vasoactivators and Placental Vasoactivity. [PubMed] [Google Scholar]
- 31.Padia S.H., Kemp B.A., Howell N.L., Fournie-Zaluski M.-C., Roques B.P., Carey R.M. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension (Dallas) 2008;51:460–465. doi: 10.1161/HYPERTENSIONAHA.107.103242. [DOI] [PubMed] [Google Scholar]
- 32.Batlle D., Wysocki J., Soler M.J., Ranganath K. Angiotensin-converting enzyme 2: enhancing the degradation of angiotensin II as a potential therapy for diabetic nephropathy. Kidney Int. 2012;81:520–528. doi: 10.1038/ki.2011.381. [DOI] [PubMed] [Google Scholar]
- 33.Varagic J., Ahmad S., Nagata S., Ferrario C.M. ACE2: angiotensin II/angiotensin-(1–7) balance in cardiac and renal injury. Curr Hypertens Rep. 2014;16:420. doi: 10.1007/s11906-014-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pieruzzi F., Abassi Z.A., Keiser H.R. Expression of renin-angiotensin system components in the heart, kidneys, and lungs of rats with experimental heart failure. Circulation. 1995;92(10):3105–3112. doi: 10.1161/01.CIR.92.10.3105. [DOI] [PubMed] [Google Scholar]
- 35.Ruzicka M., Keeley F.W., Leenen F.H.H. The renin-angiotensin system and volume overload-induced changes in cardiac collagen and elastin. Circulation. 1994;90(4):1989–1996. doi: 10.1161/01.CIR.90.4.1989. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch A.T., Talsness C.E., Schunkert H., Paul M., Dzau V.J. Tissue-specific activation of cardiac angiotensin converting enzyme in experimental heart failure. Circ Res. 1991;69(2):475–482. doi: 10.1161/01.RES.69.2.475. [DOI] [PubMed] [Google Scholar]
- 37.Hokimoto S., Yasue H., Fujimoto K. Expression of angiotensin-converting enzyme in remaining viable myocytes of human ventricles after myocardial infarction. Circulation. 1996;94(7):1513–1518. doi: 10.1161/01.CIR.94.7.1513. [DOI] [PubMed] [Google Scholar]
- 38.Sadoshima J.I., Xu Y., Slayter H.S., Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75(5):977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 39.Garabelli P.J., Modrall J.G., Penninger J.M., Ferrario C.M., Chappell M.C. Distinct roles for angiotensin-converting enzyme 2 and carboxypeptidase A in the processing of angiotensins within the murine heart. Exp Physiol. 2008;93(5):613–621. doi: 10.1113/2Fexpphysiol.2007.040246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.M Ferrario C., Chappell Mark. C., Ann Tallant E., Bridget Brosnihan K., Diz Debra. I. Counterregulatory actions of angiotensin-(1-7) Hypertension. 1997;30:535–541. doi: 10.1161/01.HYP.30.3.535. [DOI] [PubMed] [Google Scholar]
- 41.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2000;25(6):291–294. doi: 10.1016/2Fj.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W., Bodiga S., Das S.K., Lo J., Patel V., Oudit G.Y. Role of ACE2 in diastolic and systolic heart failure. Heart Fail Rev. 2012;17:683–691. doi: 10.1007/s10741-011-9259-X. [DOI] [PubMed] [Google Scholar]
- 43.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme: cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275(43):33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 44.Donoghue M., Hsieh F., Baronas E. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 45.Harmer D., Gilbert M., Borman R. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 46.Turner A.J., Hooer N.M. The angiotensin-converting enzyme gene family: genomics and pharmacology. Trends Pharmacol Sci. 2002;23(4):177–183. doi: 10.1016/s0165-6147(00)01994-5. [DOI] [PubMed] [Google Scholar]
- 47.Rice G.I., Thomas D.A., Grant P.J., Turner A.J., Hooper N.M. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383(1):45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stewart J.A., Jr., Lazartigues E., Lucchesi P.A. The angiotensin converting enzyme 2/Ang-(1–7) axis in the heart: a role for mas communication? Circ Res. 2008;103(11):1197–1199. doi: 10.1161/CIRCRESAHA.108.189068. doi: 10.1161/CIRCRESAHA.108.189068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bodiga S., Zhong J.C., Wang W. Enhanced susceptibility to biomechanical stress in ACE2 null mice is prevented by loss of the p47phox NADPH oxidase subunit. Cardiovasc Res. 2011;91(1):151–161. doi: 10.1093/cvr/cvr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crackower M.A., Sarao R., Oudit G.Y. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 51.Yagil Y., Yagil C. Hypothesis: ACE2 modulates blood pressure in the mammalian organism. Hypertension. 2003;41:871–873. doi: 10.1161/01.HYP.0000063886.71596.C8. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto K., Ohishi M., Katsuya T. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension. 2006;47(4):718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura K., Koibuchi N., Nishimatsu H. Candesartan ameliorates cardiac dysfunction observed in angiotensin-converting enzyme 2-deficient mice. Hypertens Res. 2008;31(10):1953–1961. doi: 10.1291/hypres.31.1953. [DOI] [PubMed] [Google Scholar]
- 54.Kiely D.G., Cargill R.I., Wheeldon N.M., Coutie W.J., Lipworth B.J. Haemodynamic and endocrine effects of type 1 angiotensin II receptor blockade in patients with hypoxaemic cor pulmonale. Cardiovasc Res. 1997;33(1):201–208. doi: 10.1016/s0008-6363(96)00180-0. [DOI] [PubMed] [Google Scholar]
- 55.Imai Y., Kuba K., Rao S. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donoghue M., Wakimoto M., Maguire C.T. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol. 2003;35:1043–1053. doi: 10.1016/s0022-2828(03)00177-9. [DOI] [PubMed] [Google Scholar]
- 57.Zhang H., Penninger J.M., Li Y. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu X., Chen P., Wang J. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its Spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus Spike receptor-binding domain complexed with receptor. Science. 2005;309(5742):1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 60.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020;94(7):e00127–e00220. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu K.L., Peng G.Q., Wilken M., Geraghty R.J., Li F. Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus. J Biol Chem. 2012;287:8904–8911. doi: 10.1074/jbc.M111.325803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bertram S., Heurich A., Lavender H. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PloS One. 2012;7 doi: 10.1371/journal.pone.0035876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:1–10. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding Y., He L., Zhang Q. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gu J., Gong E., Zhang B. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/2Fjem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li W., Zhang C., Sui J. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simmons G., Gosalia D.N., Rennekamp A.J. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci USA. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glowacka I., Bertram S., Müller M.A. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsuyama S., Nagata N., Shirato K. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol. 2010;84:12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iwata-Yoshikawa N., Okamura T., Shimizu Y. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J Virol. 2019;93 doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawase M., Shirato K., van der Hoek L. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 2012;86:6537–6545. doi: 10.1128/2FJVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shulla A., Heald-Sargent T., Subramanya G. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol. 2011;85:873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shirato K., Kanou K., Kawase M., Matsuyama S. Clinical Isolates of Human Coronavirus 229E Bypass the Endosome for Cell Entry. J Virol. 2016;91 doi: 10.1128/JVI.01387-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shirato K., Kawase M., Matsuyama S. Wild-type human coronaviruses prefer cell-surface TMPRSS2 to endosomal cathepsins for cell entry. Virology. 2018;517:9–15. doi: 10.1016/j.virol.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ishiyama Y., Gallagher P.E., Averill D.B. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43(5):970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 76.Ramos H.R., López L.E., Castro W.Q., Serra C.M. High-sensitivity cardiac troponins: sex-specific values in clinical practice. Precision or confusion? Hellenic J Cardiol. 2019;60(3):171–177. doi: 10.1016/j.hjc.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 77.Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients with2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou Fei, Ting Yu, Du Ronghui. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flammer A.J., Anderson T., Celermajer D.S. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonetti P.O., Lerman L.O., Lerman A. Endothelial dysfunction - a marker of atherosclerotic risk. Arterioscl Throm Vas. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 81.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng Y., Ma Y., Zhang J. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;S0033–0620(20) doi: 10.1016/j.pcad.2020.03.001. 30055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi S., Qin M., Shen B. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020:E1–E8. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ding Y., Wang H., Shen H. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oudit G.Y., Kassiri Z., Jiang C. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong C.K., Lam C.W.K., Wu A.K.L. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. https://www.escardio.org/static_file/Escardio/Education-General/Topic/20pages/Covid-19/ESC/20Guidance/20Document/ESC-Guidance-COVID-19-Pandemic.pdf [Updated. 21. Apr. 2020]. Available at:
- 93.Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arentz M., Yim E., Klaff L. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peiris J.S., Chu C.M., Cheng V.C. HKU/UCH SARS Study Group. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chong P.Y., Chui P., Ling A.E. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med. 2004;128(2):195–204. doi: 10.1043/1543-2165(2004)128%3C195:AODDTS%3E2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 97.Clayton T.C., Capps N.E., Stephens N.G. Recent respiratory infection and the risk of myocardial infarction. Heart. 2005;91:1601–1602. doi: 10.1136/hrt.2004.046920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barnes M., Heywood A.E., Mahimbo A. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart. 2015;101(21):1738–1747. doi: 10.1136/2Fheartjnl-2015-307691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guo T., Fan Y., Chen M. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020:e201017. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alexander L.K., Keene B.W., Yount B.L. ECG changes after rabbit coronavirus infection. J Electrocardiol. 1999;32(1):21–32. doi: 10.1016/s0022-0736(99)90018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boriani G., Fauchier L., Aguinaga L., Group ESCSD European Heart Rhythm Association (EHRA) consensus document on management of arrhythmias and cardiac electronic devices in the critically ill and post-surgery patient, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin American Heart Rhythm Society (LAHRS) Europace. 2019;21(1):7–8. doi: 10.1093/europace/euy110. [DOI] [PubMed] [Google Scholar]
- 103.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respirat Med. 2020;S2213–2600(20) doi: 10.1016/S2213-2600(20)30079-5. 30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chan J.W., Ng C.K., Chan Y.H. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58(8):686–689. doi: 10.1136/2Fthorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang J., Zheng Y., Gou X., etal Prevalence of comorbidities in the novel Wuhan coronavirus(COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;S1201–9712(20) doi: 10.1016/j.ijid.2020.03.01732173574. 30136-3. [DOI] [Google Scholar]
- 106.Li G., Hu R., Zhang X. Antihypertensive treatment with ACEI/ARB of patients with COVID-19 complicated by hypertension. Hypertens Res. 2020;43:588–590. doi: 10.1038/s41440-020-0433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walters T.E., Kalman J.M., Patel S.K. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace. 2017;19:1280–1287. doi: 10.1093/europace/euw246. [DOI] [PubMed] [Google Scholar]
- 108.Onder G., Rezza G., Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 109.Chen J., Qi T., Liu L. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80(5):e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ferguson N., Laydon D., Nedjati Gilani G. 2020. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tonnesen P., Marott J.L., Nordestgaard B. Secular trends in smoking in relation to prevalent and incident smoking-related disease: A prospective population-based study. Tob Induc Dis. 2019;17:72. doi: 10.18332/tid/112459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou Z., Chen P., Peng H. Are healthy smokers really healthy? Tob Induc Dis. 2016;14:35. doi: 10.1186/s12971-016-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park J.E., Jung S., Kim A. MERS transmission and risk factors: a systematic review. BMC Publ Health. 2018;18(1):574. doi: 10.1186/s12889-018-5484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arcavi L., Benowitz N.L. Cigarette smoking and infection. Arch Intern Med. 2004;164(20):2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 115.Vardavas C.I., Nikitara K. COVID-19 and smoking: A systematic review of the evidence. Tob Induc Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang Y.T., Lee Y.C., Hsiao C.J. Hospitalization for ambulatory-care-sensitive conditions in Taiwan following the SARS outbreak: a population-based interrupted time series study. J Formos Med Assoc. 2009;108:386-394. doi: 10.1016/S0929-6646(09)60082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chan-Yeung M., Xu R.H. SARS: epidemiology. Respirology. 2003;8:S9-S14. doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morra M.E., Van Thanh L., Kamel M.G. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: a systematic review and meta-analysis. Rev Med Virol. 2018;28 doi: 10.1002/rmv.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roca-Ho H., Riera M., Palau V., Pascual J., Soler M.J. Characterization of ACE and ACE2 Expression within Different Organs of the NOD Mouse. Int J Mol Sci. 2017;18:563. doi: 10.3390/ijms18030563. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wysocki J., Ye M., Soler M.J. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55:2132–2139. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- 121.Rao S., Lau A., So H.-C. medRxiv; 2020. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of 2019-nCov: A Mendelian randomization analysis (Preprint) [DOI] [PubMed] [Google Scholar]
- 122.Fernandez C., Rysä J., Almgren P. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J Intern Med. 2018;284:377–387. doi: 10.1111/joim.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen X., Hu W., Ling J. medRxiv; 2020. Hypertension and diabetes delay the viral clearance in COVID-19 patients (Preprint) [DOI] [Google Scholar]
- 124.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dietz W., Santos-Burgoa C. Obesity; 2020. Obesity and its Implications for COVID-19 Mortality. [DOI] [PubMed] [Google Scholar]
- 126.Zheng K.I., Gao F., Wang X.B. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020:154244. doi: 10.1016/2Fj.metabol.2020.154244. published online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muscogiuri G., Pugliese G., Barrea L. Obesity: the “Achilles heel” for COVID-19? Metabolism. 2020;108:154251. doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hales K., Carroll M.D., Fryar C.D., Ogden C.L. no. 360. National Center for Health Statistics; Hyattsville, MD: 2020. (Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief). [PubMed] [Google Scholar]
- 129.Li X.C., Zhang J., Zhuo J.L. The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res. 2017;125:21–38. doi: 10.1016/j.phrs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respirat Med. 2020;8 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anguiano L., Riera M., Pascual J. Circulating angiotensin-converting enzyme 2 activity in patients with chronic kidney disease without previous history of cardiovascular disease. Nephrol Dial Transplant. 2015;30:1176–1185. doi: 10.1093/ndt/gfv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hristova M., Stanilova S., Miteva L. Serum concentration of renin-angiotensin system components in association with ACE I/D polymorphism among hypertensive subjects in response to ACE inhibitor therapy. Clin Exp Hypertens. 2019;41:662–669. doi: 10.1080/10641963.2018.1529782. [DOI] [PubMed] [Google Scholar]
- 133.Klimas J., Olvedy M., Ochodnicka-Mackovicova K. Perinatally administered losartan augments renal ACE2 expression but not cardiac or renal Mas receptor in spontaneously hypertensive rats. J Cell Mol Med. 2015;19(8):1965–1974. doi: 10.1111/jcmm.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun M.L., Yang J.M., Sun Y.P., Su G.H. Inhibitors of RAS might be a good choice for the therapy of COVID-19 pneumonia. Zhonghua Jiehe He Huxi Zazhi. 2020;43:E014. doi: 10.3760/cma.j.issn.1001-0939.2020.0014. 0. [DOI] [PubMed] [Google Scholar]
- 135.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020;2020:1–4. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Patients taking ACE-I and ARBs who contract COVID-19 should continue treatment, unless otherwise advised by their physician. heart.org; 2020. press release. [Google Scholar]
- 137.Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Keyaerts E., Li S., Vigen L. Antiviral Activity of Chloroquine against Human Coronavirus OC43 Infection in Newborn Mice. Antimicrob Agents Chemother. 2008;53(8):3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ohshio G., Saluja A.K., Leli U., Sengupta A., Steer M.L. Esterase inhibitors prevent lysosomal enzyme redistribution in two noninvasive models of experimental pancreatitis. Gastroenterology. 1989;96:853–859. [PubMed] [Google Scholar]
- 140.Vincent M.J., Bergeron E., Benjannet S. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yao X., Ye F., Zhang M. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gautret P., Lagier J.C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 143.Page R.L., O'Bryant C.L., Cheng D. American Heart Association Clinical Pharmacology and Heart Failure and Transplantation Committees of the Council on Clinical Cardiology; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes Research. Drugs That May Cause or Exacerbate Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2016;134(6):e32–e69. doi: 10.1161/CIR.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 144.Basu-Ray I., Soos M.P. StatPearls. StatPearls; Treasure Island (FL): 2020. Cardiac Manifestations Of Coronavirus.https://www.ncbi.nlm.nih.gov/books/NBK556152/ (COVID-19) [Updated 2020 Apr 12]. [Internet] [Google Scholar]
- 145.Chan J.F., Yao Y., Yeung M.L. Treatment With Lopinavir/Ritonavir or Interferon-β1b Improves Outcome of MERS-CoV Infection in a Nonhuman Primate Model of Common Marmoset. J Infect Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schulman S. Inhibition of warfarin activity by ribavirin. Ann Pharmacother. 2002;36(1):72–74. doi: 10.1345/aph.1A181. [DOI] [PubMed] [Google Scholar]
- 147.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mulangu S., Dodd L.E., Davey R.T. A Randomized, Controlled Trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leung Y.Y., Yao Hui L.L., Kraus V.B. Colchicine-Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45(3):341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.The 2015 ESC Guidelines on the diagnosis and management of pericardial diseases. https://www.ncbi.nlm.nih.gov/pubmed/26547486 PubMed – NCBI, Available from: [DOI] [PubMed]
- 151.Calkins H., Hindricks G., Cappato R. HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. J Arrhythmia. 2017;33(5):369–409. doi: 10.1016/j.joa.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Toldo S., Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15(4):203–214. doi: 10.1038/nrcardio.2017.161. [DOI] [PubMed] [Google Scholar]
- 153.Grailer J.J., Canning B.A., Kalbitz M. Critical Role for the NLRP3 Inflammasome during Acute Lung Injury. J Immunol. 2014;192(12):5974–5983. doi: 10.4049/jimmunol.1400368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li D., Ren W., Jiang Z., Zhu L. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol Med Rep. 2018;18(5):4399–4409. doi: 10.3892/mmr.2018.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Castaño-Rodriguez C., Honrubia J.M., Gutiérrez-Álvarez J. Role of Severe Acute Respiratory Syndrome Coronavirus Viroporins E, 3a, and 8a in Replication and Pathogenesis. mBio. 2018;9(3) doi: 10.1128/mBio.02325-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Siu K.-L., Yuen K.-S., Castaño-Rodriguez C. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019;33(8):8865–8877. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen I.-Y., Moriyama M., Chang M.-F., Ichinohe T. Severe Acute Respiratory Syndrome Coronavirus Viroporin 3a Activates the NLRP3 Inflammasome. Front Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maxmen A. More than 80 clinical trials launch to test coronavirus treatments. Nature. 2020;578:347–348. doi: 10.1038/d41586-020-00444-3. [DOI] [PubMed] [Google Scholar]
- 159.U. S. National Library of Medicine ClinicalTrials.gov. https://clinicaltrials.gov/ct2/help/how-find/index Available at:
- 160.Úri K., Fagyas M., Mányiné Siket I. New Perspectives in the Renin-Angiotensin-Aldosterone System (RAAS) IV: Circulating ACE2 as a Biomarker of Systolic Dysfunction in Human Hypertension and Heart Failure. PloS One. 2014;9(4) doi: 10.1371/journal.pone.0087845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hajar R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views. 2017;18(3):109-114. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_106_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Geerlings Suzanne E., Andy I., Hoepelman M. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26(3-4):259–265. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 163.Lea J.P., Nicholas S.B. Diabetes mellitus and hypertension: key risk factors for kidney disease. J Natl Med Assoc. 2002;94(8):7S-15S. [PMC free article] [PubMed] [Google Scholar]
- 164.Lalande S., Johnson B.D. Diastolic dysfunction: a link between hypertension and heart failure. Drugs Today. 2008;44(7):503-513. doi: 10.1358/dot.2008.44.7.1221662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121(6):21-33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ponnappan S., Ponnappan U. Aging and immune function: molecular mechanisms to interventions. Antioxidants Redox Signal. 2011;14(8):1551-1585. doi: 10.1089/ars.2010.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Onor I.O., Stirling D.L., Williams S.R. Clinical Effects of Cigarette Smoking: Epidemiologic Impact and Review of Pharmacotherapy Options. Int J Environ Res Publ Health. 2017;14(10):1147. doi: 10.3390/ijerph14101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tragaki A., Panagiotakos D. Population ageing and cardiovascular health: the case of Greece. Hellenic J Cardiol. 2018;59(6):360–361. doi: 10.1016/j.hjc.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 169.Nikolaou M., Lazaros G., Karavidas A. Recurrent viral myocarditis: The emerging link toward dilated cardiomyopathy. Hellenic J Cardiol. 2018;59(1):60–63. doi: 10.1016/j.hjc.2017.08.003. [DOI] [PubMed] [Google Scholar]