To the Editor:
Coronavirus disease 2019 (COVID-19) is globe-trotting, and thousands of researchers and stakeholders are spending repose-less days and sleepless nights in search of effective therapies. Currently, the entire research sphere is dealing with a pandemic triad: hypes, hypotheses, and hopes. In the absence of a specific antiviral agent or vaccine against novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), “repurposing” of old time-tested medications is being tried. Famotidine is the most recent addition to this trend, creating a lot of hustle among the public and stirring criticism in the scientific arena.1 A phase 3 trial “Multi-site Adaptive Trials Using Hydroxychloroquine for COVID-19” (MATCH; ClinicalTrials.gov identifier: NCT04370262) has already been launched inconspicuously.1 , 2 This randomized double-blind clinical trial (N=1170) has been designed to compare clinical outcomes between 2 arms: one receiving hydroxychloroquine 200 mg plus famotidine (360 mg/d intravenously) and the other receiving hydroxychloroquine plus placebo. Famotidine will be administered for a maximum of 14 days or up to hospital discharge, whichever will come earlier.2 In this briefing, we will try to enlighten some facts regarding whether it is truly possible for famotidine to have a beneficial effect in COVID-19 or is it just hitting the castle in a Don Quixote way.
Antithetical to the initial belief, SARS-CoV-2 is a multisystemic illness with an array of manifestations protean in disease progression, severity, and outcome. The key pathogenesis revolves around the “cytokine storm” occurring because of the disruption of a delicate balance between proinflammatory and anti-inflammatory mediators and a depressed immune system.3 The climacteric role for the resolution of viral infection will be imparted upon the complex interplay between innate and adaptive immune systems in the host. Although an irrefutable pathogenesis and an efficacious vaccine is still a dream, attenuation of perpetual hyperinflammation is the bull’s-eye at this moment.
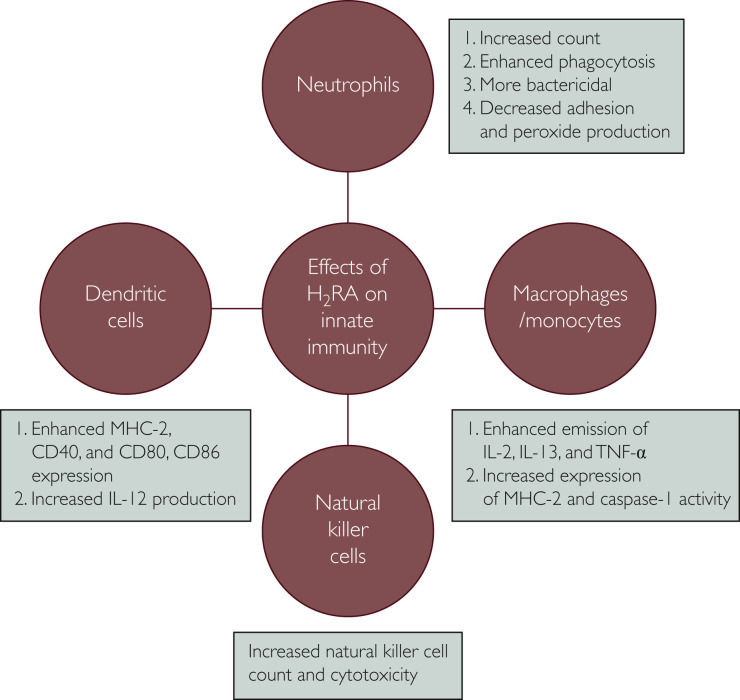
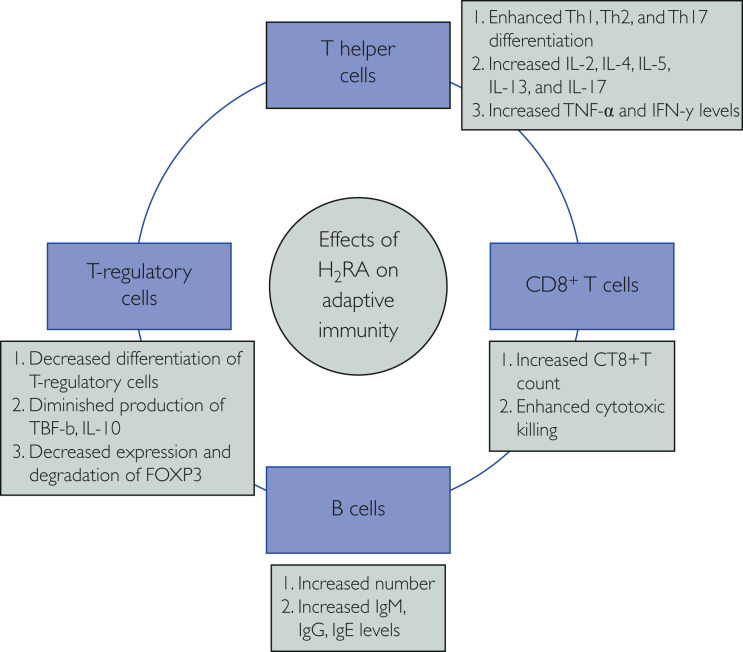
It is not the maiden time that the scientists have decided to “repurpose” the drug famotidine, an age-old antacid, to combat a viral disease. The effects of histamine on different substrates of immune system and immunomodulatory effects of H2 receptor antagonists (H2RAs) are well recognized.4 Through binding with histamine receptor 2 and modulating the effector pathways mediated by protein kinase A, famotidine potentially regulates innate and adaptive immune responses (Figures 1 and 2 ). It modulates antibody generation by B cells, cytokine release by T helper cell 1 (Th1), T-cell differentiation and proliferation, mast cell degranulation, and dendritic cell response.5 Innate immune system function is potentially boosted by stimulatory effects of H2RAs on its effectors, that is, macrophages, neutrophils, monocytes, dendritic cells, natural killer cells, and natural killer–T cells, and the adaptive system is filliped by activation of helper T cells (Th1, Th2, and Th17), regulatory T cells, and cytotoxic CD8+ T cells.6 It has been documented that famotidine completely demolishes histamine receptor 2–mediated negative effects on cytokine production, especially tumor necrosis factor-α (TNF-α) and interferon-γ7; lipopolysaccharide-induced TNF-α production; and B7-1 expression on monocytes,8 and also curtails the inhibitory effects of histamine on the production of Th1-mediated cytokine release.9 H2RAs have been used in many other conditions, such as cancer, viral infection, bone remodeling, burn management, and vaccine potency enhancer, with mixed results.6 Previously, H2RA has been used with some success against HIV,10 , 11 human papilloma virus,12 herpes simplex virus,13 Epstein-Barr virus,14 and chronic hepatitis B infection.15 Ranitidine bismuth citrate has been found to inhibit the nucleoside triphosphate hydrolase and DNA unwinding activities of the SARS-CoV helicase and hinders its replication.16
Figure 1.
Effects of H2 receptor antagonist (H2RA) on the innate immune system. IL-# = interleukin #; MHC-2 = major histocompatibility complex-2; TNF-α = tumor necrosis factor α.
Figure 2.
Effects of H2 receptor antagonist (H2RA) on the adaptive immune system. FOXP3 = forkhead box P3; IL-# = interleukin #, INF-γ = interferon-γ; TGF-β = transforming growth factor beta; Th# = T helper cell #; TNF-α = tumor necrosis factor.
Although the above mechanistic explanations sound reasonable, the real outcomes in clinical trials might be completely futile as evidenced previously.11 The unpublished Chinese data that received publicity in the press claiming that the mortality rate for patients with COVID-19 taking famotidine was 14% compared with 27% for those not taking the drug reported not to be statistically significant.1 However, before concluding anything from this, one needs to analyze actual complete data along with the confounders. Moreover, scientists’ claims of famotidine having anti-protease–like effects1 have not stemmed from any strong published evidence, but rather from the evidence of the negative pharmacokinetic effects of famotidine on protease inhibitors.17 The dosage of famotidine being used in the MATCH trial is nearly 10 times greater than the usual dosage used for severe forms of peptic ulcer diseases. Although famotidine is a time-tested and safe drug, excessive inhibition of gastric acid secretion might precipitate pneumonia.18 Cardiac failure and arrhythmias have also been reported with high doses of intravenous famotidine administration.19
Considering its relative cheapness, wide availability, and previous use as an antiviral agent, famotidine might usher some hope; however, we must wait for the trial results. Until then, hoarding and therapeutic misadventure with this drug must be condemned.
Footnotes
Potential Competing Interests: The authors report no competing interests.
Reference
- 1.Borrell B. New York clinical trial quietly tests heartburn remedy against coronavirus. Science. https://www.sciencemag.org/news/2020/04/new-york-clinical-trial-quietly-tests-heartburn-remedy-against-coronavirus Published April 26, 2020.
- 2.Multi-site Adaptive Trials Using Hydroxychloroquine for COVID-19 (MATCH). ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/show/NCT04370262
- 3.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahm K.B., Kim W.H., Lee S.I., Kang J.K., Park I.S. Comparison of immunomodulative effects of the histamine-2 receptor antagonists cimetidine, ranitidine, and famotidine on peripheral blood mononuclear cells in gastric cancer patients. Scand J Gastroenterol. 1995;30(3):265–271. doi: 10.3109/00365529509093275. [DOI] [PubMed] [Google Scholar]
- 5.Frei R., Ferstl R., Konieczna P. Histamine receptor 2 modifies dendritic cell responses to microbial ligands. J Allergy Clin Immunol. 2013;132(1):194–204. doi: 10.1016/j.jaci.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Jafarzadeh A., Nemati M., Khorramdelazad H., Hassan Z.M. Immunomodulatory properties of cimetidine: Its therapeutic potentials for treatment of immune-related diseases. Int Immunopharmacol. 2019;70:156–166. doi: 10.1016/j.intimp.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Smolinska S., Groeger D., Perez N.R. Histamine receptor 2 is required to suppress innate immune responses to bacterial ligands in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22(7):1575–1586. doi: 10.1097/MIB.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 8.Takagaki K., Osawa S., Horio Y. Cytokine responses of intraepithelial lymphocytes are regulated by histamine H(2) receptor. J Gastroenterol. 2009;44(4):285–296. doi: 10.1007/s00535-009-0019-9. [DOI] [PubMed] [Google Scholar]
- 9.Morichika T., Takahashi H.K., Iwagaki H. Histamine inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production in an intercellular adhesion molecule-1- and B7.1-dependent manner. J Pharmacol Exp Ther. 2003;304(2):624–633. doi: 10.1124/jpet.102.042515. [DOI] [PubMed] [Google Scholar]
- 10.Bourinbaiar A.S., Fruhstorfer E.C. The effect of histamine type 2 receptor antagonists on human immunodeficiency virus (HIV) replication: identification of a new class of antiviral agents. Life Sci. 1996;59(23):PL365–PL370. doi: 10.1016/s0024-3205(96)00553-x. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett J.A., Berry P.S., Bockman K.W. A placebo-controlled trial of ranitidine in patients with early human immunodeficiency virus infection. J Infect Dis. 1998;177(1):231–234. doi: 10.1086/517361. [DOI] [PubMed] [Google Scholar]
- 12.Gooptu C., Higgins C.R., James M.P. Treatment of viral warts with cimetidine: an open-label study. Clin Exp Dermatol. 2000;25(3):183–185. doi: 10.1046/j.1365-2230.2000.00608.x. [DOI] [PubMed] [Google Scholar]
- 13.Kürkçüoğlu N., Alli N. Cimetidine prevents recurrent erythema multiforme major resulting from herpes simplex virus infection. J Am Acad Dermatol. 1989;21(4, pt 1):814–815. doi: 10.1016/s0190-9622(89)80290-7. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein J.A. Cimetidine, ranitidine, and Epstein-Barr virus infection. Ann Intern Med. 1986;105(1):139. doi: 10.7326/0003-4819-105-1-139_2. [DOI] [PubMed] [Google Scholar]
- 15.Xie X., Geng S., Liu H., Li C., Yang Y., Wang B. Cimetidine synergizes with praziquantel to enhance the immune response of HBV DNA vaccine via activating cytotoxic CD8(+) T cell. Hum Vaccin Immunother. 2014;10(6):1688–1699. doi: 10.4161/hv.28517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang N., Tanner J.A., Zheng B.J. Bismuth complexes inhibit the SARS coronavirus. Angew Chem Int Ed Engl. 2007;46(34):6464–6468. doi: 10.1002/anie.200701021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Boffito M., Zhang J. Effects of the H2-receptor antagonist famotidine on the pharmacokinetics of atazanavir-ritonavir with or without tenofovir in HIV-infected patients. AIDS Patient Care STDS. 2011;25(9):509–515. doi: 10.1089/apc.2011.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eom C.S., Jeon C.Y., Lim J.W., Cho E.G., Park S.M., Lee K.S. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ. 2011;183(3):310–319. doi: 10.1503/cmaj.092129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoenwald P.K., Sprung J., Abdelmalak B., Mraović B., Tetzlaff J.E., Gurm H.S. Complete atrioventricular block and cardiac arrest following intravenous famotidine administration. Anesthesiology. 1999;90(2):623–626. doi: 10.1097/00000542-199902000-00040. [DOI] [PubMed] [Google Scholar]