Abstract
Aim
Previous studies have suggested a more frequent and severe course of novel coronavirus SARS-CoV-2 infection in cancer patients undergoing active oncologic treatment. Our aim was to describe the characteristics of the disease in this population and to determine predictive factors for poor outcome in terms of severe respiratory distress (acute respiratory distress syndrome [ARDS]) or death.
Patients and methods
Patients consecutively admitted for SARS-CoV-2 infection were prospectively collected, and retrospective statistical analysis was performed. Univariate and multivariate analyses were performed to assess potential factors for poor outcomes defined as ARDS or death.
Results
Sixty-three patients were analysed, and 34 of them developed respiratory failure (70% as ARDS). Lymphocytes/mm3 (412 versus 686; p = 0.001), serum albumin (2.84 versus 3.1); lactate dehydrogenase (LDH) (670 versus 359; p < 0.001) and C-reactive protein (CRP) levels (25.8 versus 9.9; p < 0.001) discriminate those that developed respiratory failure. Mortality rate was 25%, significantly higher among ARDS, neutropenic patients (p = 0.01) and in those with bilateral infiltrates (44% versus 0%; p < 0.001). Multivariate logistic analyses model confirmed the predictive value of severe neutropenia (odds ratio [OR] 16.54; 95% confidence interval [CI] 1.43–190.9, p 0.025), bilateral infiltrates (OR 32.83, CI 95% 3.51–307, p 0.002) and tumour lung involvement (OR 4.34, CI 95% 1.2–14.95, p 0.02).
Conclusion
Cancer patients under active treatment admitted for SARS-CoV-2 infection have worse outcomes in terms of mortality and respiratory failure rates compared with COVID-19 global population. Lymphopenia, LDH, CRP and albumin discriminate illness severity, whereas neutropenia, bilateral infiltrates and tumour pulmonary involvement are predictive of higher mortality.
Keywords: 2019 novel coronavirus infection, COVID-19 virus disease, Predictive factors, Cáncer, Treatment, Mortality, Respiratory distress syndrome, Adult
1. Introduction
Since the outbreak of the novel coronavirus disease (COVID-19) in Wuhan (China) on December 2019 [1], the spread of this disease to the rest of the world including Europe and the United States of America [2] led the World Health Organisation (WHO) to declare the infection caused by SARS-CoV-2 a public health emergency of international concern in March, 11th 2020 [3]. SARS-CoV-2 infection ranges from asymptomatic course to severe lower respiratory tract infection with pulmonary infiltrates that could evolve to acute respiratory distress syndrome (ARDS) and death.
Cancer patients, especially those undergoing active oncologic, could represent a more vulnerable group among infected population. Only a few case series or retrospective studies have been published so far with limited clinical information and small sample size. Specific data regarding cancer history, antitumour treatment, respiratory course, laboratory testing and mortality rates are scant [4,5]. Despite patients receiving active anticancer treatment are clearly underreported in those papers aforementioned, they agree in a higher incidence of infection and risk of severe events among cancer patients [6].
The objective of this work was to describe the characteristics, clinical manifestations, course and outcomes of patients under active antitumour treatment admitted due to SARS-CoV-2 infection and also to determine predictive factors of poor outcome in this specific population.
2. Material and methods
2.1. Patients and study design
Since March 9th, on-site active screening for patients attending our Outpatient clinic and Oncologic Urgent Care Unit was implemented. Until April 19th, 287 patients had been screened by means of clinical assessment, chest X-ray imaging, complete blood test and a nasopharyngeal swab for specific real-time or reverse-transcriptase PCR (RT-PCR) for SARS-CoV-2.
SARS-CoV-2–confirmed cases were considered those with positive testing by RT-PCR following WHO recommendations for viral testing in either nasopharyngeal swab or sputum. Highly suspicious patients were considered those with symptoms, blood testing results, as well as radiological infiltrates consistent with viral pneumonia despite two consecutively negative RT-PCR results. Patients showing radiologically proven pneumonia as well as those presenting with hypoxemia (defined as arterial pO2<80 mmHg) were admitted.
Patients underwent complete medical exam followed by complete blood testing, electrocardiogram (EKG) and chest X-ray at admission. Lab testing was repeated every 24 or 48 h including complete blood count, serum biochemistry (including renal and liver function testing, lactate dehydrogenase [LDH] levels, C-reactive protein levels [CRP] and ferritin) as well as coagulation profile (including fibrinogen and D-Dimer –DD-levels). Interleukin-6 (IL-6) was only measured at the time of admission as well as in case of respiratory deterioration.
2.2. Treatments for COVID-19
Specific treatment for viral infection was started according to the limited medical evidence at the time of admission and reviewed in a timely manner: in case of no contraindication, lopinavir/ritonavir (LPV/r), hydroxichloroquine (HCQ) and azithromycin (AZ), triple therapy was initiated. After the results of Cao et al. [7], patients were treated with HCQ [8,9] and AZ alone. In selected cases with ARDS and/or hyperinflammatory patient condition defined by high ferritin, D-Dimers and CRP serum levels, tocilizumab (TCZ), a monoclonal antibody against IL-6 was employed as previously described [10].
2.3. Study definitions
Metastatic status was divided in patients with either 1 metastatic location (oligometastatic) or ≥ 2metastatic locations (polymetastatic). Pulmonary tumour involvement was considered in case of documented lung metastases or a primary lung cancer. Active oncologic treatment was defined as any antitumour therapy administered within 4 weeks before admission. Oncologic treatment was divided according to its main components into highly myelosuppressant or non-myelosuppressant, chemotherapy regimens, endocrine therapy, target therapy and immunotherapy (anti-PD1 or anti-CTLA4 drugs). Eastern Cooperative Oncology Group (ECOG) score was evaluated at the moment of admission.
Significant previous medical comorbidities were recorded in terms of chronic kidney disease, cardiopathy, chronic obstructive pulmonary disease (COPD), anaemia, previous venous thromboembolic disease (VTED) and chronic glucocorticoid therapy.
Previous anticoagulation therapy was defined in every patient under chronic anticoagulation either with heparin or oral anticoagulants.
Respiratory failure was defined as the need of any supplementary oxygen therapy. ARDS was defined as any acutely developed respiratory event showing PaFi<200 mmHg on FiO2 21%. This definition correlates with moderate and severe ARDS as they are defined in the interim guidance of the WHO for COVID-19 [11]. Time to respiratory failure is defined as number of days since beginning of symptoms until development of severe hypoxemia defined as arterial pO2<60 mmHg. Time to SpO2 improvement is defined as number of days required for first decrease in oxygen requirements because instauration of respiratory failure (either as O2 flow decrease or as oxygen therapy device switch to lesser O2 flow requirement). Time to death is defined as number of days since beginning of the symptoms until death of any cause.
Laboratory findings were collected as the absolute nadir or the highest value reached during hospitalisation.
2.4. Statistical analysis
For categorical variables, we applied Pearson's chi-squared test or Fisher's exact test (if any value was n < 5). Quantitative continuous variables were expressed as means and compared with Student's t test; categorical variables were expressed as number (%) and compared by Fisher's exact test. Pie charts were dropped to describe oncologic population. We used logistic regression to calculate the odds ratio (OR) and 95% confidence interval (95% CI) for dichotomic variables. All inferential analysis were performed using two-sided methods (α = 0.05), and results were considered significant when p-values were <0.05. Statistical analysis was performed using STATA software, version, 14∙2. (StataCorp LP, College Station, TX, USA).
3. Results
3.1. Main clinical and cancer-related characteristics
Until April 19th, 287 patients had been screened, and 90 required inpatient treatment due to COVID-19. We present here the first 63 consecutive oncologic patients that were admitted. Baseline clinicopathological characteristics of patients are shown in Table 1 .
Table 1.
Main clinical and cancer-related characteristics of patients with active cancer treatment admitted due to COVID19 infection.
| Demographics | All patients (N = 63) | ||
|---|---|---|---|
| Age | <50 years | 4 (6%) | |
| 50–70 years | 36 (57%) | ||
| >70 years | 23 (37%) | ||
| Sex | Male | 34 (54%) | |
| Female | 29 (46%) | ||
| Race | Caucasian | 54 (86%) | |
| Latin American | 4 (6%) | ||
| Others | 5 (8%) | ||
| Smoking history |
Never smoker | 29 (46%) | |
| Former smoker | 27 (43%) | ||
| Current smoker |
7 (11%) |
||
| Comorbidities |
No |
Yes |
|
| Previous anaemia | 53 (84%) | 10 (16%) | |
| Hypertension | 30 (48%) | 33 (52%) | |
| Diabetes | 52 (83%) | 11 (17%) | |
| Chronic kidney disease | 58 (92%) | 5 (8%) | |
| Cardiopathy | 51 (80%) | 12 (19%) | |
| Chronic pulmonary disease | 49 (78%) | 14 (22%) | |
| Previous VTED | 50 (79%) | 13 (21%) | |
| ACEi/ARBs treatment | 53 (84%) | 10 (16%) | |
| Anticoagulant therapy | 50 (79%) | 13 (21%) | |
| Chronic corticosteroids | 48 (76%) | 15 (24%) | |
| >10 mg | NA |
12 (19%) | |
| <10 mg |
3 (5%) |
||
| Cancer-related characteristics |
No |
Yes |
|
| Metastatic disease | 11(18%) | 52(82%) | |
| Oligometastatic | NA | 36 (57%) | |
| Polymetastatic | 16 (25%) | ||
| Tumour pulmonary involvement | 38 (60%) | 25 (40%) | |
| Active cancer treatment | 2 (4%) | 61 (96%) | |
| Chemotherapy | NA | 36 (58%) | |
| Endocrine | 10 (15%) | ||
| Target therapy | 7 (11%) | ||
| I mmunotherapy | 8 (12%) | ||
Numbers are expressed as n (%).
VTE, venous thromboembolic disease; ACEi/ARBs, angiotensin converting enzyme inhibitor/angiotensin receptor blockers.
Fifty-two patients (83%) were RT-PCR confirmed cases, whereas 11 patients (17%) were diagnosed based on clinical and radiological findings. Mean age was 66 (CI 95% 63.4–68.8). Thirty-four (54%) were male, and most (86%) were Caucasian. Thirty-four patients had previous smoking history among which seven (11%) were current smokers. Regarding previous medical comorbidities, hypertension was documented in 30 patients (48%), diabetes mellitus in 11 (17%), chronic kidney disease in five (8%), cardiopathy in 12 (19%), chronic pulmonary disease in 14 (22%) and previous venous thromboembolic (VTE) in 13 patients (21%). Anaemia was present in 10 (16%), and 15 patients admitted were on chronic steroid therapy (24%), 12 of them taking a daily dose greater than 10 mg of prednisone or equivalent. The ECOG performance status was 0–1 in 54 (85%) of patients.
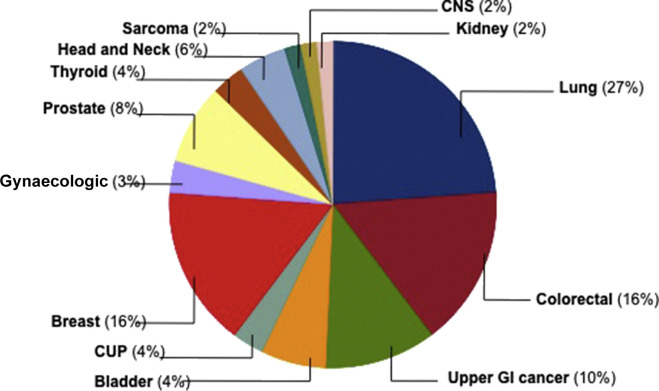
Regarding tumour characteristics and active oncologic therapies, most frequent primary tumour sites were lung cancer (27%), followed by colorectal (16%) and breast cancer (16%) [Fig. 1 ]. Disease was metastatic in 52 patients (88%) with more than two metastatic locations in 25% of them. Visceral and pulmonary tumour involvement was documented in 48% and 40% of cases, respectively. Almost all (61 cases, 96%) were under active antitumour treatment, 58% of them were receiving chemotherapy-based regimens, 15% endocrine therapy, 11% targeted therapy and 12% were under immunotherapy.
Fig. 1.
Pie chart with distribution as per primary tumour site of patients with SARS-CoV-2 infection. CUP, cancer of unknown primary; CNS, central nervous system; GI, gastroIntestinal.
3.2. COVID-19–related clinical characteristics and respiratory outcome in cancer patients
Fever (>38.5°C) was present in 89% of our cancer population, and malaise was the second most related symptom (70%) followed by cough in 66% of cases. Some grade of dyspnoea was reported by 45% of all subjects. Other previously described symptoms of SARS-CoV-2 infection such as rhinorrhea and hyposmia were less frequent symptoms in our series (8% and 18% respectively). Ninety-five percent had documented lung infiltrates in chest X-ray or CT scan, among which 55% showed bilateral pneumonia. Six percent of the patients did not receive specific treatment, 27% were treated with LPV/r + HCQ + AZ and 67% received HCQ + AZ alone [Table 2 ].
Table 2.
Symptoms, radiological pattern and treatment administered to cancer patients with SARS-CoV-2.
| n (%) | All patients (N = 63) |
|---|---|
| Symptoms | |
| Fever | 56 (89%) |
| Cough | 41 (66%) |
| Rhinorrhea | 11 (18%) |
| Malaise | 44 (70%) |
| Dyspnoea | 28 (45%) |
| Diarrhoea | 31 (50%) |
| Hyposmia/Ageusia | 5 (8%) |
| Radiological pattern | |
| No infiltrates | 3 (5%) |
| Unilobar pneumonia | 17 (28%) |
| Bilobar pneumonia | 10 (17%) |
| Bilateral pneumonia | 33 (55%) |
| Treatment | |
| No specific treatment | 4 (6%) |
| LPV/r + HCQ + AZ | 16 (27%) |
| HCQ + AZ | 41 (67%) |
LPV/r, lopinavir/ritonavir; HCQ, hydroxichloroquine; AZ, azithromycin.
Thirty-four patients (54%) experienced respiratory failure as previously defined; and ARDS was diagnosed in 24 cases (38% of global population). Twenty-nine percent of those suffering ARDS had lung cancer, 87% had metastatic disease and 46% had previously documented tumour pulmonary involvement. More than 50% of them had received chemotherapy-based oncologic treatment within 4 weeks. Those patients with bilateral pneumonia showed significantly higher rates of respiratory failure (79% versus 20%, p < 0.001) and ARDS (91% versus 8%; p < 0.001). Regarding comorbidities 90% (9/10) of patients with previously documented chronic anaemia developed respiratory failure (p = 0.01), 60% as ARDS [Table 3 ].
Table 3.
Clinical course among cancer patients with SARS-CoV-2 infection.
| n (%) | Overall respiratory failure |
ARDS |
||||
|---|---|---|---|---|---|---|
| N = 34 | p value | N = 24 | p value | |||
| Primary tumour site | ||||||
| Lung | 9 (26%) | 0.54 | 7 (29%) | 0.54 | ||
| Other site | 25 (74%) | 17 (71%) | ||||
| Metastatic status | ||||||
| Non-metastatic | 5 (14%) | 0.53 | 3 (13%) | 0.∙5 | ||
| Metastatic | 29 (86%) | 21 (87%) | ||||
| Tumour pulmonary involvement | 0.11 | |||||
| Yes | 14 (41%) | 0.18 | 11 (46%) | 0.11 | ||
| No | 20 (59% | 13 (54%) | ||||
| Cancer treatment | ||||||
| Chemotherapy | 19 (56%) | 0.∙28 | 7 (29%) | 0.64 | ||
| Endocrine | 6 (18%) | 4 (17%) | ||||
| Target therapy | 2 (6%) | 1 (4%) | ||||
| Inmunotherapy | 4 (12%) | 3 (13%) | ||||
| Radiological pattern | ||||||
| Unilobar/bilobar | 7 (20%) | <0.001 | 2 (8%) | <0.001 | ||
| Bilateral/multilobar | 27 (79%) | 22 (91%) | ||||
| Age | ||||||
| <50 | 3 (9%) | 2 (8%) | ||||
| 50–70 | 19 (56%) | 12 (50%) | ||||
| >70 | 12 (35%) | 0.8 | 10 (42%) | 0.5 | ||
| Smoking history | ||||||
| Never smoker | 15 (44%) | 0.77 | 0.32 | 11 (46%) | 0.92 | 0.41 |
| Former smoker | 14 (42%) | 9 (37%) | ||||
| Current smoker | 5 (14%) | 4 (17%) | ||||
| Sex | ||||||
| Male | 19 (56%) | 0.74 | 13 (54%) | 0.92 | ||
| Female | 15 (44%) | 11 (46%) | ||||
| Comorbidities | ||||||
| Previous anaemia | 9 (27%) | 0.01 | 6 (25%) | 0.12 | ||
| HTA | 20 (59%) | 0.44 | 14 (58%) | 0.65 | ||
| Diabetes | 6 (18%) | 0.36 | 6 (25%) | 0.16 | ||
| Chronic kidney disease | 4 (12%) | 0.22 | 3 (13%) | 0.29 | ||
| Cardiopathy | 6 (18%) | 0.76 | 2 (8%) | 0.1 | ||
| COPD | 8 (24%) | 0.78 | 5 (20%) | 0.83 | ||
| Previous VTED | 7 (21%) | 0.9 | 6 (25%) | 0.5 | ||
| Previous ACEi/ARBs | 5 (14%) | 0.6 | 3 (13%) | 0.97 | ||
| Anticoagulant therapy | 8 (24%) | 0.53 | 6 (25%) | 0.5 | ||
| Chronic corticosteroids | ||||||
| <10 mg | 2 (6%) | 0.∙32 | 1 (4%) | 0.77 | ||
| >10 mg | 8 (24%) | 5 (20%) | ||||
Numbers are expressed as n/N for each category. p values for Fisher's exact test.
VTE, venous thromboembolic disease; COPD, chronic obstructive pulmonary disease, ACEi/ARBs, angiotensin converting enzyme inhibitor/angiotensin receptor blockers; HTA, hypertension.
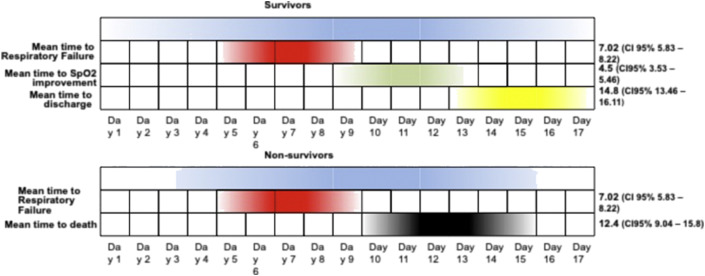
Mean time to respiratory failure was 7.02 days (CI 95% 5.83–8.22) since beginning of symptoms. Mean time to SpO2 improvement was 4.5 days (CI 95% 3.53–5.46) since respiratory failure. Mean time to hospital discharge was 14.8 days (CI 95% 13.46–16.11) since beginning of symptoms. Mean length of inpatient stay was 9.2 days (CI 95% 8.17–10.33). Among non-survivors, mean time to death since beginning of symptoms was 12.4 days (CI 95% 9.04–15.8) [Fig. 2 ].
Fig. 2.
Clinical and respiratory course of SARS-CoV-2 survivors and non-survivors among inpatient cancer population in our study. CI, confidence interval.
3.3. Laboratory findings and respiratory outcome
Neutropenia was more frequent among patients who developed ARDS (p = 0.001). On the contrary, Platelets count (Differential platelets count (DPC)) lower than 100,000/mm3 was not statistically different between patients without respiratory failure and those who developed ARDS (p = 0.06).
Mean lymphocyte count/mm3 (412, CI 95% 297–527 versus 686, CI 95% 555–817; p = 0.001) and serum albumin levels (2.84, CI 95% 2.69–2.98 versus 3.1, CI 95% 2.95–3.3, p = 0.007) were significantly lower in patients with ARDS than in patients who did not develop any grade of respiratory failure. Similar findings were obtained regarding higher LDH (670, CI 95% 529–810 versus 359, CI 95% 321–397; p < 0.001) or CRP serum levels (25.8, CI 95% 20.1–31.4 versus 9.9, 7.4–12.4; p < 0∙001) [Table 4 ].
Table 4.
Relationship between laboratory findings and respiratory outcomes in SARS-CoV-2 infected cancer patients.
| Laboratory data | Respiratory outcome |
||
|---|---|---|---|
| ARDS N = 24 |
No respiratory failure N=29 |
p value∗ | |
| Neutrophils < 500/mm3 | 13% |
0% |
0.01 |
| Mean, (CI 95%) |
Mean, (CI 95%) |
p valueTT |
|
| Lymphocytes/mm3 | 412 (297–527) | 686 (555–817) | 0.001 |
| Lactate dehydrogenase, U/L | 670 (529–810) | 359 (321–397) | <0.001 |
| Reactive C protein, mg/dL | 25.8 (20.1–31.4) | 9.9 (7.4–12.4) | <0.001 |
| Fibrinogen, mg/dL | 921 (842–999) | 804 (725–882) | 0.02 |
| D-Dimers, ng/mL | 8358 (0–17,159) | 2303 (1589–2018) | 0.06 |
| Ferritin ng/mL | 4374 (831–7914) | 1487 (917–2061) | 0.03 |
| Albumin, g/dL | 2.84 (2.69–2.98) | 3.1 (2.95–3.3) | 0.007 |
| Interleukin-6, pg/mL | 466 (0–934) | 98.7 (0–260) | 0∙06 |
Numbers are expressed as either proportions of total observations or means.
CI, confidence interval.
∗p values for Fisher's exact test.
TT p values for Student's t test.
3.4. Venous thromboembolic events
Overall incidence of new VTE in our population was 14%. Moreover, 23% of patients who were under chronic anticoagulation therapy at admittance developed new VTE event (2 as deep venous thrombosis (DVT) and 1 as pulmonay embolism (PE)).
3.5. Mortality outcome and multivariate analysis
Overall mortality was 25.4% (16/63). 37% had lung cancer, and 63% showed tumour pulmonary involvement. Mortality rate among patients that develop respiratory failure was 47% (16/34), and in case of ARDS, mortality raised up to 67%. Besides ARDS, higher mortality rates were significantly associated with tumour pulmonary involvement (p = 0.006), severe neutropenia (p = 0.01) and bilateral pneumonia (p < 0.001) [Table 5 ].
Table 5.
Mortality as outcome among admitted cancer patients with SARS-CoV-2 infection.
| n (%) | Mortality rate | p valueT |
|---|---|---|
| Primary tumour site | ||
| Lung,% (n/N) | 40% (6/15) | 0.17 |
| Other site,% (n/N) | 21% (10/48) | |
| Metastatic status | ||
| Non-metastatic, % (n/N) | 9% (1/11) | 0.17 |
| Metastatic, % (n/N) | 29% (15/52) | |
| Pulmonary extension | ||
| Yes, % (n/N) | 16% (6/38) | 0.006 |
| No, % (n/N) | 40% (10/25) | |
| Severe neutropenia | ||
| No | 0/60 | 0.01 |
| Yes | 3/3 (100%) | |
| Radiological pattern | ||
| Unilobar/bilobar, %(n/N) | 0%, (0/30) | <0∙001 |
| Bilateral, % (n/N) | 48%, (16/33) | |
| Sex | ||
| Male | 26% (9/34) | 0.83 |
| Female | 24% (7/29) | |
| Comorbidities (N = 63) | ||
| Previous anaemia, % (n/N) | 5% (3/63) | 0.71 |
| HTA | 16% (10/63) | 0.40 |
| Diabetes | 5% (3/63) | 0.70 |
| Chronic kidney disease | 3% (2/63) | 0.43 |
| Cardiomyopathy | 1.6% (1/63) | 0.13 |
| Chronic obstructive pulmonary disease | 3% (2/63) | 0.69 |
| Previous VTE | 9% (6/63) | 0.05 |
| Previous ACEi/ARBs | 1.6% (1/63) | 0.37 |
| Anticoagulant therapy | 5% (3/63) | 0.22 |
| Previous corticosteroids | 5% (3/63) | 0.15 |
Numbers are expressed as % and n/N, being n the number of deaths and N the total number of patients in that category except for comorbidities, where N is referred to the total number of patients in the study.
T p values for Fisher's exact test.
The multivariate logistic regression model, after adjusting for risk factors confirmed the association between bilateral pneumonia on baseline X-ray evaluation and development of ARDS (OR 21.4, CI 95% 4.2–108.55, p < 0.001). Previous episode of VTED (OR 4.82, CI 95% 1.14–20.3, p = 0.03), bilateral pneumonia (OR 32.83, CI 95% 3.51–307, p = 0.002), severe neutropenia (OR 16.54, CI 95% 1.43–190.9, p = 0.025) and pulmonary tumour involvement (OR 4.34, CI 95% 1.26–14.95, p = 0.02) were independently identified as predictive factors for increased risk of death. [Table 6 ].
Table 6.
Multivariable logistic predictive model for respiratory distress and mortality outcomes.
| n (%) | ARDS |
Mortality |
||||
|---|---|---|---|---|---|---|
| OR | CI 95% | p value | OR | CI 95% | p value | |
| Age, >65 years | 1.17 | 0.42–3.2 | 0.76 | 1.59 | 0.5–4.99 | 0.42 |
| Sex, Female = 1 | 0.98 | 0.35–2.74 | 0.98 | 0.88 | 0.28–2.73 | 0.83 |
| Smoking habit | ||||||
| Former smoker | 0.81 | 0.27–2.45 | 0.72 | 0.89 | 0.25–3.11 | 0.86 |
| Current smoker | 2.4 | 0.48–11.8 | 0.28 | 2.48 | 0.5–12.54 | 0.27 |
| Comorbidities | ||||||
| Hypertensiona | 1.5 | 0.5–4.43 | 0.46 | 2 | 0.68–5.88 | 0.21 |
| Diabetesa | 2.4 | 0.63–9.44 | 0.19 | 1.15 | 0.34–3.9 | 0.81 |
| CKDa | 3.5 | 0.46–26.18 | 0.22 | 2.39 | 0.29–19.85 | 0.42 |
| COPDa | 0.79 | 0.18–3.34 | 0.75 | 0.35 | 0.05–2.23 | 0.26 |
| Previous VTEDa | 1.79 | 0.48–6.7 | 0.39 | 4.82 | 1.14–20.3 | 0.03 |
| Chronic anaemiac | 2.76 | 0.62–12.3 | 0.18 | 1.32 | ||
| ACEI/ARBsIb | 0.64 | 0.11–3.5 | 0.6 | 0.23 | 0.02–2.23 | 0.2 |
| COPDd | 1.69 | 0.33–8.62 | 0.52 | 1.43 | 0.25–8.33 | 0.68 |
| Radiol infiltrates bilateral=1 | 21.4 | 4.2–108.55 | <0.001 | 32.83 | 3.51–307 | 0.002 |
| Neutropeniae | ||||||
| <1500 | 0.46 | 0.13–1.58 | 0.21 | 0.62 | 0.16–2.37 | 0.43 |
| <500 | 10.36 | 0.93–114.52 | 0.056 | 16.54 | 1.43–190.9 | 0.025 |
| Primary tumourf | ||||||
| Overall lung cancer | 1.81 | 0.49–6.7 | 0.37 | 2.35 | 0.58–9.51 | 0.22 |
| Colorectal cancer (lung=1) | 1.65 | 0.1–16.97 | 0.81 | 2.33 | 0.17–30.94 | 0.52 |
| Breast cancer (lung=1) | 0.9 | 0.12–6.54 | 0.9 | 2.47 | 0.17–34.23 | 0.5 |
| Genitourinary cancer (lung=1) | 1.56 | 0.17–14.13 | 0.69 | 2.29 | 0.17–30.73 | 0.53 |
| Other tumours (lung=1) | 1.86 | 0.37–9.19 | 0.44 | 2.13 | 0.42–10.82 | 0.36 |
| Metastatic diseaseg | 1.11 | 0.23–5.38 | 0.88 | 1.88 | 0.19–18.19 | 0.58 |
| Visceral metastatasish | 0.7 | 0.21–2.25 | 0.54 | 1.32 | 0.38–4.55 | 0.44 |
| Pulmonary involvementi | 1.96 | 0.63–6.14 | 0.24 | 4.34 | 1.26–14.95 | 0.02 |
| Cancer Treamentj | ||||||
| Chemotherapy alone | 1.36 | 0.4-4-56 | 0.68 | 1.6 | 0.4–6.33 | 0.5 |
| Immunotherapy + Chemo | 0.97 | 0.14–6.45 | 0.95 | 1,96 | 0.29–13.18 | 0.49 |
| Immnunotherapy alone | 0.26 | 0.03–1.88 | 0.18 | 0.15 | 0.01–1.65 | 0.12 |
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; VTED, venous thromboembolic disease; ACEi/ARBs, angiotensin converting enzyme inhibitor/angiotensin receptor blockers; ECOG, Eastern Cooperative Oncology Group; OR, odds ratio; CI, confidence interval.
Adjusted by age, sex, hypertension, diabetes, CKD, COPD, previous VTE, smoking habit.
Adjusted by age, sex, hypertension, diabetes, CKD, COPD, smoking habit.
Adjusted by age, sex, hypertension, diabetes, CKD, COPD, Previous VTE, smoking habit, oesophagogastric tumour.
Adjusted by age, sex, cardiopathy, previous VTED.
Adjusted by age, sex, hypertension, diabetes, CKD, COPD, Previous VTE, smoking habit.
Adjusted by age, sex, CT.
Adjusted by age, sex, COPD, previous VTE, metastasis, ECOG.
Adjusted by age, sex, previous VTE, pulmonary involvement.
Adjusted by age, sex, ECOG, metastasis, previous VTE.
Adjusted by age, sex, ECOG, metastasis, previous VTE, COPD.
4. Discussion
To the best of our knowledge, we present here the largest series of cancer patients undergoing active treatment admitted due to SARS-CoV-2 infection.
COVID-19 pandemic spread is supposed to be major risk for cancer patients because of their frequent comorbidities and more susceptibility to infections because of immunosuppression derived from both neoplasm and oncologic therapies. To our best knowledge, by mid-April 2020, only three reports have been published focus on this population [[4], [5], [6]]. They all suggest that cancer patients are more likely to be infected and/or that COVID-19 infection is more severe than in general population [12]. In these reports, there is a lack of data regarding cancer patients undergoing active oncological therapies (comprising only from 22% to 41% of cases), as well as there is no detailed information regarding possible predictive factors for poor outcome.
Our infected cancer patients were older (mean 66 years) than described both in East [13] and Western population [14] and similar to those from Yu et al. [5] and Zhang et al. [6]. Regarding previous condition, history of smoking habit was more frequent (11% current, 43% former smokers) than reported in non-cancer patients from New York City Area or from mainland China (both around 15%); as well as COPD (22% in cancer patients, 5.4% in US population 3% in China). Other comorbidities, such as hypertension, cardiopathy or chronic kidney disease, seem to be equally distributed among our cancer patients series and western population previously reported by Richardson et al. [14].
The highest incidence of COVID-19 infection was found among lung cancer subjects and colorectal cancer patients. In our series, 88% of infected patients presented with metastatic disease, and 96% had received antitumour treatment within 4 weeks before infection, being chemotherapy-based regimens in more than 50% of them. Given that previously published data have suggested a higher risk of severe events for patients who underwent chemotherapy or surgery in weeks before the infection, our results could be of special relevance.
Cancer patients under active oncological therapy show a higher mortality rate due to COVID-19 (25%) than previously reported for global population in China (1.4%) [13] but quite similar to actual death rate among hospitalised patients both in China [15] and New York City Area (24.5% and 25%, respectively). In our series, we found that only pulmonary tumour involvement (either primary or metastatic), severe neutropenia due to recent anticancer treatment and bilateral SARS-CoV-2–related pneumonia were statistically significant on multivariate analysis as independent mortality risk factors.
Interestingly, among cancer patients, we found higher incidence of both respiratory failure (54%) and ARDS (38%) than previously reported being respiratory failure more frequent in those patients with previous anaemia as well as among subjects with bilateral infiltrates (79%). In terms of primary tumour site, lung cancer patients accounted for 26% of all respiratory failure events. In our current series, no single cancer patient underwent mechanical ventilation nor admitted to intensive care unit given limited resources at disease outbreak in Madrid, Spain, that were further reserved for non-comorbid patients. This could explain the fact that while our mortality rate is similar to studies with 14,2% [14] or 26% [15] of ICU admissions, all those patients who developed severe ARDS (16/63) finally died.
COVID-19 course and severity of symptoms seems also different in cancer patients. While mean time since the beginning of symptoms until respiratory failure among cancer patients was similar (7.02 days) to hospitalised non-cancer patients [15]; mean time to death was 12∙4 days in contrast to 21.0 days. These data may suggest either that oncologic patients may present worse outcomes in a shorter period of time or that global population may have longer time since respiratory deterioration to death owing to the use of mechanical ventilation at ICU units.
Laboratory results in cancer patients were consistent with those from hospitalised non-cancer patients, with up to 92% of lymphopenia, as well 95%, 98%, 83% and 96% of cases with LDH, CRP, ferritin and IL-6 levels above upper normal limits, respectively. Hypoalbuminemia during hospitalisation was documented in 84% of cancer patients in concordance with previous data [6].
Another interesting finding in our current report is linked to higher mortality associated to coagulopathy in SARS-CoV-2 infection [16]. Anticoagulant therapy has recently proved to collaborate to a favourable outcome among COVID-19 patients [17]. VTE represents a highly frequent complication in cancer patients with SARS-CoV-2 infection with an overall rate of newly diagnosed VTE of 14% and a recurrence rate of 15% among previously diagnosed in our series. Even more concerning is that up to 20% of patients under chronic anticoagulation therapy a new VTE was found.
Our multivariate logistic regression model has been able to identify several predictive factors of poor outcome both in terms of severe ARDS and death. Thus, presence of bilateral pneumonia, severe neutropenia, previous history of VTE or pulmonary tumour involvement should be considered in evaluation of COVID-19 cancer patient.
5. Conclusion
SARS-CoV-2–infected cancer patients show worse outcomes in terms of mortality and severe respiratory failure than global population but similar death rate to non-cancer patients hospitalised for this disease. Altered lymphocyte count, LDH, CRP and albumin serum levels may discriminate illness severity. ARDS is the main cause of death, and VTED screening as well as assessment of neutropenia, lung involvement and bilateral pneumonia at diagnosis are essential for the management of this high risk and fragile population.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interest statement
None declared.
Acknowledgements
The authors wish to thank the patients, their families and all those healthcare workers involved in this study, as well as to all cancer patients and their families suffering this disease.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;(395):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization W.H. 2020. Coronavirus disease (COVID-19) pandemic. [Google Scholar]
- 4.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020 Mar 25:e200980. doi: 10.1001/jamaoncol.2020.0980. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020 Jul;31(7):894–901. doi: 10.1016/j.annonc.2020.03.296. [Epub 2020 Mar 26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020 May 7;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [Epub 2020 Mar 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Sevestre J., et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Trav Med Infect Dis. 2020:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarma P., Kaur H.A.-O., Kumar H., Mahendru D., Avti P., Bhattacharyya A., et al. Virological and clinical cure in Covid-19 patients treated with hydroxychloroquine: a systematic review and meta-analysis. J Med Virol. 2020:1096–9071. doi: 10.1002/jmv.25898. [Electronic] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18(1):164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Organization W.H. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim Guidance. March. 2020;13 [Google Scholar]
- 12.Aakash Desai S.S., Parekh Tarang, Desai Rupak. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO J Global Oncol. 2020;(6):557–559. doi: 10.1200/GO.20.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemostasis JTH. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemostasis JTH. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]