To the Editor:
We present the case of a 60-year-old previously healthy man who was admitted to the intensive care unit with a confirmed case of coronavirus disease 2019 (COVID-19) pneumonia 3 days after his initial hospitalization and 8 days after the onset of symptoms (fever, cough). The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus was detected in a nasopharyngeal swab and in bronchoalveolar lavage fluid. Reverse transcriptase–polymerase chain reaction did not show any evidence of other concurrent viral infections including influenza, parainfluenza, respiratory syncytial virus, adenovirus, human metapneumovirus, and rhinovirus. An enzyme-linked immunosorbent assay–based antibody test later confirmed the presence of immunoglobulin A and G antibodies against SARS-CoV-2. After 13 days of intensive care treatment, the patient was transferred back to a medical floor in a stable cardiopulmonary state, but with hyperactive delirium. After recovery of his mental state, he reported deafness with a loud tinnitus (white noise) bilaterally. The hearing loss was confirmed using acoustically evoked potentials, and the patient was transferred to the ear, nose, and throat service for further diagnostics and treatment.
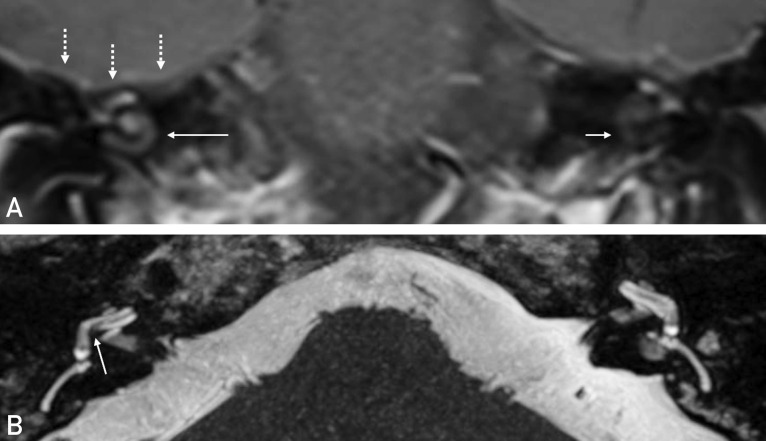
The patient had experienced no previous episodic or chronic hearing impairment. Audiologic testing revealed complete deafness on the right side and profound sensorineural hearing loss on the left side. A magnetic resonance imaging (MRI) scan showed pronounced contrast enhancement in the right cochlea (Figure A ) and a partially decreased fluid signal in the basal turn of the right cochlea (Figure B). Adjacent to the temporal bone, meningeal contrast enhancement was seen at the base of the right temporal lobe (Figure A).
Figure.
A, Coronal view in the T1 post-contrast sequence. Increased contrast enhancement of the right cochlea (long arrow). The left cochlea shows a normal hypointense pattern (short arrow). Linear contrast enhancement of the meninges at the base of the temporal lobe (dashed arrows). B, Axial view in the T2 sequence. Diminished fluid signal between the scala tympani of the basal cochlear turn and the vestibulum compared to the left side.
The MRI findings were interpreted as signs of an inflammatory process in the cochlea. Such a process can lead to soft tissue formation or even ossification of the cochlea, making the insertion of a cochlear implant (CI) electrode for hearing rehabilitation more challenging or impossible.1 Hence the need for urgent CI was given. The patient’s condition was still poor because of his recent COVID-19 infection; therefore, CI surgery was performed under local anesthesia with analgosedation instead of general anesthesia. The left ear was treated with three intratympanic triamcinolone injections to avoid the systemic immunosuppressant side effects of intravenous steroids.
During his treatment for COVID-19 pneumonia the patient had received two medications with reported ototoxic effects: azithromycin and furosemide. Independent of the individual toxicity profiles of these medications, a toxic effect is unlikely to manifest in MRI, as seen in this case. Furthermore, ototoxicity affects both ears symmetrically. Severe ototoxicity mediated by the above-mentioned drugs is therefore unlikely to be the cause of hearing loss in this patient.
Sensorineural hearing loss is a known complication of a number of viral infections. There is a plausible mechanism that may have caused virus-related hearing loss in the present case. A recent report of a series of 58 patients suggests an association between acute respiratory distress syndrome due to SARS-CoV-2 infection and encephalopathy, with 8 of 13 scanned patients showing leptomeningeal contrast enhancement,2 as seen in the patient discussed here. Hearing loss is a known possible complication of bacterial or viral meningitis3 and occurs to varying degrees in approximately 7% of cases.4 In the present case, MRI signs of inflammation of the meninges and the right cochlea were present and the patient showed clinical manifestations in the form of delirium and hearing loss. Hence, there may have been virus-triggered inflammation of the meninges with subsequent spread to the cochlea, leading to acute hearing loss. Virus-triggered, immune-mediated inflammation seems likely, considering that severe cases of COVID-19 have been associated with a dysregulation of the immune system. In these severe cases, an increased neutrophil-to-lymphocyte ratio and elevated inflammatory cytokines such as interleukin 6 were observed,5 features also seen in this case.
This stands in contrast to other sensory manifestations of the coronavirus infection such as anosmia, which can occur in otherwise asymptomatic patients. Gene expression databases have shown that the SARS-CoV-2 receptors ACE2 and TMPRSS2 are present in olfactory epithelium. With the virus replicating in the nose and nasopharynx, this represents a mechanism for direct damage of olfactory epithelium by the virus in the frame of a mild infection.
In conclusion, the case presented in this report highlights the importance of urgent audiologic and radiologic diagnostics in COVID-19 patients who report hearing loss, especially if neurologic symptoms are present.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Lenarz T. Cochlear implant — state of the art. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2017;16:Doc04. doi: 10.3205/cto000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms J., Kremer S., Merdji H. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. 2020;382:2268–2272. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenhall U., Kankkunen A. Hearing alterations following meningitis. 1. Hearing improvement. Ear Hear. 1980;1(4):185–190. doi: 10.1097/00003446-198007000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Fortnum H., Davis A. Hearing impairment in children after bacterial meningitis: incidence and resource implications. Br J Audiol. 1993;27(1):43–52. doi: 10.3109/03005369309077889. [DOI] [PubMed] [Google Scholar]
- 5.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]