Abstract
Objective:
We performed a nationwide analysis to assess the impact of adjuvant therapy on survival after a microscopically margin-positive (R1) resection for esophageal cancer.
Methods:
The National Cancer Database (NCDB) was used to identify patients with R1 resection for esophageal cancer (2004-2015). Patients were grouped by type of adjuvant therapy. Patients who had other margin status, M1 disease, neoadjuvant chemotherapy and radiation, missing survival, and no or unknown treatment were excluded. The primary outcome was overall survival. A 1:1 propensity score-matched sensitivity analysis was also performed comparing patients who received no adjuvant therapy with those who received adjuvant chemoradiation.
Results:
Of 546 patients, 279 (51%) received adjuvant therapy and 267 (49%) did not Patients receiving adjuvant therapy were more likely to be younger, have more advanced pathologic stage, have non-squamous histology, and have shorter hospitalization. In multivariable analysis, adjuvant chemotherapy, radiation, and chemoradiation were all associated with improved survival compared to no adjuvant therapy. In a propensity score-matched analysis of 123 patient pairs, adjuvant chemoradiation was associated with improved survival compared to no adjuvant therapy (adjusted HR 0.30; 95%CI [0.22, 0.40]).
Conclusion:
Adjuvant therapy is associated with improved survival compared to no adjuvant therapy in patients with R1 resection for esophageal cancer even after adjustment for pathologic stage. Adjuvant therapy should be considered in patients with incompletely resected esophageal cancer in concordance with national guidelines.
Introduction
In 2018, an estimated 17,290 people will be diagnosed with esophageal cancer, and 15,850 will die from it1. Surgery remains the mainstay treatment of stage I to III esophageal cancer. The National Comprehensive Cancer Network (NCCN) guidelines do not make specific recommendations regarding optimal margins, although retrospective cohort studies in largely gastric and esophagogastric junction populations suggest that 5 cm of proximal and distal margins may mitigate the risk of residual disease2,3. Incomplete resections occur in approximately 8-20% of patients4-14 and are associated with poor survival ranging from 9 to 17 months5,6,11,12 and an increased risk of recurrence9,12.
The NCCN guidelines recommend adjuvant chemotherapy and radiation for patients with incomplete resections who did not receive neoadjuvant therapy. However, there are few studies focused on the impact of adjuvant therapy on outcomes in patients with margin-positive esophageal resections. The largest clinical trial, the Intergroup 0116 study, that informs the administration of adjuvant therapy in esophageal cancer excluded the 8% of patients with margin-positive resections and primarily included patients with gastric adenocarcinoma7. Further, neoadjuvant rather than adjuvant therapy is considered the standard of care in patients with locally advanced esophageal cancer15. The existing literature consists of a few retrospective cohort analyses of often small samples with conflicting data on the impact of adjuvant therapy in patients with incomplete resections4,12,14,16,17. We investigated the effects of adjuvant therapy on survival in patients undergoing microscopically margin-positive (R1) resection of esophageal cancer using a large hospital-based database.
Methods
The National Cancer Database
The National Cancer Database (NCDB) is a joint effort of the American Cancer Society and the American College of Surgeons. It contains data on about 80% of cancers diagnosed annually collected in over 1500 accredited treatment centers in the United States and Puerto Rico18,19.
Study Design
The study was reviewed and deemed exempt by our Institutional Review Board. The NCDB was interrogated for patients with pathologic stage I-IVA esophageal cancer who underwent esophagectomy with a microscopic margin-positive (R1) resection between 2004 and 2015. Patients with Surveillance, Epidemiology, and End Results (SEER) ICD-O-3 codes 800-857 and 893-896 were included. Patients with either adenocarcinoma or squamous cell histology were included in this study because (1) the NCCN guidelines have similar recommendations on treatment of incompletely resected squamous cell and adenocarcinoma and (2) we developed a multivariable Cox model with an interaction term between histology and receipt of adjuvant therapy and found that the interaction term was nonsignificant (ANOVA p=0.86), suggesting that the relationship between histology and type of adjuvant therapy did not meaningfully influence survival in our cohort. The Surgical Margins variable of the NCDB was used to identify patients with an R1 resection. The total number of patients with macroscopic margin-positive (R2) resections in the database was too small for analysis (<100). Patients with indeterminate margins or positive margins not specified as R1 or R2 were excluded. Patients receiving neoadjuvant therapy, with missing data about adjuvant treatment, and who had missing survival information were excluded (Figure 1). The remaining patients were then stratified by receipt of adjuvant therapy: adjuvant therapy, or no adjuvant therapy. Adjuvant therapy was defined as chemotherapy alone, radiation alone, or chemoradiotherapy, and was identified using the Systemic Surgery Sequence and Radiation Surgery Sequence variables. The primary outcome of interest was overall survival (OS).
Figure 1:
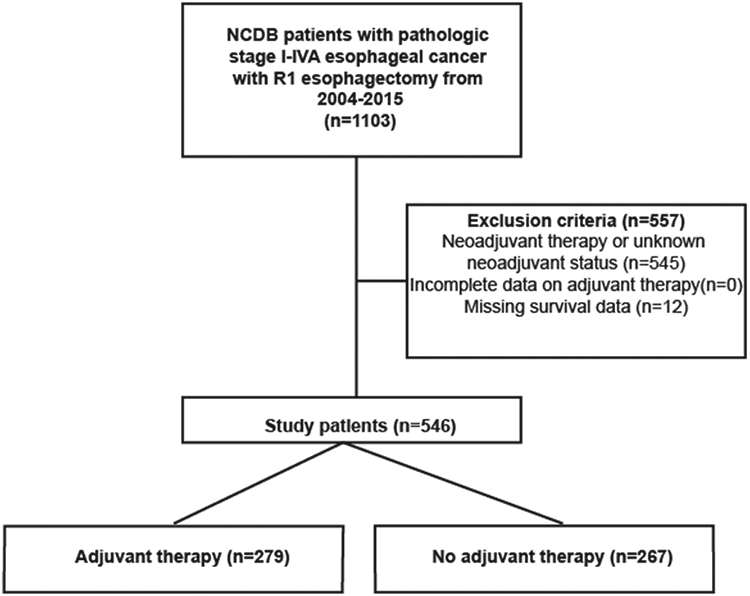
Scheme of patient selection for study
Statistical Analysis
Background characteristics between groups were compared using the Wilcoxon rank sum test and Pearson’s chi-squared test for continuous and categorical variables, respectively. OS was measured using the Kaplan-Meier method, and groups compared using the log-rank test. A Cox proportional hazards model was used for multivariable regression. Variables used for the model were selected a priori based on clinical significance and data from prior studies demonstrating their association with survival: age, sex, race, year of diagnosis, Charlson-Deyo comorbidity index (CDCC) score, insurance status, treatment at an academic center, tumor size, grade, squamous histology, and pathologic stage. Year of diagnosis was dichotomized based on the median year in our cohort (2010). Unadjusted Cox estimates of each variable were also computed. Proportional hazards assumption was checked with both visual and quantitative estimates of Schoenfeld residuals. A subgroup analysis was performed excluding all patients who suffered a postoperative 90-day mortality because these patients may have had complications of surgery that prevented them from receiving adjuvant therapy, thereby introducing selection bias. As another sensitivity analysis, a 1:1 propensity-score matched analysis using the nearest-neighbor algorithm was performed to compare patients receiving no adjuvant therapy with those who received adjuvant chemotherapy and radiation. Because the NCCN guidelines recommend adjuvant chemoradiation for most patients with incompletely resected NSCLC and few patients received adjuvant chemotherapy (n=71) or radiation (n=24) alone, patients who received chemoradiation were selected as the treatment group for propensity score-matched analysis. Patients were matched by age, sex, race, era of diagnosis, CDCC score, insurance status, histology, academic center treatment, pathologic stage, grade, and tumor size. A 1:1 nearest-neighbor algorithm20 was used that utilizes a series of logistic regressions to match control and treatment groups on distance; we used a caliper of 0.1 to eliminate differences and ensure a mean standardized difference ≤ 0.1 for each variable21,22. Statistical analysis was performed using R version 3.5.1 for Mac (Vienna, Austria). The threshold for significance was a two-sided p value less than or equal to 0.05.
Results
A total of 546 patients met study criteria, of whom 279 (51%) received adjuvant therapy and 267 (49%) did not (Figure 1). Of the patients receiving adjuvant therapy, 184 (66%) received chemoradiation, 71 (25%) chemotherapy alone, and 24 (9%) radiation alone. Compared to patients who did not receive adjuvant therapy, patients who received adjuvant therapy were more likely to be younger, privately insured, be treated at a non-academic center, have a higher pathologic stage, have non-squamous histology, have a shorter length of hospitalization, and less likely to have 30-day postoperative readmission (Table 1).
Table 1.
Background characteristics of patients with R1 resection, stratified by adjuvant treatment
| No adjuvant (n=267) (%) |
Adjuvant chemo (n=71)(%) |
Adjuvant RT (n=24)(%) |
Adjuvant chemoRT (n=184)(%) |
p value |
|
|---|---|---|---|---|---|
| Age (years, median) | 70 | 61 | 71 | 62 | <0.001 |
| Sex (female) | 50(19) | 18(25) | 8(33) | 29(16) | 0.10 |
| Race | 0.34 | ||||
| White | 233(88) | 65(91) | 20(83) | 168(92) | |
| Black | 27(10) | 4(6) | 3(13) | 8(4) | |
| Other | 6(2) | 2(3) | 1(4) | 6(3) | |
| Year of diagnosis | 0.20 | ||||
| 2004-2009 | 187(70) | 43(61) | 18(75) | 136(74) | |
| 2010-2015 | 80(30) | 28(39) | 6(25) | 48(26) | |
| CDCC Score | 0.51 | ||||
| 0 | 168(63) | 49(69) | 17(71) | 134(73) | |
| 1 | 74(28) | 16(23) | 5(21) | 38(21) | |
| 2+ | 25(9) | 6(8) | 2(8) | 12(6) | |
| Insurance status | 0.001 | ||||
| Private | 77(29) | 36(51) | 4(17) | 78(42) | |
| Government | 181(69) | 33(47) | 18(78) | 98(54) | |
| None | 5(2) | 1(2) | 1(5) | 7(4) | |
| Facility location | 0.72 | ||||
| Metro | 208(82) | 56(82) | 18(90) | 138(80) | |
| Urban | 40(16) | 9(13) | 2(10) | 31(18) | |
| Rural | 5(2) | 3(4) | 0(0) | 3(2) | |
| Academic center | 139(53) | 25(36) | 12(50) | 61(34) | 0.001 |
| Clinical stage | 0.329 | ||||
| I | 26(20) | 1(3) | 0(0) | 10(15) | |
| II | 32(24) | 10(30) | 3(43) | 16(25) | |
| III | 66(50) | 17(52) | 3(43) | 35(54) | |
| IVA | 9(7) | 5(15) | 1(14) | 4(6) | |
| Pathologic stage | 0.006 | ||||
| I | 30(11) | 2(3) | 2(8) | 6(3) | |
| II | 59(22) | 9(13) | 7(29) | 34(19) | |
| III | 139(52) | 51(72) | 13(54) | 124(67) | |
| IVA | 39(15) | 9(13) | 2(8) | 20(11) | |
| Squamous histology | 77(29) | 9(13) | 10(42) | 35(19) | 0.03 |
| 30-day readmission | 35(13) | 9(13) | 0(0) | 7(4) | 0.002 |
| Length of stay (median days with IQR) | 15(10-25) | 13(9-18) | 11(8-20) | 10(8-14) | <0.001 |
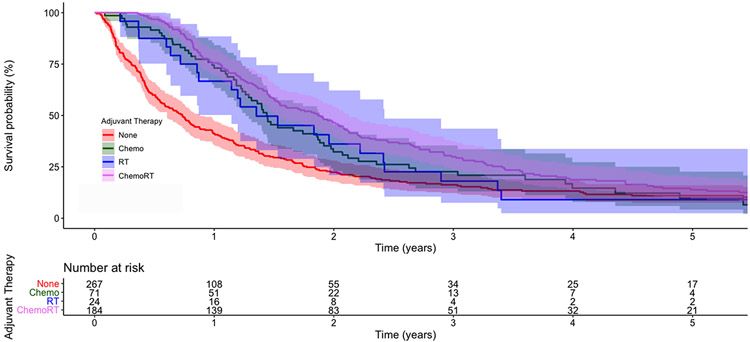
The unadjusted five-year OS of patients receiving no adjuvant therapy, adjuvant chemotherapy, adjuvant radiation, and adjuvant chemoradiation was 11% (95%CI 8,16), 10% (95%CI [4,23]), 9% (95%CI [2,34]), and 14% (95%CI [10,20]), respectively (Figure 2). In unadjusted Cox regression, adjuvant chemotherapy and chemoradiation were associated with improved survival compared to no adjuvant therapy, but adjuvant radiation alone was not associated with survival (Supplemental Table 1). In multivariable analysis, the receipt of adjuvant chemotherapy, radiation, and chemoradiation were all associated with improved survival compared to no adjuvant therapy (Table 2). Compared to adjuvant chemotherapy alone, adjuvant chemoradiation was associated with improved survival (HR 0.57; 95%CI [0.39,0.83]; p=0.004). In a subgroup analysis of 460 patients who survived 90 days following surgery, adjuvant chemotherapy (HR 0.72; 95%CI [0.50,1.04]; p=0.08) and radiation (HR 0.69; 95%CI [0.40,1.19]; p=0.19) were not associated with survival while adjuvant chemoradiation (HR 0.53; 95%CI [0.39,0.71]; p<0.001) was associated with improved survival compared to no adjuvant therapy.
Figure 2:
Kaplan-Meier survival curves for patients with R1 esophagectomy, stratified by receipt of adjuvant therapy. The shaded areas represent 95% confidence intervals. The numbers at risk are provided below the graph
Table 2.
Cox proportional hazards model of factors independently associated with survival in patients with R1 margin status following esophagectomy for esophageal cancer
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Variable | Hazard Ratio |
Lower | Upper | p-value |
| Age (per year) | 1.01 | 1.00 | 1.03 | 0.05 |
| Sex (female) | 1.10 | 0.83 | 1.44 | 0.51 |
| Race (reference: white) | ||||
| Black | 0.67 | 0.42 | 1.06 | 0.08 |
| Other | 0.73 | 0.34 | 1.56 | 0.43 |
| Year of diagnosis (reference: <2010) | ||||
| 2010-2015 | 0.69 | 0.55 | 0.88 | 0.002 |
| Charlson-Deyo Comorbidity Index (reference: 0) | ||||
| 1 | 1.03 | 0.81 | 1.32 | 0.78 |
| 2+ | 1.30 | 0.92 | 1.84 | 0.14 |
| Insurance status (reference: private) | ||||
| Government | 1.06 | 0.83 | 1.37 | 0.63 |
| None | 1.40 | 0.76 | 2.55 | 0.28 |
| Academic center (reference: non-academic) | 0.90 | 0.72 | 1.11 | 0.31 |
| Tumor size (per mm) | 1.00 | 1.00 | 1.00 | 0.09 |
| Grade (reference: low grade) | ||||
| Moderate | 1.41 | 0.86 | 2.30 | 0.17 |
| High | 1.57 | 0.97 | 2.55 | 0.07 |
| Pathologic stage (reference: 1) | ||||
| 2 | 2.47 | 1.41 | 4.33 | 0.002 |
| 3 | 3.49 | 2.04 | 5.98 | <0.001 |
| 4A | 4.98 | 2.80 | 8.88 | <0.001 |
| Thirty-day readmission (reference: none) | 0.81 | 0.57 | 1.15 | 0.24 |
| Postoperative hospital length of stay (per day) | 1.00 | 0.99 | 1.01 | 0.96 |
| Squamous histology | 1.11 | 0.85 | 1.46 | 0.43 |
| Adjuvant therapy (reference: none) | ||||
| Chemotherapy | 0.54 | 0.39 | 0.76 | <0.001 |
| Radiation | 0.54 | 0.32 | 0.90 | 0.02 |
| Chemoradiation | 0.38 | 0.29 | 0.50 | <0.001 |
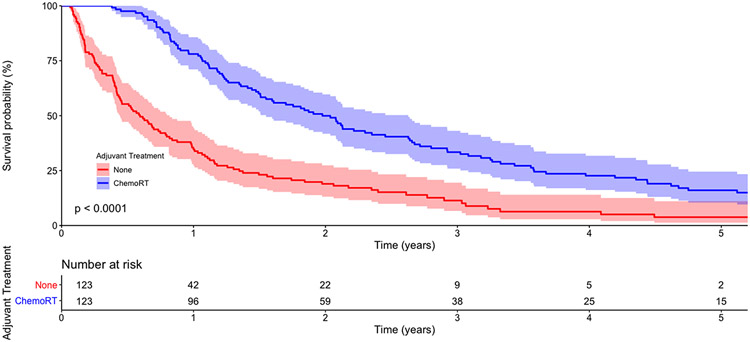
In 123 propensity score-matched pairs of patients (Table 3), patients receiving no adjuvant therapy and adjuvant chemoradiation had an unadjusted five-year OS of 4% (95%CI [1,11]) and 16% (95%CI [10,25]), respectively (Figure 3). In multivariable regression, adjuvant chemoradiation was associated with significantly improved survival compared to no adjuvant therapy (Table 4).
Table 3.
Background characteristics of propensity score-matched patients with R1 resection, stratified by adjuvant treatment
| No Adjuvant (n=123)(%) |
Adjuvant ChemoRT (n=123)(%) |
Standardized Mean Difference |
p-value | |
|---|---|---|---|---|
| Age (years, median) | 65 | 66 | 0.069 | 0.78 |
| Sex (female) | 19(15) | 23(19) | 0.090 | 0.61 |
| Race | 0.59 | |||
| White | 116(94) | 112(91) | ||
| Black | 5(4) | 7(6) | 0.083 | |
| Other | 2(2) | 4(3) | 0.097 | |
| Year of diagnosis | 0.78 | |||
| 2004-2009 | 85(69) | 88(71) | ||
| 2010-2015 | 38(31) | 35(29) | 0.055 | |
| CDCC Score | 0.79 | |||
| 0 | 87(71) | 82(67) | ||
| 1 | 26(21) | 30(24) | 0.081 | |
| 2+ | 10(8) | 11(9) | 0.031 | |
| Insurance status | 0.50 | |||
| Private | 41(33) | 42(34) | ||
| Government | 80(65) | 76(62) | 0.065 | |
| Academic center | 47(38) | 51(42) | 0.069 | 0.70 |
| Pathologic stage | 0.92 | |||
| I | 6(5) | 5(4) | ||
| II | 20(16) | 22(18) | 0.042 | |
| III | 79(64) | 81(66) | 0.035 | |
| IVA | 18(15) | 15(12) | 0.082 | |
| Grade | 0.54 | |||
| Low | 9(7) | 5(4) | ||
| Moderate | 39(32) | 39(32) | 0.000 | |
| High | 75(61) | 79(64) | 0.066 | |
| Squamous histology | 25(20) | 29(24) | 0.082 | 0.64 |
| Tumor size (median mm) | 40 | 40 | 0.073 | 0.93 |
Figure 3:
Kaplan-Meier survival curves for propensity score-matched patients with R1 esophagectomy, stratified by receipt of adjuvant therapy. The p-value represents the result of the log-rank test. The shaded areas represent 95% confidence intervals. The numbers at risk are provided below the graph
Table 4.
Cox proportional hazards model of factors independently associated with survival in propensity score-matched patients with R1 margin status following esophagectomy for esophageal cancer.
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Variable | Hazard Ratio |
Lower | Upper | p-value |
| Age (per year) | 1.04 | 1.02 | 1.06 | <0.001 |
| Sex (female) | 0.96 | 0.63 | 1.46 | 0.84 |
| Race (reference: white) | ||||
| Black | 1.06 | 0.51 | 2.19 | 0.88 |
| Other | 0.82 | 0.32 | 2.12 | 0.68 |
| Year of diagnosis (reference: <2010) | ||||
| 2010-2015 | 0.59 | 0.43 | 0.83 | 0.002 |
| Charlson-Deyo Comorbidity Index (reference: 0) | ||||
| 1 | 1.06 | 0.76 | 1.47 | 0.74 |
| 2+ | 1.46 | 0.87 | 2.45 | 0.15 |
| Insurance status (reference: private) | ||||
| Government | 0.81 | 0.57 | 1.15 | 0.24 |
| None | 2.18 | 0.96 | 4.99 | 0.06 |
| Academic center (reference: non-academic) | 0.78 | 0.59 | 1.03 | 0.08 |
| Tumor size (per mm) | 1.00 | 1.00 | 1.00 | 0.17 |
| Grade (reference: low grade) | ||||
| Moderate | 1.16 | 0.62 | 2.18 | 0.64 |
| High | 1.22 | 0.66 | 2.22 | 0.53 |
| Pathologic stage (reference: 1) | ||||
| 2 | 1.65 | 0.72 | 3.77 | 0.23 |
| 3 | 2.86 | 1.33 | 6.16 | 0.007 |
| 4A | 5.99 | 2.61 | 13.7 | <0.001 |
| Squamous histology | 0.87 | 0.58 | 1.31 | 0.50 |
| Adjuvant therapy (reference: none) | ||||
| Chemoradiation | 0.30 | 0.22 | 0.40 | <0.001 |
Discussion
In this analysis of a large national database, in patients with stage I to IVA esophageal cancer who did not receive neoadjuvant therapy, the use of adjuvant therapy after R1 esophagectomy was associated with improved overall survival compared to no adjuvant therapy.
The results of our study are consistent with NCCN guidelines, which recommend administration of adjuvant chemotherapy and radiation to patients with R1 resection for esophageal cancer who did not receive neoadjuvant chemoradiation. The use of adjuvant therapy in esophageal cancer is largely derived from the Intergroup 0116 study7, where 556 patients with T1-4N0-3M0 gastric or gastroesophageal adenocarcinoma were randomized to receive surgery alone or surgery with adjuvant chemoradiotherapy. The study found that patients receiving adjuvant therapy experienced better survival (median 36 vs. 27 months) and lower relapse (HR 1.52 compared to surgery alone). However, this study was limited by only a quarter of the cohort having gastroesophageal cancer. Additionally, the 8% of patients with margin-positive resections were excluded. As a result, the generalizability of the study’s findings to esophageal cancer and to R1 resections is poor. Two prospective trials have examined the role of adjuvant therapy in esophageal cancer. In the JCOG9204 study, Ando and colleagues compared surgery to surgery followed by chemotherapy for squamous cell cancer, and found a higher five-year disease-free survival for the adjuvant therapy cohort (55 vs. 45%)23. Adelstein and colleagues conducted a prospective single-arm trial of postoperative concurrent chemoradiation for ‘poor prognosis’ esophageal cancer, defined as N1, T3, or M1a disease8. They found a four-year overall survival of 51% that compared favorably to historical outcomes. Eighteen percent of their cohort were patients with R1 resection, but this subgroup was not specifically analyzed.
While there is an absence of prospective, randomized data on the use of adjuvant therapy for R1 resections in esophageal cancer, there is a paucity of observational studies as well. Markar and colleagues performed a retrospective cohort analysis of outcomes in 30 European centers of patients with R1 resections, finding significantly worse survival in this population compared to patients with R0 resections (median 17 vs. 28 months in a propensity-matched cohort)24. The use of adjuvant therapy was even less frequent in their cohort (36% compared to 57% in ours). Adjuvant therapy was associated with improved three-year overall survival (34 vs. 26%) compared to no adjuvant therapy, but there was no difference in locoregional recurrence. In contrast to our findings, the use of adjuvant chemoradiation conferred no survival benefit over adjuvant chemotherapy alone. Hsu and colleagues reported data from 290 patients with squamous cell cancer and found that adjuvant chemoradiation was associated with a survival beneft compared to no adjuvant therapy in the 21 patients with R1 resection (HR 0.17)25. Two NCDB analyses published in 2017 also include subsets of R1 patients in their analyses. Gao and colleagues examined the role of adjuvant therapy in incidentally node-positive cT1-2 esophageal cancer, and found that adjuvant chemoradiation conferred a survival benefit compared to adjuvant chemotherapy alone or no therapy in patients with margin-positive resection in this cohort16. Wong and colleagues examined the role of adjuvant radiation in patients with pT1-4N1-3M0 esophageal cancer, and found that adjuvant radiation alone was associated with improved survival compared to no adjuvant therapy in unadjusted analysis in a subgroup of patients with margin-positive resection17. These outcomes were not examined in multivariable analysis. A third NCDB analysis of patients with margin-positive resections in a smaller, early cohort (2003-2006) demonstrated worse five-year survival for patients with margin-positive resections compared to margin-negative resections, and a survival benefit for adjuvant chemoradiotherapy compared to no adjuvant therapy14. Our study represents the largest, contemporary, and most rigorous analysis of adjuvant therapy for margin-positive esophageal resection. Our finding that adjuvant therapy confers a survival benefit over no adjuvant therapy in patients with R1 resection for esophageal cancer despite pathologic stage is in concordance with published literature. However, our observation that adjuvant chemoradiation is associated with improved survival compared to adjuvant chemotherapy alone in R1 resection conflicts with the European data12, despite the increased proportion of squamous cell carcinoma in the European cohort (46% vs. 24% in our study).
The decision to offer adjuvant therapy is nonetheless multifactorial. Adjuvant therapy carries significant risks including systemic adverse reactions as severe as heart failure in response to chemotherapy and radiation-related pneumonitis26, mediastinitis, and fistulization of the newly created gastric conduit. Adjuvant therapy should therefore be offered after a careful consideration of its potential risks and benefits tailored to the individual patient. In our institution, the decision to offer adjuvant therapy is predicated on several patient- and tumor-related factors including age, frailty, comorbidities, pathologic stage, nodal disease burden, grade, lymphovascular invasion (LVI), and margin status. In our cohort, for instance, patients were more likely to receive adjuvant therapy if they had more advanced pathologic disease. In a multivariable Cox regression with an interaction term of pathologic stage and adjuvant therapy, however, the p-value of the interaction term was nonsignificant (ANOVA p=0.48). This suggests that in patients with incompletely resected esophageal cancer, the survival benefit observed in patients receiving adjuvant therapy is independent from the pathologic stage of their cancer.
There are limitations to this study. Importantly, this is a retrospective cohort analysis and therefore subject to confounding that we cannot fully adjust for. For instance, it is difficult to estimate the overall fitness of patients in each group in the absence of detailed health information, and while we use a crude surrogate in the form of the CDCC score, there could still be meaningful differences between the groups that might account for the difference in survival. The use of propensity score-matched analysis attempts to approximate randomization but is also inherently flawed27. We are also limited by the variables available in the database, which often do not provide detailed information to understand bias that could affect the study. For example, there is no information on why some patients in the cohort were offered adjuvant therapy while others were not. Similarly, the NCDB does not provide information on the exact chemotherapy regimen used, which can confound the analysis. The NCDB also does not provide details about the postoperative course of patients, leaving us to rely on surrogate markers like length of hospitalization and 30-day readmission rates. There is also a significant amount of missing data in the NCDB, which could skew the results in ways we cannot estimate. For instance, data about LVI are missing in 80% of patients, which prevented us from including it in our multivariable models although it is a known prognostic factor in esophageal cancer. The NCDB also does not provide information on the distance of margins obtained in surgery and the identity of positive margins, which precludes analysis of important questions like the optimal margins for esophagectomy and the significance of positive radial margins28,29. Our study was also limited by the relatively small number of patients with early disease (36 patients with stage I pathology), and our findings may therefore not accurately represent this population of patients. Our study also included a substantial number of patients with squamous histology (24%), which might limit the generalizability of our findings, especially due to the often differing tumor characteristics between squamous cell carcinoma and adenocarcinoma. However, a multivariable model with an interaction term between histology and adjuvant therapy revealed no relationship between the two variables. Finally, we excluded patients who received neoadjuvant therapy because the number of patients receiving both neoadjuvant and adjuvant therapy for R1 resection was small, so our findings cannot be generalized to patients with R1 resection following neoadjuvant therapy.
Nevertheless, using a large national database, we demonstrate that adjuvant therapy is associated with improved overall survival compared to no adjuvant therapy in patients with R1 resection for esophageal cancer even after adjustment for pathologic stage. Adjuvant chemotherapy and radiation should be considered in patients with R1 resection for esophageal cancer in concordance with national guidelines.
Supplementary Material
Acknowledgements
We thank Dr. Hanghang Wang, M.D., Ph.D., for statistical review of this manuscript.
Drs. Raman and Voigt were supported by a National Institutes of Health T-32 grant 5T32CA093245 in surgical oncology. Dr. Jawitz was supported by a National Institutes of Health T-32 grant 5T32HL069749 in clinical research.
The American College of Surgeons is in a Business Associate Agreement that includes a data use agreement with each of its Commission on Cancer accredited hospitals. The data used in the study are derived from a de-identified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigators.
Footnotes
The authors have no relevant conflicts of interest to disclose.
References
- 1.Key Statistics for Esophageal Cancer Available from: https://www.cancer.org/cancer/esophagus-cancer/about/key-statistics.html. Accessed November 9, 2018.
- 2.Casson AG, Darnton SJ, Subramanian S, et al. What is the optimal distal resection margin for esophageal carcinoma? Ann Thorac Surg. 2000;69:205–209. [DOI] [PubMed] [Google Scholar]
- 3.Barbour AP, Rizk NP, Gonen M, et al. Adenocarcinoma of the gastroesophageal junction: influence of esophageal resection margin and operative approach on outcome. Ann Surg. 2007;246:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Survival benefits of postoperative chemoradiation for lymph node-positive esophageal squamous cell carcinoma. - PubMed - NCBI Available from: https://www.ncbi.nlm.nih.gov/pubmed/24612702. Accessed November 9, 2018. [DOI] [PubMed]
- 5.Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27:5062–5067. [DOI] [PubMed] [Google Scholar]
- 6.Law S, Arcilla C, Chu K, et al. The significance of histologically infiltrated resection margin after esophagectomy for esophageal cancer. Am J Surg. 1998;176:286–290. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. [DOI] [PubMed] [Google Scholar]
- 8.Adelstein DJ, Rice TW, Rybicki LA, et al. Mature Results from a Phase II Trial of Postoperative Concurrent Chemoradiotherapy for Poor Prognosis Cancer of the Esophagus and Gastroesophageal Junction. J Thorac Oncol. 2009;4:1264–1269. [DOI] [PubMed] [Google Scholar]
- 9.Hulscher JBF, van Sandick JW, Tijssen JGP, et al. The recurrence pattern of esophageal carcinoma after transhiatal resection11No competing interests declared. J Am Coll Surg. 2000;191:143–148. [DOI] [PubMed] [Google Scholar]
- 10.Mariette C, Finzi L, Fabre S, et al. Factors predictive of complete resection of operable esophageal cancer: a prospective study. Ann Thorac Surg. 2003;75:1720–1726. [DOI] [PubMed] [Google Scholar]
- 11.Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic Tumor Regression and Lymph Node Metastases Determine Prognosis Following Neoadjuvant Radiochemotherapy for Esophageal Cancer. Ann Surg. 2005;242:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markar SR, Gronnier C, Duhamel A, et al. Significance of Microscopically Incomplete Resection Margin After Esophagectomy for Esophageal Cancer . Epub ahead of print April 2016. DOI: info:doi/ 10.1097/SLA.0000000000001325. [DOI] [PubMed] [Google Scholar]
- 13.Blewett CJ, Miller JD, Young JE, et al. Anastomotic leaks after esophagectomy for esophageal cancer: a comparison of thoracic and cervical anastomoses. Ann Thorac Cardiovasc Surg Off J Assoc Thorac Cardiovasc Surg Asia. 2001;7:75–78. [PubMed] [Google Scholar]
- 14.Javidfar J, Speicher PJ, Hartwig MG, et al. Impact of Positive Margins on Survival in Patients Undergoing Esophagogastrectomy for Esophageal Cancer. Ann Thorac Surg. 2016;101:1060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. 10.1056/NEJMoa1112088. . Epub ahead of print May 30, 2012. DOI: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 16.Gao SJ, Park HS, Corso CD, et al. Role of Adjuvant Treatment in Esophageal Cancer With Incidental Pathologic Node Positivity. Ann Thorac Surg. 2017;104:267–274. [DOI] [PubMed] [Google Scholar]
- 17.Wong SL. Lymph Node Counts and Survival Rates After Resection for Colon and Rectal Cancer. Gastrointest Cancer Res GCR. 2009;3:S33–S35. [PMC free article] [PubMed] [Google Scholar]
- 18.National Cancer Database. American College of Surgeons. Available from: https://www.facs.org/quality-programs/cancer/ncdb. Accessed August 25, 2018.
- 19.Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MatchIt: Nonparametric Preprocessing for Parametric Causal Inference Available from: https://gking.harvard.edu/matchit. Accessed April 19, 2019.
- 21.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrell FE. Multivariable Modeling Strategies In: Harrell Jr Frank E, ed. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Cham: Springer International Publishing:63–102. [Google Scholar]
- 23.Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21:4592–4596. [DOI] [PubMed] [Google Scholar]
- 24.Markar S, Gronnier C, Duhamel A, et al. Significance of Microscopically Incomplete Resection Margin After Esophagectomy for Esophageal Cancer. Ann Surg. 2016;263:712–718. [DOI] [PubMed] [Google Scholar]
- 25.Hsu P-K, Huang C-S, Wang B-Y, et al. Survival benefits of postoperative chemoradiation for lymph node-positive esophageal squamous cell carcinoma. Ann Thorac Surg. 2014;97:1734–1741. [DOI] [PubMed] [Google Scholar]
- 26.Smith JC. Radiation pneumonitis. Am Rev Respir Dis. 1963;87:647–655. [DOI] [PubMed] [Google Scholar]
- 27.King G, Nielsen R. Why Propensity Scores Should Not Be Used for Matching. 33. [Google Scholar]
- 28.Dexter SPL, Sue-Ling H, McMahon MJ, et al. Circumferential resection margin involvement: an independent predictor of survival following surgery for oesophageal cancer. Gut. 2001;48:667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Chen Q-X, Teng L, et al. Prognostic significance of positive circumferential resection margin in esophageal cancer: a systematic review and meta-analysis. Ann Thorac Surg. 2014;97:446–453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.