Abstract
Coronavirus disease-19 is a respiratory viral disease that commonly presents with mild symptoms. However, it can cause serious complications such as acute respiratory disease, especially in patients with comorbidities. As it is a new disease, the full picture of the disease and its complications are not yet fully understood. Moreover, the patients at risk of complications are not well identified; and the data about the risk in patients with hematological malignancies is limited. Here, we report a 65-year-old male with accelerated phase chronic myeloid leukemia, on dasatinib, tested positive for coronavirus disease-19, then complicated with febrile neutropenia acute respiratory distress syndrome.
Keywords: Chronic myelogenous leukemia, COVID-19, Pneumonia, Acute respiratory distress syndrome, Febrile neutropenia
Introduction
Coronavirus disease-19 is a pandemic viral illness that typically presents with respiratory symptoms and is caused by a newly discovered coronavirus, known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), previously known as 2019-nCoV [1]. In December 2019, the outbreak started in Wuhan, a city in China, then spread globally. In March 2020, the World Health Organization declared it a pandemic [2]. The disease severity extends from mild to severe, and even death in some cases. The severity and complications are more in patients with risk factors or other comorbidities such as hypertension, diabetes, cardiovascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, or chronic kidney disease [3]. Although some studies showed that the risk of complications among cancer patients is increased, the impact of the disease on cancer patients or other hematological neoplasms is still controversial [4, 5].
Here we report a case of COVID-19 pneumonia in a 65-year-old male with chronic myeloid leukemia, on dasatinib, complicated by a neutropenic fever and acute respiratory distress syndrome (ARDS).
Case Presentation
A 65-year-old male with accelerated phase chronic myelogenous leukemia (AP-CML) presented to the emergency department with a 3-day history of febrile sensation (not measured by the patient) and progressive shortness of breath, mainly with exertion. Also, he had had a productive cough with a small amount of yellowish sputum and intermittent pleuritic chest pain for a few days. He denied any history of runny nose, sore throat, loss of smell or taste sense, night sweating, or weight loss. There was no history of contact with sick patients, especially with COVID-19, nor recent travel.
He had had AP-CML for 4 years, currently in major molecular remission, and the latest BCR-ABL level was 0.04%; he was on dasatinib 140 mg but decreased to 100 mg because he had pancytopenia, which was resolved after adjusting the dose. Also, he had a chronic right-sided small loculated pleural effusion which was attributed to dasatinib (Fig. 1).
Fig. 1.
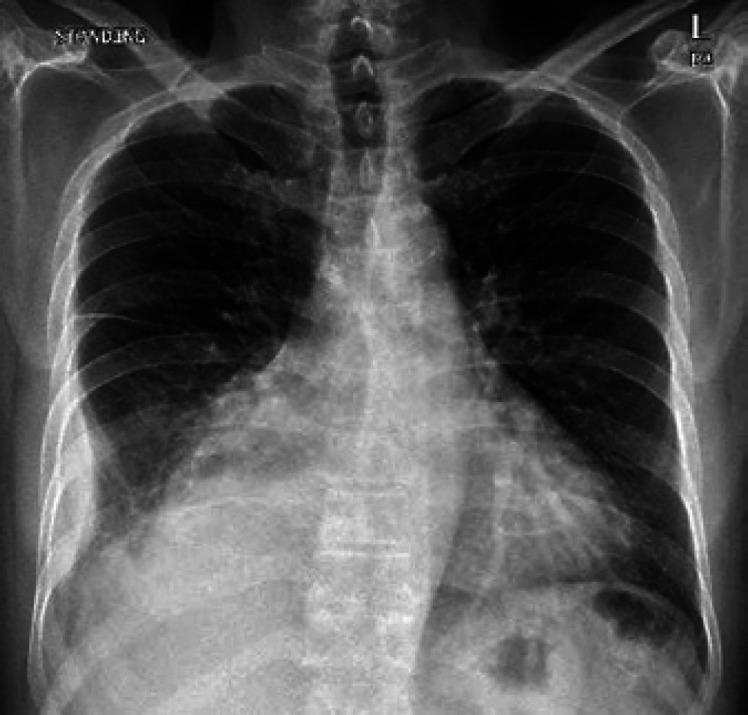
PA chest X-ray shows a pleural-based well-defined opacity which is noted at the lateral aspect of the right lower lung zone, mostly representing loculated pleural effusion.
On arrival to the emergency department, he was febrile, and his recorded temperature was 38.4°C. He had an oxygen saturation (SpO2) of 94% on 6 L via a nonrebreather mask, a respiratory rate of 19 breath per minute, a heart rate of 104 beats per minute, and a blood pressure of 138/79 mm Hg. He was conscious, oriented, and feeling well. Breath sounds were decreased on the bilateral lower lung fields with a coarse crepitation in the right middle to lower zones. The examination of other systems was unremarkable.
Laboratory findings (on the admission day) showed pancytopenia, increased PTT, INR and elevated D-dimer. The liver and kidney functions were normal. C-reactive protein level was elevated and LDH level was normal (Table 1).
Table 1.
Laboratory test results on the day of admission
| Laboratory testing on the day of admission | Value | Normal range |
|---|---|---|
| CBC | ||
| WBC | 3.8×103/µL | 4–10 |
| Lymphocytes | 2.8×103/µL | 1–3 |
| ANC | 0.9×103/µL | 2–7 |
| Hgb | 7.5 g/dL | 13–17 |
| Platelets | 42×103/µL | 150–400 |
| Coagulation | ||
| PT | 14 s | 9.4–12.5 |
| INR | 1.2 s | <1.1 |
| APTT | 39.2 s | 25.1–36.5 |
| D-dimer | 3.41 mg/L | 0.00–0.49 |
| Inflammatory marker | ||
| CRP | 74.6 mg/L | 0.0–5 |
| Others | ||
| LDH | 192 U/L | 135–125 |
| Lactic acid | 0.7 mmol/L | 0.5–2.2 |
The chest X-ray showed bilateral lower lobe collapse and consolidation with pleural effusion, more noted on the left side, which is increased compared to baseline (Fig. 2). Considering the patient's background, clinical presentation and the current pandemic, we tested him for COVID-19 and it came back positive.
Fig. 2.
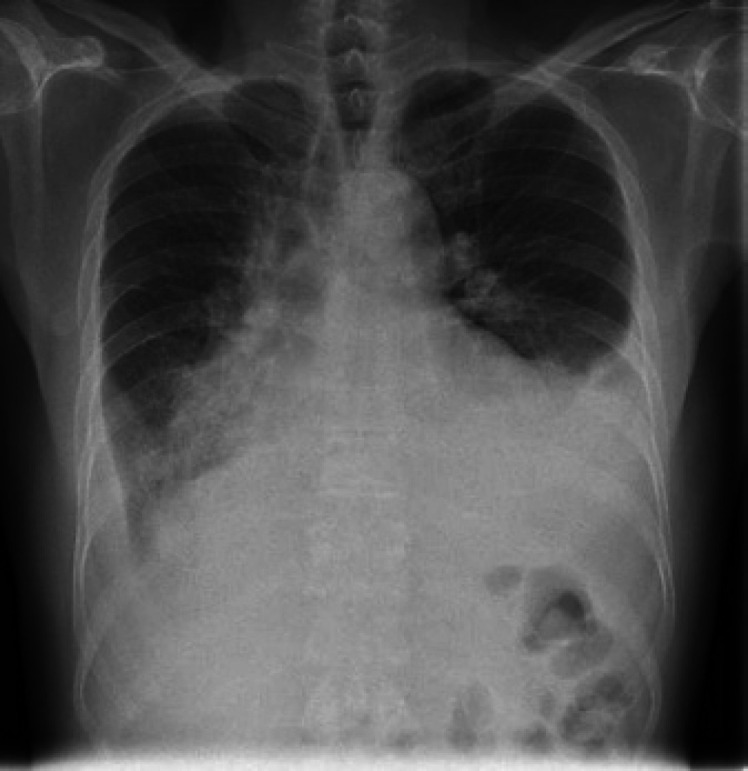
PA erect chest X-ray shows bilateral lower lobe collapse and consolidation with pleural effusion, more noted on the left side.
Chest computed tomography (CT) showed bilateral pleural effusions evident on the left side with lower lobe subsegmental collapse and consolidation as a consequence as well as likely right anterior empyema formation and bilateral lower lung lobe post-inflammatory changes (Fig. 3, 4). There was no growth in blood nor sputum cultures. Also, acid fast bacilli smears and PCR were negative.
Fig. 3.
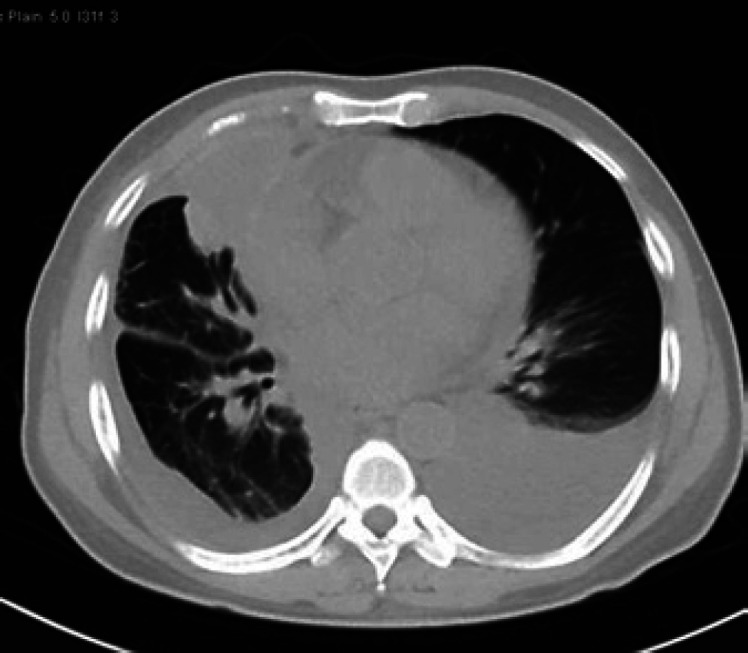
CT of the chest shows bilateral pleural effusions evident on the left side with a consequent lower lobe subsegmental collapse and consolidation.
Fig. 4.
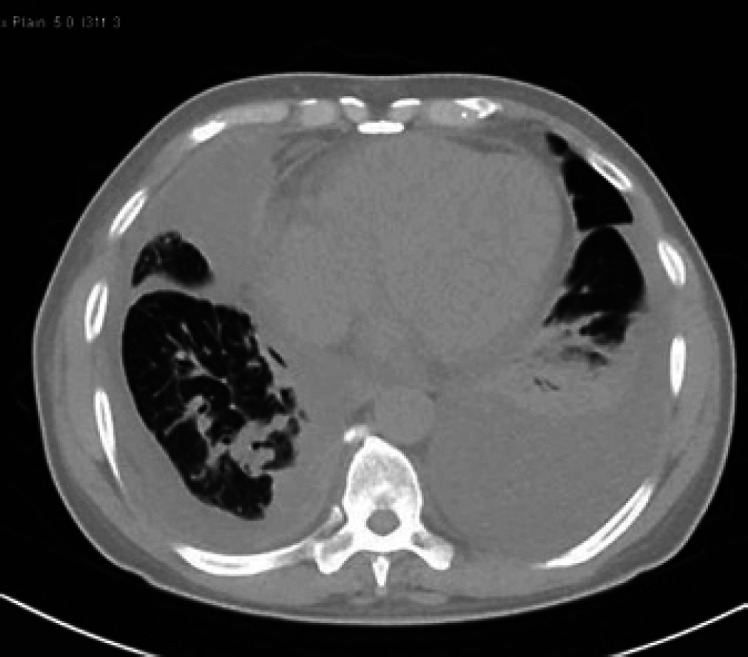
CT of the chest shows bilateral pleural effusions evident on the left side with a consequent lower lobe subsegmental collapse and consolidation.
The patient was admitted with neutropenic fever and COVID-19 and was initially started on hydroxychloroquine 400 mg orally daily, azithromycin 500 mg p.o. daily, oseltamivir 150 mg p.o. b.i.d., based on local COVID-19 management protocol, and piperacillin/tazobactam 4,500 mg i.v. every 8 h. Dasatinib was kept on hold as recommended by the hematology team.
Diagnostic thoracocentesis was done to rule out other underlying causes, 500 mL was removed, and a drain was inserted. Pleural fluid analysis showed exudative lymphocytic pleural effusion and negative acid fast bacilli smears, PCR and cultures.
On day 4, he started to improve, and he was off oxygen; his SpO2 was 95% on room air. However, he became more neutropenic (Table 2).
Table 2.
Laboratory test results on day 4
| Laboratory testing on the day of admission | Value | Normal range |
|---|---|---|
| CBC | ||
| WBC | 2.2×103/µL | 4–10 |
| Lymphocytes | 1.6×103/µL | 1–3 |
| ANC | 0.5×103/µL | 2–7 |
| Hgb | 8.7 g/dL | 13–17 |
| Platelets | 42×103/µL | 150–400 |
On day 8, he had dyspnea after coming back from the bathroom, and became tachypneic (respiratory rate: 43 breaths per minute) and tachycardic (heart rate: 115 beats per minute), requiring 10 L of oxygen via the nonrebreather mask to maintain his SpO2 level above 94%. Chest auscultation showed diffuse bilateral crackles, and there were no signs of fluid overload. Arterial blood gas results were pH 7.48 (normal range: 7.35–7.45), pO2 63 mm Hg (normal range: 83–108), HCO3 27.4 mmol/L (normal range: 23–27) and pCO2 34 mm Hg (normal range: 35–45). The patient was given oxygen and salbutamol nebulization but without significant improvement. The repeated chest X-ray (Fig. 5) showed bilateral infiltrations and his calculated PaO2/FiO2 ratio = 143 (PaO2: 63 on 6 L oxygen, FiO2 44%). Cardiac causes have been ruled out, so he fulfilled the ARDS criteria. Moreover, he was still neutropenic, and his ferritin, LDH and triglyceride levels were elevated (Table 3). So, the patient was transferred to the intensive care unit.
Fig. 5.
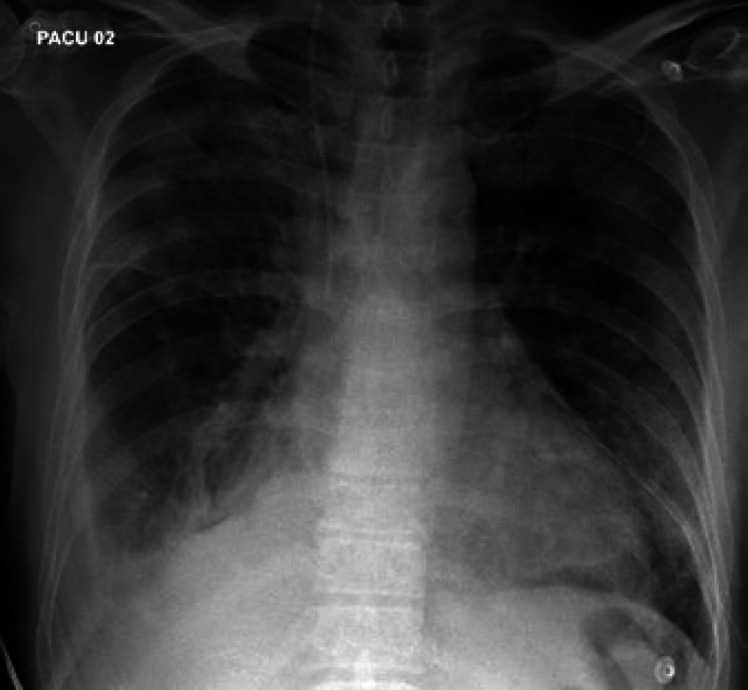
AP chest X-ray shows no significant changes in his bilateral consolidations and infiltrations.
Table 3.
Laboratory test results on the day of deterioration (day 8)
| Laboratory testing on the day of admission | Value | Normal range |
|---|---|---|
| CBC | ||
| WBC | 1.8×103/µL | 4–10 |
| Lymphocytes | 0.4×103/µµL | 1–3 |
| ANC | 0.5×103/µL | 2–7 |
| Hgb | 8.6 g/dL | 13–17 |
| Platelets | 44×103/µL | 150–400 |
| Coagulation | ||
| PT | 12 s | 9.4–12.5 |
| INR | 1.1 s | <1.1 |
| APTT | 40 s | 25.1–36.5 |
| D-dimer | 6.38 mg/L | 0.00–0.49 |
| Inflammatory marker | ||
| CRP | 295.4 mg/L | 0.0–5 |
| 7Ferritin | 1,199 µg/L | 33–553 |
| Others | ||
| LDH | 429 U/L | 135–125 |
| Lactic acid | 2.2 mmol/L | 0.5–2.2 |
| Procalcitonin | 2.87 ng/mL | <0.5 |
| Triglyceride | 2.9 mmol/L | <1.7 |
The infectious disease team recommended to manage him as a case of severe COVID-19 pneumonia complicated by ARDS, which might be due to a cytokine storm. Therefore, they added ritonavir plus lopinavir 500 mg orally daily for 14 days, tocilizumab 400 mg i.v. single dose, and methylprednisolone 40 mg i.v. every 12 h for 5 days, and BiPAP has been applied to improve his SpO2 level. After a few hours, he started to improve with this regimen, so he was shifted back to a simple face mask, and gradually the amount of oxygen has decreased. The repeated ABG showed an improvement in pO2 (100 mm Hg).
On day 12, the chest X-ray was repeated and showed satisfactory lung field expansion with no significant interval change regarding the bilateral airspace opacities. Right-sided pleural effusion is still noted with progression in the segmental right lower lung zone collapse (Fig. 6).
Fig. 6.
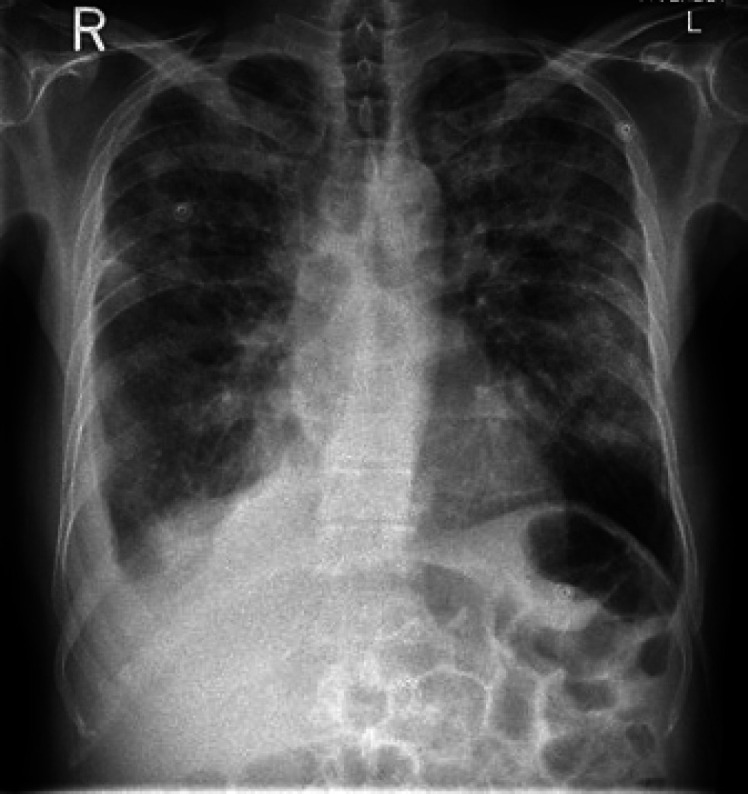
AP chest X-ray shows satisfactory lung field expansion with no significant interval change regarding the bilateral airspace opacities.
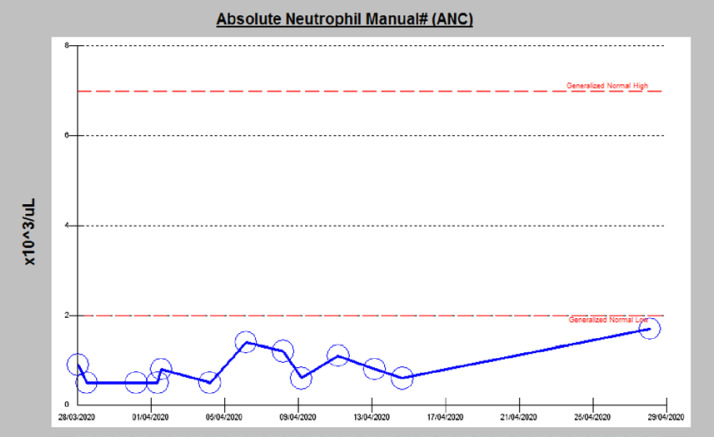
After completion of the treatment, he has gradually improved, and his SpO2 is 98% on ambient air. However, his ANC is fluctuating between 0.4 and 1.8 × 103/μL and he was on daily filgrastim (Fig. 7), so dasatinib has not been started yet. He is still in the hospital but off medications and waiting for his repeated PCR to be negative for discharge. The latest one was done on day 30 (Table 4).
Fig. 7.
Shows the fluctuating level of ANC during the hospital course.
Table 4.
Results of subsequent SARS-CoV-2 PCR tests
| Day of admission | Result |
|---|---|
| Day 1 | Positive |
| Day 9 | Positive |
| Day 12 | Positive |
| Day 19 | Positive |
| Day 26 | Negative |
| Day 27 | Positive |
Discussion
Chronic myeloid or myelogenous leukemia (CML) is a myeloproliferative neoplasm which is defined as uncontrolled and dysregulated production of mature myeloid cells, mainly granulocytes. It results from acquired abnormal chromosomal translocation, t(9; 22), known as the Philadelphia chromosome, which contains the BCR-ABL1 fused gene and produces BCR-ABL1 fusion protein with abnormal tyrosine kinase activity [6]. It has three phases: chronic, accelerated, and blast. Tyrosine kinase inhibitors (TKIs) such as dasatinib, nilotinib, and bosutinib are the first-line treatment [7].
Some studies reported that dasatinib per se might induce immunosuppression and increases the risk of infection and recurrent respiratory infection, which increases the utilization of health care resources [8, 9, 10, 11]. However, another study showed that there is no strong evidence that dasatinib could increase the risk of infection in CML patients in the chronic phase [12].
Moreover, it has been shown that TKIs have some antiviral activity by inducing an immune response and a cytotoxic activity against cytomegalovirus and decrease human immunodeficiency virus replication (in vitro) [13]. Also, they inhibit SARS and MERS coronaviruses replication (in vitro) [14]. So, the risk of infection in patients with CML on dasatinib is debatable.
SARS-CoV-2 is a zoonotic beta coronavirus, like MERS and SARS-CoV-1, which causes a respiratory illness known as COVID-19. Most cases are simple with mild symptoms such as fever, fatigue, and dry cough [15]. However, some cases are severe and might be complicated by ARDS leading to mechanical ventilation. Although the disease could be severe and the complications are more frequently found among patients with comorbidities, such as hypertension and respiratory and cardiovascular system diseases [16], there is no evidence that hematological neoplasms such as CML, even if they are on TKIs, might place the patient at high risk for COVID-19 and its complications.
The main findings of COVID-19 pneumonia on lung CT images are bilateral, subpleural, ground-glass opacities with air bronchograms and ill-defined margins [17]. In contrast, pleural effusion, septal thickening, segmental consolidation, and absence of ground-glass opacities are uncommon and atypical findings [18]. Our patient has dasatinib-related pleural effusion [19], but on admission day, it was increased, and this might be due to COVID-19 pneumonia.
Lymphopenia is the most common laboratory finding. However, leukocytosis and leukopenia have been reported [19, 20]. Thrombocytopenia is more common in patients with critical diseases, and it could distinguish between mild and severe cases [21]. Some patients might develop cytokine storm or secondary hemophagocytic lymphohistiocytosis, which is characterized mainly by cytopenias and hyperferritinemia plus the other features [22]. Usually, these complications occur after a few days of disease onset and being on immunosuppressive medications could have a protective value [23].
Our patient was found to have pancytopenia and febrile neutropenia on the day of admission, a reported side effect of dasatinib [24]. The patient initially started to improve after stopping the medication and starting the antibiotics, but on day 8, he suddenly deteriorated and developed ARDS and became more neutropenic, which is most likely due to severe COVID-19 pneumonia as it improved after administrating COVID-19 pneumonia medications, applying BiPAP, and giving one dose of tocilizumab.
While there is no evidence-based protocol for COVID-19 management, so far, we started the management based on our local protocol for a high-risk group. We administered him, initially, hydroxychloroquine, azithromycin, and oseltamivir, since there is a study showing that these combinations might have a role in viral eradication [25]. After a few days of improvement, he developed ARDS, which might be due to a severe COVID-19 cytokine storm, so the infectious disease team administered him tocilizumab, steroid, and other antiviral medications [26]. This rapid improvement supports our diagnosis of cytokine storm. However, we did not confirm the diagnosis by sending the level of interleukins because they are not available in our hospital. Also, the patient findings were not corresponding with a diagnosis of hemophagocytic lymphohistiocytosis and bone marrow aspiration was not done as the patient has improved.
The available data on COVID-19 in CML patients are very limited, and the recent recommendations are to manage them as healthy persons, especially if they are stable, as CML does not increase their risk of complications. Although there is a study about the prevalence of COVID-19 in CML patients, it is a preprint and has not been peer-reviewed yet. It showed that the risk of death is higher in patients with other comorbidities, and patients who failed to respond to CML treatment are more symptomatic [27].
To the best of our knowledge, this is the first case report in the literature about COVID-19 in AP-CML patient on dasatinib complicated by febrile neutropenia and ARDS.
Conclusion
We would like to highlight the importance of early admission and early treatment of CML patients with COVID-19, even if they present with mild symptoms or initially improved, and especially if they are on chemotherapy medications. Also, we emphasize the importance of the introduction of supportive management (oxygen with BiPAP, tocilizumab with pulse methylprednisolone) to control cytokine storm and other complications.
Although ground-glass opacities are the typical radiological features of COVID-19, it could be presented with other radiological findings like unilateral or bilateral pleural effusion and might be complicated by ARDS as in our case.
Statement of Ethics
The case was approved by the HMC Medical Research center and the patient signed a written informed consent to publish the case (including publication of images).
Disclosure Statement
The authors report no conflicts of interest in this work.
Funding Source
The authors received funding from the Qatar national library.
Author Contributions
All authors equally contributed in witting and editing.
Acknowledgment
We acknowledge the internal medicine residency program for scientific support.
References
- 1.Gorbalenya A. Severe acute respiratory syndrome-related coronavirus: The species and its viruses, a statement of the Coronavirus Study Group. BioRxiv. 2020 doi: 10.1101/2020.02.07.937862. [DOI] [Google Scholar]
- 2.WHO Director-General's opening remarks at the media briefing on COVID-19 – 11 March 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020.
- 3.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1,590 patients with Covid-19 in China: A Nationwide Analysis. Eur Respir J. 2020 May 14;55((5)):pii–2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21((3)):335–7. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21((4)):e180. doi: 10.1016/S1470-2045(20)30150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Dewik N, Morsi H, Samara M, Ghasoub R, Gnanam C, Bhaskaran S, et al. Is adherence to imatinib mesylate treatment among patients with chronic myeloid leukemia associated with better clinical outcomes in Qatar? Clin Med Insights Oncol. 2016 Jan;10:CMO–S32822. doi: 10.4137/CMO.S32822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turkina A, Wang J, Mathews V, Saydam G, Jung C, Al Hashmi H, et al. TARGET: a survey of real‐world management of chronic myeloid leukaemia across 33 countries. Br J Haematol. 2020 doi: 10.1111/bjh.16599. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez GH, Ahmed SI, Al-akhrass F, Rallapalli V, Safda A. Characteristics of, and risk factors for, infections in patients with cancer treated with dasatinib and a brief review of other complications. Leuk Lymphoma. 2012;53((8)):1530–5. doi: 10.3109/10428194.2012.656626. [DOI] [PubMed] [Google Scholar]
- 9.Obut F, Randall N, Young JA, Valent P, Ustun C. Dasatinib-induced immunosuppression and recurrent respiratory tract infections. Leuk Lymphoma. 2015;56((8)):2484–5. doi: 10.3109/10428194.2014.994179. [DOI] [PubMed] [Google Scholar]
- 10.Seiter K, Latremouille-Viau D, Guerin A, Ndife B, Habucky K, Tang DH, et al. Burden of infections among chronic myeloid leukemia patients receiving dasatinib or nilotinib: a real-world retrospective healthcare claims study in the United States. Adv Ther. 2018;35((10)):1671–85. doi: 10.1007/s12325-018-0772-3. [DOI] [PubMed] [Google Scholar]
- 11.Sillaber C, Herrmann H, Bennett K, Rix U, Baumgartner C, Böhm A, et al. Immunosuppression and atypical infections in CML patients treated with dasatinib at 140 mg daily. Eur J Clin Invest. 2009;39((12)):1098–109. doi: 10.1111/j.1365-2362.2009.02206.x. [DOI] [PubMed] [Google Scholar]
- 12.Al-Ameri AM, Kantarjian H, Burton E, O'Brien S, Ravandi F, Borthakur G, et al. Low risk of infectious events in patients (pts) with chronic myeloid leukemia (CML) in chronic phase (CP) treated with dasatinib. Blood. 2009;114((22)):3291. [Google Scholar]
- 13.Climent N, Plana M. Immunomodulatory activity of tyrosine kinase inhibitors to elicit cytotoxicity against cancer and viral infection. Front Pharmacol. 2019;10:1232. doi: 10.3389/fphar.2019.01232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman CM, Sisk JM, Mingo RM, Nelson EA, White JM, Frieman MB. Abelson kinase inhibitors are potent inhibitors of severe acute respiratory syndrome coronavirus and middle east respiratory syndrome coronavirus fusion. J Virol. 2016;90((19)):8924–33. doi: 10.1128/JVI.01429-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323((13)):1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20((4)):425–34. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020:200823. doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395((10223)):507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 Mar;12(pii):ciaa248. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395((10229)):1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395((10229)):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoury HJ, Guilhot F, Hughes TP, Kim DW, Cortes JE. Dasatinib treatment for Philadelphia chromosome-positive leukemias: practical considerations. Cancer. 2009;115((7)):1381–94. doi: 10.1002/cncr.24155. [DOI] [PubMed] [Google Scholar]
- 25.Gautret P, Lagier JC, Parola P, Van Thuan Hoang. Hoang VT, Meddeb L, Mailhe B, et al. Hydroxychloroquine and Azithromycin as a Treatment of COVID-19: Results of an Open-Label Non-Randomized Clinical Trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;202003((00026)):v1. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Guo J, Yang Z, You Y, Chen Z, Chen S, et al. The first report of the prevalence of COVID-19 in chronic myelogenous leukemia patients in the core epidemic area of China: a multicentre. Cross-Sectional Surv. 2020. [DOI]