Key Points
Question
What are appropriate dosing strategies for hydroxychloroquine and remdesivir in children with coronavirus disease 2019?
Findings
In this simulation-based dose-ranging study, pediatric dosing strategies were devised that provided similar exposures between children within different developmental stages and adults. However, the analysis raised concerns regarding hydroxychloroquine use for coronavirus disease 2019 treatment because unbound plasma concentrations were less than those postulated to mediate an antiviral effect.
Meaning
To confirm the appropriateness of the proposed dosing schemes, prospective pharmacokinetic, safety, and efficacy studies in children are required.
Abstract
Importance
Children of all ages appear susceptible to severe acute respiratory syndrome coronavirus 2 infection. To support pediatric clinical studies for investigational treatments of coronavirus disease 2019 (COVID-19), pediatric-specific dosing is required.
Objective
To define pediatric-specific dosing regimens for hydroxychloroquine and remdesivir for COVID-19 treatment.
Design, Setting, and Participants
Pharmacokinetic modeling and simulation were used to extrapolate investigated adult dosages toward children (March 2020-April 2020). Physiologically based pharmacokinetic modeling was used to inform pediatric dosing for hydroxychloroquine. For remdesivir, pediatric dosages were derived using allometric-scaling with age-dependent exponents. Dosing simulations were conducted using simulated pediatric and adult participants based on the demographics of a white US population.
Interventions
Simulated drug exposures following a 5-day course of hydroxychloroquine (400 mg every 12 hours × 2 doses followed by 200 mg every 12 hours × 8 doses) and a single 200-mg intravenous dose of remdesivir were computed for simulated adult participants. A simulation-based dose-ranging study was conducted in simulated children exploring different absolute and weight-normalized dosing strategies.
Main Outcomes and Measures
The primary outcome for hydroxychloroquine was average unbound plasma concentrations for 5 treatment days. Additionally, unbound interstitial lung concentrations were simulated. For remdesivir, the primary outcome was plasma exposure (area under the curve, 0 to infinity) following single-dose administration.
Results
For hydroxychloroquine, the physiologically based pharmacokinetic model analysis included 500 and 600 simulated white adult and pediatric participants, respectively, and supported weight-normalized dosing for children weighing less than 50 kg. Geometric mean-simulated average unbound plasma concentration values among children within different developmental age groups (32-35 ng/mL) were congruent to adults (32 ng/mL). Simulated unbound hydroxychloroquine concentrations in lung interstitial fluid mirrored those in unbound plasma and were notably lower than in vitro concentrations needed to mediate antiviral activity. For remdesivir, the analysis included 1000 and 6000 simulated adult and pediatric participants, respectively. The proposed pediatric dosing strategy supported weight-normalized dosing for participants weighing less than 60 kg. Geometric mean-simulated plasma area under the time curve 0 to infinity values among children within different developmental age-groups (4315-5027 ng × h/mL) were similar to adults (4398 ng × h/mL).
Conclusions and Relevance
This analysis provides pediatric-specific dosing suggestions for hydroxychloroquine and remdesivir and raises concerns regarding hydroxychloroquine use for COVID-19 treatment because concentrations were less than those needed to mediate an antiviral effect.
This pharmacokinetic simulation study estimates appropriate pediatric-specific dosing regimens for hydroxychloroquine and remdesivir in the treatment of pediatric patients with COVID-19.
Introduction
Children of all ages appear susceptible to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus responsible for coronavirus disease 2019 (COVID-19). Epidemiologic and clinical data in both adults and children remain limited as COVID-19 continues to spread worldwide. While most pediatric cases have been asymptomatic or mild, cases of critically ill infants and children have been reported. No drugs have been proven efficacious in clinical trials for treating SARS-CoV-2, but hydroxychloroquine and the antiviral remdesivir (GS-5743) are undergoing clinical studies in adults.
Hydroxychloroquine has immunomodulating properties and is widely used in adults and children for the prophylaxis and treatment of malaria, other intracellular infections, and rheumatologic diseases. Hydroxychloroquine has shown in vitro activity against the related severe acute respiratory syndrome coronavirus 1, and in March 2020 demonstrated in vitro activity against SARS-CoV-2. Adult pharmacokinetic (PK) data indicate hydroxychloroquine exhibits extensive tissue and red blood cell distribution (volume of distribution in plasma >44 000 L). Clearance is primarily mediated through hepatic metabolism, with kidney excretion representing a minor eliminatory pathway. Based on a previously published physiologically based PK (PBPK) modeling analysis, 400 mg twice daily for 1 day, followed by 200 mg twice daily for 4 additional days was postulated to provide optimal lung tissue concentrations in adults for treatment of SARS-CoV-2 infections.
Remdesivir (GS-5473) is a phosphoramidate prodrug of an adenine nucleotide analogue that exhibits broad-spectrum antiviral activity against coronaviruses. Antiviral activity is mediated via intracellular conversion to its active triphosphate metabolite, GS-443902, which inhibits RNA-dependent RNA polymerase. Following intravenous administration in healthy adults, plasma concentrations of remdesivir rapidly decrease (half-life of approximately 1 hour). Pathways of remdesivir clearance in humans are incompletely characterized. Remdesivir is not yet approved for clinical use, but a February 2020 in vitro study demonstrated its activity against SARS-CoV-2.
Clinical trial efforts are rapidly being mobilized to combat COVID-19, with a focus on adult patients. To support clinical research efforts in pediatric populations, we sought to provide dosing suggestions for hydroxychloroquine and remdesivir in children using PK modeling and simulation.
Methods
Based on the availability of physicochemical and drug-specific absorption, distribution, metabolism, and excretion information, different methods were adopted to scale currently investigated adult dosages to children. For hydroxychloroquine, the availability of human PK studies permitted the development of a pediatric PBPK model. Physiologically based PK models are mathematical constructs combining organism-specific and drug-specific parameters to provide a priori predictions of drug PK. This modeling strategy is routinely incorporated into regulatory submissions to support initial dosing for pediatric clinical studies. Limited published human PK studies were available for remdesivir, so pediatric dosages were derived using a modified allometric scaling approach. This analysis follows applicable sections of the Enhancing the Quality and Transparency of Health Research reporting guidelines for population PK studies, was conducted from March 2020 to April 2020, and was exempt from the Duke University Health System institutional review board review because the research did not involve human participants.
Software
Physiologically based PK modeling was performed in PK-Sim, version 8 (Open Systems Pharmacology). Postprocessing of PBPK model simulations and allometry-based dose scaling were conducted in R, version 3.4.3 (R Foundation for Statistical Computing), and RStudio, version 1.1.383 (RStudio; Integrated Development for R), with the ggplot2, cowplot, zoo, and PKNCA packages.
Hydroxychloroquine
Pediatric PBPK models were developed using a US Food and Drug Administration–supported workflow that leverages the abundance of adult PK information to construct an adult PBPK model prior to scaling toward children.
Adult PBPK Model Development
An adult PBPK model for hydroxychloroquine was developed using physicochemical and absorption, distribution, metabolism, and excretion data obtained from the literature (eTable 1 in the Supplement). Hydroxychloroquine displays affinity for albumin and α1-acid glycoprotein; however, for model parameterization, plasma protein binding was attributed toward α1-acid glycoprotein. Hepatic metabolism and kidney excretion represent major and minor pathways of hydroxychloroquine clearance, respectively. Approximately 26% of hydroxychloroquine appears unchanged in urine after intravenous dosing. Because the product of the glomerular filtration rate (120 mL/min) and fraction unbound in plasma (0.48) is less than the estimated plasma kidney clearance for hydroxychloroquine (211 mL/min), both glomerular filtration and tubular secretion were assumed to contribute toward kidney excretion. The specific mechanism of hydroxychloroquine tubular secretion is unknown; therefore, tubular secretion was attributed to multiantimicrobial extrusion protein-1 (MATE-1), an apical kidney transporter. This assumption was implemented based on an in vitro drug transport study that identified the antimalarial chloroquine, a structurally similar aminoquinoline, as a substrate for MATE-1.
Literature identifying the specific isozymes and their contribution toward hydroxychloroquine metabolism is lacking, so an initial adult PBPK model was developed using a single first-order hepatic intrinsic clearance process. Using blood and plasma concentration time data digitized from published adult PK studies, the following parameters were optimized in the initial adult model using PK-Sim’s Parameter Identification module: logarithm of the octanol-water partition coefficient (a direct predictor of drug distribution), intestinal (transcellular) permeability, and hepatic intrinsic clearance. Additionally, tubular secretion intrinsic clearance was optimized to maintain a fraction excreted unchanged in the urine of 26%. Following this parameter optimization process, the single hepatic intrinsic clearance process was segregated to proportionally account for the contributions of 3 hepatic isozymes (cytochrome P450 [CYP] 2C8, 2D6, and 3A4), defined based on in vitro metabolism data for chloroquine using human liver microsomes and recombinant cytochrome P450 enzymes (45%, 44%, and 11%, respectively). The developed adult PBPK model was evaluated against PK data from 3 studies not used for model development (eMethods in the Supplement).
Pediatric PBPK Model Development
Pediatric PBPK model development consisted of scaling anatomical (eg, organ sizes) and physiological (eg, blood flows) model parameters from the evaluated adult model toward children (birth to 18 years postnatal age). Age-specific anatomic and physiologic parameters are defined within PK-Sim. Ontogeny functions defined within PK-Sim for α1-acid glycoprotein, hepatic CYP2C8, CYP2D6, and CYP3A4 were used to facilitate pediatric PK predictions. For the apical kidney transporter, MATE-1, a previous investigation evaluating the ontogeny of mRNA expression and protein abundances of select kidney transporters failed to demonstrate an age-dependent association for MATE-1. Therefore, no ontogeny function was included for this transporter, but interpatient variability in MATE-1 expression was included in the model; variability was defined using digitized protein abundance data across all analyzed samples (geometric SD, 1.48).
Pediatric Dosing Simulations
The developed pediatric PBPK model was used to inform oral hydroxychloroquine dosing for children ranging from term neonates to adolescents. We aimed to devise a pediatric dosing regimen to provide similar unbound plasma concentrations as predicted for adults receiving 400 mg of hydroxychloroquine sulfate every 12 hours × 2 doses, followed by 200 mg every 12 hours × 8 doses. This dose was recommended based on a previously published PBPK model analysis, where simulated lung tissue concentrations (adjusted by the fraction unbound in plasma) in adults were reported to exceed an in vitro–determined effective concentration for 50% inhibition of viral replication (50% EC; 0.72μM; 242 ng/mL). Age-specific pediatric dosages were optimized to achieve similar average unbound hydroxychloroquine concentrations over 120 hours in plasma during a 5-day period as those estimated for adults. Comparability of exposures was asserted for instances where the geometric mean of average unbound hydroxychloroquine concentrations for children fell within 80% to 125% of the simulated value for adults. The dosing scheme was developed based on model simulations for a simulated population consisting of 600 children (birth to 18 years postnatal age). Adult reference simulations were generated for 500 simulated participants ranging from 20 to 50 years postnatal age. Simulated participants were generated in PK-Sim based on the demographics of a white US population. The male-to-female ratio of the generated population was 50:50. To demonstrate the relevance of hydroxychloroquine exposures in unbound plasma toward the target organ space of interest for SARS-CoV-2 infections, unbound lung interstitial fluid simulations were generated to compare concentrations between the 2 matrices.
Remdesivir
A modified allometric-based scaling approach was used to inform remdesivir dosing in children ranging from term neonates to adolescents.
Allometry With Age-Dependent Exponents
Drug clearance is a primary PK parameter that, in conjunction with dose, can provide estimates of drug exposure. To estimate age-specific clearance values for remdesivir in children, we used an allometric approach with age-dependent scalers. Using this approach, pediatric clearance values were estimated from adult values using Equation 1:
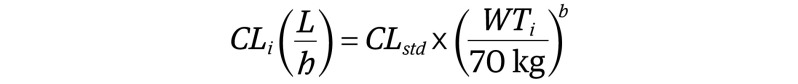 |
where CLi represents the individual clearance; CLstd, clearance of remdesivir in a healthy 70-kg adult; WTi, individual weight; and b, an age-dependent exponent with values of 1.1, 1.0, 0.9, and 0.75 for children 3 months or younger, older than 3 months to 2 years, older than 2 years to 5 years, and older than 5 years, respectively.
Pediatric Dosing Simulations
In clinical trials, the adult remdesivir dose for SARS-CoV-2 infection is a 200-mg intravenous load (day 1) followed by 100 mg intravenous daily (≥day 2). Because pediatric dosing is not yet established for SARS-CoV-2, pediatric dosing for Ebola virus disease (EVD) may provide a logical starting point. For pediatric patients weighing less than 40 kg, EVD dosing consists of 5 mg/kg intravenous load (day 1) followed by 2.5 mg/kg daily (≥day 2).
In this analysis, we aimed to (1) estimate plasma remdesivir exposure in children following administration of EVD recommended dosages and (2) devise a pediatric dosing regimen to provide similar plasma exposures as observed in adults receiving currently recommend doses. Clearance of remdesivir in adults was calculated from a dose-ranging study (10-225 mg) where areas under the plasma concentration time curve (AUC) from 0 to infinity were defined. For the evaluated dosing cohorts, each composed of 8 healthy adult participants, clearance was defined as the quotient of dose and AUC time from 0 to infinity. Mean clearance for all dosing cohorts was assumed to represent the value for a healthy 70-kg adult.
Pharmacokinetic simulations were conducted for simulated populations consisting of 6000 simulated children (birth to 18 years postnatal age) and 1000 simulated adults (age 20-50 years). Simulated participants were generated in PK-Sim based on a white US population. The male-to-female ratio of the generated population was 50:50. Simulated AUC 0 to infinity was calculated for each simulated participant according to Equation 2:
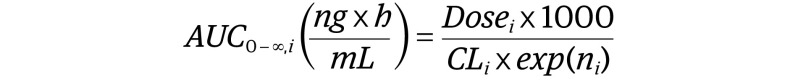 |
where Dosei is the individual dose in mg; CLi, individual clearance (liters per hour) calculated based on Equation 1; and ni, a random variable used to introduce interindividual variability into PK simulations. We empirically parameterized ni as N(0,0.3) to provide an approximate interindividual variability of 30% on remdesivir clearance. Considering the plasma half-life of remdesivir in adults is approximately 1 hour and a dosing frequency of every 24 hours, it was inferred that remdesivir would not accumulate in plasma using daily dosing. Therefore, PK simulations were computed after single doses. Pediatric doses were considered equivalent to adults if the geometric mean of plasma AUC 0 to infinity in children was within 80% to 125% of adult values.
Results
Hydroxychloroquine
Adult PBPK Model Evaluation
The developed adult PBPK model adequately characterized hydroxychloroquine disposition in plasma and blood among healthy volunteers and participants with rheumatoid arthritis following oral dosing (eTable 2 and eFigure 1 in the Supplement). Ratios of model predicted vs observed maximum concentration values ranged between 0.81 to 0.96 in blood and plasma. Physiologically based PK model-predicted blood and plasma exposures computed over 100 hours (ie, AUC, 0-100 hours) were similar to estimates derived from previously published empirically based PK models. Ratios of PBPK model predicted vs empirically derived AUC from 0 to 100 hour values were 1.03 for plasma and 1.17 for blood. Additionally, empirically derived concentration time estimates in blood and plasma were adequately described by the developed adult PBPK model (eFigure 1F and G in the Supplement).
Age-Specific Dosing Simulations
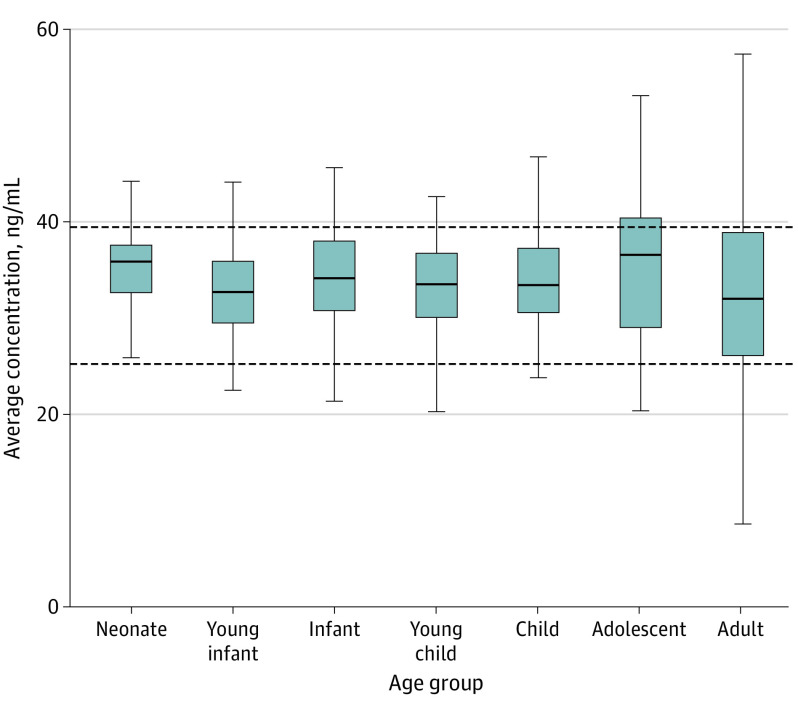
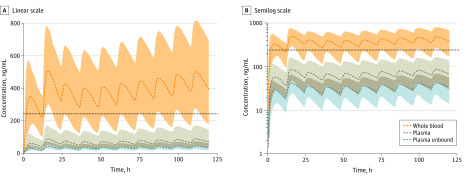
The PBPK model–informed pediatric dosing scheme classifies patients based on weight and uses weight-normalized dosing for children less than 50 kg (Table 1). Using the suggested dosing regimen, geometric mean-simulated average unbound plasma concentrations values among children within different developmental age groups (32-35 ng/mL) were congruent to adult values (32 ng/mL; Figure 1; eTable 3 in the Supplement). Additionally, the trajectory of simulated plasma unbound hydroxychloroquine concentrations within different developmental age groups were similar to those depicted in adults over 120 hours (eFigure 2 in the Supplement). Furthermore, PBPK model simulations indicated that plasma unbound concentrations approximated unbound lung interstitial fluid concentrations (eFigure 3 in the Supplement). For example, geometric mean-simulated average concentrations values in unbound plasma and unbound lung interstitial fluid were both 35 ng/mL in neonates. Lastly, to demonstrate the differences in hydroxychloroquine exposure between sampling matrices, we simulated blood, plasma, and unbound plasma exposures in adults following the administration of a previously defined 5-day dosing regimen (400 mg every 12 hours × 2 doses, followed by 200 mg every 12 hours ×8 doses; Figure 2). Geometric mean-simulated average concentration values in whole blood, plasma, and unbound plasma were 375 ng/mL, 66 ng/mL, and 32 ng/mL, respectively. Importantly, during the 5 days of dosing, unbound plasma hydroxychloroquine concentrations were less than the reference in vitro 50% EC value for viral inhibition (0.72μM; 242 ng/mL).
Table 1. Hydroxychloroquine: Suggested Oral Pediatric Dosing Algorithma,b.
| Weight, kg | Day 1 | Days 2-5 |
|---|---|---|
| <5 | 6 mg/kg every 12 h | 1.5 mg/kg every 12 h |
| 5 to <25 | 6 mg/kg every 12 h | 2.5 mg/kg every 12 h |
| 25 to <50 | 6 mg/kg every 12 h | 3 mg/kg every 12 h |
| ≥50 | 400 mg every 12 h | 200 mg every 12 h |
Dosages defined in terms of hydroxychloroquine sulfate.
Dosages expected to provide similar average unbound drug concentrations in plasma over 120 hours as those computed for adults.
Figure 1. Simulated Average Unbound Hydroxychloroquine Concentrations in Plasma for 120 Hours in Children and Adults.
Average unbound hydroxychloroquine concentrations in plasma for 120 hours in children and adults under the proposed dosing algorithm. Age classifications are based on postnatal age and are defined as follows: neonate (0 to <30 days), young infant (1 to <6 months), infant (6 to <24 months), young child (2 to <6 years), child (6 to <12 years), adolescent (12-18 years), and adult (20-50 years). Horizontal lines of the box correspond to the first quartile, median, and third quartiles. Upper whiskers extend from the third quartile to the largest observation to a maximum length of 1.5 times the interquartile range (ie, third quartile to first quartile). Lower whiskers extend from the first quartile to the lowest observation to a maximum length of 1.5 times the interquartile range (ie, third quartile to first quartile).
Figure 2. Hydroxychloroquine Adult Population Physiologically Based Pharmacokinetic (PBPK) Model Simulations.
Hydroxychloroquine adult population PBPK model simulations depicting a dosage regimen consisting of 400 mg every 12 hours × 2 doses followed by 200 mg every 12 hours × 8 doses. Linear scale (A) and semilog scale (B). Model-generated median and 95% prediction intervals are displayed as dashed lines and colored areas, respectively. An in vitro–derived effective concentration (50% EC = 0.72uM [242 ng/mL]) value for hydroxychloroquine for treatment of severe acute respiratory syndrome coronavirus 2–infected Vero cells is displayed for reference (dotted line).
Age-Specific Dosing Simulations: Remdesivir
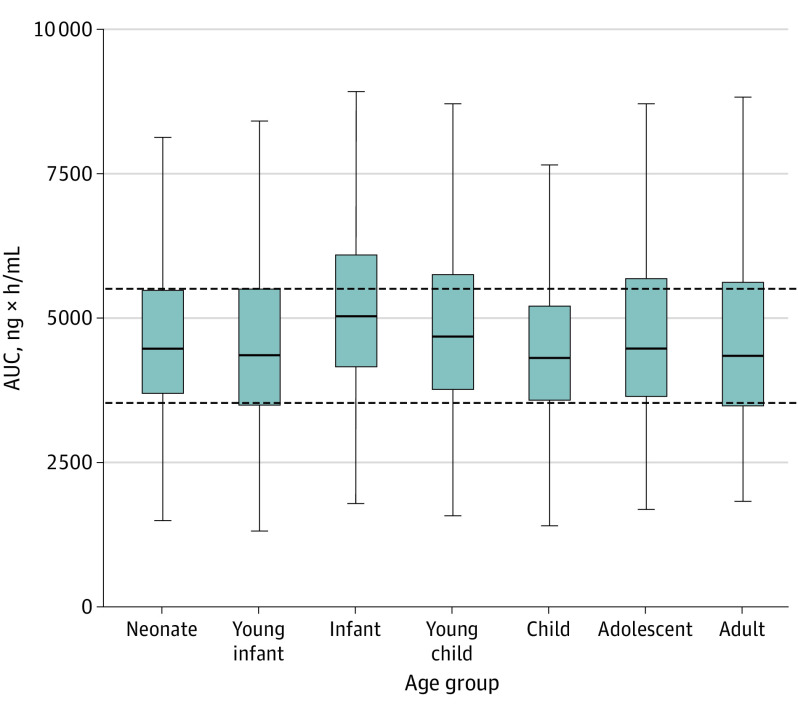
Table 2 displays the suggested pediatric dosing regimen for remdesivir, based on allometry with age-dependent exponents. The dosing scheme classifies patients by weight and provides weight-normalized dosages for patients weighing less than 60 kg. Geometric mean-simulated plasma AUC 0 to infinity values among children within different developmental age groups (4315-5027 ng × h/mL) were similar to adults (4398 ng × h/mL; Figure 3; eTable 4 in the Supplement). In contrast, for children younger than 12 years, geometric mean plasma AUC 0 to infinity values were 147% to 256% of the adult value for simulations based on dosing recommendations for EVD (eFigure 4 and eTable 5 in the Supplement).
Table 2. Pediatric Dosing Algorithm for Remdesivira.
| Weight, kg | Loading (day 1) | Maintenance (≥day 2) |
|---|---|---|
| <6 | 2 mg/kg | 1 mg/kg |
| 6 to <15 | 3 mg/kg | 1.5 mg/kg |
| 15 to <30 | 3.5 mg/kg | 1.75 mg/kg |
| 30 to <60 | 3 mg/kg | 1.5 mg/kg |
| ≥60 | 200 mg | 100 mg |
Dosages expected to provide similar plasma exposures (ie, area of the concentration-time cure) as those computed for adults.
Figure 3. Simulated Plasma Exposures for Remdesivir in Children and Adults.
Simulated plasma exposures for remdesivir (area under the plasma concentration time curve from time 0 to infinity) in children and adults for the proposed dosing algorithm. Exposures representative of loading dose administration. Age classifications are based on postnatal age and are defined as follows: neonate (0 to <30 days), young infant (1 to <6 months), infant (6 to <24 months), young child (2 to <6 years), child (6 to <12 years), adolescent (12-18 years), and adult (20-50 years). Horizontal lines of the box correspond to the first quartile, median, and third quartiles. Upper whiskers extend from the third quartile to the largest observation to a maximum length of 1.5 times the interquartile range (ie, third quartile to first quartile). Lower whiskers extend from the first quartile to the lowest observation to a maximum length of 1.5 times the interquartile range (ie, third quartile to first quartile).
Discussion
We sought to provide pediatric-specific dosing regimens for investigational therapies against COVID-19 in children. Although simulated hydroxychloroquine concentrations in unbound plasma were similar between children and adults using the model-derived dosing scheme (eTable 3 in the Supplement), a significant finding from our analysis was that unbound plasma concentrations failed to exceed the reference 50% EC value (Figure 2). This in vitro–determined value was derived based on 48-hour incubations with SARS-CoV-2-infected Vero cells (0.72μM; 242 ng/mL). Factors such as drug binding in incubation, virus input, time of infection, and cell passage number can influence drug potency in cell-based assays, so variability in in vitro–determined 50% EC values is expected. Nevertheless, separately published in vitro–derived 50% EC values for hydroxychloroquine were considerably higher, ranging from 4.06 to 17.31μM (1364-5814 ng/mL), concentrations that are unachievable in unbound plasma with current evaluated dosing. Because simulated hydroxychloroquine concentrations in unbound plasma parallel those in unbound lung interstitial fluid, our analysis indicates that target site concentrations of hydroxychloroquine for COVID-19 will be lower than those required to exert an antiviral effect; however, our findings do not deny the potential utility of hydroxychloroquine for COVID-19 treatment based on other mechanisms of action, such as immunomodulation. Additionally, because viruses are intrinsically intracellular pathogens, the reference concentration of interest may lie within the intracellular space. In contrast to a competing PBPK model-based analysis for hydroxychloroquine, we chose not to simulate lung intracellular or lung tissue concentrations for 2 reasons. First, such simulations are highly dependent on the defined lung tissue-to-plasma partition coefficient. Depending on the source (preclinical species or experimental conditions), lung tissue-to-plasma partition coefficient values can vary, influencing the outcome of model simulations. Second, in vitro studies assessing hydroxychloroquine’s antiviral effects were based on drug added to incubations containing Vero cells, where 50% EC values were defined based on drug concentrations in incubation (ie, extracellular concentration), not intracellular or tissue concentrations. Therefore, in this analysis, we simulated unbound concentrations in the lung interstitial fluid (ie, extracellular fluid), which represents the most relevant compartment for assessing hydroxychloroquine’s in vivo antiviral efficacy based on in vitro–derived 50% EC values.
Simulated hydroxychloroquine plasma concentrations for a typical 70-kg adult participant from our PBPK model were similar to estimates generated by another published PBPK model (400 mg every 12 hours × 2 doses followed by 200 mg every 12 hours × 8 doses; eFigure 5A in the Supplement). However, simulated (typical) concentration estimates from competing population PK models were relatively higher (eFigure 5A in the Supplement). For example, the maximum plasma concentration value on dosing day 5 was 7.5 times higher for the Lim et al population PK model compared with our PBPK model; despite these discrepancies, anticipated unbound plasma concentrations are expected to be less than 242 ng/mL (ie, the lowest reported 50% EC value) for all models except for the Lim et al model (eFigure 5B in the Supplement). As such, simulations based on different modeling approaches may provide alternate interpretations of the utility of hydroxychloroquine for COVID-19 treatment.
Our understanding of clearance pathways for remdesivir and its metabolites in humans are limited, so we used an empirical-based strategy, allometry with age-dependent exponents, to estimate pediatric dosing. Compared with traditional fixed-exponent allometry, the use of age-dependent exponents provides more conservative dosage estimates for younger children (<5 years), where key physiologic processes that mediate drug clearance have yet to reach maturity. In an analysis of 73 drugs, which included compounds cleared through various pathways (ie, hepatic and kidney), allometry with age-dependent exponents provided a similar predictive capacity to pediatric PBPK modeling, with 90.6% and 91.1% of clearance or exposure estimates falling within 0.5-fold to 2-fold of observed pediatric values, respectively. In our analysis, dose extrapolations for remdesivir were performed assuming the presence of dose-proportional PK (ie, linear PK) in both children and adults. Although pediatric PK information for remdesivir is currently unavailable, preliminary data from adults indicate the presence of dose-proportional PK (eFigure 6 in the Supplement).
In this analysis, simulations were based on white populations, which were used to define anatomic and physiologic parameters for the hydroxychloroquine PBPK model and the age and weight distribution of participants in the remdesivir allometric-based analysis. Owing to hydroxychloroquine’s extensive tissue distribution, we do not anticipate weight-normalized volume of distribution values to vary substantially among different races. However, CYP2D6 may contribute to hydroxychloroquine’s hepatic metabolism, so racial/ethnic differences in enzyme polymorphisms might play a role in perpetuating variability in drug clearance. For example, an analysis of 194 Korean adult patients with systemic lupus erythematosus indicated that CYP2D6 polymorphism (rs1065852) was associated with differences in whole-blood concentrations of the hydroxychloroquine metabolite, N-desethyl hydroxychloroquine. Nevertheless, no difference in whole-blood concentrations of hydroxychloroquine was observed between the different polymorphisms. For our analysis of remdesivir, dosing was segregated using weight-based cohorts. Consequently, differences in body size between different races/ethnicities were not anticipated to impart a substantial influence.
Limitations
This analysis has some limitations. The proposed pediatric dosing regimens for both compounds were based solely on PK considerations, which assumes that the concentration-effect association (ie, pharmacodynamic) is preserved between children and adults. Additionally, owing to knowledge gaps associated with hydroxychloroquine metabolism, the identity and proportional contribution of the specific isozymes responsible for hepatic metabolism were assumed based on in vitro drug metabolism data for chloroquine. To assess our model’s sensitivity to these assumptions, we formulated an alternative PBPK model based on a separate in vitro analysis of chloroquine metabolism where proportional contributions of CYP2C8, CYP2D6, and CYP3A4 toward hepatic intrinsic clearance were 59%, 16%, and 25%, respectively. Using this alternative model in conjunction with the previously proposed pediatric dosing scheme, congruent unbound plasma concentrations between children and adults were observed (eTable 6 in the Supplement). Furthermore, the proposed pediatric dosing scheme for remdesivir was based on identifying dosages that would provide equivalent plasma exposures between children and adults; however, because remdesivir is a nonactive prodrug, extrapolating pediatric dosages based on intracellular exposure of the active triphosphate moiety (GS-443902) would be more appropriate. Unfortunately, PK information defining the association between remdesivir dose, plasma exposure, and intracellular GS-443902 exposure in children is lacking. Based on a preliminary analysis of remdesivir PK studies in adults, there appears to be a linear association between remdesivir plasma exposure and intracellular peripheral blood mononuclear cell exposure of GS-441524, a nucleoside metabolite that may reflect the total amount of dephosphorylated as well as monophosphate, diphosphate, and triphosphate metabolites of remdesivir (eFigure 7 in the Supplement). To define pediatric-specific dosages, our analysis assumed the presence of a similar association in children. Lastly, owing to limited pediatric PK information for both compounds, we could not evaluate the predictive performance of the developed PK models used to inform pediatric dosing.
Conclusions
We used PK modeling and simulation to identify pediatric-specific dosages for hydroxychloroquine and remdesivir, which highlights how PK modeling and simulation can support the rapid development of pediatric clinical studies. While our study focused on investigational COVID-19 treatments, the described methods are generalizable and can be applied to other drugs and disease states. Importantly, our findings raise concerns about hydroxychloroquine for COVID-19 treatment because unbound plasma exposures were less than those needed to mediate an antiviral effect. To confirm the appropriateness of the proposed dosing schemes, prospective PK, safety, and efficacy studies in children are required.
eMethods. Adult PBPK Model Evaluation
eTable 1. Hydroxychloroquine adult PBPK model parameterization
eTable 2. Adult PBPK Model Evaluation
eTable 3. Average Unbound Hydroxychloroquine Concentrations (Cavg) in Plasma Over 120 Hours in Children and Adults Using the Proposed Dosing Scheme*
eTable 4. Simulated Remdesivir Plasma Exposures (AUC from time 0 to ∞) Following Loading Dose Administration in Children and Adults under the Proposed Dosing Algorithm (Day 1)*
eTable 5. Simulated remdesivir plasma exposures (area under the concentration time curve [AUC] from time 0 to ∞) following loading dose administration in children and adults based on dosages recommended for Ebola virus diseasea
eTable 6. Average unbound hydroxychloroquine concentrations (Cavg) in plasma over 120 hours in children and adults using the proposed dosing scheme. Simulations computed using an alternative PBPK model that assumed proportional contributions of CYP2C8, CYP2D6 and CYP3A4 to hepatic intrinsic clearance were 59%, 16%, and 25%, respectively, based on in-vitro chloroquine metabolism data17
eFigure 1. Evaluation of the Developed Adult PBPK Model in Whole Blood and Plasma
eFigure 2. Comparison of Plasma Unbound Hydroxychloroquine Concentrations
eFigure 3. Comparison of Unbound Hydroxychloroquine Concentrations in Plasma to Lung Interstitial Fluid in Adults and Different Pediatric Age Groups
eFigure 4. Simulated Plasma Exposures for Remdesivir
eFigure 5. Comparison Simulated Hydroxychloroquine Concentrations in (A) Plasma/Serum and (B) Unbound Plasma/Serum Between Published Adult Models
eFigure 6. Comparison of Mean Plasma Remdesivir Exposures Versus Dose in Healthy Adult Volunteers
eFigure 7. Mean GS441524 Peripheral Blood Mononuclear Cell Exposures Versus Mean Plasma Remdesivir Exposures
References
- 1.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;e20200702. doi: 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 2.Lu X, Zhang L, Du H, et al. ; Chinese Pediatric Novel Coronavirus Study Team . SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663-1665. doi: 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199-1207. doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020;19(3):149-150. doi: 10.1038/d41573-020-00016-0 [DOI] [PubMed] [Google Scholar]
- 6.Ko WC, Rolain JM, Lee NY, et al. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. 2020;55(4):105933. doi: 10.1016/j.ijantimicag.2020.105933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colson P, Rolain JM, Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55(3):105923. doi: 10.1016/j.ijantimicag.2020.105923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raoult D, Houpikian P, Tissot Dupont H, Riss JM, Arditi-Djiane J, Brouqui P. Treatment of Q fever endocarditis: comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch Intern Med. 1999;159(2):167-173. doi: 10.1001/archinte.159.2.167 [DOI] [PubMed] [Google Scholar]
- 9.Biot C, Daher W, Chavain N, et al. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem. 2006;49(9):2845-2849. doi: 10.1021/jm0601856 [DOI] [PubMed] [Google Scholar]
- 10.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020:ciaa237. doi: 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tett SE, Cutler DJ, Day RO, Brown KF. A dose-ranging study of the pharmacokinetics of hydroxy-chloroquine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. 1988;26(3):303-313. doi: 10.1111/j.1365-2125.1988.tb05281.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furst DE. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus. 1996;5(suppl 1):S11-S15. doi: 10.1177/0961203396005001041 [DOI] [PubMed] [Google Scholar]
- 13.Lim HS, Im JS, Cho JY, et al. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax. Antimicrob Agents Chemother. 2009;53(4):1468-1475. doi: 10.1128/AAC.00339-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez MA. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020;64(5):e00399-20. doi: 10.1128/AAC.00399-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381-385. doi: 10.1038/nature17180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agostini ML, Andres EL, Sims AC, et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9(2):e00221-e18. doi: 10.1128/mBio.00221-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Medicines Agency (EMA) . Summary on compassionate use: Remdesivir Gilead (Procedure No. EMEA/H/K/5622/CU). Published April 3, 2020. Accessed April 14, 2020. https://www.ema.europa.eu/en/documents/other/summary-compassionate-use-remdesivir-gilead_en.pdf
- 18.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi: 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maharaj AR, Barrett JS, Edginton AN. A workflow example of PBPK modeling to support pediatric research and development: case study with lorazepam. AAPS J. 2013;15(2):455-464. doi: 10.1208/s12248-013-9451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maharaj AR, Edginton AN. Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacometrics Syst Pharmacol. 2014;3:e150. doi: 10.1038/psp.2014.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimstein M, Yang Y, Zhang X, et al. Physiologically based pharmacokinetic modeling in regulatory science: an update from the U.S. Food and Drug Administration’s Office of Clinical Pharmacology. J Pharm Sci. 2019;108(1):21-25. doi: 10.1016/j.xphs.2018.10.033 [DOI] [PubMed] [Google Scholar]
- 22.Mahmood I, Tegenge MA. A comparative study between allometric scaling and physiologically based pharmacokinetic modeling for the prediction of drug clearance from neonates to adolescents. J Clin Pharmacol. 2019;59(2):189-197. doi: 10.1002/jcph.1310 [DOI] [PubMed] [Google Scholar]
- 23.Dykstra K, Mehrotra N, Tornøe CW, et al. Reporting guidelines for population pharmacokinetic analyses. J Pharmacokinet Pharmacodyn. 2015;42(3):301-314. doi: 10.1007/s10928-015-9417-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; 2009. [Google Scholar]
- 25.Wilke CO. cowplot: Streamlined Plot Theme and Plot Annotations for 'ggplot2'. rdrr.io website. Accessed April 14, 2020. https://rdrr.io/cran/cowplot/
- 26.Denney W, Duvvuri S, Buckeridge C. Simple, automatic noncompartmental analysis: the PKNCA R package. J Pharmacokinet Pharmacodyn. 2015;42:S65-S65. [Google Scholar]
- 27.Grothendieck G, Zeileis A. zoo: S3 infrastructure for regular and irregular time series. J Stat Softw. 2005;14(i06). [Google Scholar]
- 28.Leong R, Vieira ML, Zhao P, et al. Regulatory experience with physiologically based pharmacokinetic modeling for pediatric drug trials. Clin Pharmacol Ther. 2012;91(5):926-931. doi: 10.1038/clpt.2012.19 [DOI] [PubMed] [Google Scholar]
- 29.Brocks DR, Skeith KJ, Johnston C, et al. Hematologic disposition of hydroxychloroquine enantiomers. J Clin Pharmacol. 1994;34(11):1088-1097. doi: 10.1002/j.1552-4604.1994.tb01986.x [DOI] [PubMed] [Google Scholar]
- 30.McLachlan AJ, Cutler DJ, Tett SE. Plasma protein binding of the enantiomers of hydroxychloroquine and metabolites. Eur J Clin Pharmacol. 1993;44(5):481-484. doi: 10.1007/BF00315548 [DOI] [PubMed] [Google Scholar]
- 31.Müller F, König J, Glaeser H, et al. Molecular mechanism of renal tubular secretion of the antimalarial drug chloroquine. Antimicrob Agents Chemother. 2011;55(7):3091-3098. doi: 10.1128/AAC.01835-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan HW, Ma ZX, Chen J, Yang XY, Cheng JL, Li YB. Pharmacokinetics and bioequivalence study of hydroxychloroquine sulfate tablets in Chinese healthy volunteers by LC-MS/MS. Rheumatol Ther. 2015;2(2):183-195. doi: 10.1007/s40744-015-0012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tett SE, Cutler DJ, Day RO. Bioavailability of hydroxychloroquine tablets assessed with deconvolution techniques. J Pharm Sci. 1992;81(2):155-159. doi: 10.1002/jps.2600810211 [DOI] [PubMed] [Google Scholar]
- 34.Li XQ, Björkman A, Andersson TB, Gustafsson LL, Masimirembwa CM. Identification of human cytochrome P(450)s that metabolise anti-parasitic drugs and predictions of in vivo drug hepatic clearance from in vitro data. Eur J Clin Pharmacol. 2003;59(5-6):429-442. doi: 10.1007/s00228-003-0636-9 [DOI] [PubMed] [Google Scholar]
- 35.McLachlan AJ, Tett SE, Cutler DJ, Day RO. Bioavailability of hydroxychloroquine tablets in patients with rheumatoid arthritis. Br J Rheumatol. 1994;33(3):235-239. doi: 10.1093/rheumatology/33.3.235 [DOI] [PubMed] [Google Scholar]
- 36.McLachlan AJ, Tett SE, Cutler DJ, Day RO. Absorption and in vivo dissolution of hydroxycholoroquine in fed subjects assessed using deconvolution techniques. Br J Clin Pharmacol. 1993;36(5):405-411. doi: 10.1111/j.1365-2125.1993.tb00388.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tett SE, Cutler DJ, Day RO, Brown KF. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol. 1989;27(6):771-779. doi: 10.1111/j.1365-2125.1989.tb03439.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheung KWK, van Groen BD, Spaans E, et al. A comprehensive analysis of ontogeny of renal drug transporters: mRNA analyses, quantitative proteomics, and localization. Clin Pharmacol Ther. 2019;106(5):1083-1092. doi: 10.1002/cpt.1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willmann S, Höhn K, Edginton A, et al. Development of a physiology-based whole-body population model for assessing the influence of individual variability on the pharmacokinetics of drugs. J Pharmacokinet Pharmacodyn. 2007;34(3):401-431. doi: 10.1007/s10928-007-9053-5 [DOI] [PubMed] [Google Scholar]
- 40.Sciensano Epidemiology of Infectious Disease. Interim clinical guidance for adults with suspected or confirmed COVID-19 in Belgium, Version 7. sciensano website. Published April 7, 2020. Accessed April 14, 2020. https://epidemio.wiv-isp.be/ID/Documents/Covid19/COVID-19_InterimGuidelines_Treatment_ENG.pdf
- 41.Michigan Medicine, University of Michigan. Inpatient guidance for treatment of COVID-19 in adults and children. Michigan Medicine, University of Michigan website. Accessed April 14, 2020. http://www.med.umich.edu/asp/pdf/adult_guidelines/COVID-19-treatment.pdf
- 42.Kramer NI, Krismartina M, Rico-Rico A, Blaauboer BJ, Hermens JL. Quantifying processes determining the free concentration of phenanthrene in Basal cytotoxicity assays. Chem Res Toxicol. 2012;25(2):436-445. doi: 10.1021/tx200479k [DOI] [PubMed] [Google Scholar]
- 43.Postnikova E, Cong Y, DeWald LE, et al. Testing therapeutics in cell-based assays: factors that influence the apparent potency of drugs. PLoS One. 2018;13(3):e0194880. doi: 10.1371/journal.pone.0194880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou D, Dai SM, Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020:dkaa114. doi: 10.1093/jac/dkaa114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arnold SLM, Buckner F. Hydroxychloroquine for treatment of SARS-CoV-2 infection? improving our confidence in a model-based approach to dose selection. Clin Transl Sci. 2020;. doi: 10.1111/cts.12797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balevic SJ, Green TP, Clowse MEB, Eudy AM, Schanberg LE, Cohen-Wolkowiez M. Pharmacokinetics of hydroxychloroquine in pregnancies with rheumatic diseases. Clin Pharmacokinet. 2019;58(4):525-533. doi: 10.1007/s40262-018-0712-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morita S, Takahashi T, Yoshida Y, Yokota N. Population pharmacokinetics of hydroxychloroquine in Japanese patients with cutaneous or systemic lupus erythematosus. Ther Drug Monit. 2016;38(2):259-267. doi: 10.1097/FTD.0000000000000261 [DOI] [PubMed] [Google Scholar]
- 49.Tegenge MA, Mahmood I. Age- and bodyweight-dependent allometric exponent model for scaling clearance and maintenance dose of theophylline from neonates to adults. Ther Drug Monit. 2018;40(5):635-641. doi: 10.1097/FTD.0000000000000543 [DOI] [PubMed] [Google Scholar]
- 50.Lee JY, Vinayagamoorthy N, Han K, et al. Association of polymorphisms of cytochrome P450 2D6 with blood hydroxychloroquine levels in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2016;68(1):184-190. doi: 10.1002/art.39402 [DOI] [PubMed] [Google Scholar]
- 51.Projean D, Baune B, Farinotti R, et al. In vitro metabolism of chloroquine: identification of CYP2C8, CYP3A4, and CYP2D6 as the main isoforms catalyzing N-desethylchloroquine formation. Drug Metab Dispos. 2003;31(6):748-754. doi: 10.1124/dmd.31.6.748 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Adult PBPK Model Evaluation
eTable 1. Hydroxychloroquine adult PBPK model parameterization
eTable 2. Adult PBPK Model Evaluation
eTable 3. Average Unbound Hydroxychloroquine Concentrations (Cavg) in Plasma Over 120 Hours in Children and Adults Using the Proposed Dosing Scheme*
eTable 4. Simulated Remdesivir Plasma Exposures (AUC from time 0 to ∞) Following Loading Dose Administration in Children and Adults under the Proposed Dosing Algorithm (Day 1)*
eTable 5. Simulated remdesivir plasma exposures (area under the concentration time curve [AUC] from time 0 to ∞) following loading dose administration in children and adults based on dosages recommended for Ebola virus diseasea
eTable 6. Average unbound hydroxychloroquine concentrations (Cavg) in plasma over 120 hours in children and adults using the proposed dosing scheme. Simulations computed using an alternative PBPK model that assumed proportional contributions of CYP2C8, CYP2D6 and CYP3A4 to hepatic intrinsic clearance were 59%, 16%, and 25%, respectively, based on in-vitro chloroquine metabolism data17
eFigure 1. Evaluation of the Developed Adult PBPK Model in Whole Blood and Plasma
eFigure 2. Comparison of Plasma Unbound Hydroxychloroquine Concentrations
eFigure 3. Comparison of Unbound Hydroxychloroquine Concentrations in Plasma to Lung Interstitial Fluid in Adults and Different Pediatric Age Groups
eFigure 4. Simulated Plasma Exposures for Remdesivir
eFigure 5. Comparison Simulated Hydroxychloroquine Concentrations in (A) Plasma/Serum and (B) Unbound Plasma/Serum Between Published Adult Models
eFigure 6. Comparison of Mean Plasma Remdesivir Exposures Versus Dose in Healthy Adult Volunteers
eFigure 7. Mean GS441524 Peripheral Blood Mononuclear Cell Exposures Versus Mean Plasma Remdesivir Exposures