Abstract
Extracorporeal shockwave therapy (ESWT) is a popular non-invasive therapeutic modality in the medical field for the treatment of numerous musculoskeletal disorders. This technique first emerged around the 1980s as extracorporeal shockwave lithotripsy and has been studied since then for its application towards orthopedics and traumatology. ESWT works by the emission of acoustic waves (shockwaves) that carry energy and can propagate through tissues. Shockwaves can generate interstitial and extracellular responses, producing many beneficial effects such as: pain relief, vascularization, protein biosynthesis, cell proliferation, neuro and chondroprotection, and destruction of calcium deposits in musculoskeletal structures. The combination of these effects can lead to tissue regeneration and significant alleviation of pain, improving functional outcomes in injured tissue. Considering these facts, ESWT shows great potential as a useful regenerative medicine technique for the treatment of numerous musculoskeletal injuries.
Keywords: Regeneration, Inflammation, Shockwave, Musculoskeletal disorders
1. Introduction
Extracorporeal shockwave therapy (ESWT) has become a popular non-invasive therapeutic modality in the field of orthopedics and traumatology for the treatment of many musculoskeletal disorders, including problematic soft tissue wounds. ESWT first emerged around the 1980s as extracorporeal shockwave lithotripsy (ESWL), a tool used to disintegrate renal stones.1 The essential principle behind this technique revolves around the action of shockwaves, which are rapid but short duration acoustic waves that carry energy and can propagate through tissues.2 In contrast to ESWL, the application of shockwaves in orthopedics (orthotripsy) can be beneficial due to their ability to cause interstitial and extracellular responses which lead to tissue regeneration.3 In general, ESWT bears positive effects in the management of cartilage and bone diseases. This therapy has shown promising results in the treatment of various musculoskeletal disorders, including tendinitis, epicondylitis, plantar fasciitis, trocanteritis, and “jumper’s knee”, as examples.3,4 Furthermore, its utility can also be applied towards the treatment of non-union in long bone fractures, avascular necrosis of the femoral head, chronic diabetic or non-diabetic ulcers, ischemic heart disease and even erectile dysfunction.2,4 Acting as a mechanical stimulus, it is believed that ESWT promotes healing via mechanotransduction.5 Reportedly, biological responses include tissue regeneration, wound healing, angiogenesis and bone remodeling.5, 6, 7, 8, 9 Additionally, it may also alleviate pain by means of hyperstimulation analgesia.10,11 Previous investigations have suggested that mechanotransduction seems to be the major mechanism whereby ESWT triggers angiogenic and tissue regeneration responses at cellular and molecular levels, generating beneficial therapeutic effects in clinical scenarios.8,12 Although ESWT may be utilized to treat a variety of physical problems involving musculoskeletal tissues, there may be different mechanisms underpinning this technique, each playing a specific role and contributing to the regenerative processes, collectively.
The motivation for this manuscript is to review the principal mechanisms of action of shockwaves in the treatment of musculoskeletal injuries from a standpoint of the regenerative potential of ESWT.
2. Shock wave therapy: an overview
2.1. Brief history
It is well understood that shockwaves are pressure waves that travel in a tridimensional path and normally induce an increase in pressure in a matter of nanoseconds.4 As they are rapid waves, there are rising positive pressure impulses that go from 5 to 120 MPa in 5 ns. Then, there is a decrease to negative pressure values of approximately −20 MPa.13 In 1980, shockwaves were first put to use in the field of urology to disintegrate renal crystal aggregation. The promising results of urolithiasis raised researchers’ interest amongst the scientific community and the technique was soon redirected towards pathological neocalcifications in musculoskeletal disorders.1 In 1993, Loew and Jurgowski conducted initial experiments on tendinosis calcarea of the shoulder.14 Enthesiopathic neocalcifications of tendon insertion area of plantar fascia prompted the suitable intervention offered by physical principles of ESWT in chronic plantar heel pain15. Originally, the low-energy extracorporeal shock wave therapy was meant for the mechanical disintegration of painful heel spur. Although the technique did not successfully eliminate the spur, clinical studies have reported an average success rate of 81% in managing painful plantar heel spur.13,16 At least for plantar fasciitis, where ESWT is frequently applied to, the success rate remained comparably superior to other conservative and operative treatment alternatives.17 As an additional example, a randomized, placebo-controlled, double-blind clinical trial18 evaluating the treatment of plantar fasciitis with high-energy ESWT managed to demonstrate a clinically successful treatment for pain alleviation in patients suffering from chronic plantar fasciitis with safety and efficacy. Lateral epicondylits is another common inflammatory disease that became a suitable target for ESWT experimentation. In comparison to plantar fasciitis, epicondylopathy treatment was not as efficient, since it only showed a success rate of approximately 60%.19,20 With the emerging success of shockwave therapy, other tendinopathies were soon included as manageable cases by ESWT, with rates of success ranging from 60 to 80%.21 One noteworthy observation is that high-energy shockwaves disrupt tissues altogether, so the intensities had to be reduced in order to avoid damage.21
2.2. Parameters
Energy Flux Density (EFD) is the parameter referred to by professionals based on the flow of shockwave energy through an area with perpendicular orientation to the direction of propagation. The unit of measurement is given in mJ/mm2. ESWT has been previously classified based on EFD with low (<0.08 mJ/mm2), medium (<0.28 mJ/mm2) and high (<0.60 mJ/mm2) treatment intensities.22 It should be taken into consideration that there is no consensus in the classification regarding EFD since the literature shows different energy parameters reported in various studies. Despite this observation, clinicians typically resort to energy ranges from 0.001 to 0.4 mJ/mm2. Lower and medium EFD trigger the release of nitric oxide (NO), which is beneficial due to its antalgic, angiogenetic and anti-inflammatory effects in clinical settings.4 Higher EFD intensity is usually recommended for the treatment of pseudoarthrosis, for example, and yields about 72% success rate.4
2.3. Mechanics of shockwaves
It is well known that pressure waves, also called sound waves, are oscillating mechanical waves with the ability to propagate through solids, liquids and gases. As previously introduced, shockwaves are a non-linear type of pressure wave with a short rise time; a shockwave lasts up to 10 μs.23,24 The positive and negative phases of shockwaves exert certain effects on interfaces between various tissues and their different densities. In the positive phase, high pressure shockwaves may hit an interface and be reflected or they may pass through and gradually become absorbed. During the negative phase (also referred to as tensile phase), the shockwave generates cavitation at the tissue interfaces, which results in the subsequent formation of air bubbles. The air bubbles then implode with high speed, producing a second wave of shockwaves or micro-jets of fluid.23,24
2.3.1. Types of ESWT
Shockwave therapy is subdivided into two types: focused shockwave therapy (FSWT) and radial shockwave therapy (RSWT).
FSWT features the generation of a pressure field that converges in the adjustable focus at determined depths in specific tissues where maximal pressure is achieved. Focused shockwaves can be generated via three methods: electrohydraulic (EH), electromagnetic (EM) and piezoelectric (PE).24 Due to the fact that the acoustic impedance of water and biologic tissue is comparable, focused shockwaves are generated in water. Therefore, reflection is limited and propagation of waves into the body is facilitated. The similarity between EH, EM and PE is that they all utilize water for the generation of focused shockwaves. The difference, however, is the moment at which the shockwaves are formed. EH generators, for example, produce shockwaves immediately after the spark gap, whereas EM and PE generators have a slight delay in a matter of nanoseconds by means of focuzation of waves.25
RSWT is described by the diverging pressure field of RSWT devices, which reach maximal pressure at the source instead of selected depths in tissues. Radial shockwaves are not generated in water. Instead, they are generated upon acceleration of a projectile, using compressed air through a tube which has an extremity connected to an applicator. The projectile is accelerated until it collides with the applicator and, subsequently, the pressure wave that is generated is relayed into the body.26
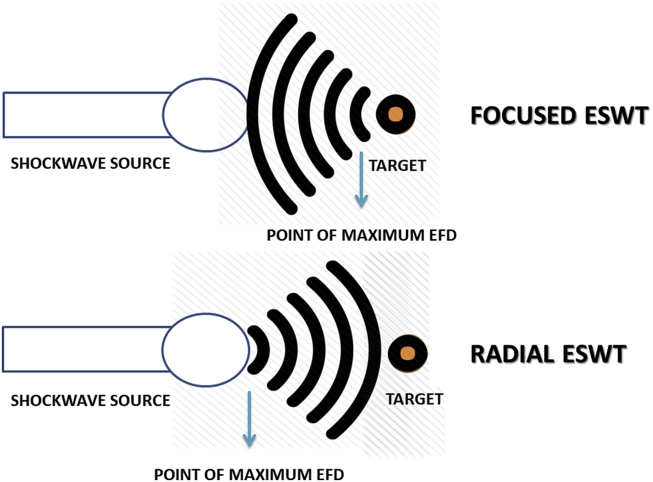
The fundamental differences between the two types of shockwave therapy, as illustrated in Fig. 1 of this manuscript, are as follows. First and foremost, focused shockwaves reach their maximal energy deeper in body tissues whereas radial shockwaves have a more superficial aspect.24,26 McClure and Dorfmuller demonstrated that RSWT devices generate pressure fields which extend to 40 mm in water. Conversely, the pressure fields generated via FSWT may penetrate a distance about twice as deep.27 These observations do depend on the device and the energy settings that are used, but the fact remains that focused shockwaves will still propagate further and have more impact on tissues located deeper in the body.
Fig. 1.
The fundamental differences between focused and radial ESWT.
On a different perspective, some research28 points out that it may be equivocal to refer to RSWT as an actual shockwave therapy because, technically, such devices do not generate “real” shockwaves. RSWT does not have the regular physical features of shockwaves, such as short rise time, a high peak pressure and non-linearity.23 This may be attributed to the fact that the speed of sound in tissue is around 1500 m/s, whereas in RSWT the acceleration of the projectile yields proximal speeds of 20 m/s, which is insufficient to generate a real shockwave.27 This is based on the study28 conducted by Chitnis and Cleveland, who found that the rise time of a wave generated from two electrohydraulic FSWT devices was 25–40 ns, whereas for the RSWT device it was 600 ns. While 25–40 ns is much longer than the usual 5 ns (as mentioned in section 2.1) for the definition of shockwave, the EH-generated waves displayed the physical features of a conventional shockwave, but the same was not true for the radial device in question.
3. ESWT mechanisms of action
From previous research,29 4 reaction phases of ESWT on tissue have been proposed, as follows: physical, physicochemical, chemical, and biological. Firstly, in the physical phase, shockwaves cause a positive pressure to generate absorption, reflection, refraction, and transmission of energy to tissues and cells.24 Additionally, it appears that cavitation increases the permeability of cell membranes and ionization of biological molecules. Secondly, in the physicochemical phase, the physical stimulus leads to biochemical reactions. ESWT triggers the release of biomolecules such as adenosine triphosphate (ATP) for the activation of cell signaling pathways.30 Thirdly, the chemical phase has shockwaves altering the functions of ion channels in cell membranes and mobilization of calcium.31 Lastly, the biological phase is where ESWT plays its role in modulating angiogenesis, anti-inflammatory effects, and healing of bone and soft tissue wounds.8,31
Interestingly, a study reported that low-energy ESWT also stimulates a polarity shift in the macrophage phenotype from M1 to M2.32 This is particularly valuable for the inflamed cellular microenvironment from a standpoint of the regenerative potential of ESWT, because the macrophage expresses two major phenotypes. These are M1 and M2, and may depend on the given chemical signal.33 Since M1 is usually stimulated by microbial agents, it takes on a pro-inflammatory role. Conversely, the M2 macrophage is produced by the T-helper type 2 (Th2) immune response and exhibits an anti-inflammatory property, typically characterized by an increase in the biosynthesis of interleukins IL-4, IL-5, IL-9 and IL-13. The type 2 response is known to be directly involved in regenerative processes after injury and macrophages elicit their protective role mainly by the promotion of angiogenesis via the release of cytokines and growth factors.33 The M2 macrophage has also shown stimulation of cell proliferation and repair through polyamine and collagen synthesis in addition to other tissue remodeling functions, releasing IL-10 and IL-4. The M1 type on the other hand displays microbicidal activity and inhibits cell proliferation, releasing the inflammatory IL-6 and tumor necrosis factor-α (TNF-α) cytokines.33
In addition to the aforementioned cellular effects, many studies on ESWT for musculoskeletal infirmities have been performed with focused shockwaves, generating promising results. The positive effects are broadly categorized as pain relief, tissue regeneration and destruction of calcifications in tissues, which will be discussed in detail in the subsequent sections of this manuscript.
3.1. Pain relief
It has been hypothesized that the biological effects of ESWT are a consequence of mechanotransduction, a phenomenon which relies on the action of ultrasonic vibrations on tissues, which then lead to regeneration and healing.34 So far, there are two principal hypotheses proposed to explain the analgesia induced by SW treatment. One of them suggests that SWs degenerate nerve fibers from small immunoreactive neurons, therefore decreasing the concentration of pro-inflammatory mediators. The second mechanism is theorized to cause analgesia via hyperstimulation, indicating that SWs trigger the release of endorphins and other analgesic molecules by activating the descending inhibitory system.35 To elaborate, conditioned pain modulation remains an experimental paradigm, dependent on the state of the descending inhibitory system function. This means that in a standard nociceptive system, for instance, the amount of pain generated by a primary nociceptive stimulus will be reduced during and after the presentation of a second nociceptive stimulus. This is due to the activation of endogenous analgesia.36,37 Since the analgesic effects of SWs are more prominent when the maximum energy density tolerable by the patient is applied, it is therefore reasonable to accredit analgesia to the activation of the descending inhibitory system.38
García-Muntión and colleagues coordinated a randomized controlled trial39 evaluating the mechanisms of action of the hypoalgesic effect of pressure under shock waves application. The authors aimed to determine if the perceived pain intensity during the application of SWs is a determinant mechanism in producing hypoalgesic changes in pressure pain thresholds (PPTs) in asymptomatic individuals before and after intervention. PPT was assessed bilaterally at the lateral epicondyle, median nerve in the flexure of the elbow, and tibia. The participants were allocated into three groups: SW-mild pain (SW-DP), SW-moderate pain (SW-MP) and cold pressor test (CPT) for conditioned pain modulation. It was found that SWs only had hypoalgesic effects if the application produced moderate pain. Also, the SW-MP group revealed a greater hypoalgesic effect in the contralateral limb of application than the CPT group, whereas the CPT group obtained local hypoalgesic changes concerning the SW-MP group. It is possible that the hypoalgesic effect obtained after the application of SW could be partially explained by the activation of the descending inhibitory system.
Other studies performed on animals, however, suggest that ESWT may have an influence on pain transmission to the brainstem by acting on substance P, calcitonin gene gene-related peptide (CGRP) expression in the dorsal root ganglion, and on neurovascular sprouting.10,11,22,40,41 In a rat model of Achilles Tendinopathy (AT), there was evidence indicating that ESWT triggered the release of tenocyte-derived growth factors and increased expression of transforming growth factor β1 (TGF-β1) and insulin-like growth factor 1 (IGF-1) in response to treatment.42 In regards to tendinopathy, there is another important molecule produced via the effects of shockwaves, which is lubricin. This mucinous glycoprotein is particularly important for tendinous structures as it facilitates tendon gliding. Lubricin expression is upregulated by both mechanical and biochemical stimuli.43 Elaborating further, the elevated expression of TGF-β1 mediated by SW has also been found to stimulate the expression of lubricin44, contributing to healing in tendinopathy.42 These observations supported the long hypothesized effect of ESWT in stimulating the expression of lubricin in tendons, prompting researchers to carry on with additional investigations. Zhang and colleagues explored the effects of shockwaves in tendons and septa of rats in 2011.45 The researchers learned that increased lubricin deposition in tendons and septa following the application of ESWT may convey beneficial effects. Their conclusion was derived from the observation that the expression of the endogenous lubricant was associated with facilitated movement among gross structures and collagen fascicles. This would therefore allow protection from the “wear and tear” process in treated tissues and alleviate pain45.
A few more studies on rats led to the hypothesis that application of low-energy SWs to skin results in nearly complete degeneration of epidermal sensory nerve fibers with a regeneration period of about 42 days.46,47 To complement, it is also known that higher levels of glutamate receptors and vascular endothelial growth factors (VEGF) exist near the nerves of patients with painful AT, implying an increase in nociceptive nerve fibers and neovascularization.47
A first prospective, randomized, double-blind, placebo controlled clinical trial conducted by Palmieri et al., in 2009 aimed to investigate the effects of ESWT in patients with peyronie’s disease (PD).48 The authors administered four weekly treatment sections with 2000 focused shock waves each. In the placebo group, a nonfunctioning transducer was employed. The results showed that after 12 weeks mean (Visual Analogue Scale) VAS score, mean (International Index of Erectile Function) IIEF-5 score, and mean QoL (quality of life) score ameliorated significantly in the treatment group. Overall, Palmieri et al. concluded that in their particular study, ESWT led to pain resolution and ameliorated erectile function and QoL in patients with PD.
3.2. Tissue regeneration
The hypothesis of ESWT leading to tissue regeneration seems plausible and does match with the principles of mechanotransduction. Mechanical load applied on the cytoskeleton stimulates cellular responses and an increase in protein biosynthesis.5
3.2.1. Protein biosynthesis and proliferation
A controlled laboratory study7 by Vetrano and colleagues on a primary culture of human tenocytes aimed to investigate whether the effects of ESWT could affect the behavior of the cells over a 12-day period. It was shown that ESWT influences the overall cell morphology by impeding the dedifferentiation of cells. This means that ESWT may impart a protective role on tenocytes, in this case maintaining their original properties. Furthermore, the authors performed an MTT colorimetric assay and learned that shockwaves mediated cellular growth due to the detection of significant levels of the Ki67 proliferation marker. Lastly, they also saw a significant increase in ESWT-induced collagen type I synthesis in tenocytes compared to controls. Overall, this particular in vitro study demonstrated the encouraging effects of ESWT on cell growth and protein synthesis, suggesting a regenerative medicine potential.
Another similar in vitro study49 evaluated the biologic effects of ESWT on affected human tenocytes. Han and colleagues hypothesized that the production of pro-inflammatory cytokines and matrix metalloproteinases (MMPs) would be down-regulated following the application of ESWT on diseased Achilles tendon tissue and healthy flexor hallucis longus tissues. The researchers found higher levels of MMPs and interleukins in affected tenocytes in comparison with normal cells. After applying ESWT, they reported a diminished expression of several tendinopathy-associated MMPs and interleukins which can interfere with regular cellular function. This suggests another important mechanism whereby ESWT may play an appreciable role in supporting restoration of diseased human tissues.
A double-blind, randomized clinical trial50 conducted by Rasmussen and colleagues in 2009 determined to compare the effect of supplementing conservative treatment of chronic AT with ESWT or placebo. The authors recruited 48 patients; the individuals assigned to nonoperative treatment of chronic AT were randomized to receive either active ESWT or sham ESWT over 4 weeks. The AOFAS (American Orthopaedic Foot and Ankle Society) score and pain were assessed before treatment, during the 4-week treatment period, and at 4, 8, and 12 weeks of follow-up. Upon interpreting results, the authors concluded that both groups improved during the treatment and follow-up period. The mean AOFAS score went from 74 to 81 in the placebo group, whereas in the treatment group it went from 70 to 88. At 8 and 12 weeks of follow-up, better results were observed (p = 0.01 and p = 0.04, respectively) in the treatment group, suggesting that ESWT may be a clinically relevant supplement to conservative treatment of tendinopathy.
Reported animal studies also show the benefits of ESWT. Bosch and collaborators previously evaluated the effect of focused shockwaves on collagen matrix and gene expression in normal tendons and ligaments51 as well as the effects on the biochemical composition and metabolic activity of tenocytes in normal tendinous structures in ponies.52 The first study demonstrates that exposure of normal tendinous tissue to ESWT triggers a disorganization of matrix structure and alterations in degraded collagen levels. Additionally, the up-regulation of collagen type I expression 6 weeks after ESWT treatment was noticed, which may further reinforce the repair mechanism. The second study revealed a transient stimulation of metabolism in equine tendinous structures shortly after treatment. In the explants harvested 3 h after shockwave application, glycosaminoglycan (GAG) and protein syntheses were significantly increased. Biochemically, the level of degraded collagen was increased 3 h post-treatment. However, at the sixth week following the procedure, all measured parameters were decreased. Metabolic activity and collagen and GAG contents all suffered a significant decrease; GAG levels turned out lower than the ones from untreated control limbs. The bottom line: the stimulating effects of ESWT might advance the initiation of the healing process in soft tissue injuries in the short-term, whereas in the long run, the effects appear to be less valuable.
Weihs et al. also made remarkable contributions regarding the effects of shockwaves on proliferation, further clarifying tissue regeneration mechanisms.30 In their in vitro study, the authors utilized an electrohydraulic shockwave device and applied a relatively low intensity to the cultured cells in suspension. Their objective was to investigate the activation of specific signaling pathways and the subsequent biological effects. It was learned that extracellular ATP was released in both an energy- and pulse number-dependent manner. In this particular setting, ESWT triggered the release of ATP, increased activation of Erk1/2 and p38 MAPK signaling pathways, enhancing proliferation of three different types of cell lines. Furthermore, the researchers praise purinergic signaling as it induces Erk1/2 pathway activation, which appears to play pivotal role in the proliferative effect.
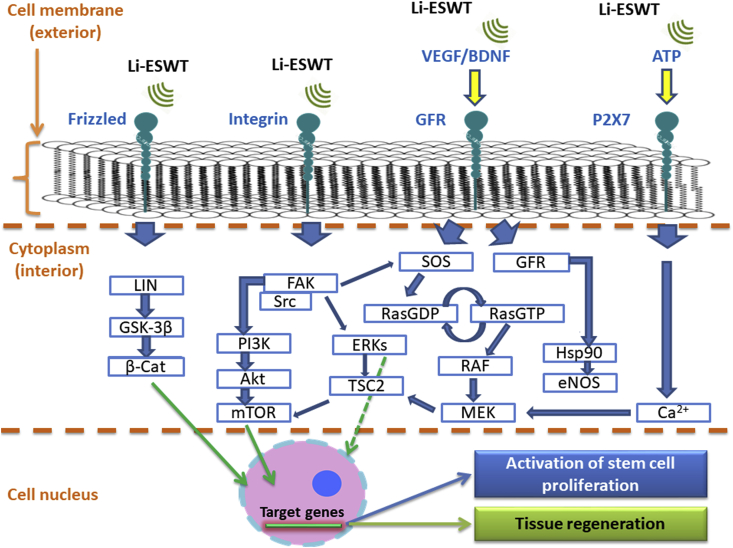
In addition to proliferation, purinergic signaling is also appreciated for its participation in other cellular events such as motility, differentiation, regeneration, and apoptosis.53 Stimulated cells release ATP, and this molecule can bind to P2X and P2Y purinoreceptors, which are known as ion channels, and G-protein coupled receptors, respectively.53 As previously mentioned, purinoreceptors are valuable to the complex signaling pathways. Adequate receptor binding secures progression of the signaling cascade, enabling eventual expression of certain target genes associated with the promotion of stem cell proliferation, for example, and tissue regeneration. This process is illustrated by Fig. 2.
Fig. 2.
The cell signaling pathways activated by ESWT application.
3.2.2. Vascularization
In addition to the effects previously discussed, ESWT-induced neovascularization and improvement of blood flow may also potentialize the regenerative properties of this technique. In painful tendinopathies, for example, it is possible to employ the use of color or power Doppler to detect blood flow in tendons. Power Doppler ultrasound (PDU) and color Doppler are simple, non-invasive tools used to analyze blood vessels, aiming to quantify neovascularization or hypervascularity.54
A study published by Santamato and colleagues55 aimed to evaluate the effect of focused ESWT on pain and function in patients suffering from non-insertional Achilles tendinopathy. The authors focused on the neovascularization aspect using PDU at 1 and 3 months post-treatment to describe the focused ESWT-mediated impacts on clinical outcome, if any. The selected participants received five sessions over 35 days, at 0.12 mJ/mm2 via electrohydraulic focused ESWT. The device applied 1600 pulses per session at a set frequency of 4Hz, with a probe with a depth of 5 mm for the applicator head to treat the affected area. After analyzing the results the authors learned that, at least in that particular occasion, even with the aid of PDU they did not detect any significant increase in number of vessels. In spite of causing significant pain reduction and improvement of functionality, only 1 of the 12 patients enrolled in the study exhibited prominent neovascularization.
While in humans it may be somewhat difficult to determine neovascularization effects, experiments in rabbits conducted by Wang CJ and co-workers revealed that ESWT application caused neovascularization associated with up-regulation of angiogenic and osteogenic growth factors, including endothelial nitric oxide synthase (eNOS), vascular endothelial growth factor (VEGF), proliferating cell nuclear antigen (PCNA), and bone morphogenetic protein-2 (BMP-2) at the tendon–bone junction of the Achilles tendon in rabbits.8,56 The authors reported that, 1 week post-procedure, there was an increase in neo-vessels, which plateaued around the fourth week and then persisted through 12 weeks. Additionally, the up-regulation of eNOS, VEGF and BMP-2 was significantly increased in one week, reaching a peak value at week 12 until it slowly dropped to baseline by the end of that period. PCNA values began to rise 1 week after ESWT treatment and reached the highest value at 12 weeks. To reiterate, these results mean that shockwaves promote the ingrowth of neovascularization one week post-treatment, an effect which persists even 12 weeks further. This is also explained by the persistent elevation of PCNA at 12 weeks after ESWT application.
3.2.3. Neuroprotection
Previous studies have demonstrated the potential mechanisms of low-energy ESWT for preservation of neuronal and vascular function in many animal models. More specifically, it has been shown that in low intensity, ESWT can: induce the release of VEGF, suppress apoptosis, and reduce the axonal damage derived from nerve injury57; promote nerve innervation in bladder and skin tissues46,58; induce the expression of growth-associated protein 43 as well as activating transcription factor 3, stimulating neurite sprouting from injured ganglia59; and activate and induce the proliferation of Schwann cells, which play a key role in axonal maintenance and support the regeneration of nerves.60 In particular, low-intensity ESWT (Li-ESWT) seems quite beneficial due to its reported potential to stimulate numerous signaling pathways, affecting transcription and modification of intracellular proteins. Examples include: focal adhesion kinase (FAK), extracellular-signal-regulated kinase (ERK), Wnt, ATP/P2X7, protein kinase R-like endoplasmic reticulum kinase/activated transcription factor (PERK/ATF), vascular endothelial growth factor (VEGF), and brain-derived neurotrophic factor (BDNF).61
In 2014, Yamaya and collaborators set out to investigate what effects, if any, low-intensity ESWT (Li-ESWT) produced on VEGF expression, neuroprotection and amelioration of locomotor recovery after spinal cord injury (SCI).62 The authors selected 60 adult female Sprague-Dawley rats which were randomly divided into 4 groups, as follows: sham group (laminectomy only), sham-SW group (low-energy ESWT applied after laminectomy), SCI group (SCI only), and SCI-SW group (low-energy ESWT applied after SCI). The rats suffered spinal cord contusion injury, which was inflicted via the use of an impactor. Low-energy ESWT was then applied to the injured area 3 times a week for 3 weeks. In order to elaborate the results, the authors performed evaluation of locomotor function, histological and immunostaining analyses, and real-time polymerase chain reactions to detect the mRNA expression of specific proteins. Overall, their results revealed that low-energy ESWT significantly increased expressions of VEGF and Flt-1 in the spinal cord without any detrimental effect. Additionally, the authors also detected significant reduction in neuronal loss in damaged neural tissue as well as the improvement of locomotor function after SCI. These observations indicate that Li-ESWT may enhance the neuroprotective effect of VEGF in reducing secondary injury, leading to better locomotor recovery following SCI.
The illustration and summary provided by Fig. 2 and Table 1, respectively, indicate the biologic potential of ESWT to provoke molecular alterations. This promotes the expression of certain target genes associated with cellular events such as growth, proliferation, migration, differentiation, survival and even angiogenic responses, which are indispensable for wound healing and tissue repair.
Table 1.
Li-ESWT signaling pathways summary.
| Signaling pathway | Biological role |
|---|---|
| LIN | Regulates cell fate and morphogenesis |
| GSK-3β | Cell proliferation, migration, glucose regulation and apoptosis |
| Β-Cat | Regulation and coordination of cell-cell adhesion |
| FAK | Cell migration and angiogenesis |
| Src | Regulates cellular growth, proliferation and differentiation |
| PI3K/AKT/mTOR | Regulates cell cycle; proliferation of neural stem cells |
| TSC2 | Regulates cell size |
| MAPK/ERK (RAS/RAF/MEK/ERK) | Promotes gene expression for cell growth and survival. Associated with cell migration and wound healing and tissue repair via stimulation of angiogenesis |
| SOS | The SOS proteins interact with Ras proteins, promoting guanine nucleotide exchange (GDP/GTP turnover) and formation of the active Ras-GTP complex |
| GFR | Induction of cell differentiation and proliferation |
| Hsp90 | Assists protein folding, intracellular transport, maintenance and facilitates other signaling pathways |
| eNOS | Promotes multiple phosphorylation of cellular targets lowering cellular Ca2+ concentrations and promoting vascular relaxation |
| Ca2+ | Exerts regulatory effects on enzymes and proteins. Activates ion channels and may act as a second messenger in wide-ranging physiological roles |
Abbreviations: Beta-catenin (β-Cat), Phosphoinositide 3-kinases (PI3Ks), Protein kinase B (also known as Akt), Lineage variant gene (LIN), Glycogen synthase kinase 3 beta (GSK-3β), Focal Adhesion Kinase (FAK), mechanistic/mammalian target of rapamycin (mTOR), Proto-oncogene tyrosine-protein kinase (Src), Tuberous Sclerosis Complex 2 (TSC2), Calcium ion (Ca2+), Endothelial nitric oxide synthase (eNOS), Rapidly Accelerated Fibrosarcoma (RAF) protein kinase, Mitogen-activated protein kinase kinase (MEK), Growth Factor Receptor (GFR), Rat Sarcoma (Ras) protein, heat shock protein 90 (Hsp90), guanosine diphosphate (GDP), guanosine triphosphate (GTP), extracellular-signal-regulated kinase (ERK), Son of Sevenless (SOS), Vascular Endothelial Growth Factor (VEGF), Adenosine triphosphate (ATP); Brain-Derived Neurotrophic Factor (BDNF); P2X7 is a purinergic receptor.
3.2.4. Chondroprotection
There are many studies evaluating the effects of ESWT on cartilage with differing opinions. In 2010, Mayer-Wagner and colleagues demonstrated in their in vivo study that high-energy ESWT (single bout; 1500 shock waves of 0.5 mJ/mm2) affected the structural integrity of articular cartilage.63 The authors analysed chitinase 3-like protein 1 (Chi3L1) and Tenascin-C, both showing expression signals indicative of reorganization in matrix protein composition connected to cartilage injury at 10 weeks after high-energy ESWT in the treated group. Collagen (II) alpha (1) (COL2A1) mRNA was increased at 1 week and 4 weeks after ESWT treatment. The researchers found alterations on the ultrastructural level, such as expansion of the rough-surfaced endoplasmatic reticulum, detachment of the cell membrane and necrotic chondrocytes. Additionally, high-energy ESWT was also responsible for hyaline cartilage alterations on a molecular and ultrastructural level that were notably different from control cartilage. Interestingly, similar alterations have been described before, matching the early phases of osteoarthritis (OA). This particular study proposes that high-energy ESWT might therefore be detrimental to cartilage due to the degenerative effects it imparted on hyaline cartilage, similar to the ones found in the onset of OA.
Speaking of OA, the biological effects of ESWT for the treatment of this disorder have been investigated by other authors. Studies revealed that application of shockwaves to the subchondral bone of the medial tibial condyle showed time dependent and site specific chondroprotective effects in the initial progression of osteoarthritic alterations of the knee in rats.64, 65, 66 In additional studies with murine subjects, Wang CJ and colleagues detected significant increases in VEGF, BMP-2 and osteocalcin levels in the subchondral bone as compared to the control at week 2, 4, 8, and 12. The most beneficial effect of ESWT in the osteoarthritic knees occurred at 4 weeks post-treatment and persisted until week 12.64
Furthermore, in 2014, Wang CJ and collaborators demonstrated that osteoporosis (OP) increased the severity of chondral damage in knee OA.67 Upon application of ESWT as a treatment modality, effectiveness in the reduction of osteoporotic osteoarthritis of the knee in rats was observed. The authors selected fifty-six 8-weeks-old female Sprague–Dawley (SD) rats, which were randomly divided into 7 groups, each containing 8 rats, according to Table 2. Regarding methodology, the authors planned 800 impulses of shockwave with an EFD of 0.22 mJ/mm2, which were delivered to the proximal medial tibial condyle at 0.5 cm below the joint line and 0.5 cm from the medial skin edge in a single session.
Table 2.
Treatment design.
| Group | Treatment |
|---|---|
| 1 – Sham | Laparotomy |
| 2 – OA | ACLT and MM of the left knee |
| 3 – OP | Bilateral ovariectomy |
| 4 – OA + OP | ACLT and MM of the left knee and bilateral ovariectomy |
| 5 – OA + ESWT | ACLT + MM of the left knee and ESWT |
| 6 – OP + ESWT | Bilateral ovariectomies + ESWT |
| 7 – OA + OP + ESWT | ACLT and MM of the left knee, bilateral ovariectomies, and ESWT |
Abbreviations: Anterior Cruciate Ligament Transection (ACLT), Medial Meniscectomy (MM).
Summarizing their results, Wang CJ et al. report that ESWT showed effectiveness in the reduction of osteoporotic osteoarthritis of the knee in rats. Via immunohistochemical analysis, they observed that Dickkopf-related protein 1 (DKK-1), a central regulator of osteoblast activity, was significantly higher in OA, and OA + OP groups, but not in the OP group as compared with the sham group. ESWT significantly decreased the DKK-1 expressions, as compared with those without ESWT. DKK-1 expression was significantly higher in the OA + OP group than in the OA group and decreased after ESWT. PCNA, VEGF and BMP-2 were significantly decreased in the OA, OP, and OA + OP groups, and the data increased after ESWT as compared with those without ESWT. Overall, their study showed that early intervention with relatively moderate energy ESWT significantly reversed osteoarthritic alterations and resulted in chondroprotective effects in the rat knee.
3.3. Bone healing
The effects of ESWT on bone have been investigated with regards to fracture healing in both human and animal models.2,8 Previous experiments involving animals revealed the positive effects of ESWT in promoting bone healing in cases of acute fracture and chronic non-union.68,69 Mechanotransduction seems to be underlying mechanism which allows ESWT to propagate energy into cells, stimulate the bone lacunae-canalicular network and convert the physical stimulus into biochemical signals.70 Additionally, it has been theorized that shockwaves produced micro-fractures which would then lead to the formation of hematomas, callus formation and, ultimately, fracture healing.56 While these principles sound plausible, the literature lacks a significant number of studies to address the theory. On the other hand, some studies have illustrated the significant promotion of bone healing mediated by ESWT after fracture and tendon-to-bone healing in bone tunnel.56,71
Previously, Wang CJ and colleagues investigated the biological effects of ESWT on bone healing in a rabbit model.56 The authors gathered 16 rabbits and randomly divided them into study and control groups with an even distribution, where the study group received ESWT treatment whereas the control group did not. Before treating the animals, an intra-medullary pin was inserted retrograde into the femur canal, and a closed fracture of the femur was created with a three-point bend method. After analysis, Wang CJ learned that the ESWT group displayed prominent bone strength in the biomechanical aspect and enhanced cortical bone formation via histomorphological examination. Additionally, the results also indicated an increase in the number of neo-vessels as well as angiogenic and osteogenic growth markers, such as VEGF, eNOS, PCNA, and BMP-2 on immunohistochemical stains. These results prove that ESWT is able to significantly improve bone healing and formation of cortical bone after induction of fracture, at least in leporine femora.
Others have evaluated the potential of ESWT for the treatment of osteonecrosis of the hip joint.72, 73, 74 In 2008, Wang CJ et al. conducted a study and revealed that ESWT promoted regeneration in hip necrosis.75 Histopathological analysis indicated that patients whose hips were treated exhibited more viable bone and less necrosis, higher cell concentration and increased cellular activity, including phagocytic cells. On the molecular level, ESWT promoted a significant increase in vWF, VEGF, CD 31, Wnt3 and PCNA, whilst decreasing vascular cell adhesion molecule (VCAM) and DKK-1 in comparison to those without ESWT before surgery.
In 2011, Yin TC and colleagues further investigated this medical tool and combined the application of harvested bone marrow stromal cells (BMSCs) and shockwaves.74 The authors collected the cells from the bone marrow cavity of the proximal femur in six patients with osteonecrosis. The ESWT treated group showed significant increases in cell proliferation, VEGF, alkaline phosphatase, BMP-2, runt-related transcription factor 2 (RUNX2) and osteoclast in mRNA expressions. This study revealed the strong potential of ESWT in significantly augmenting angiogenic and osteogenic effects of specific cells.
Regarding safety and efficacy of this technique applied to bone tissue, studies have been conducted but no shockwave-induced crack or micro-damage was noted on bone. On the contrary, it has been suggested that augmented bone formation may be attributed to shockwave-sensitive osteogenesis instead of damage to the bone architecture.76 Some authors further demonstrated the benefits of ESWT in bone healing. Remarkable observations were the acceleration of fracture healing with improvement of neovascularization and increased synthesis of angiogenic and osteogenic growth factors including eNOS, VEGF, PCNA and BMP-2.56 Wang FS et al. revealed that ESWT activates transcription of osteogenic and angiogenic factors such as Cbfal/Runx2, HIF-1α and VEGF in osteoblasts.77,78 Similarly, Martini and colleagues also evaluated the biological effects of shockwave therapy on human osteoblast-like cells. The authors reported an elevation in nitric oxide, which promoted cellular proliferation and differentiation.79 Undeniably, ESWT triggers multiple biological responses which significantly contribute to the bone healing process.
3.4. Calcified tendinopathy
Calcified tendinopathy (CT) of the rotator cuff structure seems to be the most common condition in shoulder pathology. This disorder is characterized by a reactive calcification that affects tendons which are part of the rotator cuff.80 The reported prevalence remains from 2.7% to 22% and women aged between 30 and 50 years of age are most affected by this condition.81 There are two proposed pathogenic processes that lead to the formation of calcium deposits in the rotator cuff; one process involves a degenerative calcification, where the degeneration of the tendon fibers precedes calcification; and the other event is the reactive calcification, a process which is actively mediated by cells in a viable environment.80
Calcified tendinopathy is given in 3 distinct stages: precalcific, calcific and post-calcific. In the first stage, tendinous tissue is altered into fibrocartilage via the action of several factors which stimulate a metaplastic change of the tenocytes into chondrocytes. The second stage is subdivided into three phases: formation, resting and resorption, which are characterized by deposition of amorphous calcium phosphate followed by vascularization and, lastly, by resorption, which coincides with significant clinical pain. The final stage is characterized by collagenization of the lesion by fibroblasts.82 Intra-operatively, CTs usually appear as a sandy mass or viscous fluid, or even as an amorphous mass composed of small circular bodies.83 The material identified in calcium deposits happens to be calcium carbonate apatite, which has been further classified as an A and B-type apatite.84 A study85 of the chemical components in CT revealed that both types of carbonate apatite varied in quantities during the formative, resting and resorption phases of the disease progression. Histochemically, extracellular matrix (ECM) vesicles have been detected near the calcified deposition of the rotator cuff. A possible explanation for this observation is that, normally, the vesicles are inhibited from mineralization; however, in the presence of any pathology, the inhibitory stimulus may be lost, therefore, the vesicles undergo mineralization.86,87
CT remains primarily asymptomatic in the majority of patients, but when it does become symptomatic, it is reported that the pain is extremely severe and debilitating, especially during the acute phase.88,89 In CT, nociception appears to be a result of an inflammatory response to the local chemical pathology or to direct mechanical irritation.82 Pain related to calcium deposition has been previously classified as follows. Firstly, the pain may be a result of the chemical irritation of the tissue in response to the presence of calcium. Secondly, pain is caused by tissue pressure as a result of swelling. The third type is an impingement-like pain caused by bursal thickening or irritation by the deposit itself. Lastly, the fourth type of pain is triggered by a chronic stiffening of the glenohumeral joint as a result of prolonged immobilization by the patient trying to avoid possible irritation by the deposits with abduction or overhead activities.82 A study published by Gotoh M and colleagues89 proposed that certain mechanical and chemical factors could be the source for noxious stimuli which induce increased amounts of substance P, a neuropeptide involved in pain transmission. This neuropeptide has been shown to affect the afferent nerves.
Peters and colleagues90 aimed to investigate clinical and radiological efficacy of different energy levels of ESWT in calcific tendinitis of the shoulder. The researchers selected 90 study subjects with radiographically verified calcific tendinitis of one shoulder. The individuals were allocated to three groups to receive ESWT at either E1- 0.15 mJ/mm2 or E2- 0.44 mJ/mm2, or the sham treatment for 6 weekly intervals until symptoms resolved. It was found that participants from the E1 group presented significantly less pain during treatment but more treatments compared to E2 and at 6 month follow-up showed residual calcification and recurrence of pain (87%). The individuals from E2 group had no residual calcification or recurrence of pain; sham treatment was ineffective. No extreme side effects were reported except for a small number of hematomas in some cases. Overall, their study demonstrates that the medical application of ESWT for CT of the shoulder is significantly effective and safe at 0.44 mJ/mm2.
A prospective study91 aimed to explore whether ESWT was effective and efficient over the long term by recruiting 115 patients with CT and treating them with high-energy ESWT by 2 different protocols over 4 years. The patients were divided into 2 groups in order of enrollment in the study. In group A, individuals received one session of 2000-impulse high-dose shockwaves, whereas participants from group B received 2 sessions of the same treatment modality 1 week apart; the procedure was performed with an electromagnetic lithotripter. Results showed significant relief of pain (P < 0.001) and patient satisfaction in both groups. The percentages with partial or complete resorption of the calcification in groups A and B were 30% and 52% at F1, 47% and 77% at F2, and 93% for both groups at F3 time intervals, respectively. F1, F2 and F3 represent 3 months, 6 months and 4 years after therapy, in that order. Another significant result was the difference in radiologic alterations between the 2 groups after 6 months (P < 0.046). With their study, Daecke and co-workers were able to confirm the effectiveness of ESWT in CT of the shoulder and propose the importance of dose-dependent therapy for significant results. The authors also emphasize that, much like surgical procedures, ESWT should be considered only when an adequate conservative approach to treatment of chronic pain associated with a dense and distinctly outlined calcific deposit remains unsuccessful. This seems even more promising to patients who favor non-operative alternatives.
A 2017 retrospective clinical study92 conducted by Malliaropoulos et al. aimed to evaluate an individualized radial ESWT protocol for the treatment of symptomatic calcific shoulder tendinopathy. The authors gathered 67 patients and included 76 shoulders for analysis, to which radial ESWT treatment protocol was applied. Variables considered were number of sessions, shockwave impulses, pressure and frequency. Results showed significant decreases in VAS scores in all consecutive follow up stages, with 52% reduction in mean VAS immediately post treatment, 62% at 1 month and 75% at 3 months. Additionally, it was also observed that the treatment became increasingly successful over time, going from a 12% success rate immediately post-treatment to a 92% success rate at 1-year follow-up. Improvement in symptoms was maintained at 1 year with an 88% mean VAS reduction from baseline at 12 months. The 1-year recurrence rate was 7%, where 93% of the shoulders presented no further symptoms that required additional treatments. In summary, the authors demonstrate high success rates and low recurrence rates at1 year follow up after administration of radial ESWT for CT.
A similar study93 recently published by Wu and co-workers evaluated the efficacy of ESWT on calcified and noncalcified shoulder tendinosis in a total of 20 patients. The patients were allocated into the three following groups: noncalcified tendinosis (NCTS), type I dense calcified tendinosis of shoulder (DCTS), and type II and type III translucent calcified tendinosis of shoulder (TCTS) according to Gartner and Heyer classification. Much like the previous study, the researchers learned that the VAS scores decreased significantly. Regarding functional outcome, significant differences were found in pain reduction and functional improvement 12 months after ESWT, with the TCTS group exhibiting the greatest reduction in VAS scores compared to NCTS and DCTS groups. Overall satisfaction showed that 70% of the patients were complaint-free in the TCTS group, much higher than the NCTS and DCTS groups with 15% and 25%, respectively. In summary, their study further indicates the efficacy of ESWT for the management of calcified musculoskeletal structures.
4. Conclusion
In the present study, the authors review and discuss the principal biological mechanisms triggered by the application of extracorporeal shockwaves therapy to support the treatment of musculoskeletal injuries as a regenerative medicine technique. This non-invasive, medical procedure is capable of triggering cellular and molecular alterations that assist the regeneration of injured tissues. ESWT is mainly responsible for: pain relief, by acting directly on nerve fibers; tissue regeneration by stimulating vascularization; and reduction of calcium deposits in tissues. Numerous publications have proven the efficacy and safety of both radial and focused ESWT for the treatment of many musculoskeletal disorders, including osteoarthritis and different types of tendinopathies. ESWT should be considered in severe cases where conventional treatments prove to be of little success, especially in patients who prefer non-operative alternatives.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Contributor Information
Claudio Lopes Simplicio, Email: drsimplicio@terra.com.br.
Joseph Purita, Email: jpurita@aol.com.
William Murrell, Email: doctormurrell@gmail.com.
Gabriel Silva Santos, Email: gabriel1_silva@hotmail.com.
Rafael Gonzales dos Santos, Email: rafaelgsantos01@gmail.com.
José Fábio Santos Duarte Lana, Email: josefabiolana@gmail.com.
References
- 1.Chaussy C., Eisenberger F., Forssmann B. Extracorporeal shockwave lithotripsy (ESWL): a chronology. J Endourol. 2007;21(11):1249–1254. doi: 10.1089/end.2007.9880. [DOI] [PubMed] [Google Scholar]
- 2.Cheng J., Wang C. Biological mechanism of shockwave in bone. Int J Surg. 2015;24:143–146. doi: 10.1016/j.ijsu.2015.06.059. [DOI] [PubMed] [Google Scholar]
- 3.Wang C. Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res. 2012;7(1):11. doi: 10.1186/1749-799X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Notarnicola A., Moretti B. The biological effects of extracorporeal shock wave therapy (eswt) on tendon tissue. Muscles Ligaments Tendons J. 2012;2(1):33–37. [PMC free article] [PubMed] [Google Scholar]
- 5.Khan K., Scott A. Mechanotherapy: how physical therapists’ prescription of exercise promotes tissue repair. Br J Sports Med. 2009;43(4):247–252. doi: 10.1136/bjsm.2008.054239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittermayr R., Hartinger J., Antonic V. Extracorporeal shock wave therapy (ESWT) minimizes ischemic tissue necrosis irrespective of application time and promotes tissue revascularization by stimulating angiogenesis. Ann Surg. 2011;253(5):1024–1032. doi: 10.1097/SLA.0b013e3182121d6e. [DOI] [PubMed] [Google Scholar]
- 7.Vetrano M., d’Alessandro F., Torrisi M., Ferretti A., Vulpiani M., Visco V. Extracorporeal shock wave therapy promotes cell proliferation and collagen synthesis of primary cultured human tenocytes. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2159–2168. doi: 10.1007/s00167-011-1534-9. [DOI] [PubMed] [Google Scholar]
- 8.Wang C., Wang F., Yang K. Shock wave therapy induces neovascularization at the tendon–bone junction. A study in rabbits. J Orthop Res. 2003;21(6):984–989. doi: 10.1016/S0736-0266(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 9.Ito Y., Ito K., Shiroto T. Cardiac shock wave therapy ameliorates left ventricular remodeling after myocardial ischemia–reperfusion injury in pigs in vivo. Coron Artery Dis. 2010;21(5):304–311. doi: 10.1097/mca.0b013e32833aec62. [DOI] [PubMed] [Google Scholar]
- 10.Hausdorf J., Lemmens M., Kaplan S. Extracorporeal shockwave application to the distal femur of rabbits diminishes the number of neurons immunoreactive for substance P in dorsal root ganglia L5. Brain Res. 2008;1207:96–101. doi: 10.1016/j.brainres.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Hausdorf J., Lemmens M., Heck K. Selective loss of unmyelinated nerve fibers after extracorporeal shockwave application to the musculoskeletal system. Neuroscience. 2008;155(1):138–144. doi: 10.1016/j.neuroscience.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 12.Wang C., Wang F., Yang K., Weng L., Sun Y., Yang Y. The effect of shock wave treatment at the tendon–bone interface—an histomorphological and biomechanical study in rabbits. J Orthop Res. 2005;23(2):274–280. doi: 10.1016/j.orthres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Yalcin E., Keskin Akca A., Selcuk B., Kurtaran A., Akyuz M. Effects of extracorporal shock wave therapy on symptomatic heel spurs: a correlation between clinical outcome and radiologic changes. Rheumatol Int. 2010;32(2):343–347. doi: 10.1007/s00296-010-1622-z. [DOI] [PubMed] [Google Scholar]
- 14.Loew M., Jurgowski W. Initial experiences with extracorporeal shockwave lithotripsy (ESWL) in treatment of tendinosis calcarea of the shoulder. Z Orthop Ihre Grenzgeb. 1993;131(5):470–473. doi: 10.1055/s-2008-1040056. [DOI] [PubMed] [Google Scholar]
- 15.Rompe J., Hopf C., Nafe B., Burger R. Low-energy extracorporeal shock wave therapy for painful heel: a prospective controlled single-blind study. Arch Orthop Trauma Surg. 1996;115(2):75–79. doi: 10.1007/BF00573445. [DOI] [PubMed] [Google Scholar]
- 16.Metzner G., Dohnalek C., Aigner E. High-energy extracorporeal shock-wave therapy (ESWT) for the treatment of chronic plantar fasciitis. Foot Ankle Int. 2010;31(9):790–796. doi: 10.3113/FAI.2010.0790. [DOI] [PubMed] [Google Scholar]
- 17.Cole C., Seto C., Gazewood J. Plantar fasciitis: evidence-based review of diagnosis and therapy. Am Fam Physician. 2005;72(11):2237–2242. [PubMed] [Google Scholar]
- 18.Kudo P., Dainty K., Clarfield M., Coughlin L., Lavoie P., Lebrun C. Randomized, placebo-controlled, double-blind clinical trial evaluating the treatment of plantar fasciitis with an extracoporeal shockwave therapy (ESWT) device: a North American confirmatory study. J Orthop Res. 2006;24(2):115–123. doi: 10.1002/jor.20008. [DOI] [PubMed] [Google Scholar]
- 19.Richter D., Ekkernkamp A., Muhr G. Extracorporeal shock wave therapy--an alternative concept for the treatment of epicondylitis of the humerus and radius. Orthopä. 1995;24(3):303–306. [PubMed] [Google Scholar]
- 20.Ho C. Extracorporeal shock wave treatment for chronic lateral epicondylitis (tennis elbow) Issues Emerg Health Technol. 2007;96(2):1–4. [PubMed] [Google Scholar]
- 21.Seil R., Wilmes P., Nührenbörger C. Extracorporeal shock wave therapy for tendinopathies. Expet Rev Med Dev. 2006;3(4):463–470. doi: 10.1586/17434440.3.4.463. [DOI] [PubMed] [Google Scholar]
- 22.Rompe J., Kirkpatrick C., Küllmer K., Schwitalle M., Krischek O. Dose-related effects of shock waves on rabbit tendo Achillis. J Bone Joint Surg Br. 1998;80-B(3):546–552. doi: 10.1302/0301-620x.80b3.8434. [DOI] [PubMed] [Google Scholar]
- 23.Cleveland R. The acoustics of shock wave lithotripsy. AIP Conf Proc. 2007;900(1) doi: 10.1063/1.2723592. https://aip.scitation.org/doi/abs/10.1063/1.2723590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogden J., Tóth-Kischkat A., Schultheiss R. Principles of shock wave therapy. Clin Orthop Relat Res. 2001;387:8–17. doi: 10.1097/00003086-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Coleman A., Saunders J. A survey of the acoustic output of commercial extracorporeal shock wave lithotripters. Ultrasound Med Biol. 1989;15(3):213–227. doi: 10.1016/0301-5629(89)90066-5. [DOI] [PubMed] [Google Scholar]
- 26.Magosch P., Lichtenberg S., Habermeyer P. Radial shock wave therapy in calcifying tendinitis of the rotator cuff--a prospective study. Z Orthop Ihre Grenzgeb. 2003;141(6):629–636. doi: 10.1055/s-2003-812407. [DOI] [PubMed] [Google Scholar]
- 27.McClure S., Dorfmüller C. Extracorporeal shock wave therapy: theory and equipment. Clin Tech Equine Pract. 2003;2(4):348–357. [Google Scholar]
- 28.Chitnis P., Cleveland R. Acoustic and cavitation fields of shock wave therapy devices. AIP Conf Proc. 2006;829:440–444. [Google Scholar]
- 29.Haupt G. Use of extracorporeal shockwaves in the treatment of pseudarthrosis, tendinopathy and other orthopedic diseases. J Urol. 1997;158(1):4–11. doi: 10.1097/00005392-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Weihs A.M., Fuchs C., Teuschl A.H. Shock wave treatment enhances cell proliferation and improves wound healing by ATP release-coupled extracellular signal-regulated kinase (ERK) activation. J Biol Chem. 2014;289(39):27090–27104. doi: 10.1074/jbc.M114.580936. Epub 2014 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frairia R., Berta L. Biological effects of extracorporeal shock waves on fibroblasts. A review. Muscles Ligaments Tendons J. 2012;1(4):138–147. [PMC free article] [PubMed] [Google Scholar]
- 32.Abe Y., Ito K., Hao K. Extracorporeal low-energy shockwave therapy exerts anti-inflammatory effects in a rat model of acute myocardial infarction. Circ J. 2014;78(12):2915–2925. doi: 10.1253/circj.cj-14-0230. [DOI] [PubMed] [Google Scholar]
- 33.Lana J., Macedo A., Ingrao I., Huber S., Santos G., Santana M. Leukocyte-rich PRP for knee osteoarthritis: current concepts. J Clin Orthop Trauma. 2019;10(1) doi: 10.1016/j.jcot.2019.01.011. https://www.journal-cot.com/article/S0976-5662(18)30445-4/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.d’Agostino M., Craig K., Tibalt E., Respizzi S. Shock wave as biological therapeutic tool: from mechanical stimulation to recovery and healing, through mechanotransduction. Int J Surg. 2015;24:147–153. doi: 10.1016/j.ijsu.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 35.Saggini R., Di Stefano A., Saggini A., Bellomo R. Clinical application of shock wave therapy in musculoskeletal disorders: Part I. J Biol Regul Homeost Agents. 2015;29(3):533–545. [PubMed] [Google Scholar]
- 36.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23(5):611–615. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]
- 37.Xia W., Mørch C., Matre D., Andersen O. Exploration of conditioned pain modulation effect on long-term potentiation-like pain amplification in humans. Eur J Pain. 2017;21(4):645–657. doi: 10.1002/ejp.968. [DOI] [PubMed] [Google Scholar]
- 38.Chow I., Cheing G. Comparison of different energy densities of extracorporeal shock wave therapy (ESWT) for the management of chronic heel pain. Clin Rehabil. 2007;21(2):131–141. doi: 10.1177/0269215506069244. [DOI] [PubMed] [Google Scholar]
- 39.García-Muntión A., Godefroy L., Robert H., Muñoz-García D., Calvo-Lobo C., López-de-Uralde-Villanueva I. Study of the mechanisms of action of the hypoalgesic effect of pressure under shock waves application: a randomised controlled trial. Compl Ther Med. 2018;42:332–339. doi: 10.1016/j.ctim.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Maier M., Averbeck B., Milz S., Refior H., Schmitz C. Substance P and prostaglandin E2 release after shock wave application to the rabbit femur. Clin Orthop Relat Res. 2003;406:237–245. doi: 10.1097/01.blo.0000030173.56585.8f. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi N., Wada Y., Ohtori S., Saisu T., Moriya H. Application of shock waves to rat skin decreases calcitonin gene-related peptide immunoreactivity in dorsal root ganglion neurons. Auton Neurosci. 2003;107(2):81–84. doi: 10.1016/S1566-0702(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y., Wang C., Yang K. Extracorporeal shock waves promote healing of collagenase-induced Achilles tendinitis and increase TGF-β1 and IGF-I expression. J Orthop Res. 2004;22(4):854–861. doi: 10.1016/j.orthres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Zhao C., Sun Y.L., Kirk R.L. Effects of a lubricin-containing compound on the results of flexor tendon repair in a canine model in vivo. J Bone Joint Surg Am. 2010;92:1453–1461. doi: 10.2106/JBJS.I.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S.Y., Niikura T., Reddi A.H. Superficial zone protein (lubricin) in the different tissue compartments of the knee joint: modulation by transforming growth factor beta 1 and interleukin1 beta. Tissue Eng Part A. 2008;14:1799–1808. doi: 10.1089/ten.tea.2007.0367. [DOI] [PubMed] [Google Scholar]
- 45.Zhang D., Kearney C.J., Cheriyan T., Schmid T.M., Spector M. Extracorporeal shockwave-induced expression of lubricin in tendons and septa. Cell Tissue Res. 2011 Nov;346(2):255–262. doi: 10.1007/s00441-011-1258-7. [DOI] [PubMed] [Google Scholar]
- 46.Ohtori S., Inoue G., Mannoji C. Shock wave application to rat skin induces degeneration and reinnervation of sensory nerve fibres. Neurosci Lett. 2001;315(1-2):57–60. doi: 10.1016/s0304-3940(01)02320-5. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi N., Ohtori S., Saisu T., Moriya H., Wada Y. Second application of low-energy shock waves has a cumulative effect on free nerve endings. Clin Orthop Relat Res. 2006;443:315–319. doi: 10.1097/01.blo.0000188064.56091.a7. [DOI] [PubMed] [Google Scholar]
- 48.Palmieri A., Imbimbo C., Longo N. A first prospective, randomized, double-blind, placebo-controlled clinical trial evaluating extracorporeal shock wave therapy for the treatment of peyronie’s disease. Eur Urol. 2009;56(2):363–370. doi: 10.1016/j.eururo.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Han S., Lee J., Guyton G. Effect of extracorporeal shock wave therapy on cultured tenocytes. Foot Ankle Int. 2008;30(2):93–98. doi: 10.3113/FAI-2009-0093. 2009. [DOI] [PubMed] [Google Scholar]
- 50.Rasmussen S., Christensen M., Mathiesen I., Simonson O. Shockwave therapy for chronic Achilles tendinopathy: a double-blind, randomized clinical trial of efficacy. Acta Orthop. 2008;79(2):249–256. doi: 10.1080/17453670710015058. [DOI] [PubMed] [Google Scholar]
- 51.Bosch G., Mos M., Binsbergen R., Schie H., Lest C., Weeren P. The effect of focused extracorporeal shock wave therapy on collagen matrix and gene expression in normal tendons and ligaments. Equine Vet J. 2009;41(4):335–341. doi: 10.2746/042516409x370766. [DOI] [PubMed] [Google Scholar]
- 52.Bosch G., Lin Y., Schie H., Lest C., Barneveld A., Weeren P. Effect of extracorporeal shock wave therapy on the biochemical composition and metabolic activity of tenocytes in normal tendinous structures in ponies. Equine Vet J. 2007;39(3):226–231. doi: 10.2746/042516407x180408. [DOI] [PubMed] [Google Scholar]
- 53.Burnstock G., Verkhratsky A. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 2010;1(1) doi: 10.1038/cddis.2009.11. e9-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alfredson H., Ohberg L., Forsgren S. Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? Knee Surg Sports Traumatol Arthrosc. 2003;11(5):334–338. doi: 10.1007/s00167-003-0391-6. [DOI] [PubMed] [Google Scholar]
- 55.Santamato A., Beatrice R., Micello M. Power Doppler ultrasound findings before and after focused extracorporeal shock wave therapy for Achilles tendinopathy: a pilot study on pain reduction and neovascularization effect. Ultrasound Med Biol. 2018;45(5):1316–1323. doi: 10.1016/j.ultrasmedbio.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 56.Wang C., Wang F., Yang K. Biological effects of extracorporeal shockwave in bone healing: a study in rabbits. Arch Orthop Trauma Surg. 2008;128(8):879–884. doi: 10.1007/s00402-008-0663-1. [DOI] [PubMed] [Google Scholar]
- 57.Yahata K., Kanno H., Ozawa H. Low-energy extracorporeal shock wave therapy for promotion of vascular endothelial growth factor expression and angiogenesis and improvement of locomotor and sensory functions after spinal cord injury. J Neurosurg Spine. 2016;25(6):745–755. doi: 10.3171/2016.4.SPINE15923. [DOI] [PubMed] [Google Scholar]
- 58.Wang H., Oh B., Wang B. Low-intensity extracorporeal shockwave therapy ameliorates diabetic underactive bladder in streptozotocin-induced diabetic rats. BJU Int. 2018;122(3):490–500. doi: 10.1111/bju.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murata R., Ohtori S., Ochiai N. Extracorporeal shockwaves induce the expression of ATF3 and GAP-43 in rat dorsal root ganglion neurons. Auton Neurosci. 2006;128(1-2):96–100. doi: 10.1016/j.autneu.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Griffin J., Thompson W. Biology and pathology of nonmyelinating Schwann cells. Glia. 2008;56(14):1518–1531. doi: 10.1002/glia.20778. [DOI] [PubMed] [Google Scholar]
- 61.Liu T., Shindel A.W., Lin G., Lue T.F. Cellular signaling pathways modulated by low-intensity extracorporeal shock wave therapy. Int J Inf Retr (IJIR) 2019;31:170–176. doi: 10.1038/s41443-019-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamaya S., Ozawa H., Kanno H. Low-energy extracorporeal shock wave therapy promotes vascular endothelial growth factor expression and improves locomotor recovery after spinal cord injury. J Neurosurg. 2014;121(6):1514–1525. doi: 10.3171/2014.8.JNS132562. [DOI] [PubMed] [Google Scholar]
- 63.Mayer-Wagner S., Ernst J., Maier M. The effect of high-energy extracorporeal shock waves on hyaline cartilage of adult rats in vivo. J Orthop Res. 2010;28(8) doi: 10.1002/jor.21074. https://www.ncbi.nlm.nih.gov/pubmed/20135673 [DOI] [PubMed] [Google Scholar]
- 64.Wang C., Sun Y., Wong T., Hsu S., Chou W., Chang H. Extracorporeal shockwave therapy shows time-dependent chondroprotective effects in osteoarthritis of the knee in rats. J Surg Res. 2012;178(1):196–205. doi: 10.1016/j.jss.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 65.Wang C., Weng L., Ko J. Extracorporeal shockwave shows regression of osteoarthritis of the knee in rats. J Surg Res. 2011;171(2):601–608. doi: 10.1016/j.jss.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 66.Wang C., Sun Y., Siu K., Wu C. Extracorporeal shockwave therapy shows site-specific effects in osteoarthritis of the knee in rats. J Surg Res. 2013;183(2):612–619. doi: 10.1016/j.jss.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 67.Wang C., Huang C., Hsu S., Chen J., Cheng J. Extracorporeal shockwave therapy in osteoporotic osteoarthritis of the knee in rats: an experiment in animals. Arthritis Res Ther. 2014;16(4):R139. doi: 10.1186/ar4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang C., Huang H., Chen H., Pai C., Yang K. Effect of shock wave therapy on acute fractures of the tibia. Clin Orthop Relat Res. 2001;387:112–118. doi: 10.1097/00003086-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 69.Hsu R., Tai C., Chen C., Hsu W., Hsueh S. Enhancing mechanical strength during early fracture healing via shockwave treatment: an animal study. Clin BioMech. 2003;18(6):S33–S39. doi: 10.1016/s0268-0033(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 70.van der Worp H., van den Akker-Scheek I., van Schie H., Zwerver J. ESWT for tendinopathy: technology and clinical implications. Knee Surg Sports Traumatol Arthrosc. 2012;21(6):1451–1458. doi: 10.1007/s00167-012-2009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang C., Yang K., Ko J., Huang C., Huang H., Wang F. The effects of shockwave on bone healing and systemic concentrations of nitric oxide (NO), TGF-β1, VEGF and BMP-2 in long bone non-unions. Nitric Oxide. 2009;20(4):298–303. doi: 10.1016/j.niox.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Wang C., Huang K., Sun Y. VEGF modulates angiogenesis and osteogenesis in shockwave-promoted fracture healing in rabbits. J Surg Res. 2011;171(1):114–119. doi: 10.1016/j.jss.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 73.Wang C., Yang Y., Huang C. The effects of shockwave on systemic concentrations of nitric oxide level, angiogenesis and osteogenesis factors in hip necrosis. Rheumatol Int. 2010;31(7):871–877. doi: 10.1007/s00296-010-1384-7. [DOI] [PubMed] [Google Scholar]
- 74.Yin T., Wang C., Yang K., Wang F., Sun Y. Shockwaves enhance the osteogenetic gene expression in marrow stromal cells from hips with osteonecrosis. Chang Gung Med J. 2011;34:367–374. [PubMed] [Google Scholar]
- 75.Wang C., Wang F., Ko J. Extracorporeal shockwave therapy shows regeneration in hip necrosis. Rheumatology. 2007;47(4):542–546. doi: 10.1093/rheumatology/ken020. [DOI] [PubMed] [Google Scholar]
- 76.Chen Y., Kuo Y., Yang K., Wang C., Huang H., Wang F. Shock wave application enhances pertussis toxin protein-sensitive bone formation of segmental femoral defect in rats. J Bone Miner Res. 2003;18(12):2169–2179. doi: 10.1359/jbmr.2003.18.12.2169. [DOI] [PubMed] [Google Scholar]
- 77.Wang F., Wang C., Sheen-Chen S., Kuo Y., Chen R., Yang K. Superoxide mediates shock wave induction of ERK-dependent osteogenic transcription factor (CBFA1) and mesenchymal cell differentiation toward osteoprogenitors. J Biol Chem. 2002;277(13):10931–10937. doi: 10.1074/jbc.M104587200. [DOI] [PubMed] [Google Scholar]
- 78.Wang F., Wang C., Chen Y. Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1α and VEGF-A expression in shock wave-stimulated osteoblasts. J Biol Chem. 2003;279(11):10331–10337. doi: 10.1074/jbc.M308013200. [DOI] [PubMed] [Google Scholar]
- 79.Martini L., Giavaresi G., Fini M. Effect of extracorporeal shock wave therapy on osteoblastlike cells. Clin Orthop Relat Res. 2003;413:269–280. doi: 10.1097/01.blo.0000073344.50837.cd. [DOI] [PubMed] [Google Scholar]
- 80.Pakos E., Gkiatas I., Rakkas G. Calcific deposit needling in combination with extracorporeal shock wave therapy (ESWT): a proposed treatment for supraspinatus calcified tendinopathy. SICOT-J. 2018;4:45. doi: 10.1051/sicotj/2018043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ranalletta M., Rossi L., Bongiovanni S., Tanoira I., Piuzzi N., Maignon G. Arthroscopic removal and rotator cuff repair without acromioplasty for the treatment of symptomatic calcifying tendinitis of the supraspinatus tendon. Orthop J Sports Med. 2015;3(4) doi: 10.1177/2325967115577957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merolla G., Bhat M., Paladini P., Porcellini G. Complications of calcific tendinitis of the shoulder: a concise review. J Orthop Traumatol. 2015;16(3):175–183. doi: 10.1007/s10195-015-0339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oliva F., Via A., Maffulli N. Physiopathology of intratendinous calcific deposition. BMC Med. 2012;10(1) doi: 10.1186/1741-7015-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamada J., Tamai K., Ono W., Saotome K. Does the nature of deposited basic calcium phosphate crystals determine clinical course in calcific periarthritis of the shoulder? J Rheumatol. 2006;33(2):326–332. [PubMed] [Google Scholar]
- 85.Chiou H., Hung S., Lin S., Wei Y., Li M. Correlations among mineral components, progressive calcification process and clinical symptoms of calcific tendonitis. Rheumatology. 2009;49(3):548–555. doi: 10.1093/rheumatology/kep359. [DOI] [PubMed] [Google Scholar]
- 86.Gohr C., Fahey M., Rosenthal A. Calcific tendonitis: a model. Connect Tissue Res. 2007;48(6):286–291. doi: 10.1080/03008200701692362. [DOI] [PubMed] [Google Scholar]
- 87.Merolla G., Dave A., Paladini P., Campi F., Porcellini G. Ossifying tendinitis of the rotator cuff after arthroscopic excision of calcium deposits: report of two cases and literature review. J Orthop Traumatol. 2014;16(1):67–73. doi: 10.1007/s10195-014-0309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bayam L., Ahmad M., Naqui S., Chouhan A., Funk L. Pain mapping for common shoulder disorders. Am J Orthoped. 2011;40(7):353–358. [PubMed] [Google Scholar]
- 89.Gotoh M., Hamada K., Yamakawa H., Inoue A., Fukuda H. Increased substance P in subacromial bursa and shoulder pain in rotator cuff diseases. J Orthop Res. 1998;16(5):618–621. doi: 10.1002/jor.1100160515. [DOI] [PubMed] [Google Scholar]
- 90.Peters J., Luboldt W., Schwarz W., Jacobi V., Herzog C., Vogl T. Extracorporeal shock wave therapy in calcific tendinitis of the shoulder. Skeletal Radiol. 2004;33(12):712–718. doi: 10.1007/s00256-004-0849-8. [DOI] [PubMed] [Google Scholar]
- 91.Daecke W., Kusnierczak D., Loew M. Long-term effects of extracorporeal shockwave therapy in chronic calcific tendinitis of the shoulder. J Shoulder Elbow Surg. 2002;11(5):476–480. doi: 10.1067/mse.2002.126614. [DOI] [PubMed] [Google Scholar]
- 92.Malliaropoulos N., Thompson D., Meke M. Individualised radial extracorporeal shock wave therapy (rESWT) for symptomatic calcific shoulder tendinopathy: a retrospective clinical study. BMC Muscoskel Disord. 2017;18(1) doi: 10.1186/s12891-017-1873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu K., Chou W., Wang C. Efficacy of extracorporeal shockwave therapy on calcified and noncalcified shoulder tendinosis: a propensity score matched analysis. BioMed Res Int. 2019:1–8. doi: 10.1155/2019/2958251. [DOI] [PMC free article] [PubMed] [Google Scholar]