Abstract
This pilot randomized controlled trial evaluated the feasibility and efficacy of a brief motivational enhancement intervention to improve adherence to antiretroviral therapy in persons with HIV called Personal Approach to Treatment Choices for HIV (PATCH). We compared PATCH to an active control condition on self-reported adherence, clinical outcomes, and psychosocial outcomes. Participants were 34 individuals (61.8% male, Mage = 47.1) receiving HIV-related services who were suboptimally engaged in care. Participants completed baseline measures, participated in either PATCH or a stress reduction skills control intervention, and completed post-treatment and 3-month follow-up assessments. Results revealed no differences between conditions on adherence or clinical outcomes. At post-treatment, PATCH participants reported greater improvements in alcohol use, psychiatric symptoms, subjective mental functioning, and emotion-focused coping; improvements in subjective mental functioning were maintained at 3-months. Results suggest that motivational enhancement interventions can improve psychosocial outcomes for people with HIV. That some improvements were not maintained at follow-up suggests that effects wane over time and longer treatment may be indicated for lasting effects.
Keywords: HIV, Antiretroviral therapy, Motivational interviewing, Adherence
Introduction
Over 35 million people live with human immunodeficiency virus (HIV) worldwide; approximately 21.7 million use antiretroviral therapy (ART) as part of their treatment [1]. ART suppresses viral replication and reduces viral loads, slowing HIV progression [2–4]. ART has been transformative in HIV care: its use has led to significant reductions in HIV-related mortality and morbidity [3, 4]. Proper use of ART improves immunologic response, physical health and functioning, and quality of life for people living with HIV [5, 6]. Despite its success, many patients decline to use ART, delay using it, or have difficulty adhering to it as prescribed by their treatment team [7, 8]. U.S. studies find rates of ART refusal of 28–33%; refusals account for 19–57% of clinically eligible untreated cases [9, 10]. Delaying ART use accounts for a substantial portion of untreated cases, with approximately 31% of patients waiting 180 days or longer to initiate ART following an HIV diagnosis [11]. Over a third of patients using ART do not maintain the necessary levels of adherence [7, 8, 12]. Refusal, delay, and nonadherence to ART all contribute to negative clinical outcomes, including increased viral loads and increased risk of mortality [13–16].
Many barriers contribute to refusal, delay, and nonadherence to ART. For some, the cost of treatment is high [17]. For others, following a daily medication regimen can be difficult [18]. Regimens differ by patient but can involve multiple doses of medication each day, although twice daily dosing is feasible for most patients [19]. Others may experience side effects or need to make dietary changes to reduce side effects with can be difficult adjustments for some patients to make [19, 20]. For ART to be most effective, guidelines require that patients take ART with 90–95% consistency over time [6, 21, 22]; this can be a challenge for many patients. Some patients report feeling worse when starting ART [18] and decide to stop taking it before they learn to manage side effects [23]. The stigma associated with HIV may lead some individuals not to take medications to avoid the risk of revealing their condition to others [24]. Individual factors that negatively impact adherence include substance abuse, lack of education, lack of medical insurance, and unstable housing [25–27]. Contending with more immediate needs associated with poverty, homelessness, violence, or discrimination may lead individuals to deprioritize HIV treatment [25]. Individual beliefs about medical providers and HIV treatment may also lead some individuals to refuse or forgo taking ART. A large study conducted in 10 urban centers found that “belief barriers” to HIV care, including mistrust in the medical system and the belief that ART side effects are worse than HIV, were associated with suboptimal engagement in HIV-related medical care [26]. Barriers to ART adherence may be especially challenging for African-American HIV patients, who are less likely to be prescribed ART [27], show lower adherence rates among those who do receive a prescription [28], report more reservations about using ART when compared to white patients [29] and report more reservations regarding the appropriateness of a recommendation of ART for their HIV treatment [29]. There is a need to design and evaluate interventions that address these additional challenges so that they can be useful for African-American patients and others who experience additional adherence challenges [30, 31].
Interventions for Improving ART Adherence
There has been a good amount of work aimed at developing and testing brief interventions to improve adherence to ART. A recent network meta-analysis of 85 studies of 16,271 individuals [30] found many promising interventions incorporate Motivational Interviewing (MI), an intervention that emphasizes client autonomy, honors the client’s decisions regarding change, and attempts to increase motivation for behavioral change by exploring ambivalence and guiding clients to voice arguments for change that correspond to their personal aspirations and goals [32]. As applied to ART adherence, MI creates an opportunity for clients to express ambivalence regarding adopting or adhering to a complex medication regimen, and to voice beliefs that may make them mistrustful of ART, within a collaborative and nonjudgmental discussion that may help them resolve these issues. Several randomized controlled trials have shown significant improvements in ART adherence for those receiving MI-based interventions [33–36], although others have found no improvements relative to control conditions [37, 38]. Some trials also found improved clinical outcomes for patients receiving MI-based interventions including decreased viral loads [34, 35] and increased CD4 counts [34]. Qualitative research has revealed that clients perceive brief MI interventions for ART adherence as useful and helpful [39]. Overall, these findings support the clinical implementation of MI-based interventions in real-world HIV settings.
The Present Study
We conducted a randomized controlled pilot feasibility trial of Personal Approaches to Treatment Choices for HIV (PATCH), a brief, MI-based intervention focused on ART adherence designed to support patients’ decision-making processes and enhance intrinsic motivation, that is, motivation that arises from internal rewards and a personal sense of satisfaction [32]. We included a small sample of 34 African-American patients at an urban HIV treatment program who were suboptimally engaged in care.
We compared PATCH to an active control condition on increasing ART adherence and attendance at HIV clinical appointments, improving clinical outcomes such as viral load and CD4 + cell counts, and impacting psychosocial outcomes including psychiatric symptoms, substance use, and physical and mental health functioning. Examining the feasibility and utility of an MI-based intervention to address ART adherence in a sample of individuals who experience unique adherence barriers represents a novel and important contribution to the literature on interventions to improve ART adherence. Additionally, the present study uses an MI-based approach to address suboptimal engagement with ART via a randomized controlled trial, adding to a relatively small literature in this area.
Methods
Participants
Participants were 34 individuals with HIV receiving HIV-related services at two community-based HIV treatment programs in Baltimore, Maryland. Inclusion criteria were being African-American or multiracial with African-American heritage; not taking or adhering to ART as prescribed by the treatment team at the time of enrollment as assesesed by reviewing medical records, including provider notes; and for those not taking ART, self-report that he/she would not be ready to take it if recommended or that he/she was unsure about starting it even if it was recommended. Exclusion criteria included a diagnosis of intellectual disability or dementia listed in the medical record and presence of active psychosis or suicidality evident in the initial interview. Figure 1 shows the study consort diagram. Overall, 35 individuals were screened for preliminary eligibility; 34 (97%) signed informed consent, completed baseline (BL) assessments, and were randomized to study condition. Of these, 29 (85%) completed the post-treatment assessment and 28 (82%) completed the follow-up assessment.
Fig. 1.
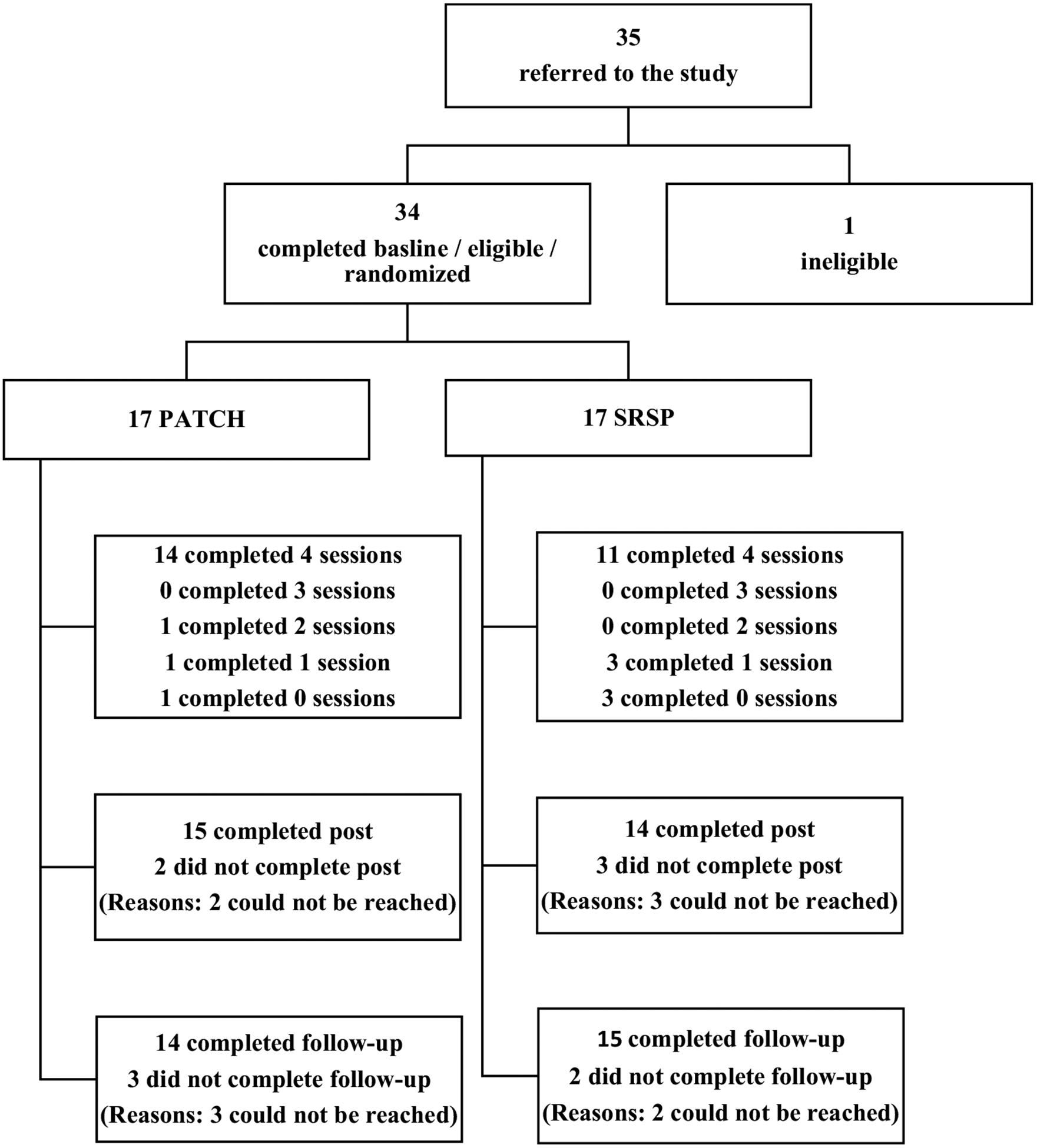
Study consort diagram
Interventions
Standard care at the recruitment site included clinicians asking patients to self-report adherence at each appointment. If patients reported that they were having trouble with adherence and/or their viral load was increasing (i.e., suggesting non-adherence), the clinician could refer the patient for a directly observed therapy evaluation and treatment monitoring at the clinic. In the present study, participants continued with standard care and were assigned to either the PATCH or a Stress Reduction Skills Program (SRSP). Both study interventions included four individual meetings that range 45 to 60 min.
PATCH is a brief intervention to enhance intrinsic motivation to initiate/adhere to ART using MI techniques focused on decreasing ambivalence and supporting individuals’ decision-making processes. Meeting 1 includes building rapport, learning about patients’ experiences with HIV diagnosis and treatment, and identifying personal values and how they connect to HIV care. Patients share personal experiences and complete a values clarification exercise in which they identify values that are most important to them and discuss how these values are connected to their health. Meeting 1 ends with the introduction of the idea of a HIV decision-making goal. Meeting 2 focuses on exploring ambivalence and its connection to personal behavior. Patients complete a decisional balance exercise in which they identify personal benefits adhering to ART and consequences of not doing so. Meeting 2 ends with a review of the decision-making goal and how achieving this goal is connected to the future goals and values. Meeting 3 involves developing discrepancy and eliciting change talk, language used by the patient that represents their personal motivations or intentions to change [32]. For those who have made changes or achieved their decision-making goal, Meeting 3 focuses on discussing the impact of these changes, identifying next steps, evoking further change talk, and problem solving as needed. For those who remain ambivalent, Meeting 3 focuses on linking values and decision-making goals with behavior change. Meeting 4 involves continued evocation of change talk, problem solving for those making changes, and connecting ART use with values and hopes for the future.
SRSP offered lessons on developing skills for coping with negative emotions. This was chosen as the comparison condition because while SRSP had the potential to be useful to patients in coping with stress it would not be expected to yield improved adherence to ART [30, 40]. Meeting 1 is focused on relaxation skills with 20 min of a simple relaxation exercise, 20 min of Progressive Muscle Relaxation, and 10–20 min of discussion about the experience and how to integrate time for relaxation into one’s life. Meeting 2 is focused on learning strategies for coping with depression and stress including increasing pleasant activities, talking to someone, getting help solving the problem that is leading to the negative feelings, or talking to one’s doctor about using medication by following structured steps that they practice by roleplaying with the interventionist. Meeting 3 is focused on learning a framework for problem solving and practicing these steps in connection with a real-life problem that the patient is experiencing in his/her life. Meeting 4 involves learning and practicing steps for expressing angry feelings and practicing them by roleplaying with the interventionist.
Measures
Participants completed assessments at BL, post-treatment (PT), and 3-month follow-up (3 M) timepoints. Demographic information collected via self-report included age, gender, race, marital status, years of education, and employment status. Inclusion criteria of nonuse of ART or ambivalence regarding its use were assessed with several questions: [1] Are you currently taking medications for HIV (yes/no; response of “no” required for eligibility); [2] At any time in the last year did a medical provider tell you that you should take medicines for HIV (yes/no; response of “yes” required for eligibility); [3] If a medical provider recommended that you start medicines for HIV tomorrow, do you think you would be ready to take them? [free response; answers reflecting ambivalence (e.g., “I’m not sure I would be willing to give it a try”), uncertainty (e.g., “I’d have to look into it”), conditions (e.g., “Yes, if I really felt I needed them”) or no for any reason other than practical concerns (e.g., lack of money or insurance coverage) were required for eligibility. Participants completed the ART Interview, a brief structured interview developed for this study to assess current and past use of ART, adherence, and attitudes towards ART use. Barriers to consistent use of ART such as toxicity, side effects, and regimen complexity were also assessed. The Brief Symptom Inventory (BSI; 44), a 53-item self-report inventory, was used to measure mental health symptoms. It yields standardized t-scores on nine symptom dimensions and three global indices: Global Severity Index (GSI, an indicator of overall psychological distress), Positive Symptom Distress Index (PSDI, a measure of symptom intensity) and Positive Symptom Total (PST, a summary of the number of reported symptoms). It demonstrates acceptable reliability and validity [41]. Index scores were used in the present analyses. Perceptions of physical and mental health were assessed with the SF-12 Health Survey [42], a standardized instrument to assess self-perceptions of general health functioning across multiple dimensions (general, physical, and emotional/psychiatric functioning). The SF-12 has shown good internal consistency, stability, and concurrent validity in populations with both physical and mental illnesses [43]. In these analyses, the physical health subscore was used as a subjective measure of somatic health functioning. The 28-item Brief COPE [44] was used to measure coping strategies across 14 domains: self-distraction, active coping, denial, substance use, use of emotional support, use of instrumental support, behavioral disengagement, venting, positive reframing, planning, humor, acceptance, religion, and self-blame. The Brief COPE has shown good reliability and validity across a range of samples [45]. All participants who attended at least one PATCH meeting completed a Program Satisfaction Interview at PT in which they were asked what they liked and disliked about PATCH and whether and how the intervention helped them think about their treatment options.
The following clinical variables were collected from participants’ medical records: CD4 + cell count (all timepoints), HIV-1 RNA viral load (all timepoints), whether or not the participant had a regular HIV primary care provider (all timepoints), number of regular outpatient HIV care appointments attended in the last month (all timepoints), and whether use of ART was initiated (PT and 3 M only).
Procedures
This study was approved by the Institutional Review Board (IRB) at the University of Maryland, School of Medicine. Participants were recruited through medical records screening, clinician referrals, and posted flyers. Following referral, the medical record was reviewed or the treatment team consulted to confirm basic inclusion criteria (HIV status, nonuse of ART); these activities were covered by a partial Health Insurance Portability and Accountability Act (HIPAA) waiver from the IRB so that we could collect a few key pieces of information so that we would only approach patients about they study who were most likely to meet eligibility criteria. A study team member then approached the individual at the recruitment site to describe the requirements of participation. Interested individuals completed written informed consent with a trained staff member. After providing informed consent, participants completed the BL assessment and were randomized to condition. The four intervention meetings were scheduled as soon as possible within the next 4 weeks. Participants could elect to do one or two meetings per week and could elect to do the second and third meetings by telephone if desired. PT assessments were completed between 2 and 3 months after BL; the 3 M assessment was completed between 5 and 6 months after BL. Assessments were administered by trained masters’ level research assistants blind to intervention condition and took approximately 1 h to complete. Assessment training included observation and co-rating of interviews administered by a trained assessor, and completion of interviews while being observed by a trained assessor. Interventionists were master’s level social workers or counselors with experience working with individuals with HIV. Interventionists received weekly training for approximately 2 months that included discussion of intervention materials, didactics related to motivational interviewing techniques (PATCH) or skills training interventions (SRSP), watching demonstrations, and conducting mock sessions with feedback. Interventionists participated in monthly supervision that involved listening to audiotaped meetings, discussing adherence to the manual and competence in delivering intervention components, and providing corrections or clarity for areas of weakness or deviation from the protocol.
Data Analysis Plan
Descriptive statistics were used to examine demographic characteristics, history of ART use at BL and participation in intervention sessions. Group means for participant characteristics at BL were compared with t-tests and Chi square tests. General linear mixed models were used to compare groups on ART adherence and readiness, clinical outcomes, and psychosocial outcomes over time. All observations from BL, PT, and 3 M assessments were included in the models. Effect sizes were interpreted using Cohen’s effect size conventions for mean differences [46] (delta: 0.25, 0.75, 1.25). All analyses were performed using SAS 9.4.
Results
Sample Characteristics
Table 1 contains descriptive characteristics for the total sample and by condition. The majority of the sample was male (Mage = 47.1). Participants were predominantly single and unemployed, and most reported receiving disability benefits. There were no differences by condition on BL characteristics.
Table 1.
Demographic characteristics of the sample at baseline
| Variable | Total (N = 34) | PATCH (n = 17) | SRSP (n = 17) |
|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |
| Age, mean (SD) | 47.1 (12.8) | 48.2 (13.2) | 46.0 (12.7) |
| n (%) | n (%) | n (%) | |
| Gender | |||
| Male | 21 (61.8%) | 10 (58.8%) | 11 (64.7%) |
| Female | 13 (38.2%) | 7 (41.2%) | 6 (35.3%) |
| Race | |||
| Black/African American | 33 (97.1%) | 17 (100.0%) | 16 (94.1%) |
| Other | 1 (2.9%) | 0 (0.0%) | 1 (5.9%) |
| Education | |||
| Grade 9–12 | 21 (70.0%) | 9 (60.0%) | 12 (80.0%) |
| Grade 12 + | 9 (30.0%) | 6 (40.0%) | 3 (20.0%) |
| Employed | |||
| Yes | 3(8.8%) | 2(11.8%) | 1(5.9%) |
| No | 31(91.2%) | 15(88.2%) | 16(94.1%) |
| Disability benefits | |||
| Yes | 22(71.0%) | 11(68.8%) | 11(73.3%) |
| No | 9(29.0%) | 5(31.3%) | |
| Marital status | |||
| Never married | 24 (70.6%) | 12 (70.6%) | 12 (70.6%) |
| Other | 10 (29.4%) | 5 (29.4%) | 5 (29.4%) |
PATCH Personal Approach to Treatment Choices for HIV, SRSP Stress Reduction Skills Program; Other marital status includes: married, divorced, separated, widowed. There were no significant differences between groups
History of ART Use
At BL, 82.4% of study participants reported currently taking ART, 26% reported difficulty taking medication all or almost all the time, and 55% reported sometimes having difficulty. Over half of the sample (55%) reported missing a dose of medication in the past week, and 58% of participants reported missing doses more than once per week. Participants reported attending an average of 1.7 (SD = 1.9) HIV clinical appointments during the 3 months prior to BL.
Feasibility and Acceptability of Intervention
Retention rates did not vary significantly by group at post-treatment or follow-up. Qualitative responses on the Program Satisfaction Interview were visually analyzed and common themes were extracted regarding participants’ perception of the PATCH intervention. A majority of participants indicated that they found PATCH appealing from the outset due to the opportunity to talk openly about difficult issues and the level of introspection that the program encouraged. For example, one participant appreciated “…the fact that [PATCH] gave me a chance to be honest with myself.” Regarding aspects of PATCH that participants found particularly helpful or meaningful, many cited the relationship with their therapist, as well as the opportunity to have someone listen to their concerns and show understanding while helping them problem-solve around specific concerns. One participant noted “It made me realize that I had some ideas already, I just had to put them into action.” Patients offered very little negative feedback about intervention, although some suggested making it a regularly available service. Additionally, when queried about the length of the intervention, most expressed satisfaction with the four-session structure, while a few others indicated that a long-term option would be preferable.
ART Use and Adherence Outcomes
Means and group comparisons for ART use and adherence outcomes at all timepoints are displayed in Table 2. There were no significant differences between conditions at PT or 3 M on adherence outcomes including, self-reported current use of ART, self-rated difficulty with taking ART, frequency of missed doses, or missing a dose in the last week. Additionally, there was no significant difference between conditions on number of visits to an HIV care provider between BL and PT or between PT and 3 M. At 3 M, there were no significant differences between conditions in the number of participants with viral load greater than 40 or on CD4 cell count.
Table 2.
ART adherence and clinical outcomes at baseline, post-treatment and follow-up assessments
| Variable | Baseline assessment | Post-treatment assessment | Follow-up assessment | |||||
|---|---|---|---|---|---|---|---|---|
| PATCH (n = 17) | SRSP (n = 17) | PATCH (n = 15) | SRSP (n = 14) | ES | PATCH (n = 14) | SRSP (n = 14) | ES | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Currently taking ART | 16 (94.1%) | 12 (70.6%) | 15 (100%) | 9 (64.3%) | 14 (100%) | 11 (78.6%) | ||
| Difficulty taking ART | 6 (35.3%) | 3 (17.6%) | 4 (26.7%) | 3 (21.4%) | 0.43 | 2 (14.3%) | 2 (14.3%) | 0.54 |
| Missed dose 1 + times per week | 11 (64.7%) | 9 (52.9%) | 2 (13.3%) | 4 (28.6%) | 0.21 | 3 (21.4%) | 4 (28.6%) | 0.50 |
| Viral load > 40 | 8 (61.5%) | 9 (69.2%) | - | - | 4 (33%) | 8 (66.7%) | 0.39 | |
| M (SD) | M (SD) | M (SD) | M (SD) | ES | M (SD) | M (SD) | ES | |
| CD4 count | 388.1 (288.4) | 325 (237.8) | - | - | 498.3 (323.2) | 377.2 (256.8) | 0.35 | |
| Clinic visits | 1.6 (1.6) | 1.8 (2.2) | 0.9 (1.2) | 0.6 (0.9) | 0.24 | 1.8 (2.2) | 2.8 (1.7) | − 0.21 |
Effect size (ES) for category variables calculated as estimated odds ratio, for continuous variables calculated as group-by-time interaction divided by raw standard deviation at baseline
Psychosocial Outcomes
Table 3 displays means for psychosocial variables at all timepoints. There was a significant group × time interaction such that PATCH participants showed greater reductions on the BSI in severity of psychiatric symptoms (t = − 2.06, p = 0.047, ES = − 0.55), greater improvements in overall perception of emotional health as measured by the Mental Health Composite Scale of the SF-12 (t = 2.13, p = 0.040, ES = 0.92), greater reductions in alcohol use (t = − 2.06, p = 0.047, ES = − 0.42), and greater reductions in emotion-focused coping on the COPE (t = − 2.33, p = 0.026, ES = − 0.77) from BL to PT compared to control participants. Effect sizes for significant differences between groups were medium; non-significant effects were generally small (see Table 3). Conditions did not differ significantly from BL to PT on positive psychiatric symptoms or distress from psychiatric symptoms on the BSI, perceptions of physical health on the SF-12 Physical Composite Scale, or the other 4 coping strategies including in the analysis. At 3 M, there was a significant group × time interaction indicating that PATCH participants reported greater improvements in overall perception of emotional health from BL to 3 M; this was a medium-sized effect (t = 2.17, p = 0.037, ES = 0.65). There were no other significant differences between conditions on changes from BL to 3 M; effect size estimates for non-significant analyses were generally small.
Table 3.
Psychosocial outcomes at baseline, post-treatment, and follow-up assessments
| Variable | Baseline assessment | Post-treatment assessment | Follow-up assessment | |||||
|---|---|---|---|---|---|---|---|---|
| PATCH (n = 17) | SRSP (n = 17) | PATCH (n = 15) | SRSP (n = 14) | ES | PATCH (n = 14) | SRSP (n = 14) | ES | |
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | ||||
| BSI global severity | 41.4 (10.1) | 46.2 (10.6) | 37.6 (8.0) | 50.3 (7.4)*** | − 0.55 | 38.22 (8.4) | 43.5 (10.2) | − 0.03 |
| BSI positive symptoms | 43.4 (11.5) | 47.5 (13.7) | 39.3 (9.3) | 51.4 (11.3) | − 0.47 | 39.8 (8.4) | 46.6 (10.6) | − 0.18 |
| BSI distress | 40.8 (9.3) | 46.3 (9.4) | 39.5 (9.7) | 50.9 (10.1) | − 0.35 | 42.2 (7.3) | 46.5 (11.3) | 0.14 |
| SF-12 physical composite | 43.9 (10.5) | 43.5 (7.9) | 42.9 (6.9) | 44.0 (9.8) | − 0.24 | 43.3 (11.8) | 46.6 (6.8) | − 0.44 |
| SF-12 mental composite | 41.9 (12.7) | 44.2 (13.3) | 53.5 (12.0) | 43.2 (13.5)*** | 0.92 | 52.8 (10.8) | 46.9 (9.6)*** | 0.65 |
| Alcohol use | 1.2 (2.5) | 5.6 (8.9) | 0.3 (0.7) | 8.4 (12.0)*** | − 0.42 | 0.3 (0.8) | 1.6 (3.6) | 0.46 |
| Drug use | 3.6 (9.9) | 0.1 (0.2) | 1.5 (4.1) | 0.4 (1.2) | − 0.36 | 0.6 (1.2) | 0.4 (0.9) | − 0.47 |
| COPE—Positive | 13.5 (2.2) | 12.4 (2.1) | 11.5 (3.2) | 12.3 (1.5) | − 0.78 | 11.9 (2.4) | 11.9 (2.9) | − 0.43 |
| COPE—Denial | 9.2 (3.1) | 9.2 (2.8) | 8.9 (2.7) | 9.4 (3.0) | − 0.14 | 8.6 (3.6) | 9.0 (2.4) | − 0.09 |
| COPE—Religious | 10.8 (2.1) | 10.9 (1.9) | 10.7 (1.9) | 11.9 (2.0) | − 0.49 | 10.6 (1.9) | 10.6 (2.3) | 0.17 |
| COPE—Emotional | 6.4 (3.0) | 6.9 (2.7) | 5.5 (1.8) | 8.8 (3.6)*** | − 0.77 | 6.0 (2.3) | 6.0 (2.1) | 0.23 |
| COPE—Acceptance | 13.1 (2.0) | 12.7 (2.6) | 13.6 (2.0) | 12.6 (2.6) | 0.30 | 12.9 (2.6) | 12.2 (2.2) | 0.18 |
Effect size (ES) calculated as group-by-time interaction divided by raw standard deviation at baseline
Significant group × time interaction indicating differences in score changes from baseline to indicated timepoint p < 0.05
Discussion
This pilot study examined the feasibility, acceptability, and preliminary efficacy of an MI-based intervention to increase ART adherence in a small sample of patients who were suboptimally engaged in care at an urban HIV treatment program. A total of 34 individuals enrolled in the trial and most completed post-intervention and follow-up assessments. Most participants were taking ART at BL but reported difficulty with adherence and frequent missed doses. Patients were highly engaged in both intervention conditions, and based on responses to the program satisfaction interview, participants valued developing a relationship with the study interventionist and the opportunity to discuss their individual mental health concerns. This degree of engagement among participants in both conditions, and the positive qualitative responses from those receiving the PATCH intervention, demonstrates that suboptimally adherent patients will engage in psychosocial interventions and perceive them as valuable. Findings that the least adherent patients report the most barriers to consistent ART use [18], illustrates the importance of implementing supports for individuals who struggle with adherence; interventions like PATCH are designed to assist patients in working through the variety of barriers they experience.
We examined differences between PATCH and an active control condition on ART adherence, clinical outcomes, and psychosocial outcomes. At post treatment and follow-up assessments, there were no differences between the PATCH and control conditions on the number of participants taking ART, frequency of missed doses, or reported difficulty with taking medication; however, the frequency of missed doses was reduced after treatment among patients in both conditions. There were also no group differences on clinical variables such as CD4 cell count and viral load at follow-up. These results are in line with the variable findings in this literature: some trials of MI-based interventions to improve ART adherence have shown improvements in CD4 count [34] and viral load [34, 35] while others have not [33, 36, 37]. Results of a recent meta-analysis [30] indicated that many types of interventions to improve ART adherence did not produce improvements in viral load compared to standard of care, while others, including cognitive-behavioral therapy and MI interventions, resulted in comparative improvements in viral load [30]. This recent meta-analysis also found that, across different types of interventions, improvements in self-reported adherence and clinical markers tended to dissipate when interventions were withdrawn. The authors concluded that individuals may need ongoing support to maintain sufficient adherence [30].
At post-treatment, PATCH participants reported fewer psychiatric symptoms, lower severity and distress related to psychiatric symptoms, improved perception of their emotional health, decreased alcohol use, and decreased emotion-focused coping relative to the control group. The magnitude of these effects was moderate but should be interpreted in light of the relatively small sample size utilized in the present study, as discussed below. These findings suggest that MI-based interventions can help individuals with HIV feel better and cope more effectively, at least during the time they are participating in the intervention. This is consistent with previous studies that have found that MI interventions can help improve psychosocial outcomes, including aiding individuals in decreasing their use of alcohol and other substances [47]. As these benefits were not found for participants in the control group who received the stress reduction skills intervention, our results suggest that the MI interventions may be particularly helpful for improving psychosocial outcomes among this population compared to other psychosocial interventions. MI involves the therapist taking a non-judgmental approach that focuses on the patient’s autonomy and individual preferences, which may have been perceived as more beneficial by participants compared to the control intervention which was primarily focused on learning specific stress-reduction skills. Many of these improvements on psychosocial outcomes were not maintained at a longer-term follow-up (i.e., once the intervention ended), suggesting that beneficial effects wane over time or must be supported by ongoing intervention to be maintained. A systematic review of MI interventions for ART adherence identified few studies reporting intervention effects on quality of life and other psychosocial outcomes, highlighting the need for future studies to consider these variables [48].
Limitations of this study include its small sample size, which reduces the generalizability of the results and may have limited the detection of significant treatment effects due to insufficient statistical power. Additional research with larger samples is needed to determine the generalizability and reproducibility of this study’s findings. Generalizability of these results may also be limited by its focus on African American individuals with HIV; the present results may not generalize to other groups. This study also relied on self-report measures of ART initiation and adherence; self-reported medication adherence may overestimate actual adherence and is vulnerable to recall bias [49]. There are other methods for measuring adherence, including some that may improve validity of assessment procedures in intervention research, such as pill counts and electronic drug monitoring methods including the Medication Event Monitoring System [49]. It is critical to utilize reliable and valid measures of adherence to see further improvements in interventions to improve ART adherence. Overall, findings of the present study suggest that implementing an MI-based intervention in an HIV clinical setting is feasible and may improve psychosocial functioning in the short-term. More research is needed to identify interventions that yield longer-term changes in these variables.
Funding
This study was funded by NIMH 5R34MH092208 (Bennett & Himelhoch, co-PIs).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.UNAIDS. Global HIV statistics. 2017. http://www.unaids.org/en/resources/documents/2018/unaids-data-2018. Accessed 23 Oct 2018.
- 2.Egger M, May M, Chêne G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. The Lancet. 2002;360(9327):119–29. [DOI] [PubMed] [Google Scholar]
- 3.Jensen-Fangel S, Pedersen L, Larsen CS, et al. Low mortality in HIV-infected patients starting highly active antiretroviral therapy: a comparison with the general population. AIDS. 2004;18(1):89–97. [DOI] [PubMed] [Google Scholar]
- 4.Pallela F, Delaney K, Moorman A, et al. Declining morbidity and mortality among HIV-infected patients with advanced HIV-infection. HIV outpatient study investigators. N Engl J Med. 1998;338(13):853–60. [DOI] [PubMed] [Google Scholar]
- 5.Mannheimer SB, Matts J, Telzak E, et al. Community programs for clinical research on AIDS. Quality of life in HIV-infected individuals receiving antiretroviral therapy is related to adherence. AIDS Care. 2005;17(1):10–22. [DOI] [PubMed] [Google Scholar]
- 6.Mannheimer S, Friedland G, Matts J, Child C, Chesney M, Beirn T. Community programs for clinical research on AIDS. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis. 2002;34(8):1115–21. [DOI] [PubMed] [Google Scholar]
- 7.Bradley H, Hall HI, Wolitski RJ, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV-United States, 2011. MMWR.2014;63(47):1113. [PMC free article] [PubMed] [Google Scholar]
- 8.Ortego C, Huedo-Medina TB, Llorca J, Sevilla L, Santos P, Rodríguez E, Warren MR, Vejo J. Adherence to highly active antiretroviral therapy (HAART): a meta-analysis. AIDS Behav. 2011;15(7):1381–96. [DOI] [PubMed] [Google Scholar]
- 9.Hsu LC, Vittinghoff E, Katz MH, Schwarcz SK. Predictors of use of highly active antiretroviral therapy (HAART) among persons with AIDS in San Francisco, 1996–1999. J Acquir Immune Defic Syndr. 2001;28:345–50. [DOI] [PubMed] [Google Scholar]
- 10.Maisels L, Steinberg J, Tobias C. An investigation of why eligible patients do not receive HAART. AIDS Patient Care STDs. 2001;15:185–91. [DOI] [PubMed] [Google Scholar]
- 11.Hoehn N, Gill MJ, Krentz HB. Understanding the delay in starting antiretroviral therapy despite recent guidelines for HIV patients retained in care. AIDS Care. 2017;29(5):564–9. [DOI] [PubMed] [Google Scholar]
- 12.Iacob SA, Iacob DG, Jugulete G. Improving the adherence to antiretroviral therapy, a difficult but essential task for a successful HIV treatment-clinical points of view and practical considerations. Front Pharmacol. 2017;8:831 10.3389/fphar.2017.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malta M, Magnanini MM, Strathdee SA, Bastos FI. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS Behav. 2010;14(4):731–47. [DOI] [PubMed] [Google Scholar]
- 14.Boussari O, Subtil F, Genolini C, et al. Impact of variability in adherence to HIV antiretroviral therapy on the immunovirological response and mortality. BMC Med Res Methodol. 2015;15:10 10.1186/1471-2288-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulett KB, Willig JH, Lin HY, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDs. 2009;23(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vrijens B, Goetghebeur E, de Klerk E, Rode R, Mayer S, Urquhart J. Modelling the association between adherence and viral load in HIV-infected patients. Stat Med. 2005;24(17):2719–31. [DOI] [PubMed] [Google Scholar]
- 17.Bangsberg DR, Mills EJ. Long-term adherence to antiretroviral therapy in resource-limited settings: a bitter pill to swallow. Antivir Ther. 2013;18(1):25–8. [DOI] [PubMed] [Google Scholar]
- 18.Walsh JC, Horne R, Dalton M, Burgess AP, Gazzard BG. Reasons for non-adherence to antiretroviral therapy: patients’ perspectives provide evidence of multiple causes. AIDS Care. 2001;13(6):709–20. [DOI] [PubMed] [Google Scholar]
- 19.Chesney MA, Ickovics J, Hecht FM, Sikipa G, Rabkin J. Adherence: a necessity for successful HIV combination therapy. AIDS. 1999;13(Suppl. A):271–8. [PubMed] [Google Scholar]
- 20.Chesney MA, Morin M, Sherr L. Adherence to HIV combination therapy. Soc Sci Med. 2000;50(11):1599–605. [DOI] [PubMed] [Google Scholar]
- 21.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43(7):939–41. [DOI] [PubMed] [Google Scholar]
- 22.Raffa JD, Tossonian HK, Grebely J, Petkau AJ, DeVlaming S, Conway B. Intermediate highly active antiretroviral therapy adherence thresholds and empirical models for the development of drug resistance mutations. J Acquir Immune Defic Syndr. 2008;47(3):397–9. [DOI] [PubMed] [Google Scholar]
- 23.Kerr T, Palepu A, Barness G, et al. Psychosocial determinants of adherence to highly active antiretroviral therapy among injection drug users in Vancouver. Antivir Ther. 2004;9(3):407–14. [PubMed] [Google Scholar]
- 24.Koenig LJ, Pals SL, Bush T, Pratt Palmore M, Stratford D, Ellerbrock TV. Randomized controlled trial of an intervention to prevent adherence failure among HIV-infected patients initiating antiretroviral therapy. Health Psychol. 2008;27(2):159. [DOI] [PubMed] [Google Scholar]
- 25.Remien RH, Hirky AE, Johnson MO, Weinhardt LS, Whittier D, Le GM. Adherence to medication treatment: a qualitative study of facilitators and barriers among a diverse sample of HIV + men and women in four US cities. AIDS Behav. 2003;7(1):61–72. [DOI] [PubMed] [Google Scholar]
- 26.Rumptz MH, Tobias C, Rajabiun S, et al. Factors associated with engaging socially marginalized HIV-positive persons in primary care. AIDS Patient Care STDs. 2007;21(S1):S–30. [DOI] [PubMed] [Google Scholar]
- 27.Gebo KA, Fleishman JA, Conviser R, Reilly ED, Korthuis PT, Moore RD, Hellinger J, Keiser P, Rubin HR, Crane L, Hellinger FJ. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. JAIDS J Acquir Immune Defic Syndr. 2005;38(1):96–103. [DOI] [PubMed] [Google Scholar]
- 28.Kleeberger CA, Phair JP, Strathdee SA, Detels R, Kingsley L, Jacobson LP. Determinants of heterogeneous adherence to HIV-antiretroviral therapies in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2001;26(1):82–92. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong K, McMurphy S, Dean LT, et al. Differences in the patterns of health care system distrust between blacks and whites. J Gen Intern Med. 2008;23(6):827–33. 10.1007/s11606-008-0561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanters S, Park JJ, Chan K, et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. Lancet HIV. 2017;4(1):e31–40. [DOI] [PubMed] [Google Scholar]
- 31.Simoni JM, Huh D, Wilson IB, Shen J, Goggin K, Reynolds NR, Remien RH, Rosen MI, Bangsberg DR, Liu H. Racial/ethnic disparities in ART adherence in the United States: findings from the MACH14 study. J Acquir Immune Defic Syndr. 2012;60(5):466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller WR, Rollnick S. Motivational interviewing-second addition. New York: Guildford; 2002. [Google Scholar]
- 33.Golin CE, Earp J, Tien HC, Stewart P, Porter C, Howie L. A 2-arm, randomized, controlled trial of a motivational interviewing-based intervention to improve adherence to antiretroviral therapy (ART) among patients failing or initiating ART. J Acquir Immune Defic Syndr. 2006;42(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46(4):443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christian P, Laurence B, Bruno S, et al. Efficacy of an educational and counseling intervention on adherence to highly active antiretroviral therapy: french prospective controlled study. HIV Clin Trials. 2003;4(2):121–31. [DOI] [PubMed] [Google Scholar]
- 36.DiIorio C, McCarty F, Resnickow K, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a randomized controlled study. AIDS Care. 2008;20:273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samet JH, Horton NJ, Meli S, et al. A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antivir Ther. 2005;10(1):83–93. [DOI] [PubMed] [Google Scholar]
- 38.van Loggerenberg F, Grant AD, Naidoo K, et al. Individualised motivational counselling to enhance adherence to antiretroviral therapy is not superior to didactic counselling in South African patients: findings of the CAPRISA 058 randomised controlled trial. AIDS Behav. 2015;19(1):145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adamian MS, Golin CE, Shain LS, DeVellis B. Brief motivational interviewing to improve adherence to antiretroviral therapy: development and qualitative pilot assessment of an intervention. AIDS Patient Care STDs. 2004;18:229–38. [DOI] [PubMed] [Google Scholar]
- 40.Duncan LG, Moskowitz JT, Neilands TB, Dilworth SE, Hecht FM, Johnson MO. Mindfulness-based stress reduction for HIV treatment side effects: a randomized, wait-list controlled trial. J Pain Symptom Manag. 2012;43(2):161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derogatis LR, Melisaratos N. The brief symptom inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 42.Ware JE Jr, Kosinski M, Keller SD. A 12-Item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;1:220–33. [DOI] [PubMed] [Google Scholar]
- 43.Salyers MP, Bosworth HB, Swanson JW, Lamb-Pagone J, Osher FC. Reliability and validity of the SF-12 health survey among people with severe mental illness. Med Care. 2000;1:1141–50. [DOI] [PubMed] [Google Scholar]
- 44.Carver CS. You want to measure coping but your protocol’too long: consider the brief cope. Int J Behav Med. 1997;4(1):92. [DOI] [PubMed] [Google Scholar]
- 45.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56(2):267. [DOI] [PubMed] [Google Scholar]
- 46.Feingold A Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychol Methods. 2009;14(1):43–53. 10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DiClemente CC, Corno CM, Graydon MM, Wiprovnick AE, Knoblach DJ. Motivational interviewing, enhancement, and brief interventions over the last decade: a review of reviews of efficacy and effectiveness. Psychol Addict Behav. 2017;31(8):862. [DOI] [PubMed] [Google Scholar]
- 48.Hill S, Kavookjian J. Motivational interviewing as a behavioral intervention to increase HAART adherence in patients who are HIV-positive: a systematic review of the literature. AIDS Care. 2012;24(5):583–92. [DOI] [PubMed] [Google Scholar]
- 49.Kreys E Measurements of medication adherence: in search of a gold standard. J Clin Pathw. 2016;2(8):43–7. [Google Scholar]


