Abstract
Parascaris spp. are major gastro-intestinal nematodes that infect foals and can lead to respiratory symptoms, poor growth, and in some cases obstruction of the small intestine and death. Ivermectin resistance has been reported for Parascaris spp. in many countries. In Poland, the knowledge of the level of resistance against ivermectin in Parascaris spp. is limited. The aim of this study was to examine the efficacy of ivermectin against Parascaris spp. in foals from south-eastern Poland. Foals (n = 225 = reared in 7 stud farms) were treated orally with ivermectin paste. Faecal samples were collected from the rectum of each foal or from the environment straight after defaecation on 1 day prior and 2 weeks after deworming. A faecal egg count (FEC) was performed using the McMaster method with a minimum detection limit of 50 eggs/g. FEC reduction (FECR) was calculated using the Faecal Egg Count Reduction Test. The statistical analysis was limited to foals excreting more than 150 eggs/g before treatment and to stud farms with at least 6 foals excreting at or above this level. Confidence intervals were determined by 1000 bootstraps at farm level and the contribution of sex and age to FECR was quantified using a generalized equation estimation procedure. Parascaris spp. eggs were found in 40% of the foals. Following ivermectin treatment, Parascaris spp. eggs were identified in 28.4% of the foals. The mean estimated FECR ranged from 44% to 97% and average efficacy was 49.3%. FECR was more pronounced in older foals (P-values = 0. 003). The FECR was more pronounced in males than in females (P value = 0.028). This study is the first to indicate a reduced efficacy of ivermectin against Parascaris spp. in foals in Poland.
Keywords: Anthelmintic resistance, Horse, Ivermectin, Nematode, Parascaris spp.
Findings
Parascaris spp. are a major threat for young horses [1]. This infection can lead to respiratory symptoms, poor growth, ill thrift accompanied by rough hair coat and bouts of diarrhoea or colic. In some cases, ascarids can lead to an obstruction and rupture of the small intestine or death of foals [2–4]. In Poland, ivermectin is the most widely used anthelmintic in equine parasite treatment [5, 6]. Drug resistance has been reported for Parascaris spp. in other countries [2, 7, 8], but this has not been investigated in Poland.
The aim of this study was to evaluate ivermectin efficacy against Parascaris spp. in foals in Poland.
The study was conducted in seven stud farms located in southern or eastern Poland from March to July 2018. The farm details are listed in Table 1. The study included 225 foals of the breeds Arabian, Malopolska, Friesian and Polish half-breed horse of both sexes and aged 3 to 6 months. The stud farms differed in terms of herd size (more than 100 horses, between 50 and 100 and, fewer than 50 horses) (Table 1) and of the management system with pastures available (farms 1, 2, 3 and 5) or not (4, 6, 7) in which case they relied on sandy paddocks with low-growing grass. In all farms, horses were dewormed using orally administered ivermectin paste 3 times a year but for this study, foals were not treated with anthelmintics prior to inclusion. The body weight was estimated visually by experienced stud workers and confirmed according to Rodríguez et al. [9]. The foals were treated orally with ivermectin paste (Paramectin® paste, ScanVet, 0.2 mg per kg of body weight + 10%). The calculated body weight was then increased up to the nearest +50 kg as the scale on the application tube is divided into 50 kg doses. For example, a foal with an estimated body weight of 163 kg was given a dose corresponding to 200 kg (163 + 16.3 = 179.3; rounded up to 200 kg). Deworming was carried out by a qualified veterinary surgeon.
Table 1.
Stud farm data and prevalence of Parascaris spp. egg excretion before and after ivermectin (IVM) treatment
| Farm | Herd size | Foals | Sex F/M | Age [3/4/5/6 months] | FEC-positive foals | EPG (min–max) | IVM efficacya | ||
|---|---|---|---|---|---|---|---|---|---|
| Pre/with EPG ≥ 150 | Post | Pre | Post | Observed FECR | |||||
| 1 | ≥ 100 | 49 | 34/15 | 6/14/10/19 | 23/22 | 20 | 471 (50–1050) | 400 (50–900) | 57.0 [36.9–74.2] |
| 2 | ≥ 100 | 40 | 26/14 | 7/10/9/14 | 28/24 | 20 | 2134 (50–13,750) | 1355 (100–5150) | 56.8 [20.3–80.3] |
| 3 | ≥ 100 | 51 | 35/16 | 4/11/17/19 | 16/15 | 16 | 572 (50–1400) | 341 (50–2050) | 44.0 [6.8–74.5] |
| 4 | 50–100 | 24 | 14/10 | 4/5/6/9 | 7/3 | 2 | 850 (50–5300) | 200 (50-350) | – |
| 5 | ≤ 50 | 16 | 9/7 | 3/0/7/6 | 12/8 | 4 | 833 (50–5550) | 325 (100-650) | 86.6 [44.8–96.4] |
| 6 | 50–100 | 34 | 0/34 | 0/10/6/18 | 6/4 | 1 | 167 (100–250) | 50 | – |
| 7 | ≤ 50 | 11 | 7/4 | 1/3/5/2 | 7/6 | 1 | 471 (50–1050) | 50 | 96.9 [92.9–100.0] |
| Total | 225 | 125/100 | 99/82 | 64 | |||||
F female, M male, FEC faecal egg count, EPG eggs per gram faeces
aObserved faecal egg count reduction (Observed FECR) with 95% confidence intervals given in square brackets
Faecal samples were collected from the rectum of each foal or from the environment straight after defaecation. Samples were collected on 1 day prior to and 2 weeks after deworming. Faecal egg counts (FEC) were done by the McMaster method (sucrose-NaCl supersaturated solution with a specific gravity of 1.25) with a minimum detection limit of 50 eggs/g [10].
Faecal egg count (FEC) reduction (FECR) was calculated by the Faecal Egg Count Reduction Test (FECRT) which is a standard method to determine anthelmintic resistance in equine cyathostomin nematodes. Although it has not been validated for Parascaris spp., it is currently the only available test for quantifying anthelmintic elimination of reproducing adult female Parascaris spp. from individual horses. We followed the guidelines of the American Association of Equine Practitioners [3] providing percent reduction thresholds for diagnosing drug resistance in strongyle populations. In general, a percent efficacy of < 95% for the macrocyclic lactones indicates resistance while a level of 95–98% is interpreted as suspected resistance. These recommendations are primarily made based on equine strongyles, but resistance evaluation for Parascaris spp. generally follows the same guidelines [11] and will have to serve until the FECRT has been validated for these.
The statistical analysis was limited to horses with an excretion level of ≥ 150 eggs per gram faeces (EPG) and stud farms with at least 6 horses shedding eggs above this threshold. FECRT 95% confidence intervals were estimated by 1000 bootstraps using the fecrtCI() function as implemented in the eggCounts package v2.1–2 [12]. To account for sex- and age-specific variation in Parascaris spp. egg excretion dynamics, bootstrapping was performed at the farm level across ages and sexes, and within farm for each age class and sex. The respective effects of these three factors on FECR were also estimated by a marginal modelling approach as previously described [13], using the geeM package v. 0.10.1 [14] assuming a negative binomial distribution for the level of EPG. Under this model, EPG counts are modelled by the sum of environmental effects (sex, age, stud farm), a binary variable coding for the treatment day (accounting for the treatment-associated change in EPG level), and their respective interactions that permits an estimation of the contribution of environmental factors to FECRs.
The detailed data concerning prevalence and EPG are presented in Table 1. Parascaris spp. eggs were found in 99 out of the 225 examined foals (40%) with a significant variation among farms ranging from 17.6 to 75%. After deworming, Parascaris spp. eggs were identified in 64 foals (28.4%). Ivermectin efficacy was estimated on farms with at least 6 foals with sufficiently high FEC (EPG ≥ 150) (Table 1). Foals from farms 4 and 6 were therefore excluded from the analyses, although a small reduction in FEC was observed in some foals after deworming.
Mean observed FECR values ranged from 44 to 97% efficacy (Table 1) and a mean observed efficacy of 71.1%. Associated confidence intervals did not span the expected 98% efficacy level in four farms, confidence interval ranged from 6.8% to 96.4%. Farm 7 was the only farm showing expected efficacy levels in single foals (> 98%). In this stable, the mean FECR was 97% and the 95% confidence interval ranged from 92.6% to 100%.
Ivermectin was least effective against Parascaris spp. infection on farms with the largest herd sizes. Drug efficacy was higher in males compared to females (relative risk of 0.49 [0.27–0.93], P-value = 0.028) (Table 2, Fig. 1), but a reduced efficacy was more common in older foals relative to 3-month-old individuals (relative risks of 3.9 [1.59–9.55] and 3.019 [1.1–8.27] and P-values of 0.003 and 0.03 for 5- and 6-month-old individuals), respectively (Table 2, Fig. 1).
Table 2.
Estimated mean Faecal Egg Count Reduction following ivermectin treatment with associated confidence intervals by stud farm, sex and age
| Study farm | 1 | 2 | 3 | 5 | 7 | By variable across stud farms | |
|---|---|---|---|---|---|---|---|
| Foals age (months) | 3 | 42.4 [− 28.63 to 77.0] | 93.2 [10.84 to 100.0] | N/A | 96.2 [95.495 to 100.0] | N/A | 72.2 [35.5 to 94.1] |
| 4 | 69.7 [27.6 to 99.6] | 82.1 [42.31 to 100.0] | 72.9 [50.0 to 100.0] | N/A | N/A | 72.3 [38.3 to 95.6] | |
| 5 | 60.0 [43.0 to 89.5] | 35.4 [14.55 to 56.5] | − 18.6 [− 45.349 to 50.0] | 85.2 [72.727 to 100.0] | 95.6 [90.476 to 100.0] | 50.80 [32.7 to 69.0] | |
| 6 | 61.4 [36.158 to 86.3] | 16.3 [0.745 to 39.7] | 49.2 [− 20.38 to 88.7] | − 62.5 [− 333.3 to 100.0] | N/A | 46.5 [22.2 to 63.5] | |
| Foals sex | Female | 50.4 [28.1 to 70.6] | 62.9 [14.2 to 87.8] | 21.7 [− 35.9 to 63.1] | 89.3 [78.6 to 100.0] | 95.5 [91.4 to 100.0] | 55.8 [36.7 to 71.8] |
| Male | 75.9 [31.0 to 100.0] | 31.8 [14.7 to 58.3] | 82.1 [67.3 to 98.3] | 84.9 [− 333.3 to 100.0] | N/A | 66.7 [40.9 to 81.9] | |
| By farm across age and sex | 57.0 [36.68 to 73.1] | 56.8 [19.61 to 79.0] | 44.0 [4.51 to 75.3] | 86.6 [45.79 to 96.6] | 96.9 [93.02 to 100.0] |
Fig. 1.
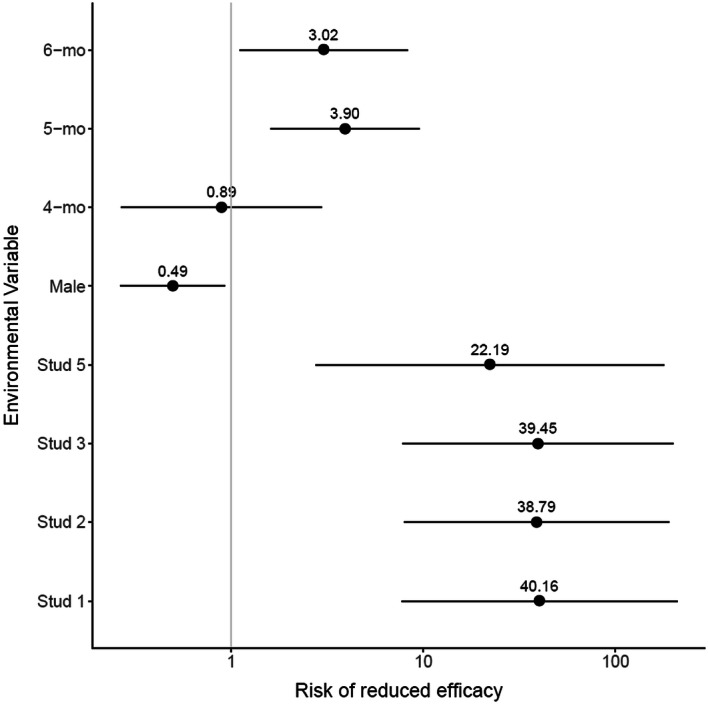
Relative risk of reduced ivermectin efficacy against Parascaris spp. was associated with sex, age and farm
Drug resistance in equine helminths has been reported across a wide range of climatic conditions and management systems [1, 5, 6, 15, 16], and limited efficacy of ivermectin against Parascaris spp. has already been described in other countries than Poland [1, 2, 8, 17–19]. This study shows for the first time that ivermectin resistance of Parascaris spp. also occurs in Poland.
The difficulty in assessing the efficacy of ivermectin is due to age of animals, their physiological behavior (coprophagia), and the biological properties of Parascaris spp. such as female fertility and resistance of eggs to environmental breakdown.
During coprophagia, foals are exposed to “eggs recycling” that can lead to 5% false-positive faecal egg counts in foals [5, 20]. The prepatent period of Parascaris spp. ranges from 70 to 110 days. Therefore, it is possible that some of the 3-month-old foals in our study were false-positives, which would explain why FECR was higher in this age group in relation to older foals. However, Parascaris spp. egg shedding usually plateaus between 4 and 5 months of age before drastic reduction [21]. This could bias FECR estimates upward in this age group, but this was not the case in our study, thereby supporting true resistance cases. Morris et al. [22] underscore the importance of a control group because Parascaris spp. FEC may be either naturally increasing or declining over the 14-day period used to conduct the FECRT. In our study, though, we were not able to create a control group as there was no possibility of isolating the foals. The main reason for this was reluctance among the owners and managers to leave the groups untreated.
The risk factors underpinning drug resistance in equine helminths are poorly characterized and the sole available estimates were quantified in strongyle populations [23, 24]. Hence it is unclear what factors are critical in the development of drug resistance in Parascaris spp. populations. Of note, Parascaris spp. populations sampled across northern Europe, North and South-America exhibited limited genetic diversity [25]. This forms a limit to maintain and select allelic variants conferring resistance to anthelmintic drugs. However, reducing the prevalence of infection would help reduce drug usage and ultimately their efficacies. Hautala et al. [16] found that Parascaris spp. infection was heavily affected by farm size and the frequency of horse movements. Horses from large breeding farms were more likely to shed Parascaris spp. eggs as found in our study. Maintaining a better environmental hygiene, e.g. faeces removal, could also contribute to a decrease in the prevalence of infections for that species [26, 27].
The genetic basis underpinning ivermectin resistance is yet to be identified. Recent work has demonstrated that the transmembrane efflux pumpP-glycoprotein-11 was associated with ivermectin resistance in Parascaris spp. [28]. This may serve as a tool for monitoring drug resistance in the field.
To limit the prevalence of drug resistance, in silico studies [29] suggested that first anthelmintic treatment should be administered only twice at the age of 2 and 5 months to allow foals to develop a sufficient immune response to Parascaris spp. while leaving enough refugia to the worm population. However, these latter results are based on simulations that have not been validated in the field to date.
The use of herbal preparations may represent a useful alternative for the management of Parascaris spp. infection. For example, extract of Artemisia dracunculus, Mentha pulegium, Zataria multiflora have potential to be used as anthelmintic for the control of ascariasis in horses [30]. This requires further research on their activity. Parascaris spp. larval culture is possible [31] but in vitro screening assays still need to be developed and validated for this species.
Acknowledgements
Not applicable.
Prior publication
Data have not been published previously.
Abbreviations
- FEC
Faecal egg count
- FECR
Faecal egg count reduction
- FECRT
Faecal egg count reduction test
Authors’ contributions
MBS planned the study and collected the samples. MBS, MRK, MDK and KSz performed the laboratory analyses and GS performed the statistical analyses. MBS, GS and KT analysed and interpreted the findings. MBS and KT coordinated the study. MBS, GS and MRK drafted the manuscript. All authors have read and approved the final manuscript.
Funding
This study was funded by University of Life Sciences in Lublin, Poland. The University was not involved in the design, collection of samples, analyses, interpretation and writing of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study did not require official or institutional ethical approval. The animals were handled according to high ethical standards and national legislation. Faecal material was collected in the farms of routine deworming. Therefore, the study did not require the consent of an ethics committee.
Consent for publication
The stud farm owners consented to collecting faecal samples from horses by a veterinarian, transferring them to the Department of Parasitology and Invasive Diseases University of Life Sciences in Lublin and using the results obtained for scientific research.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maria Bernadeta Studzińska, Email: maria.studzinska@up.lublin.pl.
Guillaume Sallé, Email: guillaume.salle@inra.fr.
Monika Roczeń-Karczmarz, Email: monika.roczen-karczmarz@up.lublin.pl.
Klaudiusz Szczepaniak, Email: k.o.szczepaniak@gmail.com.
Marta Demkowska-Kutrzepa, Email: marta.demkowska@up.lublin.pl.
Krzysztof Tomczuk, Email: krzysztof.tomczuk@up.lublin.pl.
References
- 1.Reinemeyer CR. Diagnosis and control of anthelmintic-resistant Parascaris equorum. Parasit Vectors. 2009 doi: 10.1186/1756-3305-2-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laugier C, Sevin C, Ménard S, Maillard K. Prevalence of Parascaris equorum infection in foals on French stud farms and first report of ivermectin-resistant P equorum populations in France. Vet Parasitol. 2012 doi: 10.1016/j.vetpar.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen MK. Evidence-based considerations for control of Parascaris spp. Infections in horses. Equine Vet Educ. 2016 doi: 10.1111/eve.12536. [DOI] [Google Scholar]
- 4.Tatz AJ, Segev G, Steinman A, Berlin D, Milgram J, Kelmer G. Surgical treatment for acute small intestinal obstruction caused by Parascaris equorum infection in 15 horses (2002–2011) Equine Vet J. 2012 doi: 10.1111/j.2042-3306.2012.00607.x. [DOI] [PubMed] [Google Scholar]
- 5.Kornaś S, Cabaret J, Nowosad B. Parascaris and cyathostome nematodes in foals: parasite in transit or real infection? Pol J Vet Sci. 2010 doi: 10.2478/v10181-010-0010-7. [DOI] [PubMed] [Google Scholar]
- 6.Studzińska MB, Demkowska-Kutrzepa M, Bogucki J, Roczeń-Karczmarz M, Tomczuk K. Influence of horse management systems in south-western Poland on the prevalence and intensity of gastrointestinal parasites. Med Weter. 2017 doi: 10.21521/mw.5800. [DOI] [Google Scholar]
- 7.Veronesi F, Fioretti DP, Genchi C. Are macrocyclic lactones useful drugs for the treatment of Parascaris equorum infections in foals? Vet Parasitol. 2010 doi: 10.1016/j.vetpar.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Von Samson-Himmelstjerna G, Fritzen B, Demeler J, Schürmann S, Rohn K, Schnieder T, Epe C. Cases of reduced cyathostomin egg-reappearance period and failure of Parascaris equorum egg count reduction following ivermectin treatment as well as survey on pyrantel efficacy on German horse farms. Vet Parasitol. 2007 doi: 10.1016/j.vetpar.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez C, Muñoz L, Rojas H, Briones M. New formula for bodyweight estimation of thoroughbred foals. Vet Record. 2007;161:165–166. doi: 10.1136/vr.161.5.165. [DOI] [PubMed] [Google Scholar]
- 10.Coles GC, Bauer C, Borgsteede FH, Geerts S, Klei TR, Taylor MA, Waller PJ. World Association for the Advancement of Veterinary Parasitology (W) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 1992 doi: 10.1016/0304-4017(92)90141-u. [DOI] [PubMed] [Google Scholar]
- 11.Martina F, Höglunda J, Bergströmb TF, Karlsson Lindsjöc O, Tydéna E. Resistance to pyrantel embonate and efficacy of fenbendazole in Parascaris univalens on Swedish stud farms. Vet Parasitol. 2018 doi: 10.1016/j.vetpar.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Torgerson PR, Kaplan RM, George MM, Furrer R. Modelling anthelmintic resistance by extending eggCounts package to allow individual efficacy. Int J Parasitol Drugs Drug Resist. 2018 doi: 10.1016/j.ijpddr.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker M, Churcher TS, Basanez MG. Models for measuring anthelmintic drug efficacy for parasitologists. Trends Parasitol. 2014 doi: 10.1016/j.pt.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 14.McDaniel LS, Henderson NC, Rathouz PJ. Fast pure R implementation of GEE: application of the Matrix package. R.J. 2013;1:181-7. [PMC free article] [PubMed]
- 15.Raza A, Qamar AG, Hayat K, Ashraf S, Williams AR. Anthelmintic resistance and novel control options in equine gastrointestinal nematodes. Parasitology. 2019 doi: 10.1017/S0031182018001786. [DOI] [PubMed] [Google Scholar]
- 16.Hautala K, Näreaho A, Kauppinen O, Nielsen MK, Sukura A, Rajala-Schultz PJ. Risk factors for equine intestinal parasite infections and reduced efficacy of pyrantel embonate against Parascaris sp. Vet Parasitol. 2019 doi: 10.1016/j.vetpar.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Näreaho A, Vainio K, Oksanen A. Impaired efficacy of ivermectin against Parascaris equorum, and both ivermectin and pyrantel against strongyle infections in trotter foals in Finland. Vet Parasitol. 2011 doi: 10.1016/j.vetpar.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 18.Relf VE, Lester HE, Morgan ER, Jodgkinson JE, Matthews JB. Anthelmintic efficacy on UK Thoroughbred stud farms. Int J Parasitol. 2014 doi: 10.1016/j.ijpara.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Veronesi F, Moretta I, Moretti A, Fioretti DP, Genchi C. Field effectiveness of pyrantel and failure of Parascaris equorum egg count reduction following ivermectin treatment in Italian horse farms. Vet Parasitol. 2009 doi: 10.1016/j.vetpar.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen MK, Fritzen B, Duncan JL, Guillot J, Eysker M, Dorchies P, et al. Practical aspects of equine parasite control: a review based upon a workshop discussion consensus. Equine Vet J. 2010 doi: 10.1111/j.2042-3306.2010.00065.x. [DOI] [PubMed] [Google Scholar]
- 21.Fabiani JV, Lyons ET, Nielsen MK. Dynamics of Parascaris and Strongylus spp parasites in untreated juvenile horses. Vet Parasitol. 2016 doi: 10.1016/j.vetpar.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Morris LH, Colgan S, Leathwick DM, Nielsen MK. Anthelmintic efficacy of single active and combination products against commonly occurring parasites in foals. 2019 doi: 10.1016/j.vetpar.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen MK, Branan MA, Wiedenheft AM, Digianantonio R, Scare JA, Bellaw JL, et al. Risk factors associated with strongylid egg count prevalence and abundance in the United States equine population. Vet Parasitol. 2018 doi: 10.1016/j.vetpar.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Sallé G, Cortet J, Bois I, Dubès C, Guyot-Sionest Q, Larrieu C, et al. Risk factor analysis of equine strongyle resistance to anthelmintics. Int J Parasitol Drugs Drug Resist. 2017 doi: 10.1016/j.ijpddr.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tydén E, Morrison DA, Engströma A, Nielsen MK, Eydal M, Höglund J. Population genetics of Parascaris equorum based on DNA fingerprinting. Infect Genet Evol. 2013 doi: 10.1016/j.meegid.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Gould JC, Rossano MG, Lawrence LM, Burk SV, Ennis RB, Lyons ET. The effects of windrow composting on the viability of Parascaris equorum eggs. Vet Parasitol. 2013 doi: 10.1016/j.vetpar.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Koudela B, Bodecek S. Effects of low and high temperatures on viability of Parascaris equorum eggs suspended in water. Vet Parasitol. 2006 doi: 10.1016/j.vetpar.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 28.Janssen IJ, Krücken J, Demeler J, von Samson-Himmelstjerna G. Transgenically expressed Parascaris P-glycoprotein-11 can modulate ivermectin susceptibility in Caenorhabditis elegans. Int. J Parasitol Drugs Drug Resist. 2015 doi: 10.1016/j.ijpddr.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leathwick DM, Sauermann CW, Geurden T, Nielsen MK. Managing anthelmintic resistance in Parascaris spp.: a modelling exercise. Vet Parasitol. 2017 doi: 10.1016/j.vetpar.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Rakhshandehroo E, Asadpour M, Malekpour SH, Jafari A. The anthelmintic effects of five plant extracts on the viability of Parascaris equorum larvae. Equine vet educ. 2017 doi: 10.1111/eve.12676. [DOI] [Google Scholar]
- 31.Burk SV, Dangoudoubiyam S, Brewster-Barnes T, Bryant UK, Howe DK, Carter CN, Vanzant ES, Harmon RJ, Kazacos KR, Rossano MG. In vitro culture of Parascaris equorum larvae and initial investigation of parasite excretory-secretory products. Parasitol Res. 2014 doi: 10.1007/s00436-014-4097-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


