Abstract
Background
Migraine has been recognized as one of common diseases in the world whose current treatment options are not ideal. Lasmiditan, an oral 5-hydroxytryptamine (HT)1F receptor agonist, appears more promising for the acute treatment of migraine because of considerably better effect profiles with no severe adverse events (AEs). This review aimed to systematically evaluate the efficacy and safety of lasmiditan from the results of randomized controlled trials (RCTs).
Methods
PubMed, Cochrane Library, Embase were searched on lasmiditan for the acute treatment of migraine from inception of the databases to Feb 1, 2020. Pain free and pain relief, global impression (very much/much better), and no/mild disability at 2 h in efficacy; total treatment-emergent adverse events (TEAEs), dizziness, nausea, fatigue, paraesthesia and somnolence in safety were extracted from the included studies. A systematic review and meta-analysis was performed using Review Manager Software version 5.3 (RevMan 5.3).
Results
Four RCTs with a total of 4960 subjects met our inclusion criteria. The overall effect estimate showed that lasmiditan was significantly superior to placebo in terms of pain free (RR 1.71, 95% CI 1.55–1.87), pain relief (RR 1.40, 95% CI 1.33–1.47), global impression (very much/much better) (RR 1.55, 95% CI 1.44–1.67), and no/mild disability (RR 1.15, 95% CI 1.10–1.20) at 2 h. For the safety, significant number of patients experienced TEAEs with lasmiditan than with placebo (RR 2.77, 95% CI 2.53–3.03), most TEAEs were central nervous system (CNS)-related and included dizziness (RR 5.81, 95% CI 4.72–7.14), nausea (RR 2.58, 95% CI 1.87–3.57), fatigue (RR 5.38, 95% CI 3.78–7.66), paraesthesia (RR 4.48, 95% CI 3.33–6.02), and somnolence (RR 2.82, 95% CI 2.18–3.66).
Conclusions
This meta-analysis suggests that lasmiditan is effective for the acute treatment of migraine with a higher incidence of CNS-related adverse reactions compared with placebo. Long-term, open-label, multi-dose trials are required to verify the current findings.
Keywords: Migraine, Lasmiditan, 5-HT1F receptor agonist, Meta-analysis
Background
Migraine is a common neurological disease that was ranked by the Lancet Global Burden of Disease Study as the second highest cause of disability in 328 diseases from 195 countries between 1990 and 2016, and is becoming a larger component of the global burden of disease [1]. Statistically, 45.1 million of total years lived with disability are suffered from migraine [1], which has a significant impact on quality of life and increased use of health resources [2, 3]. It is characterized by moderate-severe, unilateral, throbbing headache attacks lasting from 4 to 72 h, accompanied by additional symptoms such as nausea, vomiting, phonophobia, and/or photophobia [4]. However, the exact etiology and pathogenesis of migraine currently is unclear. Thus, to find a safety, effective and highly specific medication remains a challenge and warrants further research.
In general, the choice of acute treatment is based mainly on two classes of medicines: nonspecific (analgesics and nonsteroidal antiinflammatory drugs, NSAIDs) and specific drugs (triptans and ergot derivatives) [5]. The triptans, regarded as the gold standard in the migraine therapy, are a class of selective and effective 5-hydroxytryptamine (HT)1B/1D receptor agonists that have replaced ergot derivatives. However, 30% ~ 40% of treated patients do not respond to triptans that are also endowed with the risk of serious cardiovascular adverse events caused by vasoconstriction yielded by 5-HT1B receptor activation [6, 7]. Therefore, a new acute therapy for migraine is urgently needed, especially for those patients unable to achieve optimal outcomes with current therapies.
Lasmiditan, also known as COL-144 and LY573144, is a novel 5-HT receptor agonist with high-affinity and selectivity for the 5-HT1F receptor, which acts on the trigeminal system without causing vasoconstriction because of its low affinity for 5-HT1B receptors [8]. Representing a new class of migraine medications, lasmiditan is believed to act both centrally and peripherally, and developed as an acute therapy for migraine to address significant unmet needs in patients with cardiovascular risk factors, those with stable cardiovascular disease, or patients who respond poorly to their current treatment.
The U.S. Food and Drug Administration approved lasmiditan for the acute treatment for migraine with or without aura in adults on 11 October 2019 [9]. Data from phase II and III studies showed significant efficacy and high incidence of treatment-emergent adverse reactions (TEAEs) of this molecule versus placebo in acute treatment for migraine. However, up to now, there was no systematic review that examined the efficacy and tolerability of lasmiditan. Therefore, in this paper, we performed this systematic review and meta-analysis to evaluate the safety and efficacy of lasmiditan in the treatment of acute migraine attacks.
Methods
Literature search and inclusion criteria
Two reviewers (MH and HYX) independently searched PubMed, Cochrane Library, Embase for articles by entering “migraine” or “headache” and “lasmiditan” or “COL-144” or “LY573144” or “5-HT1F receptor agonists” as search terms. Then all articles and their reference lists were examined to expand potentially relevant articles. The bibliographic databases were searched from their respective inception to Feb 1, 2020. The articles were included in the meta-analysis if they met the following criteria: (1) included patients were adults (18–65 years of age) with migraine with or without aura which had been diagnosed according to the International Headache Society criteria (IHS) [10, 11]; (2) randomized controlled trials (RCTs) evaluating the efficacy and safety of lasmiditan for the acute treatment of migraine; (3) lasmiditan and placebo in any formulation or in any dose as treatment group and control group respectively; (4) relevant indexes of the efficacy and safety of lasmiditan were provided or could be calculated from original data in the articles. Studies were excluded when one of the following issues occurs: (1) subjects were animals; (2) interventions were drug combinations; and (3) except for RCTs, other types of trials such as cross-over designs, healthy controlled trials and self-contrast trials. Disagreement between two reviewers was settled by consensus or consultation with a third author (JHC or LC).
Quality assessment of the included studies
The methodological quality of included studies was assessed by two independent raters using Review Manager Software version 5.3 (RevMan 5.3) provided by the Cochrane Collaboration with a seven-item scale (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias) [12]. Each of the items involved assigning a judgment of high, low, or unclear risk of material bias with lower bias indicating better quality. Detailed criteria for making judgments about the risk of bias from each of the items in the tool are available in the Cochrane Handbook [13]. Any discrepancies between two reviewers were discussed and settled by consensus or consultation with a third reviewer (XFW or LC).
Statistical analysis
All extracted data syntheses were performed by RevMan 5.3 (Cochrane Collaboration, Oxford, England), and overall effects and safety of lasmiditan for the treatment of acute migraine were calculated by risk ratios (RRs) with 95% confidence intervals (CIs) with a fixed- or random-effect model. The heterogeneity analyses were conducted by using Chi-square test, I2 values smaller than 50% indicate no significant heterogeneity, and are acceptable. The fixed-effect model of analysis is then appropriate. Otherwise, the random-effect model is considered [14, 15]. In addition, representative funnel plots were not performed to detect publication bias of the meta-analysis due to the small number of RCTs.
Results
Selection and inclusion of studies
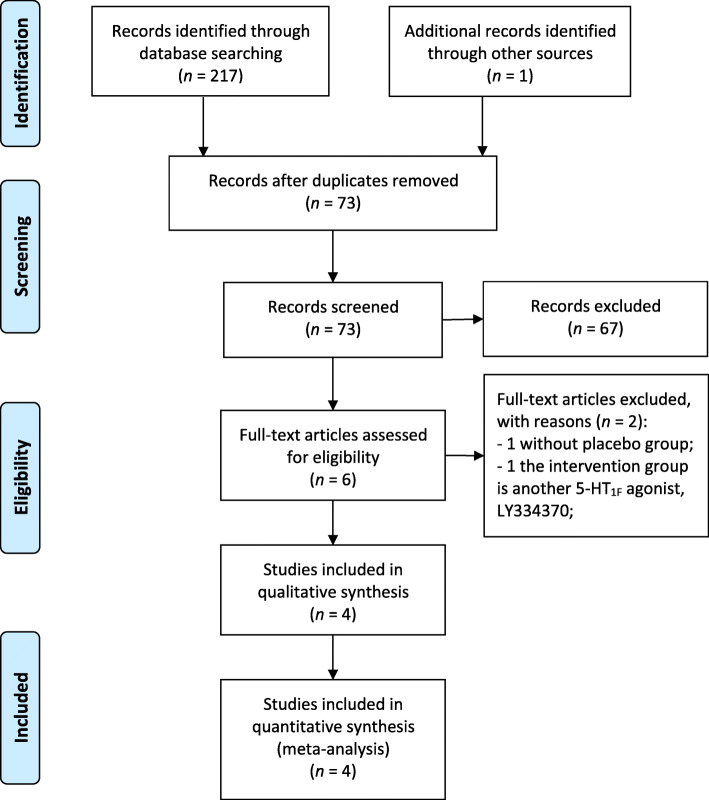
The initial search strategy retrieved 218 articles whose titles were screened for eligibility. One hundred forty-five potentially relevant studies remained after removing duplicates, then 139 reports were eliminated during abstract screening, of which full-text assessment was conducted on 6 studies. Lastly, a total of 4 RCTs involved in phase II – III (4960 participants) met the inclusion criteria and were included in this review [16–19]. A flow chart of the search strategy is shown in Fig. 1.
Fig. 1.
Process of identifying eligible studies for the meta-analysis
The baseline demographics did not differ widely among the included studies. All studies included patients with migraine classified by the IHS criteria as mentioned in the inclusion criteria. A greater percentage of subjects were female in both treatment groups: lasmiditan (84.93 ± 1.72) %, placebo (86.82 ± 2.34) %. All subjects were older than 18 years, with a mean age of 42.07 years in the lasmiditan group and 42.31 years in the placebo group. Patients had experienced a mean of 5.0 migraines per month in the lasmiditan group, and a mean of 5.1 migraines per month in the placebo group. Efficacy results were reported at primary endpoints of 2 h in placebo-controlled phase, and the safety were observed until 24 or 48 h. Details of the study characteristics were shown in Table 1.
Table 1.
Characteristics of the included studies
| Included trials | Location (s); Study design |
Eligibility criteria | Gender (male/female); mean age (years) |
Migraine attacks per month | Drug doses | Primary efficacy outcomes at 2 h | Most frequent TEAEs | ||
|---|---|---|---|---|---|---|---|---|---|
| Control | Trial | Control | Trial | ||||||
| Ferrari MD et al., 2010 [16] | Multinational; RCT | IHS 1.1 & 1.2.1 | 4/38; 40.3 | 13/75; 38.4 | 3.3 | 3.5 | 2.5–45 mg | Pain freedom, sustained pain free, other efficacy outcomes such as nausea, photophobia, phonophobia. | Dizziness, paresthesia, fatigue, sensation of heaviness, and feeling of relaxation |
| Färkkilä M et al., 2012 [17] | Multinational; RCT | IHS 1.1 & 2.1 | 11/75; 40.5 ± 10.3 | 38/267; 40.2 ± 11.0 | 3.1 ± 1.6 | 3.3 ± 1.7 | 50, 100, 200, 400 mg | Pain free, headache response, other efficacy outcomes such as nausea, photophobia, phonophobia | Dizziness, paresthesia, fatigue, nausea, vertigo and somnolence |
| Kuca B et al., 2018 [18] | USA; RCT | IHS 1.1 & 1.2.1 | 92/525; 42.4 ± 12.3 | 212/1027; 41.8 ± 11.9 | 5.1 ± 1.8 | 5.2 ± 2.1 | 100, 200 mg | Headache pain free, MBS free, other efficacy outcomes such as nausea, photophobia, phonophobia | Dizziness, paresthesia, fatigue, nausea, lethargy, and palpitations |
| Goadsby PJ et al., 2019 [19] | Multinational; RCT | IHS 1.1 & 1.2.1 |
100/545; 42.6 ± 12.9 |
309/1629; 42.7 ± 12.8 | 5.5 ± 2.4 | 5.2 ± 2.1 | 50, 100, 200 mg | Headac he pain free, MBS free, other efficacy outcomes such as nausea, photophobia, phonophobia | Dizziness, paresthesia, fatigue, nausea, lethargy and somnolence |
RCT Randomized controlled trial, IHS The International Headache Society criteria
Risk of bias and quality of the included studies
Four studies evaluating the efficacy and safety of lasmiditan for migraine were included [16–19], all of which were randomized, double-blind, placebo-controlled trials. Except that the other bias was unclear, all the reviewed trials clearly described adequate random sequence generation and allocation concealment (eg. via the Interactive Response Technology system), which were evaluated as “low” risk of bias. Blinding of participants, investigators, and outcome assessors was considered adequate in all studies. Therefore, blinding of participants and personnel, and blinding of outcome assessment in all trials were classified as having a low risk of bias. Furthermore, all studies had a low risk of incomplete outcome data and selective reporting because they provided the conclusions in detail. Using the 7-item criteria in RevMan 5.3, the assessment on risk of bias between both reviewers showed an overall agreement. As presented in Fig. 2, all trials identified as low risk of bias and high-quality assessment material.
Fig. 2.
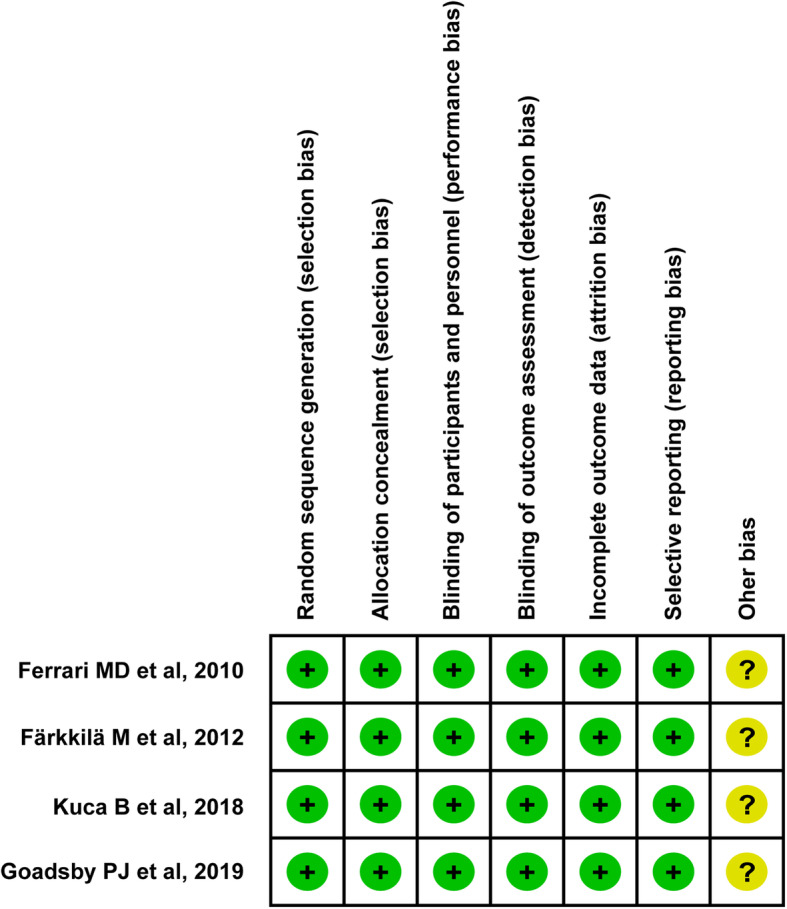
Risk of bias for included trials
Effectiveness of lasmiditan for the acute treatment of migraine
Pain free and pain relief
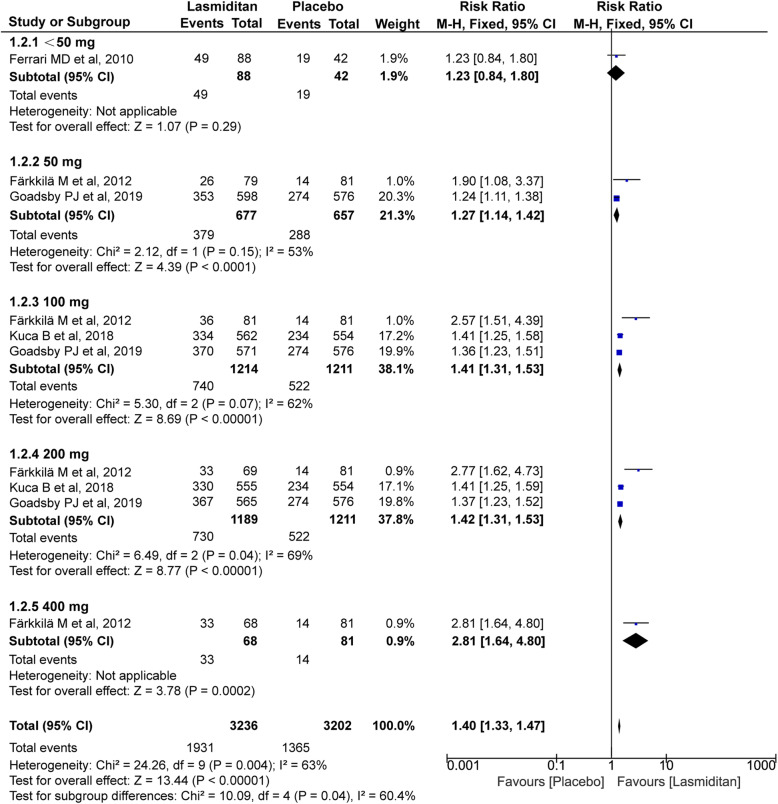
All four trials (4209 and 4489 subjects, respectively) included in this meta-analysis were evaluated for the pain free and pain relief at 2 h. As shown in Fig. 3 and Fig. 4, the significantly higher percentage of recipients treated with lasmiditan achieved pain free and pain relief after treatment compared with placebo (pain free: RR 1.71, 95% CI 1.55–1.87, P<0.00001; pain relief: RR 1.40, 95% CI 1.33–1.47, P<0.00001). Notably, there were dose-related improvements for patients who reported the pain free and pain relief across the lasmiditan treatment groups (pain free:<50 mg RR 1.19[0.57, 2.48], 50 mg RR 1.37[1.12, 1.68], 100 mg RR 1.63[1.40, 1.91], 200 mg RR 1.96[1.69, 2.27], 400 mg RR 3.77[1.60, 8.91]; pain relief:<50 mg RR 1.23[0.84, 1.80], 50 mg RR 1.27[1.14, 1.42], 100 mg RR 1.41[1.31, 1.53], 200 mg RR 1.42[1.31, 1.53], 400 mg RR 2.81[1.64, 4.80]). The I2 value (χ2 = 15.96, P = 0.07, I2 = 44%) on pain free and pain relief revealed non-significant heterogeneity among the included trials. However, there was some heterogeneity on pain relief (χ2 = 24.26, P = 0.04, I2 = 63%), which could result from the difference of evaluation criteria. Heterogeneity was best resolved by excluding the study by Färkkilä M et al (χ2 = 3.55, P = 0.62, I2 = 0%) [17].
Fig. 3.
Meta-analysis of the pain free at 2 h after therapy with lasmiditan compared with placebo. The diamond indicates the estimated relative risk with 95% confidence interval for the pooled patients. M-H, Mantel-Haenszel; CI, confidence interval
Fig. 4.
Meta-analysis of the pain relief at 2 h after therapy with lasmiditan compared with placebo. The diamond indicates the estimated relative risk with 95% confidence interval for the pooled patients. M-H, Mantel-Haenszel; CI, confidence interval
Global impression: very much/much better
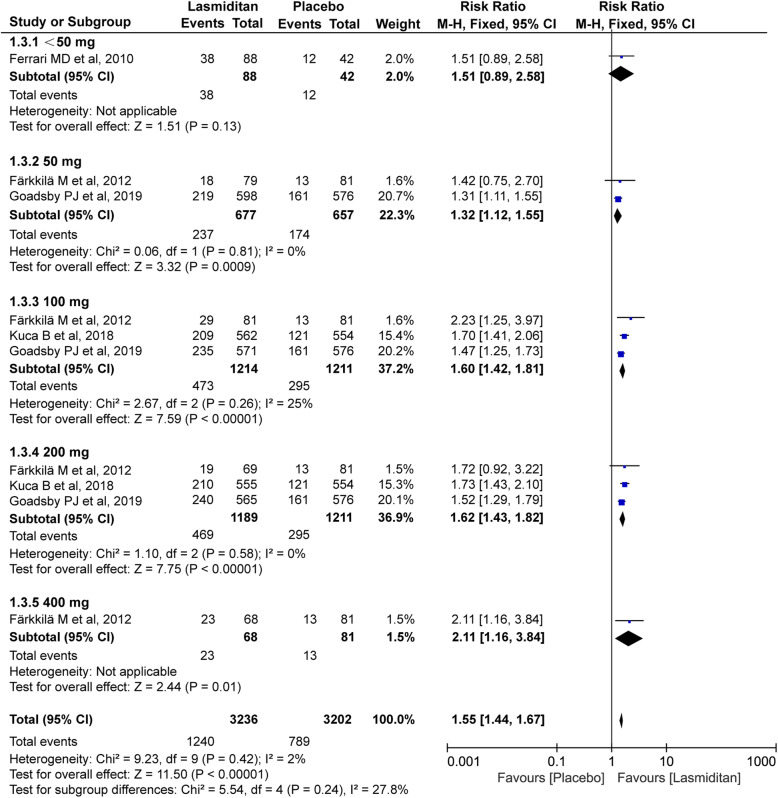
All four trials (4489 subjects) included in this meta-analysis were evaluated for the global impression (very much/much better) at 2 h. The overall RR after treatment favored lasmiditan over placebo (RR 1.55, 95% CI 1.44–1.67, P<0.00001, Fig. 5), which also had some dose-effect relation (<50 mg: RR 1.51[0.89, 2.58], 50 mg: RR 1.32[1.12, 1.55], 100 mg: RR 1.60[1.42, 1.81], 200 mg: RR 1.62[1.43, 1.82], 400 mg: RR 2.11[1.16, 3.84]). Furthermore, the I2 value (χ2 = 9.23, P = 0.42, I2 = 2%) on the global impression revealed a non-significant heterogeneity among the included trials.
Fig. 5.
Meta-analysis of the global impression (very much/much better) at 2 h after therapy with lasmiditan compared with placebo. The diamond indicates the estimated relative risk with 95% confidence interval for the pooled patients. M-H, Mantel-Haenszel; CI, confidence interval
No/mild disability
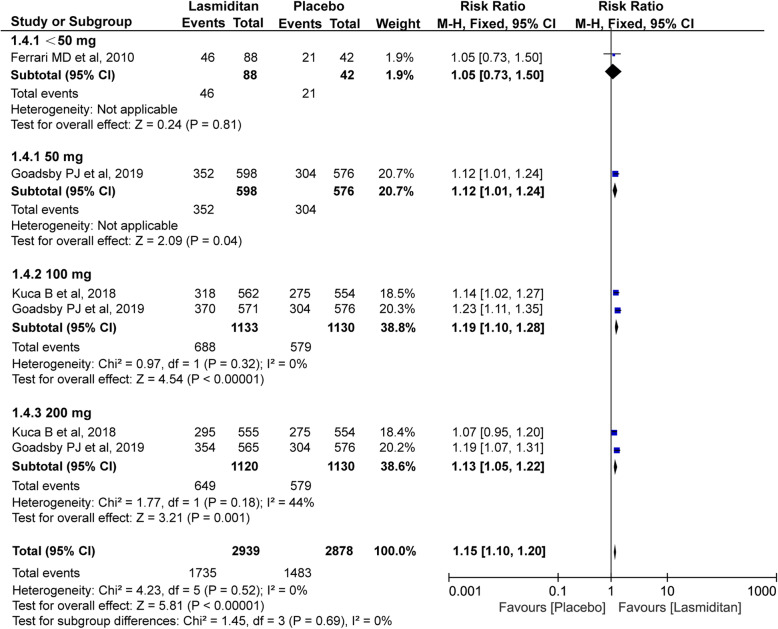
Three trials with a total of 4111 subjects included in this meta-analysis were evaluated for the no/mild disability at 2 h. As showed in Fig. 6, lasmiditan also showed benefits over placebo at 2 h in terms of the proportion of the no/mild disability patients (RR 1.15, 95% CI 1.10–1.20, P<0.00001). The I2 value (χ2 = 4.23, P = 0.52, I2 = 0%) on the no/mild disability revealed a non-significant heterogeneity among the included trials.
Fig. 6.
Meta-analysis of the disability (no/mild) at 2 h after therapy with lasmiditan compared with placebo. The diamond indicates the estimated relative risk with 95% confidence interval for the pooled patients. M-H, Mantel-Haenszel; CI, confidence interval
Safety of lasmiditan for the acute treatment of migraine
Total TEAEs
After the first dose for 24 or 48 h, more TEAEs were reported in the lasmiditan group than in the placebo group, with a statistically significant risk ratio of 2.77 (95% CI 2.53–3.03, P<0.00001). Total TEAEs rate of all subgroup also had proved this dose-response relationship for the treatment of migraine (50 mg: RR 2.33[1.88, 2.89], 100 mg: RR 2.66[2.30, 3.07], 200: mg RR: 3.01[2.61, 3.48], 400 mg: RR 3.82[2.53, 5.75]) (See Additional file 1: Figure S1). Statistical heterogeneity was significant (χ2 = 17.33, P = 0.03, I2 = 54%), which was improved when the study by Kuca B et al was removed (χ2 = 10.17, P = 0.12, I2 = 41%) [18].
Main TEAEs
The most frequently reported TEAEs in migraine with lasmiditan were associated with the CNS, which included dizziness, nausea, fatigue, paraesthesia and somnolence. As shown in Table 2, there were obvious differences between lasmiditan and placebo group in these TEAEs (dizziness: RR 5.81, 95% CI 4.72–7.14, P<0.00001 (See Additional file 1: Figure S2); nausea: RR 2.58, 95% CI 1.87–3.57, P<0.00001 (See Additional file 1: Figure S3); fatigue: RR 5.38, 95% CI 3.78–7.66, P<0.00001 (See Additional file 1: Figure S4); paraesthesia: RR 4.48, 95% CI 3.33–6.02, P<0.00001 (See Additional file 1: Figure S5); somnolence: RR 2.82, 95% CI 2.18–3.66, P<0.00001 (See Additional file 1: Figure S6)). Furthermore, the increased risk appeared to be mostly dose-related. Majority I2 value revealed a non-significant heterogeneity among the included studies except dizziness (χ2 = 7.69, P = 0.10, I2 = 48%), which was resolved by excluding the studies by Ferrari MD et al [16] and Färkkilä M et al [17].
Table 2.
Comparison of main TEAEs between different doses of lasmiditan and placebo
| Outcome or Subgroup | Studies | Participants | Risk Ratio (M-H, Fixed, 95% CI) | P | I2 |
|---|---|---|---|---|---|
| Dizziness | 4 | 7125 | 5.81 [4.72, 7.14] | <0.00001 | 67% |
| <50 mg | 1 | 130 | 1.75 [0.77, 3.99] | 0.18 | / |
| 50 mg | 2 | 1467 | 4.55 [2.70, 7.67] | <0.00001 | 70% |
| 100 mg | 3 | 2695 | 5.75 [4.10, 8.06] | <0.00001 | 69% |
| 200 mg | 3 | 2677 | 6.59 [4.72, 9.21] | <0.00001 | 57% |
| 400 mg | 1 | 156 | 64.94 [4.03, 1047.06] | 0.003 | / |
| Nausea | 3 | 6995 | 2.58 [1.87, 3.57] | <0.00001 | 0% |
| 50 mg | 2 | 1467 | 2.63 [1.20, 5.75] | 0.45 | 0% |
| 100 mg | 3 | 2695 | 2.37 [1.42, 3.94] | 0.0009 | 41% |
| 200 mg | 3 | 2677 | 2.54 [1.54, 4.21] | 0.0003 | 0% |
| 400 mg | 1 | 156 | 13.48 [0.76, 239.65] | 0.08 | / |
| Fatigue | 4 | 7125 | 5.38 [3.78, 7.66] | <0.00001 | 29% |
| <50 mg | 1 | 130 | 1.19 [0.40, 3.58] | 0.75 | / |
| 50 mg | 2 | 1467 | 3.52 [1.62, 7.64] | 0.001 | 0% |
| 100 mg | 3 | 2695 | 6.99 [3.62, 13.48] | <0.00001 | 0% |
| 200 mg | 3 | 2677 | 6.77 [3.51, 13.07] | <0.00001 | 0% |
| 400 mg | 1 | 156 | 9.83 [2.34, 41.31] | 0.002 | / |
| Paraesthesia | 4 | 7125 | 4.48 [3.33, 6.02] | <0.00001 | 13% |
| <50 mg | 1 | 130 | 20.78 [1.29, 334.92] | 0.03 | / |
| 50 mg | 2 | 1467 | 2.24 [0.98, 5.14] | 0.06 | 0% |
| 100 mg | 3 | 2695 | 3.90 [2.43, 6.26] | <0.00001 | 21% |
| 200 mg | 3 | 2677 | 5.03 [3.17, 7.99] | <0.00001 | 0% |
| 400 mg | 1 | 156 | 8.60 [2.02, 36.58] | 0.004 | / |
| Somnolence | 3 | 6995 | 2.82 [2.18, 3.66] | <0.00001 | 0% |
| 50 mg | 2 | 1467 | 2.86 [1.60, 5.09] | 0.04 | 0% |
| 100 mg | 3 | 2695 | 2.59 [1.70, 3.95] | <0.00001 | 0% |
| 200 mg | 3 | 2677 | 2.92 [1.92, 4.42] | <0.00001 | 0% |
| 400 mg | 1 | 156 | 4.91 [1.08, 22.40] | 0.0004 | / |
Discussion
With the growing knowledge of the pathogenesis on migraine, the expression of 5-HT1F receptor mRNA in neurons of the trigeminal ganglia led to the suggestion that 5-HT1F receptors could be a therapeutic target for migraine [20]. As expected, it became the potential new class of anti-migraine therapy with no vascular activity and the related issues on the vascular and neuronal aspects of migraine pathogenesis. So far two selective 5-HT1F agonists, LY334370 and lasmiditan, have been studied in clinical trials for the acute treatment of migraine. LY334370 was efficient with a much higher rate of asthenia, dizziness, somnolence, and parestesia than placebo for attenuating migraine attacks through selective trigeminovascular neuronal inhibition [21]. Unfortunately, the LY334370 project withdrew because of toxicity in animals [22]. Admittedly, the efficacy of LY334370 and lasmiditan also proved that vasoconstriction was not essential for anti-migraine therapy.
The U.S. FDA approval was based on positive results from two pivotal phase III trials (SAMURAI and SPARTAN), in which lasmiditan signifcantly improved the proportions of patients achieving freedom from headache pain and freedom from the most bothersome symptoms (photophobia, phonophobia or nausea) compared with placebo [9]. The current study is the first meta-analysis, to the best of our knowledge, to evaluate the efficacy and safety of lasmiditan for the treatment of acute migraine attacks. The results suggested the use of lasmiditan (daily doses from ≤50 mg to 400 mg) for patients who had at least a 1-year history of disabling migraine with or without aura was associated with significantly more pain freedom and pain relief at 2 h. Furthermore, lasmiditan also showed benefits over placebo at 2 h in terms of the proportion of patients in global impression of change ratings and disability level ratings. The findings of this systematic review confirmed that lasmiditan was superior to placebo in relieving migraine, however, as feared earlier, there was some concern about the relatively high incidence of CNS-related AEs (especially dizziness, nausea, and fatigue) as the published reviews discussing by Peer C et al [23] and David K et al [24]. The CNS-related AEs were reported in all included studies, and remarkably increased with increasing doses compared with placebo. Most adverse events affected the CNS probably due to the drugs lipophilic structure which leads to high permeability through the blood brain barrier [25], which prompted that the future development of 5-HT1F agonists could give more attention to the safety profile.
For the long-term efficacy and safety of lasmiditan, a phase III GLADIATOR study involved patients who had completed SPARTAN or SAMURAI [26], and received lasmiditan 100 mg or 200 mg to be used as their frst treatment (within 4 h of pain onset) for every new migraine attack with moderate to severe pain. The interim safety and efficacy results were consistent with the previous researches, which showed a benefit of lasmiditan for reducing both the headache pain and most bothersome symptoms of migraine attacks. It is interesting to note that TEAEs over time generally showed a decrease in the incidence of these events with subsequent treated migraine attacks, and no new serious safety findings were observed, with no deaths occurring and no other trends with regard to serious AEs reported during treatment with lasmiditan for up to 1 year. Despite the most frequently reported TEAEs were associated with the CNS, there were no serious accidents or injuries resulting from a CNS-related AEs during long-term intermittent treatment. Further research should be needed to support these results, and verify the efficacy and safety of lasmiditan.
Compared to previous studies [27, 28] aimed to summarize the evidence on lasmiditan for the acute treatment of migraine, this study provided a systematic and more detailed assessment on the efficacy and safety of lasmiditan. Indeed, this first meta-analysis covered a greater number of studies and larger sample size to obtain more precise estimates on the efficacy and safety. The results showed some new valuable information about lasmiditan. First, we proved that lasmiditan (daily doses from ≤50 mg to 400 mg) was effective for the acute treatment migraine with some dose-effect relationship. Then, we analyzed the safety profile of lasmiditan by comparing TEAEs across different doses, which appeared to be mostly dose-related in the increased risk. These more detailed findings will provide some references for clinical application of lasmiditan, specially for the subpopulation of patients with relative risk factors and/or disease.
Limitations
While this review was systematic and comprehensive, several limitations should be taken into account. First, although a total of 4960 participants were included in our meta-analysis, it was based on only four RCTs. That funnel plots were not performed to detect publication bias of the meta-analysis due to the small number of RCTs. However, all of these four trials were multicenter and high-quality RCTs. Second, the definitional standard of some efficacy and safety indicators were various and resulted in some heterogeneity in this meta-analysis, such as headache pain relief when defined as a reduction of moderate or severe pain to mild or no pain in Färkkilä M et al study [17], however, also included a reduction in headache severity from mild at baseline to none in Kuca B et al and Goadsby PJ et al trials [18, 19]. Third, this meta-analysis only focused on the short-term pain responses and side effects after a single dose during clinical trials and neglected the long-term efficacy and safety due to the limited data. The long-term efficacy and safety of lasmiditan remains unknown and needs to be validated following continued dosing. Furthermore, the safety evaluation period was not completely consistent in our included studies, ranging from 24 h to 48 h, which might contribute to heterogeneity. Fourth, another interesting aspect is the efficacy and tolerability of lasmiditan in patients with cardiovascular contraindications to triptans. However, the subgroup analysis was not performed due to the limited number of patients with pre-existing cardiovascular conditions in these included studies.
Conclusions
This meta-analysis suggested that lasmiditan are effective for the acute treatment of migraine, however, with a higher incidence of CNS-related adverse reactions compared with placebo. It is critical to weigh the benefits against the risk of AEs in clinical application of lasmiditan. More long-term, open-label, multi-dose trials with larger sample sizes are needed before a definitive conclusion about the efficacy and safety of lasmiditan for migraine in the future.
Supplementary information
Additional file 1: Meta-analysis of the total TEAEs and main AEs after therapy with lasmiditan compared with placebo. Figure S1, total TEAEs; Figure S2, dizziness; Figure S3, nausea; Figure S4, fatigue; Figure S5, paraesthesia; Figure S6, somnolence. The diamond indicates the estimated relative risk with 95% confidence interval for the pooled patients. M-H, Mantel-Haenszel; CI, confidence interval.
Acknowledgments
The authors thank Dr. Ji Ming Wang (Cancer and Inflammation Program, Center for Cancer Research, National Cancer Institute at Frederick, Frederick, MD 21702, USA) for reviewing the manuscript.
Abbreviations
- 5-HT
5-hydroxytryptamine
- CNS
Central nervous system
- IHS
The International Headache Society criteria
- RCTs
Randomized clinical trials
- RR
Relative risk
- CI
Confidence intervals
- M-H
Mantel-Haenszel
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- TEAEs
Treatment-emergent adverse events
Authors’ contributions
JHC and MH conceived and designed this study. MH and HYX carried out the searches, identified studies for inclusion and extracted relevant data. CL, XFW, DMD, JL and PZ revised the manuscript for intellectual content and provided essential comments to finalize the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the grants from National Major Science and Technology Projects of China (No. 2018ZX09J18109–005), and research project of Third Military Medical University (No. 2015XZH19) and Chongqing (No. CSTC2015jcyjBX0018).
Availability of data and materials
All data generated or analyzed data in study are included in this article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s10194-020-01138-x.
References
- 1.GBD Disease and injury incidence and prevalence collaborators (2017) global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2016;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saylor D, Steiner T. The global burden of headache. Semin Neurol. 2018;38(02):182–190. doi: 10.1055/s-0038-1647245. [DOI] [PubMed] [Google Scholar]
- 3.Steiner TJ, Birbeck GL, Jensen RH, Katsarava Z, Stovner LJ, Martelletti P. Headache disorders are third cause of disability worldwide. J Headache Pain. 2015;16(1):58. doi: 10.1186/s10194-015-0544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capi M, de Andrés F, Lionetto L, Gentile G, Cipolla F, Negro A, et al. Lasmiditan for the treatment of migraine. Expert Opin Investig Drugs. 2017;26(2):227–234. doi: 10.1080/13543784.2017.1280457. [DOI] [PubMed] [Google Scholar]
- 5.Antonaci F, Ghiotto N, Wu S, Pucci E, Costa A. Recent advances in migraine therapy. Springerplus. 2016;5(1):637. doi: 10.1186/s40064-016-2211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheftell F, Almas M, Weeks R, Mathew NT, Pitman V, Lipton RB. Quantifying the return of headache in triptan-treated migraineurs: an observational study. Cephalalgia. 2010;30(7):838–846. doi: 10.1177/0333102409354390. [DOI] [PubMed] [Google Scholar]
- 7.Dodick DW. Triptan nonresponder studies: implications for clinical practice. Headache. 2005;45(2):156–162. doi: 10.1111/j.1526-4610.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 8.Nelson DL, Phebus LA, Johnson KW, Wainscott DB, Cohen ML, Calligaro DO, et al. Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia. 2010;30(10):1159–1169. doi: 10.1177/0333102410370873. [DOI] [PubMed] [Google Scholar]
- 9.Lamb YN. Lasmiditan: first approval. Drugs. 2019;79(18):1989–1996. doi: 10.1007/s40265-019-01225-7. [DOI] [PubMed] [Google Scholar]
- 10.Headache Classification Subcommittee of the International Headache Society The international classification of headache disorders (second edition) Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 11.Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders, 3rd edition (beta version) Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Altman DG (2008) Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Wiley.
- 14.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid-Based Healthc. 2015;13(3):196–207. doi: 10.1097/XEB.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari MD, Färkkilä M, Reuter U, Pilgrim A, Davis C, Krauss M, et al. Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan – a randomised proof-of-concept trial. Cephalalgia. 2010;30(10):1170–1178. doi: 10.1177/0333102410375512. [DOI] [PubMed] [Google Scholar]
- 17.Färkkilä M, Diener HC, Géraud G, Láinez M, Schoenen J, Harner N, et al. Efficacy and tolerability of lasmiditan, an oral 5-HT1F receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11(5):405–413. doi: 10.1016/S1474-4422(12)70047-9. [DOI] [PubMed] [Google Scholar]
- 18.Kuca B, Silberstein SD, Wietecha L, Berg PH, Dozier G, Lipton RB. Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology. 2018;91(24):e2222–e2232. doi: 10.1212/WNL.0000000000006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goadsby PJ, Wietecha LA, Dennehy EB, Kuca B, Case MG, Aurora SK, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. 2019;142(7):1894–1904. doi: 10.1093/brain/awz134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adham N, Bard JA, Zgombick JM, Durkin MM, Kucharewicz S, Weinshank RL, et al. Cloning and characterization of the Guinea pig 5-HT1F receptor subtype: a comparison of the pharmacological profile to the human species homolog. Neuropharmacology. 1997;36(4–5):569–576. doi: 10.1016/S0028-3908(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein DJ, Roon KI, Offen WW, Ramadan NM, Ferrari MD. Selective seratonin 1F (5-HT1F) receptor agonist LY334370 for acute migraine: a randomised controlled trial. Lancet. 2001;358(9289):1230–1234. doi: 10.1016/S0140-6736(01)06347-4. [DOI] [PubMed] [Google Scholar]
- 22.Ramadan NM. Acute treatments: some blind alleys. Curr Med Res Opin. 2001;17(Suppl 1):s71–s80. doi: 10.1185/0300799039117001. [DOI] [PubMed] [Google Scholar]
- 23.Tfelt-Hansen PC, Olesen J. The 5-HT1Freceptor agonist lasmiditan as a potential treatment of migraine attacks: a review of two placebo-controlled phase II trials. J Headache Pain. 2012;13(4):271–275. doi: 10.1007/s10194-012-0428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudrow D, Krege JH, Hundemer HP, Berg PH, Khanna R, Ossipov MH, et al. Issues impacting adverse event frequency and severity: differences between randomized phase 2 and phase 3 clinical trials for Lasmiditan. Headache. 2020;60(1):1–13. doi: 10.1111/j.1526-4610.1981.hed2101001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raffaelli B, Israel H, Neeb L, Reuter U. The safety and efficacy of the 5-HT 1F receptor agonist lasmiditan in the acute treatment of migraine. Expert Opin Pharmacother. 2017;18(13):1744–7666. doi: 10.1080/14656566.2017.1361406. [DOI] [PubMed] [Google Scholar]
- 26.Brandes JL, Klise S, Krege JH, Case M, Khanna R, Vasudeva R, et al. Interim results of a prospective, randomized, open-label, phase 3 study of the long-term safety and efficacy of lasmiditan for acute treatment of migraine (the GLADIATOR study) Cephalalgia. 2019;39(11):1343–1357. doi: 10.1177/0333102419864132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knievel K, Buchanan AS, Lombard L, Baygani S, Raskin J, Krege JH, et al. Lasmiditan for the acute treatment of migraine: subgroup analyses by prior response to triptans. Cephalalgia. 2020;40(1):19–27. doi: 10.1177/0333102419889350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krege JH, Rizzoli PB, Liffick E, Doty EG, Dowsett SA, Wang J, et al. Safety findings from phase 3 lasmiditan studies for acute treatment of migraine: results from SAMURAI and SPARTAN. Cephalalgia. 2019;39(8):957–966. doi: 10.1177/0333102419855080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Meta-analysis of the total TEAEs and main AEs after therapy with lasmiditan compared with placebo. Figure S1, total TEAEs; Figure S2, dizziness; Figure S3, nausea; Figure S4, fatigue; Figure S5, paraesthesia; Figure S6, somnolence. The diamond indicates the estimated relative risk with 95% confidence interval for the pooled patients. M-H, Mantel-Haenszel; CI, confidence interval.
Data Availability Statement
All data generated or analyzed data in study are included in this article.