Abstract
Background
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is increasingly used in patients with critical cardiopulmonary failure. To investigate the association between hospital VA-ECMO procedure volume and outcomes in a large, nationwide registry.
Methods
By using administrative data from the German Federal Health Monitoring System, we analyzed all VA-ECMO procedures performed in Germany from 2013 to 2016 regarding the association of procedural volumes with outcomes and complications.
Results
During the study period, 10,207 VA-ECMO procedures were performed; mean age was 61 years, 43.4% had prior CPR, and 71.2% were male patients. Acute coronary syndrome was the primary diagnosis for VA-ECMO implantation (n = 6202, 60.8%). The majority of implantations (n = 5421) were performed at hospitals in the lowest volume category (≤ 50 implantations per year).
There was a significant association between annualized volume of VA-ECMO procedures and 30-day in-hospital mortality for centers with lower vs. higher volume per year. Multivariable logistic regression showed an increased 30-day in-hospital mortality at hospitals with the lowest volume category (adjusted odds ratio 1.13, 95% confidence interval [CI] 1.01–1.27, p = 0.034).
Similarly, higher likelihood for complications was observed at hospitals with lower vs. higher annual VA-ECMO volume (adjusted odds ratio 1.46, 95% CI 1.29–1.66, p = 0.001).
Conclusions
In this analysis of more than 10,000 VA-ECMO procedures for cardiogenic shock, the majority of implantations were performed at hospitals with the lowest annual volume. Thirty-day in-hospital mortality and likelihood for complications were higher at hospitals with the lowest annual VA-ECMO volume.
Keywords: Venoarterial extracorporeal membrane oxygenation, VA-ECMO, Procedure volume, Outcomes, Complications
Introduction
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is increasingly used for treatment of patients with critical cardiopulmonary failure and as a rescue therapy to stabilize critically ill patients with circulatory compromise [1, 2]. In the last decade, VA-ECMO therapy has seen a rapid increase in the western world, although in-hospital mortality rates remained at a high level [3, 4]. We have recently reported that utilization of VA-ECMO for cardiopulmonary support increased by more than 30-fold in Germany between 2007 and 2015, while in-hospital mortality remained unchanged around 60% [5].
As a result of the rapid increase in VA-ECMO implantations, the number of implanting centers has also increased. To what extent an increase of the VA-ECMO implanting hospitals and their procedure volume of VA-ECMO implantations has a role in terms of outcome remains unclear. Several studies have shown that higher hospital procedure volume is associated with improved outcomes in interventional procedures such as transcatheter aortic-valve replacement [6], percutaneous mitral valve repair [7], percutaneous coronary intervention [8], and coronary artery bypass grafting [9]. It is speculated that this observation is the expression of a learning curve for interventional procedures. Whether this might be translated to VA-ECMO implantation is currently unknown.
Importantly, not only mortality, but also incident complication would be an outcome of interest, as 50% of all VA-ECMO patients experience therapy-limiting complications such as major bleeding, stroke, limb and abdominal ischemia, thrombosis, and infection [10–12]. Nevertheless, whether treatment of these complications in higher volume centers affects outcome is unknown.
The aim of this study was to investigate the association between hospital VA-ECMO procedure volume and outcomes in a large, nationwide registry. Findings regarding volume-mortality association could be of importance to inform the complex decision-making processes when considering initiation of VA-ECMO therapy for cardiogenic shock.
Methods
Data source and study population
In Germany, administrative data on characteristics and outcomes of all in-patients treated in German hospitals are obligatorily and routinely collected and reported to the Federal Statistical Office. Completely anonymized patient-level data are centrally stored and managed by the Research Data Center of the Federal Bureau of Statistics (Wiesbaden, Germany).
For the present analyses, all cases treated with VA-ECMO between 2013 and 2016 were identified and selected by the primary operation and procedural (OPS) code “VA-ECMO” (OPS code 8852.3) during index-hospitalization. The study population was divided into three categories of annualized hospital procedure volume (≤ 50 procedures, > 50–100 procedures, and > 100 procedures).
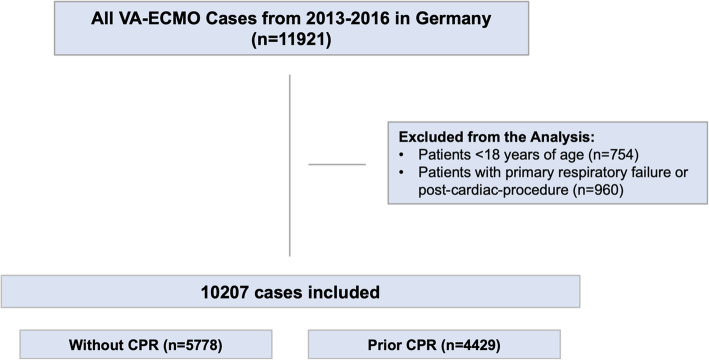
To obtain data on coexisting conditions, outcomes, and complications, we used the available German modification of international statistical classification of diseases and related health problems (ICD-10-GM) and OPS codes (Table I, II, and III in the online-only Data Supplement). Individuals younger than 18 years of age and patients with primary respiratory failure or post-cardiac-procedure as leading indication for VA-ECMO were excluded (Fig. 1 and Table II in the online-only Data Supplement). For subgroup analysis, patients with prior cardiopulmonary resuscitation (CPR) were identified and selected by OPS code 8871. The investigators did not have direct access to the raw data but were allowed to submit statistical scripts to the Research Data Center which performed the analyses and returned summarized statistical reports.
Fig. 1.
Flow chart of the study population
Indications for VA-ECMO utilization
To characterize the primary indication for VA-ECMO utilization during index-hospitalization, patients were allocated into one of three mutually exclusive categories based on suggestions of recent consensus guidelines [13]: complications post heart transplantation (abbreviated as heart transplantation), acute coronary syndrome, and acute heart failure (Table II in the online-only Data Supplement). The assignment of indication categories was hierarchical if more than one of the three indications was formally present. For example, a patient was only considered for the acute heart failure category if he did not undergo heart transplantation and did not suffer from acute coronary syndrome in parallel. To avoid categorization based on diagnoses that developed as complications of VA-ECMO placement, only diagnosis codes present at the time of admission were considered.
Statistical analysis
All individuals were divided into three hospital procedure volume groups. Binary variables were shown as absolute numbers and percentages, whereas continuous variables were shown as mean ± standard deviation (SD). For between-group comparisons, a one-way ANOVA test was used for continuous variables and the χ2 test for binary variables. For pairwise comparisons, adjusted p values by Holm were computed [14]. χ2 test was also used for comparisons between the overall cohort and the subset cohort of prior CPR patients. A multivariable logistic regression model was fitted to investigate the association of hospital procedure volume with 30-day in-hospital mortality, adjusted for age, sex, prior CPR, for the indication categories (post heart transplantation (HTX), acute coronary syndrome (ACS) and acute heart failure (AHF)), duration of VA-ECMO support, and complications.
Additionally, a multinomial logistic regression model was fitted to investigate the association between hospital procedure volume and complications. This model was adjusted for age, sex, prior CPR, for the indication categories (HTX, ACS, AHF), duration of VA-ECMO support, and time to 30-day in-hospital mortality.
Complications considered in both logistic regression analysis (either as dependent or as independent variable) were bleeding, stroke, abdominal ischemia, and limb ischemia during the index hospital stay (variable definitions are provided in Table III in the online-only Data Supplement).
All statistical methods were written in IBM SPSS Statistics 26 and were performed at the Federal Bureau of Statistics in Germany.
Results
Study population
Between 2013 and 2016, 10,207 patients ≥ 18 years of age received VA-ECMO for temporary circulatory support in Germany. Mean age of the overall cohort was 61 years (SD 17 years), 71.2% were male patients, and prior CPR was observed in 43.4%. In this cohort, the leading indication for VA-ECMO was ACS in 60.8%, AHF in 37.5%, and HTX in 1.8% (Table 1). Crude 30-day in-hospital mortality was 60.6% (Table 2).
Table 1.
Baseline characteristics of the overall cohort according to annualized hospital volume of VA-ECMO procedures
| Baseline characteristics | P value | ||||
|---|---|---|---|---|---|
| Overall (n = 10,207) | ≤ 50 procedures/Yr (n = 5421) | > 50–100 procedures/Yr (n = 2799) | > 100 procedures/Yr (n = 1987) | ||
| Demographics,n(%) | |||||
| Mean age, Yr | 61 ± 17 | 62 ± 19 | 61 ± 14 | 61 ± 14 | < 0.001 |
| Male gender | 7272 (71.2) | 3879 (71.5) | 1998 (71.4) | 1395 (70.2) | 0.515 |
| CPR | 4429 (43.4) | 2637 (48.6) | 1104 (39.4) | 668 (34.6) | < 0.001 |
| Baseline comorbidities,n(%) | |||||
| AF | 4031 (39.5) | 2849 (36.2) | 1171 (41.8) | 893 (44.9) | < 0.001 |
| CAD | 6659 (65.2) | 3713 (68.4) | 1741 (62.2) | 1205 (60.6) | < 0.001 |
| CHF | 6803 (66.7) | 3570 (65.8) | 1789 (63.9) | 1444 (72.7) | < 0.001 |
| CKD | 2441 (23.9) | 1382 (25.4) | 655 (23.4) | 404 (20.3) | < 0.001 |
| COLD | 514 (5.0) | 331 (6.1) | 136 (4.9) | 47 (2.4) | < 0.001 |
| PH | 1783 (17.5) | 761 (14.0) | 541 (19.3) | 481 (24.2) | < 0.001 |
| HTN | 4821 (47.2) | 2542 (46.8) | 1373 (49.1) | 906 (45.6) | 0.047 |
| HLD | 2531 (24.8) | 1351 (24.9) | 698 (24.9) | 482 (24.3) | 0.825 |
| Diabetes | 2571 (25.2) | 1383 (25.5) | 669 (23.9) | 519 (26.1) | 0.159 |
| Cancer | 278 (2.7) | 169 (3.1) | 79 (2.8) | 30 (1.5) | 0.001 |
| Liver disease | 507 (5.0) | 238 (4.3) | 147 (5.3) | 122 (6.1) | 0.006 |
| Mean VA-ECMO duration in h (mdn) | 116 (72) | 98 (72) | 128 (120) | 150 (120) | 0.001 |
| Indication category for VA-ECMO support,n(%) | |||||
| HTX | 179 (1.8) | 22 (0.4) | 75 (2.7) | 82 (4.1) | < 0.001 |
| ACS | 6202 (60.8) | 3528 (65.0) | 1583 (56.6) | 1091 (54.9) | < 0.001 |
| AHF | 3826 (37.5) | 1871 (34.5) | 1141 (40.8) | 814 (41.0) | < 0.001 |
Abbreviations: ACS acute coronary syndrome, AF atrial fibrillation, AHF acute heart failure, CAD coronary artery disease, CHF congestive heart failure, CPR cardiopulmonary resuscitation, CKD chronic kidney disease, COLD chronic obstructive lung disease, PH pulmonary hypertension, h hours, HTN hypertension, HLD hyperlipidemia, mdn median, n number, Yr year
Table 2.
Thirty-day in-hospital mortality and complications of the overall cohort according to annualized hospital volume of VA-ECMO procedures
| 30-day in-hospital mortality and complications | |||||
|---|---|---|---|---|---|
| Overall (n = 10,207) | ≤ 50 procedures/Yr (n = 5421) | > 50–100 procedures/Yr (n = 2799) | > 100 procedures/Yr (n = 1987) | P value | |
| 30-day in-hospital mortality,n(%) | |||||
| 30-day in-hospital mortality | 6190 (60.6) | 3550 (65.0) | 1578 (56.4) | 1062 (53.4) | < 0.001 |
| Complications,n(%) | |||||
| Bleeding | 2044 (20.0) | 798 (14.7) | 620 (22.2) | 626 (31.5) | < 0.001 |
| Stroke | 105 (1.0) | 47 (0.8) | 38 (1.4) | 20 (1.0) | 0.112 |
| Abdominal ischemia | 711 (7.0) | 277 (5.1) | 215 (7.7) | 219 (11.1) | < 0.001 |
| Limb ischemia | 751 (7.4) | 325 (5.9) | 244 (8.7) | 182 (9.2) | < 0.001 |
Abbreviations: n number, Yr year
Subsequent analyses were performed for 4429 patients (43.4%) with prior CPR. These patients had a mean age of 61 years (SD 20 years), and 72.0% were male patients. The leading indication for the VA-ECMO was ACS in 66.3%, AHF in 32.9%, and HTX in 0.9% patients with prior CPR (Table 3). Crude 30-day in-hospital mortality of patients with prior CPR was 60.6% (Table 4). Detailed reports of the baseline characteristics and comorbidities depending on VA-ECMO procedural volume of the overall cohort and patients with prior CPR are shown in Tables 1 and 3.
Table 3.
Baseline characteristics of all patients with prior CPR according to annualized hospital volume of VA-ECMO procedures
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Overall (n = 4429) | ≤ 50 procedures/Yr (n = 2637) | > 50–100 procedures/Yr (n = 1104) | > 100 procedures/Yr (n = 688) | P value | |
| Demographics,n(%) | |||||
| Mean age, Yr | 61 ± 20 | 61 ± 23 | 61 ± 14 | 61 ± 14 | 0.173 |
| Male gender | 3189 (72.0) | 1915 (72.6) | 801 (72.6) | 473 (68.8) | 0.118 |
| Baseline comorbidities,n(%) | |||||
| AF | 1510 (34.1) | 815 (30.9) | 399 (36.1) | 296 (43.0) | < 0.001 |
| CAD | 3102 (70.0) | 1875 (71.1) | 766 (69.4) | 461 (67.0) | 0.097 |
| CHF | 2780 (62.8) | 1629 (61.8) | 670 (60.7) | 481 (69.9) | < 0.001 |
| CKD | 974 (22.0) | 607 (23.0) | 238 (21.6) | 129 (18.8) | 0.051 |
| COLD | 187 (4.2) | 106 (4.0) | 50 (4.5) | 31 (4.5) | 0.004 |
| PH | 574 (13.0) | 292 (11.1) | 154 (13.9) | 128 (18.6) | < 0.001 |
| HTN | 2032 (45.9) | 1172 (44.4) | 534 (48.4) | 326 (47.4) | 0.062 |
| HLD | 1016 (22.9) | 582 (22.1) | 264 (23.9) | 170 (24.7) | 0.230 |
| Diabetes | 1115 (25.2) | 664 (25.2) | 276 (25.0) | 175 (25.4) | 0.979 |
| Cancer | 130 (2.9) | 80 (3.0) | 37 (3.4) | 13 (1.9) | 0.183 |
| Liver disease | 187 (4.2) | 106 (4.0) | 50 (4.5) | 31 (4.5) | 0.719 |
|
Mean VA-ECMO duration in h (mdn) |
98 (72) | 85 (24) | 111 (72) | 128 (72) | < 0.001 |
| Indication category for VA-ECMO support,n(%) | |||||
| HTX | 39 (0.9) | 6 (0.2) | 13 (1.2) | 20 (2.9) | < 0.001 |
| ACS | 2935 (66.3) | 1797 (68.1) | 717 (64.9) | 421 (61.2) | < 0.001 |
| AHF | 1455 (32.9) | 834 (31.6) | 374 (33.9) | 247 (35.9) | < 0.001 |
Abbreviations: ACS acute coronary syndrome, AF atrial fibrillation, AHF acute heart failure, CAD coronary artery disease, CHF congestive heart failure, CPR cardiopulmonary resuscitation, CKD chronic kidney disease, COLD chronic obstructive lung disease, PH pulmonary hypertension, h hours, HLD hyperlipidemia, HTN hypertension, HTX post heart transplantation, mdn median, n number, Yr year
Table 4.
Thirty-day in-hospital mortality and complications of patients with prior CPR according to annualized hospital volume of VA-ECMO procedures
| 30-day in-hospital mortality and complications | |||||
|---|---|---|---|---|---|
| Overall (n = 4429) | ≤ 50 procedures/Yr (n = 2637) | > 50–100 procedures/Yr (n = 1104) | > 100 procedures/Yr (n = 688) | P value | |
| 30-day in-hospital mortality,n(%) | |||||
| 30-day in-hospital mortality | 3016 (68.1) | 1866 (70.8) | 732 (66.3) | 418 (60.8) | < 0.001 |
| Complications,n(%) | |||||
| Bleeding | 817 (18.4) | 375 (14.2) | 239 (21.6) | 203 (29.5) | < 0.001 |
| Stroke | 40 (0.9) | 17 (0.6) | 15 (1.4) | 8 (1.2) | 0.080 |
|
Abdominal ischemia |
291 (6.6) | 124 (4.7) | 89 (8.1) | 78 (11.3) | < 0.001 |
| Limb ischemia | 335 (7.6) | 160 (6.1) | 106 (9.6) | 69 (10.0) | < 0.001 |
Abbreviations: n number, Yr year
Patient characteristics according to hospital procedure volume
In the overall cohort, the majority (53.1%, n = 5421) of patients was treated at hospitals in the lowest volume category (≤ 50 VA-ECMO implantations per year) in Germany. We observed significant differences in baseline characteristics of treated patients regarding the annualized procedural volume. More patients treated at hospitals in the lowest procedural volume category had ACS, prior CPR, and CAD. However, patients treated at hospitals with > 50 annual procedures had a higher percentage of AHF and CHF. There were no clinically significant differences in sex between the hospital procedure volume categories (p > 0.05). At hospitals with an annual procedure volume > 50 VA-ECMO implantations, we observed a longer VA-ECMO duration as compared to low-volume hospitals (Table 1).
In patients with prior CPR, there were significant differences in the patient characteristics regarding the annualized procedural volume, too. More patients with prior CPR treated at hospitals in the lowest volume category had ACS, CAD, and CKD as compared to the overall cohort. In regard to VA-ECMO duration, we found a similar trend in patients with prior CPR compared to the overall cohort (Table 3).
Hospital procedure volume and 30-day in-hospital mortality in the overall cohort and prior CPR
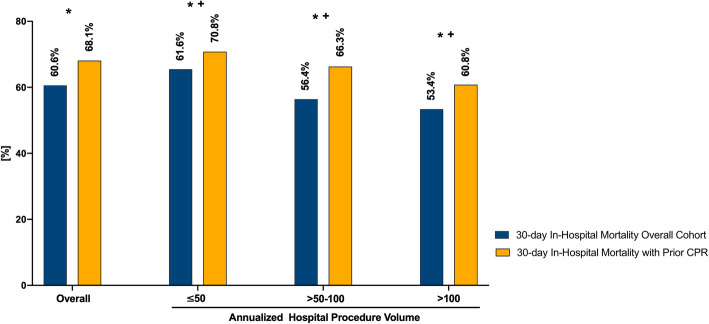
The overall 30-day in-hospital mortality was 60.6% (Fig. 2, Table 2). There was a significant difference in 30-day in-hospital mortality between the groups of annualized hospital volume of VA-ECMO procedures. In detail, 30-day in-hospital mortality was significantly lower at hospitals with a higher annualized procedural volume > 50–100 or > 100 cases (30-day in-hospital mortality > 50–100 cases: 56.4%; 30-day in-hospital mortality > 100 cases: 53.4%) than at hospitals with a lower annualized procedural volume of ≤ 50 cases (30-day in-hospital mortality ≤ 50 cases: 65.0%; both p < 0.05).
Fig. 2.
Thirty-day in-hospital mortality of the overall cohort and patients with prior CPR according to annualized hospital volume of VA-ECMO procedures. *p < 0.001: total cohort vs. prior CPR in each category (overall, ≤ 50, > 50–100, > 100). +p < 0.05: ≤ 50 vs. > 50–100, ≤ 50 vs. > 100, > 50–100 vs. > 100 for the total cohort and prior CPR
In patients with prior CPR, 30-day in-hospital mortality was 68.1%, which was significantly higher than in the overall cohort (p < 0.001) (Fig. 2, Table 4). Congruently to the results of the overall cohort, 30-day in-hospital mortality was significantly lower at hospitals with a higher annualized procedural volume of > 50–100 or > 100 cases than at hospitals with a lower annualized procedural volume of ≤ 50 cases (both p < 0.05) in patients with prior CPR.
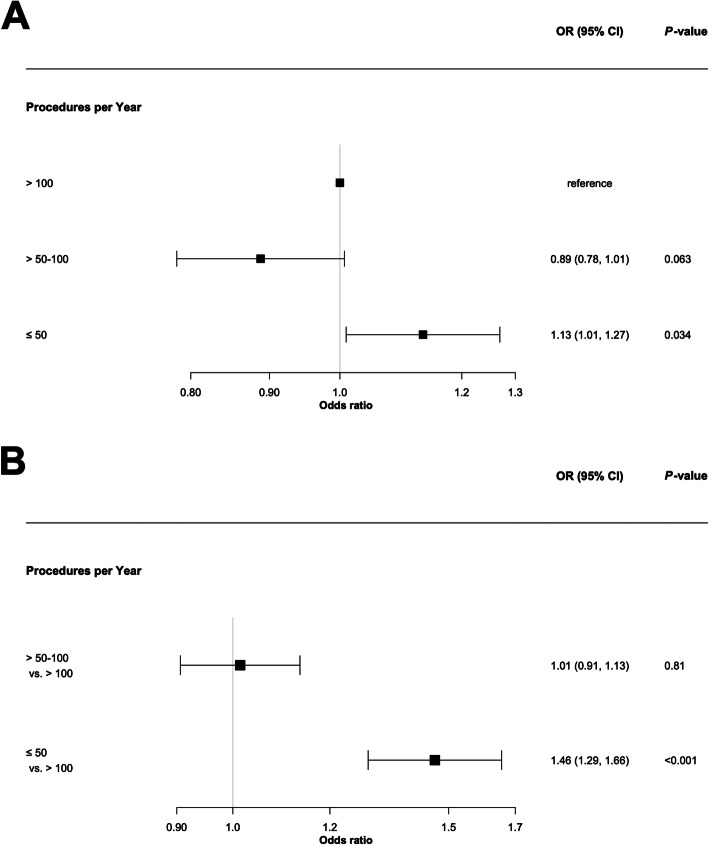
Multivariable adjusted logistic regression analysis showed a significant association of 30-day in-hospital mortality and hospital procedure volume. Patients treated at hospitals with ≤ 50 VA-ECMO implantations per year had a 13% higher relative risk of 30-day in-hospital mortality as compared to patients treated at hospitals with an annual procedure volume of > 100 (adjusted odds ratio 1.13, 95% confidence interval [CI] 1.01–1.27, p < 0.001; Fig. 3A).
Fig. 3.
a Thirty-day in-hospital mortality depending on hospital procedure volume during index-hospitalization adjusted for age, sex, prior CPR, indication category, duration of VA-ECMO support, and complications (bleeding, stroke, abdominal ischemia, and limb ischemia). b Association between hospital procedure volume and complications (bleeding, stroke, abdominal ischemia, and limb ischemia) during index-hospitalization adjusted for age, sex, prior CPR, indication category, duration of VA-ECMO support, and time to 30-day in-hospital mortality
Complications according to hospital procedure volume
Among 10,207 patients undergoing VA-ECMO implantation, 2044 (20.0%) patients had major bleeding, 751 (7.4%) limb ischemia, 711 (7.0%) abdominal ischemia, and 105 (1.0%) stroke, respectively.
Adjusted multinomial logistic regression analysis revealed a higher likelihood for complications at hospitals with lower vs. higher annual procedure volume (annual procedure volume ≤ 50 vs. > 100 VA-ECMO implantations, adjusted odds ratio 1.46, 95% CI 1.29–1.66, p = 0.001) (Fig. 3B).
Discussion
In this large, nationwide study, most VA-ECMO cases were treated at hospitals with a lower annual procedure volume. Treatment at a hospital with a lower annual procedure volume was associated with a higher mortality risk and likelihood for complications compared to higher volume centers.
Previous studies have shown that higher procedural volumes of cardiovascular interventions were associated with lower mortality rates [6, 8, 9]. Selected medical conditions including VA-ECMO in cardiac failure and critical care conditions such as mechanical ventilation are shown to have a significant volume-outcome association in which higher hospital case numbers are associated with survival benefit [15–17]. Particularly, an international study using hospital ECMO volumes from 290 international centers found strong associations of higher hospital-level ECMO volume and lower mortality for neonates and adults, but not for children [18]. A similar observation has been made in Japan; for cases involving ECMO for respiratory failure, a higher hospital procedure volume of ECMO treatment for any indications was associated with lower in-hospital mortality [19]. Contrary to the traditional volume-outcome relationship, other studies on VA-ECMO reported increased mortality associated with high-volume hospitals and in patients transferred to tertiary centers [20, 21]. However, additional information is needed about patient characteristics and selection, management strategies for VA-ECMO, and organization of care within high- and low-volume centers to improve treatment and outcomes among these critically ill patients. In the present study, the majority of patients with VA-ECMO support were treated at hospitals with a low procedural volume (≤ 50 VA-ECMO implantations per year). However, 30-day in-hospital mortality across the categories of hospital procedural volume showed a significant difference between the lowest and highest annualized hospital procedural volumes. Lowest 30-day in-hospital mortality was observed at hospitals with an annualized hospital procedural volume > 100 VA-ECMO cases, especially. However, at hospitals in the lowest procedural volume category, a greater proportion of patients with ACS, prior CPR, CAD, CKD, and advanced age underwent therapy. In contrast, patients treated in the highest procedural volume categories showed a higher proportion of AHF and CHF, whereas sex differences between the hospital procedure volume categories were not observed. As expected across all categorical divisions and compared to the overall cohort, patients with prior CPR had a higher 30-day in-hospital mortality on VA-ECMO in general. Again, differences in baseline criteria were observed between the groups. The proportion of patients suffering from ACS decreased with a higher procedural volume. At hospitals with ≥ 50 annual procedural volume, the leading indication for VA-ECMO implantation slightly shifts from ACS to AHF. Significant differences in patient characteristics indicated by a higher proportion of patients with ACS, prior CPR, and comorbidities such as CAD and CKD in lowest procedural volume category could explain the higher 30-day in-hospital mortality compared to the highest volume categories with > 50 VA-ECMO implantations per year. However, after adjusting for potential confounders, the significant association with an increased risk in patients treated at lower volume centers persisted.
VA-ECMO is a potentially life-saving therapy for reversible circulatory failure not responsive to conventional therapies [3, 22]. However, complications on VA-ECMO are very common and are associated with a significant increase in morbidity and mortality [4, 23].
In the present study, we observed high complication rates of bleeding with 20.0%, limb ischemia with 7.4%, abdominal ischemia with 7.0%, and stroke with 1.0% in the overall cohort.
Stroke occurs in approximately 4% of VA-ECMO patients [11, 24]. This is similar to our results. In addition, the rate of stroke varies by indication and cannulation technique. It has been reported that femoral artery cannulation had a noticeably lower risk compared to central access [25]. Furthermore, the cause of stroke is multifactorial and can be associated with hemodynamic instability and thromboembolic events [26].
In a meta-analysis of 1866 patients by Cheng et al., lower extremity ischemia occurred in 16.9% of patients undergoing VA-ECMO support [11]. However, our analysis showed a lower complication rate. Although this might be explained by underreporting in the used registry, the use of prophylactic antegrade perfusion catheters might have reduced the frequency of limb ischemia over time [27].
Abdominal ischemia during VA-ECMO support is a known complication. To date, incidence rate of bowel and intestinal ischemia on VA-ECMO therapy is largely unknown. Among the total cohort, the incidence rate of abdominal ischemia was 6.7%. Hence, it can be deduced that abdominal ischemia is a relevant complication on VA-ECMO therapy and should be carefully considered before initiation of this invasive treatment.
We found a strong association between hospital procedure volume and complications. Although the crude incidence of complications was higher in hospital with a higher procedural volume, after adjusting for confounders, a higher likelihood for complications was observed at hospitals with lower vs. higher annual VA-ECMO volume. This finding is in line with previous cardiovascular and cardiac surgical studies that have demonstrated a relationship between higher hospital procedure or operator volume and fewer complication rates [28–30].
The observation that higher procedural experience for VA-ECMO was associated with better in-hospital outcomes could be explained by differences in patient characteristics, technical factors, and more experienced handling of patients with complications at higher volume centers.
Limitations
This is a retrospective study based on administrative data, with its inherent limitations. Individual additional follow-up data with long-term survival and complication rates were not available. Therefore, analysis of predisposing factors for ischemic and bleeding complications might be biased. As known from other registries, incidence of complication rates is potentially misreported. Moreover, medical approaches applied prior to VA-ECMO implementation were not available (e.g., timing of inotrope and vasopressor initiation and dosing, etc.). In this regard, patient complexity and severity of illness were not adjusted for in the current model. We did not have data on complications after hospital discharge which might have impacted our results.
Moreover, differences in institutional structure, processes, and thresholds to initiate VA-ECMO therapy potentially bias outcomes and must be considered when interpreting this study. The exact time course of the different diagnoses as being prevalent at admission or incident during the hospital stay is not possible in the current data set. Finally, our data is limited to data for Germany and may not be generalizable to other health care systems.
Conclusions
In a large nationwide registry with more than 10,000 patients, a significant volume-mortality association was shown for VA-ECMO procedures in cardiogenic shock. The majority of patients with VA-ECMO support were treated at hospitals with a low procedural volume in Germany. Thirty-day in-hospital mortality risk and likelihood of complications were higher at hospitals with the lowest annual procedure volume.
Supplementary information
Acknowledgments
Code availability
Software codes are available.
Disclosures
None to declare. No relationships with industry.
Authors’ contributions
Prof. Westermann and Dr. Becher take responsibility for the integrity of the data and the accuracy of the data analysis. All persons have provided the corresponding author with permission to be named in the manuscript. Study concept and design: Becher, Twerenbold, and Westermann. Acquisition, analysis, or interpretation of data: Becher, Goßling, and Westermann. Drafting of the manuscript: Westermann, Becher, and Goßling. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Becher, Goßling, Twerenbold, and Westermann. Administrative, technical, or material support: Fluschnik, Schrage, and Westermann. The authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and material are available.
Ethics approval and consent to participate
Informed consent was obtained from legal guardians.
Consent for publication
Not applicable.
Competing interests
The authors designed the study, analyzed the data, vouch for the data, wrote the paper, and decided to publish. Analysis provided by the Research Data Center of the Federal Bureau of Statistics, Wiesbaden, Germany. All authors have read and approved the manuscript. The manuscript and its contents have not been published previously and are not being considered for publications elsewhere in whole or in part in any language, including publicly accessible web sites or e-print servers.
Dr. Twerenbold received research support from the Swiss National Science Foundation (P300PB_167803), the University Hospital Basel, the University of Basel, and the Cardiovascular Research Foundation Basel, as well as speaker honoraria/consulting honoraria from Abbott, Brahms, Siemens, Singulex, and Roche.
Dr. Westermann received honorary from Astra-Zeneca, Bayer, BöhringerIngelheim, Berlin-Chemie, and Novartis.
Dr. Schrage received speaker’s fee from AstraZeneca and is currently funded by the German Research Foundation.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Peter Moritz Becher, Email: m.becher@uke.de.
Dirk Westermann, Email: d.westermann@uke.de.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13054-020-03016-z.
References
- 1.Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372(9638):554–561. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 2.Mosier JM, Kelsey M, Raz Y, Gunnerson KJ, Meyer R, Hypes CD, et al. Extracorporeal membrane oxygenation (ECMO) for critically ill adults in the emergency department: history, current applications, and future directions. Crit Care. 2015;19:431. doi: 10.1186/s13054-015-1155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorusso R, Gelsomino S, Parise O, Mendiratta P, Prodhan P, Rycus P, et al. Venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock in elderly patients: trends in application and outcome from the Extracorporeal Life Support Organization (ELSO) registry. Ann Thorac Surg. 2017;104(1):62–69. doi: 10.1016/j.athoracsur.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Combes A, Leprince P, Luyt CE, Bonnet N, Trouillet JL, Leger P, et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36(5):1404–1411. doi: 10.1097/CCM.0b013e31816f7cf7. [DOI] [PubMed] [Google Scholar]
- 5.Becher P.M.; Schrage BS, C.R.; Schmack, B.; Fluschnik, N.; Schwarzl, M.; Waldeyer, C.; Lindner, D.; Seiffert, M.; Neumann, J.T.; Bernhardt, A.M.; Zeymer, U.; Thiele, H.; Reichenspurner, H.; Blankenberg, S.; Twerenbold, R.; Westermann, D. Venoarterial extracorporeal membrane oxygenation for cardiopulmonary support. Insights From a German Registry. Circulation. 2018;138(20):2298–300. [DOI] [PubMed]
- 6.Vemulapalli S, Carroll JD, Mack MJ, Li Z, Dai D, Kosinski AS, et al. Procedural volume and outcomes for transcatheter aortic-valve replacement. N Engl J Med. 2019;380(26):2541–2550. doi: 10.1056/NEJMsa1901109. [DOI] [PubMed] [Google Scholar]
- 7.Omran J, Enezate T, Abdullah O, Mahmud E. The volume-outcomes relationship between mitral valve surgery and MitraClip procedure in a U.S. registry. J Am Coll Cardiol. 2019;74(13 Supplement):B437. doi: 10.1016/j.jacc.2019.08.1004. [DOI] [Google Scholar]
- 8.Badheka AO, Patel NJ, Grover P, Singh V, Patel N, Arora S, et al. Impact of annual operator and institutional volume on percutaneous coronary intervention outcomes: a 5-year United States experience (2005-2009) Circulation. 2014;130(16):1392–1406. doi: 10.1161/CIRCULATIONAHA.114.009281. [DOI] [PubMed] [Google Scholar]
- 9.Nallamothu BK, Saint S, Hofer TP, Vijan S, Eagle KA, Bernstein SJ. Impact of patient risk on the hospital volume-outcome relationship in coronary artery bypass grafting. Arch Intern Med. 2005;165(3):333–337. doi: 10.1001/archinte.165.3.333. [DOI] [PubMed] [Google Scholar]
- 10.Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med. 2009;35(12):2105–2114. doi: 10.1007/s00134-009-1661-7. [DOI] [PubMed] [Google Scholar]
- 11.Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97(2):610–616. doi: 10.1016/j.athoracsur.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Zangrillo A, Landoni G, Biondi-Zoccai G, Greco M, Greco T, Frati G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013;15(3):172–178. [PubMed] [Google Scholar]
- 13.Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, et al. SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol. 2015;65(19):e7–e26. doi: 10.1016/j.jacc.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 14.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 15.Aso S, Matsui H, Fushimi K, Yasunaga H. In-hospital mortality and successful weaning from venoarterial extracorporeal membrane oxygenation: analysis of 5,263 patients using a national inpatient database in Japan. Crit Care. 2016;20:80. doi: 10.1186/s13054-016-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Combes A, Brodie D, Bartlett R, Brochard L, Brower R, Conrad S, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190(5):488–496. doi: 10.1164/rccm.201404-0630CP. [DOI] [PubMed] [Google Scholar]
- 17.Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O'Brien CR, Rubenfeld GD. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355(1):41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 18.Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191(8):894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muguruma K, Kunisawa S, Fushimi K, Imanaka Y. Epidemiology and volume-outcome relationship of extracorporeal membrane oxygenation for respiratory failure in Japan: a retrospective observational study using a national administrative database. Acute Med Surg. 2020;7(1):e486. doi: 10.1002/ams2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey KL, Downey P, Sanaiha Y, Aguayo E, Seo YJ, Shemin RJ, et al. National trends in volume-outcome relationships for extracorporeal membrane oxygenation. J Surg Res. 2018;231:421–427. doi: 10.1016/j.jss.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy FH, McDermott KM, Spragan D, Hoedt A, Kini V, Atluri P, et al. Unconventional volume-outcome associations in adult extracorporeal membrane oxygenation in the United States. Ann Thorac Surg. 2016;102(2):489–495. doi: 10.1016/j.athoracsur.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guglin M, Zucker MJ, Bazan VM, Bozkurt B, El Banayosy A, Estep JD, et al. Venoarterial ECMO for adults: JACC scientific expert panel. J Am Coll Cardiol. 2019;73(6):698–716. doi: 10.1016/j.jacc.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 23.Ouweneel DM, Schotborgh JV, Limpens J, Sjauw KD, Engstrom AE, Lagrand WK, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. 2016;42(12):1922–1934. doi: 10.1007/s00134-016-4536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Megarbane B, Deye N, Aout M, Malissin I, Resiere D, Haouache H, et al. Usefulness of routine laboratory parameters in the decision to treat refractory cardiac arrest with extracorporeal life support. Resuscitation. 2011;82(9):1154–1161. doi: 10.1016/j.resuscitation.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Wong JK, Melvin AL, Joshi DJ, Lee CY, Archibald WJ, Angona RE, et al. Cannulation-related complications on veno-arterial extracorporeal membrane oxygenation: prevalence and effect on mortality. Artif Organs. 2017;41(9):827–834. doi: 10.1111/aor.12880. [DOI] [PubMed] [Google Scholar]
- 26.Mateen FJ, Muralidharan R, Shinohara RT, Parisi JE, Schears GJ, Wijdicks EF. Neurological injury in adults treated with extracorporeal membrane oxygenation. Arch Neurol. 2011;68(12):1543–1549. doi: 10.1001/archneurol.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang WJ, Cho YH, Park TK, Song YB, Choi JO, Hahn JY, et al. Fluoroscopy-guided simultaneous distal perfusion as a preventive strategy of limb ischemia in patients undergoing extracorporeal membrane oxygenation. Ann Intensive Care. 2018;8(1):101. doi: 10.1186/s13613-018-0445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strom JB, Wimmer NJ, Wasfy JH, Kennedy K, Yeh RW. Association between operator procedure volume and patient outcomes in percutaneous coronary intervention: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2014;7(4):560–566. doi: 10.1161/CIRCOUTCOMES.114.000884. [DOI] [PubMed] [Google Scholar]
- 29.Jolly SS, Cairns J, Yusuf S, Niemela K, Steg PG, Worthley M, et al. Procedural volume and outcomes with radial or femoral access for coronary angiography and intervention. J Am Coll Cardiol. 2014;63(10):954–963. doi: 10.1016/j.jacc.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 30.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and material are available.