Abstract
Background:
Obesity is a complex disorder influenced by various genetic and environmental factors. It has been shown that gut microbiota, which colonizes gastrointestinal tract, has a substantial role as an environmental factor in the pathophysiology of obesity. Since the composition of gut microbiota alters with regard to different criteria, such as ethnicity, geographical location, diet, lifestyle, age, and gender, we aimed to determine F/B ratio and the abundance of important gut microbiota members, A. muciniphila, F. prausnitzii, Roseburia, Bifidobacterium, and Prevotella in Iranian obese and normal weight individuals, for the first time.
Methods:
In this study, 50 normal and 50 obese subjects were recruited and classified based on their BMI into normal weight and obese groups. Stool samples were collected. Following DNA extraction from the samples, qPCR was conducted based on 16s rDNA universal primers. Finally, the correlation between the bacterial abundance and obesity was analyzed by statistical analyses.
Results:
We observed a significant increase of F/B ratio in the obese group, compared to the normal weight group (p = 0.002). Although A. muciniphila (p = 0.039) and Bifidobacterium (p = 0.049) abundance significantly decreased, the abundance of F. prausnitzii (p = 0.046) significantly elevated with BMI increase in the studied groups.
Conclusion:
Owing to the importance of the gut microbiota composition in obesity development, determination and targeted restoration of gut microbiota pattern could be valuable in the control and treatment of obesity in certain populations.
INTRODUCTION
Obesity is a global health problem due to change in people’s life style. Various factors, including genetic and environmental factors, are involved in the pathophysiology of obesity[1-3]. Gut microbiota has been known as an important environmental factor for inducing and developing obesity. After birth, the gastrointestinal tract is colonized by a complex and dynamic microbial community, which is called gut microbiota. The composition of gut microbiota depends on multiple factors, including genetic background, mode of delivery, nutrition, antibiotic consumption, physical activity, geographical distribution, ethnicity, age, gender, lifestyle, and others[4-6]. This microbial community settles down during 2-3 years of life and consists of bacteria, archaea, protozoa, fungi, and viruses. Bacteria are dominant in this microbial population where the Firmicutes and Bacteroidets phyla make up the most frequency of gut microbiota. Also, Actinobacteria, Proteobacteria, and Verrucomicrobia are constituents of gut microbiota with low frequency[7,8]. The gut microbiota and its metabolites have determinative role in health and diseases due to the fact that they have significant potential in host, including regulation of inflammatory responses, energy homeostasis, and glucose/lipid metabolism. Therefore, any change in gut microbiota composition, which is termed dysbiosis, can lead to disruption in host functions and development of metabolic disorders, including obesity and type 2 diabetes . In this regard, determination of altered gut microbiota composition is an inevitable part of etiological recognition of obesity[2,9,10].
Obesity is associated with low-grade inflammation, insulin resistance, increased weight gain, and fat deposition[2,11]. It has been documented that high-fat diet induces dysbiosis, which favors the increase of energy harvest from diet, deregulation of immune responses, and metabolic pathways[1,2,12]. Hence, to achieve healthy state, the delicate arrangement of gut microbiota composition in the gastrointestinal tract is required. In this regard, many studies have shown that F/B ratio increases in obese subjects, and changes in F/B ratio have a significant role in calorie intake and have a direct correlation with obesity[13,14].
Currently, numerous investigations have shown that anaerobic intestinal commensal bacteria such as A. muciniphila, F. prausnitzii, Roseburia, Bifido-bacterium, and Prevotella have significant role in the gut microbiota-host interactions, including influence on host metabolism and immune system through anti-inflammatory properties. Thus, their relative abundance could be a potential health biomarker[15-19].
As mentioned above, dysbiosis is critical starting point in developing obesity and related complications (type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, etc.). Also, F/B ratio, A. muciniphila, F. prausnitzii, Roseburia, Bifidobacterium, and Prevotella influence the pathophysiology of metabolic disorders. As the gut microbiota is under the influence of different factors such as ethnicity, diet, lifestyle, and geographical distribution, we decided to investigate the relative abundance of these bacteria in obese Iranian population, which would be the first report in this context, to the best of our knowledge. For this purpose, fecal samples from Iranian subjects were collected and analyzed using qPCR based on 16s rDNA gene of targeted bacteria. We aimed to determine the correlation between the abundance of the aforementioned bacteria and BMI among our studied population.
MATERIALS AND METHODS
Study population
A total of 100 adult Iranian individuals (aged between 20 and 60 years) were selected for this study during October 2016 to December 2017. The subjects were equally grouped into normal weight group with BMI between 18.5 and 24.9 kg/m2 and obese group with BMI above 25 kg/m2. Exclusion criteria included the use of corticosteroids, antibiotics, alcohol, smoking, significant infection, and gastrointestinal diseases.
Fecal sampling and DNA extraction
Subjects were asked to collect their stool samples in a conventional laboratory plastic container dedicated for fecal sampling. The samples were immediately transferred to the laboratory in cold chain storage. These samples were stored at -80 °C (fresh frozen) upon arrival until further processing. DNA was extracted from the samples using QIAamp DNA stool mini kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. The quality and quantity of the extracted DNA was analyzed by agarose gel electrophoresis and NanoDrop ND-8000 (Thermo Scientific, USA), respectively[20].
qPCR analyses
The abundance of bacteria was analyzed using qPC based on SYBER green method (LightCycler® 96 SW 1.1; Roche, Germany)[13,20,21]. Each 20 µl of qPCR reaction was composed of SYBR Premix Ex Taq II
(RR820L; Takara, Japan), 0.5 µl of each of the specific 16s rDNA primers (Table 1), and 1 µ1 of the DNA template. Each qPCR reaction was performed in duplicate using LightCycler® 8-Tube Strips (white; Roche). The amplification program was designed
Table 1.
16S rRNA gene specific primers for the studied bacterial group/species
| Target organism | Forward (5´ to 3´) | Reverse (5´ to 3´) | Ref. |
|---|---|---|---|
| Firmicutes | TGAAACTYAAGGAATTGACG | ACCATGCACCTGTC | [ 13 ] |
| Bacteroidetes | AAACTCAAAKGAATTGACGG | GGTAAGGTTCCTCGCGCTAT | [ 13 ] |
|
CAGCACGTGAAGGTGGGGAC | CCTTGCGGTTGGCTTCAGAT | [ 20 ] |
| F. prausnitzii | GGAGGAAGAAGGTCTTCGG | AATTCCGCCTACCTCTGCACT | [ 21 ] |
| Prevotella | CACCAAGGCGACGATCA | GGATAACGCCYGGACCT | [ 47 ] |
| Roseburia | TACTGCATTGGAAACTGTCG | CGGCACCGAAGAGCAAT | [ 47 ] |
| Bifidobacterium | CTCCTGGAAACGGGTGG | GGTGTTCTTCCCGATATCTACA | [ 48 ] |
| Escherichia coli | CATTGACGTTACCCGCAGAAGAAGC | CTCTACGAGACTCAAGCTTGC | [ 49 ] |
according to the appropriate annealing temperature: 1 cycle of 95 °C for 60 s, followed by 40 cycles of denaturation at 95 °C for 5 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s. Melting curve analysis was carried out after amplification to control the specificity of PCR reaction, followed by 1 cycle at 95 °C for 5 s, 60 °C for 60 s, and 95 °C for 1 s.
Standard curve
The abundance of bacteria was calculated as previously described[22]. Briefly, the standard curve was prepared using serial dilutions of DNA from standard strain Escherichia coli. This curve allows us to calculate DNA concentration of each bacterium from fecal samples. The standard curve is graphically represented as a semi-log regression line plot of CT value vs. log of DNA concentration.
Statistical analyses
In this study, categorical variables are presented as numbers (percent) and continuous variables as mean ± SD. Independent t-test was employed to assess mean differences between the normal and obese groups. Chi-square analysis was used for qualitative data, and K-S analysis was applied to control the normal distribution of the data. Linear regression model and Pearson’s correlation coefficient were performed to determine correlation between BMI and the abundance of two bacteria phyla, Bacteroidetes and Firmicutes. Statistical analyses were conducted using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were 2-tailed, and a p < 0.05 was considered statistically significant.
Ethical statement
The above-mentioned sampling protocols were approved by the National Institute for Medical Research Development (NIMAD, Tehran, Iran; ethical code: IR.NIMAD.REC.1395.043). Written informed consents were provided by all the patients.
RESULTS
Demographic characteristics of the study population
Adult subjects were divided, based on BMI, into two groups: normal weight (50%) and obese (50%). Characteristics of the subjects are shown in Table 2. Obese group consisted of 32 overweight subjects with BMI between 25 and 29.9 kg/m2 and 18 subjects with BMI above 30 kg/m2. There were not significant differences in age, gender, and height between the two studied groups.
Table 2.
Characteristics of obese and normal weight adults under study
| Characteristics | Obese | Normal weight |
|---|---|---|
| Subjects | 50 | 50 |
| Gender (male/female) | 25/25 | 25/25 |
| Age (y) | 38.76 ± 1.76 | 38.74 ± 1.41 |
| Weight (kg) | 83.92 ± 1.89 | 64.8 ± 1.24 |
| Height (m) | 1.69 ± 0.015 | 1.69 ± 0.012 |
| BMI (kg/m2) | 29.36 ± 0.50 | 22.4 ± 0.26 |
| BMI s.d. score | 3.55 | 1.86 |
F/B ratio
In Table 3, the mean abundance of Firmicutes, Bacteroidetes, and F/B are presented. The results demonstrated that Firmicutes and Bacteroidetes abundance significantly increased and decreased in the obese group and the control, respectively (Fig. 1). Besides, the F/B ratio was significantly higher in the obese (p = 0.002) than control group.
Table 3.
Mean abundance of each phylum across each of the BMI categories
| Phylum |
BMI
index
|
p value | |
|---|---|---|---|
| 18.5-24.9 | ≥25 | ||
| Firmicutes | 6.59 ± 0.19 | 7.15 ± 0.18 | 0.045 |
| Bacteroidetes | 4.92 ± 08.0 | 4.64 ± 10.0 | 0.040 |
| F/B | 1.66 ± 0.18 | 2.5 ± 0.19 | 0.002 |
Fig. 1.
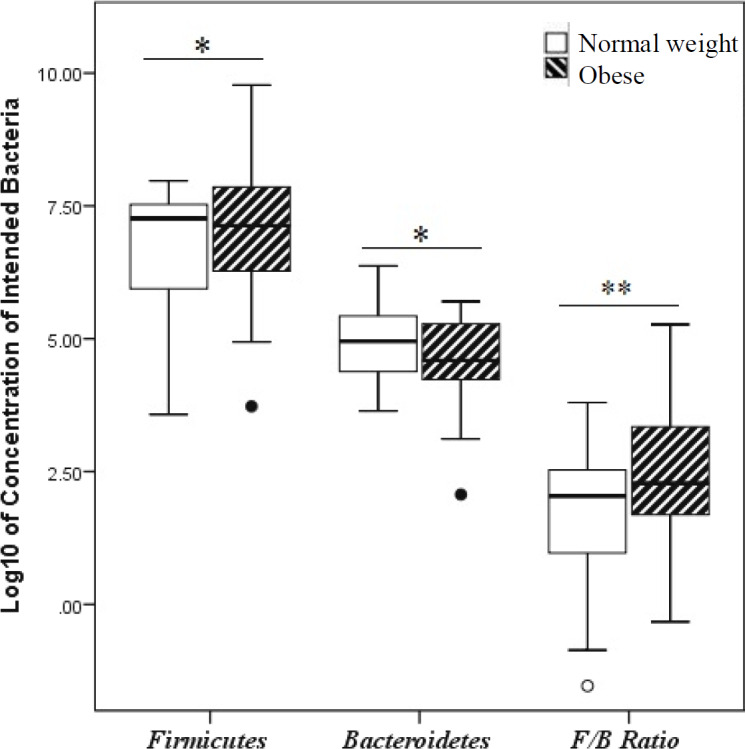
F/B ratio of obese and normal weight Iranian subjects detected by qPCR. *p < 0.05; **p < 0.01
Quantification and comparison of important gut microbiota memebers
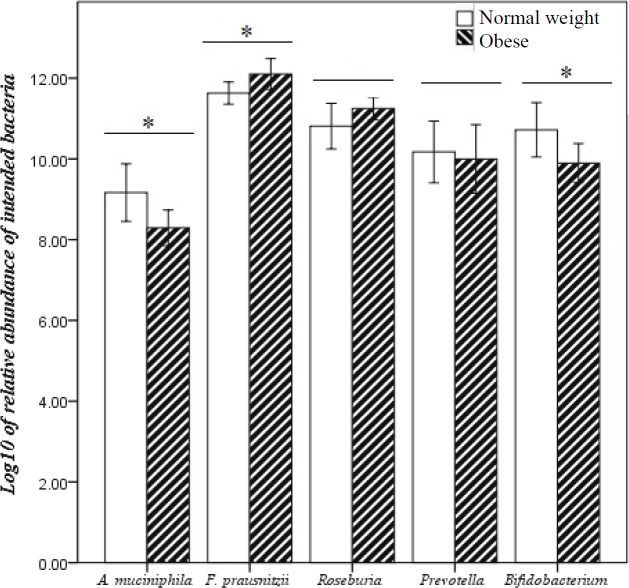
The concentrations of A. muciniphila, F. prausnitzii, Roseburia, Prevotella, and Bifidobacterium were quantified in fecal samples of the studied groups. The highest and lowest concentrations of targeted bacteria were 8.69 × 1013 and 1.88 × 105 CFU/g for A. muciniphila, 9.71 × 1013 and 1.83 × 107 CFU/g for F. prausnitzii, 2.06 × 1013 and 4.95 × 101 CFU/g for Roseburia, 7.84 × 1014 and 8.69 × 102 CFU/g for Prevotella, and 4.52 × 1015 and 1.19 × 101 CFU/g for Bifidobacterium in normal weight and obese groups, respectively (Table 4). In order to find a correlation between the bacterial abundance and BMI, differences in gut microbiota composition between the two groups were analyzed. Our results demonstrated that A. muciniphila relative abundance significantly decreased in parallel with BMI increase in obese vs. normal weight (p = 0.039) groups. Also, a significant reduction of Bifidobacterium relative abundance (p = 0.049) was observed in the obese group. In contrast, there was a significant increase of F. prausnitzii relative abundance in the obese compared to the normal weight (p = 0.460) subjects. Although the relative abundance of Roseburai (p = 0.170) and Prevotell (p = 0.756) increased and decreased with BMI increase, respectively, no significant correlation was found between their frequency and the studied groups (Fig. 2).
Table 4.
Relative abundance of A. muciniphila, F. prausnitzii, Roseburia, Prevotella, and Bifidobacterium
| A. muciniphila (CFU/g) |
F. prausnitzii
(CFU/g) |
Roseboria
(CFU/g) |
Prevotella
(CFU/g) |
Bifidobacterium
(CFU/g) |
|
|---|---|---|---|---|---|
| Mean | 1.93 × 1012 | 4.90 × 1012 | 1.05 × 1012 | 4.07 × 1013 | 6.69 × 1013 |
| Std. Error of Mean | 1.02 × 1012 | 1.13 × 1012 | 2.44 × 1011 | 1.43 × 1013 | 4.65 × 1013 |
| Std. Deviation | 1.02 × 1013 | 1.13 × 1013 | 2.44 × 1012 | 1.43 × 1014 | 4.65 × 1014 |
| Minimum | 1.88 × 105 | 1.83 × 107 | 4.95 × 101 | 8.69 × 102 | 1.19 × 101 |
| Maximum | 8.69 × 1013 | 9.71 × 1013 | 2.06 × 1013 | 7.84 × 1014 | 4.52 × 1015 |
Fig. 2.
Comparison of the abundance of important gut microbiota members in normal weight and obese Iranian subjects detected by qPCR. Data of qPCR are expressed as mean log10 CFU/g. Error bars 95% CI. *p < 0.05
DISCUSSION
The dominant roles of gut microbiota in pathophysiology of obesity has been currently evidenced[23]. Several studies have shown that the imbalance of energy homeostasis, low-grade inflammation, and insulin resistance are important determinants, which result from dysbiosis and lead to the negative regulation of host metabolism[24]. Due to the fact that gut microbiome exerts crucial functions such as the influence on energy harvest from diet, and anti-inflammatory and metabolism regulation, any change in gut microbiota composition can induce and develop obesity[24,25]. According to the importance of gut microbiota composition in obesity and its uniqueness in each population, we studied, for the first time, the differences of F/B ratio and the relative abundnce of A. muciniphila, F. prausnitzii, Roseburia, Bifidobacterium, and Prevotella in normal weight and obese Iranian subjects.
Various animal and human studies have revealed the increase of Frimicutes and decrease of Bacteroidets concentrations (increase of F/B ratio) in obese vs. normal subjects[26-29], which is in line with our study. Dominancy of Firmicutes, which is enriched by bacterial genes related to nutrient transporters and primary fermentation enzymes, could be explained by elevated calories absorption and weight gain during obesity[30,-32]. Higher frequency of bacterial genes responsible for carbohydrate metabolism, which belongs to Bacteroidetes, has been reported in Turnbaugh et al.'s research of lean and obese-twin gut microbiome[30]. Nevertheless, there are inconsistent results of F/B ratio. In this regard, Andoh et al.[33] did not observed any difference in the F/B ratio between the obese and non-obese Japanese groups.
Recently, the significant roles of some intestinal anaerobic commensal bacteria, such as A. muciniphila, F. prausnitzii, Bifidobacterium, Roseburia, and Prevotella, have been illustrated in gut microbiota-host interaction[24,25]. One important feature of these bacteria is the production of short-chain fatty acids, which have various functions, including the regulation of gut barrier integrity, regulation of metabolism, and inflammation. Thus, there is a correlation between the abundance of mentioned bacteria and obesity[24,25,34-36].
Increasing body of evidence in animal and human studies has demonstrated an inverse correlation of A. muciniphila abundance with obesity[36,37]. In agreement with other studies, A. muciniphila abundance significantly reduced in Iranian obese subjects in comparison with normal weight subjects.
This reduction of A. muciniphila abundunce is associated with impaired metabolic status during obesity, since it has many health promoting potentials, including regulation of glucose metabolism, blood lipid concentration, and fat distribution[15].
The genus Bifidobacterium has been shown to have beneficial health effects due to the effect on the gut barrier and immune system[38]. The association of Bifidobacterium abundance and obesity has been studied in several studies[39-42]. Ignacio et al.[43] have reported the negative correlation between the abundance of Bifidobacterium and BMI and showed increased abundance of Bifidobacterium spp. in the lean group. Similarly, we identified significant negative correlation of Bifidobacterium abundance with BMI increase.
There are various studies that inconsistently reported that the F. prausnitzii abundance is associated with obesity[44-46]. In accordance with the report of gut microbiota composition in obese Indian children[46], the abundance of F. prausnitzii significantly increased along with BMI increase in Iranian subjects. However, Feng et al.[45] did not observe any significant difference of F. prausnitzii levels between obese and normal Chinese subjects.
Taken together, our results demonstrated a significant increase of F/B ratio and reduction of A. muciniphila and Bifidobacterium in obese Iranian subjects vs. normal weight individuals. additionally, we observed higher F. prausnitzii abundance in obese subjects. Since gut microbiota composition is established based on various factors, including ethnicity, diet, life style, and geographical location, which induce differences between various populations, it is necessary to determine this composition to design proper strategies for obesity treatment in each targeted population.
ACKNOWLEDGEMENTS
This work was supported by the National Institute for Medical Research Development (NIMAD), Tehran, Iran (IR.NIMAD.REC.1395.043) and Iran Biotech Fund (grant no. 94/10243). The authors would like to thank the colleagues at Mycobacteriology and Pulmonary Research Department and Microbiology Research Center at Pasteur Institute of Iran, Tehran, Iran.
CONFLICT OF INTEREST.
None declared.
References
- 1.Rampelli S, Candela M, Turroni S, Biagi E, Pflueger M, Wolters M, Ahrens W, Brigidi P. Microbiota and lifestyle interactions through the lifespan. Trends in food science and technology. 2016;57(Part B):265–272. [Google Scholar]
- 2.Cani PD, Delzenne NM. Gut Microbiota, Obesity and Associated Metabolic Disorders. reterieved from: https://www.worldgastroenterology.org/UserFiles/file/WDHD-2014-handbook-FINAL.pdf. [DOI] [PubMed]
- 3.Moreno-Indias I, Cardona F, Tinahones FJ, Queipo-Ortuño MI. Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Frontiers in microbiology. 2014;5:190. doi: 10.3389/fmicb.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clinica chimica acta. 2015;451(Pt A):97–102. doi: 10.1016/j.cca.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MC, Marchesi JR, Collado MC. The composition of the gut microbiota throughout life, with an emphasis on early life. Microbial ecology in health and disease. 2015;26:26050. doi: 10.3402/mehd.v26.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nature reviews microbiology. 2013;11(4):227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 7.Houghteling PD, Walker WA. Why is initial bacterial colonization of the intestine important to the infant’s and child’s health? Journal of pediatric gastroenterology and nutrition. 2015;60(3):294–307. doi: 10.1097/MPG.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matamoros S, Gras-Leguen C, Le Vacon F, Potel G, de La Cochetiere MF. Development of intestinal microbiota in infants and its impact on health. Trends in microbiology. 2013;21(4):167–173. doi: 10.1016/j.tim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839–2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC immunology. 2017;18(1) doi: 10.1186/s12865-016-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. The lancet diabetes and endocrinology. 2015;3(3):207–215. doi: 10.1016/S2213-8587(14)70134-2. [DOI] [PubMed] [Google Scholar]
- 12.Nehra V, Allen JM, Mailing LJ, Kashyap PC, Woods JA. Gut microbiota: modulation of host physiology in obesity. Physiology (Bethesda) 2016;31(5):327–335. doi: 10.1152/physiol.00005.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, Gavalko Y, Dorofeyev A, Romanenko M, Tkach S, Sineok L, Lushchak O, Vaiserman A. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC microbiology. 2017;17(1):120. doi: 10.1186/s12866-017-1027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, Berry D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in F irmicutes populations. Environmental microbiology. 2017;19(1):95–105. doi: 10.1111/1462-2920.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L; MICRO-Obes Consortium, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 16.Hand TW, Vujkovic-Cvijin I, Ridaura VK, Belkaid Y. Linking the microbiota, chronic disease, and the immune system. Trends in Endocrinology andmetabolism. 2016;27(12):831–843. doi: 10.1016/j.tem.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hippe B, Remely M, Aumueller E, Pointner A, Magnet U, Haslberger AG. Faecalibacterium prausnitzii phylotypes in type two diabetic, obese, and lean control subjects. Beneficial microbes. 2016;7(4):511–517. doi: 10.3920/BM2015.0075. [DOI] [PubMed] [Google Scholar]
- 18.Remely M, Tesar I, Hippe B, Gnauer S, Rust P, Haslberger AG. Gut microbiota composition correlates with changes in body fat content due to weight loss. Beneficial microbes. 2015;6(4):431–439. doi: 10.3920/BM2014.0104. [DOI] [PubMed] [Google Scholar]
- 19.Schneeberger M, Everard A, Gómez-Valadés AG, Matamoros S, Ramírez S, Delzenne NM, Gomis R, Claret M, Cani PD. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Scientific reports. 2015 doi: 10.1038/srep16643. 5: Article no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Applied and environmental microbiology. 2007;73(23):7767–7770. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benus RF, van der Werf TS, Welling GW, Judd PA, Taylor MA, Harmsen HJ, Whelan K. Association between Faecalibacterium prausnitzii and dietary fibre in colonic fermentation in healthy human subjects. British journal of nutrition. 2010;104(5):693–700. doi: 10.1017/S0007114510001030. [DOI] [PubMed] [Google Scholar]
- 22.Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148(1):257–266. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- 23.Tilg H, Moschen AR. Gut Microbiome, Obesity, and Metabolic Syndrome. In: Ahima RS, editor. Metabolic Syndrome: A Comprehensive Textbook. 2016. pp. 447–459. [Google Scholar]
- 24.Newsholme P, Homem de Bittencourt PI Jr. Gut associated bacteria are critical to metabolism, inflammation and health. Current opinion in clinical nutrition and metabolic care. 2016;19(4):245–249. doi: 10.1097/MCO.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 25.de Clercq NC, Groen AK, Romijn JA, Nieuwdorp M. Gut microbiota in obesity and undernutrition. Advances in nutrition. 2016;7(6):1080–1089. doi: 10.3945/an.116.012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barlow GM, Yu A, Mathur R. Role of the gut microbiome in obesity and diabetes mellitus. Nutrition in clinical practice. 2015;30(6):787–797. doi: 10.1177/0884533615609896. [DOI] [PubMed] [Google Scholar]
- 27.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proceedings of the national academy of sciences. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathur R, Barlow GM. Obesity and the microbiome. Expert review of gastroenterology and hepatology. 2015;9(8):1087–1099. doi: 10.1586/17474124.2015.1051029. [DOI] [PubMed] [Google Scholar]
- 29.Sweeney TE, Morton JM. The human gut microbiome: a review of the effect of obesity and surgically induced weight loss. JAMA surgery. 2013;148(6):563–569. doi: 10.1001/jamasurg.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 31.Krajmalnik‐Brown R, Ilhan ZE, Kang DW, DiBaise JK. Effects of gut microbes on nutrient absorption and energy regulation. Nutrition in clinical practice. 2012;27(2):201–214. doi: 10.1177/0884533611436116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dugas LR, Fuller M, Gilbert J, Layden BT. The obese gut microbiome across the epidemiologic transition. Emerging themes in epidemiology. 2016;13:2. doi: 10.1186/s12982-015-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andoh A, Nishida A, Takahashi K, Inatomi O, Imaeda H, Bamba S, Kito K, Sugimoto M, Kobayashi T. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. Journal of clinical biochemistry and nutrition. 2016;59(1):65–70. doi: 10.3164/jcbn.15-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cani PD, Everard A, Duparc T. Gut microbiota, enteroendocrine functions and metabolism. Current opinion in pharmacology. 2013;13(6):935–940. doi: 10.1016/j.coph.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50(11):2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 36.Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microbial pathogenesis. 2017;106:171–181. doi: 10.1016/j.micpath.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proceedings of the national academy of sciences. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.AArboleya S, Watkins C, Stanton C, Ross RP. Gut bifidobacteria populations in human health and aging. Frontiers in microbiology. 2016;7:1204. doi: 10.3389/fmicb.2016.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Million M, Maraninchi M, Henry M, Armougom F, Richet H, Carrieri P, Valero R, Raccah D, Vialettes B, Raoult D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. International journal of obesity (London) 2012;36(6):817–825. doi: 10.1038/ijo.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Salazar N, Dewulf EM, Neyrinck AM, Bindels LB, Cani PD, Mahillon J, de Vos WM, Thissen JP, Gueimonde M, de Los Reyes-Gavilán CG, Delzenne NM. Inulin-type fructans modulate intestinal Bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women. Clinical nutrition. 2015;34(3):501–507. doi: 10.1016/j.clnu.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Kondo S, Xiao JZ, Satoh T, Odamaki T, Takahashi S, Sugahara H, Yaeshima T, Iwatsuki K, Kamei A, Abe K. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Bioscience, biotechnology, and biochemistry. 2010;74(8):1656–1661. doi: 10.1271/bbb.100267. [DOI] [PubMed] [Google Scholar]
- 42.QQueipo-Ortuño MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F, Casanueva F, Tinahones FJ. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS one. 2013;8(5):e65465. doi: 10.1371/journal.pone.0065465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ignacio A, Fernandes MR, Rodrigues VA, Groppo FC, Cardoso AL, Avila-Campos MJ, Nakano V. Correlation between body mass index and faecal microbiota from children. Clinical microbiology and infection. 2016;22(3):258.e1–e8. doi: 10.1016/j.cmi.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 44.Ryan PM, Delzenne NM. Gut microbiota and metabolism. The gut-brain axis. 2016;2016:391–401. [Google Scholar]
- 45.Feng J, Tang H, Li M, Pang X, Wang L, Zhang M, Zhao Y, Zhang X, Shen J. The abundance of fecal Faecalibacterium prausnitzii in relation to obesity and gender in Chinese adults. Archives of microbiology. 2014;196(1):73–77. doi: 10.1007/s00203-013-0942-2. [DOI] [PubMed] [Google Scholar]
- 46.Balamurugan R, Chittaranjan SP, Chandragunasekaran AM, Ramakrishna BS. Molecular detection of the ruminal bacterium, Butyrivibrio fibrisolvens, in feces from rural residents of southern India. Microbial ecology in health and disease. 2009;21(1):38–43. [Google Scholar]
- 47.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS one. 2010;5(2) doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuki T, Watanabe K, Fujimoto J, Kado Y, Takada T, Matsumoto K, Tanaka R. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Applied and environmental microbiology. 2004;70(1):167–173. doi: 10.1128/AEM.70.1.167-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartosch S, Fite A, Macfarlane GT, McMurdo MET. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Applied and environmental microbiology. 2004;70(6):3575–3581. doi: 10.1128/AEM.70.6.3575-3581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]