Abstract
STUDY QUESTION
What is the status of fertility treatment and birth outcomes documented over the first 6 years of the Canadian Assisted Reproductive Technologies Register (CARTR) Plus registry?
SUMMARY ANSWER
The CARTR Plus registry is a robust database containing comprehensive Canadian fertility treatment data to assist with providing evidence-based rationale for clinical practice change.
WHAT IS KNOWN ALREADY
The rate of infertility is increasing globally and having data on fertility treatment cycles and outcomes at a population level is important for accurately documenting and effecting changes in clinical practice.
STUDY DESIGN, SIZE, DURATION
This is a descriptive manuscript of 183 739 fertility treatment cycles from 36 Canadian clinics over 6 years from the CARTR Plus registry.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Canadian ART treatment cycles from 2013 through 2018 were included. This manuscript described trends in type of fertility treatment cycles, pregnancy rates, multiple pregnancy rates, primary transfer rates and birth outcomes.
MAIN RESULTS AND THE ROLE OF CHANCE
Over the 6 years of the CARTR Plus registry, the number of treatment cycles performed ranged from less than 200 to greater than 1000 per clinic. Patient age and the underlying cause of infertility were two of the most variable characteristics across clinics. Similar clinical pregnancy rates were found among IVF and frozen embryo transfer (FET) cycles with own oocytes (38.9 and 39.7% per embryo transfer cycle, respectively). Fertility treatment cycles that used donor oocytes had a higher clinical pregnancy rate among IVF cycles compared with FET cycles (54.9 and 39.8% per embryo transfer cycle, respectively). The multiple pregnancy rate was 7.4% per ongoing clinical pregnancy in 2018, which reflected a decreasing trend across the study period. Between 2013 and 2017, there were 31 811 pregnancies that had live births from all ART treatment cycles, which corresponded to a live birth rate of 21.4% per cycle start and 89.1% of these pregnancies were singleton live births. The low multiple pregnancy rate and high singleton birth rate are associated with the increase in single embryo transfers.
LIMITATIONS, REASONS FOR CAUTION
There is potential for misclassification of data, which is present in all administrative health databases.
WIDER IMPLICATIONS OF THE FINDINGS
The CARTR Plus registry is a robust resource for ART data in Canada. It provides easily accessible aggregated data for Canadian fertility clinics, and it contains data that are internationally comparable.
STUDY FUNDING/COMPETING INTEREST(S)
There was no funding provided for this study. The authors have no competing interests to declare.
Keywords: IVF, registry, ART, pregnancy outcomes, birth outcomes, Canada
WHAT DOES THIS MEAN FOR PATIENTS?
The rates of infertility treatment are increasing in many countries around the world. This is also true for Canadians. Databases that collect information about fertility treatment and the outcomes associated with the treatment are important for evaluating success. In Canada, a new database called the Canadian Assisted Reproductive Technologies Register (CARTR) Plus registry was designed to create a new resource for clinicians and scientists to report on treatment outcomes and to conduct research. Additionally, it was designed so that it could be comparable to other international registries. The CARTR Plus registry started collecting treatment cycle information in January 2013 and includes 183 739 fertility treatment cycles entered that corresponded with 31 811 pregnancies that resulted in live births. This database provides a platform for quality assurance and research to support evidence-based clinical practice and public policy in Canada.
Introduction
Globally, there is an increasing prevalence of infertility treatment, including ART (Boivin et al., 2007). ART is used as a treatment for a number of causes of infertility, including endometriosis, tubal factor, and male factor infertility (Sokol, 1995; Abrao et al., 2013). The capacity to conduct longitudinal studies on ART is limited by the availability of data. However, there is a need to examine the effects of these treatments on patients and infants born from these treatments in large epidemiologic studies, in order to inform evidence-based changes in policy and clinical practice when indicated. The Canadian Assisted Reproductive Technologies Register (CARTR) was established in 1999, to collect fertility treatment cycle data from Canadian fertility centres. This was the first national database to collect ART data in Canada, and was owned by the IVF Medical Directors Group and administered by the Canadian Fertility and Andrology Society (CFAS). From 1999 through 2012, CARTR collected data on ART treatment cycles and accompanying outcomes. This was a manual process in which data were amalgamated each year by a research coordinator and presented annually at the CFAS meeting. CARTR provided relevant data that supported a number of IVF initiatives, including the Canadian Framework for the Prevention of Multiple Births associated with Infertility (‘Making a Difference—AHRC Annual Report,’ 2011) and the IVF Improvement Committee.
In 2011, discussions began on integration between the Canadian IVF Medical Directors Group and the Better Outcomes Registry & Network (BORN) Ontario, the province’s maternal–child registry. BORN Ontario provides database infrastructure, a secure environment, privacy and security policies and procedures approved by the province’s privacy legislation, as well as epidemiologic expertise. In Ontario, integration with BORN Ontario would mean that fertility clinics would have access to birth outcome information directly from the BORN Ontario registry and would no longer need direct follow-up with patients. This collaboration involved the integration and enhancement of the existing CARTR database, in order to improve the utility and functionality of this ART registry. The vision of the CARTR-BORN collaboration was to follow pregnancies from conception through birth in order to produce accurate and reliable information for clinics with the goal of improving or facilitating care, as well as providing an avenue to facilitate research. In Ontario, the linkage to BORN Ontario allows for almost real-time birth outcomes and as BORN Ontario expands the collection of data into the childhood period, it will facilitate acquiring knowledge about ART children’s health beyond the neonatal period.
The alignment between the data repository and reporting requirements of the Canadian fertility clinics and the design, structure and capabilities of the BORN Information System led to a successful alliance. Fertility treatment data are collected and stored following strict privacy and security controls, according to industry best practices. Access to and use of these data is tightly controlled and only made available to authorized users on an as-needed basis. Access to these data is monitored with robust auditing controls that track all data access. Each fertility clinic signs a data sharing agreement with BORN Ontario that outlines which data are collected and for what purpose. Patients who have fertility treatment at Ontario clinics must consent to allow the clinic to receive the corresponding birth outcomes information from BORN Ontario.
Design and data collection
A fundamental objective of the CARTR Plus registry was to improve utility and functionality over the historical CARTR database. The design of the CARTR Plus registry was based on recommendations from several sources, including the CARTR Plus registry Steering Committee, Data Collection Subcommittee, Reporting and Outcomes Subcommittee and the Technical Subcommittee, in order to ensure a clear and comprehensive concept for the design of the database. These committees explored ART registries from other countries in order to design and develop a data capture system that could produce relevant results for Canadian ART clinics, but would also be clinically comparable to registries worldwide. Additionally, two principal objectives were as follows: first, to have access to information on babies born through ART past the first few minutes of life, and second, the ability to produce cumulative pregnancy rates.
Data collection in the CARTR Plus registry includes patient demographics, reason for infertility treatment, obstetrical history, treatment(s), pregnancy and birth outcomes. These data may be entered through two methods: manually through the secure CARTR Plus registry web-based application or directly through an upload from a clinic’s electronic medical record (EMR) system. Fertility treatment cycle data are entered for a variety of ART procedures including IVF with and without ICSI, IVM, oocyte banking, oocyte donation using both fresh oocytes and frozen donor oocyte, preimplantation genetic testing of embryos, frozen embryo transfer (FET), outcomes of the use of autologous frozen oocytes and gestational carrier cycles. These cycles can be linked by an unidentifiable patient identifier, a cycle identifier and an indicator for each batch of oocytes, the latter of which allows for linkage across all treatment cycles using oocytes retrieved in the same batch. This is a novel concept for fertility registries, which enables cumulative pregnancy rate calculations not only per patient but also per batch of oocytes. This allows for investigations to be conducted on embryos derived from a single cohort.
The CARTR Plus registry also collects detailed information on the type of stimulation protocol (i.e. agonist, antagonist), medication for controlled ovarian stimulation (i.e. clomiphene citrate, aromatase inhibitor, estrogen patch, FSH, LH), trigger medications (GnRH agonist, recombinant-hCG), estradiol levels, progesterone levels, number of follicles, number of oocytes, number of usable embryos, transfer date and donor gamete information. Birth outcome information is entered by the clinics in every province with a fertility clinic; however, in Ontario, pregnancies from ART cycles are directly linked within the BORN Information System, which contains birth outcomes and supplementary information on the labour and birth, including information on preterm birth, low birth weight, congenital anomalies and adverse maternal outcomes. Ontario fertility clinics are also able to add additional birth outcome information. This is especially relevant for capturing fetal loss prior to 20 weeks’ gestation. Using the near-automatic data entry within Ontario, the CARTR Plus registry contains robust data on the delivery and outcome, which allows access to long-term follow-up of babies born after IVF treatment. Currently, the CARTR Plus registry does not collect information on IUI; however, the database has the capacity to collect and report this information.
Data output
Clinics have near real-time access to their data through the BORN Information System. They can also view a variety of demographic, clinical and annual summary reports that allow them to view their data aggregated by time periods, as well as compare their data to aggregated data from all Canadian clinics. There are additional reports that provide clinics with the proportions and rates that they use on a daily basis in their clinic. These reports were designed by stakeholders to enable fertility clinics to better monitor the care that they provide to patients. The aim of the simplicity of these reports was to provide clinics with a succinct, but comprehensive look at how their clinic is performing. Recent reports have been designed to be representative of global ART reporting methods. CARTR Plus registry data are also available for research via a formal data request process through BORN Ontario.
The objective of this study was to describe fertility treatment and birth outcomes in Canada that have been documented throughout the first 6 years of the new CARTR Plus registry.
Materials and Methods
This study included all fertility treatment cycles from 1 January 2013 through 31 December 2018, as well as all corresponding birth outcomes from 1 January 2013 through 31 December 2017. Categorical variables were described using frequencies, and proportions and continuous variables were described using mean values. All analyses were performed using SAS version 9.4. All data elements contained within the CARTR Plus registry can be found in the Supplementary Data.
Results
The CARTR Plus registry began collecting fertility treatment cycle data in Canada on 1 January 2013. As of 31 December 2018, there were 183 739 fertility treatment cycles entered into the CARTR Plus registry. The number of fertility clinics that offer services in Canada has fluctuated from 31 to 36 clinics over the 6 years of the CARTR Plus registry. Although these clinics all offer IVF services, the number of treatment cycles performed by each clinic ranges from less than 200 to greater than 1000. Additionally, the patient population served by each clinic is equally as varied. Patient age and the underlying cause of infertility are two of the most variable characteristics across clinics.
IVF and FET treatment cycles using a patient’s own oocytes comprise the majority of treatment cycles in the CARTR Plus registry and; the majority of patients seeking fertility treatment are ≥35 years of age (Table I). IVF and FET treatment cycles were the two methods of treatment that have consistently remained the most prevalent; however, in recent years there has been a shift to performing freeze-all treatment cycles. In these cases, an IVF cycle is performed, but there is no intention to transfer a fresh embryo. Rather, all viable embryos are cryopreserved and then transferred at a later date through a FET cycle. This has led to a greater proportion of FET treatment cycles in the CARTR Plus registry database in recent years (2013, 33.3%; 2018: 48.0%). This can be further demonstrated when the numbers of freeze all cycles for preimplantation genetic testing for aneuploidy (PGT-A), preimplantation genetic testing for monogenic disorders (PGT-M) or preimplantation genetic testing for structural rearrangements (PGT-SR) are examined (Table II). Although only a relatively small proportion of treatment cycles used PGT-A or PGT-M/SR in this study, which were identified as PGT cycles at the initiation of the treatment cycle, Table II shows the dramatic change in clinical practice over a short period of time.
Table I.
Distribution of IVF and frozen embryo transfer treatment cycles in Canada by patient age.
| Year |
Patient age
(years) |
IVF
own oocytes n = 92 694 n (%) |
FET
own oocytes n = 67 089 n (%) |
IVF
donor oocytes n = 4578 n (%) |
FET
donor oocytes n = 8890 n (%) |
|---|---|---|---|---|---|
| 2013 | <35 | 5651 (39.0) | 3434 (45.6) | 98 (14.3) | 121 (13.4) |
| 35–37 | 3292 (22.7) | 1932 (25.7) | 87 (12.7) | 89 (9.9) | |
| 38–40 | 3172 (21.9) | 1411 (18.7) | 109 (15.9) | 131 (14.5) | |
| 41–42 | 1726 (11.9) | 505 (6.7) | 116 (16.9) | 122 (13.5) | |
| ≥43 | 631 (4.4) | 246 (3.3) | 276 (40.2) | 438 (48.6) | |
| Total | 14 472 | 7528 | 686 | 901 | |
| 2014 | <35 | 6024 (39.2) | 4028 (45.4) | 114 (18.0) | 127 (12.6) |
| 35–37 | 3506 (22.8) | 2235 (25.2) | 84 (13.2) | 108 (10.7) | |
| 38–40 | 3333 (21.7) | 1645 (18.5) | 98 (15.4) | 159 (15.8) | |
| 41–42 | 1811 (11.8) | 657 (7.4) | 105 (16.5) | 131 (13.0) | |
| ≥43 | 681 (4.4) | 308 (3.5) | 234 (36.9) | 484 (48.0) | |
| Total | 15 355 | 8873 | 635 | 1009 | |
| 2015 | <35 | 6200 (39.6) | 4399 (44.5) | 155 (21.2) | 185 (14.6) |
| 35–37 | 3560 (22.7) | 2552 (25.8) | 93 (12.7) | 134 (10.6) | |
| 38–40 | 3475 (22.2) | 1915 (19.4) | 119 (16.3) | 197 (15.5) | |
| 41–42 | 1730 (11.1) | 687 (6.9) | 100 (13.7) | 178 (14.0) | |
| ≥43 | 691 (4.4) | 335 (3.4) | 263 (36.0) | 576 (45.4) | |
| Total | 15 656 | 9888 | 730 | 1270 | |
| 2016 | <35 | 5971 (38.7) | 5396 (44.5) | 173 (22.2) | 260 (16) |
| 35–37 | 3567 (23.1) | 3183 (26.2) | 103 (13.2) | 194 (11.9) | |
| 38–40 | 3457 (22.4) | 2268 (18.7) | 126 (16.2) | 241 (14.8) | |
| 41–42 | 1825 (11.8) | 876 (7.2) | 118 (15.2) | 207 (12.7) | |
| ≥43 | 593 (3.8) | 415 (3.4) | 258 (33.2) | 723 (44.5) | |
| Total | 15 413 | 12 138 | 778 | 1625 | |
| 2017 | <35 | 6215 (38.8) | 6191 (44.6) | 178 (21) | 294 (15.2) |
| 35–37 | 3777 (23.6) | 3640 (26.2) | 118 (13.9) | 230 (11.9) | |
| 38–40 | 3519 (22) | 2592 (18.7) | 152 (17.9) | 290 (15) | |
| 41–42 | 1835 (11.5) | 992 (7.1) | 114 (13.4) | 277 (14.4) | |
| ≥43 | 654 (4.1) | 463 (3.3) | 287 (33.8) | 839 (43.5) | |
| Total | 16 000 | 13 878 | 849 | 1930 | |
| 2018 | <35 | 5939 (37.6) | 6335 (42.9) | 199 (22.1) | 288 (13.4) |
| 35–37 | 3710 (23.5) | 3975 (26.9) | 148 (16.4) | 287 (13.3) | |
| 38–40 | 3601 (22.8) | 2847 (19.3) | 168 (18.7) | 354 (16.4) | |
| 41–42 | 1841 (11.7) | 1083 (7.3) | 115 (12.8) | 311 (14.4) | |
| ≥43 | 707 (4.5) | 544 (3.7) | 270 (30) | 915 (42.5) | |
| Total | 15 798 | 14 784 | 900 | 2155 |
FET: frozen embryo transfer
Table II.
Proportion of IVF treatment cycles using own oocytes that did not have an embryo transferred owing to freeze all for PGT-A or PGT-M/SR.
| Year |
Freeze all for PGT-A
n (%) |
Freeze all for PGT-M/SR
n (%) |
Total fresh cycles
N |
|---|---|---|---|
| 2015 | 354 (6.7) | 407 (7.7) | 5312 |
| 2016 | 1459 (22.4) | 598 (9.2) | 6534 |
| 2017 | 1926 (24.1) | 506 (6.3) | 8006 |
| 2018 | 2585 (30.7) | 198 (2.4) | 8424 |
PGT-A: preimplantation genetic testing for aneuploidy, PGT-M/SR: PGT for monogenic disorders/structural rearrangements
The CARTR Plus registry contains 52 284 clinical pregnancies, 47 315 of which had a fetal heart beat detected on ultrasound (ongoing clinical pregnancy), and 42 170 of which were singleton pregnancies. The overall clinical pregnancy rate per embryo transfer cycle was 39.5%. When stratified by the type of treatment cycle, there was a similar clinical pregnancy rate among IVF and FET cycles with own oocytes (38.9 and 39.7% per embryo transfer cycle, respectively). Treatment cycles that used donor oocytes had a higher clinical pregnancy rate among fresh IVF cycles compared with FET cycles (54.9 and 39.8% per embryo transfer cycle, respectively). As expected, the proportion of clinical pregnancies per cycle start was greater amongst those cycles that did not use PGT-A (29.1% compared to 22.3%); however, when we compared the proportion of clinical pregnancies per embryo transfer cycle, the rate was higher amongst cycles that used PGT-A (49.1% compared to 38.9%).
The CARTR Plus registry collects detailed information on the indication for receiving fertility treatment. Female factor infertility, which included a variety of diagnoses, such as advanced female age, diminished ovarian reserve, tubal factor, polycystic ovary syndrome, endometriosis, other ovulatory disorder, other female factor and other uterine factor, was the most commonly reported reason for treatment (100 138 [54.5%]). However, male factor infertility was documented in 59 335 ART treatment cycles (32.3%). Male factor infertility was most prevalent among patients <35 years of age.
Although the majority of treatment cycles used autologous oocytes, 15 173 of treatment cycles used donor oocytes or embryos (8.26% of all ART treatment cycles). Currently, the CARTR Plus registry contains information on 3953 fertility treatment cycles that used a gestational carrier. Approximately half of these gestational carrier treatment cycles used donor oocytes or embryos (57.9%). Similar to practice changes among non-gestational carrier treatment cycles, the mean number of embryos that were transferred per embryo transfer cycle has decreased in recent years (2013: 1.79; 2018: 1.10). In total, 44.3% of these gestational carrier cycles resulted in a clinical pregnancy. Additionally, a small number of treatment cycles in the CARTR Plus registry were for oocyte or embryo banking for cancer and other medical or non-medical reasons (2016 and 1958, respectively).
The CARTR Plus registry permits the linking of treatment cycles in two ways: by patient and by batch of oocytes produced from a single retrieval cycle. This allowed for the estimation of the cumulative success rather than the success at an individual stage. Using this database feature, we first calculated cumulative pregnancy rates per patient, defined as the number of clinical pregnancies resulting from one or more retrieval cycles, including the cycle when fresh embryos were transferred and all related subsequent frozen/thawed embryo transfer cycles if the fresh embryo transfer did not result in a pregnancy, using cycles from 2013 through 2018. After one embryo transfer cycle, the probability of clinical pregnancy was 40.7% per patient including all autologous embryo transfer cycles. However, the cumulative clinical pregnancy rate per patient after two embryo transfer cycles was 53.3%. We also calculated the cumulative pregnancy rate per batch of oocytes retrieved from a single retrieval cycle and found a cumulative pregnancy rate of 39.5% after one embryo transfer cycle (fresh or frozen transfers with autologous oocytes). This cumulative pregnancy rate increased to 48.2% after the second embryo transfer cycle (Fig. 1). There were 6603 cycles that were cancelled prior to retrieval and 21 425 where no embryo transfer occurred due to a variety of factors, including no oocytes, no utilizable oocytes, no normal fertilization, no utilizable embryos and no sperm (Supplementary Data). A portion of the cycles that had no embryo transfer in this cohort were freeze all cycles where the FET cycle had not yet occurred.
Figure 1.
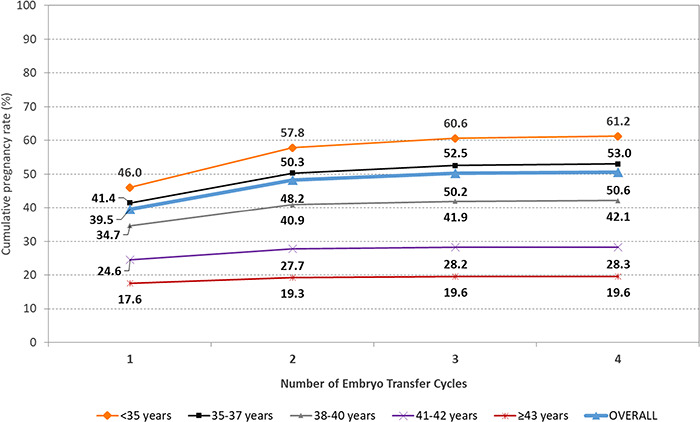
Canadian cumulative pregnancy rate by embryo transfer cycle number from embryos from a single retrieval cycle, stratified by age. For the data in this figure: a clinical pregnancy included clinical intrauterine, heterotopic or ectopic pregnancy; own oocytes were used exclusively, and patient age refers to age at oocyte retrieval. Each ‘transfer cycle’ includes the fresh transfer and subsequent frozen transfers. Cycles without transfer are not included in the dataset. Reprinted with permissions from Canadian Assisted Reproductive Technologies Registry (CARTR) Plus, September (2019).
The primary transfer rate is a clinically relevant measure of success, allowing clinicians and patients to predict their success rate if a viable embryo is achieved and transferred, especially with the increased prevalence of freeze all cycles. Primary transfer was defined as the first IVF or FET cycle in the CARTR Plus registry with an embryo transfer using a patient’s own oocytes. Patients included in this subgroup also had no documented prior treatment cycles using their own oocytes. The overall clinical pregnancy rate per primary transfer was 40.7%. As expected, when the clinical pregnancy rate was stratified by patient age, the rate for patients <35 years of age was 46.4% while the rate for patients ≥35 years was 35.5%.
Between 2013 and 2017, there were 31 811 pregnancies that resulted in live births from all ART treatment cycles, corresponding to a live birth rate (LBR) of 21.4% per cycle start and 89.1% of these pregnancies resulted in singleton live births (Table III). We further classified singleton live births delivered at ≥37 weeks’ gestation and with a birth weight ≥2500 g as a live birth with a good perinatal outcome [23 652 (83.4%)]. The greatest frequency of live births occurred from IVF treatment cycles using own oocytes [13 296 (76.2%)]. The majority of these live births (IVF with own oocytes) were singletons (87.3%). Miscarriages accounted for 19.3% of birth outcomes among ongoing clinical pregnancies, whereas stillbirth was only 0.82%. A similar proportion of live births was observed among birth outcomes for FET treatment cycles with autologous oocytes (73.0%). Singleton live births and multiple live births per pregnancy accounted for 91.2 and 8.8% of birth outcomes, respectively. There was a slightly higher rate of miscarriage among FET with own oocytes at 21.3%.
Table III.
Distribution of birth outcomes per ongoing clinical pregnancy.
|
Live birth
n = 31 811 n (%) |
Stillbirth
n = 335 n (%) |
|
|---|---|---|
| Singleton ongoing clinical pregnancy | 27 670 (87.0) | 285 (85.1) |
| Multiple pregnancy | 4141 (13.0) | 50 (14.9) |
| Twin | 4001 (12.6) | 49 (14.6) |
| Triplet or higher order | 140 (0.4) | S |
Ongoing clinical pregnancy: clinical intrauterine, heterotopic or ectopic pregnancy with ≥ 1 fetal heart beat on ultrasound. Multiple pregnancy: ongoing clinical pregnancy with > 1 fetal heart beat on ultrasound. Live birth: at least one live birth if multiple pregnancy S: frequency and percentage suppressed due to cell count less than six.
Similar to clinical pregnancy rates, birth outcome success rates were greatly affected by patient age. Among IVF treatment cycles using own oocytes, the LBR per embryo transfer cycle was 38.6% among patients <35 years compared to 5.49% among patients ≥43 years. A similar trend was seen for both singleton live births and live births with a good perinatal outcome. Birth outcome success rates were stratified by age at time of oocyte retrieval per embryo transfer cycle for FET treatment cycles using own oocytes. The LBR for patients aged <35 years at time of oocyte retrieval was 32.9% in FET cycles, while the LBR among patients ≥43 years was 12.6% for FET cycles.
The overall multiple LBR per ongoing clinical pregnancy for Canada was 8.1%. The multiple LBR was greatly affected by the number of embryos transferred for all ART treatment cycles. Among ongoing clinical pregnancies, when one embryo was transferred the twin LBR was 1.18%; when two embryos were transferred the twin LBR increased to 20.1% and the triplet or higher order LBR was 0.31%. The transfer of three or more embryos led to a twin LBR of 14.8% and a triplet or higher order LBR of 1.35%. About two-thirds (62.6%) of multiple live births following IVF were born preterm (<37 weeks), as compared to 12.2% of singleton live births. Similar rates of preterm birth were observed among multiple and singleton live births following FET treatment cycles (64.3 and 10.5%, respectively).
Discussion
This study describes the creation of the CARTR Plus registry, including the type of information collected by the registry, as well as pregnancy and birth outcomes for the first 6 years of operation. The development of this registry has allowed for more comprehensive and accurate tracking of fertility treatment cycles and their treatment outcomes, as well as birth outcomes. This is especially apparent in the province of Ontario where CARTR Plus registry data are linked to the perinatal outcomes that are documented for all births in the province.
There have been initiatives where having quality data have assisted in effectively evaluating changes in practice and communicating results. Decreasing the multiple pregnancy rate among IVF conceptions has been a priority for reproductive endocrinology and infertility specialists in Canada. In 2010, the Society of Obstetricians and Gynaecologists of Canada and the CFAS published a joint practice guideline on the importance of elective single embryo transfers (SET) following IVF in good prognosis patients (Min et al., 2010). In 2012, CFAS also published a position statement on reducing the multiple pregnancy risk associated with IVF (Canadian Fertility and Andrology Society, 2012). The goal was to reduce the multiple pregnancy rate among IVF pregnancies to 25% by 2012 and to 15% by 2015 (Canadian Fertility and Andrology Society, 2012). Figure 2 shows the decreasing trend of multi-fetal gestations among ART pregnancies from 2013 to 2018. It illustrates that the fertility centres in Canada have successfully reduced their multiple pregnancy rate. In 2018, the multiple pregnancy rate was 7.4% per ongoing clinical pregnancies and there were only 642 documented multiple gestation pregnancies in Canada following ART (twin pregnancies: 625; triplet or higher order pregnancies: 17).
Figure 2.
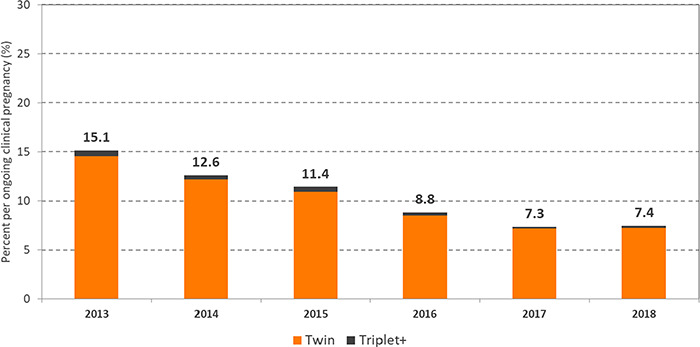
Percentage of multiple pregnancies per ongoing clinical pregnancy for all ART treatment cycles in Canada, 2013–2018. For data in this figure ongoing clinical pregnancy defined as: clinical pregnancy with ≥1 fetal heart beat on ultrasound; multiple pregnancy defined as: ongoing clinical pregnancy with >1 fetal heart beat on ultrasound. Reprinted with permissions from Canadian Assisted Reproductive Technologies Registry (CARTR) Plus, September (2019).
In addition to the CFAS position statement to decrease multiple pregnancy rates among IVF conceptions, the CARTR plus registry has also influenced provincial funding models for IVF in Quebec (2010–2015) and Ontario (2015–present) and embryo transfer practices. In both provinces, the majority of IVF cycles required that only one viable embryo was transferred through SET in order to receive public (provincial health insurance) funding for the treatment cycle. The transfer of two embryos was permitted in Quebec under the funded model, if a patient was >38 years of age. In 2018, 64.0% of embryo transfer cycles among IVF cycles using own oocytes were SET. Among FET cycles using own oocytes a larger proportion of embryo transfer cycles were SET (85.4%). Table IV illustrates the differences in the mean number of embryos transferred in Canada.
Table IV.
Mean number of embryos transferred and rate of multiple pregnancy among ART pregnancies.
| Year | Canada | Quebec | Ontario | Rest of Canada | ||||
|---|---|---|---|---|---|---|---|---|
| Mean # embryos transferred* | Multiple pregnancy rateǂ | Mean # embryos transferred* | Multiple pregnancy rateǂ | Mean # embryos transferred* | Multiple pregnancy rateǂ | Mean # embryos transferred* | Multiple pregnancy rateǂ | |
| 2013 | 1.59 | 15.2 | 1.27 | 4.7 | 1.72 | 18.8 | 1.76 | 19.4 |
| 2014 | 1.49 | 12.6 | 1.21 | 4.6 | 1.58 | 14.0 | 1.68 | 17.8 |
| 2015 | 1.40 | 11.4 | 1.20 | 4.4 | 1.46 | 13.2 | 1.54 | 15.4 |
| 2016 | 1.31 | 8.8 | 1.18 | 4.2 | 1.26 | 7.6 | 1.48 | 12.9 |
| 2017 | 1.25 | 7.3 | 1.17 | 4.6 | 1.20 | 6.0 | 1.40 | 10.7 |
| 2018 | 1.21 | 7.4 | 1.15 | 3.2 | 1.17 | 5.8 | 1.34 | 12.2 |
| Total | 1.37 | 10.3 | 1.20 | 4.3 | 1.36 | 11.1 | 1.52 | 15.0 |
*Per embryo transfer cycle (includes fresh and all FET cycles)
ǂPer ongoing clinical pregnancy following ART
The establishment of the CARTR Plus registry in 2013 has created a robust database for Canadian IVF treatment cycles. The large number of cycles contained within the CARTR Plus registry provides the ability to examine rare outcomes with the appropriate statistical power. Additionally, the comprehensive collection of hundreds of data elements by fertility treatment clinics allows for detailed reporting, evaluation and research. The collection of data directly from the medical chart provides a strong source of data. Recently, specific data elements from the CARTR Plus registry were validated. The audit involved a reabstraction of 25 data elements from medical charts at six Canadian fertility clinics, selected using a purposive sampling strategy. The mode of data entry (manual entry compared to EMR), volume of treatment cycles performed and geographic location were used to determine which clinics would be audited (Bacal et al., 2020). This audit investigated the validity of data entry within the CARTR Plus registry. It is essential that adequate data quality is ensured as CARTR Plus registry is an administrative database. The results of the Bacal et al. (2020) validation study have provided insight on the levels of misclassification bias. The results provide support for the use of these data for research and to inform health policy decisions.
Conclusion
The CARTR Plus registry was developed to provide a comprehensive resource for ART data in Canada. There was commitment from the Canadian fertility community to capture accurate and reliable data. The CARTR Plus registry allows for opportunities to increase data analysis capacity and provides easily accessible aggregated data by reducing the reporting burden through having a shared database for Canadian fertility clinics. Additionally, it was designed to incorporate features of other international databases and therefore the CARTR Plus registry contains data that are internationally comparable. This database offers greater access to accurate population-level Canadian ART data, providing a platform for quality assurance, ongoing surveillance, and research to support evidence-based clinical practice and public policymaking for ART in Canada.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Dr Matt Gysler who was essential to the creation of the CARTR Plus registry database and its acceptance among Canadian IVF Medical Directors, as well as Dr Vanessa Bacal for her work validating the CARTR Plus registry database.
Supplementary data
Supplementary data are available at Human Reproduction online.
Authors’ roles
A.L. wrote the manuscript and conducted the analyses; M.T., A.E.S., M.J., A.Y., F.B., M.C.L. and M.C.W. were involved in creating CARTR Plus registry and edited the manuscript; D.B.F., H.W., Y.G. and L.M. were involved in analyses and edited the manuscript; and M.E. was involved in the technical development of CARTR Plus registry and edited the manuscript.
Funding
There was no funding provided for this study.
Conflict of interest
The authors have no conflicts of interest.
References
- Abrao MS, Muzii L, Marana R. Anatomical causes of female infertility and their management. Int J Gynecol Obstet 2013;123:S18–S24. [DOI] [PubMed] [Google Scholar]
- Bacal V, Fell DB, Shapiro H, Lanes A, Sprague AE, Johnson M, Walker M, Gaudet LM. The Canadian Assisted Reproductive Technologies Register (CARTR) Plus database: a validation study. Hum Reprod Open 2020;2:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 2007;22:1506–1512. [DOI] [PubMed] [Google Scholar]
- Ontario BORN. Better Outcomes Registry & Network (BORN) Ontario Annual Report 2012–2013 and 2013–2014 2015.
- Canadian Fertility and Andrology Society Reduction of Multiple Pregnancy Risk Associated with IVF/ICSI IVF Medical Directors of Canada Position Statement 2012. 2012.
- Making a Difference—AHRC Annual Report 2011.
- Min J, Hughes E, Young D. Elective single embryo transfer following in vitro fertilization. J Obstet Gynaecol Can 2010;32:363–377. [DOI] [PubMed] [Google Scholar]
- Sokol R. The diagnosis and treatment of male factor infertility. Obstet Gynecol 1995;7:177–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


