Abstract
In recent decades, artificial selection has contributed greatly to meeting the demands for animal meat, eggs, and milk. However, it has also resulted in changes in behavior, metabolic and digestive function, and alterations in tissue development, including the brain and skeleton. Our study aimed to profile the behavioral traits and transcriptome pattern of chickens (broilers, layers, and dual-purpose breeds) in response to artificial selection. Broilers spent less time gathered as a group in a novel arena (P < 0.01), suggesting reduced fearfulness in these birds. Broilers also showed a greater willingness to approach a model predator during a vigilance test but had a greater behavioral response when first exposed to the vocalization of the predator. Genes found to be upregulated and downregulated in previous work on chickens divergently selected for fear responses also showed consistent differences in expression between breeds in our study and indicated a reduction in fearfulness in broilers. Gene ACTB_G1 (actin) was differentially expressed between breeds and is a candidate gene involved with skeletal muscle growth and disease susceptibility in broilers. Furthermore, breed-specific alterations in the chicken domestic phenotype leading to differences in growth and egg production were associated with behavioral changes, which are probably underpinned by alterations in gene expression, gene ontology terms, and Kyoto Encyclopedia of Genes and Genomes pathways. The results highlight the change in behavior and gene expression of the broiler strain relative to the layer and a dual-purpose native breed.
Keywords: artificial selection, behavioral traits, chicken, domestication phenotype, transcriptome profile
Introduction
Domestic chickens are under intensive artificial selection for fast growth and high egg production (Rauw et al., 1998). As a result, broilers for meat production have experienced a deliberate improvement in traits such as rapid growth and increased pectoral muscle mass (Corr et al., 2003; Schmidt et al., 2009) and leg morphology changes (Duggan et al., 2015). Unintended consequences have also occurred, such as changes in metabolic and digestive function (Jackson and Diamond, 1996) as well as changes in the brain and bone size (Agnvall et al., 2017). Despite thousands of years of domestication (Xiang et al., 2014), chickens retain the cognitive abilities of their red junglefowl ancestors (Smith et al., 2011). Similarly, the wild behavioral repertoire is largely intact in modern chickens, for example, as displayed by layer chicks that begin pecking and learning about appropriate food sources during the first 24 h of life and imprint on conspecifics and develop fear-related avoidance of people and unfamiliar objects (Dawkins, 2015). However, artificial selection has also resulted in profound changes in some aspects of behavior. For laying hens, deliberate selection against broody behavior has occurred (Price, 1999) alongside longer-term changes in animal personality such as responses to threatening stimuli (Jöngren et al., 2010) and stress tolerance (Schütz et al., 2004; Campler et al., 2009). The alternation of animal behavioral traits is known to be associated with changes in gene expression. For example, red junglefowl selected for reduced fear of humans had modifications in brain gene expression patterns (Belteky et al., 2016). Also, the early experience of acute stress has transgenerational effects on associative learning and stress responses operating through the regulation of gene expression (Goerlich et al., 2012). Despite these studies, there is little literature describing how chicken behavioral traits that have changed in response to selection are regulated by their transcriptome profiles.
This study aimed to determine the effect of the transcriptomic profile on production and behavioral traits of relevance to economic profitability and animal welfare and to provide a domestication and evolutionary insight on chickens under artificial selection. To maximize divergence in phenotypic traits, we compared phenotypic and transcriptomic patterns in breeds of chickens selected for different functions within China—globally the largest producer of eggs and the third largest producer of broilers. Specifically, we studied a broiler breed, a layer breed, and a dual-purpose native Chinese breed under a cage rearing system. The phenotypic traits recorded included productivity, response to a novel environment, and response in a test of vigilance. Thus, the study aimed to provide a comprehensive understanding of the transcriptomic profiles underlying divergent selection of chickens for different purposes.
Materials and Methods
Animals and husbandry
The experimental protocols were approved by the China Agricultural University Laboratory Animal Welfare and Animal Experimental Ethical Inspection Committee (approval no.: CAU20170605-1). The experiment was carried out at a farm located at Bijie City, Guizhou Province. Chicks (200 per selection line; all females) entered the experiment immediately after hatching (day 0). The commercial broilers and layers were bought from Guangxi Jinling Agriculture and Animal Husbandry Group Co., Ltd. (Nanning, Guangxi), and native birds were provided by Guizhou Nayong Yuanshengmuye Ltd. (Bijie, Guizhou). The broiler was a Chinese local breed selected for meat and with similar growth performance to other commercially available breeds, the layer was a highly commercialized breed from abroad, and the native breed was reared in Wumeng Mountain Area under relatively low selection pressure. The experiment compared a highly selected meat breed (Jinlinghua), a highly selected layer breed (Rowan Range), and a native dual-purpose breed (Wumeng black-bone chickens from Southwest China). The Jinlinghua breed was chosen to allow a more meaningful comparison against the native breed. Few layer breeds have been developed in China and hence the Rowan Range breed, developed outside of China, was used. All birds entered the experiment at the same age. Chickens were initially housed under brooder lamps in a single pen at a temperature of 32 to 34 °C for the first week and 28 to 30 °C for the second week. The lighting regime was 23:1 (L:D) h at hatching, reducing by 1 h light per day for the first 7 d and remaining at 16:8 (L:D) h dark thereafter.
On day 14, 100 chickens of each breed were randomly assigned into a conventional cage rearing system to give three treatments (broilers, layers, and native breed birds). All chicks were provided with food and water ad libitum. They were fed the same diet (New Hope Group, Chengdu, Sichuan, China) with a commercial broiler starter feed during the first 30 d and with a grower broiler feed from day 31 to the end of the experiment. Chicks of each breed were reared in four cages (groups of 25 birds; 0.45 m2 per cage; 0.018 m2 per chick) from day 15 to 21. Thereafter, the group size was reduced weekly by moving birds to additional cages such that the floor space allowance was 0.041 m2 per chicken at day 52. The cages were located on the top two tiers of a three-tier battery cage building.
Data collection
Production performance
From day 11, the amounts of food provided and that remaining at the end of the day were recorded daily to calculate the daily feed consumption. Fifty chickens per treatment, selected at random, were weighed on days 21, 28, 35, 42, and 51. The caged birds were randomly selected from several cages in all the tests.
Behavioral traits
Novel arena test.
On day 30, 20 randomly selected chicks from each treatment were moved to a test arena (2 × 2.5 × 1.5 m) enclosed by solid panels. Chicks were individually identifiable by colored leg rings attached from the age of 30 to 40 d. Behaviors (defined in Supplementary Table S1) were recorded by continuous observation of videos for 10 min and included standing, walking, resting, exploring, preening, aggression, feather pecking, and sham dust bathing as well as duration spent gathered together in response to the novel environment (hereafter called the “gathered duration”). Gathered duration represented the approximate duration of the 10-min novel arena test in which 15 or more chickens stood or lay in close proximity within a circle of approximately 50 cm radius. This duration was estimated from scan samples taken at 30 s intervals. A single observer extracted the data from the videos.
Vigilance test.
On day 40, 20 chickens of each group used in the novel arena test were individually placed in the same arena to test vigilance in response to a predator. Prior to testing, the birds were familiarized with live worms (200 g/d) as a highly valued food resource that were provided alongside regular food over the period from day 33 to 39 in their home pen per cage. Before the day of testing, chickens were deprived of food and water from 1800 hours the day before the test. When tested, regular feed was placed in one corner of the arena and regular feed with live worms was placed in the opposite corner where a hawk model (length 30 cm and width 30 cm) was placed 50 cm vertically above the feed. Furthermore, the vocalizations of a hawk were played three times (at 4, 8, and 12 min) during the 12-min test. The reaction of the individual was scored each time the hawk call was played on a scale from 0 to 4 according to Favati et al. (2014), where 0 represented the lowest fear response. In brief, 0 represented no visible change in the chicken’s behavior; 1 was scored if the bird lifted its head once and then immediately returned to exploration or eating; 2 was scored if the chicken lifted its head once and uttered an alarm call and/or walked rapidly for >3 s or froze for 3 to 10 s; 3 was recorded if the bird reacted as for score 2 but ran, attempted to escape or froze for 10 to 30 s; 4 was scored if the bird reacted as for score 2 but ran, attempted to escape or froze for >30 s.
Transcriptome profiles
At day 52, 10 birds from each treatment were randomly selected from those 20 birds being tested in the novel arena and vigilance tests and humanely slaughtered by rapid decapitation, and the right hemisphere of the hippocampus was collected. The rest of the birds were returned to the company of Guizhou Nayong Yuanshengmuye Ltd. (Bijie, Guizhou). The samples were immediately stored in liquid nitrogen after collection. Total RNA isolation was performed using TRIzol reagent (Invitrogen, USA). Transcriptomic data were analyzed by the support of BGI Genomics Technology Co., Ltd, Shenzhen, China. The library construction was prepared using NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, Beverly, MA, USA) and an Illumina Hiseq platform was used to generate paired-end 150 bp reads. The raw sequences were quality controlled by removing reads containing adapter and ploy-N and low-quality reads and placed with accession number PRJNA525938 in the National Center for Biotechnology Information (NCBI).
The Gallus_gallus-5.0 (Ensembl release 91) was used as the reference genome and gene model annotation file. The software HISTA v0.1.6-beta was deployed to map clean reads to the genome reference (Kim et al., 2015). The index of the reference genome was built using Bowtie v2.2.3 (Langmead and Salzberg, 2012), and then gene expression level counts were enumerated with RSEM v1.2.12 (Li et al., 2011).
Our study also analyzed several genes found to be upregulated or downregulated in the previous studies in chickens (Belteky et al., 2016) and mice (Mei et al., 2005; Ponder et al., 2010) in which different levels of fear were experimentally induced.
Statistical analyses
All data were analyzed by SAS 9.2 (SAS Inst. Inc., Cary, NC, USA) and are displayed as mean ± standard error (SE). Production performance data were checked for normality and homogeneity of variance, transformed where necessary, and analyzed by one-way ANOVA. The Duncan post hoc test was used to analyze the difference between breeds when significance (P < 0.05) was detected. Behavioral data did not meet the assumptions for parametric analysis, and no variable could be successfully transformed to meet these assumptions. Kruskal–Wallis H and post hoc tests were used to test the treatment differences. The Wilcoxon test was used to analyze the score obtained in the vigilance test. All values with P < 0.05 were regarded as statistically significant.
Differential expression analyses were performed using DESeq2, based on the negative binomial distribution (Love et al., 2014). The resulting P-values were adjusted using the Benjamini procedure for controlling the false discovery rate. Genes with a q-value < 0.05 were assigned as differentially expressed with fold change ≥ 2.0. Gene ontology (GO) enrichment was implemented by the GOseq R package (Young et al., 2010), and GO terms with corrected P-value (q-value) < 0.05 were considered to be enriched. After obtaining the GO annotation for differentially expressed genes (DEGs), the WEGO software was used to perform GO functional classification for DEGs and to understand the distribution of gene functions of the species at the macro level. Phyper of R was used for the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses using P ≤ 0.01 as the statistical criteria.
Results
Production performance
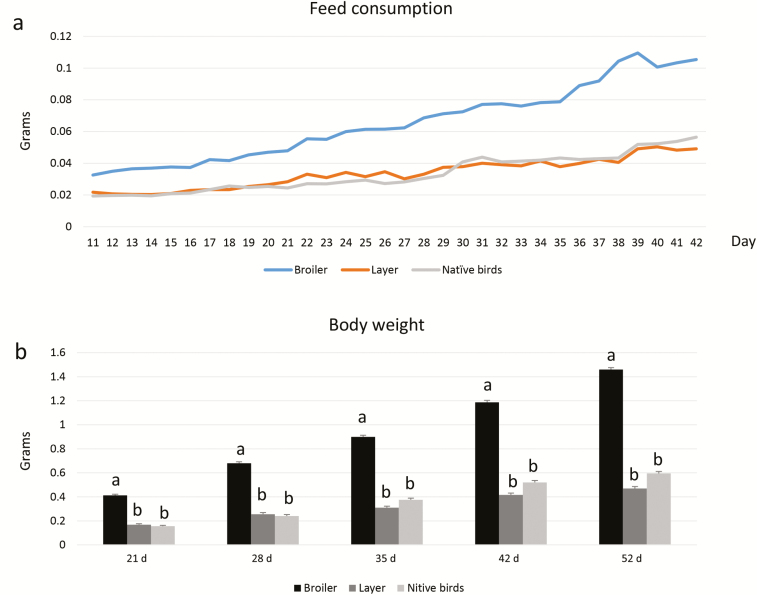
Feed consumption was greater in broilers than layers and native dual-purpose birds (Figure 1a). Body weight showed a significant effect of breed with heavier in broilers than layers and native dual-purpose birds on all weighing days (P < 0.001, Figure 1b).
Figure 1.
Feed consumption (g) from day 11 to 42 (a). Weight (g) profile of BL and NL birds at days 21, 28, 35, 42, and 52 (b). n = 100 birds per treatment for feed consumption, while n = 50 birds per treatment for the weight profile.
Behavioral traits
Novel arena test
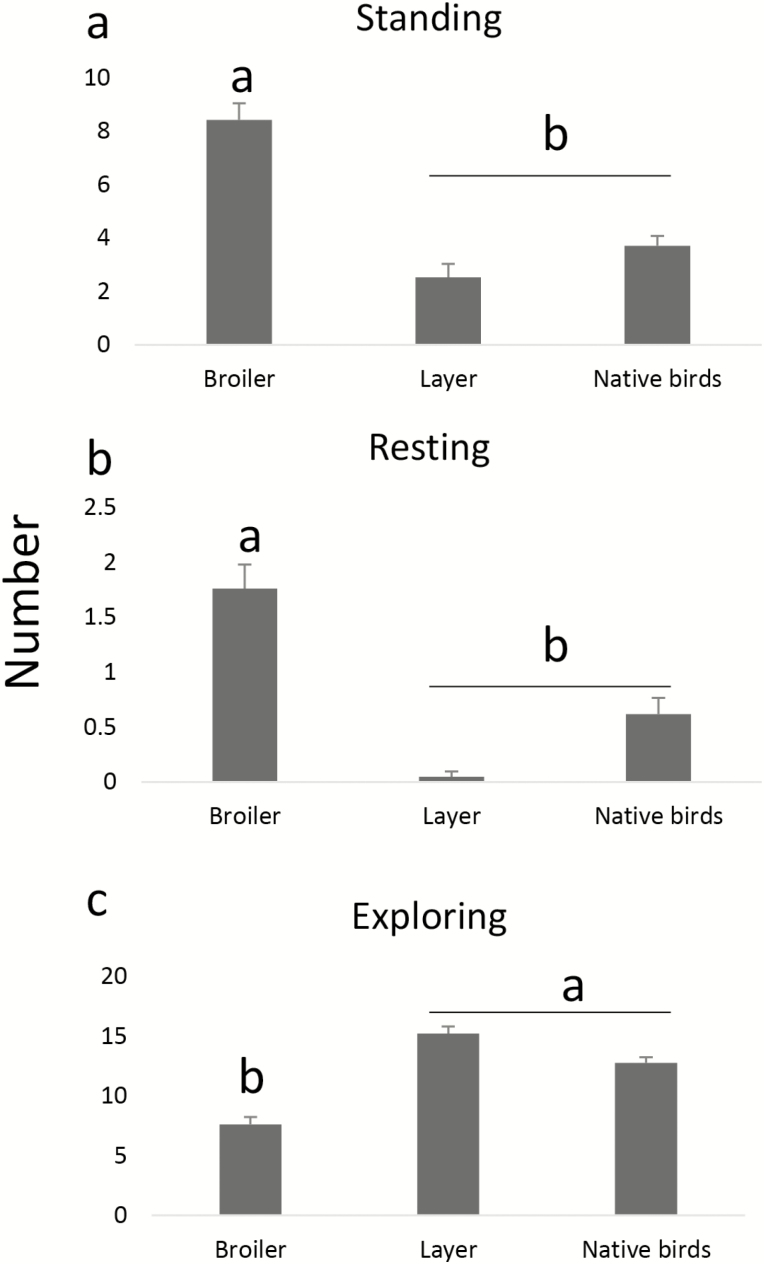
Aggression and sham dust bathing behaviors were observed in native breed birds and layers. Standing (Figure 2a) and resting (Figure 2b) occurred more frequently in broilers than the other breeds (P < 0.01). Broilers showed a lower frequency of exploratory behavior than the other breeds (P < 0.001; Figure 2c). Other behavior patterns were unaffected by breed.
Figure 2.
The duration spent gathered as a group standing (a), resting (b) and exploring (c) in response to a novel arena test. a and b represent the statistically significant differences between breeds. n = 20 birds per treatment.
Vigilance test
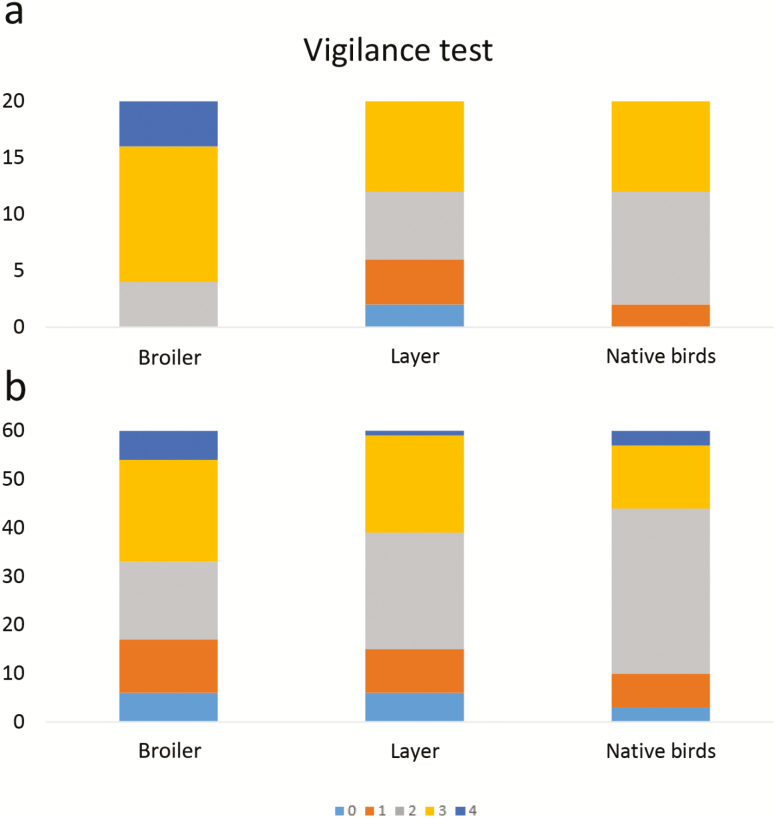
During the 12-min test, seven broilers consumed worms positioned below the model hawk while no layers or native birds did. Furthermore, a significant effect was seen under among the three breeds (P = 0.001) when birds experienced the hawk call for the first time (Figure 3a), whereby more broilers received scores 3 and 4 compared with layers and natives. These breed differences disappeared when responses to all three exposures to the hawk calls were considered together (Figure 3b).
Figure 3.
The response to the first hawk call (a) and total response to all three hawk calls (b) during the vigilance test. The scale of 0 to 4 represents the fear response, of which 0 indicates the lowest fear. n = 20 birds per treatment.
Transcriptomic profile of the hippocampus
In total, we obtained 25,273,907-32,687,371 paired-end reads for each sample (Supplementary Table S2). The Pearson correlation (R2) of the biological repeats of each group was higher than 0.99. The DEGs between different comparisons are shown in Supplementary Figure S1.
Comparison of broilers and native breed birds
The DEGs between broilers and native breed birds in cages were enriched on “developmental process,” “immune system,” “localization” and “locomotion” GO terms of the “biological process” ontology pathway, and several “cellular components” as well as “molecular activity” terms of the “molecular function” pathway (Supplementary Figure S2) but these GO terms did not significantly differ between treatments. Furthermore, several metabolism-related pathways including “global and overview maps,” “cofactors and vitamins,” “carbohydrate metabolism,” and “biosynthesis of secondary metabolites” were found to have four downregulated genes (Alpha-d-Glucose, HLCS, GCK, and ENO) in broilers vs. native birds (Table 1). The “endocrine and metabolic disease” pathway had two upregulated (IRS1 and KCNJ11) and two downregulated (GLUT4 and GK) genes in broilers birds. Furthermore, “endocrine” and “immune system” pathways were highlighted, with one upregulated gene (ERM) and three downregulated genes (EPAC, GCK, and CTNNA).
Table 1.
The differential KEGG pathways (P < 0.05) between broiler and native bird
| Level 1/level 21 | Pathway | Upregulation2 | Downregulation2 |
|---|---|---|---|
| M-Global and overview maps | Carbon metabolism | Alpha- d -Glucose | |
| M-Metabolism of cofactors and vitamins | Biotin metabolism | HLCS | |
| M-Carbohydrate metabolism | Glycolysis/gluconeogenesis | GCK, ENO | |
| M-Biosynthesis of other secondary metabolites | Neomycin, kanamycin, and gentamicin biosynthesis | HLCS | |
| H-Endocrine and metabolic diseases | Type II diabetes mellitus | IRS1, KCNJ11 | GLUT4, GK |
| O-Endocrine system | Insulin signaling pathway | GLUT4, GK, PHK | |
| O-Endocrine system | Insulin secretion | EPAC, GCK | |
| O-Immune system | Leukocyte transendothelial migration | ERM | EPAC, CTNNA |
1H, human disease; M, metabolism; O, organismal systems.
2Means candidate genes up/downregulated in broilers compared to native chickens.
Comparison of broilers and layers in cages
Between broilers and layers, the DEGs were also mainly enriched on GO terms of the “biological process” ontology pathway, including “behavior,” “growth,” “localization” and “locomotion,” and several “cellular components” as well as “molecular function” terms (Supplementary Figure S3). Specifically, the transport pathways of the “biological process” pathway and extracellular-related terms and terms in the “molecular function” pathway showed significant differences between groups (P < 0.05, Supplementary Table S3). Significant pathways with candidate genes are shown in Table 2. Two metabolism pathways, the “ether lipid” and “nitrogen metabolism” pathways with ENPP2 and CA, were upregulated and PLD_2 and CA downregulated in broilers compared with layer birds. As compared with layers, BC chickens were found to show enrichment of “organismal system” pathways, including “protein digestion and absorption,” “mineral absorption,” “leukocyte transendothelial migration,” “chemokine signaling pathway,” “platelet activation,” and the “cytosolic DNA-sensing” pathway. These pathways contained upregulated genes in BC birds (Pept, KCN, MT, TF, HEPH, ACTB_G1, P130cas, CAMs, NCF1, GRO, CAS, P47PHOX, CD182, GNB1, PAR1, CCL4, and CCL5) and downregulated genes (COL1A, CD10, CIC-2, EPAC, MLCK, and RPC1). The “cell adhesion molecules” (CAMs) and “ECM-receptor interaction” pathways with upregulation of MHC, SN, CD6, NCAM, NGL2, CLDN, MPZ, AGRN, CHAD, and CD104 and downregulation of TN were found in broilers compared with layers. The “tight junction” pathway had upregulated genes (CGN, ACTB_G1, MPP4, CFTR, CACNA1D, MYH) in broilers compared with layer chickens, whereas the “advanced glycation end-products (AGEs) RAGE signaling pathway in diabetic complications” had both upregulated and downregulated genes (COL and FN).
Table 2.
The differential KEGG pathways (P < 0.05) between broilers and layers
| Level 1/level 21 | Pathway | Upregulation2 | Downregulation2 |
|---|---|---|---|
| M-Lipid metabolism | Ether lipid metabolism | ENPP2 | PLD_2 |
| M-Energy metabolism | Nitrogen metabolism | CA | CA |
| O-Digestive system | Protein digestion and absorption | Pept, KCN | COL1A, CD10 |
| O-Digestive system | Mineral absorption | MT, TF, HEPH | CIC-2 |
| O-Immune system | Leukocyte transendothelial migration | ACTB_G1, P130cas, CAMs, NCF1 | EPAC |
| O-Immune system | Chemokine signaling pathway | GRO, CAS, P47PHOX, CD182, GNB1 | |
| O-Immune system | Platelet activation | ACTB_G1, PAR1 | MLCK |
| O-Immune system | Cytosolic DNA-sensing pathway | CCL4, CCL5 | RPC1 |
| O-Endocrine system | Relaxin signaling pathway | GNB1 | |
| H-Endocrine and metabolic diseases | AGEs RAGE signaling pathway in diabetic complications | COL, FN | COL, FN |
| E-Signaling molecules and interaction | CAMs | MHC, SN, CD6, NCAM, NGL2, CLDN, MPZ | |
| E-Signaling molecules and interaction | ECM-receptor interaction | AGRN, CHAD, CD104 | TN |
| C-Cellular community—eukaryotes | Tight junction | CGN, ACTB_G1, MPP4, CFTR, CACNA1D, MYH |
1C, cellular process; E, environmental information processing; H, human disease; M, metabolism; O, organismal systems.
2Means candidate genes up/downregulated in broilers compared with layers chickens.
Comparison between layers and native breed birds
Differences between layer and native birds were enriched on GO terms in the “biological process” ontology pathway, including “behavior,” “growth,” “localization” and “locomotion,” and several terms of the “cellular component” and “molecular function” pathways (Supplementary Figure S4). Among these GO terms, the “anion transport” and “adult behavior” terms of the “biological process” pathway and the “calcium-dependent phospholipid binding” term of the “molecular function” pathway were significantly different between layer and native birds (P < 0.05, Supplementary Table S3). Significant pathways (P < 0.05) with candidate genes are shown in Table 3. “Metabolism of cofactors and vitamins” and “amino acid metabolism” pathways with downregulation of HLC5, PCBD, ACADM, AGXT2, and MCEE and upregulation of OXCT were found in layers compared with native chickens. The “digestive” and “immune system” components of the “organismal system” pathways contained upregulated (COL1A, CD10, KIR) and downregulated genes (PepT, KCN, COL1A, CD10, ApoH, LCAT, CETP, LRP1/2, PCSK9, ABCB11, MHC1, CD8) in layer compared with native chickens. Several “environmental information processing” and one “cellular process” pathway with downregulation of immune-related genes such as MCH1 and CD8 as well as upregulation of CREB and TUBA were found in layer compared with native chickens. One immune disease system was found, namely the “graft-vs.-host disease” pathway, with downregulated MHC1 in a layer over native chickens.
Table 3.
The differential KEGG pathways (P < 0.05) between layers and native birds
| Level 1/level 21 | Pathway | Upregulation2 | Downregulation2 |
|---|---|---|---|
| M-Metabolism of cofactors and vitamins | Biotin metabolism | HLC5 | |
| M-Metabolism of cofactors and vitamins | Folate biosynthesis | PCBD | |
| M-Amino acid metabolism | Valine, leucine, and isoleucine degradation | OXCT | ACADM, AGXT2, MCEE |
| O-Digestive system | Protein digestion and absorption | COL1A, CD10 | PepT, KCN, COL1A, CD10 |
| O-Digestive system | Cholesterol metabolism | ApoH, LCAT, CETP, LRP1/2, PCSK9, ABCB11 | |
| O-Immune system | Antigen processing and presentation | KIR | MHC1, CD8 |
| E-Signaling molecules and interaction | Cell adhesion molecules | MHC1, CD8, NCAM, MAG, CLDN | |
| E-Signal transduction | cAMP signaling pathway | CREB | GPCR, PDE, VDCC, Hip1, DARPP32, CFTR |
| E-Membrane transport | ABC transporters | ABCA4, ABCB11 | |
| C-Cellular community—eukaryotes | Tight junction | TUBA | CLDN, CGN, CFTR, CACNA1D, ACTB_G1 |
1C, cellular process; E, environmental information processing; M, metabolism; O, organismal systems.
2Means candidate genes up/downregulated in layers compared with native chickens.
Discussion
In recent decades, well-documented impacts on behavior, morphology, physiology, and reproduction have occurred due to intensive artificial selection on meat and egg traits. Some of these changes have negatively affected animal welfare. However, the underlying transcriptomic changes have not been extensively studied. As expected and in agreement with previous evidence (Rauw et al., 1998; Corr et al., 2003), our study found impacts of selection on growth and feed consumption which were greater in broilers than native breed birds and layers.
Fear or anxiety is usually evoked by exposure to a novel environment (Forkman et al., 2007). Broilers showed more frequent resting, and standing behavior suggesting a greater frequency of behavioral transitions and more activity in this breed when they were placed in a novel environment. This was accompanied by less frequent exploratory behaviors that are usually performed when relaxed (Zimmerman et al., 2011) but exploration can also be driven by a desire to escape (Anselme, 2008). Interpreting these breed-specific behavioral responses in the context of fearfulness is, therefore, difficult. If this is an indication that broilers are less fearful than the other breeds, it is supported by the finding that broilers were the only birds willing to approach and consume preferential feed located below a model hawk in the vigilance test. The greater feed intake requirements of this breed may affect their risk aversiveness when presented with the opportunity of feed with a high nutritional value. This suggestion fits with the argument of Stamps (2010) that selection for high growth rates will increase potentially risky behavior across and within populations. However, broilers showed a greater behavioral response to the first call of the hawk in the vigilance test. Since they were more likely to be located close to, or directly beneath the hawk model, this first call may have carried greater salience for the broilers. When averaged across the repeated presentations of the call, no breed differences were detected suggesting that the greater fear response of broilers did not persist. Interpretation of the behavioral responses from the various tests is difficult but may suggest that broilers are less dependent upon social support in a novel environment and less risk-averse when faced with a dangerous but high-value feeding opportunity.
In the wild, threats from competition for resources and predation are common and severe (Smith and Johnson, 2012) and, therefore, fear is central to how an individual copes with its environment (Forkman et al., 2007). A reduction in fear has been suggested to be an essential step in the early domestication process (Jensen, 2014; Agnvall et al., 2015), which was linked to an increase in feed efficiency (Agnvall et al., 2015) and the production of larger offspring (Agnvall et al., 2017). Higher fear in Leghorns than broilers has been identified in social situations and when the levels of fear evoked by the test are moderate (Keer-Keer et al., 1996). Besides, the White Leghorn layer breed has been shown previously to have reduced fearfulness compared with red junglefowl (Campler et al., 2009; Agnvall et al., 2012; Belteky et al., 2016). These studies may indicate that fear responses are relative to the degree and the purpose of artificial selection. If the evidence above is indeed that broilers show reductions in fearfulness, which admittedly may be context specific, it suggests that selection on rapid meat production may have fundamentally altered a key aspect of the domestication phenotype. In contrast, the largely similar behavioral responses of the layer and dual-purpose native breed birds indicate that intense selection for egg production has affected responses to novelty and predation threat in a similar way to selection on a broader range of traits.
Genes (Supplementary Table S4) found to be up/downregulated after fear conditioning in mice (Mei et al., 2005; Ponder et al., 2010) were differently expressed between breeds in our study. The genes CAP1 (adenylate cyclase associated protein 1), CDH8 (cadherin 8, type 2), PACSIN2 (protein kinase C and casein kinase substrate in neurons 2), DGKB (diacylglycerol kinase, beta 90 kDa), PLCL2 (phospholipase C like 2), CTSD (cathepsin D), and GABRA1 (gamma-aminobutyric acid [GABA] A receptor, alpha 1) identified in mice (Mei et al., 2005; Ponder et al., 2010) to have altered expression after selection for context-specific fear conditioning were also located, with CAP1 and CTSD having lower expression in broiler than native birds while the others had higher expression in the former than the latter. The relative expression of CDH8, and PLCL2 was higher, while that of CAP1, and CTSD was lower in broilers compared to layer birds. Besides, MCM6 (minichromosome maintenance complex component 6) was downregulated and VIM (vimentin) was upregulated in layer compared with native birds. Accordingly, the expression of these fear-related genes did not follow a clear pattern with regard to the three breeds. For example, fear conditioning in mice downregulated DGKB and GABRA1 and these genes were also downregulated in native breed birds compared with broilers, possibly indicating heightened fearfulness in the former. In contrast, the upregulation of CDH8 and PACSIN2 was found after fear conditioning in mice and in broilers compared with native birds in our study, possibly indicating greater fearfulness in broilers compared with native breed birds. There has been little research to identify genes associated with differential fear responses in chickens. However, 21 DEGs were found in lines of chickens selected over five generations for high or low fear responses to human presence (Belteky et al., 2016). Of these, the relative expression of MAEA (macrophage erythroblast attacher) was upregulated and the relative expression of MR1 (class I histocompatibility antigen, F10 alpha chain-like) was downregulated in broiler birds relative to native birds, the relative expression of SAG (S-antigen; retina and pineal gland (arrestin) was downregulated, and the relative expression of MAEA was upregulated in broilers compared with layer birds, and the relative expression of fear-related genes ROPN1 L (rhophilin-associated tail protein 1 like), and DYNLRB2 (dynein, light chain, roadblock-type 2) were upregulated in layer compared with native birds. In our study, some of these genes were also found to significantly differ in expression between breeds and in all cases the direction of the upregulation or downregulation suggested reduced fearfulness in broilers. Specifically, MAEA, ROPN1L, and DYNLRB2 showed lower expression in the high fear strain of the Belteky et al. (2016) study and also lower expression in layers and native birds than broiler birds. Furthermore, MR1 and SAG were upregulated in the high fear strain and in layers and native birds compared with broilers. Thus, changes in fear response as a result of selection for fast growth in broilers compared with dual-purpose native and layer breeds seem to be underpinned by alterations in gene expression.
Between broilers and native birds, differential expression in pathways related to “metabolism,” “organismal system,” “human disease,” “environmental information processing,” and “cellular processes” were found. These systems are sensitive to amino acid metabolism and downregulation of the ACTB_G1 (actin) gene in broilers compared with native birds was found. The ACTB_G1 gene is involved in ATP synthase activity via carnosine synthase that is present in high concentrations in skeletal muscle and the olfactory bulb of vertebrates (Crush, 1970). This difference in gene expression is likely to play a major role in the divergent growth trajectories of broilers and to allow the differences in skeletal muscle growth rates of broiler and layer chickens previously found (Zheng et al., 2009). Furthermore, our study also provides molecular explanations for the substantial health costs paid by broilers for fast growth. It has long been demonstrated that artificial selection for fast growth results in increased risk of disease, higher mortality rates, and decreased physiological resilience later in life (Schantz et al., 1995; Dennis et al., 2006; Sumners et al., 2014). The carbon pathway, which was found to be differentially regulated in broilers and native birds in our study, plays a critical role in both DNA methylation and DNA synthesis and impacts both genetic and epigenetic processes in disease etiology (Wang et al., 2015) with effects on depression (Słopien, 2008) and pancreatic cancer (Suzuki et al., 2008) in humans. Evidence of regulatory changes were also found in disease pathways relating to cardiovascular disease, which is common in broiler populations (Siller and Hemsley., 1966). All the disease-related pathways involved the downregulation of the ACTB_G1 (actin) gene in broilers compared with native birds, which is known to be linked to a novel disease gene in familial hypertrophic cardiomyopathy (Mogensen et al., 1999) and immune system (Dustin, 2007; Huang et al., 2008) and cell motility processes (Ohmori et al., 1992). A higher rate of footpad dermatitis in broilers than layers and native birds was found (S. Chen, C. Yan, H. Xiang, J. Xiao, J. Liu, H. Zhang, J. Wang, H. Liu, X. Zhang, M. Ou, Z. Chen, W. Li, S. P. Turner, and X. Zhao, data are shown in a submitted manuscript), which is highly related to the quantity of moisture in the environment (Dawkins et al., 2004; Xiang et al., 2018). Thus, it is reasonable to expect differential expression of pathways such as those in the endocrine, digestive, and immune systems as well as cellular process pathways between broilers and natives as a result of the altered activity of ACTB_G1.
Differences in the immune-related pathways between broilers and layers were similar to those between broilers and native birds, which may suggest similar compromises to the broilers’ biology relative to that of the slower-growing strain selected for laying. Other basic pathways are known to have been altered by selection for fast growth. For example, the nitrogen metabolism pathway has been studied in relation to energy consumption in broiler chickens for a long time (Macleod and Dabutha, 1997) and it is not surprising to find differential regulation of genes in this pathway between breeds in our study. AGEs are regarded as the most important mechanism that triggers the pathophysiological cascades associated with diabetic complications (Chilelli et al., 2013) and may also be linked to the deliberate selection for meat in broilers compared with the laying strain. CAMs are known to be important for maintaining the proximity of cells during embryogenesis (Thiery et al., 1982) and are crucial in mediating inflammation-associated diseases (Zhong et al., 2018). Differences in expression of genes associated with CAMs and tight junction pathways were found between layers and broilers as well as between layers and native breed birds but not between broilers and native birds suggesting that changes in expression had occurred mostly in the layers. The regulation of cellular tight junctions was mostly mediated by upregulated genes in layers, suggesting greater control of molecule and ion flow between cells in layer birds. Differences between layer and native birds revealed higher rates of expression of pathways associated with cofactor, vitamin and amino acid metabolism, and immune and digestive-related functions in native birds compared with layers. The metabolism of cofactors and vitamins is important in preventing metabolic diseases (Rodrigues et al., 2012) and indeed they influence energy balance and digestibility in fast-growing broiler strains (Wallis and Balnav, 1984; Macleod and Dabutha, 1997; Schøyen et al., 2007). The upregulation of these pathways in native birds compared with layers may derive from the faster growth of the dual-purpose breed compared with the layers. The upregulation of immune and digestion-related pathways probably stems from an effort to prevent metabolic disease during the fast growth of native birds.
Conclusion
Artificial selection of chickens for different purposes was associated with changes in fear responses to novelty and in vigilance in the face of predation risk. Lower fear responses were found in broilers compared with layers and native breed birds. This is supported by evidence that genes found to be upregulated and downregulated in previous work that compared lines of chickens divergently selected for fear responses also showed consistent differences in expression between breeds in our study and indicated a reduction in fearfulness in the broiler breed. The results highlight the change in behavior and gene expression of the broiler strain relative to the layer and a dual-purpose native breed.
Supplementary Data
Supplementary data are available at Journal of Animal Science online.
Supplementary Table S1. Definitions of chicken behaviors. Behaviors in response to novel environment and vigilance tests
Supplementary Table S2. Statistical information on the transcriptomic data
Supplementary Table S3. GO term comparisons between treatments
Supplementary Table S4. Comparisons between treatments in differentially expressed fear-related genes
Supplementary Figure S1. DEGs between comparisons.
Supplementary Figure S2. Enriched on GO terms“biological process,” “cellular components,” and “molecular function” terms between broiler and native birds.
Supplementary Figure S3. Enriched on GO terms“biological process,” “cellular components,” and “molecular function” terms between broiler and layer.
Supplementary Figure S4. Enriched on GO terms“biological process,” “cellular components,” and “molecular function” terms between layer and native birds.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files. The sequences with accession number PRJNA525938 reported in this paper have been deposited in the NCBI.
Ethics Statement
The experimental protocols were approved by the China Agricultural University Laboratory Animal Welfare and Animal Experimental Ethical Inspection Committee (approval no.: CAU20170605-1).
Glossary
Abbreviations
- CAM
cell adhesion molecules
- CAP1
adenylate cyclase associated protein 1
- CDH8
cadherin 8, type 2
- CTSD
cathepsin D
- DEG
differentially expressed gene
- DGKB
diacylglycerol kinase, beta 90 kDa
- DYNLRB2
dynein, light chain, roadblock-type 2
- GABRA1
gamma-aminobutyric acid (GABA) A receptor, alpha 1
- GO
gene ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MAEA
macrophage erythroblast attacher
- MCM6
minichromosome maintenance complex component 6
- PACSIN2
protein kinase C and casein kinase substrate in neurons 2
- PLCL2
phospholipase C like 2
- ROPN1 L
rhophilin-associated tail protein 1 like;
- SAG
S-antigen
- VIM
vimentin
Acknowledgments
We express sincere thanks to all the staff from Guizhou Nayong Yuanshengmuye Ltd., Bijie, Guizhou, China for their support during the experiment and for providing the Wumeng Black-bone chicks. Many thanks to Rong and all the staff from Qianlong organic farm for the supply of the birds. This work was supported by the Joint Projects of Guizhou Nayong Professor Workstation (201705510410352), Joint fund of basic and applied basic research fund of Guangdong Province (2019A151 5110598), and by the Guangdong Provincial Key Laboratory of Animal Molecular Design and Precise Breeding (2019B030301010). The funder had no role in the conduct of the study.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors’ Contributions
X.Z., S.C., H.X., X.Z., M.O., Z.C., W.L.., and H.L. designed the project; S.C., C.Y., H.X., J.X., J.L., H.Z., J.W., H.L., X.Z., M.O., Z.C., and W.L. performed the experiment; S.C. and CY analyzed the transcriptome data; S.C. and H.Z. analyzed and interpreted the behavioral data; S.C., S.T., and X.Z. drafted and revised the manuscript. All authors have read and approved the manuscript.
Literature Cited
- Agnvall B., Bélteky J., and Jensen P... 2017. Brain size is reduced by selection for tameness in red junglefowl – correlated effects in vital organs. Sci. Rep. 7:3306. doi: 10.1038/s41598-017-03236-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnvall B., Jöngren M., Strandberg E., and Jensen P... 2012. Heritability and genetic correlations of fear-related behaviour in red junglefowl–possible implications for early domestication. PLoS One. 7:e35162. doi: 10.1371/journal.pone.0035162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnvall B., Katajamaa R., Altimiras J., and Jensen P... 2015. Is domestication driven by reduced fear of humans? Boldness, metabolism and serotonin levels in divergently selected red junglefowl (Gallus gallus). Biol. Lett. 11:20150509. doi: 10.1098/rsbl.2015.0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselme P. 2008. Abnormal patterns of displacement activities: a review and reinterpretation. Behav. Processes 79:48–58. doi: 10.1016/j.beproc.2008.05.001 [DOI] [PubMed] [Google Scholar]
- Bélteky J., Agnvall B., Johnsson M., Wright D., and Jensen P... 2016. Domestication and tameness: brain gene expression in red junglefowl selected for less fear of humans suggests effects on reproduction and immunology. R. Soc. Open Sci. 3:160033. doi: 10.1098/rsos.160033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campler M., Jöngren M., and Jensen P... 2009. Fearfulness in red junglefowl and domesticated White Leghorn chickens. Behav. Processes 81:39–43. doi: 10.1016/j.beproc.2008.12.018 [DOI] [PubMed] [Google Scholar]
- Chilelli N. C., Burlina S., and Lapolla A... 2013. AGEs, rather than hyperglycemia, are responsible for microvascular complications in diabetes: A “glycoxidation-centric” point of view. Nutr. Metab. Cardiovasc. Dis. 23:913–919. doi: 10.1016/j.numecd.2013.04.004 [DOI] [PubMed] [Google Scholar]
- Corr S. A., Gentle M. J., Mccorquodale C. C., and Bennett D... 2003. The effect of morphology on the musculoskeletal system of the modern broiler. Anim. Welfare 12:145–157(113). doi: 10.1051/animres:2003017 [DOI] [Google Scholar]
- Crush K. G. 1970. Carnosine and related substances in animal tissues. Comp. Biochem. Physiol. 34:3–30. doi: 10.1016/0010-406x(70)90049-6 [DOI] [PubMed] [Google Scholar]
- Dawkins R. 2015. The ontogeny of a pecking preference in domestic chicks. Z. Tierpsychol. 25:170–186. doi: 10.1111/j.1439-0310.1968.tb00011.x [DOI] [PubMed] [Google Scholar]
- Dawkins M. S., Donnelly C. A., and Jones T. A... 2004. Chicken welfare is influenced more by housing conditions than by stocking density. Nature 427:342–344. doi: 10.1038/nature02226 [DOI] [PubMed] [Google Scholar]
- Dennis R., Zhang H. M., and Cheng H. W... 2006. Effect of selection for resistance and susceptibility to viral diseases on concentrations of dopamine and immunological parameters in six-week-old chickens. Poult. Sci. 85:2135–2140. doi: 10.1093/ps/85.12.2135 [DOI] [PubMed] [Google Scholar]
- Duggan B. M., Hocking P. M., Schwarz T., and Clements D. N... 2015. Differences in hindlimb morphology of ducks and chickens: effects of domestication and selection. Genet. Sel. Evol. 47:88. doi: 10.1186/s12711-015-0166-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L. 2007. Cell adhesion molecules and actin cytoskeleton at immune synapses and kinapses. Curr. Opin. Cell Biol. 19:529–533. doi: 10.1016/j.ceb.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favati A., Leimar O., and Løvlie H... 2014. Personality predicts social dominance in male domestic fowl. PLoS One. 9:e103535. doi: 10.1371/journal.pone.0103535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkman B., Boissy A., Meunier-Salaün M. C., Canali E., and Jones R. B... 2007. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 92:340–374. doi: 10.1016/j.physbeh.2007.03.016 [DOI] [PubMed] [Google Scholar]
- Goerlich V. C., Nätt D., Elfwing M., Macdonald B., and Jensen P... 2012. Transgenerational effects of early experience on behavioral, hormonal and gene expression responses to acute stress in the precocial chicken. Horm. Behav. 61:711–718. doi: 10.1016/j.yhbeh.2012.03.006 [DOI] [PubMed] [Google Scholar]
- Huang Y., Comiskey E. O., Dupree R. S., Li S., Koleske A. J., and Burkhardt J. K... 2008. The c-Abl tyrosine kinase regulates actin remodeling at the immune synapse. Blood 112:111–119. doi: 10.1182/blood-2007-10-118232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S., and Diamond J... 1996. Metabolic and digestive responses to artificial selection in chickens. Evolution 50:1638–1650. doi: 10.1111/j.1558-5646.1996.tb03936.x [DOI] [PubMed] [Google Scholar]
- Jensen P. 2014. Behaviour epigenetics – the connection between environment, stress and welfare. Appl. Anim. Behav. Sci. 157:1–7. doi: 10.1016/j.applanim.2014.02.009 [DOI] [Google Scholar]
- Jöngren M., Westander J., Nätt D., and Jensen P... 2010. Brain gene expression in relation to fearfulness in female red junglefowl (Gallus gallus). Genes Brain. Behav. 9:751–758. doi: 10.1111/j.1601-183X.2010.00612.x [DOI] [PubMed] [Google Scholar]
- Keer-Keer S., Hughes B. O., Hocking P. M., Jones R. B... 1996. Behavioural comparison of layer and broiler fowl: measuring fear responses. Appl. Anim. Behav. Sci. 49:321–333. doi: 10.1016/0168-1591(96)01055-6 [DOI] [Google Scholar]
- Kim D., Langmead B., and Salzberg S. L... 2015. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12:357–360. doi: 10.1038/nmeth.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., and Salzberg S. L... 2012. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9:357–359. doi: 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., and Dewey C. N... 2011. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. Bmc Bioinformatics 12:323. doi: 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M. I., Huber W., and Anders S. J. G. B... 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15(12):550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod M. G., and Dabutha L. A... 1997. Diet selection by Japanese quail (Coturnix coturnix japonica) in relation to ambient temperature and metabolic rate. Br. Poult. Sci. 38:586–589. doi: 10.1080/00071669708418040 [DOI] [PubMed] [Google Scholar]
- Mei B., Li C., Dong S., Jiang C. H., Wang H., and Hu Y... 2005. Distinct gene expression profiles in hippocampus and amygdala after fear conditioning. Brain Res. Bull. 67:1–12. doi: 10.1016/j.brainresbull.2005.03.023 [DOI] [PubMed] [Google Scholar]
- Mogensen J., Klausen I. C., Pedersen A. K., Egeblad H., Bross P., Kruse T. A., Gregersen N., Hansen P. S., Baandrup U., and Borglum A. D... 1999. Alpha-cardiac actin is a novel disease gene in familial hypertrophic cardiomyopathy. J. Clin. Invest. 103:R39–R43. doi: 10.1172/JCI6460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Toyama S., and Toyama S... 1992. Direct proof that the primary site of action of cytochalasin on cell motility processes is actin. J. Cell Biol. 116:933–941. doi: 10.1083/jcb.116.4.933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder C. A., Kliethermes C. L., Drew M. R., Muller J., Das K., Risbrough V. B., Crabbe J. C., Gilliam T. C., and Palmer A. A... 2010. Selection for contextual fear conditioning affects anxiety-like behaviors and gene expression. Genes Brain and Behav. 6:736–749. doi: 10.1111/j.1601-183x.2007.00306.x [DOI] [PubMed] [Google Scholar]
- Price E. O. 1999. Behavioral development in animals undergoing domestication. Appl. Anim. Behav. Sci. 65:245–271. doi: 10.1016/s0168-1591(99)00087-8 [DOI] [Google Scholar]
- Rauw W. M., Kanis E., Noordhuizen-Stassen E. N., and Grommers F. J... 1998. Undesirable side effects of selection for high production efficiency in farm animals: a review. Livest. Prod. Sci. 56:15–33. doi: 10.1016/s0301-6226(98)00147-x [DOI] [Google Scholar]
- Rodrigues J. V., Henriques B. J., Lucas T. G., and Gomes C. M... 2012. Cofactors and metabolites as protein folding helpers in metabolic diseases. Curr. Top. Med. Chem. 12:2546–2559. doi: 10.2174/1568026611212220009 [DOI] [PubMed] [Google Scholar]
- Schantz T. V., Tufvesson M., Göransson G., Grahn M., Wilhelmson M., and Wittzell H... 1995. Artificial selection for increased comb size and its effects on other sexual characters and viability in Gallus domesticus (the domestic chicken). Heredity 75:518–529. doi: 10.1038/hdy.1995.168 [DOI] [Google Scholar]
- Schmidt C. J., Persia M. E., Feierstein E., Kingham B., and Saylor W. W... 2009. Comparison of a modern broiler line and a heritage line unselected since the 1950s. Poult. Sci. 88:2610–2619. doi: 10.3382/ps.2009-00055 [DOI] [PubMed] [Google Scholar]
- Schøyen H. F., Hetland H., Rouvinen-Watt K., and Skrede A... 2007. Growth performance and ileal and total tract amino acid digestibility in broiler chickens fed diets containing bacterial protein produced on natural gas. Poult. Sci. 86:87–93. doi: 10.1093/ps/86.1.87 [DOI] [PubMed] [Google Scholar]
- Schütz K. E., Kerje S., Jacobsson L., Forkman B., Carlborg O., Andersson L., and Jensen P... 2004. Major growth QTLs in fowl are related to fearful behavior: possible genetic links between fear responses and production traits in a red junglefowl × white leghorn intercross. Behav. Genet. 34:121–130. doi: 10.1023/B:BEGE.0000009481.98336.fc [DOI] [PubMed] [Google Scholar]
- Siller W. G., and Hemsley L. A... 1966. The incidence of congenital heart disease in seven flocks of broiler chickens. Vet. Rec. 79:451–454. doi: 10.1136/vr.79.16.451 [DOI] [PubMed] [Google Scholar]
- Słopien R. 2008. Polymorphic variants of genes encoding MTHFR, MTR, and MTHFD1 and the risk of depression in postmenopausal women in Poland. Maturitas 61:252–255. doi: 10.1016/j.maturitas.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Smith C. L., and Johnson J... 2012. The chicken challenge: what contemporary studies of fowl mean for science and ethics. Between Species. 15:75–102. doi: 10.15368/bts.2012v15n1.4 [DOI] [Google Scholar]
- Smith C. L., Taylor A., and Evans C. S... 2011. Tactical multimodal signalling in birds: facultative variation in signal modality reveals sensitivity to social costs. Anim. Behav. 82:521–527. doi: 10.1016/j.anbehav.2011.06.002 [DOI] [Google Scholar]
- Stamps J. A. 2010. Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol. Lett. 10:355–363. doi: 10.1111/j.1461-0248.2007.01034.x [DOI] [PubMed] [Google Scholar]
- Sumners L. H., Zhang W., Zhao X., Honaker C. F., Zhang S., Cline M. A., Siegel P. B., and Gilbert E. R... 2014. Chickens from lines artificially selected for juvenile low and high body weight differ in glucose homeostasis and pancreas physiology. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 172:57–65. doi: 10.1016/j.cbpa.2014.02.020 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Matsuo K., Sawaki A., Mizuno N., Hiraki A., Kawase T., Watanabe M., Nakamura T., Yamao K., Tajima K.,. et al. 2008. Alcohol drinking and one-carbon metabolism-related gene polymorphisms on pancreatic cancer risk. Cancer Epidemiol. Biomarkers Prev. 17:2742–2747. doi: 10.1158/1055-9965.EPI-08-0470 [DOI] [PubMed] [Google Scholar]
- Thiery J. P., Duband J. L., Rutishauser U., and Edelman G. M... 1982. Cell adhesion molecules in early chicken embryogenesis. Proc. Natl. Acad. Sci. U. S. A. 79:6737–6741. doi: 10.1073/pnas.79.21.6737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis I. R., and Balnave D... 1984. The influence of environmental temperature, age and sex on the digestibility of amino acids in growing broiler chickens. Br. Poult. Sci. 25:401–407. doi: 10.1080/00071668408454880 [DOI] [PubMed] [Google Scholar]
- Wang X., Guan Z., Chen Y., Dong Y., Niu Y., Wang J., Zhang T., and Niu B... 2015. Genomic DNA hypomethylation is associated with neural tube defects induced by methotrexate inhibition of folate metabolism. PLoS One. 10:e0121869. doi: 10.1371/journal.pone.0121869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H., Chen S., Zhang H., Zhu X., Wang D., Liu H., Wang J., Yin T., Liu L., Kong M.,. et al. 2018. Transcriptome changes provide genetic insights into the effects of rearing systems on chicken welfare and product quality. J. Anim. Sci. 96: 4552–4561. doi: 10.1093/jas/sky314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H., Gao J., Yu B., Zhou H., Cai D., Zhang Y., Chen X., Wang X., Hofreiter M., and Zhao X... 2014. Early Holocene chicken domestication in northern China. Proc. Natl. Acad. Sci. U. S. A. 111:17564–17569. doi: 10.1073/pnas.1411882111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. D., Wakefield M. J., Smyth G. K., and Oshlack A... 2010. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 11:R14. doi: 10.1186/gb-2010-11-2-r14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q., Zhang Y., Chen Y., Yang N., Wang X. J., and Zhu D... 2009. Systematic identification of genes involved in divergent skeletal muscle growth rates of broiler and layer chickens. BMC Genomics 10:87. doi: 10.1186/1471-2164-10-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L., Simard M. J., and Huot J... 2018. Endothelial microRNAs regulating the NF-κB pathway and cell adhesion molecules during inflammation. FASEB J. 32:4070–4084. doi: 10.1096/fj.201701536R [DOI] [PubMed] [Google Scholar]
- Zimmerman P. H., Buijs S. A. F., Bolhuis J. E., and Keeling L. J... 2011. Behaviour of domestic fowl in anticipation of positive and negative stimuli. Anim. Behav. 81:569–577. doi: 10.1016/j.anbehav.2010.11.028 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files. The sequences with accession number PRJNA525938 reported in this paper have been deposited in the NCBI.