Abstract
BACKGROUND
Infertility is an important side effect of treatments used for cancer and other non-malignant conditions in males. This may be due to the loss of spermatogonial stem cells (SSCs) and/or altered functionality of testicular somatic cells (e.g. Sertoli cells, Leydig cells). Whereas sperm cryopreservation is the first-line procedure to preserve fertility in post-pubertal males, this option does not exist for prepubertal boys. For patients unable to produce sperm and at high risk of losing their fertility, testicular tissue freezing is now proposed as an alternative experimental option to safeguard their fertility.
OBJECTIVE AND RATIONALE
With this review, we aim to provide an update on clinical practices and experimental methods, as well as to describe patient management inclusion strategies used to preserve and restore the fertility of prepubertal boys at high risk of fertility loss.
SEARCH METHODS
Based on the expertise of the participating centres and a literature search of the progress in clinical practices, patient management strategies and experimental methods used to preserve and restore the fertility of prepubertal boys at high risk of fertility loss were identified. In addition, a survey was conducted amongst European and North American centres/networks that have published papers on their testicular tissue banking activity.
OUTCOMES
Since the first publication on murine SSC transplantation in 1994, remarkable progress has been made towards clinical application: cryopreservation protocols for testicular tissue have been developed in animal models and are now offered to patients in clinics as a still experimental procedure. Transplantation methods have been adapted for human testis, and the efficiency and safety of the technique are being evaluated in mouse and primate models. However, important practical, medical and ethical issues must be resolved before fertility restoration can be applied in the clinic.Since the previous survey conducted in 2012, the implementation of testicular tissue cryopreservation as a means to preserve the fertility of prepubertal boys has increased. Data have been collected from 24 co-ordinating centres worldwide, which are actively offering testis tissue cryobanking to safeguard the future fertility of boys. More than 1033 young patients (age range 3 months to 18 years) have already undergone testicular tissue retrieval and storage for fertility preservation.
LIMITATIONS, REASONS FOR CAUTION
The review does not include the data of all reproductive centres worldwide. Other centres might be offering testicular tissue cryopreservation. Therefore, the numbers might be not representative for the entire field in reproductive medicine and biology worldwide. The key ethical issue regarding fertility preservation in prepubertal boys remains the experimental nature of the intervention.
WIDER IMPLICATIONS
The revised procedures can be implemented by the multi-disciplinary teams offering and/or developing treatment strategies to preserve the fertility of prepubertal boys who have a high risk of fertility loss.
STUDY FUNDING/COMPETING INTEREST(S)
The work was funded by ESHRE. None of the authors has a conflict of interest.
Keywords: cryopreservation, in vitro spermatogenesis, fertility preservation, fertility restoration, prepubertal boys, spermatogonial stem cell, testicular tissue freezing, testis, transplantation
WHAT DOES THIS MEAN FOR PATIENTS?
Future fertility is important for long-term quality of life in patients who have received chemotherapy and/or radiotherapy during childhood. New strategies to give patients hope of fathering their own child must therefore be developed and brought into clinical practice. Testicular tissue cryopreservation is currently the only possibility to preserve fertility potential in children because sperm are not produced until puberty.
Methods to obtain mature sperm from prepubertal testicular tissue are under investigation and studies in monkeys have shown the feasibility. Hence, an increasing number of centres worldwide now advocate testicular tissue cryobanking as an experimental approach for patients with the objective of obtaining mature sperm from the tissue in the future. Approaches under investigation include in vitro culture or autologous transplantation of testicular tissue or cells. This review will describe current progress in fertility preservation for prepubertal boys, while also describing the challenges that must be overcome before these approaches can be implemented in clinical practice.
Introduction
Male gonads contain spermatogonial stem cells (SSCs), which are at the foundation of spermatogenesis that gives rise to mature and fertilization-competent spermatozoa. Sperm production begins at puberty and continues throughout adulthood. Germ cell loss can be induced by cytotoxic treatments, but is also linked to hereditary conditions such as Klinefelter syndrome or Fanconi syndrome. Therefore, to prevent infertility in disorders or conditions associated with prepubertal germ cell loss, well-timed cryopreservation of testicular tissues containing SSCs is a promising experimental strategy. Various approaches for the restoration of male fertility following oncological treatments or other fertility-compromising diseases are now under investigation. These approaches include SSC transplantation, testicular tissue grafting and in vitro spermatogenesis.
Worldwide, an increasing number of institutions are offering fertility preservation to prepubertal boys facing SSC loss. The first fertility preservation programme for prepubertal males started in 2002, and the number of centres offering cryopreservation has steadily increased over recent years (Picton et al., 2015). We anticipate that, in the near future, patients will return with questions regarding clinical options to restore their fertility. Therefore, the present review will describe current clinical practices, patient inclusion strategies and experimental methods used to preserve and restore the fertility of prepubertal boys at high risk of fertility loss. In addition, the scientific, ethical and legal issues relating to fertility preservation and restoration will be discussed.
Impact of irradiation and cytotoxic treatments on testicular function
Testicular cells, especially rapidly dividing germ cells, are highly sensitive to irradiation and chemotherapy treatment. The potential for recovery will depend on survival of spermatogonial populations within the testis. Low cytotoxic doses will deplete the pool of differentiating spermatogonia but are likely to result in only temporary cessation of spermatogenesis, while reserve SSCs may survive and resume mitotic activity to produce differentiating spermatogonia. If the damage is severe and the SSC number is drastically reduced, recovery may not occur until many years later. If all SSCs are depleted, the patient becomes permanently infertile. Spermatogonia have been shown to be susceptible to such depletion at all stages of life, including childhood (Whitehead et al., 1982; Relander et al., 2000; Jahnukainen et al., 2011). Furthermore, patient age, preexisting testicular pathology and the individual susceptibility to cancer treatment toxicity may also influence the potential of the seminiferous epithelium to support spermatogenesis after treatment (Wyns et al., 2010; Rives et al., 2012).
In a systematic literature review, the International Late Effects of Childhood Cancer Guideline Harmonization Group found evidence for adverse effects of cyclophosphamide, mechlorethamine and procarbazine on spermatogenesis (Skinner et al., 2017). Alkylating agent–based hematopoietic stem cell transplant (HSCT) conditioning regimens (i.e. busulfan and cyclophosphamide, fludarabine or melphalan) and ifosfamide have also been associated with impaired spermatogenesis. Data have suggested a relationship between the dose of alkylating agents and the likelihood of developing impaired spermatogenesis (Skinner et al., 2017). Azoospermia and oligozoospermia are unlikely after cyclophosphamide equivalent doses <4000 mg/m2 (Green et al., 2014). However, a threshold dose that predicts impaired spermatogenesis, azoospermia or any completely safe lower threshold dose could not be identified (Green et al., 2014; Skinner et al., 2017). Alkylating agents used in combination are considered to have an additive adverse effect on spermatogenesis (Skinner et al., 2017).
Assessing spermatogonial quantity per tubular cross section (Masliukaite et al., 2016) offers a method to measure adverse effects of disease or its therapy on quality and fertility potential of tissue obtained from prepubertal boys (Poganitsch-Korhonen et al., 2017; Stukenborg et al., 2018a). Using this approach, it has been shown that patients exposed to alkylating agents, especially to cyclophosphamide equivalent doses >4000 mg/m2, show significantly reduced spermatogonial quantity compared to normative reference values or those for patients treated with non-alkylating agents (Poganitsch-Korhonen et al., 2017). Therefore, in order to collect sufficient amounts of spermatogonia for fertility preservation, a testicular biopsy sample should be acquired early, before the initiation of alkylating agent chemotherapy.
Testicular irradiation can impair spermatogenesis by directly damaging germ cells, spermatogenesis-supporting Sertoli cells and testosterone-producing Leydig cells (Kenney et al., 2012; Stukenborg et al., 2018b). The differentiating spermatogonia are radiosensitive to scattered doses as low as 0.1 Gy leading to short-term cessation of spermatogenesis (Rowley et al., 1974). Doses of 2–3 Gy also affect SSCs and cause long-term azoospermia. Doses in excess of 6 Gy are able to deplete the SSC pool and lead to permanent infertility (Rowley et al., 1974; Centola et al., 1994). It is also well established that even very small doses invariably cause acute impairment and that recovery does not always occur. Thus, any dose of testicular radiotherapy should be regarded as possibly increasing the risk of impaired spermatogenesis (Skinner et al., 2017). Pubertal status in humans at the time of HSCT has been shown to be an independent predictor of adult testicular volume (Wilhelmsson et al., 2014), which in turn is primarily determined by Sertoli cell number (Sharpe et al., 2003). Total body irradiation (TBI 10–12 Gy) used to prepare a patient for HSCT before initiation of pubertal maturation leads to significantly smaller adult testicular volumes compared to same therapy during or after puberty (Wilhelmsson et al., 2014). These findings provide evidence for effects of cancer treatments also on the Sertoli cell population. There are several studies suggesting that testicular radiotherapy doses ≥21–24 Gy and the majority of those ≥12 Gy, including TBI are probably associated with an increased risk of Leydig cell failure resulting in testosterone deficiency (Skinner et al., 2017). Chemotherapy-induced Leydig cell failure is instead relatively rare (Sklar, 1999).
Which patients are at risk for fertility loss?
Crucial for a successful fertility preservation practice is the selection of patients who will benefit from this service. Since the surgical removal of testicular tissue is an invasive procedure and fertility restoration strategies from those tissues are still at an experimental level, the inclusion criteria for testicular tissue cryobanking should preferably be restricted to patients at significant risk of treatment-induced testicular damage and subsequent infertility. The inclusion criteria for testicular tissue cryobanking in prepubertal and adolescent male patients differ between centres (Wyns et al., 2011; Picton et al., 2015). In some centres, testicular tissue banking is restricted to patients who have not received any previous treatment (Wyns et al., 2011). Other centres also include patients that already have had a previous round of chemo- or radiotherapy (Stukenborg et al., 2018a; Valli-Pulaski et al., 2019; Braye et al., 2019). Most testicular tissue banking is performed in prepubertal boys suffering from cancer and mainly before treatment of testicular cancer, Hodgkin’s and non-Hodgkin’s malignant lymphoma and solid tumours (Picton et al., 2015).
Testicular tissue cryopreservation has been offered to patients at significant risk of infertility as a result of a treatment that is planned or has recently been initiated and patients at risk of genetically predisposed testicular degeneration.
Patients receiving therapies associated with a high risk of infertility include those receiving allogeneic or autologous HSCT or irradiation exposure to the testes, while first-line chemotherapy with antimetabolites, vinca alkaloids, podophyllotoxins and antitumor antibiotics are associated with a low risk of infertility (Skinner et al., 2017). Therefore, the majority of childhood cancer patients do not meet the criteria of a significant risk of impaired fertility at the time of cancer diagnosis; however, poor disease response or relapse may result in reclassification of patients into treatment regimens that often involve potentially sterilising therapy (Jahnukainen et al., 2015). This means that many cancer patients at the time of fertility preservation have already received chemotherapy, which may decrease the quality of cryopreserved tissue. On the other hand, if only untreated patients are eligible for fertility preservation, this may improve the quality of cryopreserved tissue but would mean that testicular biopsy is offered to a large patient population (if not restricted to the high-risk group), many of whom might not become infertile. Furthermore, this would also mean that relapsed patients would not qualify for testicular tissue cryopreservation, even for those whose SSC population might not be affected by the previous treatment. Therefore, patients treated with non-alkylating agents having a SSC population in the normal range according to published reference values (Masliukaite et al., 2016) should with relapse get the possibility for fertility preservation after this first treatment (Poganitsch-Korhonen et al., 2017; Stukenborg et al., 2018a). However, the quality of the germ cells cryopreserved during or shortly after chemotherapy could be theoretically affected and further studies are needed to elucidate the potential impairments in detail.
Allogeneic HSCT is a curative treatment option for patients with bone marrow failure, thalassemia, sickle cell disease and many other genetic diseases. As a consequence, there are an increasing number of children with non-malignant conditions at risk of potential loss of fertility. While early studies document spontaneous puberty among children receiving reduced-intensity conditioning (Panasiuk et al., 2015), limited data are currently available on fertility effects. These studies demonstrate a 50% risk of azoospermia after HSCT or long-term treatment with hydroxyurea for sickle cell disease (Lukusa et al., 2009). Therefore, testicular tissue preservation is also indicated in this patient group.
As spermatogonial quantity is also reduced in non-malignant diseases involving single gene mutations (thalassemia majors, Fanconi anaemia and immunodeficiency caused by a variant of the forkhead box P3 (FOXP3) gene) (Stukenborg et al., 2018a), the primary disease may also impact gonadal function or increase susceptibility to the toxicity of subsequent therapy. In light of this evidence, more research is needed to understand treatment- and patient-related factors that may impact on fertility preservation practice. This will allow for optimization of patient selection and timing of testicular biopsy.
Individuals with Klinefelter syndrome face germ cell depletion during development and, hence, azoospermia in >90% cases (Franik et al., 2016; Van Saen et al., 2018). Cryopreservation of testicular biopsies from adolescent Klinefelter syndrome patients is performed as part of the fertility preservation programme in some centres (Heckmann et al., 2018; Rives et al., 2018; Van Saen et al., 2018). However, as SSCs are lost at a very early age (<4 years), the question of whether fertility preservation should be offered to prepubertal patients with Klinefelter syndrome remains highly controversial (Van Saen et al., 2018).
Fertility Preservation in Prepubertal Boys: Current Practice
Management of fertility preservation
Lack of time in the case of cancer patients and lack of care-provider knowledge on infertility risk have been identified as the main barriers to including fertility preservation in patient care. Therefore, proper and efficient management in the field requires a team of highly trained physicians, nurses and psychologists (Fig. 1). Ideally, those teams are involved in both oncology and reproductive medicine in order to consider the challenges of each diagnosis and patient. Multi-collaborative care pathways (MCCPs), including well-informed care providers, with enhanced communication between oncologists and reproductive specialists, facilitate shared decisions at the time of diagnosis and lead to increased patient referrals and acceptance rates of the procedure (Wyns et al., 2015). In the male paediatric population, the highest acceptance rates were achieved with face-to-face consultations at the time of cancer diagnosis in a small pilot study (Ginsberg et al., 2010), which was further confirmed in a larger series of patients suffering from benign and malignant haematological diseases (74% for boys under 12 years) (Wyns et al., 2015). Such MCCPs should support the treating oncologists in order to facilitate the timely implementation of fertility preservation measures and offer appropriate nurse support and paediatric psychology counselling to enhance patient and parent support at the time of diagnosis. It is therefore of paramount importance to identify and educate key staff capable of initiating discussions on fertility and fertility preservation strategies and provide clear referral pathways to facilitate fertility-related discussions in paediatric oncology (Armuand et al., 2017). Dialogue with patients and parents was also shown to be critical for informed decision-making (Nagel and Neal, 2008; Carlson et al., 2017). Discussions should not only aim to provide full and understandable information of experimental strategies but also place the emphasis on the future hope for parenthood as a positive decisional factor. However, parents and patients must be informed about the experimental nature of the procedures. Furthermore, access to appropriate educational materials is highly recommended in this process (Vadaparampil et al., 2008). Patient information and decision aids should be age-adapted and should be available in multilingual versions (Rodriguez-Wallberg et al., 2019).
Figure 1.
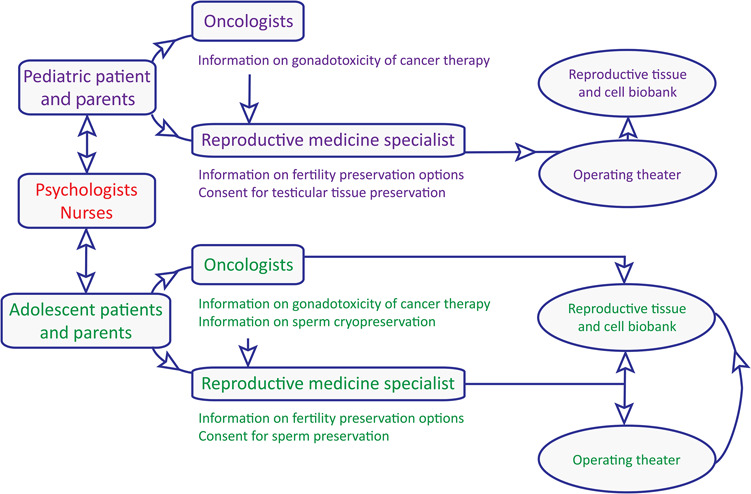
Schematic overview of a multi-collaborative care pathway for fertility preservation in boys.
Adequate management should be tailored to the individual patient. Hence, besides constraints directly linked to disease-specific care, it is essential that care providers have detailed knowledge of the hormonal events and testicular physiology before and around puberty. This is required in order to provide patients/parents with accurate information regarding the options for cryopreservation of gametes or gonadal tissue. This is especially the case for peripubertal boys as haploid germ cells and/or mature sperm may be present in the testis of these boys (even in the absence of ejaculated sperm) (Wyns et al., 2011). Considering that protocols used to preserve mature germ cells differ from those used to preserve testicular tissue containing SSCs, institutional guidelines aimed at prioritising cryobanking of testicular sperm whenever possible rather than testicular tissue should be available (Picton et al., 2015).
Patients requiring gonadotoxic therapies should be referred to a fertility specialist before gonadotoxic treatment is initiated (Redig et al., 2011). However, real-life clinical care showed that a proportion of patients and/or their parents or guardians requested fertility preservation measures while chemotherapy was already in its early phase (Abofoul-Azab et al., 2018). Moreover, as oncological therapy remains a health priority, fertility preservation should be considered and discussed where possible.
To develop further guidance in the field of fertility preservation, integration of the patients that participated in fertility preservation programmes into a systematic long-term follow-up (Melan et al., 2018) is recommended. It is important to inform the patients and their parents that, to date, the possible future use of frozen testicular tissue remains a research activity.
Besides hospital-wide MCCPs, establishment of structured networks between clinics is an important step for an optimal management of fertility preservation given that inequalities in access exist. Although oncological therapies should take priority over fertility preservation strategies, fertility and its preservation should always be discussed and considered where possible.
Procedure of fertility preservation
In peripubertal boys, it is theoretically possible to freeze spermatozoa obtained after sperm collection performed by masturbation. However, in case of failure or refusal of sperm collection, severe oligozoospermia, azoospermia or necrozoospermia (Safsaf et al., 2011; Daudin et al., 2015), an alternative procedure is to consider testicular biopsy combined with testicular sperm extraction (TESE) and possibly sperm freezing. If during surgery no sperm are retrieved, TESE can be combined with testicular tissue freezing in order to be able to store SSCs. As sperm production also stops for long periods after initiation of cancer therapy (Schrader et al., 2001), it is recommended to cryopreserve sperm before starting gonadotoxic treatment, also to avoid chemo- and radiotherapy-induced mutagenic effect(s) on the germ cell nucleus and the potential risk on the conceptus (miscarriages, malformations, genetic or chromosomal anomalies) if used in ART (Foresta et al., 2000; Picton et al., 2015). The stored spermatozoa will be usable for IUI or IVF (conventional or with ICSI) depending on sperm number and motility after thawing.
In prepubertal boys who do not produce spermatozoa or in peripubertal boys who have already started treatment (chemotherapy or radiotherapy) with low gonadotoxic doses and did not have the opportunity to bank sperm beforehand, testicular tissue freezing with the objective of preserving SSCs appears to be an acceptable solution (Picton et al., 2015). Testicular tissue biopsy before freezing is preferably unilateral and is performed surgically according to local procedures. The testicular fragments are immediately placed into a transport medium kept at 4 to 8°C and transported rapidly to the laboratory to minimize the risk of hot ischemia and to minimise microbial contamination. For longer distances between surgery room and laboratory, testicular tissue can be transported at 4°C in specialised medium (DMEM/F12) for up to 3 days (Faes and Goossens, 2016, 2017). The freezing of either testicular cell suspensions or testicular tissue has been proposed to cryopreserve SSCs. However, cryopreservation of testicular tissue pieces preserves the opportunity to pursue either cell- or tissue-based therapies in the future. Each piece of testicular tissue is cut into small fragments, which are deposited in cryotubes containing a cryoprotective medium (Rives et al., 2013). At present, no standardised protocol has been established for human testicular tissue freezing in terms of optimal cryopreservation technique or fertility restoration procedure able to generate spermatozoa. However, the most commonly used freezing protocol is controlled slow freezing with seeding (Keros et al., 2005; Keros et al., 2007; Wyns et al., 2007; Curaba et al., 2011; Babayev et al., 2013; Poels et al., 2013; Ginsberg et al., 2014; Pietzak 3rd et al., 2015; Ho et al., 2017; Valli-Pulaski et al., 2019). Dimethylsulfoxide, a penetrating cryoprotective agent, has been shown to be superior to 1,2-propanediol and glycerol in terms of cell viability (Keros et al., 2005) and is currently used in the majority of slow freezing protocols (Keros et al., 2007, Wyns et al., 2007, Wyns et al., 2008, Curaba et al., 2011, Wyns et al., 2011, Babayev et al., 2013, Baert et al., 2013, Poels et al., 2013, Ginsberg et al., 2014, Pietzak et al., 2015, Ho et al., 2017). Sucrose, a non-penetrating cryoprotective agent, is also added as a component of freezing media (Wyns et al., 2007; Wyns et al., 2008; Curaba et al., 2011; Wyns et al., 2011; Babayev et al., 2013; Baert et al., 2013; Poels et al., 2013; Ginsberg et al., 2014; Ho et al., 2017). Finally, some studies have explored vitrification (i.e. ultra-rapid freezing) of testicular tissue in a small number of patients although with no proven superiority in relation to tissue and cell integrity (Curaba et al., 2011; Baert et al., 2013; Poels et al., 2013).
Follow-Up of Patients Offered Testicular Tissue Cryopreservation
Follow-up of patients offered testicular cryopreservation serves several purposes: to determine the short- and long-term complications of testicular biopsy in this patient group; to determine the effect of potentially gonadotoxic treatments on subsequent reproductive function and fertility; to adapt selection criteria for future patients by ongoing stratification of fertility risks based on underlying diagnoses and treatments received; and to inform patients and their families about developments in the field that may allow them to use their tissue in the future.
Complications associated with testicular biopsy
Obtaining a testicular biopsy can be considered a relatively low-risk procedure. The frequency and number of anaesthetics can be minimised by combining the procedure with other necessary interventions such as placement of a central venous catheter, bone marrow aspirate or diagnostic lumbar puncture (Anderson et al., 2015). However, for specific patient groups in whom fertility preservation is offered, there may be potential complications that relate to their underlying disease or treatment. For patients receiving chemotherapy and radiotherapy, or those with conditions associated with bone marrow suppression (e.g. aplastic anaemia), there may be additional risks of bleeding and infection. These risks can be managed by identifying thrombocytopenia and neutropenia, and use of platelet transfusions to cover the procedure where indicated. Careful monitoring of the wound and clinical condition of the patient in the immediate days post-surgery is also required with prompt initiation of antibiotic treatment in the case of infection. A limited number of studies have reported the rate of intra-operative and short-term post-operative complications following testicular biopsy for the purposes of fertility preservation. To date, the overall complication rate is around 2–3% (Ginsberg et al., 2014; Ho et al., 2017; Uijldert et al., 2017; Ming et al., 2018; Valli-Pulaski et al., 2019). Reported complications include infection of the testicle or surgical wound, including one reported case of wound dehiscence. Pain is an important complication of testicular biopsy and should be regularly assessed in the post-operative period and appropriate analgesia should be administered. While current data suggest that testicular biopsy for the purposes of fertility preservation is a low-risk procedure, additional data on post-operative complications and development of standardised guidelines for the pre-, peri- and post-operative management of patients should be a subject for further research.
While obtaining testicular tissue for fertility preservation is the primary goal of testicular biopsy, it is imperative that the procedure itself does not further compromise testicular function in these patients. Follow-up of testicular function through childhood, puberty and into adulthood is an important aspect of current research. To date, only one study has reported the effects of testicular biopsy over the medium term (Uijldert et al., 2017): this prospective study in 64 boys involved ultrasound scans to determine the volume of the biopsied testis and comparison with the contralateral non-biopsied testis. Despite an initial bilateral reduction in testicular volume immediately following surgery, no differences were subsequently observed between biopsied and non-biopsied testes at 6 and 12 months of follow-up, albeit that there was evidence of fibrosis in the biopsied testes in a small proportion (~6%) of patients after 12 months of follow-up (Uijldert et al., 2017). Importantly, the testicular volumes in these patients remained in the prepubertal (<4 ml) range. Given that testicular volume does not markedly increase until puberty, further studies are required to determine the long-term effect of testicular biopsy on pubertal testicular growth and adult testicular volume in this patient group. This assessment should also take the combined effect of biopsy and gonadotoxic therapies into account.
Reproductive function in patients following testicular tissue biopsy for cryopreservation
For individual patients, follow-up is required to ensure that puberty is initiated and progresses normally. This is important for the assessment of the development of secondary sexual characteristics, maintenance of testosterone production into adulthood and fertility. Puberty can be assessed clinically by Tanner staging and measurement of testicular volume and monitoring should be commenced no later than 12 years of age in at-risk individuals (Skinner et al., 2017). LH-stimulated testosterone production is required for development of secondary sexual characteristics at puberty and the support of spermatogenesis in adulthood. While LH and testosterone production at puberty and into adulthood is usually unaffected in patients who have received cancer treatment in childhood, fertility may still be affected as a result of primary gonadal damage (reviewed in Stukenborg et al., 2018b). A raised serum FSH is indicative of Sertoli cell dysfunction and impaired spermatogenesis (Kelsey et al., 2017). Fertility potential can also be assessed post-puberty by measurement of testicular volume, serum gonadotrophins or by semen analysis (Skinner et al., 2017).
Adaptation of patient selection criteria for testicular tissue cryopreservation
A key aspect of follow-up is to validate and, if necessary, adapt patient selection criteria for testicular tissue cryopreservation. This is required to prevent unnecessary surgery in those who subsequently retain natural fertility, while ensuring that those who may benefit are offered the procedure. This approach has already been successfully applied to ovarian tissue cryopreservation, demonstrating that the current selection criteria are able to identify those at risk of premature ovarian failure (POF) with a high degree of accuracy (Wallace et al., 2014). In this study, POF occurred in 30% of those who were offered ovarian cryopreservation, while POF did not occur in the patients who were not offered fertility preservation.
A similar approach for males would require a serial follow-up of gonadotrophins and semen analysis, and a comparison between those who were offered testicular biopsy and those who were not. However, follow-up in these patients, particularly those who were not considered for fertility preservation, may be challenging.
Recommendations for follow-up in patients offered testicular tissue cryopreservation
Based on the current data and requirements for additional research, prepubertal patients receiving potentially gonadotoxic treatments should receive regular assessment of reproductive function by a paediatric endocrinologist, andrologist or urologist. Assessment of reproductive function should include pubertal staging, assessment of testicular volume (using an orchidometer or ultrasound) and, where relevant, serum gonadotrophins. Assessment of baseline Sertoli cell function (anti-Müllerian hormone, inhibin B) may also be considered. A pragmatic approach to post-treatment follow-up during prepuberty may consist of infrequent (every 1–2 years) assessment of testicular volume and gonadotrophins. From the age of 12 years, patients should be assessed (at least annually) for pubertal status including Tanner staging and testicular volume (Skinner et al., 2017). Assessment and measurement of gonadotrophins and testosterone may also be performed as part of regular pubertal assessment in order to ensure that treatment for delayed puberty is initiated when indicated. Pubertal delay may result from hypergonadotrophic hypogonadism (high gonadotrophins and low testicular volumes), indicating primary gonadal failure. Alternatively, puberty may be delayed as a result of hypogonadotrophic hypogonadism, indicating a central cause. In boys with delayed puberty (testicular volumes <3 ml at 14 years), pubertal induction with testosterone should aim to initiate and maintain the development of secondary sexual characteristics in line with their peers. Assessment of fertility in adulthood includes measurement of testicular volume, serum gonadotrophin and testosterone levels and semen analysis (Skinner et al., 2017).
Survey of Centres in Europe and the USA Offering Cryopreservation of Immature Testicular Tissues
A first survey of the European and Israeli centres offering cryopreservation of immature testicular tissues was performed in 2012 (Picton et al., 2015). In this survey, results from seven centres were taken into account. In order to determine recent developments in the field of male fertility preservation, the survey was repeated using an extended questionnaire prepared by the special interest groups (SIG) Andrology and SIG Fertility Preservation of ESHRE. The questionnaire was sent in total to 22 contact persons from Europe and the USA, and responses were received from 17. Survey data contain information from 24 sites (of which some are part of a network) offering a fertility preservation programme to cryopreserve immature testicular tissues. Four of the remaining five contact persons stated that they were indeed involved in collection of samples for cryopreservation, but that their data were reported by a co-ordinating centre, and one person was no longer situated at a clinic offering cryopreservation of testicular tissues.
The 24 sites are located in Europe (Scandinavia, Iceland, the UK, Belgium, the Netherlands, France, Spain, Germany), USA, Israel and Jordan. It is of note that some co-ordinating centres offer cryopreservation of immature testicular tissues to a number of collaborating clinics in the frame of national or even international networks: in the frame of the Nordfertil network, testicular tissues are obtained in clinics located in Finland as well as Iceland and Sweden and are then centrally stored at the University Hospital in Helsinki and at Karolinska Institutet and Karolinska University Hospital, respectively. The network in the UK stores samples in Edinburgh and Oxford, while research is currently conducted in Edinburgh using shared samples. The German Androprotect Network currently comprises three German clinics as tissue retrieval sites and the University Clinic Münster as a central location for storage of cryopreserved samples. Finally, as recently published (Valli-Pulaski et al., 2019), the North American network consisting of 11 international sites has centralised the cryopreservation of samples at the University of Pittsburgh School of Medicine.
Impressively, while the absolute number of cryopreserved tissues was around 260 at the time of the last survey in 2012, the total number was 1033 in this 2019 survey, constituting a 4-fold increase (Table I). This number adds to the recently estimated total of 700 testicular samples stored worldwide (Valli-Pulaski et al., 2019) and demonstrates the increasing efforts and acceptability for fertility preservation strategies in boys. This development is also reflected by the fact that the healthcare systems at least partially bear the costs for collection and storage of the tissues in at least half of the co-ordinating centres. This appears to be a rather recent development, as according to the survey from 2012 (Picton et al., 2015) costs were still covered by hospitals and research grants (six centres) or even by the patients (one centre). An overview of the results from the questionnaire from 2019 is provided in Table I.
Table I.
Results based on the questionnaire prepared by the Andrology and Fertility Preservation special interest groups of ESHRE.
| Results from sites collecting testicular tissues | Number of cases/samples (total) | Range of cases/samples (lowest-highest) | |
|---|---|---|---|
| (i) How many immature patients underwent testicular tissue retrieval as a strategy for fertility preservation? | 1033 | ||
| (ii) How many samples are currently stored? | 989 | ||
| (iii) What was the age range of pre-pubertal patients? | 3 months −18 years | ||
| (iv) Do you also recruit non-malignant patients? | Yes (all) | ||
| (v) What is the proportion of malignant cases? | 2–98% | ||
| (vi) Which cryopreservation protocol was applied? | Slow freezing (all) | ||
| (vii) Which cryoprotectant was used? | DMSO (5) DMSO and sucrose (1) DMSO & HSA (3)/or patient serum & sucrose (2) DMSO & ethylene glycol (1) PBS & ethylene glycol, HSA & sucrose (1) |
||
| (viii) Who pays for collection of tissue? | Health insurance (3) Combination of health insurance and charitable funds/grants/institution (3) Study (1) Study plus charitable donation (1) The hospital (3) |
||
| (xiv) Who pays for storage? | Healthcare (4) Healthcare plus charitable funds (1) Study plus the patient (after 1 year of storage) (1) Study plus charitable donation (1) The hospital/institutional money (4) |
DMSO = dimethyl sulfoxide; HSA = human serum albumin.
Numbers between brackets show the number of times this answer was given.
In line with the previous survey, the age of patients undergoing testicular biopsy for preservation of spermatogonia ranged from a few months to 18 years of age. Interestingly, the number of samples currently stored is almost identical to the number of patients who underwent testicular tissue retrieval. Considering that those samples have not yet been used for the purpose of fertility restoration, these numbers indicate that few samples have been discarded. One potential reason for discarding a sample is the death of a patient. While some sites allow for the use of these samples for research, samples have to be discarded after a death at other sites. However, with informed consent from patients or parents the tissue can be donated for research studies.
An obvious change over the past 7 years is the composition of the patient groups seeking fertility preservation. While in 2012 the majority of centres had stored tissues from patients prior to oncological treatments, only four centres had cryopreserved tissues from patients with non-malignant diseases facing the risk of germ cell loss (Picton et al., 2015). In 2019, all co-ordinating sites stated that they also recruit patients with non-malignant disease. It is also of note that the proportion of tissues from patients with non-malignant diseases ranges from 2 to 91%, while this patient group makes up ≥50% or more in four cryopreservation sites already.
In line with this development, the list of diseases mentioned as indications for testicular tissue banking in boys has become more diverse, in particular for the non-malignant diseases (Fig. 2A–C).
Figure 2.
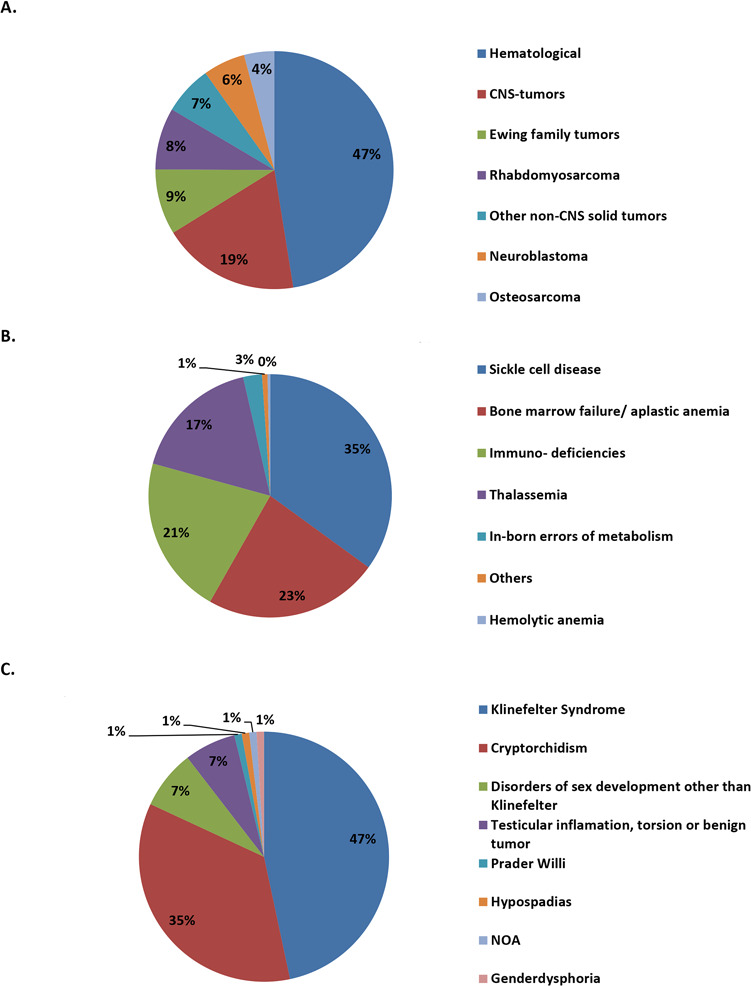
Subclassification and proportions of cryopreserved testicular tissue samples. Data are shown for patients with malignant diseases (n = 630; cancer treatment as indication for FP) (A), non-malignant diseases (n = 280; HSCT as indication for FP) (B) and genital, testicular or sexual disorders (n = 105; testicular pathology or risk for it as indication for FP) (C). CNS = central nervous system; FP = fertility preservation; HSCT = hematopoietic stem cell transplantation; NOA = non-obstructive azoospermia.
Taking 1015 of the 1033 reported testicular biopsies into account for which clinical information was provided, patients were generally categorised as having malignant diseases (n = 630), non-malignant diseases (n = 280) and non-haematological genetic abnormalities (including gonadal dysgenesis syndromes and disorders of sex development; n = 105). Within these three subcategories, the most prominent diseases were further classified. Among the malignant disorders, diagnosis of haematological disorders and tumours of the central nervous system were the most common diseases, whereas sickle cell disease and Klinefelter syndrome were the most common indications for fertility preservation among the non-malignant and genetic diseases, respectively (Fig. 2A–C).
The questionnaire also inquired about the legal restrictions concerning the cryopreserved testicular tissues, which are regulated in the informed consent forms. While most sites do not have any specific restrictions regarding the maximum storage time, individual sites do require annual systematic contact with the patient’s parents or the patient once he has turned 18 years of age. None of the sites has reported a minimum age for fertility preservation measures.
Finally, all sites stated that the gonadal tissues are considered to be tissues and not gametes, which has implications with regard to the legal regulations, including reimbursement and storage costs.
Fertility Restoration: Future Options
Although fertility preservation programmes for prepubertal boys are in place in several medical centres around the world, methods to restore fertility with the cryopreserved testicular tissue are still in development. Currently, research is focused on three approaches: SSC propagation and auto-transplantation, testicular tissue auto-grafting and in vitro spermatogenesis.
SSC propagation and auto-transplantation
In vitro propagation of SSCs followed by auto-transplantation into the seminiferous tubules via the rete testis is considered the only method for restoration of fertility with the potential to father a child via natural conception. In short, the proposed future clinical cell therapy will consist of isolation of testicular cells from the cryopreserved testicular biopsy to propagate the SSC subpopulation in vitro. Subsequently, these propagated SSCs will be auto-transplanted into the testis of the childhood cancer survivor who was rendered infertile due to previous treatment(s). After transplantation, the SSCs will colonize the testis, undergo spermatogenesis and provide continuous sperm production allowing natural conception to occur (Brinster, 2007). This concept of sperm generation after SSC transplantation was first shown to be successful in 1994 in a mouse model (Brinster and Zimmermann, 1994) and has since been translated to other animal models, including non-human primates (Hermann et al., 2012): the latter demonstrated the proof-of-principle for colonization of non-human primate SSCs after transplantation (Schlatt et al., 1999) and successful embryo development using transplanted SSC-derived spermatozoa (Hermann et al., 2012). This method was reproduced and further optimised using isolated human cadaver testes (Ning et al., 2012; Faes et al., 2013; Faes et al., 2017). Of interest, natural conception after SSC transplantation was successfully achieved in a mouse model and healthy offspring were obtained for two generations, as indicated by normal general development and absence of numerical chromosomal abnormalities or abnormal epigenetic marks (Goossens et al., 2009; Goossens et al., 2010).
Because of the limited number of SSCs in biopsied prepubertal testicular tissue, propagation of SSCs will be required for successful clinical application of SSC auto-transplantation. Long-term propagation of SSCs was established for the first time in 2003 in a mouse model (Kanatsu-Shinohara et al., 2003). Even after long-term (2 years) propagation in vitro, these mouse SSCs were able to colonise the testis upon transplantation and produce differentiating germ cells, which could be used to generate healthy offspring after round spermatid injection and ICSI. Subsequently, in vitro SSC propagation was achieved in various animal models including rat (Hamra et al., 2005), bovine (Aponte et al., 2008), porcine (Zhang et al., 2017) and tree shrew (Li et al., 2017). More importantly, for future restoration of fertility using auto-transplantation, human SSCs from adult and prepubertal testis tissue have been propagated in vitro and their ability to home into their niches within the seminiferous tubules of a recipient testis has been demonstrated by xeno-transplantation (Sadri-Ardekani et al., 2009; Sadri-Ardekani et al., 2011). Similar human SSC propagation cultures, with minor adaptations in culture medium, have subsequently been reported (He et al., 2010; Koruji et al., 2012; Conrad et al., 2014; Zheng et al., 2014; Dong et al., 2019). Although there is proof-of-principle on the ability of human SSCs to propagate in vitro, the remaining somatic cells also propagate in this cell culture method resulting in somatic cell overgrowth and a very low percentage of SSCs. Therefore, further characterization of human SSC culture conditions is required to improve SSC isolation and propagation. Several specific molecular markers have been suggested to quantify human SSCs in culture (He et al., 2010; Kossack et al., 2013; Smith et al., 2014; Zheng et al., 2014), and recent studies described the gene expression profile of several spermatogonial subtypes (Guo et al., 2018; Hermann et al., 2018; Wang et al., 2018; Sohni et al., 2019), but none of these can specifically identify SSCs. SSCs can only be conclusively distinguished from the undifferentiated spermatogonial population by their stem cell properties of self-renewal and differentiation using the transplantation assay, where an almost linear correlation between the extent of spermatogenesis and the number of transplanted SSCs was demonstrated in mice (Dobrinski et al., 1999). A limited number of papers have reported the capacity of cultured human SSCs sorted for a selected marker to migrate to the SSC niche on the seminiferous membrane after xeno-transplantation in the mouse testis (Langenstroth et al., 2014; Nickkholgh et al., 2014a). Identifying specific markers (or a combination of markers) for SSCs will be pivotal for optimising SSC propagation in vitro and for developing standardised protocols for SSC transplantation therapy.
Clinical translation of SSC therapy is also dependant on the stability and safety of propagated SSCs. Data on genetic and epigenetic stability of SSCs during culture or upon differentiation are limited and mainly involve animal models (Kanatsu-Shinohara et al., 2005; Langenstroth-Rower et al., 2017). On the other hand, in several studies it has been shown that transplantation of uncultured or cultured SSCs did not lead to tumours in the testes of recipients 3 months after transplantation in a mouse model or in a human-to-mouse xeno-transplantation model (Nagano et al., 2002; Sadri-Ardekani et al., 2009; Sadri-Ardekani et al., 2011; Nickkholgh et al., 2014b) indicating no tumorigenic transformation of the SSCs in these studies. Also, after transplantation of cultured SSCs in a mouse model, no differences were observed in health effects compared to non-transplanted control mice in terms of increased risk of tumorigenesis and life expectancy (Mulder et al., 2018). However, more sensitive methods are needed to address the safety aspect more in detail, and exclude potential genetic and epigenetic changes in the cultured cells.
A drawback of transplanting cells from a testicular biopsy is the potential risk to reintroduce cancerous cells that have been infiltrating the testis before biopsy. Such a risk might occur in patients with non-solid tumours such as leukaemia or blood-metastasising tumours. Although, several papers have reported promising results for cell sorting of leukemic cells from testicular cell suspensions (Dovey et al., 2013; Sadri-Ardekani et al., 2014), other studies highlight the risk of cancer cell contamination in such testicular cell preparations (Hou et al., 2007, 2009; Kilcoyne and Mitchell 2019). In conclusion, currently there are no clinical methods available to exclude the possibility of potential cancer contamination in testicular cells or tissue samples. Therefore, these samples should not be used in clinical re-implantation strategies.
Taken together, during the last decade progress has been made on several aspects of a future SSC transplantation therapy to restore fertility in childhood cancer survivors. The most critical step in this therapy will be optimising SSC propagation in vitro to establish a safe and feasible SSC auto-transplantation therapy.
Testicular tissue auto-grafting
Transplantation of fragments of testicular tissue provides an alternative strategy to the use of SSC suspensions. This approach maintains the SSCs within their unexposed natural niche, thus preserving the interactions between the germ cells and their supporting somatic cells. Autologous transplantation of the testicular biopsy can however only be proposed for restoring spermatogenesis if the presence of malignant cells can be excluded (Kilcoyne and Mitchell, 2019).
Tissue can be grafted to an ectopic or homotopic location. Initially, in mouse models, grafting was performed to ectopic sites, such as in the peritoneal space, the ear or under the back skin (Boyle et al., 1975; Schlatt et al., 2002). Full spermatogenesis was reported in xenografts from several species (Honaramooz et al., 2002; Schlatt et al., 2002; Schlatt et al., 2003; Honaramooz et al., 2004; Oatley et al., 2004; Snedaker et al., 2004; Rathi et al., 2006; Zeng et al., 2006; Abrishami et al., 2010; Liu et al., 2016), but never in xenografts from marmoset (Schlatt et al., 2002; Wistuba et al., 2004) or human (Goossens et al., 2008). The higher temperature at these ectopic sites compared with the scrotum was suggested to be the cause of either sclerosis of the graft or meiotic arrest. Consequently, tissue was grafted to the scrotum of castrated animals. Xeno-transplantation of immature human testicular tissue to the scrotum of immunodeficient mice maintained SSC proliferation (Wyns et al., 2007) and permitted initiation of differentiation up to the spermatid stage on histological morphological examination, although differentiation appeared abnormal based on electron microscopy characteristics and immunocytochemical markers (Wyns et al., 2008). The potential to successfully restore fertility might be improved by transplanting human tissue under the tunica albuginea of the testis (intratesticular grafting) as, in mice, this technique proved highly efficient with the re-establishment of full spermatogenesis in all of the grafts (Van Saen et al., 2009). Spermatogenesis has also been achieved using prepubertal primate tissue xenografted into the mouse testis. So far, the most advanced germ cell type obtained after xenografting non-human primate immature testis tissue to mouse testis is secondary spermatocytes specified by Boule Homolog and phosphorylated H2A histone family member X expression (Ntemou et al., 2019b).
Compared with ectopic xenografting, xenotransplantation of prepubertal human testis tissue into the mouse testis also resulted in better graft survival and initiation of germ cell differentiation (Van Saen et al., 2011). Although meiosis could be observed after intratesticular grafting of human testis tissue into the mouse, a considerable loss of SSCs was reported. This loss resulted from degeneration of tubules in the centre of the graft, possibly due to hypoxia during the first days after grafting (Van Saen et al., 2013). To reduce this SSC loss, early blood supply to the grafted tissue should be stimulated. Vascular endothelial growth factor (VEGF) is an important regulator of angiogenesis during development as well as in adulthood (Holmes and Zachary, 2005). In bovine, VEGF-treated grafts showed increased graft weight and more tubules contained elongating spermatids. These VEGF-treated grafts tended to have a better vasculature compared with control grafts (Schmidt et al., 2006). In addition to its role in angiogenesis, VEGF also regulates SSC homeostasis (Caires et al., 2012). In vitro treatment with VEGF prior to grafting prevented germ cell death and stimulated differentiation in bovine grafts (Caires et al., 2009). When immature human testicular tissue was embedded in hydrogel loaded with VEGF-nanoparticles before transplantation to the mouse scrotum, revascularisation and spermatogonial survival improved (Poels et al., 2016). This was also the case when immature human testicular tissue was pretreated with VEGF in vitro before intratesticular xenotransplantation. However, VEGF did not influence germ cell differentiation (Ntemou et al., 2019a).
Autologous transplantation has been performed in non-human primates. When testicular fragments were grafted under the skin of adult marmosets or rhesus macaques, meiotic arrest was observed in the grafts (Luetjens et al., 2008; Jahnukainen et al., 2012). However, fragments grafted to the scrotum re-established full spermatogenesis, although spermatogenic efficiency remained poor (Luetjens et al., 2008, Jahnukainen et al., 2012). In 2016, healthy monkeys were born after ICSI using sperm from ectopic xenografts (Liu et al., 2016). In 2019, transplantation of macaque testicular fragments to castrated immature macaques (under the skin and in the scrotum) showed very high efficiency of fertility restoration, with the birth of a healthy female baby after ICSI (Fayomi et al., 2019). Neither the addition of Matrigel nor cryopreservation nor graft location had an impact on the percentage of tubules displaying complete spermatogenesis. Interestingly, compared to other studies, in this study, unusually large fragments were transplanted (9–20 mm3).
Ectopic or scrotal grafting always has to be combined with ICSI in order to generate offspring. Also, for intratesticular grafts, it is still an open question whether connections can be established between grafted and endogenous tubules, ensuring a functional excretory system. However, grafting studies in rodent models have never reported the presence of donor-derived spermatozoa in the epididymis and, thus, the chance for natural reproduction may be very small. Due to the lack of an excretory system in case of the intratesticular/scrotal grafts, spermatozoa and fluid may accumulate and cause damage to the testis epithelium (Pilsworth et al., 1981). Therefore, spermatogenesis in grafts may not occur indefinitely.
In vitro differentiation of male germ cells obtained before and after puberty
Until robust decontamination protocols have been established, options to restore fertility in patients with malignant haematological diseases are limited to strategies enabling the isolation and cryopreservation of sperm generated in vitro.
Studies in the late 1990s revealed a potential use of in vitro generated sperm for fertility restoration in adult azoospermic patients. In these studies, the in vitro differentiation of primary spermatocytes into haploid cells could be observed after 48 h of culture (Tesarik et al., 1999). Subsequently, these haploid cells were reported to be functional, resulting in the birth of healthy babies after ICSI (Tesarik et al., 1999). Later studies performed by other research groups revealed similar results in terms of germ cell differentiation. In a study published in 2003, co-cultures with Vero cells allowed the division of spermatocytes into four cells, identified as haploid spermatids (Tanaka et al., 2003).
In 2014, functional haploid spermatids were generated by culturing SSCs from adult patients with cryptorchidism in conventional conditions (DMEM/F12 medium supplemented with 10% foetal bovine serum, retinoic acid and stem cell factor) for 7 to 10 days. The functionality of these in vitro generated haploid cells was tested by injecting them into murine oocytes, resulting in the production of eight-cell embryos (Yang et al., 2014).
The combination of functional somatic cells in direct contact with germ cells cultured in vitro has been reported by several groups to be an important factor to ensure survival and differentiation of male germ cells (for review, see Alves-Lopes and Stukenborg, 2017). In addition to the direct contact of different cell populations, the importance of a functional microenvironment resembling the 3D organization of the seminiferous epithelium in situ has been highlighted in several studies. Three-dimensional conditions, such as explant testicular tissue cultures or testicular organoids, are today the most promising strategies for establishing a functional in vitro tool for fertility preservation (Alves-Lopes and Stukenborg 2017; Oliver and Stukenborg 2019).
Historically, the first organotypic culture of testicular tissue from prepubertal rats was performed in 1964. The testicular fragments were deposited on a thin layer of agarose positioned on a metal grid allowing simultaneous contact between the culture medium and the air (Steinberger et al., 1964). This method resulted in meiotic entry and appearance of pachytene spermatocytes in testicular tissue from 12-day-old rats after 2 to 3 weeks of culture (Steinberger and Steinberger, 1965). The organotypic culture system then evolved by removing the metal grid and replacing it with a semi-permeable membrane. This new model has made it possible to maintain tissue architecture over short periods of culture. Organotypic culture using this membrane system allowed the evaluation of freezing protocols for prepubertal mouse testicular tissue. This work has shown the growth of seminiferous tubules, the maintenance of intra-tubular cell proliferation and the initiation of meiosis up to zygotene spermatocytes (Milazzo et al., 2008). In humans, the culture of prepubertal testicular tissue has allowed the survival of spermatogonia and the maintenance of seminiferous tubule architecture (Kvist et al., 2006; Keros et al., 2007). However, semi-permeable membrane culture did not produce complete spermatogenesis.
In 2010, the use of agarose blocks on which the testicular fragments are deposited combined with a modification of the culture medium allowed the progression of spermatogenesis up to round spermatids from prepubertal mouse testicular tissue (Gohbara et al., 2010). Complete spermatogenesis was reported in fresh prepubertal mouse testicular tissue by replacing foetal bovine serum with Knockout Serum Replacement. Viable and fertile progeny were subsequently obtained after ICSI (Sato et al., 2011). Similar results have been reported after culture of prepubertal mouse testicular tissue previously frozen by uncontrolled slow freezing or vitrification (Yokonishi et al., 2014). The yield of in vitro spermatogenesis has been enhanced after culture of fresh or frozen prepubertal mouse testicular tissue with retinol in the culture medium (Arkoun et al., 2015). More recently, a microfluidic culture system that allows the culture of testicular tissue in monolayer for more than 6 months with continuous circulation of the culture medium significantly increased the production of post-meiotic cells from fresh prepubertal mouse testicular tissue (Komeya et al., 2017). To date, the mouse is the only species in which complete spermatogenesis has been obtained from testicular fragments resulting in functional sperm. However, one study reported complete meiosis after culture on agarose gel of fresh rat prepubertal testicular tissue, without reaching elongated sperm production (Reda et al., 2016). In the same year, culture of seminiferous tubules using chitosan produced haploid cells from prepubertal rat testicular tissue and human testicular tissue from adult males treated with oestrogen within the context of gender dysphoria. However, spermatids were observed in male seminiferous tubules as early as 34 days of culture instead of the usual 72 days in physiological conditions (Perrard et al., 2016). More recently, meiotic (Medrano et al., 2018) and post-meiotic (Abofoul-Azab et al., 2018) cells have also been obtained from children aged 7 to 14 years, after organotypic culture of testicular tissues or 3D-culture conditions using dissociated cells, respectively. In addition, a study using frozen testicular tissue from young boys aged 2 to 12 years revealed the production of haploid cells after organotypic culture (de Michele et al., 2018b). However, to date, no studies have reported the in vitro production of spermatozoa from human prepubertal testicular tissue. Studies on the molecular mechanisms during organotypic culture that could explain the absence of spermiogenesis showed incomplete establishment of the blood-testis barrier (de Michele et al., 2018a).
The lack of a reliable functional approach and experimental conditions that mimic specific endocrine and paracrine pathways needed for male germ cell differentiation highlight the need for more research to establish robust in vitro conditions for fertility preservation in males.
Ethical Issues of Testicular Tissue Cryopreservation
The main ethical issue regarding fertility preservation in prepubertal boys remains the experimental nature of the intervention. Clinical research involving children is subject to a strict balance between burdens and risks on the one hand and the anticipated benefit for the child on the other hand (Lee, 2018). Given the fact that efficacy of the subsequent clinical applications has not been proven, it is crucial to offer the procedure only if the risks and burdens for the child are kept to a minimum. Thus, contrary to the situation for postpubertal patients where cryopreservation of sperm should be proposed, offering testicular tissue cryopreservation in prepubertal boys is ethically permissible in some cases but is not ethically required (McDougall et al., 2018). Some authors suggest that testicular tissue cryopreservation should not be restricted to the experimental setting (Ruutiainen et al., 2013). However, in a field that is notorious for applying new technologies without the necessary safeguards, it seems premature to move testicular tissue cryopreservation out of the experimental setting because of possible negative effects on patients (including the parents) and because all three steps of the technology (collection, cryopreservation and re-transplantation) do not fulfil the basic criteria to be considered as innovative treatment: no proof-of-principle in humans and, as a consequence, no data on safety, procedure and effectiveness (Provoost et al., 2014). The proposal to allow the application outside the experimental setting was meant to increase access. However, problems of access can be solved in other ways than by changing the scientific status (Mertes and Pennings, 2013).
The benefit of the technology is determined by the importance attributed to genetic parenthood and by the belief in scientific progress. Parents’ beliefs in scientific progress that will enable the use of the stored material influences their willingness to have their child undergo the tissue collection (Ginsberg et al., 2014). This belief changes the assumed balance of harms and benefits. Still, one may wonder when belief in scientific progress turns into therapeutic misconception or false hope. Moreover, as soon as some degree of scientific progress is envisioned, other alternative developments should also be considered. Stem cell-derived gametes, from induced pluripotent stem cells for instance, may be a possibility in the future, thus making the surgical intervention to harvest testicular tissue redundant (Hayashi et al., 2011). However, today there is no robust protocol available allowing the differentiation of human pluripotent stem cells into functional germ cells.
The main justification for offering fertility preservation is the possibility to preserve the ability to have genetically related children. The importance of this possibility is corroborated by studies indicating the importance of fertility in (surviving) cancer patients. However, another indicator of the importance could be the utilization rate of the stored material. While the majority of the cancer patients indicate that having genetically related children is important, the present utilization rate of frozen sperm in cancer patient populations is very low (Ferrari et al., 2016). The general investment needed for re-transplantation makes it highly likely that even fewer men will avail themselves of this option after testicular tissue cryopreservation. Follow-up research on future use is necessary for the ethical evaluation.
The Next Challenges
Since testicular tissue grafting is a relatively easy technique and has been proven successful in primates, the next step is to obtain proof-of-concept in humans. Some experts in the field have recommended transplanting multiple tissue fragments to different sites on the body in order to have a realistic chance of retrieving spermatozoa. However, it should be noted that testicular tissue grafting would include a second surgery to retrieve spermatozoa to be used in ART.
For patients at risk of testicular tissue contamination by cancer cells, effective strategies to ensure the removal of malignant cells from the tissues need further study. As the efficiency of SSC transplantation is rather low in mice and because biopsies taken from prepubertal boys are small and contain few SSCs, the SSCs should be propagated in vitro prior to transplantation to increase the chance of fertility restoration. However, in vitro culture systems need further optimization concerning efficiency and safety before they are suitable for clinical application.
As an alternative approach to transplantation, in vitro spermatogenesis using patient tissue may be a future option; however, this methodology requires extensive further research before it could be suitable for clinical application (Table II).
Table II.
Current challenges in the field of male fertility preservation.
| Clinical challenges |
| 1. Optimization of patient inclusion and exclusion criteria |
| 2. Standardization of protocols for the collection and cryopreservation of testicular tissue |
| 3. Optimization of protocols for the management and transportation of tissue between the procurement site and cryopreservation site |
| 4. Development of protocols for quality assurance before and after storage |
| 5. Development of protocols for minimal residual disease testing of testicular tissue |
| 6. Development of protocols for follow-up of patients after testicular biopsy |
| Research challenges |
| 1. Development of protocols for cell sorting to isolate spermatogonial stem cells (SSCs) and/or exclude cancer cells |
| 2. Optimization of protocols for propagation of SSCs |
| 3. Obtaining proof-of-concept of auto-transplantation methodologies for testicular tissue and SSCs in human |
| 4. Development of protocols for IVM of human SSCs |
| 5. Evaluation of the genetic and epigenetic stability and hence safety of cryopreserved, cultured and transplanted human SSCs and in vitro-derived sperm |
| 6. Assessment of the fertilising capacity of sperm obtained after fertility restoration |
Conclusion
Worldwide, fertility preservation is increasingly being offered to prepubertal male patients who are at risk of germ cell loss. Since the first survey in 2012, the number of patients with stored testicular tissue has increased from 266 to 1033. At the same time, important scientific progress has been made concerning fertility restoration methods. The technique of SSC transplantation has been translated to human cadaver testis, proof-of-concept has been obtained for testicular tissue grafting in non-human primates, and important steps have been taken in establishing spermatogenesis in vitro.
Considering the progress that has been made and the barriers that still have to be overcome, the first patients eligible for autologous transplantation of testicular tissue or cell suspensions are likely to be those treated for solid non-metastatic tumours or non-malignant diseases, as for these patients there is no known risk of re-introducing malignant cells into the testis. However, until successful clinical trials demonstrating safety and efficacy of fertility restoration have been conducted, testicular tissue cryopreservation for fertility preservation should remain experimental.
Acknowledgements
We are grateful to the International Society of Andrology (ISA), the European Society of Human Reproduction and Embryology (ESHRE) and all centres who contributed to the data collection in the current survey. The authors acknowledge the support of their respective departments and institutions during the preparation of this manuscript.
R.B., K.J. and J.-B.S. represent the NORDFERTIL research project on fertility preservation in boys performed in Iceland, Finland and Sweden. K.E.O. is a representative of the American Society for Reproductive Medicine.
Authors’ roles
E.G., N.N. and J.B.S. led on the preparation, drafting and editing of this review. N.N. distributed and analysed the survey data. All other authors contributed to data collection and to manuscript drafting and critical review.
Funding
European Society of Human Reproduction and Embryology; MRC Centre for Reproductive Health funded by MRC Centre Grant (MR/N022556/1 to R.T.M.); Swedish Childhood Cancer Foundation (TJ2016-0093 to J.-B.S.); Research Programme of the Research Foundation—Flanders (FWO-G010918N to E.G.); Kom op tegen kanker (KOTK, to E.G.); Netherlands Organisation for Health Research and Development (ZonMw 116003002 to A.M.M.v.P.); Belgian Fondation contre le cancer (FDC 2016-141 to C.W.); FNRS-Televie (7.4596.13, 7.4554.14F and 7.6511.16 to C.W.).
Conflict of interest
None declared.
References
- Abofoul-Azab M, AbuMadighem A, Lunenfeld E, Kapelushnik J, Shi Q, Pinkas H, Huleihel M. Development of postmeiotic cells in vitro from spermatogonial cells of prepubertal cancer patients. Stem Cells Dev 2018;27:1007–1020. [DOI] [PubMed] [Google Scholar]
- Abrishami M, Abbasi S, Honaramooz A. The effect of donor age on progression of spermatogenesis in canine testicular tissue after xenografting into immunodeficient mice. Theriogenology 2010;73:512–522. [DOI] [PubMed] [Google Scholar]
- Alves-Lopes JP, Stukenborg JB. Testicular organoids: a new model to study the testicular microenvironment in vitro? Hum Reprod Update 2018;24:176–191. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol 2015;3:556–567. [DOI] [PubMed] [Google Scholar]
- Aponte PM, Soda T, Teerds KJ, Mizrak SC, van de Kant HJ, de Rooij DG. Propagation of bovine spermatogonial stem cells in vitro. Reproduction 2008;136:543–557. [DOI] [PubMed] [Google Scholar]
- Arkoun B, Dumont L, Milazzo JP, Way A, Bironneau A, Wils J, Mace B, Rives N. Retinol improves in vitro differentiation of pre-pubertal mouse spermatogonial stem cells into sperm during the first wave of spermatogenesis. PLoS One 2015;10:e0116660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armuand GM, Nilsson J, Rodriguez-Wallberg KA, Malmros J, Arvidson J, Lampic C, Wettergren L. Physicians’ self-reported practice behaviour regarding fertility-related discussions in paediatric oncology in Sweden. Psychooncology 2017;26:1684–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babayev SN, Arslan E, Kogan S, Moy F, Oktay K. Evaluation of ovarian and testicular tissue cryopreservation in children undergoing gonadotoxic therapies. J Assist Reprod Genet 2013;30:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert Y, Van Saen D, Haentjens P, In't Veld P, Tournaye H, Goossens E. What is the best cryopreservation protocol for human testicular tissue banking? Hum Reprod 2013;28:1816–1826. [DOI] [PubMed] [Google Scholar]
- Boyle PF, Fox M, Slater D. Transplantation of interstitial cells of the testis: effect of implant site, graft mass and ischaemia. Br J Urol 1975;47:891–898. [DOI] [PubMed] [Google Scholar]
- Braye A, Tournaye H, Goossens E. Setting up a cryopreservation programme for immature testicular tissue: lessons learned after more than 15 years of experience.. Clin Med Insights: Reprod Health 2019. 10.1177/1179558119886342 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL. Male germline stem cells: from mice to men. Science 2007;316:404–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA 1994;91:11298–11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caires KC, de Avila J, McLean DJ. Vascular endothelial growth factor regulates germ cell survival during establishment of spermatogenesis in the bovine testis. Reproduction 2009;138:667–677. [DOI] [PubMed] [Google Scholar]
- Caires KC, de Avila JM, Cupp AS, McLean DJ. VEGFA family isoforms regulate spermatogonial stem cell homeostasis in vivo. Endocrinology 2012;153:887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CA, Kolon TF, Mattei P, Hobbie W, Gracia CR, Ogle S, Ginsberg JP. Developing a hospital-wide fertility preservation service for pediatric and young adult patients. J Adolesc Health 2017;61:571–576. [DOI] [PubMed] [Google Scholar]
- Centola GM, Keller JW, Henzler M, Rubin P. Effect of low-dose testicular irradiation on sperm count and fertility in patients with testicular seminoma. J Androl 1994;15:608–613. [PubMed] [Google Scholar]
- Conrad S, Azizi H, Hatami M, Kubista M, Bonin M, Hennenlotter J, Renninger M, Skutella T. Differential gene expression profiling of enriched human spermatogonia after short- and long-term culture. Biomed Res Int 2014;2014:138350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curaba M, Poels J, van Langendonckt A, Donnez J, Wyns C. Can prepubertal human testicular tissue be cryopreserved by vitrification? Fertil Steril 2011;952123:e2129–e2112. [DOI] [PubMed] [Google Scholar]
- Daudin M, Rives N, Walschaerts M, Drouineaud V, Szerman E, Koscinski I, Eustache F, Saias-Magnan J, Papaxanthos-Roche A, Cabry-Goubet R et al. . Sperm cryopreservation in adolescents and young adults with cancer: results of the French national sperm banking network (CECOS). Fertil Steril 2015;103:478–486e471. [DOI] [PubMed] [Google Scholar]
- de Michele F, Poels J, Giudice MG, De Smedt F, Ambroise J, Vermeulen M, Gruson D, Wyns C. In vitro formation of the blood-testis barrier during long-term organotypic culture of human prepubertal tissue: comparison with a large cohort of pre/peripubertal boys. Mol Hum Reprod 2018a;24:271–282. [DOI] [PubMed] [Google Scholar]
- de Michele F, Poels J, Vermeulen M, Ambroise J, Gruson D, Guiot Y, Wyns C. Haploid germ cells generated in organotypic culture of testicular tissue from prepubertal boys. Front Physiol 2018b;9:1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrinski I, Ogawa T, Avarbock MR, Brinster RL. Computer assisted image analysis to assess colonization of recipient seminiferous tubules by spermatogonial stem cells from transgenic donor mice. Mol Reprod Dev 1999;53:142–148. [DOI] [PubMed] [Google Scholar]
- Dong L, Kristensen SG, Hildorf S, Gul M, Clasen-Linde E, Fedder J, Hoffmann ER, Cortes D, Thorup J, Andersen CY. Propagation of spermatogonial stem cell-like cells from infant boys. Front Physiol 2019;10:1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovey SL, Valli H, Hermann BP, Sukhwani M, Donohue J, Castro CA, Chu T, Sanfilippo JS, Orwig KE. Eliminating malignant contamination from therapeutic human spermatogonial stem cells. J Clin Invest 2013;123:1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faes K, Goossens E. Short-term hypothermic preservation of human testicular tissue: the effect of storage medium and storage period. Fertil Steril 2016;105:1162–1169e1165. [DOI] [PubMed] [Google Scholar]
- Faes K, Goossens E. Short-term storage of human testicular tissue: effect of storage temperature and tissue size. Reprod Biomed Online 2017;35:180–188. [DOI] [PubMed] [Google Scholar]
- Faes K, Lahoutte T, Hoorens A, Tournaye H, Goossens E. In search of an improved injection technique for the clinical application of spermatogonial stem cell transplantation. Reprod Biomed Online 2017;34:291–297. [DOI] [PubMed] [Google Scholar]
- Faes K, Tournaye H, Goethals L, Lahoutte T, Hoorens A, Goossens E. Testicular cell transplantation into the human testes. Fertil Steril 2013;100:981–988. [DOI] [PubMed] [Google Scholar]
- Fayomi AP, Peters K, Sukhwani M, Valli-Pulaski H, Shetty G, Meistrich ML, Houser L, Robertson N, Roberts V, Ramsey C et al. . Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 2019;363:1314–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Paffoni A, Filippi F, Busnelli A, Vegetti W, Somigliana E. Sperm cryopreservation and reproductive outcome in male cancer patients: a systematic review. Reprod Biomed Online 2016;33:29–38. [DOI] [PubMed] [Google Scholar]
- Foresta C, Bettella A, Marin P, Galeazzi C, Merico M, Scandellari C. Analysis of sperm aneuploidy in infertile subjects after chemotherapy treatment. Ann Ital Med Int 2000;15:189–194. [PubMed] [Google Scholar]
- Franik S, Hoeijmakers Y, D'Hauwers K, Braat DD, Nelen WL, Smeets D, Claahsen-van der Grinten HL, Ramos L, Fleischer K. Klinefelter syndrome and fertility: sperm preservation should not be offered to children with Klinefelter syndrome. Hum Reprod 2016;31:1952–1959. [DOI] [PubMed] [Google Scholar]
- Ginsberg JP, Carlson CA, Lin K, Hobbie WL, Wigo E, Wu X, Brinster RL, Kolon TF. An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: a report of acceptability and safety. Hum Reprod 2010;25:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg JP, Li Y, Carlson CA, Gracia CR, Hobbie WL, Miller VA, Mulhall J, Shnorhavorian M, Brinster RL, Kolon TF. Testicular tissue cryopreservation in prepubertal male children: an analysis of parental decision-making. Pediatr Blood Cancer 2014;61:1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohbara A, Katagiri K, Sato T, Kubota Y, Kagechika H, Araki Y, Araki Y, Ogawa T. In vitro murine spermatogenesis in an organ culture system. Biol Reprod 2010;83:261–267. [DOI] [PubMed] [Google Scholar]
- Goossens E, De Rycke M, Haentjens P, Tournaye H. DNA methylation patterns of spermatozoa and two generations of offspring obtained after murine spermatogonial stem cell transplantation. Hum Reprod 2009;24:2255–2263. [DOI] [PubMed] [Google Scholar]
- Goossens E, de Vos P, Tournaye H. Array comparative genomic hybridization analysis does not show genetic alterations in spermatozoa and offspring generated after spermatogonial stem cell transplantation in the mouse. Hum Reprod 2010;25:1836–1842. [DOI] [PubMed] [Google Scholar]
- Goossens E, Geens M, De Block G, Tournaye H. Spermatogonial survival in long-term human prepubertal xenografts. Fertil Steril 2008;90:2019–2022. [DOI] [PubMed] [Google Scholar]
- Green DM, Liu W, Kutteh WH, Ke RW, Shelton KC, Sklar CA, Chemaitilly W, Pui CH, Klosky JL, Spunt SL et al. . Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. Lancet Oncol 2014;15:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Grow EJ, Mlcochova H, Maher GJ, Lindskog C, Nie X, Guo Y, Takei Y, Yun J, Cai L et al. . The adult human testis transcriptional cell atlas. Cell Res 2018;28:1141–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra FK, Chapman KM, Nguyen DM, Williams-Stephens AA, Hammer RE, Garbers DL. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc Natl Acad Sci U S A 2005;102:17430–17435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011;146:519–532. [DOI] [PubMed] [Google Scholar]
- He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod 2010;82:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann L, Langenstroth-Rower D, Pock T, Wistuba J, Stukenborg JB, Zitzmann M, Kliesch S, Schlatt S, Neuhaus N. A diagnostic germ cell score for immature testicular tissue at risk of germ cell loss. Hum Reprod 2018;33:636–645. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Cheng K, Singh A, Roa-De La Cruz L, Mutoji KN, Chen IC, Gildersleeve H, Lehle JD, Mayo M, Westernstroer B et al. . The mammalian spermatogenesis single-cell transcriptome, from spermatogonial stem cells to spermatids. Cell Rep 2018;25:1650–1667e1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G et al. . Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 2012;11:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WLC, Bourne H, Gook D, Clarke G, Kemertzis M, Stern K, Agresta F, Heloury Y, Clark H, Orme L et al. . A short report on current fertility preservation strategies for boys. Clin Endocrinol (Oxf) 2017;87:279–285. [DOI] [PubMed] [Google Scholar]
- Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol 2005;6:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaramooz A, Li MW, Penedo MC, Meyers S, Dobrinski I. Accelerated maturation of primate testis by xenografting into mice. Biol Reprod 2004;70:1500–1503. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature 2002;418:778–781. [DOI] [PubMed] [Google Scholar]
- Hou M, Andersson M, Zheng C, Sundblad A, Soder O, Jahnukainen K. Decontamination of leukemic cells and enrichment of germ cells from testicular samples from rats with Roser’s T-cell leukemia by flow cytometric sorting. Reproduction 2007;134:767–779. [DOI] [PubMed] [Google Scholar]
- Hou M, Andersson M, Zheng C, Sundblad A, Soder O, Jahnukainen K. Immunomagnetic separation of normal rat testicular cells from Roser’s T-cell leukaemia cells is ineffective. Int J Androl 2009;32:66–73. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Nurmio M, Schlatt S. Autologous ectopic grafting of cryopreserved testicular tissue preserves the fertility of prepubescent monkeys that receive sterilizing cytotoxic therapy. Cancer Res 2012;72:5174–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnukainen K, Heikkinen R, Henriksson M, Cooper TG, Puukko-Viertomies LR, Makitie O. Semen quality and fertility in adult long-term survivors of childhood acute lymphoblastic leukemia. Fertil Steril 2011;96:837–842. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Mitchell RT, Stukenborg JB. Testicular function and fertility preservation after treatment for haematological cancer. Curr Opin Endocrinol Diabetes Obes 2015;22:217–223. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod 2003;69:612–616. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Iwano T, Lee J, Kazuki Y, Inoue K, Miki H, Takehashi M, Toyokuni S, Shinkai Y et al. . Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development 2005;132:4155–4163. [DOI] [PubMed] [Google Scholar]
- Kelsey TW, McConville L, Edgar AB, Ungurianu AI, Mitchell RT, Anderson RA, Wallace WHB. Follicle stimulating hormone is an accurate predictor of azoospermia in childhood cancer survivors. PLoS One 2017;12:e0181377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney LB, Cohen LE, Shnorhavorian M, Metzger ML, Lockart B, Hijiya N, Duffey-Lind E, Constine L, Green D, Meacham L. Male reproductive health after childhood, adolescent, and young adult cancers: a report from the Children’s Oncology Group. J Clin Oncol 2012;30:3408–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keros V, Hultenby K, Borgstrom B, Fridstrom M, Jahnukainen K, Hovatta O. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod 2007;22:1384–1395. [DOI] [PubMed] [Google Scholar]
- Keros V, Rosenlund B, Hultenby K, Aghajanova L, Levkov L, Hovatta O. Optimizing cryopreservation of human testicular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Hum Reprod 2005;20:1676–1687. [DOI] [PubMed] [Google Scholar]
- Kilcoyne K, Mitchell R. Testicular transplantation for fertility preservation - clinical potential and current challenges. Reproduction 2019;158:F1–F14. [DOI] [PubMed] [Google Scholar]
- Komeya M, Hayashi K, Nakamura H, Yamanaka H, Sanjo H, Kojima K, Sato T, Yao M, Kimura H, Fujii T et al. . Pumpless microfluidic system driven by hydrostatic pressure induces and maintains mouse spermatogenesis in vitro. Sci Rep 2017;7:15459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koruji M, Shahverdi A, Janan A, Piryaei A, Lakpour MR, Gilani Sedighi MA. Proliferation of small number of human spermatogonial stem cells obtained from azoospermic patients. J Assist Reprod Genet 2012;29:957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossack N, Terwort N, Wistuba J, Ehmcke J, Schlatt S, Scholer H, Kliesch S, Gromoll J. A combined approach facilitates the reliable detection of human spermatogonia in vitro. Hum Reprod 2013;28:3012–3025. [DOI] [PubMed] [Google Scholar]
- Kvist K, Thorup J, Byskov AG, Hoyer PE, Mollgard K, Yding AC. Cryopreservation of intact testicular tissue from boys with cryptorchidism. Hum Reprod 2006;21:484–491. [DOI] [PubMed] [Google Scholar]
- Langenstroth D, Kossack N, Westernstroer B, Wistuba J, Behr R, Gromoll J, Schlatt S. Separation of somatic and germ cells is required to establish primate spermatogonial cultures. Hum Reprod 2014;29:2018–2031. [DOI] [PubMed] [Google Scholar]
- Langenstroth-Rower D, Gromoll J, Wistuba J, Trondle I, Laurentino S, Schlatt S, Neuhaus N. De novo methylation in male germ cells of the common marmoset monkey occurs during postnatal development and is maintained in vitro. Epigenetics 2017;12:527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. Ethical considerations of ovarian and testicular tissue cryopreservation in pre-pubertal children who cannot assent. Law & Inequality 2018;36:95–116. [Google Scholar]
- Li CH, Yan LZ, Ban WZ, Tu Q, Wu Y, Wang L, Bi R, Ji S, Ma YH, Nie WH et al. . Long-term propagation of tree shrew spermatogonial stem cells in culture and successful generation of transgenic offspring. Cell Res 2017;27:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Nie YH, Zhang CC, Cai YJ, Wang Y, Lu HP, Li YZ, Cheng C, Qiu ZL, Sun Q. Generation of macaques with sperm derived from juvenile monkey testicular xenografts. Cell Res 2016;26:139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetjens CM, Stukenborg JB, Nieschlag E, Simoni M, Wistuba J. Complete spermatogenesis in orthotopic but not in ectopic transplants of autologously grafted marmoset testicular tissue. Endocrinology 2008;149:1736–1747. [DOI] [PubMed] [Google Scholar]
- Lukusa AK, Vermylen C, Vanabelle B, Curaba M, Brichard B, Chantrain C, Dupont S, Ferrant A, Wyns C. Bone marrow transplantation or hydroxyurea for sickle cell anemia: long-term effects on semen variables and hormone profiles. Pediatr Hematol Oncol 2009;26:186–194. [DOI] [PubMed] [Google Scholar]
- Masliukaite I, Hagen JM, Jahnukainen K, Stukenborg JB, Repping S, van der Veen F, van Wely M, van Pelt AM. Establishing reference values for age-related spermatogonial quantity in prepubertal human testes: a systematic review and meta-analysis. Fertil Steril 2016;106:1652–1657e1652. [DOI] [PubMed] [Google Scholar]
- McDougall RJ, Gillam L, Delany C, Jayasinghe Y. Ethics of fertility preservation for prepubertal children: should clinicians offer procedures where efficacy is largely unproven? J Med Ethics 2018;44:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano JV, Vilanova-Perez T, Fornes-Ferrer V, Navarro-Gomezlechon A, Martinez-Triguero ML, Garcia S, Gomez-Chacon J, Povo I, Pellicer A, Andres MM et al. . Influence of temperature, serum, and gonadotropin supplementation in short- and long-term organotypic culture of human immature testicular tissue. Fertil Steril 2018;110:1045, e1043–1057. [DOI] [PubMed] [Google Scholar]
- Melan K, Amant F, Veronique-Baudin J, Joachim C, Janky E. Fertility preservation healthcare circuit and networks in cancer patients worldwide: what are the issues? BMC Cancer 2018;18:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertes H, Pennings G. Testicular tissue cryopreservation: combining access with safeguards. Am J Bioeth 2013;13:46–48. [DOI] [PubMed] [Google Scholar]
- Milazzo JP, Vaudreuil L, Cauliez B, Gruel E, Masse L, Mousset-Simeon N, Mace B, Rives N. Comparison of conditions for cryopreservation of testicular tissue from immature mice. Hum Reprod 2008;23:17–28. [DOI] [PubMed] [Google Scholar]
- Ming JM, Chua ME, Lopes RI, Maloney AM, Gupta AA, Lorenzo AJ. Cryopreservation of testicular tissue in pre-pubertal and adolescent boys at risk for infertility: a low risk procedure. J Pediatr Urol 2018;14:274 e271–274 e275. [DOI] [PubMed] [Google Scholar]
- Mulder CL, Catsburg LAE, Zheng Y, de Winter-Korver CM, van Daalen SKM, van Wely M, Pals S, Repping S, van Pelt AMM. Long-term health in recipients of transplanted in vitro propagated spermatogonial stem cells. Hum Reprod 2018;33:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Patrizio P, Brinster RL. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil Steril 2002;78:1225–1233. [DOI] [PubMed] [Google Scholar]
- Nagel K, Neal M. Discussions regarding sperm banking with adolescent and young adult males who have cancer. J Pediatr Oncol Nurs 2008;25:102–106. [DOI] [PubMed] [Google Scholar]
- Nickkholgh B, Mizrak SC, Korver CM, van Daalen SK, Meissner A, Repping S, van Pelt AM. Enrichment of spermatogonial stem cells from long-term cultured human testicular cells. Fertil Steril 2014a;102:558–565e555. [DOI] [PubMed] [Google Scholar]
- Nickkholgh B, Mizrak SC, van Daalen SK, Korver CM, Sadri-Ardekani H, Repping S, van Pelt AM. Genetic and epigenetic stability of human spermatogonial stem cells during long-term culture. Fertil Steril 2014b;102:1700–1707e1701. [DOI] [PubMed] [Google Scholar]
- Ning L, Meng J, Goossens E, Lahoutte T, Marichal M, Tournaye H. In search of an efficient injection technique for future clinical application of spermatogonial stem cell transplantation: infusion of contrast dyes in isolated cadaveric human testes. Fertil Steril 2012;98:1443–1448e1441. [DOI] [PubMed] [Google Scholar]
- Ntemou E, Kadam P, Van Laere S, Van Saen D, Vicini E, Goossens E. Effect of recombinant human vascular endothelial growth factor on testis tissue xenotransplants from prepubertal boys: a three-case study. Reprod Biomed Online 2019a;39:119–133. [DOI] [PubMed] [Google Scholar]
- Ntemou E, Kadam P, Van Saen D, Wistuba J, Mitchell RT, Schlatt S, Goossens E. Complete spermatogenesis in intratesticular testis tissue xenotransplants from immature non-human primate. Hum Reprod 2019b;34:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, de Avila DM, Reeves JJ, McLean DJ. Spermatogenesis and germ cell transgene expression in xenografted bovine testicular tissue. Biol Reprod 2004;71:494–501. [DOI] [PubMed] [Google Scholar]
- Oliver E, Stukenborg JB. Rebuilding the human testis in vitro. Andrology 2019. (Epub ahead of print). doi: 10.1111/andr.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasiuk A, Nussey S, Veys P, Amrolia P, Rao K, Krawczuk-Rybak M, Leiper A. Gonadal function and fertility after stem cell transplantation in childhood: comparison of a reduced intensity conditioning regimen containing melphalan with a myeloablative regimen containing busulfan. Br J Haematol 2015;170:719–726. [DOI] [PubMed] [Google Scholar]
- Perrard MH, Sereni N, Schluth-Bolard C, Blondet A, DE SG, Plotton I, Morel-Journel N, Lejeune H, David L, Durand P. Complete human and rat ex vivo spermatogenesis from fresh or frozen testicular tissue. Biol Reprod 2016;95:89. [DOI] [PubMed] [Google Scholar]
- Picton HM, Wyns C, Anderson RA, Goossens E, Jahnukainen K, Kliesch S, Mitchell RT, Pennings G, Rives N, Tournaye H et al. . A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod 2015;30:2463–2475. [DOI] [PubMed] [Google Scholar]
- Pietzak EJ 3rd, Tasian GE, Tasian SK, Brinster RL, Carlson C, Ginsberg JP, Kolon TF. Histology of testicular biopsies obtained for experimental fertility preservation protocol in boys with cancer. J Urol 2015;194:1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsworth LM, Hinton BT, Setchell BP. Effects of obstruction of the flow of seminiferous tubule fluid on the germinal epithelium in the rat. J Reprod Fertil 1981;63:347–353. [DOI] [PubMed] [Google Scholar]
- Poels J, Abou-Ghannam G, Decamps A, Leyman M, Rieux A, Wyns C. Transplantation of testicular tissue in alginate hydrogel loaded with VEGF nanoparticles improves spermatogonial recovery. J Control Release 2016;234:79–89. [DOI] [PubMed] [Google Scholar]
- Poels J, Van Langendonckt A, Many MC, Wese FX, Wyns C. Vitrification preserves proliferation capacity in human spermatogonia. Hum Reprod 2013;28:578–589. [DOI] [PubMed] [Google Scholar]
- Poganitsch-Korhonen M, Masliukaite I, Nurmio M, Lahteenmaki P, van Wely M, van Pelt AMM, Jahnukainen K, Stukenborg JB. Decreased spermatogonial quantity in prepubertal boys with leukaemia treated with alkylating agents. Leukemia 2017;31:1460–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provoost V, Tilleman K, D'Angelo A, De Sutter P, de Wert G, Nelen W, Pennings G, Shenfield F, Dondorp W. Beyond the dichotomy: a tool for distinguishing between experimental, innovative and established treatment. Hum Reprod 2014;29:413–417. [DOI] [PubMed] [Google Scholar]
- Rathi R, Honaramooz A, Zeng W, Turner R, Dobrinski I. Germ cell development in equine testis tissue xenografted into mice. Reproduction 2006;131:1091–1098. [DOI] [PubMed] [Google Scholar]
- Reda A, Hou M, Winton TR, Chapin RE, Soder O, Stukenborg JB. In vitro differentiation of rat spermatogonia into round spermatids in tissue culture. Mol Hum Reprod 2016;22:601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redig AJ, Brannigan R, Stryker SJ, Woodruff TK, Jeruss JS. Incorporating fertility preservation into the care of young oncology patients. Cancer 2011;117:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relander T, Cavallin-Stahl E, Garwicz S, Olsson AM, Willen M. Gonadal and sexual function in men treated for childhood cancer. Med Pediatr Oncol 2000;35:52–63. [DOI] [PubMed] [Google Scholar]
- Rives N, Milazzo JP, Travers A, Arkoun B, Bironneau A, Sibert L, Liard-Zmuda A, Marie-Cardine A, Schneider P, Vannier JP et al. . Cryopreservation of testicular tissue in children. Bull Acad Natl Med 2013;197:877–886discussion 886. [PubMed] [Google Scholar]
- Rives N, Perdrix A, Hennebicq S, Saias-Magnan J, Melin MC, Berthaut I, Barthelemy C, Daudin M, Szerman E, Bresson JL et al. . The semen quality of 1158 men with testicular cancer at the time of cryopreservation: results of the French National CECOS Network. J Androl 2012;33:1394–1401. [DOI] [PubMed] [Google Scholar]
- Rives N, Rives A, Rondanino C, Castanet M, Cuny A, Sibert L. Fertility preservation in Klinefelter syndrome patients during the transition period. Endocr Dev 2018;33:149–157. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Wallberg KA, Borgstrom B, Petersen C, Thurin-Kjellberg A, Morse H, Giwercman A, Jarfelt M, Work Group UftSAoLA, Regions S. National guidelines and multilingual age-adapted patient brochures and videos as decision aids for fertility preservation (FP) of children and teenagers with cancer-a multidisciplinary effort to improve children’s information and access to FP in Sweden. Acta Obstet Gynecol Scand 2019;98:679–680. [DOI] [PubMed] [Google Scholar]
- Rowley MJ, Leach DR, Warner GA, Heller CG. Effect of graded doses of ionizing radiation on the human testis. Radiat Res 1974;59:665–678. [PubMed] [Google Scholar]
- Ruutiainen T, Miller S, Caplan A, Ginsberg JP. Expanding access to testicular tissue cryopreservation: an analysis by analogy. Am J Bioeth 2013;13:28–35. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S, van Pelt AM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA 2011;305:2416–2418. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Homburg CH, van Capel TM, van den Berg H, van der Veen F, van der Schoot CE, van Pelt AM, Repping S. Eliminating acute lymphoblastic leukemia cells from human testicular cell cultures: a pilot study. Fertil Steril 2014;101:1072–1078e1071. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Mizrak SC, van Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M, Hovingh S, de Reijke TM, de la Rosette JJ, van der Veen F et al. . Propagation of human spermatogonial stem cells in vitro. JAMA 2009;302:2127–2134. [DOI] [PubMed] [Google Scholar]
- Safsaf A, Sibert L, Cleret JM, Perdrix A, Milazzo JP, Gobet F, Mace B, Rives N. Concomitant unilateral and synchronous bilateral testis cancer in azoospermic dizygotic twins: differential management of fertility preservation. Fertil Steril 2011;95:2434:e2411–e2433. [DOI] [PubMed] [Google Scholar]
- Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011;471:504–507. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Honaramooz A, Boiani M, Scholer HR, Dobrinski I. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biol Reprod 2003;68:2331–2335. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Kim SS, Gosden R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction 2002;124:339–346. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Rosiepen G, Weinbauer GF, Rolf C, Brook PF, Nieschlag E. Germ cell transfer into rat, bovine, monkey and human testes. Hum Reprod 1999;14:144–150. [DOI] [PubMed] [Google Scholar]
- Schmidt JA, de Avila JM, McLean DJ. Effect of vascular endothelial growth factor and testis tissue culture on spermatogenesis in bovine ectopic testis tissue xenografts. Biol Reprod 2006;75:167–175. [DOI] [PubMed] [Google Scholar]
- Schrader M, Muller M, Straub B, Miller K. The impact of chemotherapy on male fertility: a survey of the biologic basis and clinical aspects. Reprod Toxicol 2001;15:611–617. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003;125:769–784. [DOI] [PubMed] [Google Scholar]
- Skinner R, Mulder RL, Kremer LC, Hudson MM, Constine LS, Bardi E, Boekhout A, Borgmann-Staudt A, Brown MC, Cohn R et al. . Recommendations for gonadotoxicity surveillance in male childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Lancet Oncol 2017;18:e75–e90. [DOI] [PubMed] [Google Scholar]
- Sklar C. Reproductive physiology and treatment-related loss of sex hormone production. Med Pediatr Oncol 1999;33:2–8. [DOI] [PubMed] [Google Scholar]
- Smith JF, Yango P, Altman E, Choudhry S, Poelzl A, Zamah AM, Rosen M, Klatsky PC, Tran ND. Testicular niche required for human spermatogonial stem cell expansion. Stem Cells Transl Med 2014;3:1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedaker AK, Honaramooz A, Dobrinski I. A game of cat and mouse: xenografting of testis tissue from domestic kittens results in complete cat spermatogenesis in a mouse host. J Androl 2004;25:926–930. [DOI] [PubMed] [Google Scholar]
- Sohni A, Tan K, Song HW, Burow D, de Rooij DG, Laurent L, Hsieh TC, Rabah R, Hammoud SS, Vicini E et al. . The neonatal and adult human testis defined at the single-cell level. Cell Rep 2019;26:1501–1517e1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger A, Steinberger E. Differentiation of rat seminiferous epithelium in organ culture. J Reprod Fertil 1965;9:243–248. [DOI] [PubMed] [Google Scholar]
- Steinberger A, Steinberger E, Perloff WH. Mammalian testes in organ culture. Exp Cell Res 1964;36:19–27. [DOI] [PubMed] [Google Scholar]
- Stukenborg JB, Alves-Lopes JP, Kurek M, Albalushi H, Reda A, Keros V, Tohonen V, Bjarnason R, Romerius P, Sundin M et al. . Spermatogonial quantity in human prepubertal testicular tissue collected for fertility preservation prior to potentially sterilizing therapy. Hum Reprod 2018a;33:1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukenborg JB, Jahnukainen K, Hutka M, Mitchell RT. Cancer treatment in childhood and testicular function: the importance of the somatic environment. Endocr Connect 2018b;7:R69–R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Nagayoshi M, Awata S, Mawatari Y, Tanaka I, Kusunoki H. Completion of meiosis in human primary spermatocytes through in vitro coculture with Vero cells. Fertil Steril 2003;79:795–801. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Bahceci M, Ozcan C, Greco E, Mendoza C. Restoration of fertility by in-vitro spermatogenesis. Lancet 1999;353:555–556. [DOI] [PubMed] [Google Scholar]
- Uijldert M, Meissner A, de Melker AA, van Pelt AMM, van de Wetering MD, van Rijn RR, van Wely M, van der Veen F, Repping S. Development of the testis in pre-pubertal boys with cancer after biopsy for fertility preservation. Hum Reprod 2017;32:2366–2372. [DOI] [PubMed] [Google Scholar]
- Vadaparampil S, Quinn G, King L, Wilson C, Nieder M. Barriers to fertility preservation among pediatric oncologists. Patient Educ Couns 2008;72:402–410. [DOI] [PubMed] [Google Scholar]
- Valli-Pulaski H, Peters KA, Gassei K, Steimer SR, Sukhwani M, Hermann BP, Dwomor L, David S, Fayomi AP, Munyoki SK et al. . Testicular tissue cryopreservation: 8 years of experience from a coordinated network of academic centers. Hum Reprod 2019;34:966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Saen D, Goossens E, Aerts JL, Haentjens P, Tournaye H. Does early cell death cause germ cell loss after intratesticular tissue grafting? Fertil Steril 2013;99:1264–1272e1261. [DOI] [PubMed] [Google Scholar]
- Van Saen D, Goossens E, Bourgain C, Ferster A, Tournaye H. Meiotic activity in orthotopic xenografts derived from human postpubertal testicular tissue. Hum Reprod 2011;26:282–293. [DOI] [PubMed] [Google Scholar]
- Van Saen D, Goossens E, De Block G, Tournaye H. Regeneration of spermatogenesis by grafting testicular tissue or injecting testicular cells into the testes of sterile mice: a comparative study. Fertil Steril 2009;91:2264–2272. [DOI] [PubMed] [Google Scholar]
- Van Saen D, Vloeberghs V, Gies I, Mateizel I, Sermon K, De Schepper J, Tournaye H, Goossens E. When does germ cell loss and fibrosis occur in patients with Klinefelter syndrome? Hum Reprod 2018;33:1009–1022. [DOI] [PubMed] [Google Scholar]
- Wallace WH, Smith AG, Kelsey TW, Edgar AE, Anderson RA. Fertility preservation for girls and young women with cancer: population-based validation of criteria for ovarian tissue cryopreservation. Lancet Oncol 2014;15:1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Liu X, Chang G, Chen Y, An G, Yan L, Gao S, Xu Y, Cui Y, Dong J et al. . Single-cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell 2018;23:599–614e594. [DOI] [PubMed] [Google Scholar]
- Whitehead E, Shalet SM, Jones PH, Beardwell CG, Deakin DP. Gonadal function after combination chemotherapy for Hodgkin’s disease in childhood. Arch Dis Child 1982;57:287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson M, Vatanen A, Borgstrom B, Gustafsson B, Taskinen M, Saarinen-Pihkala UM, Winiarski J, Jahnukainen K. Adult testicular volume predicts spermatogenetic recovery after allogeneic HSCT in childhood and adolescence. Pediatr Blood Cancer 2014;61:1094–1100. [DOI] [PubMed] [Google Scholar]
- Wistuba J, Mundry M, Luetjens CM, Schlatt S. Cografting of hamster (Phodopus sungorus) and marmoset (Callithrix jacchus) testicular tissues into nude mice does not overcome blockade of early spermatogenic differentiation in primate grafts. Biol Reprod 2004;71:2087–2091. [DOI] [PubMed] [Google Scholar]
- Wyns C, Collienne C, Shenfield F, Robert A, Laurent P, Roegiers L, Brichard B. Fertility preservation in the male pediatric population: factors influencing the decision of parents and children. Hum Reprod 2015;30:2022–2030. [DOI] [PubMed] [Google Scholar]
- Wyns C, Curaba M, Martinez-Madrid B, Van Langendonckt A, Francois-Xavier W, Donnez J. Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Hum Reprod 2007;22:1603–1611. [DOI] [PubMed] [Google Scholar]
- Wyns C, Curaba M, Petit S, Vanabelle B, Laurent P, Wese JF, Donnez J. Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Hum Reprod 2011;26:737–747. [DOI] [PubMed] [Google Scholar]
- Wyns C, Curaba M, Vanabelle B, Van Langendonckt A, Donnez J. Options for fertility preservation in prepubertal boys. Hum Reprod Update 2010;16:312–328. [DOI] [PubMed] [Google Scholar]
- Wyns C, Van Langendonckt A, Wese FX, Donnez J, Curaba M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum Reprod 2008;23:2402–2414. [DOI] [PubMed] [Google Scholar]
- Yang S, Ping P, Ma M, Li P, Tian R, Yang H, Liu Y, Gong Y, Zhang Z, Li Z et al. . Generation of haploid spermatids with fertilization and development capacity from human spermatogonial stem cells of cryptorchid patients. Stem Cell Reports 2014;3:663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokonishi T, Sato T, Komeya M, Katagiri K, Kubota Y, Nakabayashi K, Hata K, Inoue K, Ogonuki N, Ogura A et al. . Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat Commun 2014;5:4320. [DOI] [PubMed] [Google Scholar]
- Zeng W, Avelar GF, Rathi R, Franca LR, Dobrinski I. The length of the spermatogenic cycle is conserved in porcine and ovine testis xenografts. J Androl 2006;27:527–533. [DOI] [PubMed] [Google Scholar]
- Zhang P, Chen X, Zheng Y, Zhu J, Qin Y, Lv Y, Zeng W. Long-term propagation of porcine undifferentiated spermatogonia. Stem Cells Dev 2017;26:1121–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Thomas A, Schmidt CM, Dann CT. Quantitative detection of human spermatogonia for optimization of spermatogonial stem cell culture. Hum Reprod 2014;29:2497–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]


