Abstract
Background
microRNAs (miRNAs) play important roles in abnormal proliferation and migration of vascular smooth muscle cells (VSMCs), which lead to restenosis in coronary artery disease. Nevertheless, the role of miR-18a-5p and how it works in VSMCs remain unknown.
Material/Methods
miR-18a-5p expression was determined by fluorescence quantitative real-time polymerase chain reaction (qRT-PCR) analysis of tissues from 20 patients with stent restenosis, and rats with carotid artery injury, as well as VSMCs. A cell viability assay was used to measure cell proliferation. Cell migration abilities were assessed by transwell migration assay and wound healing assays. To identify miR-18a-5p targets, a dual-luciferase reporter assay was performed. Western blot analysis and immunofluorescence techniques were used to assess the protein expression levels of AKT and ERK. The rescue effects of miR-18a-5p on the proliferation or migration of VSMCs were evaluated after exposure to the AKT inhibitor MK-2206 and ERK inhibitor PD98059.
Results
The expression level of miR-18a-5p was significantly higher in the blood serum of patients with stent restenosis and in rats with carotid artery injury, and the expression of AKT and ERK was higher after carotid artery injury. The proliferation and migration abilities of VSMCs were accelerated by the overexpression of miR-18a-5p. It was found that miR-18a-5p directly modulates AKT/ERK signaling. Upregulated miR-18a-5p increased the protein expression levels of AKT and ERK and we found a positive correlation between miR-18a-5p expression level and expression of AKT and ERK. Additionally, the promoting effect of miR-18a-5p on VSMCs proliferation, migration, and invasion was reversed by ERK inhibitor or AKT inhibitor.
Conclusions
miR-18a-5p can promote proliferation of VSMCs by activating the AKT/ERK signaling pathway.
MeSH Keywords: Coronary Restenosis, MAP Kinase Signaling System, Proto-Oncogene Proteins c-akt
Background
Coronary artery disease (CAD) is the primary cause of major cardiovascular events and is associated with a high mortality rate worldwide. In 2016 alone, 17 million deaths were caused by cardiovascular diseases, and in the past decade the number has increased by 14.5% [1]. Common methods of treatment for CAD include stent implantation and percutaneous coronary intervention (PCI) [2,3]. However, stent implantation often leads to restenosis due to (neo)intimal hyperplasia [4,5], which can result in short-term or long-term failure of such treatments. Some studies indicate that proliferation and migration of vascular smooth muscle cells (VSMCs) have a vital role in neointimal hyperplasia [6–8]. It is well established that foreign substances like scaffolds can promote the aberrant proliferation and migration of VSMCs [6,8], resulting in the transfer of these cells from the tunica media to tunica intima. There is also abundant secretion of extracellular matrix from the contractile to the secretory phenotype, which causes gradual thickening of the intima, resulting in stenosis [9,10]. Unfortunately, the latent mechanism of proliferation and migration of VSMC in atherosclerosis and stent restenosis is still not well understood.
MicroRNAs (miRNA), which are small, non-coding RNAs ~22 nucleotides in length, can negatively modulate gene expression by repressing mRNA translation or by inducing instability of target mRNA [11]. Previous research revealed that miRNAs such as miR-3646 [12], miR-1260b [13], and miR-93 [14], have crucial roles in regulating VSMC proliferation, differentiation, and apoptosis. Wang et al. reported that miR-92a promotes VSMCs proliferation and migration through the ROCK/MLCK signaling pathway [15], and another study found that miR-133b controls VSMC growth and migration by targeting matrix metallopeptidase9 [9]. Therefore, the differential expression profile of miRNAs might provide a basis for the preoperative clinical diagnosis and postoperative treatment of stent restenosis.
miR-18a-5p, which is part of the miRNA 17–92 cluster located at 13q31.3, has an vital role in multiple biological processes, such as proliferation, migration, and invasion, in cells of certain human cancers, including osteosarcoma [16], nasopharyngeal carcinoma [17], and non-small cell lung cancer [18]. Chen et al. found that downregulation of miR-18a-5p suppressed cell proliferation and invasion through inhibition of the protein kinase B (AKT)/extracellular regulated protein kinases (ERK) signaling pathway [19]. Importantly, the AKT/ERK signaling pathway is also involved in VSMC proliferation and migration in humans and animals with atherosclerosis [20,21]. Sun et al. found that inhibition of the AKT/ERK pathway reversed the effects of miR-21, promoting VSMC proliferation and migration [20]. However, due to the tissue specificity linked with miR-18a-5p, its role in regulating biological processes in VSMCs is uncertain. It remains unclear whether the AKT/ERK pathway has an important role in neutralizing the effect of miR-18a-5p in VSMC regulation.
Accordingly, we hypothesized that the AKT/ERK signaling pathway is activated in the unfavorable progression of VSMCs, due to the higher expression of miR-18a-5p. Our objective here was to explore the potential activity of miR-18a-5p in VSMCs. We aimed to confirm the molecular mechanisms by which miR-18a-5p controls cell proliferation and migration in VSMCs, and to provide an additional theoretical basis for improvement of treatment strategies for patients with stent restenosis.
Material and Methods
Serum samples of patients
Twenty patients with stent restenosis who underwent PCI procedures between May 2015 and May 2018 at the Third Hospital of Wuhan, Hubei, China were included in our study. In addition, 20 healthy volunteers who did not have cancer, autoimmune diseases, ankylosing spondylitis (AS) disease, inflammatory diseases, or recent infection (less than 1 month) were also included in this study as the control group. Whole venous blood (5–10 mL) was drawn from the elbow vein and were incubated at 4°C for 1 h. Serum fractions were then separated by centrifugation at 3000 rpm for 5 min. After phase separation, plasma was collected, and stored at −80°C until the extraction of RNA. The experimental protocol of this study was approved by the Ethics Committee of the Third Hospital of Wuhan, Hubei, China, and written informed consent was obtained from all patients.
Establishment of animal model and grouping of animals
Twenty-four 8-week-old Sprague-Dawley rats, weighing 200–250 g, were randomly divided into 2 groups: a negative control (NC) group (n=12) and an injury group (n=12). The NC group rats were not subjected to any treatment, while the injury group rats were subjected to carotid artery balloon injury. The basic flow of the study was as follows. Twelve 8-week-old male Sprague-Dawley rats with carotid artery balloon injury were obtained from Peking University (Beijing, China). Rats were anesthetized with isoflurane and the left carotid artery was exposed under a dissecting microscope and a 2-mm-diameter catheter was inserted in the external carotid artery, then the balloon was inflated at 1.5 atm and pulled through the carotid artery 3 times. Rats were killed 14 days after injury. All rats were maintained in a specific pathogen-free facility with a 12-h lighting schedule (8: 00–20: 00 hours).
Cell culture and transfection
VSMCs were purchased from Shanghai Cell Biochemical Institute (Shanghai, China). VSMCs were cultivated in smooth muscle cell growth medium (SMCGM) from Sigma-Aldrich (Saint Louis, MO, USA) with 10% fetal bovine serum (FBS) from Thermo Fisher Scientific, Inc. (Waltham, MA, USA), at 37°C with 5% CO2 in a damp petri dish. For transfection, VSMCs were cultured in 6-well plates and transfected with NC miRNA, miR-18a-5p mimic, and miR-18a-5p inhibitor by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. Cells were also treated with the ERK inhibitor PD98059 (25 μmol/L) and AKT inhibitor MK-2206 (20 μmol/L) to further test the role these 2 kinases play in mediating the effect of miR-18a-5p. After transfection for 48 h, cells were observed under a microscope to confirm the efficiency of transfection. The miR-18a-5p mimics and miR-18a-5p inhibitor were synthesized by ZoonBio Biotechnology Co. (Nanjing, Jiangsu, China).
Cell proliferation assay
Cell viability was measured using the Cell Counting Kit-8 (CCK-8) assay (Dojindo Laboratories, Kumamoto, Japan). After transfection with miR-18a-5p mimics or miR-18a-5p inhibitor, VSMCs were incubated, either in the presence or absence of AKT and ERK inhibitors, at 37°C with 5% CO2 in a damp petri dish. Subsequently, VSMCs were seeded in 96-well plates at 5×103 cells per well and incubated for 4 h. The cell viability was assessed by the CCK-8 assay at 12, 24, 48, and 72 h. The absorbance of each well was measured with a microplate reader at 450 nm and the measured values were used to calculate the cell viability.
VSMCs were seeded in 6-well plates at 1×104 cells/plate and subsequently transfected and treated with AKT or ERK inhibitor for 24 h. Afterwards, scratch wounds were made in each well with a sterile pipette tip. The wounded areas were observed and photographed under a microscope after 0 and 24 h. The ability of the cells to migrate was measured based on the scratch’s healed area. The scratch width and percentage of wound closure was measured with the Image Pro Plus software (Media Cybernetics, Rockville, MD, USA).
Transwell migration assay
Transwell migration assay was used to assess the cell migratory potential of VSMCs. VSMCs were seeded into the upper chamber of the Transwell® cell culture inserts with 8 μm pore size (Costar-Corning, Inc., Corning, NY, USA) containing SMCGM (without FBS). The lower chamber was filled with SMCGM with chemoattractant (10% FBS). After an incubation period of 24 h, the cells on the upper surface insert membrane were cleaned by a cotton swab. Then, 70% ethanol was used to fix the migrated cells, followed by staining with 0.5% crystal violet for 30 min at room temperature. A light microscope was used for counting the number of migrated cells (5 random fields per chamber).
Dual-luciferase reporter assay
TargetScan online software “(http://www.targetscan.org)” was used to identify miR-18a-5p targets. The AKT 3′-UTR-luciferase reporter plasmid (PTEN-WT) or ERK 3′-UTR-luciferase reporter plasmid (ERK -WT) containing the target sequence for miR-18a-5p and seed site-directed mutation plasmid (AKT-MUT and ERK-MUT) were designed and synthesized by Applied Biosystems (Waltham, MA, USA). Recombinant plasmids were created by insertion into the pGL3-Control vector (Promega Corp., Madison, WI, USA), following the instructions of the manufacturer. Luciferase activity detection was performed with VSMCs. After 48 h of incubation, the relative luciferase activity was determined by the dual-luciferase assay system (Promega Corp.) following the protocol of the manufacturer. The relative luciferase activity was determined using the activity ratio of firefly and Renilla luciferase.
Western blot analysis
Four rats from each group were sacrificed under anesthesia. Tissue was dissected and used to extract protein. Total cell lysate was prepared by resuspending and lysing the tissue in RIPA lysis buffer (Beyotime, Shanghai, China) supplemented with a proteinase inhibitor cocktail (Beyotime). The bicinchoninic acid (BCA) protein assay kit was used for determining protein concentration (Beyotime). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were used to separate proteins. After transferring the proteins to a polyvinylidene difluoride (PVDF) membrane, the unoccupied unspecific binding sites on the membrane were blocked with 5% milk in TBST buffer at room temperature. The membrane was incubated overnight at 4°C with the appropriate primary antibody (anti-AKT, ab38449; anti-ERK, ab17942; anti-GAPDH, ab8245; Abcam, Shanghai, China). Subsequently, the membrane was incubated with the corresponding peroxidase-conjugated secondary antibody. Chemiluminescent detection was performed using the ECL system (Millipore, Billerica, MA, USA).
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from blood samples using TRIzol reagent (Yaji Mall, Minhang, Shanghai, China). The extracted total RNA was stored at −80°C until used to prepare miR-18a-5p by reverse transcription into cDNA using the TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific, Inc.) followed by PCR amplification of the cDNA. The PrimeScript RT Reagent Kit was used for reverse transcription to cDNAs (Takara Bio, Inc., Shiga, Japan) according to the protocol of the manufacturer. miR-18a-5p expression was normalized against U6 small nuclear RNA (snRNA). The specific sequences of the primers used were as follows:
-
miR-18a-5p forward: 5′-UAAGGUGCAUCUAGUGCAGAUAG-3′ and
reverse: 5′-CUAUCUGCACUAGAUGCACCUUA-3′;
-
U6 snRNA forward: 5′-CTCGCTTCGGCAGCACA-3′ and
reverse: 5′-AACGCTT CACGAATTTGCGT-3′.
Immunofluorescence and immunohistochemistry assay
Four rats were anesthetized with 8% chloral hydrate (400 mg/kg, i.p.) and perfused transcardially with 200 ml of normal saline followed by 200–300 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Then, the tissue was embedded and sliced for immunofluorescence staining. Pre-cooled acetone was used for fixation of cells on glass coverslips, followed by rinsing 3 times with phosphate-buffered saline (PBS). Cells were permeabilized with 0.1% Triton X-100 and then incubated with 1% BSA/PBS to block non-specific binding. Subsequently, tissue samples were stained with the appropriate primary antibody (anti-AKT, ab38449; anti-ERK, ab17942; Abcam) at 4°C overnight. Subsequently, the stained tissue samples were washed with PBS for 5 min, and then incubated with the corresponding secondary anti-rabbit antibody (ZSGB-Bio, Beijing, China) for 30 min. Images were acquired with a Nikon Ni-U microscope (Nikon Corp., Tokyo, Japan). Then, after staining cells with a DAPI solution in the dark for 5 min, AKT-, and ERK-positive cells were counted using ImageJ 1.8.0 software (Media Cybernetics).
Hematoxylin-eosin staining
Tissue samples from 4 rats from each group were fixed in 4% paraformaldehyde for 24 h, followed by dehydration with gradient alcohol. Then, after the tissue was embedded, sliced, dewaxed, and rehydrated, sliced sections were stained with hematoxylin for 3–8 min, followed by eosin staining for 1–3 min. Subsequently, the sections were sequentially gradient dehydrated and sealed for fluorescence imaging to observe the pathological changes of the arteries.
Statistical analysis
The data were analyzed by using SPSS 18.0 software (IBM Corp., Armonk, NY, USA) and the GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Data are shown as the mean±standard deviation (SD). The t test was used to analyze differences. Analysis of variance (ANOVA) with a post hoc test was used to compare multiple groups. Correlations between 2 variables were assessed by correlation analysis. P-value <0.05 was considered statistically significant.
Results
The expression of miR-18a-5p was increased in CAD patients and rats
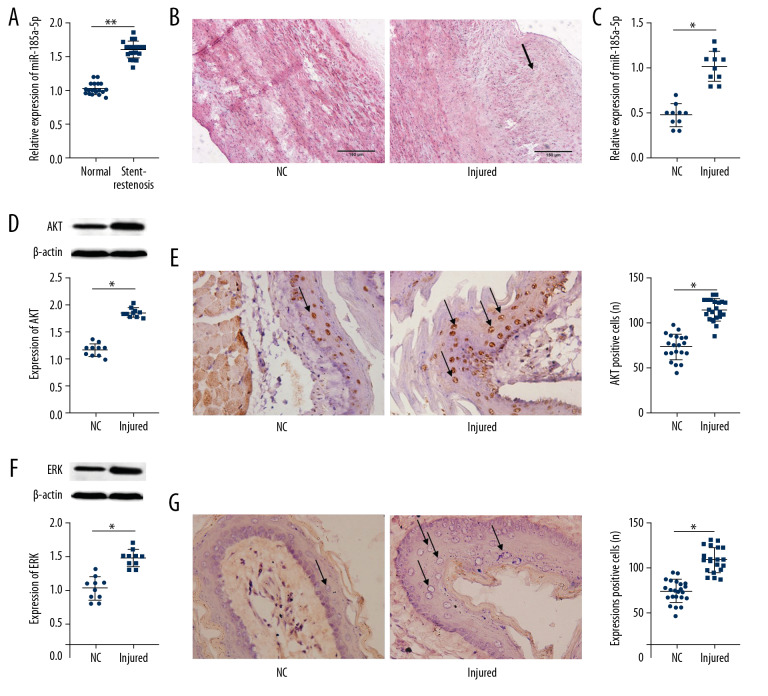
The serum expression of miR-18a-5p in CAD and control groups was determined by qRT-PCR analysis. The results shown in Figure 1A reveal that miR-18a-5p expression level significantly rose in the blood serum of the patient with stent restenosis (p<0.01). After carotid artery balloon injury, the artery exhibited abnormal stenosis and pathological thickening (Figure 1B), which indicated that the model of the carotid artery balloon injury was successfully established. The result of the miR-18a-5p expression analysis in rats (Figure 1C) revealed that its expression level was significantly higher in the injury group in comparison with the NC group (p<0.01). In addition, the carotid artery balloon injury increased the protein expression levels of AKT (p<0.01; Figure 1D) and ERK (p<0.01; Figure 1F) in rats. Also, the number of AKT-positive (p<0.01, Figure 1E) and ERK-positive (p<0.01, Figure 1G) cells was increased in the injury group rats in comparison with those in the NC group.
Figure 1.
miR-18a-5p was overexpressed in patients with restenosis and in rats with carotid artery injury. (A, C) Expression of miR-18a-5p in serum was determined by qRT-PCR. (B) The degree of arterial wall thickening in the rats was assessed using hematoxylin and eosin staining. (D, F) Expression analysis of AKT and ERK protein was performed by Western blotting. (E, G) Numbero f AKT-positive (p<0.01, Figure 3E) and ERK-positive cells were determined by immunofluorescence analysis. Representative images of the VSMCs by photomicrographs (200×). Data represent the mean±S.E.M. ** p<0.01 was significant; * p<0.05 was significant.
miR-18a-5p accelerated the proliferation and migration of VSMCs
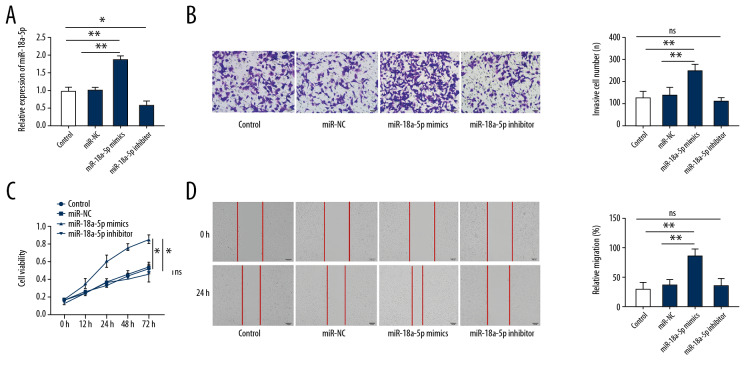
The qRT-PCR expression analysis after transfection with miR-18a-5p mimics (25 nM) revealed (Figure 2A) that miR-18a-5p expression level was higher in the miR-18a-5p mimics group than in the miR-NC group (p<0.01). The CCK-8 assay revealed (Figure 2C,) that the increased expression of miR-18a-5p significantly promoted cell proliferation (p<0.01). In contrast to the control group and NC-transfected group, VSMCs overexpressing miR-18a-5p (Figure 2B) had increased migratory capacity (p<0.01). In addition, the results of the wound healing assay indicated that VSMCs overexpressing miR-18a-5p had significantly increased migration capacity (Figure 2D) in comparison with VSMCs transfected with miR-NC (p<0.01). As anticipated, the expression of miR-18a-5p was reduced in VSMCs transfected with the miR-18a-5p inhibitor (25 nM) (Figure 2A) in comparison with the expression in VSMCs transfected with control and miR-NC (p<0.05). However, the lower level of miR-18a-5p had no significant effect on the proliferation (Figure 2C, p>0.05) and migration (Figure 2B, 2D, p>0.05) of VSMCs in comparison with the cells transfected with the control and miR-NC.
Figure 2.
Overexpression of miR-18a-5p promotes proliferation and migration in VSMCs. (A) Expression of miR-18a-5p in VSMCs was determined by qRT-PCR. (B) The migration ability of VSMCs was evaluated by transwell invasion assays. (C) Cell viability of VSMCs was evaluated by CCK-8 assay. (D) Migration ability of VSMCs was evaluated by wound healing assay. Representative image of VSMCs (200×). Data are expressed as the mean±S.E.M. ** p<0.01 was significant; * p<0.05 was significant; ns was not significant.
AKT/ERK is a target pathway of miR-18a-5p
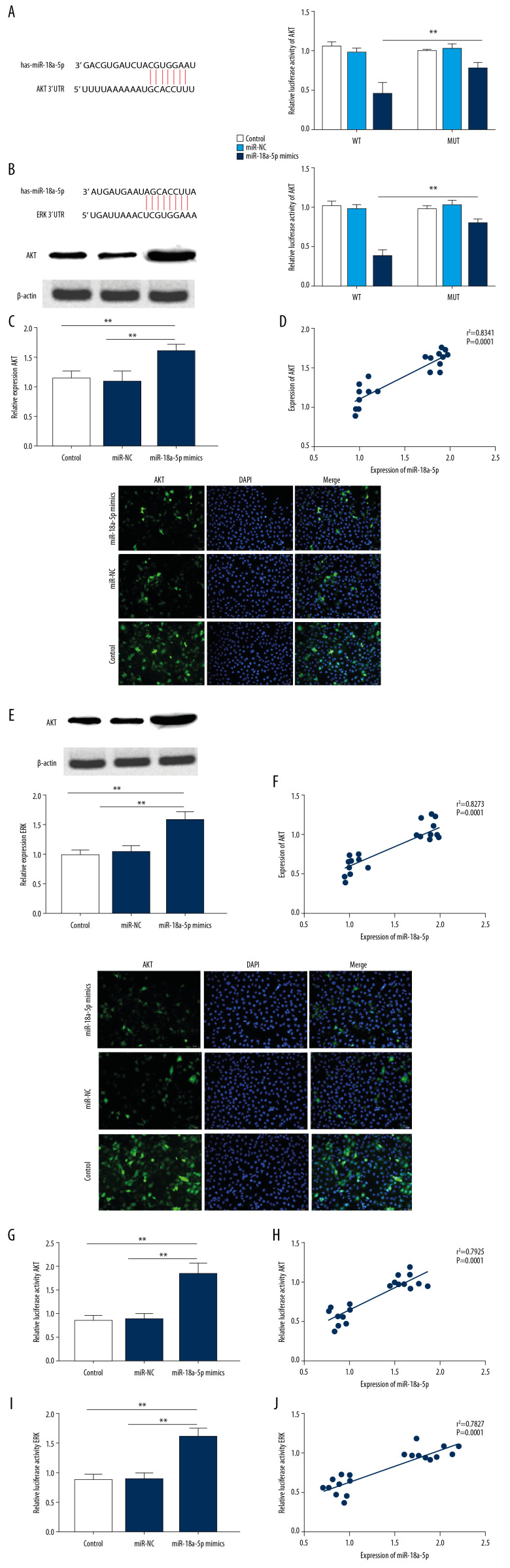
The TargetScan 7.2 target gene prediction software predicted that miR-18a-5p targeted the AKT (Figure 3A) and ERK (Figure 3B). In addition, the luciferase assay results (Figure 3A) indicated that miR-18a-5p significantly repressed pGL3-AKT-WT activity (p<0.05) and pGL3- ERK-WT (Figure 3B, p<0.05). Additionally, Western blot analysis revealed that cells transfected with miR-18a-5p mimics had significantly increased protein levels of AKT (Figure 3C, p<0.05) and ERK (Figure 3E, p<0.05). Additionally, miR-18a-5p expression was found to be strongly positively correlated with AKT protein expression (Figure 3D, p<0.05) and ERK protein expression (Figure 3F, p<0.05). Immunofluorescence analysis of protein expression revealed that the expression level of AKT (Figure 3G, p<0.01) and ERK (Figure 3I, p<0.01) was higher in cells transfected with miR-18a-5p mimics than in cells transfected with control or miR-NC. The expression level of miR-18a-5p was found to be significantly positively correlated with the protein expression levels of AKT (Figure 3H, p<0.01) and ERK (Figure 3J, p<0.01).
Figure 3.
The AKT/ERK signaling pathway is targeted and modulated by miR-18a-5p. (A, B) Luciferase activity of the AKT and ERK were measured by the dual-luciferase reporter assay. (C, E) Expression of AKT and ERK in VSMCs was measured by Western blot analysis. (D, F) Correlation between miR-18a-5p level with the protein expression level of AKT and ERKs. (G and I) Luciferase activity of AKT and ERK in VSMCs was examined by immunofluorescence analysis. (H, J) Correlation between the miR-18a-5p expression level with the AKT and ERK luciferase activity. Representative images of the VSMCs are shown (200×). Data are expressed as the mean±S.E.M. ** p<0.01 were significant.
miR-18a-5p promoted proliferation and migration via the AKT/ERK pathway
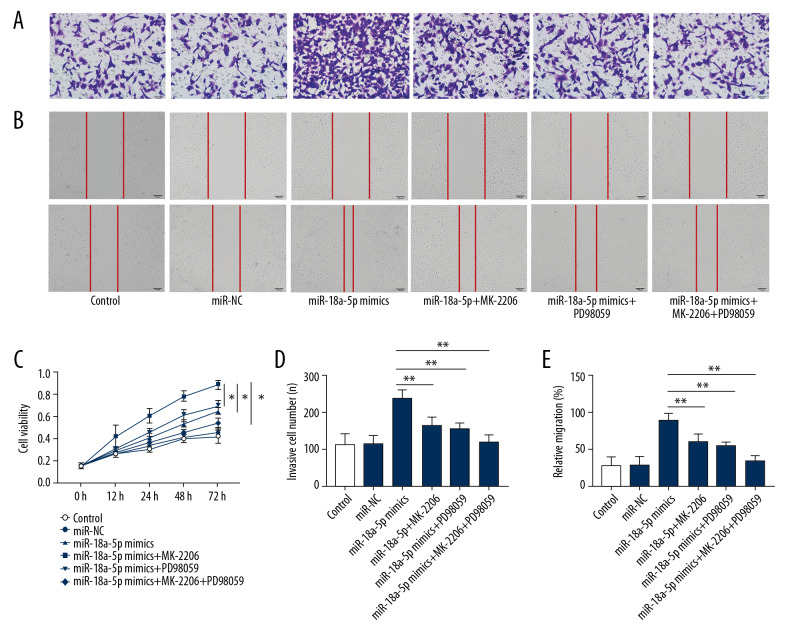
Given that AKT is a direct target of miR-18a-5p, we further investigated whether the promotion of proliferation and migration of VSMCs by increased level of miR-18a-5p was balanced through the AKT/ERK signaling pathway. To this end, we evaluated the effects of treatment with the ERK inhibitor PD98059 or AKT inhibitor MK-2206 on VSMCs transfected with miR-18a-5p mimics. The results of the CCK-8 assay indicated that the proliferation of VSMCs was greatly reduced by the inhibitors of these 2 kinases (Figure 4C, p<0.01). Meanwhile, the wound healing (Figure 4B, 4E, p<0.01) and Transwell migration assay (Figure 4A, 4D, p<0.01) indicated that the stimulatory effect of miR-18a-5p on VSMCs migration was rescued by reduced expression of ERK inhibitor or AKT inhibitor.
Figure 4.
MiR-18a-5p promotes proliferation and migration through the AKT/ERK signaling. (A, D) Migration capacity of VSMCs was evaluated by the Transwell invasion assays. (B, E) Migration capacity of VSMCs was evaluated by the wound healing assay. (C) Cell viability of VSMCs was measured by the CCK-8 assays. Representative images of the VSMCs (200×). Data are expressed as the mean±S.E.M. ** p<0.01 was significant; * p<0.05 was significant.
Discussion
The high incidence of in-stent restenosis (10%) counteracts the beneficial effects of PCI and limits its wider application for unstable CAD [4]. Some studies have suggested that miRNAs contribute to the determination of the phenotype and modulation of the proliferation of VSMCs, which results in intimal regeneration, stenosis of the lumen, and in-stent restenosis [22–24]. We found that miR-18a-5p expression level dramatically rose in blood serum of patients with stent restenosis and rats with carotid artery balloon injury, and also found increased expression of AKT and ERK after carotid artery injury in rats. In addition, functional in vitro experiments revealed that upregulation of miR-18a-5p accelerated the proliferation, migration, and invasion of VSMCs. We also confirmed that miR-18a-5p directly modulates the AKT/ERK signaling pathway, and its effect on VSMC proliferation and migration is mediated through the AKT/ERK signaling pathway. The study is the first to determine the expression, function, and mechanism of action of miR-18a-5p in patients with restenosis after PCI.
Previous research revealed that miR-18a-5p is mainly related to the recurrence and development of tumors. In particular, miR-18a-5p was shown to be a good predictor of clinical recurrence of osteosarcoma [16], nasopharyngeal carcinoma [17], and non-small cell lung carcinoma [18]. Liu et al. reported that miR-18a-5p controls the proliferation, migration, and invasion of glioma cells [25]. Unfortunately, the effect of miR-18a-5p on the function of VSMCs remains to be elucidated. In this study, we found that miR-18a-5p was dramatically increased in serum of CAD patients and rats with carotid artery balloon injury. In addition, further analysis demonstrated that miR-18a-5p overexpression significantly accelerated cell proliferation and migration of VSMCs in vitro. However, downregulation of the level of miR-18a-5p by its inhibitor did not lead to significant changes in VSMC biological processes in vitro, such as proliferation and migration capacity. A review of the related literature found no direct evidence of is a biological inhibiting effect of downregulation of miR-18a-5p in cell (including tumor cells without any pretreatment) proliferation or migration. These results suggest that the upregulation of miR-18a-5p expression is involved in abnormal VSMC biological processes/functions that are associated with the development of stent restenosis.
ERK and AKT are protein kinases that regulate multiple biological processes, including atherosclerosis, early atherosclerosis progression, vascular remodeling, and the development of arterial plaque rupture [26,27]. Sun et al. found that miR-21 can alleviate the hyperproliferative reaction of VSMCs in arterial vascular diseases and maintain plaque stability via the AKT/ERK signaling pathway [20]. The expression of AKT and ERK in the carotid artery balloon injury rat model was found to be increased, which further demonstrates the key role of the AKT/ERK signaling pathway in arterial vascular diseases. Recently, some studies reported that inhibition of the AKT/ERK signaling pathway suppresses the proliferation and migration of VSMCs in atherosclerosis [20,28]. However, there is no definitive evidence showing the relationship between miR-18a-5p and the AKT/ERK signaling pathway in VSMCs. The present study further investigated the molecular mechanism of the effect of miR-18a-5p on VSMCs biological functions through various approaches. The online miRNA target prediction software TargetScan predicted that AKT and ERK were direct targets of miR-18a-5p. Remarkably, Western blot analysis and immunofluorescence analysis of protein expression indicated that the upregulated expression of miR-18a-5p increased the protein expression level of AKT and ERK. We found that the expression of miR-18a-5p was positively correlated with the expression of AKT and ERK in VSMCs. We also determined the biological association between the expression of miR-18a-5p and activation of the AKT/ERK signaling pathway in VSMCs, which provides an avenue for further research into potential therapeutic treatment for patients with restenosis.
The role of the AKT/ERK signaling pathway in mediating the miR-18a-5p-promoting effect on the proliferation and migration of VSMCs was further investigated through the use the ERK inhibitor PD98059 and the AKT inhibitor MK-2206. We found that the effect of miR-18a-5p on the proliferation and migration of VSMCs was markedly reversed by the ERK or AKT inhibitor, and the simultaneous treatment with the 2 inhibitors had synergistic effects. This reveals that miR-18a-5p accelerates the proliferation and migration of VSMCs through activation of the AKT/ERK signaling pathway. Some studies indicated that activation of the AKT/ERK signaling pathway is involved in the switch from contractile to secretory phenotype of VSMCs in large vessels [29]. Chen et al. found that AKT, which promotes cell cycle progression from G0/G1 to the S phase, also participates in the proliferation and migration of VSMCs [30]. These are all potential mechanisms by which miR-18a-5p exerts its effect on the abnormal VSMC biological functions that are linked to development of stent restenosis by modulating the AKT/ERK signaling pathway.
This study confirmed that miR-18a-5p promotes VSMCs proliferation and migration through activation of the AKT/ERK signaling pathway. Humphreys et al. reported that miR-18a-5p regulates tumor cell cycle progression by targeting CDC42, but whether CDC42 is involved in the VSMCs cycle control is unclear [31]. Further research is needed to determine the role of upstream regulatory proteins of the AKT/ERK signaling pathway, including phosphatidylinositol-3-hydroxykinase [32], platelet-derived growth factor B [33], and mitogen-activated protein kinase [34] in mediating the impact of miR-18a-5p on the abnormal VSMCs biological functions.
Conclusions
This study demonstrated that miR-18a-5p was upregulated in patients with restenosis, and miR-18a-5p was shown to accelerate VSMC proliferation and migration by activating the AKT/ERK signaling pathway. This study is the first to show the role of miR-18a-5p in restenosis after PCI, providing novel targets for diagnosis and development of biotherapy for restenosis.
Footnotes
Conflict of interests
None.
Source of support: This work was sponsored by the Clinical Medicine Research Project of the Wuhan Health Bureau (grant no. WX12C17)
References
- 1.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feinberg MW. Healing the injured vessel wall using microRNA-facilitated gene delivery. J Clin Invest. 2014;124(9):3694–97. doi: 10.1172/JCI77509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buccheri D, Piraino D, Andolina G, Cortese B. Understanding and managing in-stent restenosis: A review of clinical data, from pathogenesis to treatment. J Thorac Dis. 2016;8(10):E1150–62. doi: 10.21037/jtd.2016.10.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pleva L, Kukla P, Hlinomaz O. Treatment of coronary in-stent restenosis: A systematic review. J Geriatr Cardiol. 2018;15(2):173–84. doi: 10.11909/j.issn.1671-5411.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Jiang M, Xu Z, et al. miR-146b-5p promotes VSMC proliferation and migration. Int J Clin Exp Pathol. 2015;8(10):12901–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Afzal TA, Luong LA, Chen D, et al. NCK associated protein 1 modulated by miRNA-214 determines vascular smooth muscle cell migration, proliferation, and neointima hyperplasia. J Am Heart Assoc. 2016;5(12):e004629. doi: 10.1161/JAHA.116.004629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Deng B, Jiang X, et al. All-trans-retinoic acid suppresses neointimal hyperplasia and inhibits vascular smooth muscle cell proliferation and migration via activation of AMPK signaling pathway. Front Pharmacol. 2019;10:485. doi: 10.3389/fphar.2019.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Yang Y, Jiang S, et al. MiR-378a-5p regulates proliferation and migration in vascular smooth muscle cell by targeting CDK1. Front Genet. 2019;10:22. doi: 10.3389/fgene.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Xiong W, Liu F, et al. MicroRNA-133b regulates the growth and migration of vascular smooth muscle cells by targeting matrix metallopeptidase 9. Pathol Res Pract. 2019;215(5):1083–88. doi: 10.1016/j.prp.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- 11.Hsu PY, Hsi E, Wang TM, et al. MicroRNA let-7g possesses a therapeutic potential for peripheral artery disease. J Cell Mol Med. 2017;21(3):519–29. doi: 10.1111/jcmm.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang X, Cao S, Ji Z, et al. miR-3646 promotes vascular inflammation and augments vascular smooth muscle cell proliferation and migration in progression of coronary artery disease by directly targeting RHOH. Int J Clin Exp Pathol. 2018;11(12):5830–39. [PMC free article] [PubMed] [Google Scholar]
- 13.Seong M, Kang H. Hypoxia-induced miR-1260b regulates vascular smooth muscle cell proliferation by targeting GDF11. BMB Rep. 2019;53(4):206–11. doi: 10.5483/BMBRep.2020.53.4.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng S, Gao L, Zhang D, et al. MiR-93 regulates vascular smooth muscle cell proliferation, and neointimal formation through targeting Mfn2. Int J Biol Sci. 2019;15(12):2615–26. doi: 10.7150/ijbs.36995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Zhang C, Li C, et al. L-92a promotes vascular smooth muscle cell proliferation and migration through the ROCK/MLCK signalling pathway. J Cell Mol Med. 2019;23(5):3696–710. doi: 10.1111/jcmm.14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu C, Peng K, Guo H, et al. miR-18a-5p promotes cell invasion and migration of osteosarcoma by directly targeting IRF2. Oncol Lett. 2018;16(3):3150–56. doi: 10.3892/ol.2018.9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Wei X, Wu B, et al. Tumor-educated platelet miR-34c-3p and miR-18a-5p as potential liquid biopsy biomarkers for nasopharyngeal carcinoma diagnosis. Cancer Manag Res. 2019;11:3351–60. doi: 10.2147/CMAR.S195654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhang Y, Yin Y, Li S. Detection of circulating exosomal miR-17-5p serves as a novel non-invasive diagnostic marker for non-small cell lung cancer patients. Pathol Res Pract. 2019;215(8) doi: 10.1016/j.prp.2019.152466. 152466. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Deng X, Shi X, Dong X. Silencing H19 regulated proliferation, invasion, and autophagy in the placenta by targeting miR-18a-5p. J Cell Biochem. 2019;120(6):9006–15. doi: 10.1002/jcb.28172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun P, Tang LN, Li GZ, et al. Effects of MiR-21 on the proliferation and migration of vascular smooth muscle cells in rats with atherosclerosis via the Akt/ERK signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(5):2216–22. doi: 10.26355/eurrev_201903_17269. [DOI] [PubMed] [Google Scholar]
- 21.Xie X, Urabe G, Marcho L, et al. ALDH1A3 regulations of matricellular proteins promote vascular smooth muscle cell proliferation. iScience. 2019;19:872–82. doi: 10.1016/j.isci.2019.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng S, Zhang Y, Wang Y, et al. MicroRNA-92 regulates vascular smooth muscle cell function by targeting KLF4 during vascular restenosis and injury. Int J Clin Exp Pathol. 2019;12(12):4253–62. [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Zhang Z, Yang S. Circ_RUSC2 upregulates the expression of miR-661 target gene SYK and regulates the function of vascular smooth muscle cells. Biochem Cell Biol. 2019;97(6):709–14. doi: 10.1139/bcb-2019-0031. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Yang F, Zhao H, et al. Circular RNA circCHFR facilitates the proliferation and migration of vascular smooth muscle via miR-370/FOXO1/Cyclin D1 pathway. Mol Ther Nucleic Acids. 2019;16:434–41. doi: 10.1016/j.omtn.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Yu W, Zhu S, et al. Long noncoding RNA GAS5 regulates the proliferation, migration, and invasion of glioma cells by negatively regulating miR-18a-5p. J Cell Physiol. 2018;234(1):757–68. doi: 10.1002/jcp.26889. [DOI] [PubMed] [Google Scholar]
- 26.Stratton MS, Yang X, Sreejayan N, Ren J. Impact of insulin-like growth factor-I on migration, proliferation and Akt-ERK signaling in early and late-passages of vascular smooth muscle cells. Cardiovasc Toxicol. 2007;7(4):273–81. doi: 10.1007/s12012-007-9006-7. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimura H, Nariai Y, Terashima M, et al. Taurine suppresses platelet-derived growth factor (PDGF) BB-induced PDGF-beta receptor phosphorylation by protein tyrosine phosphatase-mediated dephosphorylation in vascular smooth muscle cells. Biochim Biophys Acta. 2005;1745(3):350–60. doi: 10.1016/j.bbamcr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Yu S, Chen Y, Chen S, et al. Klotho inhibits proliferation and migration of angiotensin II-induced vascular smooth muscle cells (VSMCs) by modulating NF-kappaB p65, Akt, and extracellular signal regulated kinase (ERK) signaling activities. Med Sci Monit. 2018;24:4851–60. doi: 10.12659/MSM.908038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casella S, Bielli A, Mauriello A, Orlandi A. Molecular pathways regulating macrovascular pathology and vascular smooth muscle cells phenotype in type 2 diabetes. Int J Mol Sci. 2015;16(10):24353–68. doi: 10.3390/ijms161024353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S, Dong S, Li Z, et al. Atorvastatin calcium inhibits PDGF-betabeta-induced proliferation and migration of VSMCs through the G0/G1 cell cycle arrest and suppression of activated PDGFRbeta-PI3K-Akt signaling cascade. Cell Physiol Biochem. 2017;44(1):215–28. doi: 10.1159/000484648. [DOI] [PubMed] [Google Scholar]
- 31.Humphreys KJ, McKinnon RA, Michael MZ. miR-18a inhibits CDC42 and plays a tumour suppressor role in colorectal cancer cells. PLoS One. 2014;9(11):e112288. doi: 10.1371/journal.pone.0112288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei S, Zhang J, Han B, et al. Novel zinc finger transcription factor ZFP580 facilitates all-trans retinoic acid -induced vascular smooth muscle cells differentiation by raralpha-mediated PI3K/Akt and ERK signaling. Cell Physiol Biochem. 2018;50(6):2390–405. doi: 10.1159/000495098. [DOI] [PubMed] [Google Scholar]
- 33.Li JY, Liu CP, Shiao WC, et al. Inhibitory effect of PDGF-BB and serum-stimulated responses in vascular smooth muscle cell proliferation by hinokitiol via up-regulation of p21 and p53. Arch Med Sci. 2018;14(3):579–87. doi: 10.5114/aoms.2018.75085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang F, Ren X, Zhao M, et al. Angiotensin-(1–7) abrogates angiotensin II-induced proliferation, migration and inflammation in VSMCs through inactivation of ROS-mediated PI3K/Akt and MAPK/ERK signaling pathways. Sci Rep. 2016;6:34621. doi: 10.1038/srep34621. [DOI] [PMC free article] [PubMed] [Google Scholar]