Abstract
Alveolar epithelial type II cells (AEC2s) are the facultative progenitors of lung alveoli and serve as the surfactant-producing cells of air-breathing organisms. Although primary human AEC2s are difficult to maintain stably in cell cultures, recent advances have facilitated the derivation of AEC2-like cells from human pluripotent stem cells (hPSCs) in vitro. Here, we provide a detailed protocol for the directed differentiation of hPSCs into self-renewing AEC2-like cells that can be maintained for up to 1 year in culture as epithelial-only spheres without the need for supporting mesenchymal feeder cells. The month-long protocol requires recapitulation of the sequence of milestones associated with in vivo development of the distal lung, beginning with differentiation of cells into anterior foregut endoderm, which is followed by their lineage specification into NKX2–1+ lung progenitors and then distal/alveolar differentiation to produce progeny that express transcripts and possess functional properties associated with AEC2s.
Introduction
AEC2s are found in all air-breathing organisms and serve as the facultative progenitors of lung alveoli while also performing additional critical functions required for survival. They generate surfactant, reducing surface tension and preventing air-space collapse; proliferate; differentiate into type I alveolar epithelial cells (AEC1s) in response to lung injury; and play a key role in pulmonary innate immune defense. Despite their well-documented proliferative capacity in injury models in vivo, primary AEC2s placed in cell culture have not been shown to self-renew for more than a few days without mesenchymal support and, even in the presence of feeder cells, have been expanded for only a few passages in vitro to date, thus limiting our understanding of AEC2 biology1. Because AEC2 dysfunction has been implicated as an inciting event for many incurable diseases affecting the lung parenchyma, such as pulmonary fibrosis2, use of a model to study AEC2 biology in patient-derived cells should facilitate study of the pathogenesis of these diseases. Because hPSCs proliferate indefinitely in culture, PSC-derived or induced AEC2s (iAEC2s) represent an attractive new model for studying alveolar biology. Here, we provide a detailed protocol we have recently developed3 to accomplish the derivation from PSCs of self-renewing iAEC2s that can be maintained as epithelial-only spheres (alveolospheres) in 3D culture. All conditions are feeder free and use defined, serum-free media. Using this approach, the resulting alveolospheres can be serially passaged for up to 1 year, yielding epithelial cells that retain proliferative potential and continue to display a differentiated, ultrastructural, and molecular phenotype comparable to that of primary AEC2s, thus providing a valuable source of patient-derived AEC2-like cells that are otherwise difficult to access or expand in culture.
Development of the protocol
To derive iAEC2s from PSCs, we used the ‘directed differentiation’ approach, which involves the in vitro recapitulation of known developmental milestones associated with in vivo lung development. Because the lung epithelium developmentally derives from the ventral anterior foregut endoderm, we adapted3,4 the protocol of Snoeck and colleagues5,6 to use directed differentiation to differentiate hPSCs into ventral anterior foregut endodermal cells with lung epithelial competence.
We found it useful to engineer reporter cell lines to track and purify lung epithelial progenitors as they first emerge from PSC-derived foregut endodermal cells in culture (Fig. 1a). We targeted fluorochrome reporter cDNAs to the endogenous NKX2–1 locus in mouse7 or human PSCs8 because NKX2–1 is the earliest known marker to be expressed at the time of lung epithelial lineage specification. Using these reporters, we optimized two protocols for the distinct differentiation of foregut precursors into either lung or thyroid epithelia4,9, the two known Nkx2–1+ lineages that derive from the definitive endoderm germ layer. To remove the need for targeted GFP reporters to purify PSC-derived NKX2–1+ human lung progenitors, we and others identified cell-surface proteins that can be used to accomplish the same goal via antibody-based sorting using the cell-surface phenotype CD47hi/CD26− (ref. 8) or CPM+ (ref. 10). To further differentiate the first emerging NKX2–1+ lung progenitors derived from PSCs, we used approaches for proximal (airway) versus distal (alveolar) patterning of these NKX2–1+ progenitors3,11,12. Further maturation and expansion of distal alveolar-patterned derivatives as functional, self-renewing iAEC2s was concurrently reported by us3 and by Gotoh and colleagues13. Here, we also demonstrate that the carboxypeptidase M (CPM) marker or an additional marker, CKIT, can alternatively be used for the purification of iAEC2s at the later stages of differentiation.
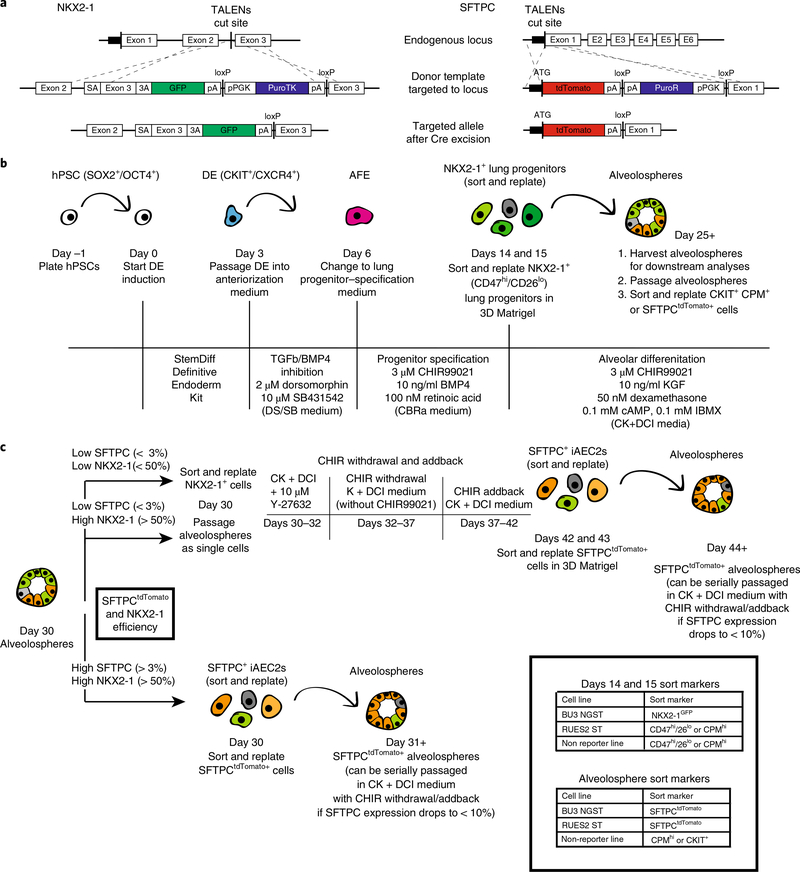
Fig. 1 |. Overview of PSC-derived alveolosphere differentiation protocol.
a, TALENs targeting strategy and edited NKX2–1GFP and SFTPCtdTomato loci after Cre-mediated antibiotic cassette excision. a adapted with permission from ref. 3, Elsevier. b, Schematic of differentiation protocol with arrows representing steps involving passaging or sorting. Markers for intermediate cell types are noted where relevant. Media components and concentrations are listed for each respective part of the protocol. c, Schematic of options for post day 30 culture of alveolospheres with recommended sort markers for days 14, 15 and 30+ cells. 3A, 3′ acceptor site; AFE, anterior foregut endoderm; ATG, ATG translation start site; DE, definitive endoderm; E, exon; hPSC, human pluripotent stem cell; pA, poly A; pPGK, phosphoglycerate kinase I promoter; puroTK, puromycin and thymidine kinase cassette; PuroR, puromycin resistance gene; SA, splice acceptor; TALEN, transcription activator-like effector nuclease.
To initiate the derivation of iAEC2s, we first induced definitive endoderm from PSCs, then patterned the endoderm into anterior foregut, specified primordial NKX2–1+ lung progenitors, and finally differentiated them into distal lung SFTPC+ alveolar epithelial cells (Fig. 1b,c). To enable real-time monitoring of this sequence of alveolar differentiation, we used our previously described dual-reporter system3 composed of fluorochrome-encoding cassettes targeted to both the NKX2–1 and SFTPC loci of an induced PSC (iPSC) line (BU3 NGST; NG=NKX2–1GFP and ST=SFTPCtdTomato, Fig. 1a). This system facilitated identification of both early lung progenitors (NKX2–1GFP+) and their anticipated descendants, putative iAEC2s (NKX2–1GFP+/SFTPCtdTomato+). We recommend the use of this bifluorescent system for those first learning this protocol because it provides visual, quantifiable feedback in real time for the investigator. The initial 72 h of endoderm induction8 relied on stimulation of activin/Nodal signaling with activin A and additional less critical published supplements5,8. However, we recommend replacement with a convenient, commercially available ‘definitive endoderm kit’ (Figs. 1b and 2a,b).
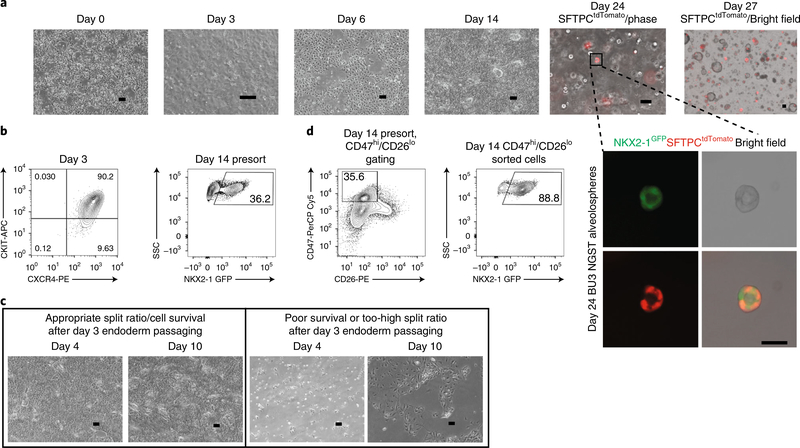
Fig. 2 |. Representative images and flow cytometric profiles throughout PSC-derived alveolosphere differentiation protocol.
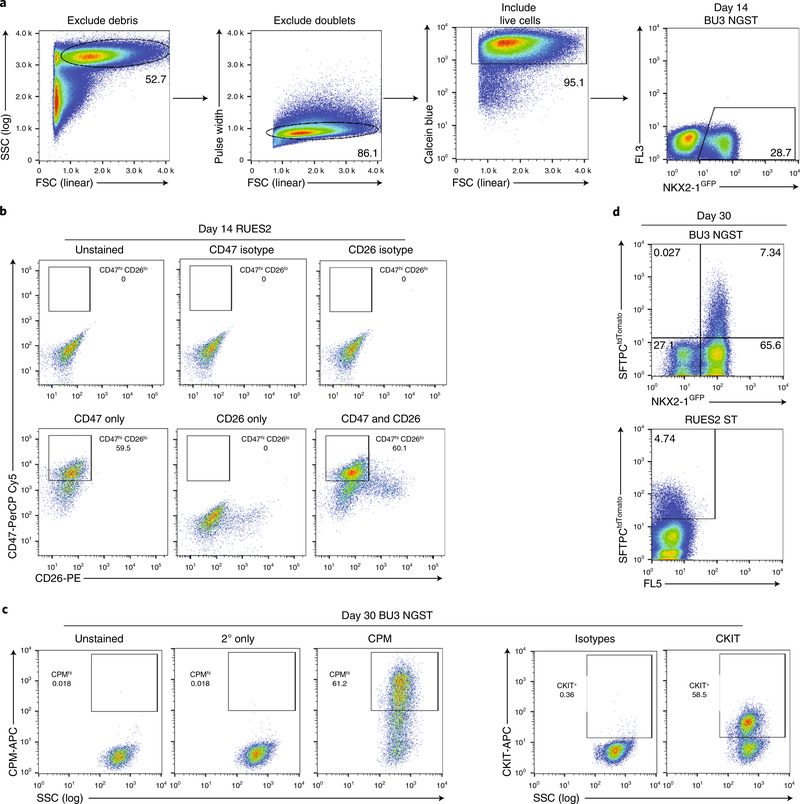
a, Representative phase-contrast (phase) images of cells at various days of differentiation, including day 0 (24 h after plating PSCs), day 3 (definitive endoderm), day 6 (anterior foregut endoderm), day 14 (NKX2–1+ lung progenitors), and days 24 and 27 (alveolospheres). Scale bars, 200 μm. Inset, higher-magnification image of alveolosphere from day 24 shows expression of NKX2–1GFP (green) and SFTPCtdTomato (red). Scale bar, 50 μm. b, Representative day 3 endoderm and day 14 presort flow cytometry. c, Left panels show morphology of cells from days 4 to 10 with good cell survival and appropriate passaging density on day 3 of differentiation, whereas right panels show morphology from days 4 to 10 with either poor cell survival or a too-sparse plating density on day 3. Scale bars, 200 μm. d, Representative flow cytometry gates for either CD47hi/CD26lo (RUES2 ST) or NKX2–1GFP + (BU3 NGST) gating strategies on day 14. APC, allophycocyanin; PE, phycoerythrin.
Once definitive endoderm had been patterned into anterior foregut endoderm via inhibition of bone morphogenetic protein (BMP) and transforming growth factor-β (TGFB) signaling5, we activated Wnt [14] using CHIR99021 (CHIR) along with BMP4 and retinoic acid supplementation. These were the minimal factors required for lung lineage specification of NKX2–1+ lung progenitors (CBRa medium; Figs. 1b and 2a,c)4. These NKX2–1+ progenitor cells were identified either by expression of an NKX2–1GFP reporter or by high expression of the cell-surface marker CD47 and low expression of the cell-surface marker CD26 (ref. 8) (Fig. 2d). After sorting and replating pure populations of either NKX2–1GFP+ or CD47hi/CD26lo cells in 3D Matrigel, monolayered epithelial spheres emerged over the next 10 d. By culturing these progenitor cells in CHIR, keratinocyte growth factor (KGF), dexamethasone, 3′,5′-cyclic adenosine monophosphate (cAMP), and 3-isobutyl-1-methylxanthine (IBMX) (CK+DCI medium), we generated NKX2–1GFP+/SFTPCtdTomato+ iAEC2s within 25–30 d of total differentiation time (Figs. 1b and 2a). SFTPCtdTomato+ cells sorted into 3D Matrigel in CK+DCI medium could proliferate for many passages and could be maintained in culture for several months3.
Applications of the protocol
Alveolospheres can be used to model alveolar development, function, and disease. By attempting to recapitulate developmental biology studies in vitro, we found that the entirety of the iAEC2 population came from early NKX2–1+ progenitor cells15, that WNT, fibroblast growth factor (FGF), cAMP, and corticosteroid signaling could promote alveolar differentiation in the absence of mesenchymal feeders16–18, and that oscillation of Wnt signaling resulted in improved AEC2 maturation3,19. Further studies of AEC2 development, injury response, and differentiation into other cell types could be performed using this model.
Alveolospheres are able to synthesize, process, and secrete surfactant, enabling future studies of the surfactant synthesis and recycling pathways in vitro. They are also able to upregulate innate immune signaling mediators in response to immune cytokines, suggesting they could be used to model AEC2 response to infection or toxin-mediated disease3.
We used alveolospheres to model a monogenic alveolar disease, surfactant protein B (SFTPB) deficiency, by studying iPSC-derived alveolospheres from the same patient both pre- and post-CRISPR–Cas9-mediated correction of the SFTPB mutation. We found that all AEC2 defects we observed in pre-correction alveolospheres were absent in post-correction alveolospheres3. Given the well-established protocols for gene editing in iPSCs, this model should enable the study of other monogenic lung diseases involving mutations in AEC2 genes. Ultimately, alveolospheres could be used to screen therapeutics for these diseases, to predict the role of disease-modifying polymorphisms, or even as a source of AEC2s for cell-based therapies to regenerate lung tissue in vivo.
Comparison with other methods
Several differentiation protocols have reported generation of alveolar cells from PSCs using defined serum-free media. Since a protocol was established to generate NKX2–1+ lung progenitor cells from PSCs5, several groups have made attempts to derive AEC2s from this population. These include Huang et al.6,20, who showed differentiation of PSCs into distal lung cells in 2D culture systems; Chen et al.21, who showed differentiation of clumps of NKX2–1+ cells into lung bud organoids that express several mesenchymal and lung epithelial cell markers; and Dye et al., who reported generation of human lung organoids in which multiple lung epithelial lineage markers were also expressed22,23. These protocols do not involve sorting of pure populations of NKX2–1+ lung progenitors, which may result in their differentiation into more heterogeneous or multilineage populations. Such populations might be desirable, depending on the goals of a project. These alternative approaches did not report long-term proliferation or function of the AEC2-like cells being produced.
Gotoh and colleagues used a technique similar to ours, in which they derived AEC2s, identifiable by a fluorescent reporter in the endogenous SFTPC locus, from a relatively pure population of NKX2–1+ progenitors using the CPM sort marker. A detailed comparison of CPM versus CD47hi/CD26lo sorting strategies to purify NKX2–1+ progenitors was recently published by this group24, validating the use of either strategy to purify iPSC-derived lung progenitors. We found that CPM was also effective in specifically identifying NKX2–1+ cells in either day 15 NKX2–1+ cells in our protocol8 or in later alveolosphere outgrowths derived from sorted progenitors (Fig. 3). Yamamoto et al.13 showed that after culturing CPM+ cells with lung mesenchyme in 3D culture with K+DCI medium, alveolar organoids emerged that expressed both AEC2 and AEC1 markers. CPM+ cells could be differentiated into alveolar organoids in the absence of mesenchymal feeders, with either CK+DCI medium + ROCK inhibitor (RI) or CK+DCI medium + RI + SB431542 (SB), a TGFb inhibitor. We found no difference in SFTPCtdTomato expression after culturing day 14 NKX2–1+ cells in CK+DCI medium with or without SB3. Of note, Yamamoto et al.13 use an additional distalizing step before sorting CPMhi cells (7 d in CHIR, FGF10, KGF, and the γ-secretase, an indirect Notch inhibitor, DAPT), which could explain the difference between their finding and ours. This study also shows long-term proliferation of alveolar organoids, which maintain alveolar gene expression over several passages, but in contrast to our protocol, mesenchymal feeders are used to promote this proliferation. In our opinion, either protocol (Yamamoto et al. or ours) can be used to achieve the goal of generating PSC-derived pure iAEC2s with high proliferative and functional capacities.
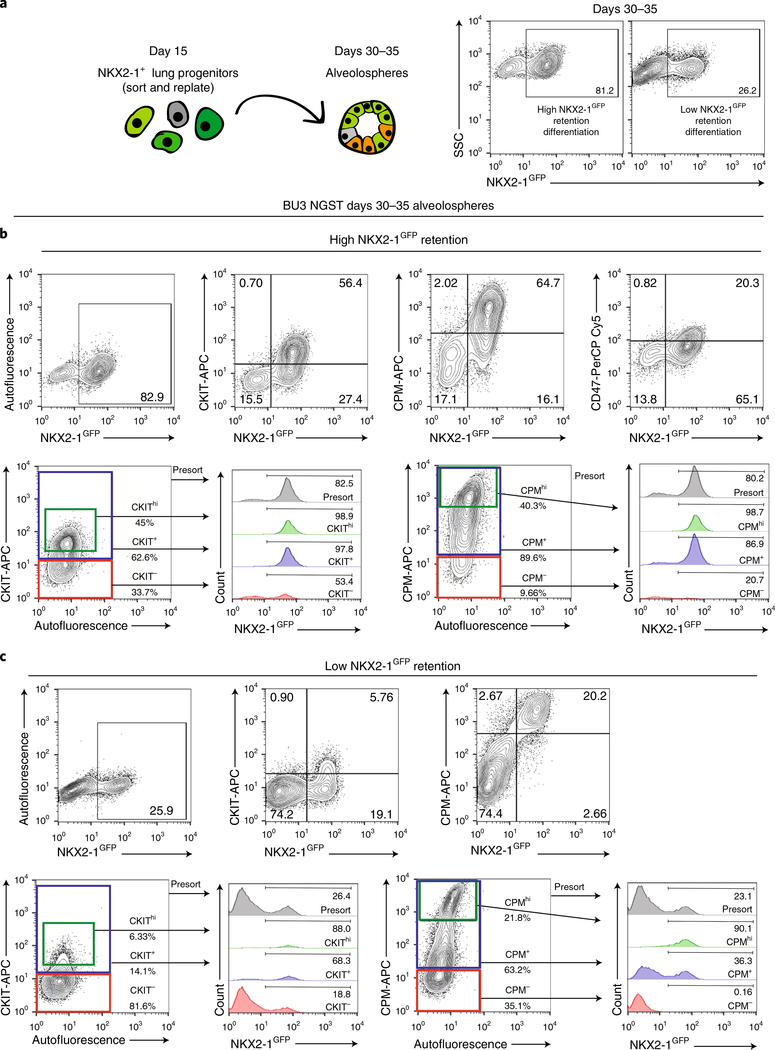
Fig. 3 |. Identification of new cell-surface markers for NKX2–1+ cells.
a, Schematic of sorted day 14 NKX2–1GFP+ lung progenitors analyzed as day 30–35 alveolospheres with flow cytometry representative of two separate differentiations with high versus low NKX2–1GFP retention. b, Top, flow cytometry in a high NKX2–1GFP retention run with coexpression of CKIT and CPM (but not CD47), together with NKX2–1GFP in days 30–35 BU3 NGST alveolospheres. Bottom, suggested sort gates for CPM and CKIT and histograms, showing expression of NKX2–1GFP in cells not gated on the basis of cell-surface markers (Presort), gated for high CPM or CKIT expression (CPMhi or CKIThi), gated for all CPM+ or CKIT+ cells and gated for all CPM− or CKIT− cells. c, Top, flow cytometry in a low NKX2–1GFP retention run with co-expression of CKIT or CPM together with NKX2–1GFP in days 30–35 alveolospheres. Bottom, suggested sort gates for CPM and CKIT and histograms showing expression of NKX2–1GFP in cells not gated on the basis of cell-surface markers (Presort), gated for high CPM or CKIT expression, gated for all CPM+ or CKIT+ cells, or gated for all CPM− or CKIT− cells.
In contrast to other differentiation protocols using either PSC-derived cells or primary AEC2s, our alveolospheres do not contain AEC1s or mesenchymal cells13,25. However, AEC1 expression in primary alveolospheres may be dependent on species or growth media, as there is also evidence from Barkauskas et al.26 that AEC1 markers are absent in human primary alveolospheres, in contrast to their presence in mouse alveolospheres. Furthermore, although primary AEC2s do not proliferate in the absence of mesenchymal feeders, we did not observe any difference in iAEC2 proliferation when cultured with or without mesenchymal feeders (Fig. 4). This makes the study of AEC2-specific biology simpler, but methods for differentiating our cells into AEC1s or co-culture methods may need to be developed to use alveolospheres to study AEC2 interactions with other lung cells.
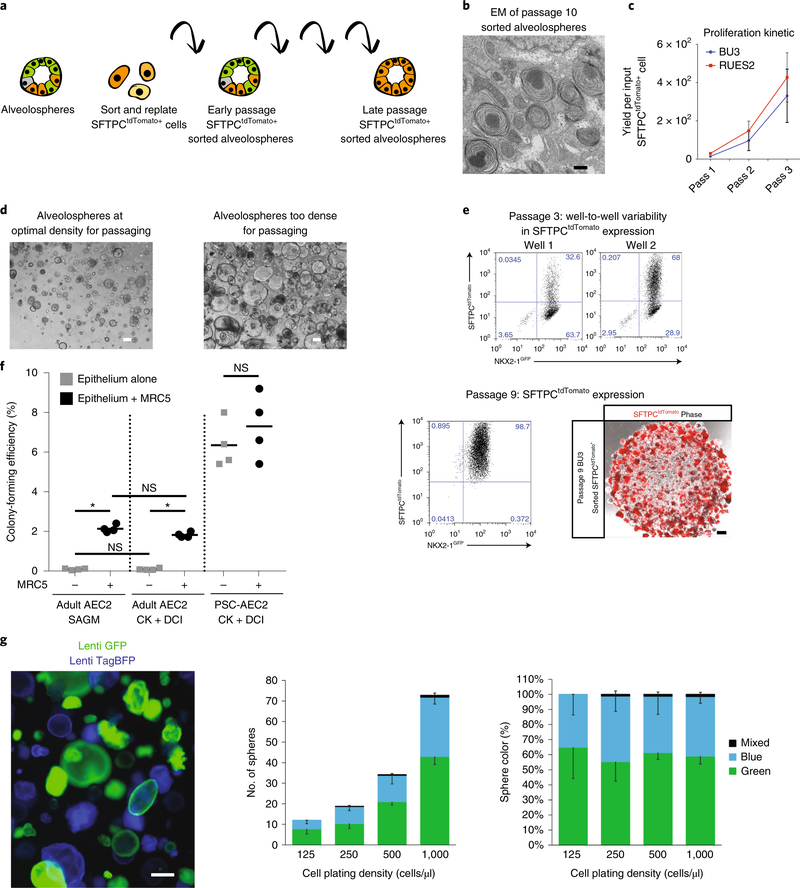
Fig. 4 |. Maintaining alveolospheres in long-term culture.
a, Schematic showing method to sort SFTPCtdTomato cells and passage them sequentially, after which the majority of cells within alveolospheres are SFTPCtdTomato+. b, Electron micrograph of passage 10 alveolospheres shows expression of lamellar body–like inclusions. c, Proliferation kinetic of cell yield per sorted and replated SFTPCtdTomato+ cell in BU3 NGST and RUES2 ST lines over three passages. d, Representative phase-contrast images of alveolospheres at an optimal density for passaging (left) versus too dense for reliable passaging (right). Scale bars, 200 μm. e, Representative flow cytometry of BU3 NGST alveolospheres at passages 3 and 9 after SFTPCtdTomato+ sorting. The percentage of SFTPCtdTomato+ is more variable in early passages (top), although the majority of cells maintain expression of NKX2–1GFP. Representative image of passage 9 alveolospheres expressing SFTPCtdTomato. f, Mean colony-forming efficiency ± s.d., n = 4 biological replicates for RUES2 SFTPCtdTomato+-sorted alveolospheres (~95% SFTPCtdTomato+) and primary adult human AEC2 cells (Adult AEC2) in either SAGM or CK+DCI medium ± MRC5 fibroblast feeder cells. *P ≤ 0.05, ANOVA. g, Assessment of clonality of alveolosphere outgrowth by fluorescent tagging of iAEC2s in separate wells (~day 100) using lentiviral vectors constitutively expressing either TagBFP (blue) or GFP (green). Subsequent mixing of flow cytometry–sorted GFP+ and TagBFP+ iAEC2s at a 1:1 ratio at various densities in 3D Matrigel produces sphere outgrowths that are predominantly monocolored. Photomicrograph represents merged BFP (blue) and GFP (green) channels. Bars represent average sphere numbers and color percentages across five random fields, n = 689 spheres scored, and downward facing error bars represent s.d. for each color; upward error bars represent mixed color scores. Results are representative of two repeated independent experiments on spheres that were >90% SFTPCtdTomato+ at the time of mixing. NS, nonsignificant; Pass, passage; Phase, phase-contrast. Scale bars, 500 nm (b); 100 μm (d); 200 μm (e); 300 μm (g). c, f adapted with permission from ref. 3, Elsevier.
Finally, to our knowledge, ours is the only published evaluation of the key AEC2 functions in surfactant synthesis, processing of surfactant protein intermediates, and secretion of surfactant proteins and phospholipids in PSC-derived iAEC2s. As demonstrated in our model of SFTPB deficiency using gene-edited alveolospheres, our protocol enables study of lamellar body function and surfactant protein processing in patient-derived cells, making it a valuable tool for studying alveolar disease.
Experimental design
PSC maintenance
Before differentiation, PSCs should be maintained in feeder-free conditions for three to four passages. PSC colonies should be up to 70–80% confluent on the day when a differentiation is started. Different PSC lines will have different kinetics in reaching confluence, and passaging PSCs at different densities (1:5, 1:10 and 1:20) before scheduling the start of a differentiation can help ensure that the cells are ready at the appropriate density. We recommend using a bifluorescent reporter system comprising iPSC-containing fluorochrome-encoding cassettes targeted to both the NKX2–1 and SFTPC loci when using the protocol first time, because this enables real-time monitoring of the sequence of alveolar differentiation.
Differentiation of definitive endoderm
In our most recent publications8, we used a modified and simplified endodermal differentiation in which PSCs are differentiated into definitive endoderm using the StemDiff Definitive Endoderm Kit. After plating PSCs dissociated into single cells in mTeSR1 + Y27632 for 24 h, the medium is changed to MR+CJ for 24 h, then to CJ only for an additional 48 h. After 72 h, ~90–100% of the cells should be CKIT+/CXCR4+. Although CKIT/CXCR4 co-expression is not specific to definitive endoderm in vivo, and therefore does not ensure definitive endoderm efficiency with complete specificity, as discussed in detail by others27–29, it is helpful in the in vitro PSC model system for monitoring activin A responses because it is highly correlated with the upregulation of definitive endoderm markers by RT–qPCR as well as by global transcriptomics. FOXA2 and SOX17 expression could alternatively be used to monitor definitive endoderm induction efficiency30. The relationship between CKIT/CXCR4 expression and later lung competence is detailed further in Hawkins et al.8. If the CKIT+/CXCR4+ efficiency is <80%, we recommend restarting the protocol or optimizing endodermal differentiation for the particular PSC line being used, as detailed in our prior protocol publication11 (Fig. 2a,b).
Differentiation of NKX2–1+ lung progenitor cells
After 72 h of endoderm induction (days 0–3; note that this differs from the StemDiff Definitive Endoderm Kit time point nomenclature, which refers to this time period as days 1–4), the cells should be confluent and each well of endoderm can be passaged at a ratio of 1:2–1:6, depending on the PSC line. When starting to work with a new line, we recommend passaging at different densities at this stage (day 3) and scoring subsequent NKX2–1 efficiency on day 14 to determine which split ratio to use (Fig. 2c,d). Day 3 endoderm is passaged into ‘DS/SB’ medium + Y27632 for 24 h and DS/SB medium for an additional 48 h (until day 6). Occasionally, there is substantial cell death at this stage, but the cells should recover with time in CBRa medium after day 6. Of note, we do not routinely monitor SOX2 kinetics as a readout of anterior foregut endoderm differentiation efficiency here, because interpretation of its kinetics is challenging because SOX2 is expressed at higher levels in both undifferentiated cells and early NKX2–1+ lung progenitor cells as compared to these day 6 cells (full transcriptomic kinetics published by Hawkins et al.8, Green et al.5, and Loh et al.31) After 72 h of anteriorization (day 6), the medium is changed to CBRa. Cells can remain in CBRa medium for 8–9 d (total differentiation time: days 14 and 15). NKX2–1 expression is upregulated by day 8, and on days 14 and 15, 20–90% of the cells should be NKX2–1+, as scored by expression of an endogenous reporter (e.g., NKX2–1GFP) or by intracellular flow cytometry (Fig. 2d). It is critical that the cells not be too confluent early during CBRa outgrowth. Thus, an optional alternative frequently used by our lab is to split the cells during this period (e.g., on day 9 at an ~1:3 split ratio), which may increase day 14 NKX2–1 efficiency, as detailed previously11.
Differentiation of SFTPC+ alveolospheres
On days 14 and 15 of differentiation, cells should be dissociated into a single-cell suspension and prepared for cell sorting. Although proceeding without any cell sorting will also effectively yield subsequent iAEC2s, albeit at diminished efficiencies3, our preference is to sort cells at this stage in order to understand lineage relationships and minimize heterogeneity of lineages in subsequent steps. Regardless, cells should be monitored at this stage to score the efficiency of NKX2–1 induction, and the protocol should be abandoned and restarted if efficiency is lower than expected for that line. NKX2–1+ cells can be identified either with an endogenous reporter (NKX2–1GFP) or with a combination of cell-surface markers (CD47hi/CD26lo, Fig. 2d). If the NKX2–1+ cell-differentiation efficiency is low, using the CD47hi/CD26lo sort markers may not result in a 100% pure population of NKX2–1+ cells8. Post-sort purity can be verified with intracellular NKX2–1 flow cytometry. We have previously published detailed protocols for both CD47/CD26 staining and intracellular NKX2–1 protein flow cytometry for scoring lung progenitor specification11. Day 14 and 15 sorted cells should ideally be replated at a density of ~65–125 cells/μl in 3D Matrigel, because data from cells grown in a similar medium (CK+DCI+Fgf10) suggest that lower plating densities improve differentiation of sorted NKX2–1GFP+ progenitors into SFTPCtdTomato+ cells (Fig. 5; ref. 8). After 2 d of culture in CK +DCI medium, SFTPCtdTomato+ cells emerge, but spheres do not form until ~5–10 d of further culture (Fig. 2a). Between days 25 and 35 of culture, alveolospheres contain iAEC2s and can be isolated for various analyses, including electron microscopy, flow cytometry, western blot, RNA extraction, and analysis of surfactant secretion3.
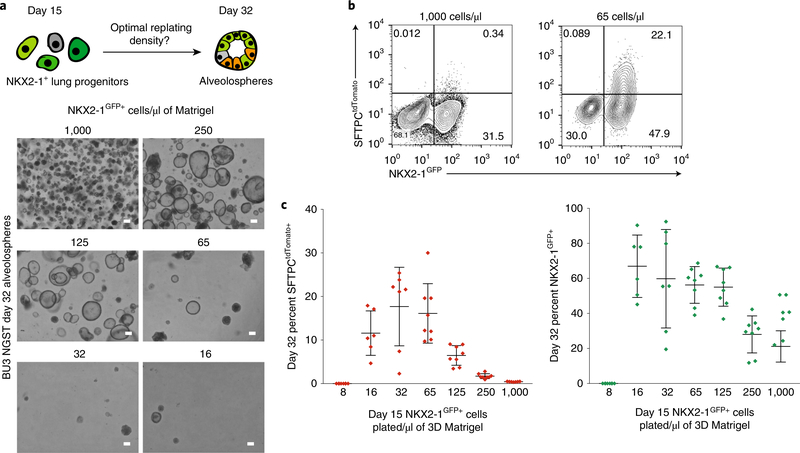
Fig. 5 |. Plating NKX2–1+ cells at lower densities in Matrigel results in improved specification of SFTPCtdTomato+ cells.
a, Schematic of experimental plan and bright-field images of spheres analyzed on day 32 after day 14 NKX2–1+ lung progenitor plating at densities ranging from 8 to 1,000 cells/μl of Matrigel. Scale bars, 200 μm. b, Representative flow cytometry of day 32 alveolospheres originally plated at 1,000 versus 65 cells/μl of Matrigel. c, Graphs show day 32 percentage of SFTPCtdTomato+ and NKX2–1GFP+ cells for each plating density, with error bars showing mean ± s.d.
If NKX2–1 retention or SFTPC expression is low, alveolospheres can be re-sorted using the NKX2–1GFP reporter or the cell-surface markers CPM or CKIT. These cells can be replated in 3D culture with CK+DCI medium. As we have previously described3, CHIR withdrawal for a period of 4–6 d can be used to mature iAEC2s, quantifiable as augmented SFTPCtdTomato fluorescence levels and intensity. Because growth kinetics slow upon CHIR withdrawal, CHIR addback after a period of 6 d stimulates proliferation without loss of SFTPCtdTomato expression, allowing further serial passaging of alveolospheres for prolonged periods3. Our preferred current version of this protocol involves distal differentiation into spheres until day 30, at which time spheres are passaged once without sorting, followed by CHIR withdrawal on days 32–36 and sorting by tdTomato after 2 weeks of sphere outgrowth (~day 44), followed by replating to generate spheres that can be serially passaged for culture expansion of iAEC2s. If using a PSC line that contains the SFTPCtdTomato reporter, sorting SFTPCtdTomato+ cells at any time (on day 30 before passaging or on any subsequent passage) and replating them in 3D culture with CK+DCI medium results in a pure population of alveolospheres that proliferates extensively over time, although SFTPC or tdTomato expression may subside or remain variable for several passages before returning, depending on the line (Fig. 4a–c)3. We found that if by day 25–30, the efficiency of differentiation into SFTPCtdTomato+ cells is too low for at least 10,000 cells to be replated after sorting (<3% SFTPCtdTomato+ population), the efficiency can be improved by passaging the alveolospheres as single cells and 2 d later withdrawing CHIR for a brief (~5 d) period to induce maturation, followed by adding back CHIR for a similar time (~5–7 d) to recover proliferation kinetics, resulting in a population of SFTPCtdTomato+ cells sufficient for sorting and replating (Fig. 1c)3. If the NKX2–1+ retention in the population drops to <50%, NKX2–1+ cells identified by the NKX2–1GFP reporter or CPM/CKIT immunostaining can be sorted and replated with a period of CHIR withdrawal and addback, which has improved the percentage of SFTPCtdTomato+ cells by up to 14-fold3. Even after sorting SFTPCtdTomato+ cells, if there is a drop in SFTPCtdTomato expression, this process of CHIR withdrawal and addback can be repeated.
Passaging of alveolospheres
Once sorted SFTPCtdTomato+ cells have been plated in 3D culture in CK+DCI medium, they can be maintained for up to 1 year in culture (Fig. 4b,c). They should be plated at a density of 200–400 cells/μl, and should be passaged when the Matrigel appears confluent with spheres (Fig. 4b), approximately every 14 d. Although our experiments show that sparser plating densities (65–125 cells/μl) may be more optimal for retention of tdTomato expression, because they require larger amounts of Matrigel and other reagents, we opt to use a slightly higher plating density to maximize cell-number yields (there are higher growth kinetics at higher plating densities) while minimizing cost. After enzymatic digestion, spheres are passaged as single cells and re-form spheres over a period of ~7–10 d. At early passages, SFTPCtdTomato+ expression can vary and is often <50%; however, at later passages the percentage of SFTPCtdTomato+ is nearly 100% (Fig. 4c). Using this approach, over a period of 225 d of serially passaging spheres, we have been able to generate a yield of 1020 SFTPCtdTomato+ iAEC2s per input sorted SFTPCtdTomato+ starting cell.
We have monitored genomic integrity using comparative genomic hybridization (CGH) Agilent DNA microarrays for three different iPSC lines and to date have found maintenance of integrity for prolonged periods with normal chromosome numbers in both starting iPSCs and their resulting alveolospheres after multiple passages (we assessed this on days 100 and 214 of differentiation; Supplementary Fig. 1). With two lines to date, including SPC2 and BU3 NGST, small copy-number variations (CNVs) (<2MB, mostly present in the starting iPSCs) of unclear importance have been documented at these time points. After more extensive continuous expansion in culture (12–15 serial passages approximately every 18 d for 266 d after the initial tdTomato+ sort), alveolospheres from one of these two lines, BU3 NGST, developed large genomic deletions and amplifications (>2 Mb), indicating loss of genomic integrity with time (Supplementary Fig. 1). Thus, we recommend that if iAEC2s are to be extensively passaged (for >100 d), genomic integrity should be monitored.
We have evaluated the clonality of alveolospheres during serial passaging, using integrating GFP-expressing and TagBFP-expressing lentiviral vectors to color SFTPCtdTomato+ alveolospheres in separate wells with either GFP or TagBFP fluorochromes under regulatory control of ubiquitous, constitutive promoter elements (pHAGE lentivirus EF1a-GFP-W versus pHAGE EF1a-TagBFP-W, with plasmid maps available at www.kottonlab.com). As shown in Fig. 4d, after 1:1 mixing of single-cell suspensions of GFP+ with TagBFP+ iAEC2s for outgrowth in 3D cultures, the resulting alveolospheres at a variety of plating densities are predominantly monocolored at both 8 and 14 d after outgrowth, suggesting the majority of SFTPCtdTomato+ spheres result from clonal proliferation of single iAEC2s at each serial passage.
Characterization of derived cells
Importantly, after alveolar differentiation, the NKX2–1GFP+ population expresses an AEC2 transcriptomic program (e.g., ABCA3, LPCAT1, LAMP3) in both tdTomato-bright and tdTomato-dim or negative cells3. This means tdTomato expression in our system serves mainly to distinguish NKX2–1+ alveolar cells that express higher levels of SFTPC mRNA from those expressing lower levels, but there is no statistically significant difference in the expression levels of most other AEC2 transcripts by bulk RNA sequencing3.
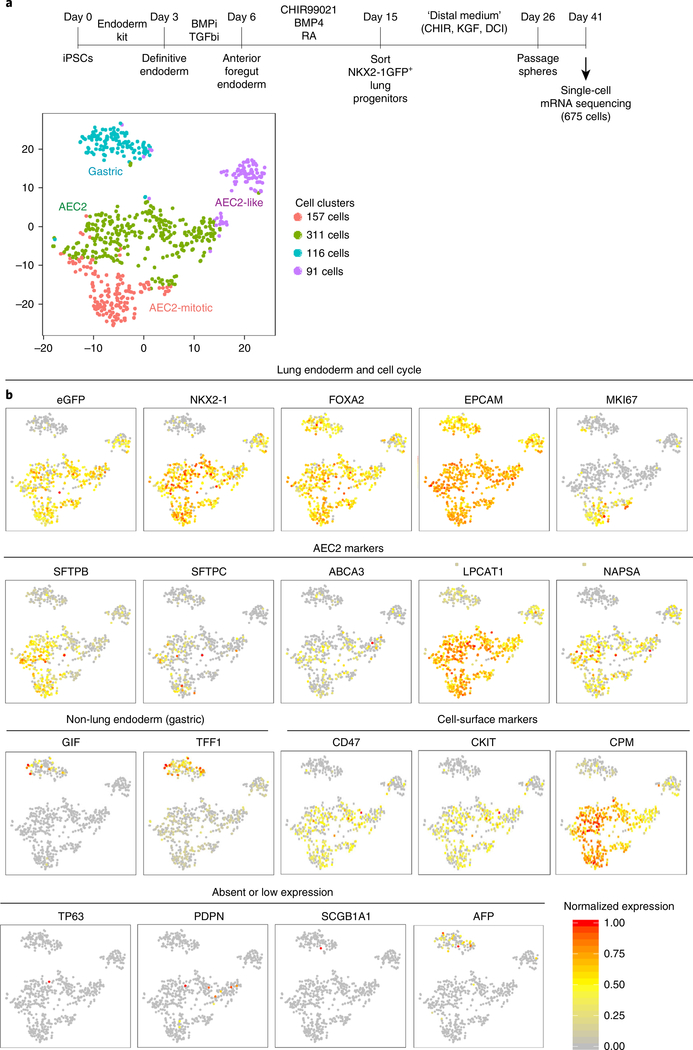
We have previously described single-cell RNA sequencing of alveolospheres versus proximal lung spheres14. In Fig. 6, we reanalyze only the 675 cells produced by the distal medium in our protocol (Fig. 6a), providing profiles of gene markers of cell identity and an assessment of the heterogeneity of cells produced on day 41 of our distal sphere protocol (Fig. 6b). After a single sphere passage (day 41 of total differentiation time, Fig. 6a), distal spheres produced in this protocol could be visualized by t-distributed stochastic neighbor embedding (tSNE) plot and grouped into four clusters, three of which corresponded to cells that express NKX2–1 (and could be identified by retention of NKX2–1GFP reporter expression), with the fourth cluster representing non-lung endoderm, probably predominantly composed of gastric-lineage cells (TFF1+, GIF+; Fig. 6b; detailed 10x Genomics cell capture, library preparation, bioinformatics analysis, and computational methods were previously published14, with raw datasets downloadable under GEO accession no. GSE103918). NKX2–1+ lung epithelial sub-clusters could be further distinguished by expression of SFTPC and other AEC2 markers without markers of proliferation (AEC2), expression of AEC2 markers and markers of proliferation (mitotic AEC2), or expression of lower SFTPC and other lung markers along with higher expression of vimentin, a marker of epithelial-to-mesenchymal transition (AEC2-like)14. This, along with our previous bulk RNA sequencing data3, suggests that the majority of NKX2–1+ cells within alveolospheres expressed AEC2 genes, regardless of levels of expression of the SFTPCtdTomato reporter. A detailed dataset and discussion of the kinetics and interpretation of the SFTPCtdTomato reporter in this protocol is available in Jacob et al.3. It is important for users to understand that the reporter identifies only the highest SFTPC locus expressing iAEC2s with high specificity and serves to effectively exclude any non-lung endodermal cells (NKX2–1−/tdTomato−) that might be present in heterogeneous cultures; however, the sensitivity of the reporter for identifying all iAEC2s is limited, as we have observed that the majority of NKX2–1+/tdTomato− cells produced in this protocol are also iAEC2s, albeit with lower (but positive) expression levels of SFTPC mRNA and higher expression levels of some proliferative markers, such as TOP2A.
Fig. 6 |. Single-cell sequencing analysis of alveolospheres.
a, Protocol schematic and tSNE plot of 675 cells captured on day 41 of distal differentiation by 10x Genomics platform for single-cell RNA sequencing. Cells were derived from BU3 iPSCs sorted on day 15 of differentiation on the basis of NKX2–1GFP expression and were further differentiated to iAEC2s in ‘distal medium (CK+DCI)’ in 3D Matrigel with a single sphere passage before harvest for computational analysis, as described in McCauley et al.14. Cells in ‘distal media (CK+DCI)’ from McCauley et al. have been reanalyzed to generate the tSNE plots and normalized gene expression overlays shown in a and b, whereas cell cluster identities have all been maintained from McCauley et al.14, where further extensive discussion is available. b, tSNE plots with overlaid normalized expression of indicated marker genes. Datasets are available for download under GEO accession no. GSE103918 or through the bioinformatics portal at www.kottonlab.com. BMPi, inhibition of BMP signaling; RA, retinoic acid; TGFbi, inhibition of TGF signaling.
Because the high specificity and low sensitivity of the tdTomato reporter mean that the SFTPCtdTomato+ population probably does not represent the entirety of the iAEC2 population, we have searched for other ways to identify all NKX2–1+ cells within alveolospheres. To purify NKX2–1+ lung progenitors, our group had previously used expression of CD47 and CD26 (ref. 8), and Gotoh et al.10 had previously used expression of CPM to purify lung epithelial progenitors. Our single-cell RNA sequencing suggested that both C-KIT and CPM were enriched in their progeny, the subsequently differentiated AEC2 and proliferative AEC2 clusters (Fig. 6b). By contrast, we found that CD47 had lost specificity by day 41 (Fig. 6b). We performed FACS analyses of day 30–35 alveolospheres using antibodies against these three surface proteins to quantify whether cell-surface phenotypes specifically identified PSC-derived cells expressing the NKX2–1GFP reporter. We found that both CKIT+ and CPM+ populations were highly enriched for NKX2–1+ cells (Fig. 3b) in alveolospheres that maintained NKX2–1 expression well, but only CPMhi gating specifically identified NKX2–1GFP+ cells regardless of whether retention of NKX2–1GFP expression was high or low (Fig. 3b,c). CKIT and CPM can thus be used to identify and sort NKX2–1+ cells to near purity from alveolospheres later in the differentiation protocol, although CPM should be used if the NKX2–1+ cell percentage is low (Fig. 1c). In our experience, CPM can be used as a reliable distal lung epithelial sort marker at most time points in the protocol, starting at day 15 and continuing for at least 100 subsequent days of alveolosphere passaging.
Controls
For flow cytometry and immunofluorescence analysis of NKX2–1 or SFTPC, we recommend using cells that are known not to express the gene of interest as negative controls to ensure that the correct gates for positive cell expression are determined (i.e., day 0 iPSCs or differentiated cells sorted on CD47 low or CPM low on day 15 and replated for sphere expansion). For RT–qPCR analyses, we recommend running all experiments with both a negative control of ‘day 0’ iPSCs and a positive control of either freshly preserved lung tissue, week 21+ primary fetal AEC2s, or adult HTII-280-sorted primary AEC2s. These are appropriate controls for most other analyses as well.
Limitations
As with most directed differentiation protocols, this is a multistage protocol that occurs over weeks, during which any number of issues could arise, leading to decreased differentiation efficiency or cell yield. Fortunately, the use of reporters or cell-surface markers can help to monitor differentiation efficiencies and enable a poor differentiation to be salvaged in many cases. We have described cell-surface markers that identify NKX2–1+ cells at both early and late time points in differentiation, but we have not identified a cell-surface marker specific for cells that express high levels of SFTPC. We hypothesize that most NKX2–1+ cells within alveolospheres are AEC2-like in gene expression and function, but further studies are needed to evaluate whether there is a functional difference between SFTPChi and SFTPClo cells.
Although our alveolospheres perform some of the key functions of AEC2s, including surfactant synthesis and secretion and innate immune responses, they do not contain AEC1s, the key differentiated progeny of AEC2s. We have not identified a mesenchyme-free system with AEC1-containing alveolospheres, although AEC1s have been observed in both iPSC-based and primary cell–based AEC2/mesenchymal co-culture systems13,25. For these reasons, our laboratory refers to our PSC-derivatives as iAEC2s, and the term ‘alveolospheres,’ although a convenient descriptor, is frequently debated as being less preferable, because these spheres currently consist exclusively of iAEC2s without other key lineages, such as AEC1s, that would be present in lung alveoli in vivo.
For studies that rely on measuring luminal secretions or environmental exposures within our 3D culture system, we acknowledge that spherical cultures may not meet these needs, because polarized epithelial cells are embedded in a thick matrix with apical cell surfaces and luminal contents of the alveolosphere forming on the inside. Limited access to the luminal portion of alveolospheres makes measuring secreted molecules and infecting the cells with viruses or other pathogens more complicated. Although we have experimented with growing alveolospheres in 2D air–liquid interface cultures3, further work needs to be done to fully characterize this system.
Finally, although day 30 alveolospheres are similar to primary AEC2s, in that the top 300 most differentially upregulated genes by RNA sequencing are also seen in primary AEC2s, these cells cluster far from primary AEC2s when their entire transcriptomes are compared3. Although these differences can be partially explained by differences in fresh primary versus cells exposed to culture media or differences in exposure to toxins and microbes, there are probably important differences in gene expression between PSC-derived alveolospheres and primary AEC2s that would benefit from further exploration and could be eliminated by further optimization of this protocol.
Materials
Biological materials
PSCs. We have performed this protocol on seven PSC lines to date, both with and without fluorescent reporters. We have previously published work with BU3, a normal iPSC line (RRID: CVCL_JW26); C17, an iPSC line from a cystic fibrosis patient (RRID: CVCL_WN83); SP212 and SP212Corr (RRID: CVCL_WN80, CVCL_WN81), iPSCs from a patient with an SFTPB mutation; and RUES2, an embryonic stem cell line (generous gift from A. Brivanlou, Rockefeller University, RRID: CVCL_B810). In generating the figures in this paper, we used the iPSC line BU3, from a healthy donor. BU3 was targeted with an NKX2–1GFP reporter, and BU3 and RUES2 were targeted with an SFTPCtdTomato reporter. We refer to these lines as BU3 NKX2–1GFP/SFTPCtdTomato (NGST; RRID: CVCL_WN82) and RUES2 ST (RRID: CVCL_WN79). Although the protocol should work with any PSC line, using these reporters facilitates monitoring of differentiation efficiency. !CAUTION The use of PSC lines is subject to restrictions on the basis of national law, institutional review board, and individual funding agency requirements !CAUTION PSC lines should be regularly karyotyped before use. Although karyotyping of alveolospheres is not available commercially, array-based CGH (aCGH) DNA microarrays (Agilent) serve as a surrogate for karyotyping that provides more detailed information about chromosomal aberrations, including CNVs, and that can be performed on alveolosphere cell pellets. Alveolospheres should be analyzed by aCGH before long-term use !CAUTION All cell lines should be tested to ensure the absence of mycoplasma contamination before use.
Reagents
Media components
mTeSR1 medium (StemCell Technologies, cat. no. 05850)
Primocin (Invitrogen, cat. no. NC9141851)
Iscove’s Modified Dulbecco’s Medium (IMDM; Thermo Fisher Scientific, cat. no. 12440053)
Ham’s F12 medium (Cellgro, cat. no. 10–080-CV)
Dulbecco’s modified Eagle’s medium and Dulbecco’s Modified Eagle’s Media: Nutrient Mixture F-12 (DMEM and DMEM/F12; Thermo Fisher Scientific, cat. nos. 11995–073 and 11330–057)
N2 supplement (Invitrogen, cat. no. 17502–048)
B27 supplement (Invitrogen, cat. no. 15260–037)
BSA 7.5% stock (7.5 g/100 ml); Thermo Fisher Scientific, cat. no. 15260037)
GlutaMAX (100×; Thermo Fisher Scientific, cat. no. 35050–061)
1-Thioglycerol (MTG; Sigma-Aldrich, cat. no. M6145)
Ascorbic acid (Sigma-Aldrich, cat. no. A4544)
SB431542 (Tocris, cat. no. 1614)
Dorsomorphin (Stemgent, cat. no. 04–0024)
CHIR99021 (Tocris, cat. no. 4423)
rhBMP4 (R&D Systems, cat. no. 314-BP-050)
Retinoic acid (RA; Sigma-Aldrich, cat. no. R2625)
rhKGF (R&D Systems, cat. no. 251-KG-010)
Dexamethasone (Sigma-Aldrich, cat. no. D4902)
8-Bromo-cAMP (8-Br-cAMP; Sigma-Aldrich, cat. no. B7880)
3-Isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich, cat. no. I5879)
Rho-associated kinase inhibitor (Y-27632; Tocris, cat. no. 1254)
StemDiff Definitive Endoderm Kit (StemCell Technologies, cat. no. 05210)
Small Airway Epithelial Cell Growth Medium BulletKit (SAGM; Lonza, cat. no. CC-3118)
Cell dissociation reagents
0.05% Trypsin–EDTA (Gibco, cat. no. 25–300-062)
Dispase II (Thermo Fisher Scientific, cat. no. 17105–041)
Gentle Cell Dissociation Reagent (StemCell Technologies, cat. no. 07174)
Extracellular matrix
Growth factor–reduced Matrigel (Corning, cat. no. 356231)
-
Human embryonic stem cell (hESC)-qualified Matrigel (5-ml vial; Corning, cat. no. 354277)
▲CRITICAL Use the dilution factor recommended by the manufacturer on the certificate of analysis, because the concentration varies from lot to lot.
Antibodies
Mouse monoclonal to human CD47, peridinin–chlorophyll protein–cyanin 5.5 (PerCP–Cy5.5) conjugated (clone CC2C6) (BioLegend, cat. no. 323110, RRID: AB_940463)
Mouse monoclonal to human CD26, phycoerythrin (PE) conjugated (clone BA5b) (BioLegend, cat. no. 302705, RRID: AB_314289)
Mouse IgG1 isotype, PE conjugated (BioLegend, cat. no. 400113, RRID: AB_326435)
Mouse IgG1 isotype, PerCP–Cy5–5 conjugated (BioLegend, cat. no. 400149, RRID: AB_2801481)
Mouse monoclonal antibody to human CKIT, allophycocyanin (APC) conjugated (Life Technologies, cat. no. CD11705, RRID: AB_1463361)
Mouse IgG1 isotype, APC conjugated (Life Technologies, cat. no. MA5–18093, RRID: AB_2539476)
Mouse monoclonal IgG2a against human CD184 (CXCR4), clone 12G5, PE (Thermo Fisher Scientific, cat. no. 17–9991-82, RRID: AB_10670878)
Mouse monoclonal to human CPM (Wako, cat. no. 014–27501; RRID: AB_2801482)
Rabbit monoclonal to human NKX2–1 (clone EP1584Y) (Abcam, cat. no. ab76013; RRID: AB_1310784)
AffiniPure donkey anti-mouse IgG (H+L), Alexa Fluor 647 conjugated (Jackson Immunoresearch, cat. no. 715–605-150; RRID: AB_2340862)
AffiniPure donkey anti-rabbit IgG (H+L), Alexa Fluor 647 conjugated (Jackson Immunoresearch, cat. no. 711–605-152; RRID: AB_2492288)
AffiniPure donkey anti-rabbit IgG (H+L), Alexa Fluor 488 conjugated (Jackson Immunoresearch, cat. no. 711–545-152; RRID: AB_2313584)
Other reagents
Calcein blue (Life Technologies, cat. no. C1429)
Paraformaldehyde (16% (aqueous); Ted Pella, cat. no. 18505)
Dulbecco’s PBS (no calcium, no magnesium; Gibco, cat. no. 14190144)
Hank’s Buffered Saline Solution (HBSS, no calcium, no magnesium, no phenol red; Gibco, cat. no. 14175095)
Dimethyl sulfoxide (DMSO; Sigma-Aldrich, cat. no. D2650)
Intracellular staining permeabilization wash buffer (10×; BioLegend, cat. no. 421002)
Hyclone FBS (characterized; GE Healthcare Life Sciences, cat. no. SH30071.03)
Hydrochloric acid (HCl; Fisher Scientific, cat. no. A481–212)
Ethanol (200 proof) Molecular Biology Grade (Fisher Scientific, cat. no. BP28184)
Trypan blue (Thermo Fisher Scientific, cat. no. 15250061)
Liquid nitrogen (Airgas, cat. no. NI NF160LT22)
Equipment
Tissue culture–treated 6- and 12-well plates (Corning, cat. nos. 07–200-83 and 07–200-82)
Disposable sterile filter systems (500 ml; Millipore, cat. no. 09–761-107)
Sterile 15- and 50-ml Falcon conical tubes (Corning, cat. nos. 352097 and 352098)
Snap-cap microcentrifuge safe-lock tubes (1.5 ml; Eppendorf, cat. no. 022363204)
SteriFlip sterile disposable vacuum filter units (EMD Millipore, cat. no. SCGP00525)
DMSO-qualified SteriFlip sterile disposable vacuum filter units (EMD Millipore, cat. no. SE1M179M6)
TipOne Sterile Filter Tips (1,000 μl, XL graduated; USA Scientific, cat. no. 1122–1730)
TipOne Sterile Filter Tips (200 μl, graduated; USA Scientific, cat. no. 1120–8710)
TipOne Sterile Filter Tips (10/20 μl, XL; USA Scientific, cat. no. 1120–3710)
TipOne Sterile Filter Tips (20 μl, XL; USA Scientific, cat. no. 1120–1780)
Pipettes (5, 10, and 25 ml; Fisher Scientific, cat. nos. 13–678-11D, 13–678-11E, and13–678-11)
1.5-ml microcentrifuge tubes (USA Scientific, cat. no. 05–402-25)
Cryovials (Fisher Scientific, cat. no. 430487)
CoolCell cryopreservation containers (Fisher Scientific, cat. no. 07–210-004)
Round-bottom polystyrene tubes (Fisher Scientific, cat. no. 06666C)
Round-bottom polypropylene tubes (Fisher Scientific, cat. no. 02–993-342). Use polystyrene or polypropylene tubes depending on flow cytometer specifications
New Classic MF Balance (Mettler Toledo, model no. MS303S)
Light microscope (inverted microscope; Olympus, model no. CKX53)
Flow cytometer able to detect PE, APC, FITC, PerCP–Cy5.5 channels (Stratedigm, model no. S1000EXi)
Cell sorter (MoFlo Astrios; Beckman Coulter, cat. no. B52102)
Humidified 37 °C incubator with 5% CO2 (Thermo Fisher Scientific, model no. HERAcell VIOS 160i)
Multipurpose centrifuge (Eppendorf, model no. 5810 R)
Manual or automated cell-counting system (Hausser Bright-Line Phase Hemacytometer; Fisher Scientific, cat. no. 02–671-6 or Luna II automated cell counter; Logos Biosystems, cat. no. L40001)
P200 pipette (Thomas Scientific, cat. no. P3960–200A-B)
P1000 pipette (Thomas Scientific, cat. no. P3960–1000A-B)
Cell scraper (Fisher Scientific, cat. no. 08–100-241)
Shaker or rocker (UltraCruz 2D Rocker; Santa Cruz Biotechnology, cat. no. sc-358757)
Reagent setup
Human PSCs
Before starting a differentiation, maintain PSCs in feeder-free conditions in mTeSR1 and passage PSCs at least once after thawing frozen vials of cells. PSCs are ready for differentiation when they have reached 70–80% confluence. Each PSC line has different growth kinetics, but passaging at a split ratio of 1:6–1:20 should result in cells ready to passage or start differentiating around once per week.
Matrigel-coated plates
Thaw a 5-ml vial of hESC-qualified Matrigel on ice. Divide Matrigel into 1.5-ml microcentrifuge tubes, also on ice, according to the dilution factor provided on the Corning website for each individual lot number. Store aliquots at −80 °C until expiration date specified by Corning according to individual lot number. One hour before plating cells, thaw an aliquot of Matrigel on ice and resuspend it in DMEM/F12 according to the manufacturer’s instructions. Place 1 ml/well in 6-well plates and incubate at room temperature (20–24 °C) for 1 h or at 37 °C for 30 min. After incubation, aspirate the Matrigel solution and wash with 2 ml of DMEM/F12. Add desired medium to plate immediately before use. Alternatively, you can store plates at 4 °C immediately after coating for up to 1 week.
3D Matrigel
Thaw 10 ml of growth factor–reduced Matrigel on ice. Divide 500 μl of Matrigel into 1.5-ml microcentrifuge tubes, also on ice. Store aliquots at −80 °C until expiration date specified by Corning according to individual lot number. On the day of use, thaw aliquots on ice 30 min before using them !CAUTION If Matrigel warms to room temperature, it will polymerize and cannot be suspended in dilution medium. Keep 3D Matrigel on ice during use to prevent this premature solidification.
Ascorbic acid
Make a stock solution of 50 mg/ml by dissolving 500 mg ascorbic acid in tissue culture–grade water. Filter with a SteriFlip and then store 500-μl aliquots at −20 °C for up to 6 months. Keep protected from light.
Diluted MTG
Make a 13 μl/ml stock solution by adding 26 μl of MTG to 2 ml of DMEM/F12 just before making complete serum-free differentiation medium (cSFDM).
Dorsomorphin
Make a 2 mM stock solution by diluting dorsomorphin according to the listed molecular weight in an appropriate volume of DMSO. Store 100-μl aliquots at −20 °C for up to 1 year.
SB431542
Make a 1,000× (10 mM) stock solution by reconstituting SB431542 according to the listed molecular weight in an appropriate volume of DMSO. Store 100-μl aliquots at −20 °C for up to 1 year.
CHIR99021
Make a 1,000× (3 mM) stock solution by reconstituting CHIR99021 according to the listed molecular weight in an appropriate volume of DMSO. Store 100-μl aliquots at −20 °C for up to 1 year.
rhBMP4
Make a 0.1% BSA solution by diluting 666 μl of 7.5% BSA in 50 ml of 4 mM HCl. Filter with a SteriFlip. Make a 10 μg/ml stock solution by diluting rhBMP4 in 0.1% BSA in HCl. Store 250-μl aliquots at −80 °C for up to 1 year.
Retinoic acid
Make a 1,000× (100 μM) stock solution by reconstituting RA according to the listed molecular weight in an appropriate volume of 200 proof ethanol. Store 500-μl aliquots at −20 °C for up to 1 year. Keep protected from light.
rhKGF
Make 0.1% BSA by diluting 666 μl of 7.5% BSA in 50 ml of PBS. Filter with a SteriFlip. Make a 10 μg/ml stock solution by diluting rhKGF in 0.1% BSA. Store 100-μl aliquots at −80 °C for up to 1 year.
Dexamethasone
Make a 2,000× (1 × 10−4 M) stock solution by reconstituting dexamethasone according to the listed molecular weight in an appropriate volume of 200 proof ethanol. Store 500-μl aliquots at −20 °C for up to 1 year.
IBMX
Make a 1,000× (0.1 M) stock solution by reconstituting IBMX according to the listed molecular weight in an appropriate volume of DMSO. Store 500-μl aliquots at −20 °C for up to 1 year.
Y-27632
Make a 1,000× (10 mM) stock solution by reconstituting Y-27632 according to the listed molecular weight in an appropriate volume of sterile water. Store 200-μl aliquots at −20 °C for up to 1 year.
Primocin
Store 1-ml aliquots at −20 °C for up to 1 year.
Calcein blue
Make a 1,000× (10 mM) stock solution by reconstituting calcein blue according to the listed molecular weight in the appropriate volume of DMSO. Store 10-μl aliquots at −20 °C for up to 1 year.
Complete serum-free differentiation medium
For ~500 ml of cSFDM, first combine 375 ml of IMDM and 125 ml of Ham’s F12. Then add 5 ml of GlutaMAX, 5 ml of B27 supplement, 3.33 ml of 7.5% BSA, 2.5 ml of N2 supplement, 500 μl of ascorbic acid (final concentration, 50 μg/ml), 1.5 ml of diluted MTG (final concentration, 4.5 × 10−4 M), and 500 μl of Primocin (final concentration, 200 ng/ml). Filter the medium and store protected from light at 4 °C for up to 1 month.
Definitive endoderm differentiation medium (StemDiff Definitive Endoderm Kit)
For day −1 medium, use mTeSR1 + 10 μM Y-27632. Add 2 μl of Y-27632 to 2 ml of mTeSR1 for each well of endoderm. For day 0 medium, follow the manufacturer’s instructions precisely. Use this medium for the next 72 h (but note that we call the initial day ‘day 0’, whereas the manufacturer may refer to this as ‘day 1’). Add 20 μl of MR supplement and 20 μl of CJ supplement to 2 ml of definitive endoderm basal medium (all from the kit; MR+CJ medium) for each well of endoderm. For day 1 medium, add 20 μl of CJ supplement to 2 ml of definitive endoderm basal medium for each well of endoderm. For day 2 medium, add 20 μl of CJ supplement to 2 ml of definitive endoderm basal medium for each well of endoderm. These media should be kept protected from light and warmed (to 37 °C) immediately before use. When starting a differentiation, make appropriate amounts of the media for days 0–2 and store them in separate 15-ml conical tubes such that only the medium for each day is warmed before use.
DS/SB medium
Make at least 4 ml of DS/SB (anteriorization) medium for each well that the definitive endoderm will be passaged into for foregut patterning by adding the 1,000× stocks of dorsomorphin and SB431542 to the appropriate volume of cSFDM. Final working concentrations of the anteriorization medium will be 10 μM SB431542 and 2 μM dorsomorphin in cSFDM. Split the medium in half and add Y-27632 to a final concentration of 10 μM to one half of the medium. You will use DS/SB + Y-27632 on the day of replating (day 3) and then refeed once with DS/SB without Y-27632 (day 4).
Lung progenitor–specification medium (CBRa)
After anteriorization (typically day 6 of total differentiation), prepare lung progenitor–specification medium without RA (CB) by adding CHIR and rhBMP4 (both from 1,000× stock solutions) to the appropriate volume of cSFDM. As cells are typically fed every other day, we recommend making at least 8–10 ml of medium for each well of cells. CB medium can be stored at 4 °C for up to 2 weeks. On the day of medium change, supplement the prepared CB medium with 1,000× RA to generate ‘CBRa’ medium. As RA is highly light sensitive, this and all subsequent steps using this medium should be performed in low lighting (turn lights off in tissue culture hood, cover media container with foil). Final concentrations will be 3 μM CHIR99021, 10 ng/ml rhBMP4, and 100 nM RA in cSFDM.
10× cAMP/IBMX stock medium
To make 50 ml of 10× cAMP/IBMX (CI) stock medium, dissolve 21.5 mg of 8-Br-cAMP in 50 ml of cSFDM (1 mM). Add 500 μl of IBMX stock for a 1 mM concentration. Once the 8-Br-cAMP has dissolved, filter the solution through a SteriFlip. Store protected from light at 4 °C for up to 1 month.
CK+DCI medium
For 50 ml of CK+DCI medium (alveolosphere medium), add 5 ml of 10× CI stock medium to 45 ml of cSFDM. Then add 50 μl of 1,000× CHIR stock and 50 μl of 1,000× rhKGF stock. Finally, add 25 μl of 2,000× dexamethasone stock. Final working concentration of CK+DCI medium will be 3 μM CHIR99021, 10 ng/ml rhKGF, 50 nM dexamethasone, 0.1 mM 8-Br-cAMP, and 0.1 mM IBMX in cSFDM. Filter through a SteriFlip and store protected from light at 4 °C for up to 2 weeks.
CK+DCI+Y-27632 medium
For 50 ml of CK+DCI+Y-27632 medium (alveolosphere medium with rho-associated kinase inhibitor), add 5 ml of 10× CI stock medium to 45 ml of cSFDM. Then add 50 μl of CHIR (1,000× stock; final concentration, 3 μM) and 50 μl of rhKGF (1,000× stock; final concentration, 10 ng/ml). Finally, add 25 μl of dexamethasone (2,000× stock; final concentration, 50 nM) and 50 μl of Y-27632 rho-associated kinase inhibitor (1,000× stock; final concentration, 10 μM). Filter through a SteriFlip and store protected from light at 4 °C for up to 2 weeks.
K+DCI medium
For 50 ml of K+DCI medium (alveolosphere medium without CHIR99021), add 5 ml of 10× CI stock medium to 45 ml of cSFDM. Then add 50 μl of rhKGF (1,000× stock; final concentration, 10 ng/ml). Finally, add 25 μl of dexamethasone (2,000× stock; final concentration, 5 × 10−8 M). Filter through a SteriFlip and store protected from light at 4 °C for up to 2 weeks.
FACS buffer (PBS+)
For 50 ml of a 2% FBS FACS staining buffer, add 1 ml of FBS to 49 ml of PBS. Filter through a SteriFlip and store at 4 °C for up to 1 month.
Flow cytometry sorting buffer (HBSS+)
To make 10 ml of a 2% FBS sorting buffer, first add 200 μl of Hyclone FBS to 10 ml of HBSS. Filter through a SteriFlip and store at 4 °C for up to 1 month. On the day of sorting, add 10 μl of Y-27632 (from 1,000× stock; final concentration, 10 μM) and 10 μl of calcein blue (from 1,000× stock; final concentration, 10 μM).
Intracellular FACS staining buffer
First, make FACS buffer as detailed above. To make 10 ml of intracellular FACS staining buffer, add 1 ml of 10× intracellular staining permeabilization wash buffer to 9 ml of FACS buffer. This buffer can be stored at 4 °C for up to 1 month.
Collection tubes for sorting
This can depend on the specifications of the specific cell sorter. For cell-collection numbers <1 × 105, add 200 μl of sorting buffer to 1.5-ml microcentrifuge tubes. For larger cell-collection numbers, add 1 ml of sorting buffer to a 15-ml conical tube. Prepare enough tubes to collect all of your samples.
Trypsin-stopping medium
For 50 ml, add 5 ml of FBS to 45 ml of DMEM. This medium can be stored at 4 °C for up to 1 month.
Freeze medium
For 10 ml, add 1 ml of DMSO to 9 ml of FBS. Filter through a DMSO-qualified SteriFlip. This medium should be made fresh just before use.
Dispase
Add dispase II powder at a concentration of 2 mg/ml to DMEM/F12. Filter through SteriFlip and store at 4 °C for up to a month.
Procedure
Preparation of PSCs for differentiation ● Timing ~3–5 d (~20 min hands-on)
-
1
Grow PSC (ESC or iPSC) colonies to ~70–80% confluency with minimal numbers of spontaneously undifferentiated cells.
!CAUTION Abundance of spontaneously differentiated PSCs in this stage could lead to inefficient induction of definitive endoderm and NKX2–1+ endodermal progenitors. If spontaneously differentiated colonies are present, remove them by scraping them off the plate with a P200 pipette.
-
2
Prepare a Matrigel-coated 6-well plate (‘Reagent setup’).
-
3
Prepare day −1 mTeSR1 + Y-27632 medium (‘Reagent setup’).
-
4
Aspirate Matrigel solution from the plate and rinse the Matrigel-coated plate with DMEM/F12.
-
5
Aspirate the DMEM/F12 wash and add 2 ml of mTeSR1 + Y-27632 medium to each well.
-
6
Aspirate the mTeSR1 culture medium from the PSCs (from Step 1) and rinse with 1 ml of DMEM/F12.
-
7
Add 1 ml of Gentle Cell Dissociation Reagent to the cells and incubate at 37 °C for 8–10 min.
-
8
Check for detachment of ES or iPS colonies from the Matrigel-coated plate. When cells are detached, large holes will be visible in the ES or iPS colonies, and there will be floating cells.
-
9
Use a P1000 pipette to gently pipette the cells five to six times to detach any cells from the plate and ensure cell aggregates are broken into single cells. If cells do not come off the plate easily, instead use a cell scraper to release the cells and then pipette with a P1000 one to two times to break up aggregates.
-
10
Transfer the Gentle Cell Dissociation Reagent containing the PSCs to a 15-ml Falcon tube.
-
11
Wash the plate previously containing PSCs with 1 ml of DMEM/F12 and transfer the medium to the 15-ml Falcon tube containing the dissociated ES or iPS colonies.
-
12
Pellet the cells by centrifuging for 5 min at 300g at 4°C. Aspirate the supernatant.
-
13
Resuspend the cells in 1 ml of mTeSR1 + Y-27632 medium and pipette no more than five times to break the pellet into single cells. Count the number of live cells.
-
14
Plate 2 × 106 PSCs into each well of the Matrigel-coated 6-well plate (from Step 5).
-
15
Ensure that the cells are evenly distributed in the wells by gently rocking the plates from side to side, forward and backward, and in figure 8 motions. Incubate the cells at 37 °C overnight.
Definitive endoderm induction ● Timing ~3 d (10 min/d hands-on)
-
16
After overnight incubation, wash cells once with 1 ml of DMEM/F12 and add 2 ml of day 0 MR+CJ StemDiff definitive endoderm medium according to the manufacturer’s instructions (‘Reagent setup’). Incubate the cells for 24 h at 37 °C.
▲ CRITICALSTEP After plating, cells may exhibit a webbed network appearance or ~100% confluence. Floating cells and substantial cell death are normal on each day of definitive endoderm induction.
-
17
Aspirate the medium and add 2 ml of the day 1 CJ-only StemDiff definitive endoderm medium (‘Reagent setup’). Incubate the cells for 24 h at 37 °C.
-
18
Aspirate the medium and add 2 ml of the day 2 CJ-only StemDiff definitive endoderm medium (‘Reagent setup’). Incubate the cells for 24 h at 37 °C.
Assessment of definitive endoderm induction efficiency using human CKIT and CXCR4 ● Timing ~2.5 h
-
19
On day 3, aspirate the medium and wash one well of cells with 1 ml of DMEM/F12. Add 1 ml of Gentle Cell Dissociation Reagent and incubate the cells at 37 °C for 4–5 min.
▲ CRITICAL STEP When first differentiating a cell line, you should prepare one well of endoderm for day 3 FACS analysis only as described here and one or more wells for passaging and continued differentiation as described in Steps 31–38. If you are restricted to differentiating only one well of endoderm, you can take 3/4 of the well for passaging as described in the next section and save 1/4 of the well for subsequent FACS analysis, limiting the samples to two: (i) isotypes and (ii) CKIT-PE/ CXCR4-APC. In this case, if the FACS analysis shows CKIT/CXCR4 expression <80%, you should discard the differentiation.
-
20
Wash, collect, and pellet cells by repeating Steps 10–12.
-
21
Gently resuspend cells from the one well in 600 μl of FACS buffer (‘Reagent setup’).
-
22
Divide the medium containing cells into five 1.5-ml microcentrifuge tubes; each tube should contain 100 μl of cell-containing medium.
-
23
Add ~2 μl of antibody or isotype (‘Reagent setup’) as appropriate to the following tubes: unstained; isotypes; CKIT-PE, CXCR4-APC, and CKIT-PE/CXCR4-APC.
-
24
Vortex all 1.5-ml microcentrifuge tubes briefly and incubate them on ice for 15 min.
-
25
After 15 min, briefly vortex the 1.5-ml microcentrifuge tubes and incubate them on ice for an additional 15 min (30 min total).
▲ CRITICAL STEP It is important to protect the 1.5-ml microcentrifuge tubes from light during incubation on ice to allow for unaffected staining. Containers used can be wrapped in foil during incubation.
-
26
Add 1 ml of FACS buffer (‘Reagent setup’) to each tube and centrifuge at 300g for 5 min at room temperature.
-
27
Aspirate the supernatant from each tube and wash the cells by repeating Step 26.
-
28
Aspirate the supernatant from each tube and resuspend the cells in 350 μl of FACS buffer (‘Reagent setup’).
-
29
Collect the 350 μl of cell solution and strain through the cell strainer cap of a polystyrene tube to ensure single-cell suspension.
-
30
Perform FACS analysis for expression of CKIT and CXCR4.
▲ CRITICAL STEP >More than 80 percent of cells should co-express CKIT and CXCR4 at this stage (typically 90–100% for the BU3 NGST line). Definitive endoderm efficiency varies from cell line to cell line. For low efficiencies, checking CKIT/CXCR4 expression by FACS at 24-h intervals from day 1 on can help to delineate the endodermal differentiation kinetics of new lines.
? TROUBLESHOOTING
Anterior foregut endoderm induction ● Timing ~3 d (10 min/d hands-on)
-
31
On day 3, preferably after FACS analysis of one well of definitive endoderm showing >80% CKIT/ CXCR4 expression, prepare fresh Matrigel-coated 6-well plates and DS/SB + Y-27632 medium (see ‘Reagent setup’ section).
-
32
Add 2 ml of DS/SB + Y-27632 medium (‘Reagent setup’) to each well of the above Matrigel-coated plates (the number of wells depends on the number of wells of endoderm and the split ratio you intend to use).
-
33
Aspirate the StemDiff definitive endoderm medium from cells and gently wash the cells with 1 ml of DMEM/F12 medium.
-
34
Add 1 ml of Gentle Cell Dissociation Reagent and incubate at room temperature for 2–3 min.
-
35
Aspirate the Gentle Cell Dissociation Reagent and add 1 ml of DS/SB + Y-27632 medium to each well (‘Reagent setup’). Pipette the medium up and down with a P1000 to ensure detachment of cells from the plate and to generate cell fragments.
-
36
Passage the well at a density of 1:2–1:6 into the waiting Matrigel-coated plate containing DS/SB + Y-27632 medium (‘Reagent setup’). Incubate at 37 °C for 24 h.
!CAUTION The size of the colony fragments influences the cell survival after beginning anterior foregut induction. If cells are single cells or dissociated into small fragments at this stage, high cell death has been observed. Optimize the incubation time in Gentle Cell Dissociation Reagent for each line to reduce cell death.
▲ CRITICAL STEP Appropriate passaging density varies greatly from cell line to cell line. Successful NKX2–1+ lung specification is typically observed when the cells are plated at a density of 200,000 cells/cm2.
? TROUBLESHOOTING
-
37
After 24 h, aspirate the medium and add 2 ml of DS/SB medium without Y-27632 (‘Reagent setup’).
? TROUBLESHOOTING
-
38
Incubate at 37 °C for 48 h.
? TROUBLESHOOTING
NKX2–1 lung progenitor induction ● Timing 8–9 d (10 min/d hands-on)
-
39
After anterior foregut endoderm induction, aspirate the medium and wash the cells with 1 ml of DMEM/F12.
-
40
Add 2 ml of CBRa medium (‘Reagent setup’).
-
41
Aspirate the medium and refeed the cells with CBRa medium (‘Reagent setup’) every 48 h for 8–9 d.
Sorting and replating NKX2–1+ lung progenitors (days 14–16) ● Timing ~2 d (~3–6 h hands-on)
▲ CRITICAL Cell sorting relies on having a single-cell suspension of live cells. Vigorous pipetting can lead to increased cell death. We recommend leaving cells in trypsin for up to 15–20 min to enzymatically digest cell–cell connections, rather than relying on mechanical pipetting.
▲ CRITICAL Dissociation in trypsin in a tissue culture plate will allow visualization of the cells throughout the process. Do not prepare cells for sorting until you see under a microscope that the majority of cells are in single-cell suspension.
-
42
Prepare FACS buffer and collection tubes (‘Reagent setup’).
-
43
Thaw 3D Matrigel and prepare CK+DCI medium (‘Reagent setup’).
-
44
Aspirate the CBRa medium from each well and add 1 ml of warm 0.05% trypsin–EDTA.
-
45
Use a P200 pipette to scratch the cell monolayer in a crosshatch pattern.
!CAUTION Crosshatching of the cell monolayer should allow for increased penetration of trypsin–EDTA and for more effective enzymatic dissociation. This process will yield substantial amounts of floating cells.
-
46
Incubate the cells at 37 °C for 10–15 min. Pipette two to three times with a P1000 pipette and then visualize the cells under microscope. If single cells are observed, move to Step 47. If there are small clumps, leave the cells in the 37 °C incubator for an additional 5 min. If the cells have not dissociated into single cells after 15 min, centrifuge the cells for 5 min at 300g at 4 °C, aspirate the supernatant, add fresh 0.05% trypsin–EDTA, and leave the cells in the 37 °C incubator for another 5 min.
? TROUBLESHOOTING
-
47
Pipette trypsinized single cells into a 15-ml conical tube and add 10 ml of trypsin-stopping medium (‘Reagent setup’) to inactivate trypsin–EDTA and keep the tube at 4 °C until replated.
!CAUTION At this point, if you do not have access to a cell sorter, you can replate the cells without sorting by continuing to Step 50.
!CAUTION If you sort cells that have not been fully dissociated, you will lose a large percentage of the cell population. If the cells are dissociated for too long, some cells will lyse and release DNA into the medium. This DNA can be very sticky and can also contribute to cell loss. Monitoring cells closely throughout this process can prevent unintended cell loss.
-
48
Centrifuge the cells at 300g for 5 min at 4 °C and resuspend 1 × 106 cells per 100 μl of FACS buffer. If using a PSC line with an NKX2–1 knockin reporter, you are ready to move to Step 54. If using a PSC line without an NKX2–1 knockin reporter, proceed to the next step.
-
49
Prepare cells without an NKX2–1 knockin reporter for CD47/CD26 immunostaining. Set aside 2 × 106 cells for use as controls and set compensation as follows (5 × 105 cells each): unstained, isotypes, CD47-PerCP–Cy5 only (1:200 dilution), and CD26-PE only (1:200 dilution).
-
50
To the remaining cells, add both CD47-PerCP–Cy5 and CD26-PE antibodies at a dilution of 1:200. For example, per million cells, add 0.5 μl of each antibody to 100 μl of FACS buffer for staining.
-
51
After staining on ice for 30 min, wash the cells in 10 ml of PBS.
-
52
Centrifuge the cells at 300g for 5 min at 4 °C and aspirate the supernatant.
-
53
Resuspend 1 × 106 cells per 500 μl of sorting buffer.
-
54
Filter cells through a 40-μm filter and transfer to round-bottom polypropylene tubes (or polystyrene tubes, depending on cell-sorter specifications) for sorting either NKX2–1GFP+ cells or CD47hi/CD26lo cells. Keep on ice protected from light.
▲ CRITICAL STEP Cell sorting requires a high level of expertise and is best performed by a well-trained operator. Our sort algorithm is as follows (Fig. 7). First, debris is gated out using forward scatter (FSC) versus side scatter (SSC). Then doublets are gated out using FSC area (FSC-A) versus FSC height (FSC-H) or pulse width versus FSC. Then live cells are gated in using calcein blue staining. Finally, cells can be gated for NKX2–1GFP or CD47hi/CD26lo. Although most of the cells will be positive for CD47 as compared to isotype controls, by using the intersection of the CD47hi and CD26+ populations as a guide for where to end the CD47hi gate, we typically obtain good NKX2–1+ cell enrichment. The more conservative your CD47hi gate, the higher the percentage of NKX2–1+ cells you will obtain in the CD47hi population8,11.
▲ CRITICAL STEP To evaluate NKX2–1+ cell-differentiation efficiency or enrichment for NKX2–1+ cells in the CD47hi/CD26lo sorted cells, fix at least 3 × 105 cells from each sample in 1.6% paraformaldehyde to analyze by intracellular flow cytometry for NKX2–1. See Step 82B(i–xii).
? TROUBLESHOOTING
-
55
After collecting NKX2–1GFP+ cells or CD47hi/CD26lo cells from the sorter, resuspend 200–400 cells/μl of undiluted 3D Matrigel in droplets ranging from 20 μl (96-well plate) to 1 ml (10-cm dish).
!CAUTION 3D Matrigel can be quite difficult to work with because it rapidly solidifies at room temperature. For cells to be successfully resuspended in Matrigel, always leave 3D Matrigel on ice before and during use, and consider either freezing pipette tips before Matrigel use or coating the inside of the pipette tips with cold PBS before pipetting the Matrigel.
▲ CRITICAL STEP Replating density on days 14–16 can substantially influence the percentage of SFTPCtdTomato+ or NKX2–1GFP cells at later time points. Hence, the plating density should be optimized for new cell lines being tested, if efficiency is low at later time points (Fig. 5a–c). There is a trade-off between percentage efficiency and total cell number yield by day 30, if plating densities are very low on day 15. Figure 5c shows that when 62–150 cells/μl of Matrigel are plated on day 15, the SFTPCtdTomato+ cell efficiency on day 32 is optimal. Although the percentage efficiency of SFTPCtdTomato+ expression is high at this density, the total cell number yields and sphere number yields are often low on day 32 in these conditions, given the low starting number of cells. Thus, we recommend scoring and passaging cells earlier (day 30) when optimizing a line for distal alveolar yields. For the BU3 NGST line, we suggest a day-15 plating density of 200–400 cells/μl. If the SFTPCtdTomato+ cell yield is low for any line, try plating cells at lower densities (Fig. 5a–c) and, as a general rule, consider re-optimizing earlier stages of the protocol to ensure foregut endodermal cells with optimal lung competence are produced.
▲ CRITICAL STEP We typically maintain alveolospheres in 50-μl 3D Matrigel droplets in 12-well plates. Spread 3D droplets on the bottom of the plate rather than leaving them as domes, because this will make it easier to image the cells and to ensure that the growth medium covers the entirety of the Matrigel drop.
-
56
Place droplets in a 37 °C incubator for 20–30 min without adding medium to allow the 3D Matrigel to solidify.
-
57
Add the appropriate amount of warm CK+DCI+Y-27632 medium to each well (for example, 200 μl of medium per well of a 96-well plate up to 10 ml of medium per 10-cm dish).
-
58
After 48 h, aspirate the medium and refeed cells with CK+DCI without Y-27632.
Fig. 7 |. Recommended sorting strategies for day 14 lung progenitors and day 30 alveolospheres.
a, Representative flow cytometry of day 14 cells and sorting gates used to exclude debris (SSC versus FSC), exclude doublets (pulse width versus FSC), include live cells (calcein blue versus FSC), and include NKX2–1GFP+ cells (FL3 versus NKX2–1GFP). b, Representative flow cytometry of day 14 lung progenitor cells already gated using strategy in a with recommended gates for CD47hi/CD26lo cells (RUES2 ST ESC line) with isotype and single-color controls. c, Representative flow cytometry of day 30 alveolospheres stained with CPM or CKIT antibodies with secondary only and isotype controls and recommended sort gates. d, Representative flow cytometry of day 30 alveolospheres already gated using strategy in a with recommended gates for NKX2–1GFP+/SFTPCtdTomato+ cells in BU3 NGST and SFTPCtdTomato+ cells in RUES2 ST. FL3 and FL5, additional fluorescence channels used to measure autofluorescence.
Alveolosphere maintenance and passaging (day 14+) ● Timing ~10 d (~2–3 h hands-on)
-
59
Refeed the cells every 48–72 h with fresh CK+DCI medium without Y-27632 (‘Reagent setup’). You should begin to see SFTPCtdTomato+ cells within 3–5 d, and by day 30, the emerging spheres should be passaged as described in the next steps to avoid overgrowth and consequent loss of SFTPC.
!CAUTION Be careful when aspirating medium to avoid dislodging the Matrigel drop. We suggest aspiration from the sides of wells furthest away from 3D Matrigel droplet.
▲ CRITICAL STEP Passage alveolospheres before they become confluent within the Matrigel drop; otherwise, SFTPCtdTomato induction efficiency will decrease (Fig. 4b,c). After the first sphere passage, at a minimum, alveolospheres should be repassaged every 2–3 weeks thereafter (typically every 14 d for the BU3 NGST line).
? TROUBLESHOOTING
-
60
When alveolospheres are ready to be passaged, aspirate the medium from Matrigel drop.
-
61
Add 1 ml of dispase (‘Reagent setup’) and incubate at 37 °C for 45 min–1 h, pipetting up and down once with a P1000 pipette after ~30 min.
-
62
Transfer 1 ml of dissociated organoids in dispase to a 15-ml conical flask and add 10 ml of DMEM/F12 to wash.
-
63
Centrifuge at 200g for 4 min at 4 °C and aspirate the supernatant. If you wish to passage alveolospheres as complete spheres, add 10 ml of DMEM/F12 to wash, centrifuge again at 200g for 4 min at 4 °C, and proceed to Step 67. If you wish to passage alveolospheres as single cells, proceed with the next step.
!CAUTION Brief centrifugation produces a poorly packed pellet. Aspirate gently, using a P1000 or P200 pipette, to reduce the possibility of loss of the pellet through aspiration; however, it is imperative at this time to remove as much of the supernatant as possible. Any remaining dispase left on the cell pellet can dissolve the 3D Matrigel used to resuspend these cells in Step 64.
!CAUTION If a semi-transparent haze is seen above the pellet, the 3D Matrigel has not totally dissolved. More dispase can be added to the pellet to dissolve the excess 3D Matrigel. After addition of more dispase, incubate the cells at 37 °C for another 20 min.
▲ CRITICAL STEP Centrifugation is brief because alveolospheres are heavier than the single cells and debris within the Matrigel. In fact, if you see large amounts of cell death and debris in the Matrigel droplet, you can allow the alveolospheres to separate from the single cells/debris by centrifuging more gently (200g for 1 min at 4 °C). You can aspirate these components away so that you analyze only cells from alveolospheres.
-
64
Resuspend the cells in 1 ml of 0.05% trypsin–EDTA per well.
-
65
Repeat Steps 46 and 47.
-
66
Centrifuge at 300g for 5 min at 4 °C, aspirate the supernatant, and resuspend the cells in 10 ml of DMEM/F12 to wash. Centrifuge again at 300g for 5 min at 4 °C.
? TROUBLESHOOTING
-
67
Aspirate the supernatant and resuspend the cells in undiluted 3D Matrigel at the desired concentration. A passaging ratio of 1:3–1:10 is reasonable, with cells at densities varying from 100 to 1,000 cells/μl of undiluted 3D Matrigel.
▲ CRITICAL STEP If the cells are in single-cell suspension, you can also perform FACS analysis to evaluate the percentage of NKX2–1+ (if using BU3 NGST, with NKX2–1GFP fluorescent reporter, or in other lines, intracellular FACS for NKX2–1 protein— see Step 82B(i–xii)) or SFTPC+ (if using BU3 NGST, RUES2 ST, or another line targeted, with SFTPCtdTomato fluorescent reporter) cells, which can help determine whether to sort or passage the cells at this stage (Fig. 1c).
? TROUBLESHOOTING
-
68
Place the plate in a 37 °C incubator for 20–30 min without adding medium to allow the Matrigel to solidify.
-
69
Add the appropriate amount of warm (37 °C) CK+DCI+Y-27632 medium to each well (200 μl of medium per 96-well plate to 10 ml of medium per 10-cm dish). After 24–48 h, aspirate the medium and refeed the cells with CK+DCI without Y-27632 medium.
Sorting alveolospheres (day 25+) ● Timing ~2 d (~3–6 h hands-on, depending on cell volume)
-
70
Prepare sorting buffer and collection tubes (‘Reagent setup’)
-
71
Dissociate the alveolospheres as described above in Steps 60–66.
▲ CRITICAL STEP Dissociate the cells in a tissue culture plate so that you can visualize them in a microscope. Cells are sensitive to over-pipetting at this stage, so leave them in trypsin–EDTA long enough for most of the dissociation to be enzymatic. If the alveolospheres have not dissociated into single cells by 12 min, spin down (300g, 4 °C, 5 min), add fresh 0.05% trypsin–EDTA, and incubate at 37 °C for another 5 min.
-
72
If the cells contain an NKX2–1GFP+or SFTPCtdTomato+ reporter, resuspend 1 × 106 cells per 500 μl in sorting buffer and take them to the sorter. If the cells do not contain a reporter, first stain the cells with CPM (option A) or CKIT (option B) antibody.
- Staining for CPM
- Stain 1 × 105 cells in 100 μl of FACS buffer with mouse anti-human CPM antibody at a dilution of 1:200 (0.5 μl) for 15 min at 4 °C.
- Set aside additional cells for use as unstained and secondary-only controls (1 × 105 cells in each) that will not be stained with primary antibody (the manufacturer does not produce a specific isotype control, and you will gate on the basis of high expression, making isotype controls less relevant).
- Wash the cells by adding 1 ml of PBS and centrifuge at 300g for 5 min at 4 °C.
- Stain 1 × 105 cells in 100 μl of FACS buffer with Alexa Fluor 647–conjugated anti-mouse IgG at a dilution of 1:300 (0.33 μl) for 15 min at 4 °C.
- Wash the cells by adding 1 ml of PBS and centrifuge at 300g for 5 min at 4 °C. Resuspend ~1 × 106 cells/500 μl in sorting buffer.
- Staining for CKIT
- Stain 1 × 105 cells in 100 μl of FACS buffer with APC-conjugated mouse anti-human CKIT antibody at a dilution of 1:50 (2 μl).
- Set aside additional cells for use as unstained and isotype controls (1 × 105 cells in each). You will use the isotype control (mouse IgG) to set the positive gate.
- Wash the cells by adding 1 ml of PBS and centrifuge at 300g for 5 min at 4°C. Resuspend ~1 × 106 cells/500 μl in sorting buffer.
-
73
Filter the cells through a 40-μm filter, transfer to round-bottom polypropylene tubes (or polystyrene tubes, depending on cell-sorter specifications) for sorting NKX2–1GFP+ cells, SFTPCtdTomato+ cells, CPM+ cells, or CKIT+ cells.
▲ CRITICAL STEP Cell sorting requires a high level of expertise and is best performed by a well-trained operator. Our sort algorithm is as follows (Fig. 7). First, debris is gated out using FSC versus SSC. Then doublets are gated out using FSC-A versus FSC-H or pulse width versus FSC. Then live cells are gated in using calcein blue staining. Finally, cells can be gated for NKX2–1GFP and SFTPCtdTomato+ expression. It can be helpful to bring a day-14 population of cells for a single-color compensation control (only NKX2–1GFP+). The entire SFTPCtdTomato+ population should be NKX2–1GFP+. The SFTPCtdTomato+ typically appears as more of a smear of cells than a separate cloud, so bringing a non-tdTomato-expressing control is helpful when first familiarizing yourself with this sorting strategy.
-
74
After collecting cells from the sorter, resuspend 200–400 cells/μl of undiluted 3D Matrigel in droplets ranging from 20 μl (96-well plate) to 1 ml (10-cm dish).
▲ CRITICAL STEP We typically maintain alveolospheres in 50 μl of 3D Matrigel droplets in 12-well plates. Spread 3D droplets on the bottom of the plate rather than leaving them as domes, because this will make it easier to image the cells and to ensure the growth medium covers the entirety of the Matrigel drop.
-
75
Place the plates in a 37 °C incubator for 15–30 min without adding medium to allow the Matrigel to solidify.
-
76
Add the appropriate amount of warm CK+DCI+Y-27632 medium to each well (200 μl of medium per 96-well plate to 10 ml of medium per 10-cm dish). After 24–48 h, aspirate the medium and refeed cells with CK+DCI without Y-27632 medium.
CHIR withdrawal and addback (days 30–42+) ● Timing ~10 d (10 min/d hands-on)
-
77
Either passage alveolospheres as single cells or prepare alveolosphere cells for sorting, depending on NKX2–1 retention. If >50% of cells express NKX2–1, passage and separate into single cells as described in Steps 60–69. If <50% of cells express NKX2–1, sort and replate NKX2–1GFP+ cells or CPMhi cells in 3D Matrigel culture as described above in Steps 70–76 and Fig. 1c.
-
78
Incubate the cells for 24–72 h in CK+DCI+Y-27632 medium.
-
79
Aspirate the medium surrounding the 3D Matrigel drop, wash the drop with 1 ml of DMEM/F12, and change the medium to K+DCI medium (no CHIR99021) (‘Reagent setup’).
▲ CRITICAL STEP Cells fed with K+DCI medium do not grow as fast as they do in CK+DCI medium. Alveolospheres cultured in K+DCI medium are much smaller than their CK+DCI medium counterparts.
-
80
Aspirate the medium surrounding the 3D Matrigel drop and refeed with K+DCI. Repeat this process every 48 h for 5 d.
-
81
After 5 d, aspirate the medium surrounding the 3D Matrigel drop and add the appropriate amount of CK+DCI medium to the cells (200 μl of medium per 96-well plate to 10 ml of medium per 10-cm dish).
▲ CRITICAL STEP You should see an increase in the size of the alveolospheres over this time, and should achieve a SFTPCtdTomato+ percentage of ~30–50%.
Analysis and storage of alveolospheres
-
82
Alveolospheres can now be frozen and thawed as described in option A, subjected to further flow cytometric analysis as described in option B, or analyzed in multiple further ways, as detailed in Jacob et al.3, including RT–qPCR, western blot, immunostaining, electron microscopy, and lipidomics. Mature iAEC2s should express NKX2–1, SFTPC, SFTPB, and other genes related to surfactant synthesis, such as ATP-binding cassette subfamily A member 3 (ABCA3), pepsinogen C (PGC), napsin A (NAPSA), and cathepsin H (CTSH). They also should contain functional lamellar bodies and produce and secrete surfactant.
▲ CRITICAL Although SFTPC is the most specific marker for AEC2s, because it is a highly hydrophobic protein, commercially available antibodies are often non-specific. We recommend using both negative controls of non-SFTPC expressing cells and positive controls of primary lung tissue whenever possible in these analyses, especially when they involve use of an SFTPC antibody.
- Alveolosphere freezing method ● Timing ~3 d (~2 h hands-on)
-
Dissociate alveolospheres into a single-cell suspension as described above in Steps 60–69.▲ CRITICAL STEP Alveolospheres will not survive freeze–thaw cycles if frozen as spheres rather than as single cells.
- Resuspend single cells at a concentration of at least 1 × 106 cells in 1 ml of freeze medium and freeze in a cryovial (‘Reagent setup’).
-
Leave the cryovial in cryo-safe container at −80 °C overnight and then transfer to −150 °C or a liquid nitrogen freezer for long-term storage.■ PAUSE POINT Cells can be left in liquid nitrogen indefinitely.
- Alveolosphere thawing. Thaw 3D Matrigel on ice.
-
After thawing cryopreserved cells, immediately add the cells to a conical tube containing10 ml of DMEM/F12 to dilute the DMSO.? TROUBLESHOOTING
- Centrifuge the cells at 300g for 5 min at 4 °C. Aspirate the supernatant and resuspend the cells in 1 ml of PBS.
- Count live cells using trypan blue staining and then centrifuge the cells at 300g for 5 min at 4 °C.
- Because the cells are more fragile at this stage, aspirate the supernatant and resuspend500 live cells/μl in undiluted 3D Matrigel (we suggest 50-μl Matrigel droplets in a 12-well plate).
- Place the plate in a 37 °C incubator for 20–30 min without adding medium to allow the Matrigel to solidify.
- Add 1 ml of warm (37°C) CK+DCI+Y-27632 medium over Matrigel drop. After 48 h, aspirate medium and refeed cells with CK+DCI without Y-27632 medium.
-
-
Intracellular flow cytometry for NKX2–1 protein ● Timing ~3 h
▲ CRITICAL NKX2–1 intracellular flow cytometry can be performed on day 14 NKX2–1+ lung progenitor cells (unsorted or sorted CD47hi/CD26lo) or alveolospheres (unsorted or sorted).
Prepare intracellular FACS staining buffer (‘Reagent setup’).
Prepare a negative control (we typically use undifferentiated PSCs, but any cell notexpressing NKX2–1 would be appropriate).
Dissociate cells into single cells. Day 14 NKX2–1+ lung progenitor cells should be dissociated into a single-cell suspension as described in Steps 42–48. Alveolospheres should be dissociated into single-cell suspension as described in Steps 60–69. Cells can also be obtained after cell sorting.
-
Resuspend 1 × 106 cells in 1 ml of 1.6% paraformaldehyde and incubate in a shaker or rocker at 37 °C for 10 min.
■ PAUSE POINT Cells can be resuspended in FACS buffer and stored at 4 °C for up to2 weeks after fixation.
-
Centrifuge the cells at 300g for 5 min at room temperature and resuspend 1 × 105 cells per 100 μl of intracellular FACS staining buffer to a volume of at least 300 μl.
▲ CRITICAL STEP It will not be possible to perform NKX2–1 intracellular FACS reliably without at least 3 × 105 cells at the start.
Transfer 100 μl of cell suspension to each of three tubes.
Stain 1 × 105 cells in 100 μl of intracellular FACS staining buffer with rabbit anti-human NKX2–1 antibody at a dilution of 1:200 (0.5 μl) for 30 min at room temperature.
Wash by adding 1 ml of PBS to the cell suspension and centrifuge at 300g for 5 min at room temperature. Aspirate the supernatant and repeat this wash step twice.
Resuspend each sample in 100 μl of intracellular FACS staining buffer with Alexa Fluor647– or Alexa Fluor 488–conjugated anti-rabbit IgG at a dilution of 1:300 (0.33 μl) for 30 min protected from light at room temperature.
Wash by adding 1 ml of PBS to the cell suspension and centrifuge at 300g for 5 min at room temperature. Aspirate the supernatant and repeat this wash step twice.
Resuspend in 300 μl of FACS buffer and transfer to round-bottom polypropylene tubes(or polystyrene tubes, depending on the flow cytometer’s specifications).
-
Analyze NKX2–1 expression on a flow cytometer.
▲ CRITICAL STEP Using a negative cell control is important in NKX2–1 staining because the isotype controls regularly underestimate where the gates should be drawn for NKX2–1+ cells. Cells known to be negative for NKX2–1 expression stained with NKX2–1 antibody are more optimal as a control to determine where to draw the NKX2–1+ gate.
Troubleshooting
Troubleshooting advice can be found in Table 1.
Table 1 |.
Troubleshooting table
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 30 | <90% of cells are CKIT/CXCR4+ endoderm | The specific PSC line requires longer definitive endoderm induction | Measure endodermal differentiation kinetics every 12–24 h by CKIT/CXCR4 FACS to identify peak expression, expected to be on days 3 and 4 of differentiation with the StemDiff kit. |
| Too much spontaneous differentiation in day −1 PSCs | Restart the protocol from day −1 | ||
| 30, 54, 59, 67 | Poor definitive endoderm, poor day 15 NKX2-1 efficiency, or poor NKX2-1 retention or SFTPC reporter expression in alveolospheres | One of the key reagents is not working as expected or cells at earlier stages were suboptimally patterned, thus limiting subsequent competence | Always score intermediate stages in the protocol to catch issues with reagents early (CKIT/CXCR4 on day 3, NKX2-1 on day 14, NKX2-1 and SFTPC after day 25), as FACS scores should drop if reagents stop working. Troubleshoot reagents on standard lines such as RUES2 and BU3, which are known to work well rather than troubleshooting reagent quality on new lines. A WNT reporter cell line can be used to monitor CHIR99021 function, if this reagent is suspected to be of poor quality12 |
| 36–38 | Poor cell survival after definitive endoderm passaging in DS/SB medium | Endoderm was dissociated for too long Split ratio was too high Overtrituration |
Optimize day −1 plating density (5 × 105–2 × 106 iPSCs), day 3 split ratio (1:2–1:6), and time of endoderm dissociation (1–3 min) each time you differentiate a new PSC line |
| 46, 66 | Cells remain in clumps during trypsinization | Too many cells in trypsin Trypsin has been inactivated (occurs if left at 37 °C for too long) |
If trypsin turns yellow during incubation, add 2 ml of fresh trypsin to cells If cells remain in clumps after 15 min of trypsinization, centrifuge at 300g for 5 min at 4 °C and resuspend in fresh trypsin |
| 54 | Poor NKX2-1 enrichment after CD47 sorting or poor efficiency of NKX2-1 day 14 induction | Overall low-efficiency differentiation CD47 was gate drawn too liberally |
The day before sorting, perform intracellular NKX2-1 FACS to determine differentiation efficiency and draw gate on the basis of predicted percentage of NKX2-1+ cells. If NKX2-1 efficiency is low, consider adjusting day 6 plating density or add an optional step of a 1:3 split on day 9 to avoid overgrown cultures Consider using CPM rather than CD47 to sort cells on day 15, depending on user preference8,24 |
| 54, 73 | Low cell yield during sorting | Cells not in single-cell suspension Poor cell survival due to harsh dissociation Cells were digested too long, resulting in DNA in cell suspension |
Monitor cells under microscope during dissociation; you should not need to pipette more than three to four times to reach a single-cell suspension, if cells were appropriately enzymatically digested Monitor cells at least every 5 min to ensure that there is not a high concentration of DNA in the cell suspension (white, sticky material). Consider adding DNAse to the flow cytometry sorting buffer |
| 59 | Low percentage of NKX2-1+ cells or sFTPCtdTomato+ cells on days 25–30 | NKX2-1+ cells were plated at too high a density (Fig. 5) and became confluent too early Poor retention of NKX2-1 in cell population |
Sort and replate SFTPCtdTomato+ cells or NKX2-1+ cells (GFP+, CPMhi, or CKIThi) and perform CHIR withdrawal and addback Passage alveolospheres as soon as they become confluent (Fig. 4) |
| 67 | Low percentage of NKX2-1+ cells or SFTPCtdTomato+ cells after passaging alveolospheres | It is expected that early-passaged cells tend to lose expression of SFTPCtdTomato in the first few sphere passages before expression returns, but if NKX2-1 retention continues to drop, the SFTPC expression will not return Cells were not passaged before they became confluent |
Continue passaging if earlier than passage 3 and percentage of SFTPCtdTomato cells is >30%. If percentage of SFTPCtdTomato+ cells <30%, check NKX2-1 efficiency by FACS (should be >50%) and consider sorting and replating SFTPCtdTomato+ cells (or NKX2-1GFP+ cells) again with CHIR withdrawal and addback Passage alveolospheres every 2–3 weeks, regardless of morphology, to ensure you do not let them overgrow |
| 82A(v) | Poor cell survival after freeze-thaw cycle | Cells were dissociated too harshly or do not recover well in DMSO (this happens frequently) | Any time you freeze alveolospheres, thaw a test vial within 2–3 d to ensure there are viable cells Although survival may be poor, if some cells do survive, wait for them to become alveolospheres and then passage just the spheres at a ratio of 1:1 |
Timing
The entire procedure can take 30–44 d to produce sorted SFTPCtdTomato+ alveolospheres, which can be maintained for up to 1 year (passaging spheres every 2 weeks).
Steps 1–15, preparation of PSCs for differentiation: ~3–5 d (~20 min/d hands-on)
Steps 16–18, differentiation of PSCs into definitive endoderm: ~3 d (10 min/d hands-on)
Steps 19–30, passaging definitive endoderm and assessment of definitive endoderm induction efficiency: ~2.5 h
Steps 31–38, differentiation of definitive endoderm into anterior foregut endoderm: ~3 d (10 min/d hands-on)
Steps 39–41, differentiation of anterior foregut endoderm into NKX2–1+ lung progenitor cells: ~8–9 d (10 min/d hands-on)
Steps 42–58, sorting and replating NKX2–1+ lung progenitor cells: ~2 d (3–6 h hands-on)
Step 59, differentiation of NKX2–1+ lung progenitor cells into alveolospheres: ~10 d (10 min/d hands-on)
Steps 60–69, passaging alveolospheres: ~2–3 h
Steps 70–76, sorting and replating alveolospheres: ~2 d (~3–6 h hands-on)
Steps 77–81, CHIR withdrawal and addback to sorted alveolospheres: ~10 d (10 min/d hands-on)
Step 82A(i–x), cryopreservation of alveolospheres: ~3 d (~2 h hands-on)
Step 82B(i–xii), NKX2–1 intracellular FACS: ~3 h
Anticipated results
Alveolospheres generated from PSCs using this protocol should exhibit several mature functions of primary AEC2s and can be used to model alveolar development and disease. Although alveolospheres are generated within 30 d, they can be maintained in culture for several months without losing their AEC2 phenotype. Because cells are in culture for long periods of time, several issues can arise that compromise differentiation efficiency. Our goal was to develop a protocol that allows for rescue of otherwise suboptimal differentiations using PSCs with reporter lines or lineage-specific cell-surface markers within the differentiation protocol.
We have optimized this protocol using primarily PSC lines with NKX2–1 and SFTPC reporters, although the use of cell-surface markers CD47, CPM, and CKIT enable isolation of pure populations of early or late NKX2–1+ cells as well. When starting to differentiate a new line, looking at checkpoints of differentiation efficiency, including CKIT/CXCR4+ definitive endoderm and day 14 NKX2–1+ cell scores, can help determine whether early steps of differentiation need to be optimized. In general, poor differentiation efficiencies should prompt the investigator to return to checking and optimizing the prior stage of differentiation, because low differentiation efficiencies at any stage tend to predict poor later yields of subsequently specified fates. Overall, we expect to see 30–90% NKX2–1+ cells on day 14 of differentiation, depending on which PSC line is used, and 1–10% SFTPCtdTomato+ cells on days 25–30 of differentiation. If a pure population of NKX2–1+ cells is sorted and replated on day 30, >50% of cells should retain expression of NKX2–1.
After they have been stabilized as passaged spheres (beyond day 40, either with or without CHIR withdrawal and addback), SFTPCtdTomato+ iAEC2s can be sorted to purity and maintained in culture for several passages. These cells are ideal for use in assays of AEC2 function, because within three to six passages tdTomato expression should have returned and stabilized, and 90–100% of cells should maintain expression of SFTPCtdTomato for several months of passaging. Because the cells proliferate in culture long term, requiring passaging only every 2 weeks, once differentiated, they are a relatively low-maintenance source of primary-like AEC2s.
Although iAEC2s share many functional characteristics of primary AEC2s, and the top 300 most upregulated genes have the same expression pattern3, they differ in their global gene expression profile, specifically expressing pathways related to innate immune signaling at lower levels than primary AEC2s. Similar to primary human AEC2s, they do not readily transdifferentiate into AEC1 cells in 3D culture26. They proliferate at higher levels than primary AEC2s and, unlike primary cells, they can be passaged even in the absence of mesenchyme. Given these differences, iAEC2s are probably best suited to being used in studies of surfactant processing and function, as well as studies of cell proliferation and monogenetic alveolar diseases requiring precise manipulation of the genome.
Although alveolospheres probably mature further with time in culture, even day 30 alveolospheres express functional lamellar bodies that process SFTPB to its fully mature 8 kDa form, and they express most AEC2-specific genes at levels similar to those of primary lung cells. They secrete surfactant into the luminal portion of alveolospheres, and they can upregulate immune mediators in response to cytokine signaling. Illustrative of their utility for studying patient-specific biology, we were able to analyze surfactant protein expression and processing, as well as lamellar body morphology, in alveolospheres, using them to model a monogenic human lung disease, SFTPB deficiency.
Supplementary Material
Acknowledgements
We wish to thank all members of the Kotton and Hawkins laboratories for helpful discussions and optimization of the presented protocol. We are grateful to B.R. Tilton of the BUSM Flow Cytometry Core, supported by NIH grant 1UL1TR001430, for technical assistance; and G. Miller, A. Iyer, and M. James of the CReM, supported by grants R24HL123828 and U01TR001810. This work was supported by grants TL1TR001410 and F31HL134274 (A.J.), HAWKIN15XX0 and R01HL139799 (F.H.), the I.M. Rosenzweig Junior Investigator Award from the Pulmonary Fibrosis Foundation (K.-D.A.), and grants U01HL099997, U01HL134745, R01HL095993, R01HL122442, R01HL128172, and U01HL134766 (D.N.K.). We thank A. Brivanlou, Rockefeller University, for the generous gift of RUES2, an embryonic stem cell line.
Footnotes
Competing interests
The authors declare no competing interests.
Additional information
Supplementary information is available for this paper at https://doi.org/10.1038/s41596-019-0220-0.
Peer review information Nature Protocols thanks Amy Ryan (Firth) and other anonymous reviewer(s) for their contribution to the peer review of this work.
Reprints and permissions information is available at www.nature.com/reprints.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
This article includes weblinks to publicly available datasets, such as bioinformatics files deposited at the Gene Expression Ombinus (GEO accession number: GSE103918). There are no restrictions on data sharing, and any raw data, cell lines, or protocol downloads are available through the corresponding author by email request or through his website portal at www.kottonlab.com.
References
- 1.Mason RJ & Williams MC Type II alveolar cell. Defender of the alveolus. Am. Rev. Respir. Dis. 115, 81–91 (1977). [DOI] [PubMed] [Google Scholar]
- 2.Whitsett JA, Wert SE & Weaver TE Diseases of pulmonary surfactant homeostasis. Annu. Rev. Pathol. 10, 371–393 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacob A et al. Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell 21, 472–488.e10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serra M et al. Pluripotent stem cell differentiation reveals distinct developmental pathways regulating lung-versus thyroid-lineage specification. Development 144, 3879–3893 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green MD et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat. Biotechnol. 29, 267–272 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang SXL et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 32, 84–91 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longmire TA et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 10, 398–411 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkins F et al. Prospective isolation of NKX2–1-expressing human lung progenitors derived from pluripotent stem cells. J. Clin. Invest. 127, 2277–2294 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurmann AA et al. Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell 17, 527–542 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotoh S et al. Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Rep. 3, 394–403 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCauley KB, Hawkins F & Kotton DN Derivation of epithelial-only airway organoids from human pluripotent stem cells. Curr. Protoc. Stem Cell Biol. 45, e51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCauley KB et al. Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell 20, 844–857.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto Y et al. Long-term expansion of alveolar stem cells derived from human iPS cells in organoids. Nat. Methods 14, 1097–1106 (2017). [DOI] [PubMed] [Google Scholar]
- 14.McCauley KB et al. Single-cell transcriptomic profiling of pluripotent stem cell-derived SCGB3A2+ airway epithelium. Stem Cell Rep. 10, 1579–1595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minoo P, Su G, Drum H, Bringas P & Kimura S Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(−/−) mouse embryos. Dev. Biol. 209, 60–71 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Mucenski ML et al. Beta-catenin regulates differentiation of respiratory epithelial cells in vivo. Am. J. Physiol. Lung Cell Mol. Physiol 289, L971–L979 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Liu Y & Hogan BLM Differential gene expression in the distal tip endoderm of the embryonic mouse lung. Gene Expr. Patterns 2, 229–233 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Gonzales LW, Guttentag SH, Wade KC, Postle AD & Ballard PL Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am. J. Physiol. Lung Cell Mol. Physiol 283, L940–L951 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Frank DB et al. Emergence of a wave of Wnt signaling that regulates lung alveologenesis by controlling epithelial self-renewal and differentiation. Cell Rep. 17, 2312–2325 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang SXL et al. The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat. Protoc. 10, 413–425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y-W et al. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat. Cell Biol. 19, 542–549 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dye BR et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife 4, 1999 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller AJ et al. In vitro induction and in vivo engraftment of lung bud tip progenitor cells derived from human pluripotent stem cells. Stem Cell Rep. 10, 101–119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korogi Y et al. In vitro disease modeling of Hermansky-Pudlak syndrome type 2 using human induced pluripotent stem cell-derived alveolar organoids. Stem Cell Rep. 12, 431–440 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zacharias WJ et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 555, 251–255 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barkauskas CE et al. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 123, 3025–3036 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Amour KA et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534–1541 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Gouon-Evans V et al. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat. Biotechnol. 24, 1402–1411 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Ogawa S et al. Three-dimensional culture and cAMP signaling promote the maturation of human pluripotent stem cell-derived hepatocytes. Development 140, 3285–3296 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christodoulou C et al. Mouse ES and iPS cells can form similar definitive endoderm despite differences in imprinted genes. J. Clin. Invest. 121, 2313–2325 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loh KM et al. Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell Stem Cell 14, 237–252 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.