Significance
Epstein–Barr virus (EBV)-associated diseases represent a major cause of morbidity worldwide, accounting for at least 1.5% of the global cancer burden, and contributing to autoimmunity. Viral reactivation from latency is associated with an increased risk of EBV-driven cancers, and thus may be a target for disease prevention. Anti-EBV agents that are safe, effective, and suitable for continuous long-term treatment are needed to address questions of whether suppression of lytic reactivation can reduce the incidence of EBV-associated diseases. Here we report that tenofovir disoproxil fumarate and tenofovir alafenamide, drugs with excellent safety profiles used for HIV prophylaxis, are potent inhibitors of EBV. This study identifies compounds that enable clinical investigations of antiviral therapy for EBV in efforts toward disease prevention.
Keywords: Epstein–Barr virus, antivirals, tenofovir disoproxil fumarate, tenofovir alafenamide
Abstract
Epstein–Barr virus (EBV) is a ubiquitous human γ-herpesvirus that establishes life-long infection and increases the risk for the development of several cancers and autoimmune diseases. The mechanisms by which chronic EBV infection leads to subsequent disease remain incompletely understood. Lytic reactivation plays a central role in the development of EBV-driven cancers and may contribute to other EBV-associated diseases. Thus, the clinical use of antivirals as suppressive therapy for EBV lytic reactivation may aid efforts aimed at disease prevention. Current antivirals for EBV have shown limited clinical utility due to low potency or high toxicity, leaving open the need for potent antivirals suitable for long-term prophylaxis. In the present study, we show that tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF), drugs with excellent safety profiles used clinically for HIV prevention, inhibit EBV lytic DNA replication, with respective IC50 values of 0.30 μM and 84 nM. In a cell-based assay, TAF was 35- and 24-fold and TDF was 10- and 7-fold more potent than acyclovir and penciclovir, respectively, and TAF was also twice as potent as ganciclovir. The active metabolite of tenofovir prodrugs, tenofovir-diphosphate, inhibited the incorporation of dATP into a primed DNA template by the EBV DNA polymerase in vitro. In contrast to acyclovir, treatment of cells during latency for 24 h with TAF still inhibited EBV lytic DNA replication at 72 h after drug was removed. Our results suggest that tenofovir prodrugs may be particularly effective as inhibitors of EBV lytic reactivation, and that clinical studies to address critical questions about disease prevention are warranted.
Epstein–Barr virus (EBV) is a double-stranded DNA γ-herpesvirus that infects >90% of humans by adulthood, with cell tropism for B cells and epithelial cells (1). While primary EBV infection is typically self-limiting in children, it can become pathogenic later in life. EBV causes infectious mononucleosis, which is a significant cause of morbidity in adolescents and young adults (1). In addition, molecular and epidemiologic studies have established a causal role for EBV in the development of several malignancies, accounting for at least 1.5% of the global cancer burden (2). EBV infection is also strongly linked to autoimmune diseases like multiple sclerosis (3). The mechanisms behind the development of disease even years to decades following primary infection are unclear, however.
Viral lytic reactivation from latency has been implicated in the development of EBV-associated cancers. Animal models of EBV lymphoma or lymphoproliferative disease demonstrate that lytic replication-defective mutants are impaired for tumorigenesis (4, 5). Associations of cancer risk with markers of lytic reactivation are also seen in human disease. Children with increased antibody titers to the viral capsid antigen (VCA) are at increased risk of developing Burkitt’s lymphoma (6). Similarly, antibody titers to VCA are higher in individuals who develop EBV-positive gastric carcinoma compared with controls (7). Elevations in antibodies to lytic and latent antigens precede the development of Hodgkin’s lymphoma by several years and carry a threefold to fourfold relative risk of disease (8). Increased IgA titers to VCA precede the diagnosis of nasopharyngeal cancer by a mean of 3 y, and rising antibody levels confer a 21-fold increased risk (9, 10). Patients at risk for posttransplantation lymphoproliferative disorder (PTLD) have high viral loads (11). Intriguingly, preemptive treatment with inhibitors of herpesvirus lytic DNA replication has been reported to show possible efficacy for reducing the incidence of PTLD (12). These studies suggest that there exists a time window, often spanning several years before the onset of disease, during which continuous suppressive antiviral therapy may aid in disease prevention.
Existing antiviral agents repurposed from the treatment of other herpesviruses are problematic for long-term suppressive therapy for EBV due to either low potency or high toxicity. Acyclovir (ACV) and its prodrug valacyclovir (VACV), inhibitors of herpesviral polymerases, are effective against herpes simplex virus (HSV)-1 and -2. However, the half-maximal inhibitory concentration (IC50) of ACV in vitro is significantly higher for EBV than for HSV (13–15), and ACV has poor selectivity for the EBV DNA polymerase compared with the HSV DNA polymerase (16). VACV is considered safe for long-term continuous treatment as suppressive therapy for HSV (17) but has low potency against EBV. Ganciclovir (GCV) and its orally available prodrug valganciclovir (VGCV) are potent inhibitors of EBV (13). However, these drugs are associated with significant adverse effects, including severe hematologic toxicity, and carry risks of carcinogenesis and mutagenesis (17). GCV and VGCV are clinically effective for the treatment of cytomegalovirus (CMV), but the toxicity of these drugs likely precludes their continuous use over a period of several years as prophylaxis for EBV lytic reactivation.
Like ACV and GCV, tenofovir (TFV) is an acyclic nucleoside/nucleotide analog (18). It is the primary metabolite of the prodrugs tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF) (19), which are clinically licensed for the treatment of HIV infection and hepatitis B infection and HIV prophylaxis, where they act as inhibitors of the viral reverse transcriptase. Both TDF and TAF are orally bioavailable drugs with highly favorable safety profiles (20). A significant difference between ACV and TFV lies in the initial phosphorylation step: ACV requires a viral kinase, while TFV does not, because it is a monophosphorylated nucleotide (18). TDF and TAF contain chemical modifications that mask the negatively charged phosphate group of TFV, allowing the drugs to reach higher intracellular concentrations. In contrast to ACV, TFV prodrugs are metabolized independently of viral enzymes to their active form, tenofovir-diphosphate (TFV-DP) (19). Of the two prodrugs, TAF has better distribution into lymphoid tissues, including lymphocytes, lymph nodes, and spleen (21).
In addition to targeting the HIV reverse transcriptase, TFV-DP can inhibit the HSV-1/2 DNA polymerase (22). However, TFV and TAF are weak inhibitors of HSV-1 with modest activity against HSV-2 in vitro (23) and have not demonstrated clear efficacy in clinical studies for HSV-2 (24). Here we show that prodrugs of TFV—TDF and TAF—are highly potent inhibitors of EBV lytic DNA replication and may be better suited for suppressive therapy for EBV lytic reactivation.
Results
TDF and TAF Inhibit EBV Lytic DNA Replication.
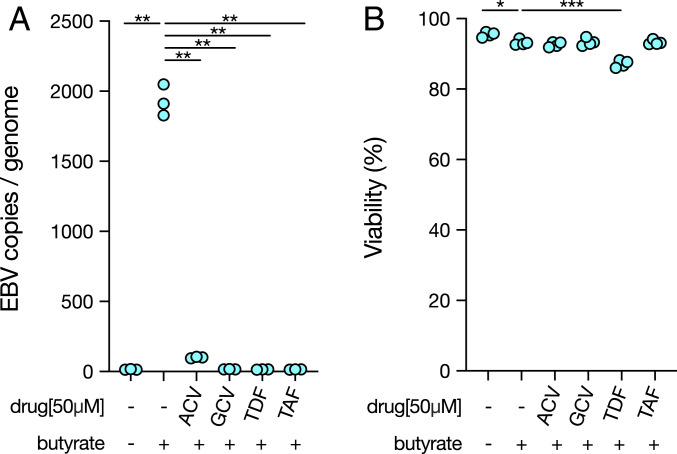
Since compounds that have efficacy against α-herpesviruses (25, 26) can inhibit EBV lytic DNA replication in vitro (13), we asked whether TFV prodrugs can have antiviral activity against EBV. In the EBV+ HH514-16 cell line, a subclone of P3HR-1 cells selected for low spontaneous EBV reactivation (27), we induced lytic EBV DNA replication with sodium butyrate. After 96 h of butyrate treatment, the average number of viral copies per genome was increased by 128-fold (Fig. 1A). We used the nucleoside analogs ACV and GCV, two known inhibitors of EBV lytic DNA replication, as positive controls (13). At a concentration of 50 μM, both ACV and GCV effectively suppressed lytic DNA replication, reducing the number of new viral copies produced by >95.5% and >99.9%, respectively (Fig. 1A). In line with previous studies, GCV was more inhibitory than ACV (13). The TFV prodrugs TDF and TAF also reduced the number of viral copies produced after lytic induction, each by >99.9% (Fig. 1A).
Fig. 1.
TDF and TAF inhibit EBV lytic DNA replication. (A) HH514-16 cells were induced with 3 mM butyrate for 96 h and simultaneously treated with the established anti-herpesvirus drugs ACV and GCV or the TFV prodrugs TDF and TAF. EBV copies per genome were measured by qPCR after 96 h of treatment at a 50 μM dose. Results were analyzed from three independent experiments; each value shown. One-way ANOVA (P < 0.05) was followed by multiple hypothesis testing between butyrate and each condition. Statistical significance is highlighted by P values as follows: **P < 0.01. (B) Viability was measured by trypan blue exclusion after 96 h. Results were analyzed from four independent experiments; each value shown. One-way ANOVA (P < 0.05) was followed by multiple hypothesis testing between butyrate and each condition. Statistical significance is highlighted by P values as follows: *P < 0.05; ***P < 0.001.
EBV DNA replication occurs both during the lytic cycle and during latency but is mediated by distinct mechanisms during each state. In latency, the EBV episome is replicated by the host DNA replication machinery; however, lytic viral DNA replication is mediated by the viral replication machinery (1). To determine whether the reduction in EBV copy number mediated by TDF and TAF was specific to the lytic cycle, we also treated cells with each drug in the absence of butyrate induction. We saw no significant reduction in viral copy number at a 50 μM dose with either drug during latency (SI Appendix, Fig. S1A), supporting roles for TDF and TAF specifically during lytic DNA replication.
We next asked whether the reduction in viral copies could be due to general cytotoxicity of TDF and TAF. While lytic induction with butyrate slightly reduced viability compared with latency, we saw no significant additional reduction in viability during ACV or GCV treatment at a 50 μM dose compared with butyrate alone (Fig. 1B). These drugs’ lack of toxicity may be due in part to the absence of cellular proliferation after butyrate treatment (SI Appendix, Fig. S2). We also observed no reduction in viability with TAF treatment, but there was a decrease in viability with TDF (Fig. 1B). Unlike TAF, TDF is known to be highly unstable in vitro in the presence of serum (23), leading to cytotoxicity. To check whether the toxicity of TDF is specific to cells in the lytic phase, we treated cells in the absence of butyrate (SI Appendix, Fig. S1B). TDF-treated cells had reduced viability, suggesting the toxicity of TDF is independent of lytic induction. Thus, toxicity alone cannot account for the ability of TFV prodrugs to inhibit EBV lytic DNA replication.
TAF Is a Highly Potent Inhibitor of EBV Lytic DNA Replication in Cell-Based Assays.
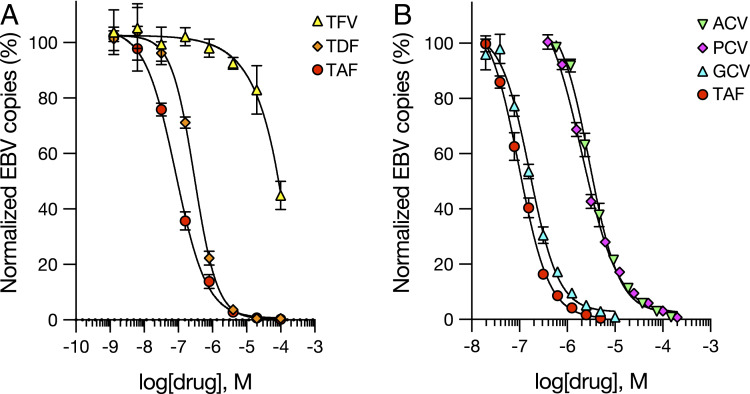
TFV is classified as ineffective against EBV (28), but TDF and TAF have not been previously tested. TFV is also known to have poor intracellular uptake compared with TDF and TAF and is less potent against HIV in vitro, with a 100- and 1,000-fold higher IC50, respectively, compared with the two prodrugs (21). We sought to determine whether HH514-16 cells exhibit differential sensitivity to TFV compared with its prodrugs. In agreement with previously reported results (28), we found TFV to have little effect against EBV DNA replication, with an IC50 of ∼100 μM (Fig. 2A), while TDF was 300-fold more potent than TFV (IC50 = 0.30 μM) and TAF was 1,200-fold more potent than TFV (IC50 = 84 nM) (Fig. 2A).
Fig. 2.
TAF is a highly potent inhibitor of EBV lytic DNA replication in cell-based assays. HH514-16 cells were induced with 3 mM butyrate for 96 h and treated with drugs over a range of concentrations. (A) TFV compared with the TFV prodrugs TDF and TAF. (B) TAF directly compared with the anti-herpesviral drugs ACV, PCV, and GCV. Each point represents the mean and SD obtained from three independent experiments. Dose–response curves (black lines) were generated by four-parameter logistic regression analysis.
To rank drug potency in the context of established inhibitors, we compared TFV prodrugs with standard anti-herpesviral drugs. ACV, GCV, and penciclovir (PCV) are clinically licensed for the treatment of α- and β-herpesviruses. These compounds also have efficacy against EBV in vitro (13, 29). In direct comparison, TAF had a lower IC50 than GCV by 2-fold (0.16 μM), PCV (2.0 μM) by 24-fold, and ACV (2.9 μM) by 35-fold (Fig. 2B). TDF had a 7-fold lower IC50 than PCV and a 10-fold lower IC50 than ACV. Thus, both TDF and TAF were more potent than standard α-herpesviral drugs.
Since TDF and TAF are metabolized by cellular enzymes to their active form, we asked whether these drugs might be selective for viral DNA replication. The cytotoxic concentration required to inhibit total cell viability during latency by 50% (CC50) was 180 μM for TAF and 40 μM for TDF (SI Appendix, Fig. S3), a 2,000-fold and 133-fold difference , respectively, compared with the IC50 for inhibition of EBV lytic DNA replication. Therefore, TFV prodrugs were selective for EBV replication.
TAF Acts Downstream of Induction to Block EBV Lytic DNA Replication.
We first asked whether TAF could block lytic induction. To make direct comparisons between TAF and other anti-herpesviral drugs, we standardized the concentration of each drug to the dose required to inhibit 95% of EBV lytic DNA replication (IC95) at 96 h: 2.5 μM for TAF, 62.5 μM for ACV, and 5 μM for GCV. We examined protein expression of the viral early antigen Ea-D by Western blot analysis after 72 h of drug treatment. Ea-D expression was not affected by TAF treatment, suggesting that TAF was not blocking induction (Fig. 3A). Consistent with this view, when we added TAF late (24 h) after lytic induction and measured viral copy number at 96 h after drug addition, we saw a similar decrease in lytic DNA replication as when the drug was added early (0 h), further suggesting that TAF acts downstream of induction (Fig. 3B).
Fig. 3.
TAF acts downstream of induction to block EBV lytic DNA replication. (A) HH514-16 cells were induced with 3 mM butyrate in the presence of drugs at the IC95. Total protein was collected at 72 h and stained for the early viral antigen Ea-D or β-actin by Western blot. Results are shown from one experiment representative of three independent experiments. (B) HH514-16 cells were induced with 3 mM butyrate in the presence of drugs at the IC95. Drugs were added at either 0 h or 24 h following butyrate addition, and the number of EBV copies per genome was quantified by qPCR. Results were analyzed from three independent experiments; each value shown. One-way ANOVA (P < 0.05) was followed by multiple hypothesis testing between butyrate and each condition at time 0 or 24 h. Statistical significance is highlighted by P values as follows: **P < 0.01.
TAF Inhibits Transcription of Late Lytic Viral Genes.
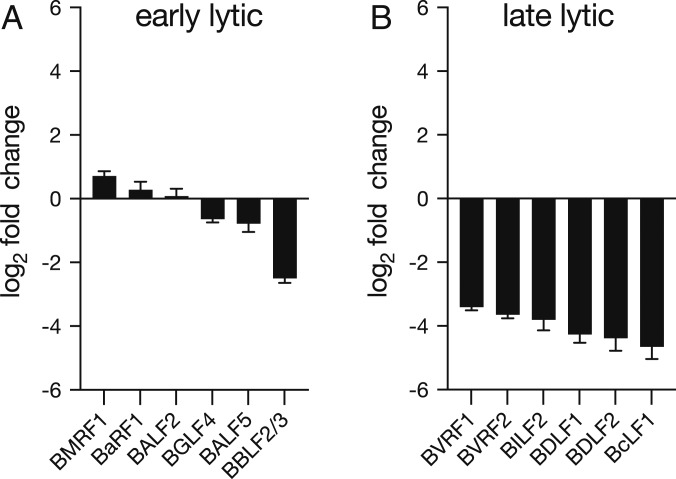
We hypothesized that TAF might be directly blocking viral DNA replication. During γ-herpesviral lytic DNA replication, continuous DNA synthesis is required for the transcription of late lytic viral genes, but not for early lytic genes (30). We measured levels of six late lytic viral transcripts—BVRF1, BVRF2, BILF2, BDLF1, BDLF2, and BcLF1—and six early lytic viral transcripts—BMRF1, BaRF1, BALF2, BGLF4, BALF5, and BBLF2/3 (31)—after 72 h of drug treatment. Expression of all six late lytic viral genes decreased with TAF treatment at IC95 by more than 10-fold (Fig. 4B); however, five of the six early viral transcripts were only minimally altered by TAF treatment (less than twofold change) (Fig. 4A). We obtained the same patterns of gene expression during ACV and GCV treatment (SI Appendix, Fig. S4), suggesting that TAF may act by a similar mechanism.
Fig. 4.
TAF inhibits transcription of late lytic viral genes. HH514-16 cells were induced with 3 mM butyrate and treated with TAF (2.5 μM) or no drug. Total RNA was collected at 72 h and used to measure the expression of six early (A) and six late (B) lytic genes by RT-qPCR. Gene expression was normalized to the housekeeping gene HPRT1. Each column represents the mean and SD obtained from three independent experiments.
TFV-DP Inhibits the EBV DNA Polymerase by Competing with dATP.
Since herpesviral DNA polymerases contain motifs conserved across subfamilies (32), we investigated whether TFV prodrugs can inhibit the EBV DNA polymerase. To do so, we carried out in vitro polymerase assays. The EBV polymerase catalytic subunit (BALF5) and processivity factor (BMRF1) were produced using a transcription/translation system in rabbit reticulocyte lysates. Protein expression was confirmed by gel electrophoresis (Fig. 5A). Polymerase assays were performed by measuring the incorporation of [3H]dNTPs into activated calf thymus DNA. Both BALF5 and BMRF1 were required for viral polymerase activity (Fig. 5B), as reported previously (32).
Fig. 5.
TFV-DP inhibits the EBV DNA polymerase by competing with dATP. (A) The EBV polymerase catalytic subunit (BALF5) and processivity factor (BMRF1) were cloned into the pcDNA3.1+ vector and produced using a transcription/translation system in rabbit reticulocyte lysates. Protein expression was confirmed using a fluorophore-labeled lysine-charged tRNA, followed by gel electrophoresis and imaging. (B) In vitro polymerase assays were performed by measuring the incorporation of 1 μM [3H]dGTP into activated calf thymus DNA during a 40-min reaction, followed by DEAE filter-binding and scintillation counting. Each column represents the mean and SD of cpm obtained from three independent experiments. (C–E) ACV-TP (C) and GCV-TP (D) were added to reactions containing 1 μM [3H]dGTP from 0 to 50 μM. TFV-DP (E) was added to reactions containing 1 μM [3H]dATP from 0 to 50 μM. Every 5 min, aliquots were removed and quenched with EDTA. The cpm values were normalized to maximum counts obtained at 40 min. Results were analyzed from two independent experiments; each value shown. (F) Dose–response curves were generated from mean inhibition of dATP or dGTP incorporation at 40 min by five-parameter logistic regression analysis. (G) Effective doses were calculated from reported concentrations of TFV-DP in PBMCs during directly observed daily therapy with TDF or TAF (*ref. 33). Black lines represent the median and IQR. Effective inhibitory concentrations (red lines) were calculated using the EC50 and Hill slope from the best-fitted line generated for TFV-DP in F.
Measurements of dNTP levels in human primary B cells have not been reported. However, concentrations of dNTP contents of resting human primary T cells range from 0.28 to 0.35 pmol/106 and are significantly lower in other nondividing cell types (e.g., macrophages) (34). Using the mean volume of lymphocytes as 206 fL (35), we calculated the range of mean concentrations of dNTPs in T cells as 1.36 to 1.70 μM. From these values, we estimated near-physiological levels of dNTPs in lymphocytes as 1 μM. In our polymerase assays, we used 1 μM [3H]dATP as the competing nucleotide for TFV-DP and 1 μM [3H]dGTP as the competing nucleotide for ACV-triphosphate (TP)/GCV-TP. Noncompeting unlabeled dNTPs were added to each reaction in excess. Drug-TPs were added at a range of concentrations up to 50 μM (Fig. 5 C–E). Aliquots were removed every 5 min for filter-binding assays. Dose–response curves for each drug were generated from the mean inhibition of DNA replication after 40 min. We observed that GCV-TP was the most potent inhibitor of the EBV DNA polymerase (Fig. 5F). Compared with GCV-TP (IC50 = 0.65 μM), TFV-DP was 5-times less potent (IC50 = 3.4 μM) and ACV-TP was 13-times less potent (IC50 = 8.6 μM) (Fig. 5F).
We then asked whether TDF and TAF at standard clinical doses could reach intracellular concentrations of TFV-DP that are relevant for inhibition of EBV. We used the reported values of TFV-DP concentrations in peripheral blood mononuclear cells (PBMCs) in human subjects following directly observed daily treatment with TDF (median, 71 fmol/106 cells; IQR, 53 to 97 fmol/106 cells) or TAF (median, 685 fmol/106 cells; IQR, 566 to 751 fmol/106 cells) (33), and the reported mean corpuscular volume of PMBCs (282.9 fL) (36). From these values, we calculated that the concentration of intracellular TFV-DP in PBMCs following TAF treatment would reach a concentration needed to block ∼40% of DNA replication (EC40) mediated by the EBV polymerase after 40 min in our in vitro assay (Fig. 5G). Intracellular concentrations of TFV-DP in PBMCs on daily TDF treatment may be less effective (Fig. 5G).
We performed similar assays using the HSV-1 polymerase catalytic subunit UL30 (SI Appendix, Fig. S5). The IC50 of TFV-DP for EBV was 1.3-fold higher than the IC50 of ACV-TP for HSV-1 (IC50 = 2.6 μM), suggesting that TFV-DP for EBV is comparable to ACV-TP for HSV-1 as an inhibitor of the EBV DNA polymerase.
TAF, but Not ACV, Is Effective Even when Treatment Precedes Lytic Induction.
The intracellular metabolism of TAF is well established. In lymphocytes, TAF is first cleaved to TFV by the lysosomal enzyme cathepsin A (CTSA) and subsequently phosphorylated by AMP kinase and nucleotide diphosphate kinases to TFV-DP (37). Since neither HIV nor hepatitis B encodes a viral kinase, the metabolism of TAF is mediated by the host. Conversely, ACV requires a viral enzyme, the EBV protein kinase BGLF4, for antiviral activity (13). The requirement of a virus-encoded kinase is thought to provide more specificity and thus decreased toxicity (38); however, TFV-class drugs bypass the requirement for a viral kinase and yet still show a highly favorable safety profile (20).
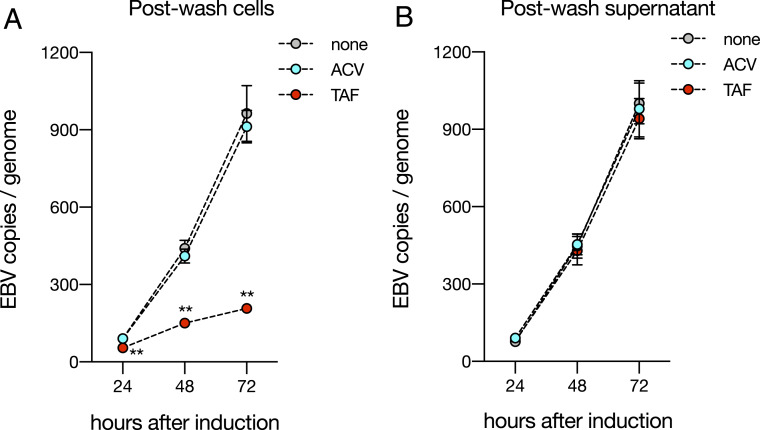
Since TAF is metabolized by CTSA, we hypothesized that it might be possible to pretreat cells before lytic induction. We confirmed protein expression of CTSA during both latency and lytic induction (SI Appendix, Fig. S6). We then added either TAF or ACV at the IC95 for 24 h. Before butyrate induction, drug was washed away until the final supernatant did not inhibit lytic DNA replication in cells unexposed to drug (Fig. 6B). After removing extracellular drug, TAF-treated cells were minimally permissive to lytic viral DNA replication, even after 72 h, but pretreatment of cells with ACV showed no effect (Fig. 6A). This suggests that host metabolism of antiviral drugs confers the advantage of being able to initiate treatment before the lytic cycle.
Fig. 6.
TAF, but not ACV, is effective even when treatment precedes lytic induction. (A) HH514-16 cells were treated with ACV (62.5 μM), TAF (2.5 μM), or no drug for 24 h in the absence of butyrate, after which cells were washed five times with fresh medium without drug. After the final wash, cells were resuspended at a concentration of 4 × 105 cells/mL in medium containing 3 mM butyrate. Genomic DNA was removed for measurements of EBV copy number every 24 h. Each point represents the mean and SD obtained from three independent experiments. Two-way ANOVA (P < 0.05) was followed by multiple hypothesis testing between butyrate and each drug. Statistical significance is highlighted by P values as follows: **P < 0.01. (B) After the final wash, a sample of the butyrate-containing supernatant was removed, filtered, and tested for inhibitory activity in cells previously unexposed to drug. Each point represents the mean and SD obtained from three independent experiments. Two-way ANOVA was performed (P > 0.05).
Discussion
EBV infection is associated with subsequent risk for the development of both cancers and autoimmune diseases (2, 3); however, there exists a delay of years to decades between primary EBV infection and onset of diseases associated with EBV. Subclinical lytic reactivation may contribute to the risk for EBV-associated diseases (6–10), and thus clinical interventions with antiviral agents may be effective in disease prevention. Here we show that the TFV prodrugs TDF and TAF are highly potent inhibitors of EBV lytic DNA replication. In cell-based assays, we demonstrate that TDF and TAF are significantly more potent than ACV and PCV. TAF is also more potent than GCV. Furthermore, we provide strong evidence suggesting that, like standard herpesviral drugs, these compounds target the EBV DNA polymerase.
While standard herpesviral drugs have activity against EBV in vitro, they are limited in their clinical utility by either low potency or high toxicity. ACV is a weak inhibitor of EBV. In cell-based assays, the reported IC50 of ACV for wild-type EBV ranged from 4.1 to 10 μM (13, 14), significantly higher values than seen for clinical isolates of HSV (0.084 to 0.34 μM) (15), and strains of HSV with an IC50 above 8.8 to 13.2 μM have been classified as clinically resistant (39, 40). Previous studies have shown that ACV-TP is a weaker inhibitor of the EBV DNA polymerase compared with the HSV-1 DNA polymerase (16). In line with this, ACV is not effective in animal models using the related pathogen murine γ-herpesvirus-68, where its in vitro IC50 is comparable to that of EBV and where more potent compounds demonstrate the capacity to achieve dramatic effectiveness (41). In a pilot study, VACV demonstrated a modest reduction in the number of symptoms reported and the severity of infectious mononucleosis (42). While VACV can reduce shedding of EBV in saliva (42) and ACV is effective in EBV-driven oral hairy leukoplakia (1), both of these responses reflect processes occurring in epithelial cells. Lymphocytes contain higher dNTP concentrations than other nondividing cells (34), and thus EBV-infected B cells may be intrinsically more resistant to ACV.
GCV and its orally available prodrug VGCV are more potent inhibitors of EBV than ACV (13). However, a major limitation to extended clinical use of these drugs is toxicity. GCV and VGCV carry warning labels for hematologic toxicity, impaired fertility, fetal toxicity, mutagenesis, and carcinogenesis (17). In a recent study examining the tolerability of VGCV for CMV prophylaxis in pediatric kidney transplant recipients for a maximum of 6.5 mo, the rate of severe neutropenia was 30.4% (43). The range of potential adverse events associated with long-term use of VGCV poses a significant challenge for extended clinical treatment.
In contrast, TFV prodrugs are compounds that are both highly potent against EBV (as we demonstrate in this study) and have highly favorable safety profiles. In a 3-y clinical trial evaluating TDF as preexposure prophylaxis (PrEP) for HIV, there were no significant differences in the risk of clinical or laboratory adverse events compared with placebo (44). TAF shows a similar safety profile as TDF (20). To date, more than 200,000 patients have been prescribed PrEP without any reports of serious toxicity (45). As they are both potent and safe, these drugs answer a clinical need for EBV antivirals that can be used for continuous treatment as suppressive therapy for lytic reactivation.
TFV prodrugs are distinguished by host-mediated drug metabolism. This permits treatment during latency, which is relevant for lymphocyte trafficking to a compartment that contains lower drug concentrations, such as blood to cerebrospinal fluid (46). Furthermore, global measurements of intracellular concentrations of TFV-DP in vivo at steady state (47) can be used for dose monitoring and correlation with clinical effects. TDF and TAF also address issues of EBV cell tropism, since TAF is metabolized by CTSA, an enzyme preferentially expressed in lymphoid tissues and PBMCs (21), while TDF reaches higher concentrations of TFV-DP in epithelial tissues (48). Combination antiviral therapy may be warranted.
Patients being treated with TFV prodrugs for PrEP provide an excellent preliminary study cohort for evaluation of EBV virologic parameters, such as viral load and antibody titers, as well as epidemiologic studies to investigate the incidence of EBV-associated diseases. A recent study in HIV-positive patients treated predominantly with TFV-based combination antiretroviral therapy showed a significant decrease (>16-fold) in EBV viral load in PBMCs after 96 wk of treatment (49). As HIV is a confounding variable in this association, HIV-negative PrEP users represent an ideal cohort for establishing the potential effectiveness of TFV prodrugs against EBV in immunocompetent persons.
Overall, our study has evaluated candidate drugs that may serve as effective and safe antiviral agents for EBV. Studies demonstrating an increased risk of cancers and autoimmune diseases after EBV infection pinpoint EBV as a significant contributor to disease. Our results suggest that TDF and TAF are potent inhibitors of EBV lytic reactivation, raising the question as to whether these drugs could reduce the burden of disease linked to EBV.
Methods
The methodology used in this study is described in more detail in SI Appendix.
Lytic Induction and Drug Treatment.
The EBV+ HH514-16 cell line was a kind gift from George Miller’s laboratory at Yale School of Medicine. Cells were seeded at a concentration of 4 × 105 cells/mL in RPMI-10% fetal bovine serum supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), and Amphotericin-B (1 μg/mL). Sodium butyrate (Sigma-Aldrich) was used to induce EBV lytic DNA replication at a concentration of 3 mM. TFV disoproxil fumarate (Sigma-Aldrich), TFV alafenamide (Selleck Chemicals), ACV (Sigma-Aldrich), ganciclovir (Sigma-Aldrich), and penciclovir (Selleck Chemicals) were added at the concentrations and time points indicated in the text. Working solutions of drugs were dissolved in dimethyl sulfoxide (DMSO); for all experiments, the final concentration of DMSO was <0.1% (vol/vol).
Recombinant EBV DNA Polymerase Assays.
The EBV DNA polymerase subunits BMRF1 and BALF5 were separately cloned by PCR from HH514-16 genomic DNA into a pcDNA3.1+ vector. Recombinant proteins were expressed using the Promega TnT T7 Coupled Reticulocyte Lysate System according to the manufacturer’s instructions. Protein expression in reticulocyte lysates was validated using the FluoroTect GreenLys in vitro translation labeling system (Promega), gel electrophoresis, and fluorescence imaging using an Amersham Typhoon 9400 scanner (GE Healthcare Life Sciences). The recombinant polymerase proteins in reticulocyte lysates were then desalted using PD-10 Sephadex-G25 columns to remove any potential interfering salts or nucleotides present in reticulocyte lysates. Polymerase assays were set up as follows in a total volume of 100 μL on ice: 50 mM Tris⋅HCl pH 7.5; 100 mM ammonium sulfate (Sigma-Aldrich); 50 μg/mL BSA (Sigma-Aldrich); 1 mM DTT (Sigma-Aldrich); 3 mM MgCl2 (Sigma-Aldrich); 10 μg/mL activated calf thymus DNA (GE Healthcare Life Sciences); 100 μM nonlimiting dNTPs (New England BioLabs); 1 μM limiting 3H-dNTP (dGTP for ACV-TP/GCV-TP or dATP for TFV-DP) (Moravek); 0 to 50 μM ACV-TP (Moravek), TFV-DP (Moravek), and GCV-TP (TriLink BioTechnologies); and 10 μL of reticulocyte lysate (desalted) containing both recombinant EBV polymerase proteins mixed at a 1:1 ratio. Reactions were incubated at 37 °C for 40 min in a heating block. Aliquots (10 μL) were removed every 5 min, mixed with 5 μL of ethylenediaminetetraacetic acid (EDTA) (200 mM), and incubated on ice to stop the reaction. Reactions (7 μL) were spotted on DEAE anion-exchange filter paper (PerkinElmer) and dried for 10 min. Filters were washed twice with 5% (wt/vol) dibasic sodium phosphate for 5 min and water for 5 min, then rinsed with ethanol. Filters were allowed to dry for 10 min and then added to 10 mL of Ultima Gold Scintillation Mixture (PerkinElmer) and counted on a Beckman LS6000 Scintillation Counter. All values were normalized to maximum counts observed with no drug at 40 min. Dose–response curves for inhibition of DNA replication by the EBV polymerase were fit by a five-parameter logistic regression model using GraphPad Prism 8 software, and EC50 measurements were calculated by the software using best-fit values.
Data Availability Statement.
All relevant data are included in the paper. Requests for data, protocols, or reagents should be directed to N.C.D.
Supplementary Material
Acknowledgments
We thank George Miller for kindly providing the HH514-16 cell line, as well as experimental protocols for cell culture and lytic induction.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2002392117/-/DCSupplemental.
References
- 1.Longnecker R. M., Kieff E., Cohen J. I., “Epstein-Barr virus” in Fields Virology, Knipe D. M., Howley P. M., Eds. (Lippincott Williams & Wilkins, Philadelphia, PA, ed. 6, 2013), pp. 1898–1959. [Google Scholar]
- 2.Farrell P. J., Epstein-Barr virus and cancer. Annu. Rev. Pathol. 14, 29–53 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Ascherio A., Munger K. L., “EBV and autoimmunity” in Epstein-Barr Virus, Münz C., Ed. (Springer International Publishing, Cham, Switzerland, 2015), Vol. 1, pp. 365–385. [Google Scholar]
- 4.Ma S.-D., et al. , A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J. Virol. 85, 165–177 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong G. K., et al. , Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model. J. Virol. 79, 13993–14003 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de-Thé G., et al. , Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt’s lymphoma from a Ugandan prospective study. Nature 274, 756–761 (1978). [DOI] [PubMed] [Google Scholar]
- 7.Imai S., et al. , Gastric carcinoma: Monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc. Natl. Acad. Sci. U.S.A. 91, 9131–9135 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller N., et al. , Hodgkin’s disease and Epstein-Barr virus: Altered antibody pattern before diagnosis. N. Engl. J. Med. 320, 689–695 (1989). [DOI] [PubMed] [Google Scholar]
- 9.Ji M. F., et al. , Sustained elevation of Epstein-Barr virus antibody levels preceding clinical onset of nasopharyngeal carcinoma. Br. J. Cancer 96, 623–630 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao S. M., et al. , Fluctuations of Epstein-Barr virus serological antibodies and risk for nasopharyngeal carcinoma: A prospective screening study with a 20-year follow-up. PLoS One 6, e19100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner H. J., et al. , Patients at risk for development of posttransplant lymphoproliferative disorder: Plasma versus peripheral blood mononuclear cells as material for quantification of Epstein-Barr viral load by using real-time quantitative polymerase chain reaction. Transplantation 72, 1012–1019 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Darenkov I. A., et al. , Reduced incidence of Epstein-Barr virus-associated posttransplant lymphoproliferative disorder using preemptive antiviral therapy. Transplantation 64, 848–852 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Meng Q., et al. , The Epstein-Barr virus (EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase (EBV-TK), is required for ganciclovir and acyclovir inhibition of lytic viral production. J. Virol. 84, 4534–4542 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zacny V. L., Gershburg E., Davis M. G., Biron K. K., Pagano J. S., Inhibition of Epstein-Barr virus replication by a benzimidazole L-riboside: Novel antiviral mechanism of 5, 6-dichloro-2-(isopropylamino)-1-beta-L-ribofuranosyl-1H-benzimidazole. J. Virol. 73, 7271–7277 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins P., Appleyard G., Oliver N. M., Sensitivity of herpes virus isolates from acyclovir clinical trials. Am. J. Med. 73, 380–382 (1982). [DOI] [PubMed] [Google Scholar]
- 16.Allaudeen H. S., Descamps J., Sehgal R. K., Mode of action of acyclovir triphosphate on herpesviral and cellular DNA polymerases. Antiviral Res. 2, 123–133 (1982). [DOI] [PubMed] [Google Scholar]
- 17.Drugs for non-HIV viral infections. Treat. Guidel. Med. Lett. 5, 59–70 (2007). [PubMed] [Google Scholar]
- 18.De Clercq E., Holý A., Acyclic nucleoside phosphonates: A key class of antiviral drugs. Nat. Rev. Drug Discov. 4, 928–940 (2005). [DOI] [PubMed] [Google Scholar]
- 19.De Clercq E., Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF). Biochem. Pharmacol. 119, 1–7 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Gallant J. E., et al. , Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: A randomised, double-blind, active-controlled phase 3 trial. Lancet HIV 3, e158–e165 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Lee W. A., et al. , Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse-transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 49, 1898–1906 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrei G., et al. , Topical tenofovir, a microbicide effective against HIV, inhibits herpes simplex virus-2 replication. Cell Host Microbe 10, 379–389 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callebaut C., Stepan G., Tian Y., Miller M. D., In vitro virology profile of tenofovir alafenamide, a novel oral prodrug of tenofovir with improved antiviral activity compared to that of tenofovir disoproxil fumarate. Antimicrob. Agents Chemother. 59, 5909–5916 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celum C., et al. ; ACTG PEARLS/A5175 Team , Herpes simplex virus type 2 acquisition among HIV-1-Infected adults treated with tenofovir disoproxyl fumarate as part of combination antiretroviral therapy: Results from the ACTG A5175 PEARLS study. J. Infect. Dis. 215, 907–910 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaeffer H. J., et al. , 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature 272, 583–585 (1978). [DOI] [PubMed] [Google Scholar]
- 26.Elion G. B., et al. , Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc. Natl. Acad. Sci. U.S.A. 74, 5716–5720 (1977). [DOI] [PubMed] [Google Scholar]
- 27.Rabson M., Heston L., Miller G., Identification of a rare Epstein-Barr virus variant that enhances early antigen expression in Raji cells. Proc. Natl. Acad. Sci. U.S.A. 80, 2762–2766 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Clercq E., Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections. Clin. Microbiol. Rev. 16, 569–596 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bacon T. H., Boyd M. R., Activity of penciclovir against Epstein-Barr virus. Antimicrob. Agents Chemother. 39, 1599–1602 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li D., Fu W., Swaminathan S., Continuous DNA replication is required for late gene transcription and maintenance of replication compartments in gammaherpesviruses. PLoS Pathog. 14, e1007070 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young L. S., Arrand J. R., Murray P. G., “EBV gene expression and regulation” in Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis, Arvin A., Ed. et al. (Cambridge University Press, 2007). [PubMed] [Google Scholar]
- 32.Narita Y., et al. , A herpesvirus-specific motif of Epstein-Barr virus DNA polymerase is required for the efficient lytic genome synthesis. Sci. Rep. 5, 11767 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yager J., et al. , “Tenofovir-diphosphate in PBMC following increasing TAF vs. TDF dosing under directly observed therapy” in Reviews in Antiviral Therapy and Infectious Diseases: 20th International Workshop on Clinical Pharmacology of HIV Hepatitis & Other Antiviral Drugs, (Virology Education, Noordwijk, The Netherlands, 2019). [Google Scholar]
- 34.Diamond T. L., et al. , Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J. Biol. Chem. 279, 51545–51553 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuse R., Schuster S., Schübbe H., Dix S., Hausmann K., Blood lymphocyte volumes and diameters in patients with chronic lymphocytic leukemia and normal controls. Blut 50, 243–248 (1985). [DOI] [PubMed] [Google Scholar]
- 36.Simiele M., et al. , Evaluation of the mean corpuscular volume of peripheral blood mononuclear cells of HIV patients by a coulter counter to determine intracellular drug concentrations. Antimicrob. Agents Chemother. 55, 2976–2978 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birkus G., et al. , Intracellular activation of tenofovir alafenamide and the effect of viral and host protease inhibitors. Antimicrob. Agents Chemother. 60, 316–322 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elion G. B., Mechanism of action and selectivity of acyclovir. Am. J. Med. 73, 7–13 (1982). [DOI] [PubMed] [Google Scholar]
- 39.Collins P., Ellis M. N., Sensitivity monitoring of clinical isolates of herpes simplex virus to acyclovir. J. Med. Virol. 1 (suppl. 1), 58–66 (1993). [DOI] [PubMed] [Google Scholar]
- 40.Bacon T. H., Levin M. J., Leary J. J., Sarisky R. T., Sutton D., Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin. Microbiol. Rev. 16, 114–128 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neyts J., De Clercq E., In vitro and in vivo inhibition of murine gamma herpesvirus 68 replication by selected antiviral agents. Antimicrob. Agents Chemother. 42, 170–172 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balfour H. H., Jr, et al. , A virologic pilot study of valacyclovir in infectious mononucleosis. J. Clin. Virol. 39, 16–21 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Varela-Fascinetto G., et al. , Tolerability of up to 200 days of prophylaxis with valganciclovir oral solution and/or film-coated tablets in pediatric kidney transplant recipients at risk of cytomegalovirus disease. Pediatr. Transplant. 21, e12833 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Baeten J. M., et al. ; Partners PrEP Study Team , Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 367, 399–410 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krakower D. S., Daskalakis D. C., Feinberg J., Marcus J. L., Tenofovir alafenamide for HIV preexposure prophylaxis: What can we DISCOVER about its true value? Ann. Intern. Med. 172, 281 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lycke J., Malmeström C., Ståhle L., Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob. Agents Chemother. 47, 2438–2441 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawkins T., et al. , Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J. Acquir. Immune Defic. Syndr. 39, 406–411 (2005). [DOI] [PubMed] [Google Scholar]
- 48.US Food and Drug Administration , FDA briefing document: Meeting of the Antimicrobial Drugs Advisory Committee, August 7, 2019. www.fda.gov/media/129607/download. Accessed 6 March 2020.
- 49.Petrara M. R., et al. , Impact of monotherapy on HIV-1 reservoir, immune activation, and co-infection with Epstein-Barr virus. PLoS One 12, e0185128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are included in the paper. Requests for data, protocols, or reagents should be directed to N.C.D.