Significance
The exploitation of induced plant defenses for pest control is a promising strategy to reduce the use of pesticides in agriculture. We describe how a newly identified plant strengthener, 4-fluorophenoxyacetic acid (4-FPA), enhances the resistance of rice and other major cereals to sap-sucking insects by triggering the formation of flavonoid polymers in plant cells. We demonstrate that the application of 4-FPA suppresses the population densities of the white-backed planthopper and thereby increases rice yield in the field. This study reveals a mechanism by which plants resist piercing and sucking herbivores and opens avenues for the design of a sustainable generation of plant strengtheners to control insect pests in agriculture.
Keywords: 4-fluorophenoxyacetic acid, phenolic polymer, rice planthopper, chemical elicitor, induced plant defense
Abstract
Synthetic chemical elicitors, so called plant strengtheners, can protect plants from pests and pathogens. Most plant strengtheners act by modifying defense signaling pathways, and little is known about other mechanisms by which they may increase plant resistance. Moreover, whether plant strengtheners that enhance insect resistance actually enhance crop yields is often unclear. Here, we uncover how a mechanism by which 4-fluorophenoxyacetic acid (4-FPA) protects cereals from piercing-sucking insects and thereby increases rice yield in the field. Four-FPA does not stimulate hormonal signaling, but modulates the production of peroxidases, H2O2, and flavonoids and directly triggers the formation of flavonoid polymers. The increased deposition of phenolic polymers in rice parenchyma cells of 4-FPA-treated plants is associated with a decreased capacity of the white-backed planthopper (WBPH) Sogatella furcifera to reach the plant phloem. We demonstrate that application of 4-PFA in the field enhances rice yield by reducing the abundance of, and damage caused by, insect pests. We demonstrate that 4-FPA also increases the resistance of other major cereals such as wheat and barley to piercing-sucking insect pests. This study unravels a mode of action by which plant strengtheners can suppress herbivores and increase crop yield. We postulate that this represents a conserved defense mechanism of plants against piercing-sucking insect pests, at least in cereals.
Plants respond to herbivore attack through the production of specific defense responses via the recognition of damage- and herbivore-associated molecular patterns (1–4). These induced defense responses are regulated by multiple signaling pathways, including those mediated by jasmonic acid (JA), salicylic acid (SA), ethylene (ET), and H2O2. In addition, plant growth-related phytohormones, such as gibberellins, brassinosteroids, auxins, and cytokinins are also involved in defense responses against herbivores (1, 5, 6). Cross-talk between these signaling pathways causes large changes in the transcriptomes, proteomes, and metabolomes, which often reduces plant growth, but enhances resistance to herbivores by reducing herbivore feeding and attracting herbivore natural enemies (1–7).
Insect pests cause considerable costs and yield losses globally. Currently synthetic insecticides are used extensively to control these insect pests but often with undesirable effects on both the environment and nontarget organisms, including humans themselves. Therefore, alternative environment-friendly measures for insect pest management are urgently required. Boosting plant immunity through the application of chemical elicitors that induce plant defenses but themselves have no harmful effects on herbivores provides an attractive, and potentially viable, approach (8, 9). To date, many chemical elicitors that enhance the resistance of plants to pathogens have been identified and several, such as benzo (1–3) thiadiazole-7-carbothioic acid S-methyl ester (BTH), acibenzolar-S-methyl and β-aminobutyric acid, are in commercial use (9, 10). Similarly, chemical elicitors that enhance the resistance of plants to insect herbivores have been characterized, including synthetic jasmonates and jasmonate homologs, auxin homologs such as 2,4-dichlorophenoxyacetic acid (2,4-D), silicon, selenium, BTH, laminarin, and plant volatiles such as green leaf volatiles and indole (7, 8, 11–14). A few of these chemical elicitors, such as JA and cis-jasmone, have also been reported to reduce herbivore damage in the field (15–17). Most of the tested elicitors of insect resistance act by boosting the canonical defense signaling pathways (8–11, 13–17). A drawback of the activation of these pathways is that they can reduce plant growth through hormonal cross-talk and, in some cases, enhance the susceptibility of plants to nontarget insects (5, 18, 19). Furthermore, none of these elicitors provide complete protection against herbivores, and many of them, such as the jasmonates, are expensive to produce. These factors may explain why yield benefits of chemical elicitors targeting insect pests have rarely been demonstrated, and why none of them have been commercialized so far.
Rice, one of the most important staple crops worldwide, suffers from many insect pests, including rice planthoppers such as brown planthopper (BPH) Nilaparvata lugens (Stål), white-backed planthopper (WBPH) Sogatella furcifera (Horvath), and small brown planthopper (SBPH) Laodelphax striatellus (Fallén) (20). Rice planthoppers can damage plants directly by feeding on phloem sap and ovipositing in plant tissues; heavy infestation can cause the complete drying and wilting of plants known as “hopperburn.” These three planthoppers can also cause plant damage indirectly by transmitting viral diseases. These three planthopper species cause yield reductions of up to 40% if not controlled (21). Previous studies have revealed that rice plants can recognize signals derived from herbivores and then activate corresponding signaling pathways mediated by signaling molecules such as JA, JA-Ile, SA, H2O2, and ET; these pathways, in turn, lead to the production of specific defense responses, including the release of herbivore-induced volatiles and the accumulation of trypsin proteinase inhibitors (TPIs) (13, 22, 23). JA- and ET-mediated pathways have been reported to positively regulate the resistance of rice to chewing insects such as the striped stem borer Chilo suppressalis and the leaf folder Cnaphalocrocis medinalis, but negatively regulate resistance to piercing and sucking insects such as BPH (22, 23). In contrast, the H2O2-mediated pathway positively modulates the resistance of rice to BPH (24). We have recently identified five candidate chemical elicitors from a collection of 29 diversity-oriented molecules using a specific high-throughput screening system for chemical elicitors (25). One of these candidate chemical elicitors is 4-fluorophenoxyacetic acid (4-FPA), a chemical analog of the herbicide 2,4-D, which can induce rice plants to repel WBPH nymph feeding (25).
In this study we elucidate the mechanisms underlying 4-FPA-induced rice defense responses against piercing and sucking insect pests, by combining molecular biology and chemistry, and laboratory and field trials. We demonstrate that 4-FPA does not induce canonical insect resistance pathways, but directly triggers the formation of flavonoid polymers in parenchyma cells of cereals. This effect is associated with enhanced resistance to planthoppers and increased rice yield in planthopper-infested fields. This plant strengthener therefore represents a potentially viable class of defense enhancers to control devastating rice pests and improve rice production while avoiding negative effects of hormonal reprogramming. Moreover, our findings open pathways for the design of plant strengtheners, which effectively exploit endogenous defense mechanisms for the control of insect pests, thus benefitting crop production.
Results
Treatment with 4-FPA Reduces Performance of WBPH.
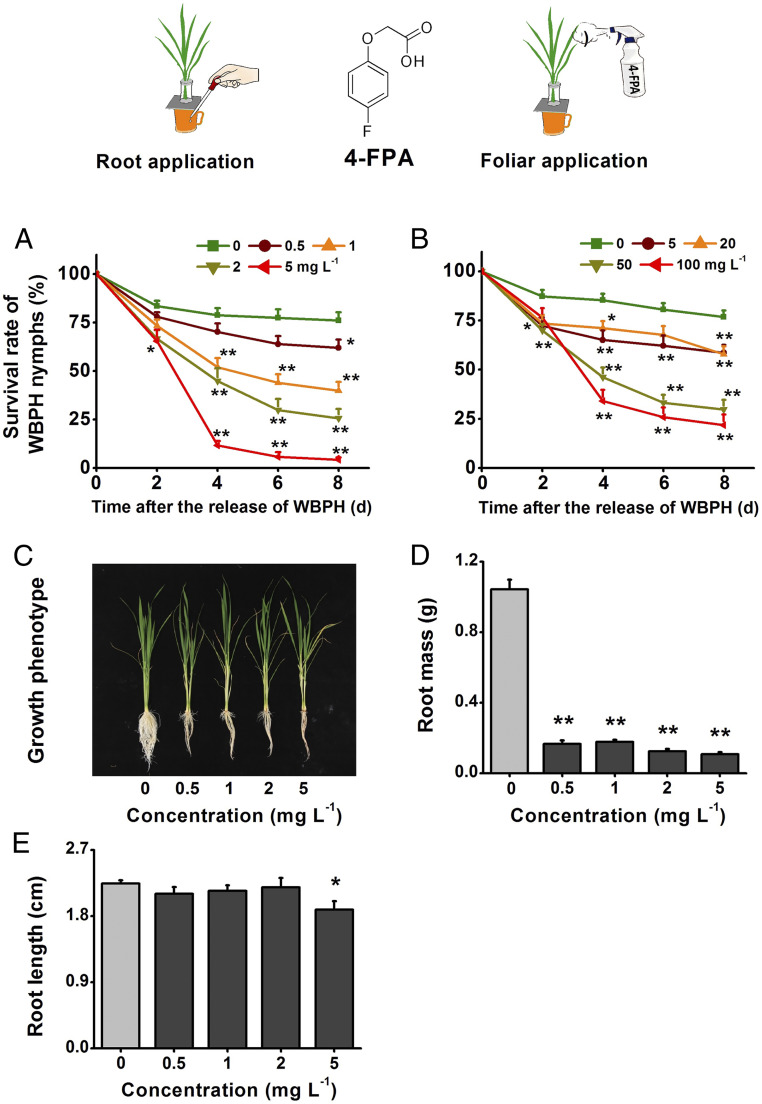
A chemical screen indicated that 4-FPA may increase WBPH resistance in rice (25). To explore the potential of 4-FPA as a chemical elicitor of insect resistance, we conducted a series of experiments using hydroponically grown 39- to 40-d-old rice plants; the 4-FPA was applied either by spraying at a range of concentrations (5 to 100 mg L−1 4-FPA) or delivered at concentrations ranging from 0.5 to 5 mg L−1 via the nutrient solution. Exogenous application of 4-FPA to rice plants reduced the survival rate of WBPH nymphs in a dose-dependent manner, irrespective of the method of application. At all concentrations tested, nymph survival on 4-FPA plants was significantly reduced compared to their respective controls, with the greatest increase in mortality rates occurring 3 to 4 d post 4-FPA treatment (Fig. 1 A and B). Moreover, nymph mortality was greatest when the roots were treated with 4-FPA (4-FPA-R) as opposed to foliar spraying (4-FPA-S). We found no evidence of contact or ingested toxicity of 4-FPA on WBPH nymphs (SI Appendix, Fig. S1), suggesting that the observed effects of 4-FPA on the survival rate is due to changes in rice physiology rather than the chemical per se.
Fig. 1.
Four-FPA inhibits root growth and reduces the survival rate of WBPH nymphs. (A and B) Mean survival rate (+SE, n = 8) of 15 newly hatched WBPH nymphs fed on plants that had been grown in nutrient solution with 0 to 5 mg L−1 4-FPA for 12 h (A); or that had been sprayed with 4 mL of 0 to 100 mg L−1 4-FPA for 12 h (B); 1 to 8 d after exposure. (C) Growth phenotypes of plants grown in nutrient solution containing 0 to 5 mg L−1 4-FPA for 10 d. (D and E) Mean root mass (+SE, n = 15) (D); and root length (+SE, n = 15) (E) of plants grown in nutrient solution containing 0 to 5 mg L−1 4-FPA for 10 d. Asterisks indicate significant differences between treatments and controls (*P < 0.05, **P < 0.01, Duncan’s multiple range test). Upper indicates treatment methods of 4-FPA and its structure.
Exogenous application of 4-FPA also inhibited root growth of rice (Fig. 1C). Ten days after growing in nutrient solution containing 4-FPA, plants showed significantly lower root mass compared to control plants, with the effects being dose dependent (0.5 to 5 mg L−1) (Fig. 1D). However, the length of the roots only decreased when plants were grown in nutrient solution containing the highest level of 4-FPA (Fig. 1E), and although statistically significant the difference was small. In contrast, none of the concentrations of 4-FPA tested had any significant effects on the mass of the vegetative tissues (above-ground parts) or on plant height (SI Appendix, Fig. S2).
Four-FPA Is Converted into 4-Fluorophenol in Plants.
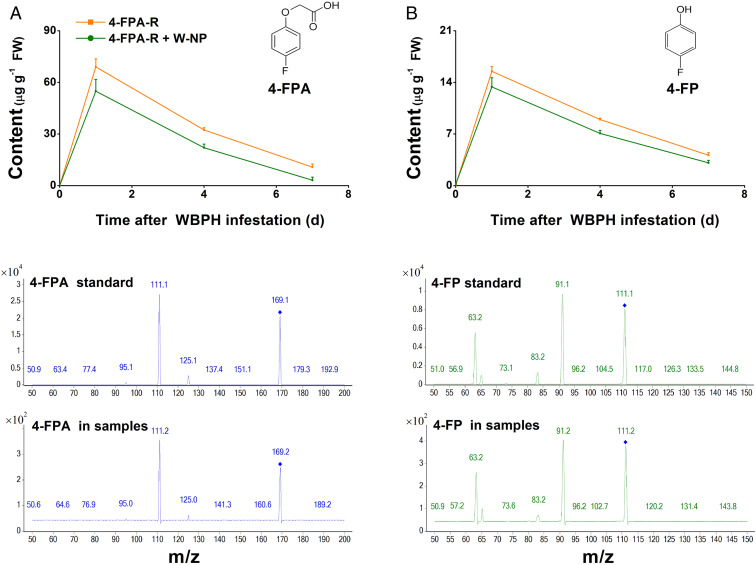
To understand the mechanism underlying 4-FPA-mediated resistance of rice to WBPH nymphs, we first investigated the metabolism of 4-FPA in plants after plant roots were exposed to 4-FPA. Four-FPA was absorbed by the roots and then transported to leaf sheaths (Fig. 2). During the sampling period, the level of 4-FPA in plant leaf sheaths was highest (approximately 69.0 μg g−1 fresh weight) 1 d posttreatment, decreasing to approximately 15.5% by day 7 (Fig. 2A). Moreover, 4-FPA was immediately converted into its metabolite, 4-fluorophenol (4-FP), within the plant, with similar metabolic dynamics as 4-FPA (Fig. 2B and SI Appendix, Fig. S3); however, 4-FP accumulated at lower concentrations in plants, being only approximately 22.5% 1 d postexposure compared to 4-FPA accumulation in leaf sheaths (Fig. 2B). WBPH infestation did not influence the absorption, transport, or conversion of 4-FPA by the plants (Fig. 2 A and B).
Fig. 2.
Four-FPA can be absorbed by rice plants and is immediately converted into 4-FP. (Top) Mean levels (+SE, n = 5) of 4-FPA (A) and 4-FP (B) in plant leaf sheaths at 0, 1, 4, and 7 d after growing in nutrient solution containing 5 mg L−1 4-FPA (4-FPA-R) or 5 mg L−1 4-FPA and simultaneously infested with 20 third instar WBPH (4-FPA-R+NP). (Middle) Mass spectra of 4-FPA (Left) and 4-FP (Right) standards. (Lower) Mass spectra of 4-FPA (Left) and 4-FP (Right) in samples.
Four-FPA Treatment Alters Levels of H2O2 and Flavonoids.
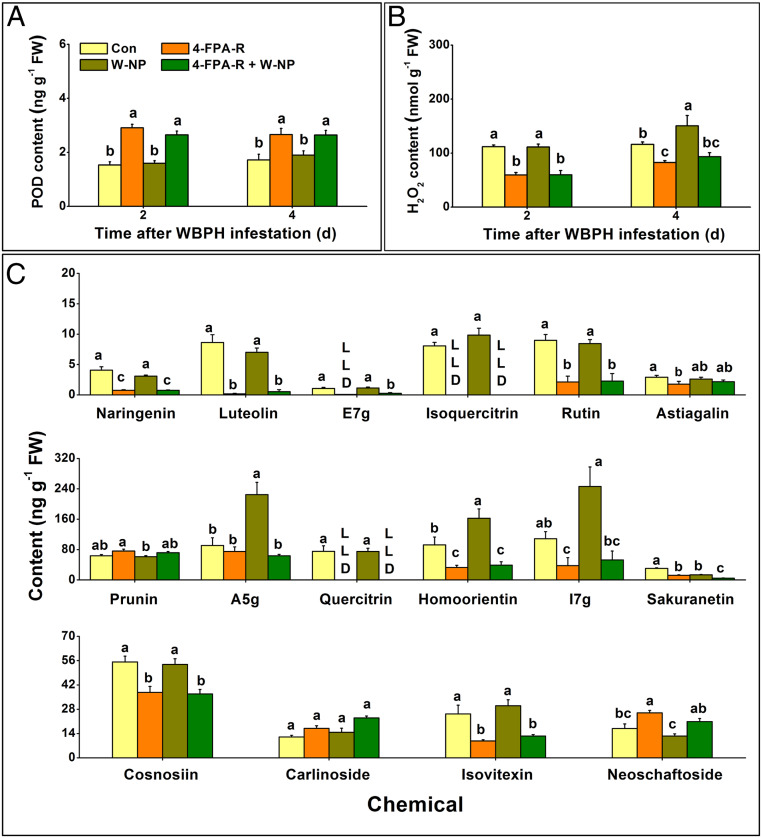
Since JA, JA-Ile, SA, and ET play important roles in the defense response in plants, including rice (22, 23), we investigated the effects of 4-FPA treatment on levels of these defense-related signals in rice. Contrary to our initial expectation, 4-FPA treatment did not influence basal and WBPH nymph-induced levels of JA, JA-Ile, ethylene, and SA (SI Appendix, Fig. S4). To put forward alternative hypotheses regarding the mode of action of 4-FPA, we analyzed transcriptional changes in plants following treatment with 4-FPA. Differentially expressed genes (DEGs) were identified based on the criteria of absolute values of log2 (fold change) ≥1 and the P value ≤0.05. In total, we observed 1,133 DEGs, of which 811 were up-regulated and 322 down-regulated, post 4-FPA treatment. To validate gene expression data, we compared the expression profiles of treated and nontreated plants using qRT-PCR. Of the 16 randomly selected genes, all showed concordant fold differences between the types of analyses (SI Appendix, Table S1), indicating that our results were reliable. Genes related to the following three pathways were most strongly up-regulated by 4-FPA: the auxin signaling pathway, the H2O2 generation and scavenging system, and the phenylpropanoid pathway (SI Appendix, Table S2–S4). We thus investigated levels of H2O2, peroxidases (PODs) that scavenge H2O2, phenolic acids, flavonoids, and lignin in response to both 4-FPA and WBPH nymph infestation. Compared to control plants, levels of PODs increased in both 4-FPA-treated and 4-FPA-treated nymph-infested plants (Fig. 3A) while the levels of H2O2 decreased in these same treatments at 2 and 4 d, but did not change at 0.5 to 1 h post WBPH infestation (Fig. 3B and SI Appendix, Fig. S5). Interestingly, in contrast to the transcriptomic data, the levels of most flavonoids (12 of the detected 16 compounds) were reduced in both 4-FPA-treated and 4-FPA+nymph-infested plants compared to their corresponding control plants (Fig. 3C). Four-FPA treatment did not influence levels of phenolic acids or lignin in the rice plants (SI Appendix, Fig. S6).
Fig. 3.
Exogenous application of 4-FPA influences levels of PODs, H2O2, and flavonoids in rice. Mean levels (+SE, n = 5 to 6) of PODs (A); H2O2 (B); and flavonoids (C) in plants at 2 and/or 4 d post the following treatments: W-NP, 4-FPA-R, 4-FPA-R+W-NP, and control (Con). Details of treatments are described in Methods. LLD, lower limit of detection; e7g, eriodictyol 7-O-glucoside; a5g, apigenin 5-O-glucoside; l7g, luteolin 7-O-glucoside. Letters indicate significant differences among treatments (P < 0.05; Duncan’s multiple range test).
Four-FPA Induces the Deposition of Phenolic Polymers.
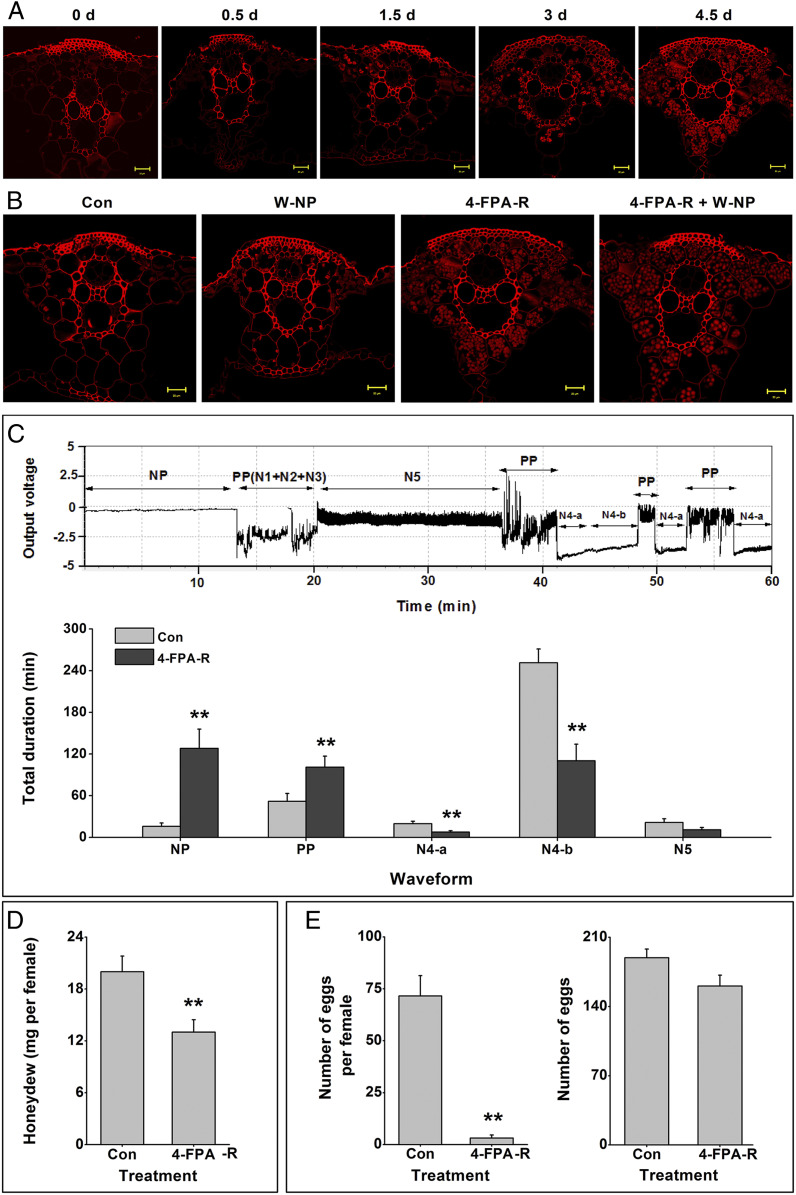
PODs can consume H2O2 to produce phenolic polymers from flavonoids (26). As we observed an increase in POD levels together with a decrease in H2O2 and a decrease in flavonoids in 4-FPA-treated plants (Fig. 3), we hypothesized that 4-FPA treatment may induce the formation of phenolic polymers. Confocal fluorescence microscopy revealed the presence of phenolic polymer deposition in parenchyma cells of leaf sheaths 1.5 d post 4-FPA treatment, with high levels of deposition in the parenchyma 4.5 d posttreatment (Fig. 4A). WBPH nymph infestation did not influence this pattern (Fig. 4B). In vitro experiments were carried out to elucidate the potential involvement of 4-FP, flavonoids, H2O2, and PODs in the formation of the polymers. Two flavonoids, naringenin and quercitrin, were selected as representative flavonoids, as their free form was most strongly suppressed in 4-FPA-treated plants. In the presence of H2O2 and POD, neither the addition of naringenin nor quercitrin resulted in particle deposition; however, when 4-FP was present, the presence of both flavonoids resulted in substantial particle formation (SI Appendix, Fig. S7). Four-FP together with H2O2 and POD also formed particles, but to a much smaller extent than when added together with the flavonoids (SI Appendix, Fig. S7). When H2O2 or POD was absent, no deposition was observed. These data indicate that the polymers found in parenchyma cells of 4-FPA-treated plants are the result of flavonoid polymerization that is triggered by 4-FP and involves H2O2 and PODs.
Fig. 4.
Four-FPA induces deposition of phenolic polymers in parenchyma cells of rice and impairs the feeding capacity of WBPH female adults. (A) Fluorescence images of cross-sections of leaf sheaths of rice plants at 0 to 4.5 d after growing in nutrient solution containing 5 mg L−1. (B) Fluorescence images of cross-sections of leaf sheaths of rice plants at 4.5 d after receiving the following treatments: W-NP, 4-FPA-R, 4-FPA-R+W-NP, and control (Con). Details of treatments are described in Methods. Sections were stained with safranine and fast green. High red fluorescence intensity corresponds to high phenolic polymer content. Yellow arrows indicate polymer particles. (Scale bars, 20 µm.) (C) Typical image of EPG waveforms generated by WBPH female adults showing the feeding behavior (Upper) and mean duration (+SE, n = 15 to 20) of the different feeding phases on control (Con) or 4-FPA-R (grown in nutrient solution containing 5 mg L−1 4-FPA for 4.5 d) rice plants (Lower). NP, nonpenetration; PP, pathway phase (N1 + N2 + N3), which is described in detail in Methods; N4-a, intracellular activity in the phloem region; N4-b, phloem sap ingestion; N5, xylem phase. EPGs were recorded for 6 h per insect. (D) Mean weight (+SE, n = 20) of honeydew excreted by a WBPH female adult feeding on control (Con) or 4-FPA-R (for 4.5 d) rice plants. (E) Mean number (+SE, n = 20) of eggs laid by a newly emerged female adult (Left) for 8 d or 15 gravid female adults (Right) for 12 h on control or 4-FPA-R (for 4.5 d) rice plants. Asterisks indicate significant differences between treatments and controls (*P < 0.05, **P < 0.01; Student’s t tests).
Four-FPA Inhibits Penetration and Feeding by Planthoppers.
WBPH is a piercing and sucking herbivore abstracting phloem sap from the leaf sheaths and main leaf veins, while vectoring plant diseases. We hypothesize that the deposition of 4-FPA-induced phenolic polymers will inhibit WBPH stylet penetration into the phloem cells and thus interfere with feeding. To test this hypothesis, we first used an electrical penetration graph (EPG) to record the feeding behavior of newly emerged WBPH females on rice plants that had been grown in nutrient solution containing 4-FPA (at 5 mg mL−1) for 4.5 d, the time taken for phenolic polymers to form (Fig. 4A). We were able to distinguish five main phases of WBPH feeding (Fig. 4C, Upper). Four-FPA treatment significantly increased the time spent by gravid females in the nonpenetration (NP) and the pathway phase (PP), but significantly decreased time spent in phloem intracellular activity (N4-a), the ingestion phase (N4-b) and the xylem phase (N5) (Fig. 4C, Lower), suggesting reduced feeding activity. In support of this interpretation, 4-FPA treatment consistently and significantly reduced the amount of honeydew excreted by the females, again indicative of reduced food intake (Fig. 4D). These data support our hypothesis that 4-FPA treatment reduces the capacity of WBPH to reach the phloem and thus reduces food intake, and, by consequence, WBPH survival and fecundity.
To investigate whether 4-FPA-induced deposition of phenolic polymers also interferes with oviposition, egg deposition was investigated. While egg deposition by newly emerged females over a period of 8 d was significantly lower on 4-FPA-treated (5 mg mL−1 for 4.5 d) compared to control plants (Fig. 4E, Left), there was no difference in the number of eggs laid by WBPH gravid females between the treated and control plants (Fig. 4E, Right) over the first 12 h. This finding suggests that the decrease in the number of eggs laid over the 8-d period on 4-FPA-treated plants is primarily due to reduced food intake by WBPH female adults compared to those on control plants and not due to difficulties in inserting the ovipositor into plant tissues.
Four-FPA Enhances Plant Resistance to Other Sucking Insects.
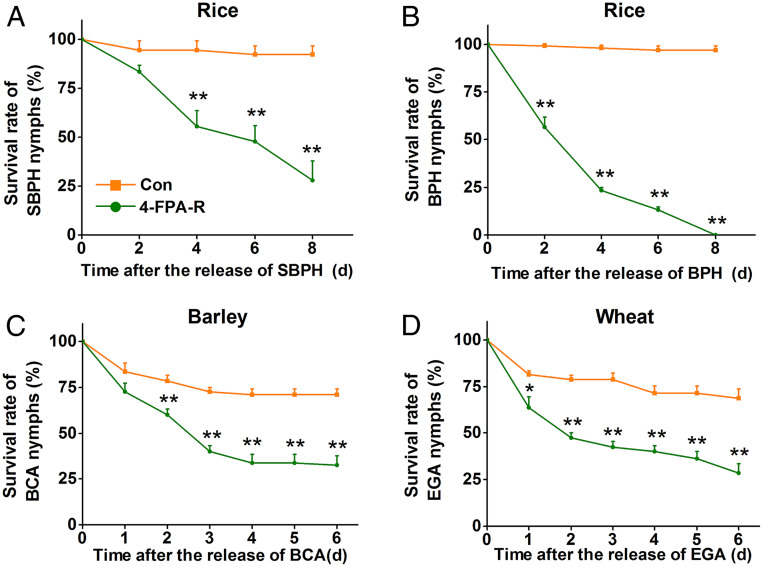
To investigate whether 4-FPA is also effective in other cereals and against other piercing-sucking insect pests, we investigated its effect on resistance of rice, barley, and wheat to BPH, SBPH, the English grain aphid (EGA; Sitobion avenae) and the bird cherry-oat aphid (BCA; Rhopalosiphum padi). Similar to WBPH nymphs, survival of all selected herbivore species on the different crop plants was significantly reduced (Fig. 5), suggesting that 4-FPA provides broad-spectrum resistance in cereals.
Fig. 5.
Exogenous application of 4-FPA reduces survival rates of other piercing and sucking herbivores. Mean survival rate (+SE, n = 8) of 15 newly hatched nymphs of small brown planthopper (SBPH, A), brown planthopper (BPH, B), bird cherry-oat aphid (BCA, C), and English grain aphid (EGA, D) fed on rice, barley, or wheat plants grown in nutrient solution with 5 mg L−1 4-FPA for 12 h, 1 to 8 d after exposure compared to their respective controls. Asterisks indicate significant differences between treatments and controls (*P < 0.05, **P < 0.01, Student’s t tests).
Four-FPA Reduces the Density of WBPH Populations in the Field.
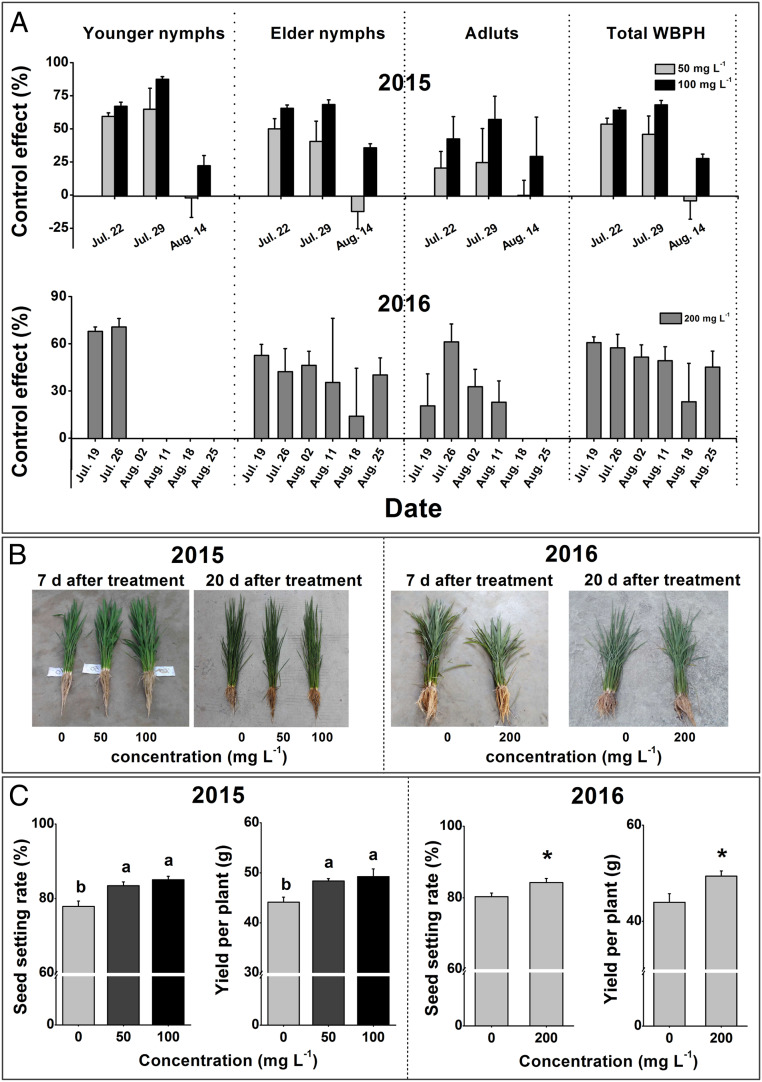
To determine the potential of 4-FPA to protect crops against herbivore pests, a series of glasshouse and field experiments were carried out. Glasshouse studies of 40-d-old soil-grown plants showed that when applied as a spray, 2% 4-FPA sodium salt aqueous solution (AS) was highly effective at inducing the deposition of phenolic polymers and suppressing WBPH survival (SI Appendix, Fig. S8). These studies were extended to the field in 2015, 2016, and 2019 using 12, 8, and 8 blocks, respectively (each of 7 m × 7 m), as detailed in SI Appendix, Table S6. In the field, formulations of 50 mg L−1 and 100 mg L−1 4-FPA sodium salt AS was effective at controlling WBPH populations. Treatment reduced the population by 46 to 54% and 64 to 68%, respectively, in 2015 over a 2-wk period; in 2016, the control effect of a 200 mg L−1 4-FPA sodium salt AS on the total WBPH population was in the range of 49 to 60% after 4 wk (Fig. 6A). Similar to our laboratory experiments, 4-FPA sodium salt AS had the greatest effect on younger WBPH nymphs within 2 wk, causing a population reduction of approximately 59 to 65% (50 ppm) and 67 to 87% (100 ppm) in 2015 and 68 to 71% (200 ppm) in 2016 (Fig. 6A). Four-FPA showed no harmful effects to spiders (SI Appendix, Fig. S9), which represent major predatory natural enemies of insect pests of rice.
Fig. 6.
Application of 4-FPA decreases the population density of WBPH and increases rice yields in the field. (A) Mean planthopper control effect (+SE, n = 3) of 4-FPA at different concentrations ranging from 50 to 200 mg L−1 on WBPH in 2015 (Upper) and 2016 (Lower). Four-FPA was sprayed on July 15 and July 12, respectively, in 2015 and 2016. (B) Growth phenotype of rice plants 7 and 20 d postspraying of 4-FPA at concentrations of 50 and 100 mg L−1 in 2015 (Left) and 200 mg L−1 in 2016 (Right). (C) Mean seed setting rate and yield of rice seed per plant (+SE, n = 3) of plants that were sprayed with different concentrations of 4-FPA in 2015 (Left) and 2016 (Right). Letters indicate significant differences among treatments (P < 0.05; Duncan’s multiple range test). Asterisks indicate significant differences between treatments and controls (*P < 0.05, **P < 0.01; Student’s t tests).
Finally we investigated the effects of 4-FPA on rice growth and yield in the field. In 2015, application levels of 50 and 100 mg L−1 4-FPA had no effect on plant growth (Fig. 6B, Left). In 2016, when applied at a much higher concentration (200 mg L−1 4-FPA) a small but significant reduction in plant growth was observed compared to control plants 7 d after the application of 4-FPA (Fig. 6B, Right). However, after 20 d the 4-FPA-treated plants had recovered and grew better than control plants (Fig. 6B, Right). Rice yield measurements revealed that all of the 4-FPA treatments increased the seed setting rate and yield per plant both in 2015 and 2016. No difference was observed in other yield parameters (Fig. 6C and SI Appendix, Fig. S10). Furthermore, no difference in yield between control and 4-FPA-treated plants was observed when plants were treated with the insecticide pymetrozine, suggesting that 4-FPA increases yield by suppressing insect damage (SI Appendix, Fig. S11). Thus, 4-FPA treatment reduces herbivore abundance and increases rice yield.
Discussion
Chemical elicitors that boost plant innate immunity and plant defenses are widely regarded as promising tools for plant protection and sustainable agriculture. Yet, most chemical elicitors reported so far are either analogs of stress hormones or compounds that enhance stress hormone signaling. Possibly because of the negative effects of defense induction on plant growth, benefits of chemical elicitors against herbivores have rarely been demonstrated in the field. Here, we report on a mechanism by which a chemical elicitor enhances insect resistance. Instead of boosting defense signaling, 4-FPA directly triggers the deposition of phenolic polymers. This phenomenon is associated with reduced survival of piercing-sucking insects and increased rice yield in a 2-y field trial. Below, we discuss the mode of action of 4-FPA together with the potential of this elicitor to be employed for sustainable agriculture.
Using a custom chemical screening procedure, we previously identified 4-FPA as a compound that renders rice plants less attractive to WBPH (25). Here, we demonstrate that 4-FPA, a phenoxyalkanoic acid derivative, exhibiting structural similarity with 2,4-D and auxin, can induce resistance in plants to piercing and sucking insects. We propose that the increase in resistance is due to the increased deposition of phenolic polymers in parenchyma cells of plants, which impairs feeding of these insect pests. Like its chemical analogs auxin and 2,4-D, 4-FPA is easily absorbed by rice roots, translocated throughout the plant, and rapidly degraded into 4-FP (Fig. 2). Moreover, 4-FPA treatment also enhances the levels of 25 POD gene transcripts (SI Appendix, Table S3), the activity of PODs (Fig. 3A), and the transcript levels of most genes related to the H2O2-generating system (SI Appendix, Table S3), although the H2O2 level in 4-FPA-treated plants was only approximately 50% of that of control plants at 2 d after 4-FPA treatment (Fig. 3B). In agreement with previous studies (26–29), we show in vitro that PODs and H2O2 are able to catalyze the deposition of phenolic polymers (SI Appendix, Fig. S7), supporting our hypothesis that the observed deposition of phenolic polymers in 4-FPA-treated plants was related to the induction of PODs and H2O2 by 4-FPA. Similar results were reported by Graham and Graham (30) who found that a cell wall glucan elicitor from Phytophthora megasperma could induce rapid and massive accumulation of phenolic polymers in soybean cotyledon cells accompanied by large increases in activity of a specific group of peroxidases. This mechanism may also explain the discrepancy between the H2O2 level and the transcript levels of H2O2-generating-related genes in 4-FPA-treated plants: 4-FPA induced the production of H2O2 by enhancing the expression of H2O2-generating-related genes, whereas the deposition of phenolic polymers requires H2O2 (26, 27).
Transcriptome analysis revealed that 4-FPA treatment up-regulated transcript levels of most genes related to the phenylpropanoid pathway (SI Appendix, Table S4), and in particular those relating to the biosynthesis of phenylpropanoid compounds, such as flavonoids, phenolic acids, and lignin (31, 32). However, in contrast to the transcriptome data, the levels of most flavonoids decreased, while the levels of phenolic acids and lignin did not change either in 4-FPA-treated or 4-FPA+WBPH nymph-infested plants (Fig. 3C and SI Appendix, Fig. S6). The finding that both the 4-FPA metabolite, 4-FP, and selected flavonoids participate in the formation of phenolic polymers in the presence of POD and H2O2 (SI Appendix, Fig. S7) leads us to propose that phenolic polymer deposition observed in 4-FPA-treated plants involves a complex biomolecular network, including different phenolic substrates, such as 4-FP and flavonoids, and different types of linkages between phenolic units, as seen in lignin (33, 34). Our conclusion is further supported by the findings that levels of 4-FP decreased rapidly in plants and that levels of most flavonoids in 4-FPA-treated plants were significantly lower than those in their control plants, although transcript levels of their biosynthesis-related genes were significantly higher in the former than in the latter (Figs. 2B and 3C and SI Appendix, Table S4); clearly there is a need to elucidate the chemical composition of this polymer in future studies.
We show that 4-FPA treatment of rice, barley, and wheat plants significantly reduced the survival of nymphs of piercing and sucking herbivores, including planthoppers (WBPH, BPH, and SBPH) and aphids (EGA and BCA) and propose that this observed resistance is primarily due to 4-FPA-induced phenolic polymer deposition and not due to direct toxicity of 4-FPA per se. Several lines of evidence support our hypothesis. First, 4-FPA (SI Appendix, Fig. S1) and its metabolite 4-FP (SI Appendix, Fig. S12A) have no direct contact and/or acute toxicity on WBPH nymphs following ingestion, and 4-FP treatment did not induce resistance of rice to WBPH nymphs (SI Appendix, Fig. S12B), suggesting that 4-FPA plays an important role in inducing defense. Second, JA, JA-Ile, SA, ET, and H2O2 signaling have all been reported to play a key role in regulating resistance of rice to planthoppers (13, 22, 23). In this study, we did not find any influence of 4-FPA treatment on either basal or WBPH nymph-induced levels of JA, JA-Ile, SA, and ET, although H2O2 was reduced by 4-FPA treatment (SI Appendix, Fig. S4 and Fig. 3B). Four-FPA treatment also had no effect on the levels of phenolic acids and lignin (SI Appendix, Fig. S6), but did enhance levels of amino acids (SI Appendix, Table S5) and decrease most flavonoid levels (Fig. 3C), both of which have been shown to influence planthopper performance on rice (35–37). Furthermore, methanol extracts of 4-FPA-treated plants had no harmful effects on WBPH nymphs compared to those from control plants (SI Appendix, Fig. S13). These findings suggest that both 4-FPA and 4-FP themselves, as well as their induced compounds, did not play an important role in the 4-FPA-induced resistance of rice to planthoppers but rather induces the observed defense. Third, the time when nymph mortality was greatest on the 4-FPA-treated plants (Fig. 1 A and B) directly corresponded with the time when large amounts of phenolic polymers were being deposited (Fig. 4A). Fourth, our data not only showed that WBPH females spent longer in the nonpenetration and the pathway phase, and a shorter time in phloem intracellular activity and the ingestion phase on 4-FPA-treated plants (Fig. 4C, Lower), but also excreted lower amounts of honeydew, an indicator of reduced food intake (Fig. 4D). Planthoppers are piercing and sucking insects feeding on phloem sap. Before abstracting the phloem sap they need to insert their stylets into vascular tissues by passing through parenchymal cells and will sample cell sap during this process. Since the stylet diameter of WBPH is about 2 μm and since the diameter of the induced phenolic polymer particles in 4-FPA-treated plants ranges from 1 to 5 μm (Fig. 4 A and B), it is highly likely that these phenolic polymers physically prevent the stylets from reaching the phloem and may also block the stylet itself, thereby decreasing food intake. Finally, 4-FPA-induced rice resistance was effective toward three different species of planthopper all of which exhibit different levels of sensitivity to different host-plant resistance mechanisms in rice (20), suggesting the possibility that other factors beyond chemicals play a pivotal role. These data, taken together, strongly suggest that 4-FPA-induced phenolic polymer deposition plays a central role in the 4-FPA-mediated resistance in plants to piercing and sucking insects by physically inhibiting the access of stylets to phloem and/or by blocking up the food canal.
As mentioned above, relatively few studies have investigated the potential of chemical elicitors in increasing host-plant resistance to herbivorous insects in the field, and even fewer studies have reported yield benefits, potentially because of the negative effects of many elicitors on plant growth. Here, we demonstrate that application of 4-FPA sodium salt AS in the field (ranging from 37.5 to 150 g per hectare) conferred effective control against WBPH: reducing population density of WBPH by 46 to 68% within 2 wk compared to that in the control field. Importantly, application of 4-FPA was not harmful to natural enemies of herbivores (SI Appendix, Fig. S9), most likely due to the fact that they do not share the same mode of feeding as planthoppers. We also found that 4-FPA-induced plant resistance is effective toward two other species of planthopper (BPH and SBPH) and two species of aphid (EGA and BCA) in the laboratory, and given the efficacy of this strategy toward controlling WBPH in the field, it is likely to be equally effective toward these other species of sap-sucking insect pests in the field, all of which are major pests. Although 4-FPA negatively affects plant growth in the laboratory (Fig. 1C–E) and early growth stages in the field (Fig. 6B), we show that the field-grown plants not only recover, but also produce significantly higher yields to that of control plants (Fig. 6C). The enhancement of resistance by 4-FPA was independent of the rice genotype (SI Appendix, Fig. S14). Four-FPA is a commercially used herbicide of low environmental toxicity. Thus, it is a promising chemical elicitor for the commercial control of piercing and sucking insect pests in crop production.
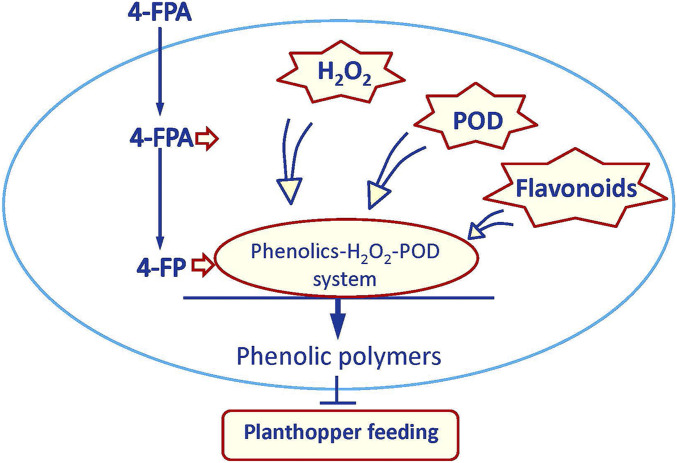
In summary, we propose the following model: 4-FPA can be absorbed by plants and then is immediately converted into 4-FP. Four-FPA alone, or together with its metabolite 4-FP, induce the production of H2O2, PODs, and flavonoids. PODs then catalyze 4-FP and flavonoids, using H2O2, as electron acceptors to form phenolic polymers in the parenchyma cells, which we hypothesize, inhibits the planthopper’s stylet from reaching the phloem and simultaneously blocks the food canal, thereby impairing feeding and significantly decreasing survival (Fig. 7). These findings open pathways for controlling piercing and sucking herbivores, many of which are important vectors of plant disease, by designing and exploiting chemical elicitors that have similar modes of action to 4-FPA.
Fig. 7.
Proposed model demonstrating the mode of action of 4-FPA in inducing the observed resistance of plants to piercing and sucking herbivores. Four-FPA can be absorbed by plants and is then immediately converted into 4-FP. Four-FPA alone or together with 4-FP induce the production of H2O2, peroxidases (PODs), and flavonoids. Four-FP and flavonoids are then catalyzed by PODs to form polymers using H2O2 as electron acceptors. Phenolic polymers in the parenchyma cells may inhibit the stylets of piercing and sucking from reaching the phloem sap in the plant sieve elements and/or may block the esophagus in the stylets, and hence inhibit feeding thus decreasing performance and survival of piercing and sucking herbivores.
Methods
Plant Growth.
Rice genotypes used in this study were Xiushui 110, TN1, Babawee, and Tianfeng, all of which are susceptible to BPH and WBPH; Xiushui 110 is a japonica rice variety while the other three varieties are indica rice varieties. Pregerminated seeds of the different lines were cultured in plastic bottles (diameter 8 cm, height 10 cm) in a glasshouse (28 ± 2 °C, 14 h light phase). Ten-day-old seedlings were transferred to 20-L hydroponic boxes filled with rice nutrient solution (38) and after 25 d individually transferred into opaque 400-mL hydroponic plastic pots. Plants were used for experiments 4 to 5 d posttransplanting.
Barley (Hordeum vulgare) and wheat (Triticum aestivum) genotypes used in this study were Eunova and Bobwhite. Seeds were pregerminated, and seedlings were individually cultured in 200-mL plastic pots (11 cm depth and 4 cm diameter) and fed with nutrient solution (39). Two-week-old seedlings were used for experiments.
Insects.
Colonies of BPH, WBPH, and SBPH were originally obtained from rice fields in Hangzhou, China and subsequently maintained on TNI rice seedlings in a controlled climate room at 26 ± 2 °C, 12 h light phase, and 80% relative humidity for at least 30 generations prior to use.
Colonies of EGA (S. avenae) and BCA (R. padi) were reared on barley seedlings in a growth chamber at 24 ± 2 °C with 16 h light phase.
Plant Treatment.
For 4-FPA treatment, two different methods were used: root treatment and spray treatment. For 4-FPA-R, plants were grown in nutrient solution, and 4-FPA was added (first dissolved in acetone, 200 mg per mL of acetone) to give final concentrations ranging from 0.5 to 5 mg L−1 (see details for different experiments below). Control (Con) plants were grown in control nutrient solution (without 4-FPA but with the same volume of acetone as in treatments). For 4-FPA-S, 4-FPA was dissolved in acetone (200 mg per mL of acetone) and diluted in distilled water to give a range of concentrations (5 to 100 mg L−1). Each plant (above-ground part) was sprayed with 4 mL of 4-FPA using an atomizer; control plants were sprayed with 4 mL of distilled water containing the same volume of acetone as in treatments. For 4-FP-R, we used the same method as stated above, with a final concentration of 4-FP in nutrient solution of 5 mg L−1; control plants (Con) were grown in control nutrient solution. For W-NP treatment, plants were individually confined in a glass cage (diameter 4 cm, height 8 cm; with 48 small holes [diameter 0.8 mm]) into which 20 third instar nymphs of WBPH were released. Plants with an empty cage were used as controls (noninfested).
Quantification of 4-FPA and 4-FP in Plants.
As the side chain of 2,4-D can be oxidized to yield 2,4-dichlorophenol in plants (40), we investigated the change in levels of 4-FPA and 4-FP in plants with the following treatments: 1) plants were first grown in nutrient solution with 4-FPA at a concentration of 5 mg L−1 for 1 d and then transferred to nutrient solution without 4-FPA; 2) plants were grown in nutrient solution with 4-FPA at a concentration of 5 mg L−1 and simultaneously each plant was infested with 20× third instar W-NP using the same method as stated above; 1 d later, these plants were transferred to nutrient solution without 4-FPA. Leaf sheaths from individual plants were harvested for chemical analysis at 0, 1, 4, and 7 d after the start of 4-FPA treatment; leaf sheaths harvested at 0 d were used as controls. Four-FPA and 4-FP were analyzed using a method as described in SI Appendix, SI Materials and Methods.
Bioassays: Controlled Environmental Conditions.
Herbivore performance on plants treated with 4-FPA or 4-FP.
To investigate the effect of 4-FPA or 4-FP treatment on the survival rate of nymphs of WBPH, BPH, SBPH, EGA, or BCA, 15 (planthopper) or 10 (aphid) neonates were allowed to feed on individual 4-FPA-R plants (rice, barley, or wheat) (grown in nutrient solution with different concentrations of 4-FPA for 12 h), 4-FPA-S plants (sprayed with different concentrations of 4-FPA for 12 h), or 4-FP-R plants (grown in nutrient solution with 5 mg L−1 4-FP for 12 h) and their corresponding control plants (see details in Figs. 1 and 5 and SI Appendix, Fig. S12). Nymph survival per plant was recorded daily for 6 to 9 d (6 d for aphids; 8 to 9 d for planthoppers) postplant infestation. Eight replicates were carried out per treatment for each of the different herbivore species.
To assess the effect of 4-FPA treatment on WBPH feeding, a newly emerged female adult was placed into a small parafilm bag (6 × 5 cm) attached to the leaf sheath of a 4-FPA-R plant (grown in nutrient solution with 5 mg L−1 4-FPA for 4.5 d) or a control plant. After feeding for 24 h, the amount of honeydew excreted by the female adult was weighed. Twenty replicates were carried out per treatment (n = 20).
The effect of 4-FPA treatment on fecundity and oviposition behavior of WBPH female adults was also investigated. Four-FPA-R (grown in nutrient solution with 5 mg L−1 4-FPA for 4.5 d) or control plants were individually confined within the glass cylinders into which a newly emerged WBPH female adult and a male adult were released and allowed to lay eggs for 8 d or into which 15 gravid WBPH female adults were released and allowed to lay eggs for 12 h. The number of eggs laid by female adults on each plant was counted using a microscope. Twenty and 10 replicates per treatment were carried out for the first (a pair of female and male adults) and second (15 gravid females) study, respectively.
Direct effect of 4-FPA or 4-FP on the survival of WBPH.
To detect direct effects of 4-FPA and its metabolite 4-FP on the survival rate of neonate WBPH nymphs, contact and/or stomach poisoning toxicity of 4-FPA, and 4-FP were measured using the method as described in SI Appendix, SI Materials and Methods.
Effect of 4-FPA on plant growth.
To investigate the effect of 4-FPA treatment on plant growth, plants were grown in nutrient solution at final concentrations ranging from 0.5 to 5 mg L−1 (see details in Fig. 1). Control plants were grown in control nutrient solution (without 4-FPA but with the same volume of acetone as in treatments). Ten days later, plants were photographed and plant height, root length, and the mass of above- and below-ground tissues were measured.
Effect of rice variety on 4-FPA-mediated plant defenses.
Survival rates of neonate WBPH were investigated on 4-FPA-R (grown in nutrient solution with 5 mg L−1 4-FPA for 12 h) and control plants of varieties XS110, Tianfeng, TN1, and Babawee. The number of nymphs surviving on each plant was recorded daily for 8 d. Eight replicates were carried out per treatment.
Effect of extracts of 4-FPA-R plants on WBPH survival.
To detect effects on the survival rate of WBPH nymphs due to chemicals in rice plants induced by 4-FPA, 15 second instar WBPH nymphs were fed on artificial diet containing the methanol extract of 4-FPA-treated plants in a 30-mL double-ended open glass cylinder (diameter 2 cm, length 9 cm) as described (41); controls were fed on artificial diet containing the methanol extract from control plants. Ten replicates per treatment were carried out. The number of surviving nymphs was recorded daily. The methanol extract of 4-FPA-treated or control plants was prepared as described in SI Appendix, SI Materials and Methods.
EPG recording of WBPH feeding behavior.
The feeding behavior of individual newly emerged WBPH adult females on 4-FPA-R plants (5 mg mL−1 for 4.5 d) or control plants was monitored for 6 h using an EPG as previously described (42). For each treatment, 15 to 20 females (replications) were performed. The signals recorded were analyzed using PROBE version 3.4 software (Wageningen Agricultural University). The output signals from EPG recordings were classified into five typical waveforms (43), including NP for nonpenetration, PP (N1 + N2 + N3) for the pathway phase (including penetration initiation [N1], salivation and stylet movement [N2], and extracellular activity near the phloem [N3]), N4-a for intracellular activity in the phloem, N4-b for phloem sap ingestion, and N5 for the xylem phase. The durations of each sequential waveform event for each insect were measured, and the average waveform duration per insect (in minutes) for each waveform was calculated for each treatment (42). Each treatment was replicated 15 to 20 times.
Bioassays: Glasshouse Studies.
To investigate the effect of 4-FPA on the survival rate of WBPH nymphs in the greenhouse, 20-d-old hydroponically grown seedlings were individually transplanted into opaque 400-mL plastic pots with soil that was obtained from rice fields in Hangzhou, China. After 20 d, plants were individually sprayed with 4 mL of 2% 4-FPA sodium salt AS (prepared in H2O/alkyl polyglycoside/isopropanol/NaOH and the final components of this solution were 2% 4-FPA, 0.46% NaOH, 10% alkyl polyglycoside, 5% isopropanol, and 82.54% H2O) at a concentration of 50 mg L−1; control plants were sprayed with 4 mL of water without 4-FPA sodium salt but with the same volumes of other components as in treatments. Twelve hours postspraying, each plant was infested with 15 neonates (as above) and the number of nymphs surviving on each plant was recorded daily for 8 d. Each treatment was replicated 10 times. In addition, leaf sheathes of plants that had been sprayed with 4-FPA sodium salt for 4.5 or 7 d, were harvested for paraffin sections and histochemical staining, as described below (seven replicates/treatment).
Bioassays: Field Studies.
To evaluate the control efficiency of 4-FPA on WBPH in the field, studies were carried out in Kaihua, Zhejiang, China from June to October in 2015 and 2016. The field plot was divided into 12 blocks (7 m × 7 m) in 2015 or 8 blocks (7 m × 7 m) in 2016, and each block was surrounded by a 1-m rice buffer zone. A total of 2% 4-FPA sodium salt AS was diluted to specific concentrations and sprayed on the above-ground parts of rice plants using a backpack sprayer at 18 to 22 d (at the tillering stage) after transplanting (SI Appendix, Table S6) (each block was sprayed with 3,700 mL of 4-FPA solution or water). In the plot, three treatments (spraying with 50 mg L−1 of 4-FPA sodium salt, 100 mg L−1 of 4-FPA sodium salt, or water [control]) in 2015 and two treatments (spraying with 200 mg L−1 of 4-FPA sodium salt or water) in 2016 were randomly assigned to 12 or 8 blocks, each treatment with four independent replicate blocks. Rice varieties used were Chunyou 84 in 2015 and Yongyou 15 in 2016, respectively. In general, in Zhejiang province, the main piercing and sucking insect pests in rice fields are BPH and WBPH (20). However, during the field experiments in 2015 and 2016, only the WBPH population reached a sufficiently high density (designated “intermediate density”), while the density of the BPH population was low. Therefore, we only performed field experiments for WBPH. The number of young and late-stage nymphs, and adults of WBPH and the number of predatory spiders in each block were recorded 1 d before and 7, 14, 21, and 30 d postapplication of 4-FPA by randomly sampling 15 hills of plants in each block using the following method: WBPHs and spiders on above-ground parts of each hill of plants were collected into a plastic tray (length 45 cm × width 33 cm × depth 0.8 cm) by softly tapping plants and then they were counted. Based on these data, the control efficiency of 4-FPA on WBPH and its effect on predatory spiders were calculated. To measure the effect of 4-FPA on rice yield, 10 hills of completely mature plants in each block were harvested and the number of panicles per hill, number of grains per panicle, seed setting rate, 1,000 seed weight, and seed yield per plant were recorded.
To evaluate the direct effect of 4-FPA on rice yields in the field in the absence of WBPH, we performed additional field studies in Kaihua, Zhejiang, China from June to October in 2019. The field plot was divided into eight blocks (7 m × 7 m), and each block was surrounded by a 1-m rice buffer zone. Two treatments (spraying with 100 mg L−1 of 4-FPA sodium salt plus 833 mg L−1 of 50% pymetrozine [Syngenta; used for controlling WBPH] or spraying with 833 mg L−1 50% pymetrozine; each block was sprayed with 3,700 mL solution) were randomly assigned to eight blocks, each treatment with four independent replicate blocks. The rice variety used was Yongyou 15. The investigation on the direct effect of 4-FPA on rice yield was carried out using the same method as described above. Management measurements for rice growth in the field, including the amount, times, and names of fertilizers and pesticides used in 2015, 2016, and 2019 are provided in SI Appendix, Table S6.
Transcriptome Analysis.
Plants were randomly assigned to 4-FPA-R (grown in nutrient solution with 5 mg L−1 4-FPA for 1 d) and control groups. Five individual plants were harvested as biological replicates and three independent biological replicates were performed for each group. Total RNA was isolated using the RNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s instructions. Construction of an Illumina library and sequencing on an IlluminaHisEq. 2000 platform were conducted by Oebiotech (Shanghai, China, https://www.oebiotech.com/). Data were analyzed as described in ref. 44.
qRT-PCR.
To confirm the results of the transcriptomic analyses, the expression levels of 16 randomly selected genes (SI Appendix, Table S7) were measured in 4-FPA-R plants and control plants by qRT-PCR. Total RNA samples were from samples used for transciptome analysis as stated above. For each total RNA sample, 1 mg of RNA was reverse transcribed using the PrimeScript RT-PCR Kit (TaKaRa). qRT-PCR was carried out in a CFX96TM Real-Time System (Bio-Rad) using iQ SYBR Green (Bio-Rad). Primers used for QRT-PCR are listed in SI Appendix, Table S7. Each gene was analyzed in three biological replications. Gene expression levels were calculated using a standard curve as previously described (45). The rice actin gene OsACTIN (accession no. Os03g50885) was used as an internal standard to normalize cDNA concentrations.
JA, JA-Ile, SA, and ET Analysis.
Plants were randomly assigned to W-NP, 4-FPA-R, 4-FPA-R+W-NP, and control groups. For W-NP treatment, plants were grown in control solution for 12 h, followed by infestation with 20 third instar WBPH for each plant as stated above. For 4-FPA-R treatment, plants were grown in nutrient solution containing 5 mg L−1 4-FPA for 12 h, with no infestation (each plant was confined with an empty cage). For 4-FPA+W-NP treatment, plants were grown in nutrient solution containing 5 mg L−1 4-FPA for 12 h, followed by infestation with 20× third instar WBPH per plant. For controls, plants were grown in control solution for 12 h, but with no subsequent infestation. For JA, JA-Ile, and SA analysis, leaf sheaths from individual plants were harvested at 0.5, 1, 3, 8, and 24 h following the start of WBPH infestation. Samples (about 100 mg each) were ground in liquid nitrogen and JA, JA-Ile, and SA were extracted with ethyl acetate spiked with labeled internal standards ([2D6]JA, [2D6]JA-Ile, and [2D4]SA) and then analyzed by high performance liquid chromatography-mass spectrometry-mass spectrometry (HPLC-MS-MS) as previously described (46). For ET, plants were individually covered with sealed glass cylinders (diameter, 4 cm; height, 50 cm) and ET production determined at 12, 24, and 48 h after the start of WBPH infestation as previously described (47). Six replicates were carried out for each treatment at each time interval.
Hydrogen Peroxide Analysis.
Plants were randomly assigned to W-NP, 4-FPA-R, 4-FPA-R+W-NP, and control groups as stated above. Leaf sheaths from individual plants were harvested 0.5 and 1 h as well as 2 and 4 d after the start of nymph infestation. Samples (about 100 mg each) were ground in liquid nitrogen and H2O2 concentration determined using an Amplex Red Hydrogen Peroxide/Peroxidase Assay kit as described in ref. 48. Six replicates were carried out for each treatment at each time interval.
POD Analysis.
Plants were randomly assigned to W-NP, 4-FPA-R, 4-FPA-R+W-NP, and control groups as above. Leaf sheaths from individual plants were harvested 2 and 4 d after the start of nymph infestation. Samples (about 100 mg each) were ground in liquid nitrogen and 1 mL of 0.1 M potassium phosphate buffer (pH 7.0) containing 5% insoluble polyvinylpolypyrrolidone added to each. Samples were vortexed for 5 min and centrifuged (18,000 × g) for 15 min at 4 °C, and the supernatants were collected. POD level was determined by ELISA (ELISA Lab, Wuhan, China) according to the manufacturer’s instructions. For each treatment, five replicates were carried out.
Phenolic Acid, Flavonoid, and Lignin Analysis.
Plants were randomly assigned to W-NP, 4-FPA-R, 4-FPA-R+W-NP, and control groups, as above. Leaf sheaths from individual plants were harvested 4 d after the start of nymph infestation. Totally, six phenolic acids, syringic acid, ferulic acid, caffeic acid, cinnamic acid, gallic acid, and coumarice acid and 16 flavonoids, naringenin, homoorientin, isovitexin, apigenin 5-O-glucoside (a5g), isoquercitrin, eriodictyol 7-O-glucoside (e7g), prunin, astragalin, sakuranetin, cosmosiin, rutin, luteolin, quercitrin, luteolin 7-O-glucoside (l7g), carlinoside, and neoschaftoside were analyzed. Phenolic acid, flavonoid, and lignin were extracted and quantified using methods described in SI Appendix, SI Materials and Methods.
Lignin Localization by Laser Scanning Confocal Microscopy.
Plants were randomly assigned to W-NP, 4-FPA-R, 4-FPA-R+W-NP, and control groups, as above. Leaf sheaths (about 2-cm length of the lower part) taken at different time intervals after the start of treatment (see details in Fig. 4 and SI Appendix, Fig. S8) were harvested, embedded in paraffin blocks, sectioned, and stained with safranine and fast green as described in ref. 49. Lignin and lignin-like polymers fluoresce red with safranine staining under green light excitation. Cells were observed using laser scanning confocal microscopy (LSCM). All treatments were replicated three times.
Polymerization Reaction of 4-FP and Flavonoids In Vitro.
To elucidate the potential involvement of 4-FP, flavonoids, H2O2, and PODs in the formation of phenolic polymers, in vitro studies using a series of reaction systems (see details in SI Appendix, Table S8) were carried out following the method as described in SI Appendix, SI Materials and Methods.
Data Analysis.
Differences in the levels of JA, JA-Ile, SA, ET, H2O2, phenolic compounds, and POD for different treatments were analyzed by one-way ANOVAs. In the case of significant treatment effects (P < 0.05), Duncan’s multiple range tests were used to test for significant differences between the groups. Differences in experiments involving two treatments were determined by Student’s t test. All tests were carried out with Statistica 6 (Statistica, SAS Institute Inc.).
Data Availability.
The RNA-seq data have been deposited in Sequence Read Archive (SRA) with accession no. SRP162374. All other data supporting the findings of this study are available within the article and its SI Appendix.
Supplementary Material
Acknowledgments
We thank Guilan Dong and Shen-gen Xie for their assistance with plant growth and insect rearing and Tobias Züst (University of Bern) for supplying aphids. This work was jointly supported by the Special Fund for Agro-Scientific Research in the Public Interest (201403030), and the earmarked fund for China Agriculture Research System (CARS-01-40).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. J.S.T. is a guest editor invited by the Editorial Board.
Data deposition: The RNA-seq data have been deposited in Sequence Read Archive (SRA) with accession number: SRP162374.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2003742117/-/DCSupplemental.
References
- 1.Erb M., Reymond P., Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 70, 527–557 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Hilker M., Fatouros N. E., Plant responses to insect egg deposition. Annu. Rev. Entomol. 60, 493–515 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Schuman M. C., Baldwin I. T., The layers of plant responses to insect herbivores. Annu. Rev. Entomol. 61, 373–394 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Heil M., Land W. G., Danger signals–Damaged-self recognition across the tree of life. Front Plant Sci 5, 578 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huot B., Yao J., Montgomery B. L., He S. Y., Growth-defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 7, 1267–1287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bürger M., Chory J., Stressed out about hormones: How plants orchestrate immunity. Cell Host Microbe 26, 163–172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turlings T. C. J., Erb M., Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 63, 433–452 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Sobhy I. S., Erb M., Lou Y., Turlings T. C. J., The prospect of applying chemical elicitors and plant strengtheners to enhance the biological control of crop pests. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20120283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bektas Y., Eulgem T., Synthetic plant defense elicitors. Front Plant Sci 5, 804 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gozzo F., Faoro F., Systemic acquired resistance (50 years after discovery): Moving from the lab to the field. J. Agric. Food Chem. 61, 12473–12491 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Quintana-Rodriguez E., Duran-Flores D., Heil M., Camacho-Coronel X., Damage-associated molecular patterns (DAMPs) as future plant vaccines that protect crops from pests. Sci. Hortic. (Amsterdam) 237, 207–220 (2018). [Google Scholar]
- 12.Erb M., et al. , Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 6, 6273 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lou Y. G., Hu L. F., Li J. C., “Herbivore-induced defenses in rice and their potential application” in Rice Planthopper Management, Heong K. L., Cheng J. A., Escalada M. M., Eds. (Zhejiang University press, Hangzhou, China, 2015), pp. 91–115. [Google Scholar]
- 14.Jimenez-Aleman G. H., Machado R. A. R., Baldwin I. T., Boland W., JA-Ile-macrolactones uncouple growth and defense in wild tobacco. Org. Biomol. Chem. 15, 3391–3395 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Thaler J. S., Stout M. J., Karban R., Duffey S. S., Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J. Chem. Ecol. 22, 1767–1781 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Baldwin I. T., Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc. Natl. Acad. Sci. U.S.A. 95, 8113–8118 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruce T. J. A., et al. , cis-Jasmone treatment induces resistance in wheat plants against the grain aphid, Sitobion avenae (Fabricius) (Homoptera: Aphididae). Pest Manag. Sci. 59, 1031–1036 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Karasov T. L., Chae E., Herman J. J., Bergelson J., Mechanisms to mitigate the trade-off between growth and defense. Plant Cell 29, 666–680 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R., et al. , Prioritizing plant defence over growth through WRKY regulation facilitates infestation by non-target herbivores. eLife 4, e04805 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng J., He J., Rice Insect Pests, (China Agricultural Press, Beijing, China, 1996). [Google Scholar]
- 21.Liu W., et al. , Statistics and analysis of crop yield losses caused by main diseases and insect psets in recent 10 years. Plant Prot. 42, 1–9 (2016). [Google Scholar]
- 22.Zhou G., et al. , Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 60, 638–648 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Lu J., et al. , Contrasting effects of ethylene biosynthesis on induced plant resistance against a chewing and a piercing-sucking herbivore in rice. Mol. Plant 7, 1670–1682 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Hu L., Ye M., Li R., Lou Y., OsWRKY53, a versatile switch in regulating herbivore-induced defense responses in rice. Plant Signal. Behav. 11, e1169357 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He X., et al. , Finding new elicitors that induce resistance in rice to the white-backed planthopper Sogatella furcifera. Bioorg. Med. Chem. Lett. 25, 5601–5603 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Yamasaki H., Sakihama Y., Ikehara N., Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol. 115, 1405–1412 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreres F., et al. , Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: An H2O2 affair? J. Exp. Bot. 62, 2841–2854 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Reihmann M., Ritter H., Synthesis of phenol polymers using peroxidases. Adv. Polym. Sci. 194, 1–49 (1970). [Google Scholar]
- 29.Zou H., Taylor K. E., Products of oxidative coupling of phenol by horseradish peroxidase. Chemosphere 28, 1807–1817 (1994). [Google Scholar]
- 30.Graham M. Y., Graham T. L., Rapid accumulation of anionic peroxidases and phenolic polymers in soybean cotyledon tissues following treatment with Phytophthora megasperma f. sp. Glycinea wall glucan. Plant Physiol. 97, 1445–1455 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxena M., Saxena J., Pradhan A., Flavonoids and phenolic acids as antioxidants in plants and human health. Int. J. Pharm. Sci. Rev. Res. 16, 130–134 (2012). [Google Scholar]
- 32.Ali M. B., McNear D. H. Jr., Induced transcriptional profiling of phenylpropanoid pathway genes increased flavonoid and lignin content in Arabidopsis leaves in response to microbial products. BMC Plant Biol. 14, 84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goni M. A., Hedges J. I., Lignin dimers–Structures, distribution, and potential geochemical applications. Geochim. Cosmochim. Acta 56, 4025–4043 (1992). [Google Scholar]
- 34.Martínez A. T., et al. , Monolignol acylation and lignin structure in some nonwoody plants: A 2D NMR study. Phytochemistry 69, 2831–2843 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Sogawa K., Studies on the feeding habits of the brown planthopper: III. Effects of amino acids and other compounds on the sucking response. Jap. J. Appl. Entomol. Zool. 16, 1–7 (1972). [Google Scholar]
- 36.Shigematsu Y., et al. , Sterols and asparagine in the rice plant, endogenous factors related to resistance against the brown planthopper (Nilaparvata-Lugens). Agr. Biol. Chem. Tokyo 46, 2877–2879 (1982). [Google Scholar]
- 37.Alamgir K. M., et al. , Systematic analysis of rice (Oryza sativa) metabolic responses to herbivory. Plant Cell Environ. 39, 453–466 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Yoshida S., Forno D. A., Cock J. H., Gomez K. A., Laboratory Manual for Physiological Studies of Rice, (International Rice Research Institute, Los Baños, Philippines, 1976). [Google Scholar]
- 39.Singh G., Biswas D. R., Marwaha T. S., Mobilization of potassium from waste mica by plant growth promoting rhizobacteria and its assimilation by maize (Zea Mays) and wheat (Triticum Aestivum L.): A hydroponics study under phytotron growth chamber. J. Plant Nutr. 33, 1236–1251 (2010). [Google Scholar]
- 40.Peterson M. A., McMaster S. A., Riechers D. E., Skelton J., Stahlman P. W., 2,4-D past, present, and future: A review. Weed Technol. 30, 303–345 (2016). [Google Scholar]
- 41.Fu Q., Zhang Z., Hu C., Lai F., Sun Z., A chemically defined diet enables continuous rearing of the brown planthopper, Nilaparvata lugens (Stal) (Homoptera: Delphacidae). Appl. Entomol. Zool. 36, 111–116 (2001). [Google Scholar]
- 42.Cao T. T., Lü J., Lou Y. G., Cheng J. A., Feeding-induced interactions between two rice planthoppers, Nilaparvata lugens and Sogatella furcifera (Hemiptera: Delphacidae): Effects on feeding and honeydew excretion. Environ. Entomol. 42, 1281–1291 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Ji R., et al. , A salivary endo-b-1,4-glucanase acts as an effector that enables the brown planthopper to feed on rice. Plant Physiol. 173, 1920–1932 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X., et al. , Comparative transcriptome analysis reveals defense-related genes and pathways against downy mildew in Vitis amurensis grapevine. Plant Physiol. Biochem. 95, 1–14 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Wong M. L., Medrano J. F., Real-time PCR for mRNA quantitation. Biotechniques 39, 75–85 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Hu L., et al. , The rice transcription factor WRKY53 suppresses herbivore-induced defenses by acting as a negative feedback modulator of map kinase activity. Plant Physiol. 169, 2907–2921 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu Y., Wang X., Lou Y., Cheng J., Role of ethylene signaling in the production of rice volatiles induced by the rice brown planthopper Nilaparvata lugens. Chin. Sci. Bull. 51, 2457–2465 (2006). [Google Scholar]
- 48.Lou Y., Baldwin I. T., Silencing of a germin-like gene in Nicotiana attenuata improves performance of native herbivores. Plant Physiol. 140, 1126–1136 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma Y., Sawhney V. K., Steeves T. A., Staining of paraffin-embedded plant material in safranin and fast green without prior removal of the paraffin. Can. J. Bot. 71, 996–999 (1993). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data have been deposited in Sequence Read Archive (SRA) with accession no. SRP162374. All other data supporting the findings of this study are available within the article and its SI Appendix.