Significance
We recently demonstrated that the effects of the β2AR agonist clenbuterol to enhance skeletal muscle strength and hypertrophy are mediated via β-arrestin1-dependent signaling. The present study reports the unique ability of carvedilol, previously identified as a β-arrestin-biased agonist at the β2AR, to enhance skeletal muscle contractility. This observation was unique among βAR antagonists tested as metoprolol and nadolol failed to stimulate skeletal muscle contractility. Our findings provide insight into the mechanism of carvedilol’s effect on skeletal muscle contractility and demonstrate the unconventional potential utility of a commonly used pharmacologic agent for a previously unappreciated clinical use. We hypothesize that the observed β-arrestin-dependent signaling pathway could be targeted in various clinical conditions associated with decreased functional exercise capacity.
Keywords: adrenergic receptor, β-arrestin, β-blocker, carvedilol, skeletal muscle contractility
Abstract
A decrease in skeletal muscle strength and functional exercise capacity due to aging, frailty, and muscle wasting poses major unmet clinical needs. These conditions are associated with numerous adverse clinical outcomes including falls, fractures, and increased hospitalization. Clenbuterol, a β2-adrenergic receptor (β2AR) agonist enhances skeletal muscle strength and hypertrophy; however, its clinical utility is limited by side effects such as cardiac arrhythmias mediated by G protein signaling. We recently reported that clenbuterol-induced increases in contractility and skeletal muscle hypertrophy were lost in β-arrestin 1 knockout mice, implying that arrestins, multifunctional adapter and signaling proteins, play a vital role in mediating the skeletal muscle effects of β2AR agonists. Carvedilol, classically defined as a βAR antagonist, is widely used for the treatment of chronic systolic heart failure and hypertension, and has been demonstrated to function as a β-arrestin-biased ligand for the β2AR, stimulating β-arrestin-dependent but not G protein-dependent signaling. In this study, we investigated whether treatment with carvedilol could enhance skeletal muscle strength via β-arrestin-dependent pathways. In a murine model, we demonstrate chronic treatment with carvedilol, but not other β-blockers, indeed enhances contractile force in skeletal muscle and this is mediated by β-arrestin 1. Interestingly, carvedilol enhanced skeletal muscle contractility despite a lack of effect on skeletal muscle hypertrophy. Our findings suggest a potential unique clinical role of carvedilol to stimulate skeletal muscle contractility while avoiding the adverse effects with βAR agonists. This distinctive signaling profile could present an innovative approach to treating sarcopenia, frailty, and secondary muscle wasting.
Reduced skeletal muscle strength presents a significant public health problem across a variety of relatively common conditions such as aging, malignancies (1), heart failure (2), chronic kidney disease, and chronic obstructive pulmonary disease (3). Multiple pharmacologic agents have been trialed in such circumstances including myostatin inhibitors (4), testosterone (5), insulin like growth factor 1 (IGF1) analogs (6), and ghrelin modulating agents (7, 8), have been evaluated in clinical trials, but these agents have not demonstrated clear evidence of benefit (9) and adverse side effects have limited their use (10, 11).
β-Adrenergic receptors (βARs) are members of the largest family of cell surface receptors, G protein-coupled receptors (GPCRs). βARs have important roles in a wide variety of physiologic and pathologic processes, and chronic β2AR stimulation in particular has previously been demonstrated to promote anabolic, hypertrophic, and strength increasing responses in skeletal muscle in response to agonist stimulation in several species (12). Clenbuterol, a selective β2AR agonist, has been shown to increase lean muscle mass and strength in patients with chronic heart failure; however, arrhythmogenic side effects have limited its use (13).
β-Arrestins (βarrs) are ubiquitously expressed proteins that mediate GPCR desensitization, internalization, and ubiquitination. βarrs are also important intracellular scaffold proteins that function as signal transducers to initiate signaling cascades independent of, or collaboratively with G proteins downstream of GPCR activation (14, 15). Carvedilol is a commonly used β-blocker displaying biased signaling properties as a functional antagonist for G protein-mediated signaling while simultaneously functioning as an agonist for βarr-mediated signaling. Other β-blockers, such as metoprolol and nadolol, function as classical antagonists for both G protein and βarr-dependent signaling (16, 17). Recently we demonstrated that the positive effects of the balanced (i.e., unbiased) β2AR agonist clenbuterol on skeletal muscle contractility and growth are dependent on βarr1 (18). We, therefore, hypothesized that a βarr-biased ligand such as carvedilol might serve as an attractive pharmacologic agent in patients with frailty and diminished skeletal muscle strength by inducing positive contractile and/or hypertrophic responses in skeletal muscle without eliciting common side effects observed with β2AR agonists.
To test this hypothesis, we generated skeletal muscle (sm)-specific βarr1 knockout mice (KO) utilizing the Cre-loxP system and studied the effect of carvedilol on skeletal muscle contractility and performance. We assessed and compared the effects of carvedilol with those from the β2AR agonist clenbuterol and the βAR antagonists nadolol and metoprolol. In addition to confirming the role of βarr1 in β2AR-mediated skeletal muscle contractility, we demonstrate a unique salutary effect of carvedilol on skeletal muscle strength that was not reproduced with other βAR antagonists.
Results
Carvedilol Enhances Skeletal Muscle Contractility.
To determine the role of βarr1-dependent signaling on skeletal muscle contractility, βarr1Flox mice (18) served as the wild type (WT) controls and were treated continuously for 2 wk with βAR antagonists (carvedilol, metoprolol, or nadolol), a β2AR agonist (clenbuterol), or vehicle via implanted s.c. alzet osmotic pumps. We measured contractile force of isolated extensor digitorum longus (EDL) muscle suspended in an organ bath chamber supplemented with 20% oxygen to maintain physiological skeletal muscle pO2 as previously described (18, 19). Contractile properties measured included twitch, tetanic force, and fatigue, in addition to the muscle weight of EDL muscle excised from mice following 2 wk of treatment. EDL muscles coded in a blinded manner were excised and underwent contractility testing for force–frequency relationship. The vehicle-treated group was compared to all treatments for statistical analysis.
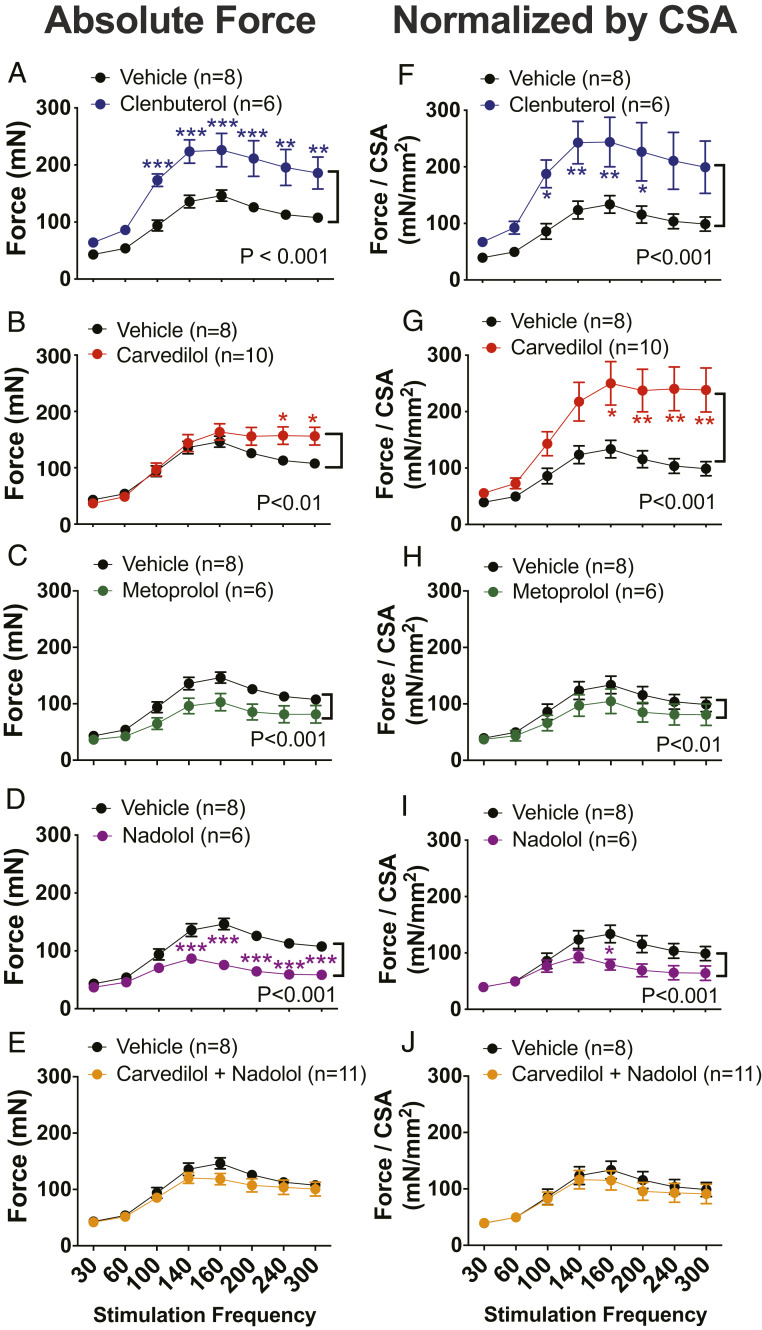
The absolute nonnormalized contractile amplitude at each frequency and for each treatment group is presented in Fig. 1 A–E and contractile amplitude normalized by cross-sectional area (CSA) is presented in Fig. 1 F–J. Wild type βarr1Flox mice treated with clenbuterol show enhanced EDL muscle contractility with increasing stimulation frequency as determined by absolute nonnormalized force (Fig. 1A) and when force is normalized to CSA (Fig. 1F; P < 0.001). This level of increase in force generation produced by clenbuterol was similar to the level we previously observed in EDL muscles from C57/B6 mice treated with clenbuterol (18).
Fig. 1.
β-Arrestin-biased agonist carvedilol enhanced contractility of skeletal muscle. Force–frequency curves for EDL muscles from βarr1Flox mice after 2 wk of clenbuterol (1 mg/kg/day) (A and F), carvedilol (1 mg/kg/day) (B and G), metoprolol (10 mg/kg/day) (C and H), nadolol (10 mg/kg/day) (D and I), nadolol (10 mg/kg/day) combined with carvedilol (1 mg/kg/day) (E and J) compared to vehicle (10% DMSO, 300 mM ascorbic acid in saline solution) treatment. The absolute nonnormalized contractile amplitude at each frequency and for each treatment group is presented (A–E) and the contractile amplitudes normalized by CSA are presented (F–J). Data represent the mean ± SEM for n independent EDL muscles as marked in the figure. A vehicle-treated group was used to compare to all treatments. Statistical significance versus vehicle-treated group was performed by using a two-way ANOVA with Sidak’s multiple comparison test (*P < 0.05; **P < 0.01; ***P < 0.001).
We next assessed the effect of the βarr-biased ligand carvedilol on EDL muscle contractility. The β-blocker, carvedilol, induced enhancement of the force–frequency response, both in absolute terms (Fig. 1B; P < 0.001) and when normalized to CSA compared to vehicle treatment (Fig. 1G; P < 0.001). When force generation was normalized by CSA, carvedilol-induced enhancement of the force–frequency response (Fig. 1G) reached levels similar to that observed in clenbuterol-treated mice (Fig. 1F). The effect of carvedilol was most prominent at stimulation frequencies above 200 Hz (Fig. 1B; P < 0.05), frequencies that typically result in decreases in force generation in WT and clenbuterol-treated EDL muscles (Fig. 1A). Taken together, these data demonstrate that carvedilol stimulates enhancement of EDL contractility when measured as absolute force generation (Fig. 1B) or when normalized for the smaller muscle size (Fig. 1G) observed in carvedilol-treated animals.
To assess whether the effect observed with carvedilol was unique among βAR antagonists, we tested the effects of two other βAR antagonists, the β1AR selective antagonist metoprolol and the nonselective βAR antagonist nadolol, on skeletal muscle contractility. In contrast to the positive contractile effect observed with carvedilol treatment, EDL muscles isolated from metoprolol-treated mice showed a decrease in force generation in force–frequency experiments compared to vehicle-treated control (Fig. 1 C and H; P < 0.01). Similar to the effect observed with metoprolol, mice treated with nadolol also demonstrated significantly reduced contractile responses compared to vehicle (Fig. 1 D and I; P < 0.001).
To confirm that the salutary effect of carvedilol on skeletal muscle function is mediated through its action on βARs, we tested the force–frequency relationship in EDL muscle from animals cotreated with both carvedilol and nadolol (Fig. 1 E and J). The enhanced EDL contractility observed with carvedilol treatment was abrogated in the presence of nadolol cotreatment and also resulted in diminished contractile response compared to EDL treated with vehicle, indicating that the enhanced contractile effect seen with carvedilol could be blocked by another antagonist competing for βARs (Fig. 1 E and J; P < 0.001 compared to carvedilol alone). These observations suggest the EDL contractility increase in response to carvedilol was unique among the three βAR antagonists tested.
Carvedilol Enhances Skeletal Muscle Contractility but Does Not Lead to Hypertrophy of Skeletal Muscle.
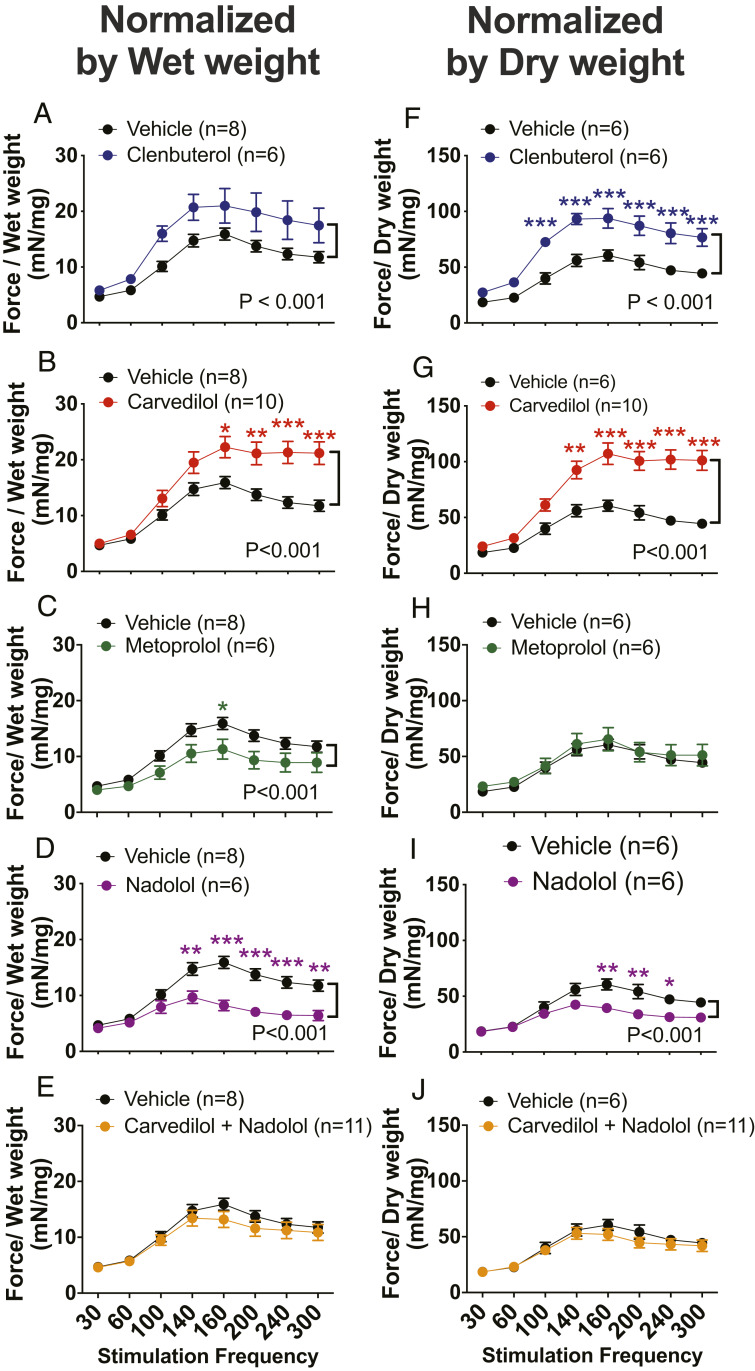
We examined the effects of βAR antagonists on βAR-mediated hypertrophic response of EDL muscles after chronic administration of drugs by measuring the wet and dry weights after drug treatment. We observed an increase of normalized EDL wet weight with chronic treatment of clenbuterol compared to the vehicle treatment (6.1 ± 0.2 mg/cm vs. 5.2 ± 0.2 mg/cm normalized by tibia length, SI Appendix, Table S1; P < 0.05) in concordance with previous reports (18, 20, 21). Despite the enhanced EDL contractility determined by the force–frequency relationship (Fig. 1 B and G), carvedilol treatment was associated with a decreased EDL wet weight normalized by tibia length by 17% compared to vehicle treatment (4.3 ± 0.2 mg/cm vs. 5.2 ± 0.2 mg/cm normalized by tibia length, SI Appendix, Table S1; P < 0.01). Neither metoprolol nor nadolol affected EDL muscle weight, and nadolol blocked the effects of carvedilol treatment to reduce EDL weight (SI Appendix, Table S1). There was no significant decrease in EDL dry weight within the carvedilol-treated group compared to vehicle-treated controls.
Next, we normalized absolute force by both EDL wet and dry weights (Fig. 2). Contractile forces normalized either to wet or dry weight from wild-type βarr1Flox mice showed an increase in EDL muscle contractility when treated with clenbuterol (Fig. 2 A and F; P < 0.001). Enhanced EDL contractility was also observed when mice were treated with carvedilol regardless of whether the force data were normalized by wet (Fig. 2B; P < 0.001) or dry weight (Fig. 2G; P < 0.001). This effect of carvedilol on potentiating muscle contractility was not observed for two other β-blockers, metoprolol and nadolol, and could be abolished with the concomitant administration of carvedilol with nadolol (Fig. 2 C–E and H–J).
Fig. 2.
β-Arrestin-biased agonist carvedilol enhanced contractility of skeletal muscle. Force–frequency curves for EDL muscles from βarr1Flox mice after 2 wk of clenbuterol (1 mg/kg/day) (A and F), carvedilol (1 mg/kg/day) (B and G), metoprolol (10 mg/kg/day) (C and H), nadolol (10 mg/kg/day) (D and I), nadolol (10 mg/kg/day) combined with carvedilol (1 mg/kg/day) (E and J) compared to vehicle (10% DMSO, 300 mM ascorbic acid in saline solution) treatment. The contractile amplitudes at each frequency and for each treatment group normalized by EDL wet weight were presented (A–E). The contractile amplitudes normalized by EDL dry weight were presented (F–J). Data represent the mean ± SEM for n independent EDL muscles as marked in the figure. Vehicle-treated group was used to compare to all treatments. Statistical significance versus vehicle-treated group was performed by using a two-way ANOVA with Sidak’s multiple comparison test (*P < 0.05; **P < 0.01; ***P < 0.001).
To further investigate the effects of carvedilol on skeletal muscle fiber composition and fiber size hypertrophy, we analyzed the fiber sizes and types in histological cross-sections of muscles labeled with anti-dystrophin, BF-F3 (major histocompatibility complex [MHC] IIB), Sc-71 (MHC IIA), and BA-F8 (MHC I) from carvedilol-treated mice and vehicle-treated control (22) (SI Appendix, Fig. S1A). We failed to identify a change in the relative numbers and sizes of three fiber types in carvedilol-treated EDL muscle compared to the vehicle-treated control (SI Appendix, Fig. S1 B–D). In particular, carvedilol administration did not alter the abundance of fast glycolytic fibers (IIb and IIx) in EDL muscle (SI Appendix, Fig. S2E). Similarly, the diameters of the fibers did not increase in carvedilol-treated muscles, in contrast to the diameters of type IIa and IIx fibers seen in clenbuterol-treated muscles (SI Appendix, Fig. S1 B and D). Taken together, these data indicate that although carvedilol and clenbuterol both enhance contractility in EDL muscles, these agents modulate skeletal muscle hypertrophy and remodeling in distinct ways (20, 23).
Carvedilol Enhances Tetanic Force of Skeletal Muscle.
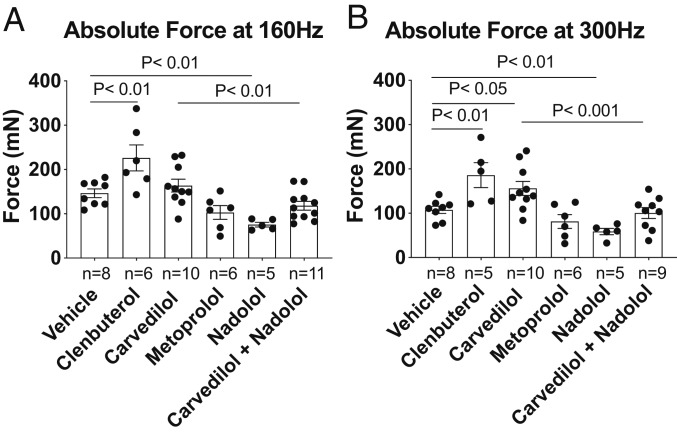
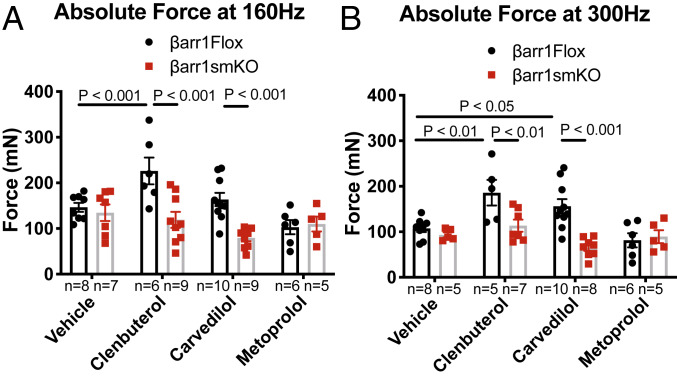
To rigorously characterize the effect of various βAR antagonists on skeletal muscle function, we analyzed tetanic contractile forces at 160 Hz and 300 Hz. βarr1Flox mice treated with clenbuterol showed enhanced tetanic force at both 160-Hz and 300-Hz stimulation frequency compared to vehicle-treated mice (Fig. 3 A and B; P < 0.01). Carvedilol-treated βarr1Flox mice showed an enhanced tetanic response at 300 Hz (156.6 ± 15.8 mN) compared to vehicle control (119.3 ± 8.8 mN; Fig. 3B; P < 0.05) but not at 160 Hz. In contrast, mice treated with nadolol or metoprolol did not exhibit any enhancement in EDL tetanic contraction compared to vehicle (Fig. 3 A and B). The enhanced response to tetanic stimulation with carvedilol was abolished by cotreatment with nadolol (Fig. 3B; P < 0.001 vs. carvedilol alone).
Fig. 3.
Effects of βAR ligands on EDL tetanic force. Contractile properties and muscle weights of EDL muscles from βarr1Flox mice were examined after 2 wk of carvedilol (1 mg/kg/day), metoprolol (10 mg/kg/day), nadolol (10 mg/kg/day), nadolol (10 mg/kg/day) combined with carvedilol (1 mg/kg/day), and clenbuterol (1 mg/kg/day) in the vehicle (10% DMSO, 300 mM ascorbic acid in saline solution). The absolute forces at 160 Hz (A) and at 300 Hz (B) responses elicited in intact EDL muscles were presented. Data represent the mean ± SEM for n independent EDL muscles as marked in the figure. Statistical comparison was performed by using a one-way ANOVA with Tukey’s (carvedilol alone vs. carvedilol with nadolol) and Dunnett’s multiple comparison test (vs. vehicle treatment).
β-Arrestin 1 Mediates Carvedilol-Enhanced Skeletal Muscle Contractility.
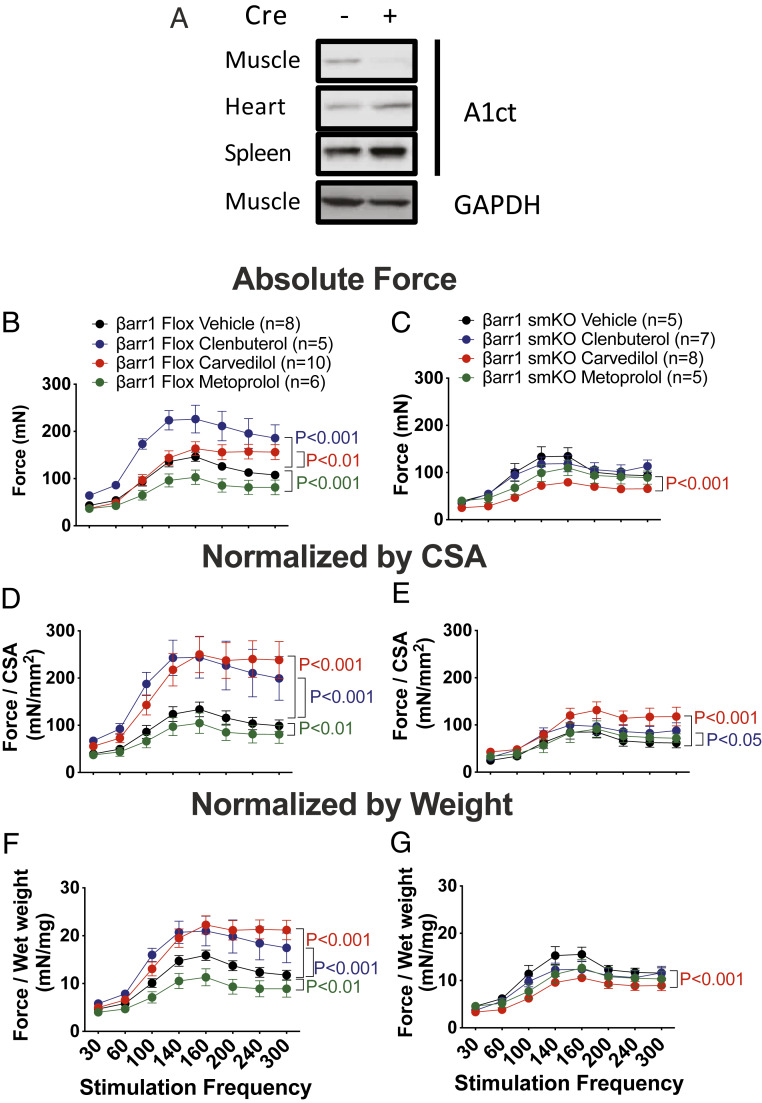
To determine the contribution of βarr1 to skeletal muscle contractility, we generated skeletal muscle-specific βarr1 KO mice (βarr1smKO) by crossing βarr1Flox mice with mice expressing myogenin cre recombinase (24) to delete βarr1 in skeletal muscle specifically (Fig. 4A). βarr1smKO mice were then treated with βAR antagonists (carvedilol and metoprolol), the βAR agonist (clenbuterol), or vehicle for 2 wk via implanted osmotic pump. We then excised the EDL muscles and measured the force–frequency relationships. Absolute nonnormalized contractile amplitude at each frequency and for each treatment group are presented in Fig. 4B (βarr1Flox mice) and Fig. 4C (βarr1smKO mice). We observed that both clenbuterol- and carvedilol-stimulated enhancement of EDL contractility was abolished in βarr1smKO mice (Fig. 4C). We found that drug treatment also notably affected muscle size and weight in both βarr1Flox mice and βarr1smKO mice (SI Appendix, Table S1). Since skeletal muscle force is a function of both muscle mass and the intrinsic contractile properties of the muscle, we compared the specific force normalized by either CSA (Fig. 4 D and E) or EDL muscle wet weight (Fig. 4 F and G). Clenbuterol and carvedilol treatments enhanced the absolute contractile force of skeletal muscle (Fig. 4B), and when normalized by either CSA (Fig. 4D) or wet weight (Fig. 4F). The nonnormalized effects of carvedilol and clenbuterol were abolished in βarr1smKO mice (Fig. 4C), and were markedly diminished (carvedilol) or completed abrogated (clenbuterol) when normalized to CSA (Fig. 4E) or wet weight (Fig. 4G).
Fig. 4.
β-Arrestin 1 is required for enhanced EDL force frequency upon carvedilol-mediated βAR activation. (A) Tissue lysates from muscle, heart, spleen of βarr1flox mice and βarr1smKO mice subjected to measure β-arrestin 1 expression by immunoblotting using anti-β-arrestin 1 antibody (A1ct). Equal amounts of tissue lysates loaded were probed by immunoblotting using anti-GAPDH antibody. Force–frequency curves for EDL muscles from βarr1flox and βarr1smKO mice after 2 wk of carvedilol (1 mg/kg/day), metoprolol (10 mg/kg/day), and clenbuterol (1 mg/kg/day) compared to vehicle (10% DMSO, 300 mM ascorbic acid in saline solution) treatment. Absolute force relationship was presented in βarr1Flox mice (B) and βarr1smKO mice (C). Specific force normalized to CSA was presented inβarr1Fox (D) and βarr1smKO mice (E). Specific force normalized to muscle weight was presented in βarr1Flox mice (F) and βarr1smKO mice(G). Data represent the mean ± SEM for n independent EDL muscles as marked in the figure. Statistical significance versus vehicle-treated group was performed by using a two-way ANOVA with Sidak’s multiple comparison test. P value from vehicle vs. clenbuterol in blue, vehicle vs. carvedilol in red, and vehicle vs. metoprolol in green.
Vehicle-treated βarr1smKO mice showed decreased EDL contractility as measured by the force–frequency relationship compared to vehicle-treated βarr1Flox mice (P < 0.01 for absolute; P < 0.001 for normalized by CSA). Clenbuterol-treated βarr1smKO mice showed no increase in EDL contractility either in the absolute force (Fig. 4C) or force normalized to wet weight (Fig. 4G), but did demonstrate a slight increase in contractile force when normalized by CSA (Fig. 4E; P < 0.05) compared to vehicle-treated βarr1smKO mice. Consistent with our previous work with a global βarr1 KO mice, we observed reduced force of muscles from clenbuterol-treated βarr1smKO compared to clenbuterol-treated βarr1Flox mice (P < 0.001). βarr1smKO mice treated with carvedilol showed decreased absolute nonnormalized or normalized by weight EDL contractility compared to vehicle-treated βarr1smKO mice (Fig. 4 C and G; P < 0.001). In contrast, when normalized to CSA (Fig. 4E), we observed a small enhancement in contractile force in response to carvedilol treatment compared to the vehicle treatment (P < 0.001). However, this carvedilol-stimulated enhanced contractile force in βarr1smKO mice was markedly diminished relative to the effect of carvedilol in βarr1Flox mice treated with carvedilol (P < 0.001) in either nonnormalized or normalized conditions, confirming the vital role of βarr1 in carvedilol-stimulated enhanced skeletal muscle contractility. Metoprolol treatment decreased contractility in the βarr1Flox mice but did not affect contractility in βarr1smKO mice compared to vehicle control (Fig. 4 C, E, and G). We also observed muscles from carvedilol-treated βarr1smKO mice were smaller than vehicle-treated mice, consistent with our observations in βarr1Flox mice (SI Appendix, Table S1). While the effect of carvedilol on enhancing contractile force was lost in βarr1smKO mice (Fig. 4 C and G), when force is normalized to CSA a small increase in contractility at high stimulation frequencies was observed due to the effect of carvedilol on skeletal muscle size (Fig. 4E). Taken together, our data show that β2AR-mediated clenbuterol and carvedilol-induced increases in force–frequency contractility are mediated by βarr1 specifically in skeletal muscle.
β-Arrestin 1 Is Required for Carvedilol-Mediated Tetanic Force of Skeletal Muscle.
We next analyzed additional measures of skeletal muscle contractility in EDL muscles from βarr1smKO mice following drug treatment. Muscle weight and the contractile relationships of EDL muscle from βarr1Flox and βarr1smKO mice are summarized in SI Appendix, Table S1. As expected, enhanced EDL tetanic responses mediated by clenbuterol compared to vehicle control in βarr1Flox mice were eliminated in βarr1smKO mice exhibiting a marked reduction compared to βarr1Flox mice treated with clenbuterol (Fig. 5 A and B; P < 0.001 at 160 Hz and P < 0.01 at 300 Hz). Consistent with our findings on contractility, the enhanced contractile effect of carvedilol was abolished in βarr1smKO mice treated with carvedilol as seen by the marked reduction in EDL tetanic contractility at 300 Hz compared to βarr1Flox mice treated with carvedilol (Fig. 5B; P < 0.001). Metoprolol treatment showed no enhancement in contractility responses compared to vehicle treatment in EDL muscle from either βarr1Flox or βarr1smKO mice (Fig. 5 A and B). We observed that the clenbuterol-induced hypertrophic response was abolished in EDL from βarr1smKO mice (SI Appendix, Table S1; P < 0.01). There was no significant difference found in muscle weights from either βarr1Flox or βarr1smKO mice when carvedilol or metoprolol was administrated (SI Appendix, Table S1). Taken together, these data indicate that βarr1 is essential in mediating the β2AR-stimulated enhancement in contractile properties of skeletal muscle, and among several well-known β-blockers, carvedilol shows unique properties as a contractile agent for skeletal muscle.
Fig. 5.
Carvedilol-mediated enhanced skeletal muscle tetanic force requires β-arrestin 1. The absolute forces at 160 Hz (A) and at 300 Hz (B) of EDL muscles from βarr1Flox and βarr1smKO mice were examined after 2 wk of carvedilol (1 mg/kg/day), metoprolol (10 mg/kg/day), and clenbuterol (1 mg/kg/day) in the vehicle (10% DMSO, 300 mM ascorbic acid in saline solution). Data represent the mean ± SEM for n independent EDL muscles as marked in the figure. Statistical comparison was performed by using a one-way ANOVA with Tukey’s multiple comparison test (vs. vehicle treatment).
Discussion
In this study, we investigated the hypothesis that the β-blocker carvedilol, a βarr-biased agonist at the βAR could enhance skeletal muscle contractile function. We examined the contractile properties of EDL skeletal muscle after chronic administration of the βAR-βarr-biased agonist carvedilol in βarr1Flox and βarr1smKO mice. Remarkably, we observed that carvedilol stimulates an increase in contractile force in EDL to a similar extent as that observed with the classic β2AR agonist clenbuterol. This increase was not observed with two other βAR antagonists, metoprolol and nadolol, and was inhibited by cotreatment with nadolol, demonstrating that the action of carvedilol was mediated through βARs. Carvedilol-induced increase in tetanic force was abrogated in βarr1smKO mice, demonstrating cell-type specificity for this βarr1-dependent response. Taken together, these data provide strong evidence that carvedilol, a widely used βAR antagonist can induce an increase in tetanic force in skeletal muscles. This effect of carvedilol is unique among βAR antagonists tested and is directly attributable to the ability of carvedilol to function as a biased agonist on βarr-dependent signaling downstream of the βAR.
βARs are ubiquitously expressed receptors that have vital functions in cardiovascular, pulmonary, and skeletal muscle physiology (25, 26). Although both βAR subtypes are expressed in skeletal muscle, β2ARs are the more prevalent βAR subtype, comprising over 90% of all βARs in skeletal muscle (27, 28). Upon activation with epinephrine, β2ARs stimulate multiple signaling pathways leading to anabolic and hypertrophic responses in skeletal muscles (12). Since this property is not shared by carvedilol it is likely that it is mediated through stimulation of G proteins. In skeletal muscle, activation of these signaling pathways has been shown to increase synthesis of contractile proteins and decrease proteasomal and lysosomal proteolysis (29). Activation of the β2AR can enhance both structural and functional characteristics of regenerating skeletal muscle such that it has been suggested that β2AR agonists may serve as useful agents in the prevention and treatment of skeletal muscle weakness and wasting secondary to aging and certain disease processes (30–32). One such agent, the selective β2AR agonist clenbuterol, has been shown to increase skeletal muscle weight (21) and has been proposed as an improved therapeutic alternative relative to anabolic steroids or IGF1 for skeletal muscle wasting disorders (26, 33). While the use of clenbuterol to enhance skeletal muscle mass in patients with chronic heart failure has been investigated (13), its use for this indication is not currently approved by the US Food and Drug Administration.
βarrs are important intracellular scaffold proteins that mediate GPCR desensitization and internalization and promote intracellular signaling independent of or in concert with G protein activation. Our own work has established that carvedilol functions as a βarr-biased agonist at the β2AR (17) and that increases in skeletal muscle strength induced by the β2AR agonist clenbuterol are dependent upon βarr1 (18). In this study, we observed that the βarr-biased carvedilol induces increases in skeletal muscle contraction mediated via βARs and βarr1. Though the detailed mechanisms of βarr’s effects on skeletal muscle contraction are not clearly understood, there are several plausible mechanisms to consider.
GPCR–arrestin complexes have been shown to initiate nondesensitized signaling at the plasma membrane by coupling with ion channels, specifically the transient receptor potential cation channel subfamily (TRP). A βarr-biased agonist of the angiotensin II receptor type 1 receptor (AT1R) stimulates acute catecholamine secretion through coupling with the TRPC3 by recruiting of TRPC3 or phosphoinositide-specific phospholipase C to the AT1R–βarr1 signaling complex (34). Angiotensin stimulation also leads to recruitment of βarr1 to the TRPV4 that results in ubiquitination and internalization of TRPV4 (35). These data provide a functional link between βarrs and TRP channels and might explain a potential mechanism by which βarrs could regulate Ca2+ influx in skeletal muscle, and by extension skeletal muscle contractility. Additionally, D3 dopamine receptors regulate axon initial segment excitability modifying CaV3 voltage dependence to suppress high-frequency action potential generation in a β-arrestin-dependent manner (36, 37). In this way, these types of βarr-mediated fast communication pathways could also provide an explanation for how βarrs contribute to skeletal muscle contractility.
In our study, we observed that carvedilol was unique among βAR antagonists tested (metoprolol and nadolol) in its ability to stimulate contractile responses in skeletal muscles. We found that carvedilol can stimulate an increase in skeletal muscle contractility, both in absolute terms as well as when normalized for muscle weight or size. In contrast to the biased agonist signaling properties observed with carvedilol, metoprolol functions as a classic antagonist of βARs with no effects on skeletal muscle contractility. Interestingly, carvedilol failed to induce an increase in skeletal muscle weight or fiber type switching, suggesting the positive contractile βarr1-dependent signaling pathways of carvedilol are independent of prohypertrophic and remodeling signaling pathways induced by clenbuterol-stimulated β2AR downstream signaling. This is further supported by the finding that carvedilol may preserve skeletal muscle fatigability, whereas clenbuterol was observed to reduce time to fatigue in skeletal muscle. Although this observation did not reach statistical significance in our study, the finding is intriguing and could be a demonstration of a unique pattern of enhanced skeletal muscle performance induced by carvedilol compared to clenbuterol. A larger sample size would be necessary to confirm this finding. Taken together, these results further demonstrate that the effects observed with carvedilol are directly attributable to βarr1-dependent signaling and suggest possible clinical potential for carvedilol in the treatment of reduced skeletal muscle strength related to aging or other muscle-wasting disorders.
Decreased skeletal muscle strength contributes to increased disability, fatigue, diminished quality of life, and reduced survival. In particular, decreased skeletal muscle strength associated with aging and other pathologic conditions presents a significant medical problem associated with high morbidity and costs and without adequate therapeutic options currently. Aged muscle mass declines by 30 to 50% accompanied by a decrease in muscle strength and an increase in fatigability, and an increase in the susceptibility to contraction-induced damage (38). Further, it has been demonstrated that lower and declining skeletal muscle strength is associated with increased mortality independent of muscle mass (39). Loss of skeletal muscle strength occurs in association with malignant disease and with multiple chronic nonmalignant diseases, including heart failure, kidney disease, chronic obstructive pulmonary disease, neurological disease, AIDS, and rheumatoid arthritis (2, 3, 7). Clinically, treatment with carvedilol has been associated with a partial reversal of skeletal muscle loss in patients with severe chronic heart failure (40). Additionally, espindolol (the s‐enantiomer of pindolol), an agent that functions clinically as a nonselective βAR antagonist but which displays weak, partial agonism for both G protein and βarr-dependent signaling at the β2AR in vitro, has been shown to reverse weight loss, improve fat-free mass, and maintain fat loss in cachectic cancer patients (41). These results are consistent with our hypothesis that βarr-dependent signaling downstream of the βAR may impart unique positive clinical outcomes in skeletal muscle.
There are some limitations of this study that should be considered. First, while it is likely the effects of carvedilol are mediated through the β2AR, we have not directly determined the specific βAR subtype responsible for the effects of carvedilol. We have observed the ability of carvedilol to enhance skeletal muscle contractility can be inhibited by the nonselective βAR antagonist nadolol. Combined with the previous observation that the β2AR subtype makes up >90% of all βARs in skeletal muscle (28, 42) and a lack of a previous reported role for β1ARs in mediating skeletal muscle contractility, we hypothesize the effects we observe with carvedilol on skeletal muscle contractility are primarily mediated via the β2AR.
Second, there is evidence in support of alternative explanations that other β-blockers may attenuate cachexia in specific situations (4, 43, 44). While of potential interest from a clinical standpoint, in this study we found that carvedilol enhances skeletal muscle contractility. This is a unique observation among βAR antagonists, and appears to be a direct result of carvedilol’s ability to stimulate signaling via βarr, which is a distinctive signaling property of carvedilol among βAR antagonists (17). More clinical studies will likely provide more insight on the roles of β-blockers on skeletal muscle contractility.
GCPR signaling bias, also known as functionally selective signaling, is a burgeoning concept in pharmacologic design and discovery (45). The concept—that a specific ligand could stimulate a subset of a given receptor’s signal transducers and thereby stimulate only desired downstream effects while avoiding potential adverse effects—is currently being tested at various stages of drug development across multiple GPCRs. The function of βarr1 in modulating β2AR signaling differs across disease conditions. An in depth understanding of the molecular mechanism(s) by which β2AR stimulation augments skeletal muscle performance may serve as the basis for drug discovery initiatives focused on therapeutic agents that induce βarr1 signaling in the treatment of age and disease-related skeletal muscle wasting. We propose that these observations could lay the groundwork for clinical trials investigating this question in selected clinical populations. Our work highlights the potential for one such biased ligand, carvedilol, that is well tolerated across a variety of clinical populations, to target an unmet clinical need.
Materials and Methods
Animals.
All animal experimental protocols were approved by the Institutional Animal Care and Use Committee at Duke University Medical Center and were performed in accordance with the standards established by the US Animal Welfare Acts.
Skeletal Muscle-Specific Deletion of Targeted Gene.
βarr1flox mice were generated using recombineering techniques as previously described (18). Two loxP cassettes were inserted into gene flanking coding exon 2. Breeding of myogenin-cre mice (Tg[Myog-cre]1Eno, The Jackson Laboratory) with βarr1flox mice led to generation of skeletal muscle specific deletion mice (βarr1smKO). Littermate βarr1flox mice lacking Cre were used as WT control.
Chronic Drug Delivery Using Isotonic Pump.
Twelve-week-old mice of either sex of the following genotypes were used. After anesthetizing with isoflurane, the animal with s.c. injection of ketamine/xylazine (100 and 2.5 mg/kg, respectively), an s.c. osmotic pump (Alzet 2002: Durect), containing either vehicle (10% dimethyl sulfoxide [DMSO] and 0.3 mM ascorbic acid), clenbuterol (1 mg/kg/day), carvedilol (1 mg/kg/day), metoprolol (10 mg/kg/day), and nadolol (10 mg/kg/day) was placed in the tissue immediately lateral to the spine on the posterior of the animal. We used 10 times more dose of metoprolol and nadolol considered to their affinities (46).
Measurement of the Contractile Property of EDL.
The contractile property of EDL was measured as previously described (18, 47). The isolated EDL was suspended in modified Krebs buffer (118 mM NaCl, 4.8 M KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 25 mM NaHCO3, and 11 mM glucose, pH 7.4) and bubbled with a premixed gas consisting of 20% O2, 5% CO2, and balance N2 (19, 48). After the optimal length of the isolated EDL was set, the twitch responses at 40 volts with a 0.5-ms square pulse and tetanus responses at 160 Hz with a 300-ms train were measured using LabChart 7 (ADInstruments). The force–frequency relationship was determined from 30-Hz up to 300-Hz trains at 40 volts with a 3-min rest between each train. The amplitude for each treatment group was normalized by CSA or muscle weight. Time to fatigue was determined to be the time the EDL took to contract at 50% of the amplitude obtained at the initial 100-Hz stimulus for 300 ms.
Statistical Analyses.
Data are presented as mean ± SEM. Statistical analysis was performed using a one-way ANOVA with Tukey’s multiple comparison test. Statistical analysis was performed using a one-way ANOVA with Dunnett’s multiple comparison test compared to its vehicle treatment control. Statistical analysis was performed using a two-way ANOVA with Sidak’s multiple comparison test compared between βarr1Flox and βarr1smKO under the same drug treatment. Statistical significance in each time point of kinetic graphs was determined by using a two-way analysis with Sidak’s or Tukey’s multiple comparison tests. For the statistical comparison of two conditions, the one sample t test was used (Prism). The level of significance is indicated as follows: ***P < 0.001, **P < 0.01, *P < 0.05.
Data Availability.
All data generated or analyzed during this study are within the paper and SI Appendix.
Supplementary Material
Acknowledgments
R.J.L. is an investigator with the Howard Hughes Medical Institute. This work was supported in part by grants from the NIH to R.J.L. (HL16037-47), C.A.G. (HD070872 and HD096385), J.W.W. (HL133488), P.B.R. (DK109911 and HD096385), and H.A.R. (HL056687 and HL075443). We are grateful to Chris Ingersol for supervising the mouse colony; Victoria Bryson for technical assistance; Ali Su and Andrew Ahn for counting fibers and measuring fiber size; Dr. Neil Freedman, Dr. Richard Premont, Dr. Julia Walker, and Dr. Chris Kontos (Duke University) for discussing data; and Donna Addison, Joanne Bisson, Victoria Brennand, and Quivetta Lennon for secretarial assistance.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1920310117/-/DCSupplemental.
References
- 1.Wallengren O., Lundholm K., Bosaeus I., Diagnostic criteria of cancer cachexia: Relation to quality of life, exercise capacity and survival in unselected palliative care patients. Support. Care Cancer 21, 1569–1577 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Saitoh M., et al. , Sarcopenia, cachexia, and muscle performance in heart failure: Review update 2016. Int. J. Cardiol. 238, 5–11 (2017). [DOI] [PubMed] [Google Scholar]
- 3.von Haehling S., Anker S. D., Cachexia as a major underestimated and unmet medical need: Facts and numbers. J. Cachexia Sarcopenia Muscle 1, 1–5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parise G., Snijders T., Myostatin inhibition for treatment of sarcopenia. Lancet Diabetes Endocrinol. 3, 917–918 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Bhasin S., et al. , Drug insight: Testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat. Clin. Pract. Endocrinol. Metab. 2, 146–159 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musarò A., et al. , Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 27, 195–200 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Cohen S., Nathan J. A., Goldberg A. L., Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 14, 58–74 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Sakuma K., Yamaguchi A., Sarcopenia and age-related endocrine function. Int. J. Endocrinol. 2012, 127362 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martone A. M., et al. , Treating sarcopenia in older and oldest old. Curr. Pharm. Des. 21, 1715–1722 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Pope H. G., Jr, et al. , Adverse health consequences of performance-enhancing drugs: An endocrine society scientific statement. Endocr. Rev. 35, 341–375 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Cola G., Cool M. H., Accili D., Hypoglycemic effect of insulin-like growth factor-1 in mice lacking insulin receptors. J. Clin. Invest. 99, 2538–2544 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams J. H., Barnes W. S., The positive inotropic effect of epinephrine on skeletal muscle: A brief review. Muscle Nerve 12, 968–975 (1989). [DOI] [PubMed] [Google Scholar]
- 13.Kamalakkannan G., et al. , Clenbuterol increases lean muscle mass but not endurance in patients with chronic heart failure. J. Heart Lung Transplant. 27, 457–461 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Thomsen A. R. B., et al. , GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell 166, 907–919 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J., et al. , Gαi is required for carvedilol-induced β1 adrenergic receptor β-arrestin biased signaling. Nat. Commun. 8, 1706 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim I. M., et al. , Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc. Natl. Acad. Sci. U.S.A. 105, 14555–14560 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wisler J. W., et al. , A unique mechanism of beta-blocker action: Carvedilol stimulates beta-arrestin signaling. Proc. Natl. Acad. Sci. U.S.A. 104, 16657–16662 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J., et al. , β-arrestin 1 regulates β2-adrenergic receptor-mediated skeletal muscle hypertrophy and contractility. Skelet. Muscle 8, 39 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eu J. P., et al. , Concerted regulation of skeletal muscle contractility by oxygen tension and endogenous nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 100, 15229–15234 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sirvent P., et al. , Effects of chronic administration of clenbuterol on contractile properties and calcium homeostasis in rat extensor digitorum longus muscle. PLoS One 9, e100281 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinkle R. T., et al. , Skeletal muscle hypertrophy and anti-atrophy effects of clenbuterol are mediated by the beta2-adrenergic receptor. Muscle Nerve 25, 729–734 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Bloemberg D., Quadrilatero J., Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7, e35273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maltin C. A., Delday M. I., Reeds P. J., The effect of a growth promoting drug, clenbuterol, on fibre frequency and area in hind limb muscles from young male rats. Biosci. Rep. 6, 293–299 (1986). [DOI] [PubMed] [Google Scholar]
- 24.Lepper C., Conway S. J., Fan C. M., Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 460, 627–631 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomiyama H., Yamashina A., Beta-blockers in the management of hypertension and/or chronic kidney disease. Int. J. Hypertens. 2014, 919256 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryall J. G., Lynch G. S., The potential and the pitfalls of beta-adrenoceptor agonists for the management of skeletal muscle wasting. Pharmacol. Ther. 120, 219–232 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Kim Y. S., Sainz R. D., Molenaar P., Summers R. J., Characterization of beta 1- and beta 2-adrenoceptors in rat skeletal muscles. Biochem. Pharmacol. 42, 1783–1789 (1991). [DOI] [PubMed] [Google Scholar]
- 28.Williams R. S., Caron M. G., Daniel K., Skeletal muscle beta-adrenergic receptors: Variations due to fiber type and training. Am. J. Physiol. 246, E160–E167 (1984). [DOI] [PubMed] [Google Scholar]
- 29.Gonçalves D. A., et al. , Clenbuterol suppresses proteasomal and lysosomal proteolysis and atrophy-related genes in denervated rat soleus muscles independently of Akt. Am. J. Physiol. Endocrinol. Metab. 302, E123–E133 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Ryall J. G., et al. , Intramuscular beta2-agonist administration enhances early regeneration and functional repair in rat skeletal muscle after myotoxic injury. J. Appl. Physiol. (1985) 105, 165–172 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Beitzel F., et al. , Beta2-adrenoceptor agonist fenoterol enhances functional repair of regenerating rat skeletal muscle after injury. J. Appl. Physiol. (1985) 96, 1385–1392 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Schertzer J. D., et al. , Beta2-agonist administration increases sarcoplasmic reticulum Ca2+-ATPase activity in aged rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 288, E526–E533 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Lynch G. S., Ryall J. G., Role of beta-adrenoceptor signaling in skeletal muscle: Implications for muscle wasting and disease. Physiol. Rev. 88, 729–767 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Liu C. H., et al. , Arrestin-biased AT1R agonism induces acute catecholamine secretion through TRPC3 coupling. Nat. Commun. 8, 14335 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukla A. K., et al. , Arresting a transient receptor potential (TRP) channel: Beta-arrestin 1 mediates ubiquitination and functional down-regulation of TRPV4. J. Biol. Chem. 285, 30115–30125 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang S., et al. , β-Arrestin-Dependent dopaminergic regulation of calcium channel activity in the axon initial segment. Cell Rep. 16, 1518–1526 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carr R., 3rd, et al. , β-arrestin-biased signaling through the β2-adrenergic receptor promotes cardiomyocyte contraction. Proc. Natl. Acad. Sci. U.S.A. 113, E4107–E4116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janssen I., Shepard D. S., Katzmarzyk P. T., Roubenoff R., The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 52, 80–85 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Metter E. J., Talbot L. A., Schrager M., Conwit R., Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J. Gerontol. A Biol. Sci. Med. Sci. 57, B359–B365 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Clark A. L., et al. , Effect of beta-adrenergic blockade with carvedilol on cachexia in severe chronic heart failure: Results from the COPERNICUS trial. J. Cachexia Sarcopenia Muscle 8, 549–556 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart Coats A. J., et al. ; for and on behalf of the ACT‐ONE study group , Espindolol for the treatment and prevention of cachexia in patients with stage III/IV non-small cell lung cancer or colorectal cancer: A randomized, double-blind, placebo-controlled, international multicentre phase II study (the ACT-ONE trial). J. Cachexia Sarcopenia Muscle 7, 355–365 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choo J. J., Horan M. A., Little R. A., Rothwell N. J., Anabolic effects of clenbuterol on skeletal muscle are mediated by beta 2-adrenoceptor activation. Am. J. Physiol. 263, E50–E56 (1992). [DOI] [PubMed] [Google Scholar]
- 43.Ouzounian M., Hassan A., Cox J. L., Johnstone D. E., Howlett J.; Improving Cardiovascular Outcomes in Nova Scotia Study Investigators , The effect of spironolactone use on heart failure mortality: A population-based study. J. Card. Fail. 13, 165–169 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Springer J., et al. , Prevention of liver cancer cachexia-induced cardiac wasting and heart failure. Eur. Heart J. 35, 932–941 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wisler J. W., Rockman H. A., Lefkowitz R. J., Biased G protein-coupled receptor signaling: Changing the paradigm of drug discovery. Circulation 137, 2315–2317 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Westhuizen E. T., Breton B., Christopoulos A., Bouvier M., Quantification of ligand bias for clinically relevant β2-adrenergic receptor ligands: Implications for drug taxonomy. Mol. Pharmacol. 85, 492–509 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Oishi P. E., Cholsiripunlert S., Gong W., Baker A. J., Bernstein H. S., Myo-mechanical analysis of isolated skeletal muscle. J. Vis. Exp. 48, 2582 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Honig C. R., Gayeski T. E., Resistance to O2 diffusion in anemic red muscle: Roles of flux density and cell PO2. Am. J. Physiol. 265, H868–H875 (1993). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are within the paper and SI Appendix.