Abstract
Recent evidence for the effects of metallic engineered nanoparticles (ENPs) on plants and plant systems was examined together with its implications for other constituents of the Society-Environment-Economy (SEE) system. In this study, we were particularly interested to determine whether or not metallic ENPs have both stimulatory and inhibitory effects upon plant performance. An emphasis was made to analyze the scientific evidence on investigations examining both types of effects in the same studies. Analysis of evidence demonstrated that metallic ENPs have both stimulatory and inhibitory effects mostly in well-controlled environments and soilless media. Nano zero-valent iron (nZVI) and Cu ENPs have potential for use as micronutrients for plant systems, keeping in mind the proper formulation at the right dose for each type of ENP. The concentration levels for the stimulatory effects of Cu ENPs are lower than for those for nZVI. Newer findings showed that extremely smaller concentrations of Au ENPs (smaller than those for nZVI and Cu ENPs) induce positive effects for plant growth, which is attributed to effects on secondary metabolites. Ag ENPs have demonstrated their usage as antimicrobial/pesticidal agents for plant protection; however, precautions should be taken to avoid higher concentrations not only for plant systems, but also, other constituents in the SEE. Further research is warranted to investigate the stimulatory and inhibitory effects of metallic ENPs in soil media in order to broaden the horizon of sustainable agriculture production in terms of higher and safer yields so as to meet the food requirements of human population.
Keywords: Metallic engineered nanoparticles, Metal ions, Plant system, Society-Environment-Economy, Stimulatory effects, Inhibitory effects
Graphical Abstract

1. Introduction
In today’s world, one is confronted with global challenges having their roots in human actions such as over-population, resource depletion, and human and ecologic health. Therefore, emerging technologies have been continually called upon as an avenue to present opportunities to tackle these global challenges. By virtue of their occurrence in economic systems, there is always a need to keep the constituents of the larger SEE System in consideration so as to augment the benefits and reduce the risks for all, the founding principle for the planet’s sustainability (Tolaymat et al., 2015a, Tolaymat et al., 2015b).
Metallic nanomaterials have been introduced as emerging technologies to respond to some of the aforementioned challenges. For example, iron and copper ENPs have been utilized as plant micronutrients. Compared with the macronutrients (N, P, K), only trace levels of micronutrients are required for the healthy growth of crops and other plants. In this respect, micronutrients are often added to the macronutrients at low rates (e.g., 5 mg/l) as soluble salts for crop uptake (Liu and Lal, 2015). Furthermore, due to their success as antifungal and antibacterial agents, Ag ENPs have been introduced in applications to control phytopathogens and to extend the vase life of some flowers (Nair et al., 2010). Metallic ENPs have also been used as nanopesticides to increase the dispersion and wettability of agricultural formulations, and unwanted pesticide movement (Khot et al., 2012).
With the above in mind, while some metallic ENPs may have positive effects upon plant systems, it is also possible that this class of ENPs have negative implications for plant systems (Anjum et al., 2015, Arruda et al., 2015, Ma et al., 2015, Ma et al., 2010, Miralles et al., 2012, Van Aken, 2015). For example, barriers to growth for plant systems may occur if the concentration level exceeds the threshold for which the nanomaterial is needed as a micronutrient (Dimkpa, 2014). Metallic ENPs may also impact soil microbial structure or function, influencing nutrient turnover in soil over longer time scales. In addition, metallic ENPs may directly or indirectly alter the formation of symbiotic associations with root fungi and bacteria, influencing nutrient availability and uptake, and plant growth (Dimkpa, 2014). Specific applications for some metallic nanomaterials may also prove to be detrimental to plant systems. nZVI, for example, is emerging as an option for the treatment of contaminated soil and groundwater targeting chlorinated organic contaminants and inorganic anions or metals (Bennett et al., 2010, Gomes et al., 2014, Kocur et al., 2014, Mueller et al., 2012). It is possible that specific ENPs at concentration levels in the harmful or toxic range find their way to plant systems, hence, impacting plant performance and growth.
With the founding principle for planet Earth’s sustainability in mind, the main goal of this study is to examine the evidence in the published literature for the positive and negative effects of metallic ENPs, if any, upon plant systems and to provide insights for these implications in the broader context of the SEE system. An emphasis was made to analyze investigations examining both effects in the same studies. The following specific aims are designed to achieve the study objective: (1) to document the evidence for the effects of metallic ENPs on plants and plant systems; (2) to analyze the evidence in terms of exposure, subject population, study outcomes, study design, and main results; and, (3) to provide insights on the evidence and uncertainties for the constituents of the SEE system. With the above in mind, we were particularly interested to determine whether or not metallic nanoparticles have stimulatory and inhibitory effects upon plants and plant systems so as to broaden the horizon for sustainable agriculture production in terms of higher and safer yields; to our knowledge, this subject has not been addressed in the published literature as evident by recent reviews (Ditta and Arshad, 2016, Sarmast and Salehi, 2016, Schwab et al., 2016). Finally, suggestions are made for future research to improve our understanding of the potential for these materials to be used in agricultural systems.
2. Methods
The methodology employed in this research consisted of the five standard steps deployed in evidence-based medicine (Sackett et al., 1996). As a first step in this process, the work reported herein attempted to provide answers to the stimulatory and inhibitory effects of metallic nanomaterials, if any, upon plant systems. Finding the evidence, the second step in the process was sought using electronic search as the primary source and investigating the bibliographies of gathered experimental and reviewed articles as the secondary source. For the electronic search, five databases were utilized: ACS, Scopus, Academic Search Complete, Pubmed, and WebofKnowledge. The following keyword combinations and Boolean operators were employed in the search: (nanoparticle OR nanoparticle OR nanomaterial OR nanomaterial) AND (metal OR silver OR gold OR iron OR aluminum OR copper OR Ag OR Au OR nZVI OR Al OR Cu OR Mn OR Zn) AND (plant OR botany). This keyword manipulation allowed a larger number of articles to be included in the first output of electronic search. The idea was to err on having more than less. The initial list was further subjected to inclusion and exclusion criteria on the basis of reviewing the titles first, then the abstract, and finally the retrieved full articles. Thereafter, bibliographies of the final list of experimental studies as well as those from review articles were fully retrieved and applied the same inclusion and exclusion criteria. The final articles meeting the primary study goal constituted the source of evidence. This process ended on May 25, 2016 and only studies in English were included.
Appraising the evidence, the third step in the evidence base methodology, analyzed the experimental studies contributing evidence in terms of: (a) exposure, (b) subject population, (c) study outcome, and (d) main results. This was followed by applying the evidence to an analysis of the concentration levels in the gathered evidence with respect to the positive (e.g., stimulatory) and negative (e.g., inhibitory) effects of metallic ENPs. As part of the final step in the analysis, we evaluated responses to ENPs in the context of possible explanatory mechanisms for the stimulatory/inhibitory effects of metallic nanoparticles. This included an inquiry into the nano versus the ionic form to determine the root causes of the observed effects. In addition, gaps in the scientific literature were pointed out for consideration in future research.
3. Results
3.1. General analysis of evidence
Analysis of evidence suggests that there were studies on nZVI (Canivet et al., 2015, Jessick et al., 2013, Kim et al., 2014a, Kim et al., 2014b, Li et al., 2015), Ag (Ardakani, 2013, Barrena et al., 2009, De La Torre-Roche et al., 2013, Dimkpa et al., 2013, Feng et al., 2013, García-Sánchez et al., 2015, Geisler-Lee et al., 2012, Gubbins et al., 2011, Jo et al., 2015, Kaveh et al., 2013, Krishnaraj et al., 2012, Kumari et al., 2009, Larue et al., 2014, Lee et al., 2012, Li et al., 2012, Musante and White, 2012, Mustafa et al., 2015, Nair and Chung, 2014, Parveen and Rao, 2014, Pokhrel and Dubey, 2013, Qian et al., 2013, Sharif et al., 2013, Song et al., 2013, Stampoulis et al., 2009, Syu et al., 2014, Thuesombat et al., 2014, Wang et al., 2013, Yin et al., 2011, Yin et al., 2012), Cu (Lee et al., 2008, Musante and White, 2012, Pradhan et al., 2015, Shah and Belozerova, 2009, Stampoulis et al., 2009), Au (Barrena et al., 2009, Gopinath et al., 2014, Kumar et al., 2013, Shah and Belozerova, 2009, Taylor et al., 2014, Zhu et al., 2012), Al (Doshi et al., 2008), Pt (Asztemborska et al., 2014), Mn (Pradhan et al., 2013), and Zn (Tarafdar et al., 2014) ENPs examining both positive and negative effects upon plant performance. Ag ENPs were the most studied metallic nanoparticles, followed by nZVI, Cu ENPs, Au ENPs, Mn ENPs and Zn ENPs.
Table 1 shows an analysis of exposure by particle size (nm), exposure duration (days) and concentration (e.g., mg/l). The great majority of studies reported the ENP size in the nano range, being < 100 nm in most cases. Exposure duration was usually less than or equal to 4 weeks, with only one study reporting a duration of 80 days. The ENP concentration range was also very wide, and in the case of micronutrients, was not necessarily provided in proportion to the known requirements for these plant nutrients.
Table 1.
Analysis of exposure parameters in terms of particle size, exposure and concentration.
| Type of metallic ENPs | Exposure | Growth medium | Source | ||
|---|---|---|---|---|---|
| Size (nm) | Duration (days) | Concentration | |||
| nZVI | 300 to 400 | 28 | 25, 50, 200, 500, 1000 mg/l | Plants were grown hydroponically in a greenhouse. Healthy Typha seedlings were placed into brown bottles containing N2-sparged solution with different concentrations of nZVI. Each bottle contained two seedlings. | (Jessick et al., 2013) |
| 20 to 80 | 5 | 0.56, 1.12, 2.24, 4.48, 8.96, 17.92 mg/l | Peanut seeds of similar sizes were germinated and grown in the acid-cleaned sand media for 18 days in a growth chamber. nZVI doses were added to modified Hoagland nutrient solutions. | (Li et al., 2015) | |
| NR | 14 | 500 mg/l | Plants were grown in a synthetic nutrient media (i.e., a half strength of standard Murashige and Skoog medium) placed in a series of 9 cm diameter Petri dishes. nZVI doses were added to the growth media. | (Kim et al., 2014a) | |
| 20 to 80 | ≤ 7 | 50 ng, 500 ng, 5 μg, 50 μg per plant | Prior to exposure to nZVI NPs: Plants were grown axenically on a solid BCD medium, in a culture chamber under irradiance and a light/dark periodicity of 16/8 h. Young plants were cultivated for 3 weeks in culture dish, then were transplanted into a new 6-well plates and were acclimatized for 1 week. Exposure to nZVI ENPs: The nanoparticles were suspended in commercial mineral water. nZVI suspension (500 μl) was applied by pipette (foliar exposure) onto the plants. |
(Canivet et al., 2015) | |
| 54 | ≤ 3 | 0.1 g/l | Hydroponic Culture: Plants were grown in a growth chamber in half strength of Murashige and Skoog medium. Exposure to nZVI was performed by mixing with the medium. Soil Culture: Plants were cultivated in a greenhouse, in an autoclaved soil mixed with nZVI. |
(Kim et al., 2014b) | |
| Ag ENPs | 5 to 10 | ≤ 11 | 0.01, 0.1, 1, 10, 100 mg/l | Plants were grown hydroponically in ¼ strength Hoagland solution. Exposure to Ag NPs was performed by mixing the required ENP concentration with the medium. | (Wang et al., 2013) |
| 6 to 25 | 7 | 1, 5, 10, 20, 40 mg/l | Plant seeds were grown in filter paper placed in sterilized Petri dishes. Medium solution including the desired concentration from Ag ENPs was added to the growth system in a greenhouse. | (Yin et al., 2011) | |
| 10 | 14 | 0.5, 1.5, 2.5, 3.5, 5 mg/l | Plants were grown a box consisting of sand matrix characterized with water-soluble trace elements that may influence plant growth. Ag ENPs were amended in the growth medium before seeding. Three independent growth studies were performed, each comprising of 3 plants per box for 5 boxes. | (Dimkpa et al., 2013) | |
| < 20 | 19 | 500, 2000 mg/l | Plants were grown in a series of jars containing vermiculite clay and 25% Hoagland’s solution mixed with Ag NPs. Or 2000 mg/l of bulk or NP Ag |
(De La Torre-Roche et al., 2013) | |
| 21 | 80 | 0.01, 0.1, 1 mg/kg | Plants were grown in a mixture of fine sand (< 2 mm diameter) and perlite (1:1 [v/v]). The growth medium and Ag NPs mixture were transferred into a series of plastic pots where three two-week-old clover seedlings were transplanted. | (Feng et al., 2013) | |
| 6 to 20 | 28 | 1, 10, 40 mg/l | Plant seeds were grown in a greenhouse. Seeds of each species were soaked in jars containing Ag ENPs suspensions, then transferred into Petri dishes containing filter paper. Petri dishes were covered and sealed with tape and randomly placed in daytime (16 h) and at night (8 h). | (Yin et al., 2012) | |
| 39 | 7 | 1, 10, 100 g/l | Prior to exposure to Ag ENPs: Young lettuce plantlets were grown in fertilized compost free of Ag in a growth chamber (day/night photoperiod 16 h/8 h) until they reached the four-leaf stage. Exposure to Ag ENPS: Plants were exposed to droplets of Ag ENPs on the side of their leaves (foliar exposure). |
(Larue et al., 2014) | |
| 10 to 15 | 35 | 50, 100, 500, 1000, 5000 mg/l | Plant germination and root elongation: Seeds were soaked in Ag ENPs solutions, then were transferred into a series of Petri dishes equipped with filter papers in a growth chamber. | (Song et al., 2013) | |
| 10 | 14 | 0.2, 0.5, 3 mg/l | Plant seed germinated on Murashige and Skoog agar plates with or without various concentrations of Ag ENPs. | (Qian et al., 2013) | |
| 10 | 2 to 5 | 100, 300, 500, 1000, 2000 mg/kg dry soil | The plant assays were conducted in the agar and soil media. Ag ENPs were added to both media. | (Lee et al., 2012) | |
| Ag ENPs | 20 to 150 | NR | 0.1, 1, 10, 100, 1000 mg/l | Plant seed germination and seedling growth were performed hydroponically in sand soil, in a greenhouse under natural light and temperature. Plant seeds were exposed to Ag ENPs by soaking the ENPs and then by adding to the medium. | (Thuesombat et al., 2014) |
| 7.5 | ≤ 1 | 0.00015, 0.0015, 0.015, 0.15, 1.5, 15, 150 μg/ml | NR | (Jo et al., 2015) | |
| 2 to 50 | ≤ 30 | 10, 100 ppb; 10, 100 ppm | Plants were grown in a green house. Seed germination and seedlings were monitored after soaking plant seeds in Ag ENP suspension and were raised in cotton beds. | (Krishnaraj et al., 2012) | |
| NR | NR | 5, 10, 20, 50 mg/l | Plants were grown in a vase life evaluation room. Bacteria and Ag ENPs were mixed and added to the medium in the vase. | (Li et al., 2012) | |
| 13 | 5 | 20, 50 mg/l | Plant seeds were grown in a series of Petri dishes containing filter paper. Ag ENPs water suspension was added to the system to test its impact on the plant. | (Parveen and Rao, 2014) | |
| < 20 nm | NR | NR | Plants were placed in microcosms by batch sorption studies. Ag ENPs were pulsed to the decaying plant material. | (Sharif et al., 2013) | |
| NR | NR | 1.5, 3, 6, 12.5, 25, 50, 100, 400, 800 mg/ml | Plants were grown in both Petri dishes and pots. The pots were filled with a mixture of autoclaved loamy soil mixed with Ag ENPs. | (Ardakani, 2013) | |
| 20 to 100 | ≤ 14 | 5, 10, 20, 40, 80, 160 μg/l | Plants were grown in test vessels containing nutrition medium mixed with Ag ENPs. | (Gubbins et al., 2011) | |
| 15 | NR | 2 ppm | Plants were grown in a series of glass tubes containing reverse osmosis water supplemented with different concentrations of Ag ENPs and particle sizes. | (Mustafa et al., 2015) | |
| 11 | NR | < 73.4 μg/l | Ten seeds of maize and cabbage plants were allowed to germinate randomly in a series of Petri dishes containing filter papers. Citrate-Ag ENP solutions were then added to each Petri dish. | (Pokhrel and Dubey, 2013) | |
| < 100 | NR | 25, 50, 75, 100 ppm | Onion bulbs were grown under dark conditions in a cylindrical glass beaker at room temperature and a daily renewed water supply. Ag ENP suspension was added to the growing medium to test its impact on the plant for 4 h. | (Kumari et al., 2009) | |
| 8 to 47 | 3 | 1, 2, 10, 100 μM | Arabidopsis seeds were germinated on ½ Murashige and Skoog media. Primarily seedlings were transferred into new ½ Murashige and Skoog solid medium and 4-days old seedlings were exposed to Ag ENPs. | (Syu et al., 2014) | |
| 20 | 10 | 5 mg/l | Arabidopsis thaliana seeds were germinated under sterile conditions in Magenta boxes filled with semi-solid nutrient medium. The boxes were incubated under dark (8 h) and white fluorescent light (16 h) photoperiod. Ag ENPs were added to the nutrient medium for investigation of its impact on the plant growth. | (Kaveh et al., 2013) | |
| NR | 7 | 0.2, 0.5, 1 mg/l | Germination of rice caryposes was performed in growth chamber in dark conditions for 48 h. Germinated caryposes were transferred onto a series of test tubes containing filter papers amended with ¼ strength Hoagland’s medium supplemented with Ag ENPs and different concentrations. | (Nair and Chung, 2014) | |
| 20 to 80 | NR | 0.2–25 μg/ml | Arabidopsis thaliana seeds were sown on Petri dishes containing ½ Murashige and Skoog medium in a growth chamber. Seedlings were transferred to test tubes and incubated until roots had grown. Plants with developed aerial rosettes were then selected for further treatment with Ag ENPs. The latter was added to the medium to test its impact on the plant health. | (García-Sánchez et al., 2015) | |
| < 100 | 14 | 100, 500 mg/l | Cucurbita pepo plant was grown hydroponically and exposed to batches of Ag ENPs. Seeds were pre-germinated in moistened germination paper, and seedlings were transferred into amber vials containing 25% Hoagland’s solution. The seedlings were placed in a growth medium amended with Ag ENPs. | (Musante and White, 2012) | |
| NR | 15 | 100, 500, 1000 mg/l | Zucchini seeds were grown in batch hydroponic system. Seeds were added to moist germination paper and seedlings were subsequently added to amber vials containing 25% Hoagland solution. The seedlings were placed in a growth medium amended with Ag ENPs. | (Stampoulis et al., 2009) | |
| Ag ENPs | 20 to 80 | 14 | 66.84, 133.68, 267.36, 534 μg/l | Arabidopsis thaliana seeds were germinated and grown hydroponically in plates containing 25% Hoagland media amended with Ag ENPs. | (Geisler-Lee et al., 2012) |
| 2 | 7 | 100 mg/l | Cucumber and lettuce seeds were germinated for 7 days in an incubator in Ag ENPs amended medium. | (Barrena et al., 2009) | |
| Cu ENPs | < 100 | 14 | 100, 500 mg/l | Cucurbita pepo plant was grown hydroponically and exposed to batches of Cu ENPs. Seeds were pre-germinated in moistened germination paper, and seedlings were transferred into amber vials containing 25% Hoagland’s solution. The seedlings were placed in a growth medium amended with either the Cu ENPs. | (Musante and White, 2012) |
| NR | 15 | 100, 500, 1000 mg/l | Zucchini seeds were grown in batch hydroponic system. Seeds were added to moist germination paper and seedlings were subsequently added to amber vials containing 25% Hoagland solution. The seedlings were placed in a growth medium amended with Cu ENPs. | (Stampoulis et al., 2009) | |
| NR | 2 | 200, 400, 600, 800, 1000 mg/l | Mung bean and wheat seeds were germinated in wet cotton at a controlled temperature under dark conditions for 24 h. Toxicity tests were conducted in a series of Petri dish test units containing agar culture media at specific concentration of Cu ENPs. | (Lee et al., 2008) | |
| NR | 15 | 0.05, 0.1, 0.5, 1 mg/l | Mung bean seeds were impeded in Cu ENP solutions for 4–6 h, then kept in the Petri dishes for germination in dark. Seeds were then transferred in pots filled with perlite supplemented with Hoagland solution. They were grown for 15 days in a growth cabinet. | (Pradhan et al., 2015) | |
| NR | 0 and 15 | 0.013 (w/w dry soil) | Lettuce seeds were grown in jars containing soil. Potting was added the soil to mimic the agriculture soil. Cu ENPs were added to the soil at different concentrations. The jars were incubated at 25 °C under static conditions. | (Shah and Belozerova, 2009) | |
| Au ENPs | 6 to 10 | 5 | 31.25 nM | Plants seeds were allowed to germinate in a series of Petri dishes placed in an incubator in the dark. The seedlings were then transferred into jars amended with hydroponic solution containing major nutrients and Au ENPs. The plants were grown in a plant growth chamber. | (Zhu et al., 2012) |
| 24 | 15 | 10, 80 μg/l | Seeds of Arabidopsis thaliana were germinated in Petri dishes containing Murashige and Skoog media amended with certain Au ENP concentrations. | (Kumar et al., 2013) | |
| 7 to 108 | NR | 25, 50, 75, 100, 200, 300, 400 mg/l | Seeds of Arabidopsis thaliana were cultivated in agar plates containing ½ strength Murashige and Skoog media amended with Au ENPs. Seedlings were grown under light with day and night photoperiods. | (Taylor et al., 2014) | |
| NR | 0 and 15 | 0.013, 0,066 (w/w dry soil) | Lettuce seeds were grown in jars containing soil. Potting was added the soil to mimic the agriculture soil. Au ENPs were added to the soil at different concentrations. The jars were incubated at 25 °C under static conditions. | (Shah and Belozerova, 2009) | |
| 10 | 7 | 62 mg/l | Cucumber and lettuce seeds were germinated for 7 days in an incubator in Au ENP amended medium. | (Barrena et al., 2009) | |
| 25 | 20 to 50 | 500, 1000 mg/l | Gloriosa superba seeds were soaked in Au ENPs suspensions and kept in the dark at room temperature for 1 day. Au ENP-treated seeds were transferred into seedling trays containing sterile sand and were kept in a mist house. | (Gopinath et al., 2014) | |
| Al ENPs | NR | NR | 10, 100, 1000, 10,000 mg/kg | California red kidney bean plants and rye grass were grown in natural soil amended with increasing concentrations Al ENPs. The plants were watered daily. | (Doshi et al., 2008) |
| Pt ENPs | < 50 | NR | 1, 10, 50, 100 mg/l | Sinapis alba and Lepidium sativum plants were cultivated in containers. The growth medium was supplemented with Pt ENPs at different concentrations. | (Asztemborska et al., 2014) |
| Mn ENPs | 20 | 15 | 0.05, 0.1, 0.5, 1 mg/l | Seeds of mung bean were Mn ENP solutions for 4–6 h, then kept in the Petri dishes for germination in the dark. Seeds were then transferred in pots filled with perlite supplemented with Hoagland solution amended with Mn ENPs. They were grown for 15 days in a growth cabinet. | (Pradhan et al., 2013) |
| Zn ENPs | 18.5 | 42 | 10 mg/l | The seeds of pearl millet were grown in natural soil under rainfed condition. Zn ENPs were sprayed by foliar after 2 week of germination at a Zn ENP concentration of 10 mg/l. | (Tarafdar et al., 2014) |
Footnote: NR – not reported.
A wide range of plant species was examined in the evidence. Arabidopsis thaliana was the most investigated plant among the studies included in the evidence (22.5%). In all studies plants and plant systems were examined under controlled environmental conditions in incubators and greenhouses, using artificial planting media, sand culture or hydroponics. Only a handful of studies took into account the role of bacterial and microbial activities into plant performance, an area of needed future research. The study outcomes included growth and biomass parameters, as well as genetic responses.
3.2. Analysis of effects
In general, the effects of nanoparticles on plants and plant systems are typified with growth promotion and growth inhibitory outcomes. A brief account of the analysis of effects is first presented for each study followed by an assessment of effects across all studies, with the analysis of individual studies grouped by the type of metallic ENP (Fig. 1).
Figure 1.
Analysis of evidence by type of metallic ENPs: (a) Ag – n = 28; (b) Au – n = 3; (c) nZVI – n = 5; (d) Al – n = 1; (e) Cu – n = 4; (f) Pt – n = 1; and Zn – n = 1.
Footnote: n = number of studies in the evidence compiled in this research.
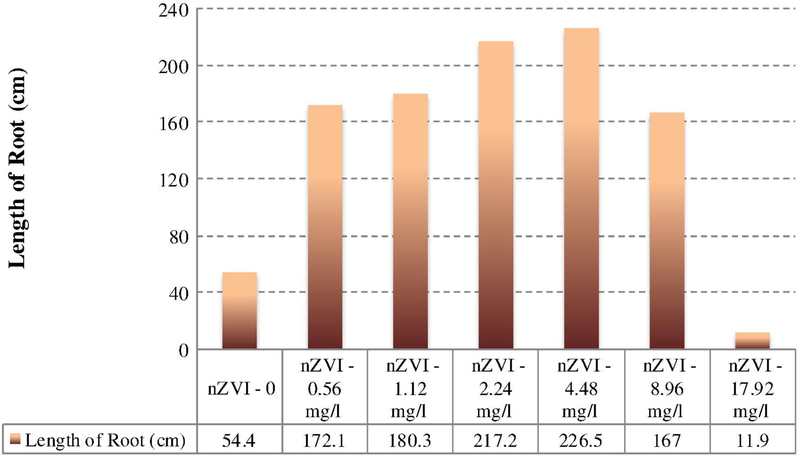
For nZVI Ma et al. (2013) found both protective and risk effects for cattail and hybrid poplar plant species grown hydroponically in a greenhouse for 4 weeks. In comparison to controls, growth rates up to 40% were obtained for concentrations between 25 and 1000 mg/l for the plant species. The threshold between stimulation and inhibition was different for both plants. Li et al. (2015) investigated the stimulatory effects of nZVI on peanut seedling development and growth up to 18 days of exposure. Fig. 2 illustrates the relationship between the nZVI concentration and total length of peanut root. It is evident that the root length increased monotonically with an increase in the nZVI concentration from 0 to 4.48 mg/l then there was a decline. A drastic inhibitory effect was detected around 17.92 mg/l. The peak of growth effect, which was obtained at 4.48 mg/l, demonstrated an increase of > 400% in the root length compared to controls. Similar findings were obtained for the projected surface area, root volume, number of tips and number of forks (Fig. 3). The following observations were obtained on average: (a) the surface area increased from 16.68 cm2 at 0 mg/l to 75.6 cm2 at 4.48 mg/l then decreased thereafter; (b) the root volume went up from 0.4 cm3 at 0 mg/l to 2.1 cm3 at 4.48 mg/l then decreased; (c) there was an increase in the number of tips from 97 at 0 mg/l to 782 at 2.24 mg/l then there was a gradual decrease; and (d) the number of forks was elevated from 235 at mg/l to a peak of 1786 at 4.48 mg/l, then went downward.
Figure 2.
Stimulatory and inhibitory effects of nZVI on peanut root growth after 18 day exposure. (Results obtained from Li et al. (2015)).
Figure 3.
Stimulatory and inhibitory effects or nZVI upon projected surface area, root volume, and number of tips and forks (Li et al., 2015).
Pradhan et al. (2015) examined the use of Cu NPs in lieu of copper sulfate (CuS) as a substitute for copper deficiency in the soil containing mung beans. Table 2 shows a comparative assessment of Cu ENP and CuS for root and shoot length, as well as, fresh and dry weight. As shown, Cu ENPs demonstrated better stimulatory effects for plant growth relative to CuS. One should note that the stimulatory effects of Cu ENPs in the Pradhan study were achieved at much lower concentration levels than those obtained for nZVI in the aforementioned studies, consistent with the lower plant requirements for Cu than for Fe (Jones, 1998).
Table 2.
Average stimulatory and inhibitory effects of Cu NPs on mung bean performance after 15-day exposure.
| Outcome | Treatment | Concentration | ||||
|---|---|---|---|---|---|---|
| Control | 0.05 mg/l | 0.1 mg/l | 0.5 mg/l | 1 mg/l | ||
| Root length (cm) | Cu ENPs | 4.4 | 6.62 | 6.5 | 5.8 | 5.63 |
| CS | 4.4 | 5.7 | 4.86 | 4 | 3.14 | |
| Shoot length (cm) | Cu ENPs | 12.84 | 15.47 | 13.93 | 13.59 | 12.87 |
| CS | 12.84 | 14.63 | 12.56 | 11.37 | 8.728 | |
| Fresh weight (g) | Cu ENPs | 0.1 | 0.147 | 0.142 | 0.126 | 0.132 |
| CS | 0.1 | 0.135 | 0.131 | 0.104 | 0.09 | |
| Dry weight (g) | Cu ENPs | 0.013 | 0.016 | 0.014 | 0.012 | 0.011 |
| CS | 0.013 | 0.014 | 0.012 | 0.01 | 0.009 | |
(Results obtained from Pradhan et al. (2015)).
Lee et al. (2008) found inhibitory effects for Cu ENPs on mung bean (cited as Phaseolus radiatus, recently renamed Vigna radiata) and wheat (Triticum aestivum) after 2 day exposure under higher concentration levels (200 to 800 mg/l) relative to those in the Pradhan et al. (2015). Stampoulis et al. (2009) provided additional evidence for the phytotoxicity effects of Cu ENPs on zucchini after 15 day exposure at high concentration levels in hydroponic culture (100 to 1000 mg/l). Although seed germination was unaffected by any of the treatments, Cu ENP-treated samples reduced shoot length by 77% and 64% relative to controls and seeds exposed to bulk Cu. Furthermore, the transpiration volume illustrated similar responses. In a field experiment using natural soils, Manceau et al. (2008) found that two wetland species (Phragmites australis and Iris pseudacorus) transformed Cu into metallic nanoparticles in the rhizosphere, which protected the plants from Cu toxicity. Additional research is needed to understand the complex chemistry of metallic ENP micronutrient availability and uptake in natural soils.
Kumar et al. (2013) observed the growth effects for Au ENPs on Arabidopsis thaliana after 15 day exposure for two extremely small concentration levels (0.01 and 0.08 mg/l) relative to controls. Even though Au is not considered a micronutrient required for plant growth, root length was observed at 1.75 and 2.45 times longer at the two aforementioned concentrations, respectively, relative to the controls. The total fresh weight of the examined plant increased by 3.68 and 6.3 times in comparison to controls. Although the mode of action was unknown, Au could have served as an enzyme co-factor facilitating secondary metabolite synthesis, similar to other metals (Jones, 1998). One major effect the Au appeared to have was to increase germination rate, followed by accelerated development throughout the lifecycle of Arabidopsis, which could also explain some of the measured differences among treatments. Although it is difficult to compare across metals because of the potentially different roles they play in stimulating plant growth, the stimulatory effects for Au ENPs were obtained at the lowest concentration levels, followed by Cu ENPs, then nZVI.
Taylor et al. (2014), who examined the effects of extremely high concentrations (25, 50, 75, 100, 200, 300, 400 mg/l) of Au ENPs on the same plant species (i.e., Arabidopsis thaliana), complemented the findings of Kumar et al. (2013). An inhibitory effect was detected for all concentration levels, with the root length reduced by 75% relative to controls. There was an up-regulation of genes involved in plant stress response such as glutathione transferases, glucosyl transferases and peroxidases. Furthermore, there was genetic down-regulation specific to metal transporters to reduce gold uptake. Even though there were fewer studies that examined iron, copper and gold ENPs, they demonstrated similar results. That is, under certain experimental conditions there can be a stimulatory effect for metallic ENPs on plant systems. However, the effective concentration range varies widely based on the type of ENP, suggesting that different mechanisms may be involved in the stimulatory responses observed.
Wang et al. (2013) also found a stimulation of Arabidopsis thaliana growth in response to low concentration levels of Ag ENPs (0.01, 0.1, 1 mg/l). Increased root growth was observed for concentration levels below 0.1 mg/l. Much higher concentrations (i.e., 100 mg/l) demonstrated complete inhibitory effects. In the same study by Wang et al., similar effects were observed for hybrid poplars (Populus deltoides × nigra). One can thus conclude that it is possible to observe stimulatory effects for Ag ENPs across different plant species. The findings of Syu et al. (2014) illustrated similar stimulatory effects for spherical and decahedral shapes of Ag ENPs for Arabidopsis thaliana.
Yin et al. (2011) examined gum arabic-coated Ag ENPs, used as an antimicrobial additive in consumer products, to follow changes in the common grass (Lolium multiflorum) exposed to concentrations varying from 1 to 40 mg/l during a 7 day exposure. Root length decreased consistently with an increase in Ag ENPs concentration. Moreover, there was no significant difference between the controls and the lowest concentration of 1 mg/l. In a similar study, Dimkpa et al. (2013)investigated a 0.5–5 mg/l concentration range of uncoated Ag ENPs on wheat (Triticum aestivum) in a sand matrix during a 14 day exposure period. Increased inhibitory effects were demonstrated with an increase in Ag ENP concentration level. Yin et al. (2012) confirmed the toxic effects of Ag ENPs to four other plant species. Although Thuesombat et al. (2014) investigated Ag ENPs over a wide range of concentration (0.1 to 1000 mg/l) with consistent inhibitory effects on jasmine rice (Oryza sativa), the results were in agreement with those of Wang et al. (2013) as to the 0.1 mg/l being approximately the lower threshold of inhibitory effects independent of plant species.
The antimicrobial properties of Ag ENPs also may be beneficial to plants and plant systems. Ardakani (2013) demonstrated the potential importance of using Ag ENPs as a deterrent against pests and pathogens. In this investigation, a 1.5 to 800 mg/ml concentration range of Ag ENPs was applied to tomatoes (Lycopersicum esculentum) in comparison to two controls: infected with root-knot nematode (Meloidogyne incognita) and non-nanotreated plants, as well as non-infected and non-treated plants. The lowest concentration level of Ag ENP was comparable to the second control with minimal inhibitory changes, yet, provided superior performance to the first control. This study and others (Prasad et al., 2014) demonstrated the potential use of Ag ENPs as a pesticide agent for plant protection.
Following the analysis of individual studies grouped by each type of metallic ENP, it is quite clear that, across all studies, metallic ENPs may have both stimulatory and inhibitory effects on plants (Table 3, Table 4). Indeed, nZVI and Cu ENPs may have potential for use as micronutrients in plant systems, keeping in mind the proper formulation of the right dose for each type of ENP, plant species, and soil type. Unfortunately, most of these studies were conducted in soilless media, without native microbial populations or soil organic components and often without control of pH, all of which are known to affect micronutrient availability. As suggested by Dimkpa (2014), the evidence indicates that ENPs may have negative effects for soil microbes and therefore agricultural processes that are microbially driven. According to Dimkpa et al. (2015), a study by Priester et al. (2012) found that soybean exposed to cerium oxide nanoparticles had lowered bacterial nitrogen fixation in their roots leading to reduced plant growth; another investigation by Feng et al. (2013)demonstrated that iron oxide nanoparticles lowered the glomalin level in clover roots with abuscular mycorrhizal fungi leading to lowered nutrient acquisition and biomass. Dimkpa et al. (2015) observed that soybean plant exposed to CuO nanoparticles and colonized by a root bacterium had inhibited growth that was more apparent in the roots.
Table 3.
Stimulatory and inhibitory concentration levels for metallic nanoparticle effects on root length.
| Type of Metallic ENPs | Plant species | Effect on plant health | Source | |||
|---|---|---|---|---|---|---|
| Stimulation | No effect | Inhibition | Note | |||
| nZVI | Cattail (Typha latifolia) | – | 25–50 mg/l | > 50–1000 mg/l | In terms of healthy roots (root gets darker in color with increased nZVI dose) | (Jessick et al., 2013) |
| Hybrid poplars (Populous deltoids × Populous nigra) | – | 25–50 mg/l | > 50–1000 mg/l | |||
| Peanut (Arachis hypogaea) | 0.56–17.92 mg/l | – | – | 4-Day exposure (longest seedling recorded at 2.24 mg/l) | (Li et al., 2015) | |
| Arabidopsis thaliana | 5–500 mg/l | – | – | 7- and 14-day exposure | (Kim et al., 2014a) | |
| Ag ENPs | Poplars (Populous deltoids × nigra) | > 0.1–<6 mg/l | ~ 6 mg/L | > 6–100 mg/l | 25 nm ENPs (in terms of root fresh weight) | (Wang et al., 2013) |
| >~0.05–<0.4 mg/l | ~ 0.4 mg/l | > 0.4–1 mg/l | 10 nm ENPs (in terms of root fresh weight) | |||
| Arabidopsis thaliana | ~ 0.004–<0.09 mg/l | ~ 0.09 mg/l | > 0.09–1 mg/l | 5 nm ENPs (in terms of root growth, mm/day) | ||
| ~ 0.008–<0.08 mg/l | ~ 0.08 mg/l | > 0.08–1 mg/l | 10 nm ENPs (in terms of root growth, mm/day) | |||
| Lolium multiflorum | – | – | 1–40 mg/l | 6 nm ENPs (in terms of root mass and length) | (Yin et al., 2011) | |
| Wheat (Triticum aestivum L.) | – | – | 0.5–5 mg/kg sand | 10 nm ENPs | (Dimkpa et al., 2013) | |
| Soybean (Glycinemax L.) | 500–2000 mg/l | – | – | In terms of wet biomass | (De La Torre-Roche et al., 2013) | |
| Zucchini (Cucurbita pepo L.) | 500–2000 mg/l | – | – | |||
| Eleven plant species, direct exposure | 40 mg/l (Carex lurida, Panicum virgatm, and Phytolacca americana only) | – | 40 mg/l (for all remaining species) | Using PVP-Ag ENPs | (Yin et al., 2012) | |
| 40 mg/l (Phytolacca americana only) | – | 40 mg/l (for all remaining species) | Using GA-Ag ENPs | |||
| Tomatoes (Lycopersicon esculentum) | – | – | 50–5000 mg/l | In terms of root elongation and biomass (rate of inhibition increases with increased ENP concentration) | (Song et al., 2013) | |
| Arabidopsis thaliana | – | – | 0.2–3 mg/l | 1- and 2-week exposure | (Qian et al., 2013) | |
| Phaseolus radiatusand Sorghum bicolor | – | – | 5–40 mg/l | Experiments conducted in agar for 2 days | (Lee et al., 2012) | |
| – | – | 100–2000 mg/kg dry soil | Experiments conducted in soil for 5 days | |||
| Jasmine rice (Oryza sativa L. cv. KDML 105) | – | – | 0.1–1000 mg/l | Root length and weight decreased with increased ENP size | (Thuesombat et al., 2014) | |
| Pennisetum glaucum | – | 20, 50 mg/l | – | (Parveen and Rao, 2014) | ||
| Tomato (Solanum lycopersicum) | – | – | 0.0007–0.02% (w/w) | In terms of root length and fresh weight | (Ardakani, 2013) | |
| Maize (Zea maysL.) and cabbage (Brassica oleraceavar. capitata L.) | – | 0.1–1 mg/l | 0–<0.1 and > 1–73.4 mg/l | – | (Pokhrel and Dubey, 2013) | |
| Cabbage (Brassica oleracea var. capitata L.) | 0.05–0.1 mg/l | ~ 0.11 mg/l | > 0.11–73.4 mg/l | – | ||
| ArabidopsisColumbia ecotype (Col-0) | 0.001–0.1 mg/l (decahedral NPs) and 0.001–0.01 mg/l (spherical NPs) | 0.1 mg/l (spherical NPs) | – | – | (Syu et al., 2014) | |
| Rice (Oryza sativaL.) | – | – | 0.2–1 mg/l | – | (Nair and Chung, 2014) | |
| Arabidopsis thaliana | – | 0.2 mg/l | 20, 40 and 80 nm NPs (in terms of root hairs) | (García-Sánchez et al., 2015) | ||
| Zucchini (Cucurbita pepo) | – | 1000 mg/l | – | – | (Stampoulis et al., 2009) | |
| Arabidopsis thaliana | 0.06684 mg/l (80 nm ENPs) | – | 0.06684–0.53472 mg/l (except for 80 nm NPs) | – | (Geisler-Lee et al., 2012) | |
| Cucumber (Cucumis sativus) | – | – | 100 mg/l | 2 nm ENPs, 7 days of incubation (in terms of root elongation) | (Barrena et al., 2009) | |
| Lettuce (Lactuca sativa) | – | – | 100 mg/l | 2 nm NPs (in terms of seed root elongation) | ||
| Cu ENPs | Mung bean (Phaseolus radiatus) and Wheat (Triticum aestivum) | – | – | 200–1000 mg/l | In terms of seedling growth | (Lee et al., 2008) |
| Mung bean seeds (Vigna radiata var. Sonali) | 0.05–1 mg/l | – | – | 15-Day exposure (in terms of length and fresh weight) | (Pradhan et al., 2015) | |
| Lettuce | – | – | 0.013% (w/w soil) | In 0 days incubation (in terms of shoot/root ratio) | (Shah and Belozerova, 2009) | |
| 0.013% (w/w soil) | – | – | In 15 days incubation (in terms of shoot/root ratio) | |||
| Au ENPs | Arabidopsis thaliana | 10–80 mg/l | – | – | In terms of root length (15-day exposure) | (Kumar et al., 2013) |
| Lettuce (Lactuca sativa) | 100 mg/l | – | – | 10 nm ENPs (in terms of root elongation) | (Barrena et al., 2009) | |
| Cucumber (Cucumis sativus) | 100 mg/l | – | – | 10 nm ENPs (in terms of root elongation) | ||
| Gloriosa superba | 500–1000 mg/l | – | – | In terms of number of lateral roots | (Gopinath et al., 2014) | |
| Lettuce | – | – | 0.013 & 0.066% (w/w soil) | In 0 days incubation (in terms of shoot/root ratio) | (Shah and Belozerova, 2009) | |
| 0.013 & 0.066% (w/w soil) | – | – | In 15 days incubation (in terms of shoot/root ratio) | |||
| Al ENPs | California red kidney bean (Phaseolus vulgaris) and rye grass (Lolium perenne) | – | 10–10,000 mg/kg dry soil | – | In terms of visual plant growth | (Doshi et al., 2008) |
| Pt ENPs | Sinapis alba and Lepidium sativum | – | 1–100 mg/l | – | In terms of visually inspected growth (e.g., color of plants, biomass production, root system or tissue hydration) | (Asztemborska et al., 2014) |
| Mn ENPs | Mung bean (Vigna radiata) | 0.05–0.5 mg/l | – | > 0.5 — 1 mg/l | In terms of root length | (Pradhan et al., 2013) |
| Zn ENPs | Pearl millet (Pennisetum americanum L.) | 10 mg/l | – | – | 18.5 nm ENPs, 6 weeks crop age (in terms of root length) | (Tarafdar et al., 2014) |
Table 4.
Stimulatory and inhibitory concentration levels for metallic nanoparticle effects on shoot length.
| Type of metallic ENPs | Plant species | Effect on plant health | Source | |||
|---|---|---|---|---|---|---|
| Stimulation | No effect | Inhibition | Note | |||
| nZVI | Cattail (Typha latifolia) | ~ 30–<150 mg/l | ~ 150 mg/l | > 150–1000 mg/l | 4-Week exposure | (Jessick et al., 2013) |
| Hybrid poplars (Populous deltoids × Populous nigra) | 25–50 mg/l | – | 200–1000 mg/l | 3-Week exposure | ||
| Peanut (Arachis hypogaea) | 0.56–<6.72 mg/l | ~ 6.72 mg/l | > 6.72–17.92 mg/l | 18-Day exposure (in terms of stem length) | (Li et al., 2015) | |
| Ag ENPs | Poplars (Populous deltoids × nigra) | 0.1–<40 mg/l | ~ 40 mg/l | > 40–100 mg/l | 25 nm ENPs (in terms of stem weight) | (Wang et al., 2013) |
| 0.1–<3 mg/l | ~ 3 mg/l | > 3–100 mg/l | 25 nm ENPs (in terms of leaf weight) | |||
| 0.01–<0.7 mg/l | ~ 0.7 mg/l | > 0.7–1 mg/l | 10 nm ENPs (in terms of stem weight) | |||
| 0.01–<0.8 mg/l | ~ 0.8 mg/l | > 0.8–1 mg/l | 10 nm ENPs (in terms of stem weight) | |||
| Arabidopsis thaliana | 0.008–<0.3 mg/l | ~ 0.3 mg/l | > 0.3–1 mg/l | 5 nm ENPs (in terms of stem growth mm/day) | ||
| 0.008–<0.2 mg/l | ~ 0.2 mg/l | > 0.2–1 mg/l | 10 nm ENPs (in terms of stem growth mm/day) | |||
| Lolium multiflorum | – | – | 1–40 mg/l | 6 nm ENPs (in terms of shoot mass and length) | (Yin et al., 2011) | |
| Wheat (Triticum aestivum L.) | – | – | 0.5–5 mg/kg sand (10 nm ENPs) | – | (Dimkpa et al., 2013) | |
| Soybean (Glycinemax L.) | – | – | 500–2000 mg/l | In terms of wet (stem and leaves) biomass | (De La Torre-Roche et al., 2013) | |
| Zucchini (Cucurbita pepo L.) | – | – | 500–2000 mg/l | |||
| Eleven plant species, direct exposture | 40 mg/l (Carex Lurida, Carex crinita, Scirpus cyperinusonly) | – | 40 mg/l (for all remaining species) | PVP-Ag ENPs | (Yin et al., 2012) | |
| 40 mg/l (Phytolacca americanaonly) | – | 40 mg/l (for all remaining species) | GA-Ag ENPs | |||
| Eleven plant species, soil exposture | – | 1–40 mg/l (GA-Ag NPs for all species except Lolium multiflorum), and (PVP-Ag NPs for Lolium multiflorum only) | 40 mg/l (for Carex spp., Phytolacca americana and Lolium multiflorumonly) | In terms of seedling growth | ||
| 40 mg/l (Lolium multiflorumonly) | – | 40 mg/l (for all remaining species) | In terms of seedling growth (using GA-Ag ENPs) | |||
| Arabidopsis thaliana | – | – | 0.2–3 mg/l | 1-Week exposure (in terms of leaf fresh weight) | (Qian et al., 2013) | |
| 0.5–3 mg/l | – | – | 2-Week exposure (in terms of leaf fresh weight) | |||
| Phaseolus radiatusand Sorghum bicolor | – | 100 mg/kg dry soil (for Phaseolus radiatus only) | 100–2000 mg/kg dry soil (for Sorghum bicoloronly); > 100–2000 mg/kg (for Phaseolus radiatus only) | Study conducted in soil for 5 days | (Lee et al., 2012) | |
| Jasmine rice (Oryza sativa L. cv. KDML 105) | – | – | 0.1–1000 mg/l | Shoot length and weight decrease with increased NP size | (Thuesombat et al., 2014) | |
| Pennisetum glaucum | – | – | 20, 50 mg/l | – | (Parveen and Rao, 2014) | |
| Tomato (Solanum lycopersicum) | – | – | 0.0007–0.02% (w/w) | In terms of shoot length and fresh weight | (Ardakani, 2013) | |
| Lemna minor L. clone St | – | – | 0.005–0.160 mg/l | In terms of frond (leaf-like) number (Inhibition increases by NP concentration and exposure time) | (Gubbins et al., 2011) | |
| Rice (Oryza sativaL.) | – | – | 0.2–1 mg/l | – | (Nair and Chung, 2014) | |
| Cu ENPs | Mung bean seeds (Vigna radiata var. Sonali) | 0.05–1 mg/l | – | – | 15-Day exposure (in terms of length and fresh weight) | (Pradhan et al., 2015) |
| Au ENPs | Arabidopsis thaliana | 10–80 mg/l | – | – | In terms of shoot length (15-day exposure) | (Kumar et al., 2013) |
| Gloriosa superba | 500–1000 mg/l | – | – | In terms of shoot length | (Gopinath et al., 2014) | |
| Al ENPs | California red kidney bean (Phaseolus vulgaris) and rye grass (Lolium perenne) | – | 10–10,000 mg/kg dry soil | – | In terms of visual plant growth | (Doshi et al., 2008) |
| Pt ENPs | Sinapis alba and Lepidium sativum | – | 1–100 mg/l | – | In terms of visually inspected growth (e.g., color of plants, biomass production, root system or tissue hydration) | (Asztemborska et al., 2014) |
| Mn ENPs | Mung bean (Vigna radiata) | 0.05–1 mg/l | – | – | 100 nm ENPs, 15-day exposure (in terms of shoot length) | (Pradhan et al., 2013) |
| Zn ENPs | Pearl millet (Pennisetum americanum L.) | 10 mg/l | – | – | 18.5 nm ENPs, 6 weeks crop age (in terms of shoot length) | (Tarafdar et al., 2014) |
In addition, improved growth resulting from metallic ENP treatment could be attributed to disease suppression as well as nutrient enhancement (Giannousi et al., 2013, Kanhed et al., 2014, Servin et al., 2015). Nonetheless, several patterns seemed to emerge from the papers examined. For example, the required concentration level for Cu ENPs was found to be lower than that for nZVI to achieve the same stimulatory effect, generally consistent with the nearly 20 fold greater plant requirement for Fe than Cu for adequate plant growth (Jones, 1998).
One question that needs to be addressed in the context of the SEE framework is whether the metallic ENP micronutrient addition is cost effective, given the alternatives available via existing fertilizer formulations. Since micronutrients are often lacking in soils, this area deserves further research. Very recent research by Dimkpa and Bindraban (2016) suggests that the positive effects of micronutrients range from 10% to 70% depending on the type of micronutrient occurring with and without NPK fertilization. It was argued that packaging of micronutrients as nanoparticles may have a vital role in crop responses and fertilizer use efficiency compared to conventional salts or bulk oxides. Another report by Dimkpa and coworkers further maintain that micronutrients added in microcapsules and nanocapsules are taken up and translocated within plants upon growth to maturity, thereby, improving crop yield and micronutrient concentrations in plants (Monreal et al., 2016).
The literature also suggests that other, non-micronutrient metallic ENPs may improve plant growth. For example, low concentrations of Au ENPs (much smaller than those for nZVI and Cu ENPs) may induce positive effects for plant growth. Indeed, these are newer findings for Au ENPs as they have not been considered in typical micronutrient applications because they are not required for plant growth. The Ag ENPs have also demonstrated their usage as pesticidal and potentially antimicrobial agents for plant protection, however, precautions should be taken to avoid higher concentrations of Ag ENPs not only for other organisms in plant ecosystems, but also for other constituents in the SEE system. One should note that the stimulatory effects for Ag ENPs occurs at higher concentrations than those reported for Au ENPs, but definitely lower than that for Cu ENPs.
Despite the scarcity of evidence in the published literature, the effects of metallic ENPs on plant systems may be related to the physical-chemical characteristics of ENPs. As mentioned earlier, Syu et al. (2014) demonstrated that spherical and decahedral shapes of Ag ENPs exhibited stimulatory effects for Ag ENPs on Arabidopsis thaliana species in comparison to triangular ENPs. The size of Ag NPs may have effects on plant performance as demonstrated by Geisler-Lee et al. (2012)for three sizes (i.e., 20, 40, 80 nm). Particle surface charge also appears to influence particle uptake and distribution in plants. Zhu et al. (2012) found greater uptake of positively charged Au ENPs in comparison to negatively charged particles; however, the results were species dependent. Koelmel et al. (2013) found that, when rice plants were hydroponically exposed to positively-, neutrally and negatively-charged Au nanoparticles, translocation to different plant organs and bioaccumulation differed depending on the surface charge of nanoparticles. Negatively-charged Au nanoparticles accumulated the most in above ground organs. From the aforementioned discussion, it appears that research in this area offers promising opportunities because of the ability to engineer or ‘tailor’ metallic ENPs to specific applications for specific plant species.
Metallic ENP coating type has also been shown by Yin et al. (2012) to have effects on plant performance. Gum arabic-coated Ag ENPs had better performance than PVP-coated ENPs for three wetland plant species (i.e., Lolium multiflorum, Carex spp., Eupatorium fistulosum) in terms of germination rate, as well as, the controls. It is recommended that future research address the parameterization of physical-chemical characteristics on plant system in regards to respective applications such as micronutrients and antimicrobial agents.
Additional supporting information has been provided in Table 1, Table 3, Table 4, respectively.
3.3. Effects of growth medium
From the aforementioned results based on evidence drawn from studies examining both stimulatory and inhibitory effects, one can observe that metallic ENPs may have both stimulatory and inhibitory effects. Upon a close scrutiny of the experimental conditions reported in these studies, however, it appears that the majority of these studies (64%) have used hydroponic/soilless systems lacking the presence of insects and nematodes and other natural predators (Resh, 2016). This is clearly a gap of knowledge in the published literature. It is expected that soil media may present challenges to plant growth due to biological species such as insects and nematodes. Therefore, further research is warranted so as to determine the areas of stimulatory effects in soil media for plant growth.
4. Discussion and concluding remarks
On the basis of observations drawn from the evidence in this research, one can deduce that metallic nanoparticles have both stimulatory and inhibitory effects on plant performance mostly in soilless media, and may prove useful for certain agricultural applications. The results have been surprisingly consistent and repeatable across different types of metallic ENPs and plant species, despite the limitations in growth conditions such as soil media and methodologies employed, as well as the instrumentations utilized. Another important point to be kept in mind is the use of nanoparticles as micronutrients for their effects upon plants (Dimkpa and Bindraban, 2016). As such, only traces of these nanoparticles should be used. Most of the studies reported in this analysis utilized a range of large concentrations, hence producing inhibitory effects.
The vast majority of the literature available on metallic ENPs has been conducted in well-controlled laboratory conditions via the use of hydroponics, sand, or artificial soil media. While this simplifies the experimental design and allows greater control of the growth conditions, it removes complex root-soil interactions known to occur in natural soils. For example, nutrient availability and uptake is determined by both soil physical and chemical characteristics, as well as microbial constituents in the bulk soil and the rhizosphere (Read et al., 1992, Walker et al., 2003). Roots synthesize and exude a broad array of compounds that regulate the uptake of nutrients, communicate with and control rhizosphere microbial communities, and provide defense from pests and pathogens. In the case of metals such as Fe, as well as other trace metal oxides and hydroxides known to have low solubility in soils, plants have adapted by deploying several mechanisms to acquire these nutrients. One mechanism involves acidifying the rhizosphere to make these metals more soluble and available for plant uptake (Tinker and Nye, 2000). In the case of Fe and Cu, plants and soil microbes can also exude ligands (e.g., siderophores) that bind these metals and make them available to plants for active uptake (Tinker, 1986). Most of the studies that have examined metallic ENP effects on plants have not included natural soil or microbial communities, and therefore the effects on nutrient availability and uptake in natural soil is unknown. This area represents a great opportunity for future metallic ENP research.
There is also a need for additional research on metallic ENP effects on beneficial, symbiotic associations between roots and soil bacteria and fungi, which influence nutrient availability and uptake (Read et al., 1992). Mycorrhizal fungi form symbiotic associations with plant roots that increase the effective root surface area and increase uptake of both macro and micronutrients. Likewise, species such as soybean form root nodules with Rhizobium sp. that are capable of reducing atmospheric N and making it available for plant use. To date, there are very few metallic ENP studies that have examined (1) the effect of ENPs on the formation of these important symbiotic associations, (2) how the symbiotic associates influence ENP chemistry and binding in the rhizosphere, and (3) whether these associates may preferentially take up/exclude metal ENPs because of their unique surface structures or chemistries. Clearly, complex interactions that occur in natural soils are difficult to predict and may be site/species specific. While controlled laboratory experiments using artificial rooting substrate provided important baseline information, additional research is needed using natural soils and a range of plant species to fully understand the potential for using ENPs for plant improvement.
Another example of study limitations includes the notion of experimental design principles which have not been greatly adhered to in most experiments (Box et al., 2005, Montgomery, 2012). An important principle is a randomization plan to conduct the experimental treatments in a particular order to minimize any bias and errors due to factors not accounted for in the experimentation. Consequently, failure to follow some or all of these guidelines in the examined evidence may bias the obtained results and mask any inhibitory and stimulatory effects. However, the larger effects obtained in the examined evidence may be attributed to, among others, the strong unique effects induced by the physical-chemical characteristics of metallic NPs at the subatomic levels as well as the sufficient number of replicates used for each treatment combination. At least four replicates were employed for each treatment combination.
4.1. Mechanisms for metallic nanoparticle effects
nZVI is a potential micronutrient of special value to seed and plant growth. Li et al. (2015) found that low concentrations of nZVI in quartz sand stimulated not only the seeds, but also, the growth of peanut plants. Furthermore, the stimulation of nZVI treated samples was better at certain concentrations (e.g., 2.24, 4.48 mg/l) than the ethylenediaminetetraacetate-iron solution (EDTA-Fe), a chelating agent used to make Fe plant-available in alkaline soils where Fe is usually insoluble and unavailable. This may suggest that nZVI was more plant-available than EDTA-Fe under conditions employed in the experiment; unfortunately, the pH of nutrient solutions was not reported. Additional studies will be needed to determine the availability of nZVI in natural soils under a range of physical and chemical conditions.
It was postulated that the nZVI particles could penetrate the peanut seed coats to increase the water uptake, hence, promote seed germination activity by increasing seedling development and thus the yield (Li et al., 2015). This hypothesis was validated with the results of seed moisture. It was found that seeds treated with 2.24 mg/l nZVI contained much more water than that of controls or EDTA-Fe group (moisture levels were 30%, 45% and 55%, respectively for the controls, EDT-Fe and nZVI groups). Images of seed coats showed that nZVI was inside the cell wall, therefore, it appears that the nanoparticles can loosen or open the peanut seed coat, giving the seedlings a ‘head start’ compared to non-treated seeds.
Kim et al. (2014a) determined in similar experiments on Arabidopsis thaliana that the oxidation capacity of nZVI lead to the release of H2O2 allowing it to cause OH radical-induced cell wall loosening in the roots. This was confirmed by the degradation of pectin polysaccharides in the roots. Furthermore, rapid root elongation led to structural changes in the root cell walls, that is, the reduction of cell wall thickness altering the orientation of cellulose microfibrils. In subsequent experiments, Kim et al. (2014b) found that nZVI-treated Arabidopsis thaliana species triggered high plasma membrane H+-ATPase activity. This resulted in a decrease in apoplastic pH, an increase in leaf area, and a wider stomatal aperture, leading possibly to an increased CO2 uptake. The investigators indicated that these findings may have implications for the possibility of eco-friendly alternatives to CO2reduction.
Pradhan et al. (2015) found that Cu ENPs may be a safer and more effective way to provide Cu to plants than traditional fertilizers. Cu is an important plant micronutrient involved in electron transport and chloroplast function, as well as protein and carbohydrate metabolism. Using mung bean (Vigna radiata), Pradhan et al. (2015) found that Cu ENPs positively enhanced the efficiency of chloroplasts via light-mediated electron-transfer. They also found improved pigment contents and nitrogen assimilation compared to CuS supplied controls. While higher concentrations of CuS above 0.5 mg/l showed toxic responses, the Cu ENPs did not exhibit similar toxicity, suggesting that Cu ENPs may be a safer and more effective fertilizer than traditional CuS salts.
Kumar et al. (2013) found that the seed germination rates of Arabidopsis thaliana species was markedly increased upon exposure to low levels of Au ENPs. Although Au is not a micronutrient, fresh biomass and relative water content of seedlings were higher in Au ENP-treatments compared to controls. The results demonstrated that Au ENPs enhanced the water uptake capacity of seeds, hence, increased seed germination rate and subsequently fresh biomass. In addition, root elongation and shoot length was enhanced, as was the radical scavenging potential of the Arabidopsis thaliana seedlings, similar to results with other non-essential ENP metals (Tamburo et al., 2015).
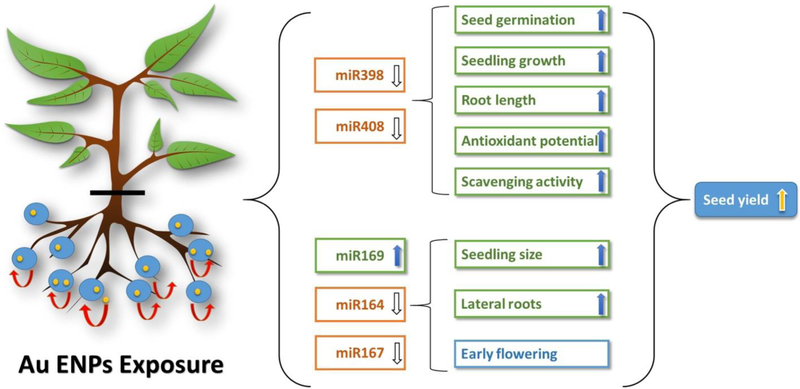
To better understand the growth mechanisms involved in response to Au ENP treatment, an analysis of the expression of microRNAs was additionally made (Fig. 4). Au ENP treated seedlings demonstrated a significant decrease in the expression level of miR398 and miR408 in comparison to control seedlings. These two miRs have been postulated to participate in the regulation of seed germination, seedling growth and root length. Prior research showed that overexpression of miR398 and miR408 in transgenic plants inhibited seed germination, seedling growth and root length (Box et al., 2005, Feng et al., 2010). Therefore, the decrease in expression level of miR398 and miR408 in Au ENP-exposed seedlings was possibly responsible for the higher rate of seed germination, seedling growth, and root elongation compared to control seedlings observed.
Figure 4.
Model demonstrating possible links between gene expression and changes in plant species upon exposure to low concentrations of Au ENPs. (Adapted from Kumar et al., 2013).
The results also suggested that the increase in lateral root emergence in response to Au ENPs was related to the disruption of auxin signals (Kumar et al., 2013). miR164 is known to target the transcription factor associated with auxin signals; the loss of miR164 in related studies has shown increases in the emergence of lateral roots (Montgomery, 2012). Therefore, it is possible that the decrease in the level of miR164 is responsible for the increase in lateral roots in Au ENP-treated A. thaliana. Lastly, the model in Fig. 4 attributes the increase in seedling size due to the increase in expression in miR169, as well as the early flowering, to the decrease in expression of miR167. It should be noted that while lateral root induction is related to auxin regulation, this phenomenon is not unique to nanoparticles. Stewart et al. (2015)have reported similar findings for ZnO NPs and metal ions.
Similar to the study by Kumar et al. (2013) examining Au ENPs, Syu et al. (2014) found stimulatory effects for Ag ENPs on Arabidopsis species. It was also postulated that Ag ENPs altered Arabidopsis gene expression associated with specific cellular events, including cell proliferation, metabolism, and hormone signaling pathways. Although their results demonstrated reactive oxygen species (ROS) accumulation, Ag ENPs enhanced root growth and increased the accumulation of proteins that are related to the cell cycle, chloroplast biogenesis, and carbohydrate metabolism. One must note that silver nanoparticles are established toxins with adverse effects on biological species (Iavicoli et al., 2010, Sarmast and Salehi, 2016). In this review, the evidence suggests stimulatory effects may occur for Ag ENPs at low doses. This may be explained at least in part due to the protective effects due to the antibacterial properties of Ag ENPs. The major mechanism through which silver nanoparticles demonstrate antibacterial properties is via anchoring and penetration to the bacterial cell wall, and modulation of cellular signaling by dephosphorylating putative key peptide substrates on tyrosine residues. Water uptake by the plant would possibly be another explanation for the positive effects of Ag ENPs at low doses.
Dimkpa (2014) found that ZnO ENPs had dose-dependent (100, 250, 500, 750 and 1000 mg/kg) effects upon the growth of bean seedlings in a sand matrix, particularly inhibitory growth for the roots. In particular, the 250 mg Zn/kg exposure from ENPs was in agreement with the reduction in cowpea root growth in aqueous media by ZnO ENPs at 25 mg/L in a study by Wang et al. (2013). It appears that these concentration levels are much higher than the range examined by Li et al. (2015) for nZVI. The study by Dimkpa (2014) clearly showed that ZnO ENPs impaired root growth and led to high bioaccumulation of Zn in the shoot with antagonistic effects on the fate of Fe and Mn via lowered solubilization and shoot uptake. The decrease in ferric reductase activity was attributed to localized release of high concentrations of Zn ions, thereby, affecting plant Zn and Fe homeostasis.
The few studies conducted to date do not provide an adequate explanation of the mechanisms involved in growth stimulation/inhibition in response to metal ENPs. In some cases, there may be direct uptake and nutritional effects on plant metabolism, particularly with plant micronutrients Cu and Fe. In other cases, the effects may be direct or indirect owing to the non-essential nature of the metal for plant growth and development. One important indirect effect that needs to be considered is the potential elimination of fungal/bacterial pathogens by metal ENP exposure (Prasad et al., 2014, Servin et al., 2015). An additional complication related to the efficacy of their use and our understanding the mechanisms is whether the metallic ENP solubilizes in the soil, or whether it remains in the metallic nano form. This issue is addressed in the following section.
4.2. Comparative effects of nano versus ionic forms of metals upon plant systems
The ionic form of metallic nanoparticles is commonly cited in the published literature as the likely factor attributed to the toxic effects upon biological species (Navarro et al., 2008, Xiu et al., 2012). Yet, the evidence gathered from the published literature in this study suggests that the stimulatory and inhibitory effects of metallic nanoparticles are influenced by both forms of metals depending upon the physical-chemical characteristics of nanoparticles, environmental conditions, soil media, and plant species (Asztemborska et al., 2014, Dimkpa et al., 2013, Gubbins et al., 2011, Kaveh et al., 2013, Kim et al., 2014b, Mustafa et al., 2015, Pokhrel and Dubey, 2013, Pradhan et al., 2015, Qian et al., 2013, Taylor et al., 2014, Wang et al., 2013, Yin et al., 2011, Yin et al., 2012).
By way of example, Qian et al. (2013) demonstrated, in a comparative assessment of the nano and ionic forms of silver on Arabidopsis thaliana, that Ag0 was much more toxic than Ag+ at the physiological, ultra-structural, and molecular levels. The results demonstrated that Ag ENPs could be accumulated in leaves, disrupt thylakoid membrane structure and decrease chlorophyll content leading to plant growth inhibition. In comparison, a small amount of Ag+ was adsorbed by seedlings and did not markedly affect chloroplast structure and other metal ion absorption as Ag ENPs did. Compared with Ag+, Ag ENPs could alter the transcription of antioxidant and aquaporin genes, indicating that Ag ENPs changed the balance between the oxidant and antioxidant systems, and also influenced the homeostatis of water and other small molecules within the plant body. It should be noted that Qian and co-workers did not include any information in regards to the purity of employed Ag ENP suspension, a concern in many of the studies. In the event of not being purified after synthesis, Ag ENP suspension may contain dissolved Ag+ together with Ag0. In this case, the study outcomes may be different.
Yin et al. (2011) found that the toxicity of 6 nm gum arabic-coated Ag ENPs was greater compared to the same concentration of dissolved Ag+ in Lolium multiflorum, with greater bioaccumulation occurring in the case of Ag ENP treatment relative to Ag. Another study by Pokhrel and Dubey (2013) observed that Citrate-coated Ag ENPs had lower Ag biouptake compared to AgNO3 treatment in maize. Collectively, these findings greatly highlight the deep influence of the physical-chemical properties of metallic nanoparticles.
Kaveh et al. (2013) examined changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. A ten-day exposure to a 5 mg/l concentration of 20 nm silver nanoparticles produced upregulation of 286 genes and downregulation of 81 genes relative to non-exposed plants. Under the same conditions, silver ion resulted in upregulation of 84 genes and downregulation of 53 genes relative to non-exposed plants. It was found that (a) the upregulated genes were associated with the response to metals and oxidative stress and (b) the downregulated genes were linked to responses to pathogens and hormonal stimuli. A considerable overlap was observed between genes differentially expressed in response to Ag0 and Ag+, that is, 13% and 21% of total up- and downregulated genes, respectively. It was deduced that Ag ENP-induced stress resulted partly from silver toxicity and partly from nanoparticle-specific effects. Results further demonstrated that three highly upregulated genes belonged to the thalianol biosynthetic pathway due to the presence of Ag ENPs and not Ag+.
In sum, the above examples demonstrate that the stimulatory and inhibitory effects of metallic nanoparticles upon plant systems depend among other things upon the unique physical-chemical characteristics of metallic nanoparticles, as well as the ionic form of dissolved nanoparticles. Clearly additional research is needed to improve our understanding of the underlying mechanisms, and to provide predictive information for the development of new metallic NPs appropriate for use in promoting plant growth and development.
4.3. Implications of metallic nanoparticle effects for all SEE constituents
The results have shown that in some cases metallic ENPs hold promise to improve plant growth and development; however, the results have also shown inhibition in response to ENP treatment, often related to the high concentrations employed. Therefore, it is important to keep in mind the effects of concentration level not only on the plant systems, but also on other constituents of the larger SEE system (e.g., humans, animals, aquatic species, bacteria and microbes, natural environments). Research suggests that the concentration levels examined in several of the reviewed studies may be detrimental to non-target organisms and mammalian cells. Bondarenko et al. (2013) found in a recent critical review that (a) the median L(E)C50 values of Ag ENPs (mg/l) were 0.01 for crustaceans, 0.36 for algae, and 1.36 for fish. The Ag ENPs were less toxic to bacteria, with the median minimal inhibitory concentration value being 7.1 mg/l. In comparison, the respective L(E)C50 value for mammalian cells was 11.3 mg/l. Metallic ENP use as fertilizers and soil amendments in terrestrial systems may adversely affect other important ecological species and processes, such as decomposition and nutrient turnover. Clearly, better understanding of threshold concentrations and modes of action of ENPs in plants and plant systems is needed to ensure safe use and tailor their use for specific applications.
In addition, analyses are needed to determine whether the use of metallic NPs is cost effective relative to existing conventional fertilizers and amendments in terms of the return on investment for newly developed micronutrients such as Ag ENPs. In this respect, detailed computations should be made to determine the relative worth of metallic ENPs on plant growth and yield. Based on preliminary and pioneering efforts by Dimkpa and Bindraban (2016), it is recommended to further explore the relative influence of each micronutrient under specific and agro-ecological situations to guide advances in the design and formulation of fertilizer products.
As the advancement of metallic ENP technologies in meeting the challenges of today’s world hinges upon the consideration of the planet’s sustainability principle, one should operate in light of the above discussion under the principle of sustainable nanomaterials as contributing value-added benefits and minimal risks to the SEE stakeholders (Tolaymat et al., 2015a, Tolaymat et al., 2015b). Although the application of emerging technologies is applied to one stakeholder herein (that is, plant systems), others along the life cycle trajectory should also be considered at the pre-design stage. This is essential to promote human and ecologic health, consequently, to ensure that the larger system remains efficient and effective.
4.4. Concluding remarks
In conclusion, despite some limitations in experimental design, the literature suggests that metal ENPs cause both stimulatory and inhibitory effects on plant performance. Metal ENPs have been used as micronutrients, antimicrobial/pesticide agents and other applications, but the underlying mechanisms are still largely unknown. There are several deciding factors that must be kept in mind in order to capitalize on the opportunities provided by metallic ENPs as emerging technologies and to reduce the harm to the constituents of SEE system, consequently, produce sustainable nanomaterials. The type of nanomaterial and the site conditions such as soil type and plant species must be considered when evaluating the effective particle concentration. Since most soils contain background levels of the metals examined, it will be critical to tailor metallic ENP application to the site in question. Future research should focus on providing a better mechanistic understanding of the underlying responses, and scaling the laboratory results to more realistic field systems where chemical and biological complexity increases. Given the potential for engineering particles with highly specific physicochemical characteristics, it may be possible to design metallic ENPs for specific applications that are cost effective and minimize detrimental effects in the context of SEE (e.g., green chemistry).
Acknowledgments
This manuscript has been subjected to the Agency’s review process. The opinions expressed in this paper are those of the author(s) and do not, necessarily, reflect the official positions and policies of the USEPA. Any mention of products or trade names does not constitute recommendation for use by the USEPA.
Abbreviations
- ENPs
engineered nanoparticles
- SEE
Society-Environment-Economy
- nZVI
nano zero-valent iron
- EDTA-Fe
ethylenediaminetetraacetate-iron solution
- ROS
reactive oxygen species
References
- Anjum NA, Adam V, Kizek R, Duarte AC, Pereira E, Iqbal M, et al. , Nanoscale copper in the soil–plant system–toxicity and underlying potential mechanisms Environ. Res, 138 (2015), pp. 306–325 [DOI] [PubMed] [Google Scholar]
- Ardakani AS, Toxicity of silver, titanium and silicon nanoparticles on the root-knot nematode, Meloidogyne incognita, and growth parameters of tomato Nematology, 15 (2013), pp. 671–677 [Google Scholar]
- Arruda SCC, Silva ALD, Galazzi RM, Azevedo RA, Arruda MAZ, Nanoparticles applied to plant science: a review Talanta, 131 (2015), pp. 693–705 [DOI] [PubMed] [Google Scholar]
- Asztemborska M, Steborowski R, Kowalska J, Bystrzejewska-Piotrowska G, Accumulation of platinum nanoparticles by Sinapis alba and Lepidium sativum plants Water Air Soil Pollut, 226 (2014), pp. 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrena R, Casals E, Colón J, Font X, Sánchez A, Puntes V, Evaluation of the ecotoxicity of model nanoparticles Chemosphere, 75 (2009), pp. 850–857 [DOI] [PubMed] [Google Scholar]
- Bennett P, He F, Zhao D, Aiken B, Feldman L, In situ testing of metallic iron nanoparticle mobility and reactivity in a shallow granular aquifer J. Contam. Hydrol, 116 (2010), pp. 35–46 [DOI] [PubMed] [Google Scholar]
- Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A, Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review Arch. Toxicol, 87 (2013), pp. 1181–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box GEP, H JS, Hunter WG, Statistics for Experimenters: Design, Innovation, and Discovery John Wiley & Sons, New York, NY: (2005) [Google Scholar]
- Canivet L, Dubot P, Garçon G, Denayer F-O, Effects of engineered iron nanoparticles on the bryophyte, Physcomitrella patens (Hedw.) Bruch & Schimp, after foliar exposure Ecotoxicol. Environ. Saf, 113 (2015), pp. 499–505 [DOI] [PubMed] [Google Scholar]
- De La Torre-Roche R, Hawthorne J, Musante C, Xing B, Newman LA, Ma X, et al. , Impact of Ag nanoparticle exposure on p, p′-DDE bioaccumulation by Cucurbita pepo(zucchini) and Glycine max (soybean) Environ. Sci. Technol, 47 (2013), pp. 718–725 [DOI] [PubMed] [Google Scholar]
- Dimkpa CO, Can nanotechnology deliver the promised benefits without negatively impacting soil microbial life? J. Basic Microbiol, 54 (2014), pp. 889–904 [DOI] [PubMed] [Google Scholar]
- Dimkpa CO, Bindraban PS, Fortification of micronutrients for efficient agronomic production: a review Agron. Sustain. Dev, 36 (2016), pp. 1–26 [Google Scholar]
- Dimkpa CO, McLean JE, Martineau N, Britt DW, Haverkamp R, Anderson AJ, Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix Environ. Sci. Technol, 47 (2013), pp. 1082–1090 [DOI] [PubMed] [Google Scholar]
- Dimkpa CO, McLean JE, Britt DW, Anderson AJ, Nano-CuO and interaction with nano-ZnO or soil bacterium provide evidence for the interference of nanoparticles in metal nutrition of plants Ecotoxicology, 24 (2015), pp. 119–129 [DOI] [PubMed] [Google Scholar]
- Ditta A, Arshad M, Applications and perspectives of using nanomaterials for sustainable plant nutrition Nano. Rev, 5 (2016), pp. 209–229 [Google Scholar]
- Doshi R, Braida W, Christodoulatos C, Wazne M, O’Connor G, Nano-aluminum: transport through sand columns and environmental effects on plants and soil communities Environ. Res, 106 (2008), pp. 296–303 [DOI] [PubMed] [Google Scholar]
- Feng X-M, Qiao Y, Mao K, Hao Y-J, Ectopic overexpression of AtmiR398b gene in tobacco influences seed germination and seedling growth Plant Cell Tissue Organ Cult, 102 (2010), pp. 53–59 [Google Scholar]
- Feng Y, Cui X, He S, Dong G, Chen M, Wang J, et al. , The role of metal nanoparticles in influencing arbuscular mycorrhizal fungi effects on plant growth Environ. Sci. Technol, 47 (2013), pp. 9496–9504 [DOI] [PubMed] [Google Scholar]
- García-Sánchez S, Bernales I, Cristobal S, Early response to nanoparticles in the arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling BMC Genomics, 16 (2015), p. 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler-Lee J, Wang Q, Yao Y, Zhang W, Geisler M, Li K, et al. , Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana Nanotoxicology, 7 (2012), pp. 323–337 [DOI] [PubMed] [Google Scholar]
- Giannousi K, Avramidis I, Dendrinou-Samara C, Synthesis, characterization and evaluation of copper based nanoparticles as agrochemicals against Phytophthora infestans RSC Adv, 3 (2013), pp. 21743–21752 [Google Scholar]
- Gomes HI, Dias-Ferreira C, Ribeiro AB, Pamukcu S, Influence of electrolyte and voltage on the direct current enhanced transport of iron nanoparticles in clay Chemosphere, 99 (2014), pp. 171–179 [DOI] [PubMed] [Google Scholar]
- Gopinath K, Gowri S, Karthika V, Arumugam A, Green synthesis of gold nanoparticles from fruit extract of Terminalia arjuna, for the enhanced seed germination activity of Gloriosa superba Journal of Nanostructure in Chemistry, 4 (2014), pp. 1–11 [Google Scholar]
- Gubbins EJ, Batty LC, Lead JR, Phytotoxicity of silver nanoparticles to Lemna minor L Environ. Pollut, 159 (2011), pp. 1551–1559 [DOI] [PubMed] [Google Scholar]
- Iavicoli I, Calabrese EJ, Nascarella MA, Exposure to nanoparticles and hormesis Dose-Response, 8 (2010), 10.2203/dose-response.10-016.Iavicoli [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessick AM, Moorman TB, Coats JR, Fate of erythromycin in sediment-containing surface water microcosms: how does aged erythromycin in sediment influence bioavailability? Evaluating veterinary pharmaceutical behavior in the environment Am. Chem. Soc, 1126 (2013), pp. 161–178 [Google Scholar]
- Jo Y-K, Cromwell W, Jeong H-K, Thorkelson J, Roh J-H, Shin D-B, Use of silver nanoparticles for managing Gibberellic fujikuroi on rice seedlings Crop. Prot, 74 (2015), pp. 65–69 [Google Scholar]
- Jones JB, Plant Nutrition Manual CRC Press, LLC, Boca Raton, FL: (1998) [Google Scholar]
- Kanhed P, Birla S, Gaikwad S, Gade A, Seabra AB, Rubilar O, et al. , In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi Mater. Lett, 115 (2014), pp. 13–17 [Google Scholar]
- Kaveh R, Li Y-S, Ranjbar S, Tehrani R, Brueck CL, Van Aken B, Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions Environ. Sci. Technol, 47 (2013), pp. 10637–10644 [DOI] [PubMed] [Google Scholar]
- Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW, Applications of nanomaterials in agricultural production and crop protection: a review Crop. Prot, 35 (2012), pp. 64–70 [Google Scholar]
- Kim J-H, Lee Y, Kim E-J, Gu S, Sohn EJ, Seo YS, et al. ,. Exposure of iron nanoparticles to Arabidopsis thaliana enhances root elongation by triggering cell wall loosening Environ. Sci. Technol, 48 (2014a), pp. 3477–3485 [DOI] [PubMed] [Google Scholar]
- Kim J-H, Oh Y, Yoon H, Hwang I, Chang Y-S, Iron nanoparticle-induced activation of plasma membrane H +-ATPase promotes stomatal opening in Arabidopsis thaliana Environ. Sci. Technol, 49 (2014b), pp. 1113–1119 [DOI] [PubMed] [Google Scholar]
- Kocur CM, Chowdhury AI, Sakulchaicharoen N, Boparai HK, Weber KP, Sharma P, et al. , Characterization of nZVI mobility in a field scale test Environ. Sci. Technol, 48 (2014), pp. 2862–2869 [DOI] [PubMed] [Google Scholar]
- Koelmel J, Leland T, Wang H, Amarasiriwardena D, Xing B Investigation of gold nanoparticles uptake and their tissue level distribution in rice plants by laser ablation-inductively coupled-mass spectrometry Environ. Pollut, 174 (2013), pp. 222–228 [DOI] [PubMed] [Google Scholar]
- Krishnaraj C, Jagan E, Ramachandran R, Abirami S, Mohan N, Kalaichelvan P Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism Process Biochem, 47 (2012), pp. 651–658 [Google Scholar]
- Kumar V, Guleria P, Kumar V, Yadav SK,Gold nanoparticle exposure induces growth and yield enhancement in Arabidopsis thaliana Sci. Total Environ, 461 (2013), pp. 462–468 [DOI] [PubMed] [Google Scholar]
- Kumari M, Mukherjee A, Chandrasekaran N, Genotoxicity of silver nanoparticles in Allium cepa Sci. Total Environ, 407 (2009), pp. 5243–5246 [DOI] [PubMed] [Google Scholar]
- Larue C, Castillo-Michel H, Sobanska S, Cécillon L, Bureau S, Barthès V, et al. , Foliar exposure of the crop Lactuca sativa to silver nanoparticles: evidence for internalization and changes in Ag speciation J. Hazard. Mater, 264 (2014), pp. 98–106 [DOI] [PubMed] [Google Scholar]
- Lee WM, An YJ, Yoon H, Kweon HS, Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles Environ. Toxicol. Chem, 27 (2008), pp. 1915–1921 [DOI] [PubMed] [Google Scholar]
- Lee W-M, Kwak JI, An Y-J, Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: media effect on phytotoxicity Chemosphere, 86 (2012), pp. 491–499 [DOI] [PubMed] [Google Scholar]
- Li H, Huang X, Li J, Liu J, Joyce D, He S, Efficacy of nano-silver in alleviating bacteria-related blockage in cut rose cv. Movie Star stems Postharvest Biol. Technol, 74 (2012), pp. 36–41 [Google Scholar]
- Li X, Yang Y, Gao B, Zhang M, Stimulation of peanut seedling development and growth by zero-valent iron nanoparticles at low concentrations PLoS One, 10 (2015), Article e0122884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Lal R, Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions Sci. Total Environ, 514 (2015), pp. 131–139 [DOI] [PubMed] [Google Scholar]
- Ma X, Geiser-Lee J, Deng Y, Kolmakov A, Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation Sci. Total Environ, 408 (2010), pp. 3053–3061 [DOI] [PubMed] [Google Scholar]
- Ma X, Gurung A, Deng Y, Phytotoxicity and uptake of nanoscale zero-valent iron (nZVI) by two plant species Sci. Total Environ, 443 (2013), pp. 844–849 [DOI] [PubMed] [Google Scholar]
- Ma C, White JC, Dhankher OP, Xing B, Metal-based nanotoxicity and detoxification pathways in higher plants Environ. Sci. Technol, 49 (2015), pp. 7109–7122 [DOI] [PubMed] [Google Scholar]
- Manceau A, Nagy KL, Marcus MA, Lanson M, Geoffroy N, Jacquet T, et al. , Formation of metallic copper nanoparticles at the soil–root interface Environ. Sci. Technol, 42 (2008), pp. 1766–1772 [DOI] [PubMed] [Google Scholar]
- Miralles P, Church TL, Harris AT, Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants Environ. Sci. Technol, 46 (2012), pp. 9224–9239 [DOI] [PubMed] [Google Scholar]
- Monreal C, DeRosa M, Mallubhotla S, Bindraban P, Dimkpa C Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients Biol. Fertil. Soils, 52 (2016), pp. 423–437 [Google Scholar]
- Montgomery D, Design and Analysis of Experiments John Wiley & Sons, New York, NY: (2012) [Google Scholar]
- Mueller NC, Braun J, Bruns J, Černík M, Rissing P, Rickerby D, et al. , Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe Environ. Sci. Pollut. Res, 19 (2012), pp. 550–558 [DOI] [PubMed] [Google Scholar]
- Musante C, White JC, Toxicity of silver and copper to Cucurbita pepo: differential effects of nano and bulk-size particles Environ. Toxicol, 27 (2012), pp. 510–517 [DOI] [PubMed] [Google Scholar]
- Mustafa G, Sakata K, Hossain Z, Komatsu S, Proteomic study on the effects of silver nanoparticles on soybean under flooding stress J. Proteome, 122 (2015), pp. 100–118 [DOI] [PubMed] [Google Scholar]
- Nair PMG, Chung IM, Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings Chemosphere, 112 (2014), pp. 105–113 [DOI] [PubMed] [Google Scholar]
- Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS, Nanoparticulate material delivery to plants Plant Sci, 179 (2010), pp. 154–163 [Google Scholar]
- Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, et al. , Toxicity of silver nanoparticles to Chlamydomonas reinhardtii Environ. Sci. Technol, 42 (2008), pp. 8959–8964 [DOI] [PubMed] [Google Scholar]
- Parveen A, Rao S, Effect of nanosilver on seed germination and seedling growth in Pennisetum glaucum J. Clust. Sci, 26 (2014), pp. 693–701 [Google Scholar]
- Pokhrel LR, Dubey B, Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles Sci. Total Environ, 452 (2013), pp. 321–332 [DOI] [PubMed] [Google Scholar]
- Pradhan S, Patra P, Das S, Chandra S, Mitra S, Dey KK, et al. , Photochemical modulation of biosafe manganese nanoparticles on Vigna radiata: a detailed molecular, biochemical, and biophysical study Environ. Sci. Technol, 47 (2013), pp. 13122–13131 [DOI] [PubMed] [Google Scholar]
- Pradhan S, Patra P, Mitra S, Dey KK, Basu S, Chandra S, et al. , Copper nanoparticle (CuNP) nanochain arrays with a reduced toxicity response: a biophysical and biochemical outlook on Vigna radiata J. Agric. Food Chem, 63 (2015), pp. 2606–2617 [DOI] [PubMed] [Google Scholar]
- Prasad R, Kumar V, Prasad KS, Nanotechnology in sustainable agriculture: present concerns and future aspects Afr. J. Biotechnol, 13 (2014), pp. 705–713 [Google Scholar]
- Priester JH, Ge Y, Mielke RE, Horst AM, Moritz SC, Espinosa K, et al. , Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption Proc. Natl. Acad. Sci, 109 (2012), pp. E2451–E2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Peng X, Han X, Ren J, Sun L, Fu Z, Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana J. Environ. Sci, 25 (2013), pp. 1947–1956 [DOI] [PubMed] [Google Scholar]
- Read DJ, L DH, Fitter AH, Alexander IJ, Mycorrhizas in Ecosystems CAB International, Wallingford, UK: (1992) [Google Scholar]
- Resh HM, Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower (seventh ed.), CRC Press; (2016) [Google Scholar]
- Sackett DL, Rosenberg WM, Gray J, Haynes RB, Richardson WS, Evidence based medicine: what it is and what it isn’t BMJ [Br. Med. J.], 312 (1996), p. 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmast MK, Salehi H, Silver nanoparticles: an influential element in plant nanobiotechnology Mol. Biotechnol, 1–9 (2016) [DOI] [PubMed] [Google Scholar]
- Schwab F, Zhai G, Kern M, Turner A, Schnoor JL, Wiesner MR, Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants–critical review Nanotoxicology, 10 (2016), pp. 257–278 [DOI] [PubMed] [Google Scholar]
- Servin A, Elmer W, Mukherjee A, De la Torre-Roche R, Hamdi H, White JC, et al. , A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield J. Nanopart. Res, 17 (2015), pp. 1–21 [Google Scholar]
- Shah V, Belozerova I, Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds Water Air Soil Pollut, 197 (2009), pp. 143–148 [Google Scholar]
- Sharif F, Westerhoff P, Herckes P, Sorption of trace organics and engineered nanomaterials onto wetland plant material Evnviron. Sci. Process. Impacts, 15 (2013), pp. 267–274 [DOI] [PubMed] [Google Scholar]
- Song U, Jun H, Waldman B, Roh J, Kim Y, Yi J, et al. , Functional analyses of nanoparticle toxicity: a comparative study of the effects of TiO2and Ag on tomatoes (Lycopersicon esculentum) Ecotoxicol. Environ. Saf, 93 (2013), pp. 60–67 [DOI] [PubMed] [Google Scholar]
- Stampoulis D, Sinha SK, White JC, Assay-dependent phytotoxicity of nanoparticles to plants Environ. Sci. Technol, 43 (2009), pp. 9473–9479 [DOI] [PubMed] [Google Scholar]
- Stewart J, Hansen T, McLean JE, McManus P, Das S, Britt DW, et al. , Salts affect the interaction of ZnO or CuO nanoparticles with wheat Environ. Toxicol. Chem, 34 (2015), pp. 2116–2125 [DOI] [PubMed] [Google Scholar]
- Syu Y.-y., Hung J-H, Chen J-C, Chuang H.-w., Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression Plant Physiol. Biochem, 83 (2014), pp. 57–64 [DOI] [PubMed] [Google Scholar]
- Tarafdar J, Raliya R, Mahawar H, Rathore I, Development of zinc nanofertilizer to enhance crop production in pearl millet (Pennisetum americanum) J. Agric. Res, 3 (2014), pp. 257–262 [Google Scholar]
- Taylor AF, Rylott EL, Anderson C, Bruce NC, Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold PLoS One, 9 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuesombat P, Hannongbua S, Akasit S, Chadchawan S, Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth Ecotoxicol. Environ. Saf, 104 (2014), pp. 302–309 [DOI] [PubMed] [Google Scholar]
- Tinker P, Trace elements in arable agriculture J. Soil Sci, 37 (1986), pp. 587–601 [Google Scholar]
- Tinker PB, Nye PH, Solute Movement in the Rhizosphere Oxford University Press, NY: (2000) [Google Scholar]
- Tolaymat T, El Badawy A, Sequeira R, Genaidy A, An integrated science-based methodology to assess potential risks and implications of engineered nanomaterials J. Hazard. Mater. (2015a) [DOI] [PubMed] [Google Scholar]
- Tolaymat T, El Badawy A, Sequeira R, Genaidy A, A system-of-systems approach as a broad and integrated paradigm for sustainable engineered nanomaterials Sci. Total Environ, 511 (2015b), pp. 595–607 [DOI] [PubMed] [Google Scholar]
- Tumburu L, Andersen CP, Rygiewicz PT, Reichman JR, Phenotypic and genomic responses to titanium dioxide and cerium oxide nanoparticles in Arabidopsis germinants Environ. Toxicol. Chem, 34 (2015), pp. 70–83 [DOI] [PubMed] [Google Scholar]
- Van Aken B, Gene expression changes in plants and microorganisms exposed to nanomaterials Curr. Opin. Biotechnol, 33 (2015), pp. 206–219 [DOI] [PubMed] [Google Scholar]
- Walker TS, Bais HP, Grotewold E, Vivanco JM, Root exudation and rhizosphere biology Plant Physiol, 132 (2003), pp. 44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Koo Y, Alexander A, Yang Y, Westerhof S, Zhang Q, et al. , Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag + at sublethal concentrations Environ. Sci. Technol, 47 (2013), pp. 5442–5449 [DOI] [PubMed] [Google Scholar]
- Xiu Z.-m., Zhang Q.-b., Puppala HL, Colvin VL, Alvarez PJ, Negligible particle-specific antibacterial activity of silver nanoparticles Nano Lett, 12 (2012), pp. 4271–4275 [DOI] [PubMed] [Google Scholar]
- Yin L, Cheng Y, Espinasse B, Colman BP, Auffan M, Wiesner M, et al. , More than the ions: the effects of silver nanoparticles on Lolium multiflorum Environ. Sci. Technol, 45 (2011), pp. 2360–2367 [DOI] [PubMed] [Google Scholar]
- Yin L, Colman BP, McGill BM, Wright JP, Bernhardt ES, Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants PLoS One, 7 (2012), Article e47674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z-J, Wang H, Yan B, Zheng H, Jiang Y, Miranda OR, et al. , Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species Environ. Sci. Technol, 46 (2012), pp. 12391–12398 [DOI] [PubMed] [Google Scholar]