Significance
From mammalian cells to microbes, cells need to be able to quickly respond to changes in their environment, e.g., to changes in nutrient availability. Since metabolic pools can rapidly deplete and fill within seconds and cellular metabolism is one of the first layers of response to nutrient starvation, we characterize here the immediate metabolic response after glucose starvation in yeast cells within seconds as well as hours. Our results reveal the predominant cellular metabolic pathways that are affected by the acute glucose withdrawal, and we demonstrate that bulk autophagy and lipid degradation via β-oxidation act in parallel to ensure energy maintenance and are critical for survival under acute glucose starvation conditions.
Keywords: Saccharomyces cerevisiae, acute glucose starvation, metabolomics, lipidomics, β-oxidation
Abstract
The ability to tolerate and thrive in diverse environments is paramount to all living organisms, and many organisms spend a large part of their lifetime in starvation. Upon acute glucose starvation, yeast cells undergo drastic physiological and metabolic changes and reestablish a constant—although lower—level of energy production within minutes. The molecules that are rapidly metabolized to fuel energy production under these conditions are unknown. Here, we combine metabolomics and genetics to characterize the cells’ response to acute glucose depletion and identify pathways that ensure survival during starvation. We show that the ability to respire is essential for maintaining the energy status and to ensure viability during starvation. Measuring the cells’ immediate metabolic response, we find that central metabolites drastically deplete and that the intracellular AMP-to-ATP ratio strongly increases within 20 to 30 s. Furthermore, we detect changes in both amino acid and lipid metabolite levels. Consistent with this, both bulk autophagy, a process that frees amino acids, and lipid degradation via β-oxidation contribute in parallel to energy maintenance upon acute starvation. In addition, both these pathways ensure long-term survival during starvation. Thus, our results identify bulk autophagy and β-oxidation as important energy providers during acute glucose starvation.
Cellular life depends on metabolic substrates for growth and survival. Glucose is a common substrate and convenient energy source that feeds directly into glycolysis, leading to rapid cell growth and proliferation in many microbes. Budding yeast, for example, depend highly on glucose as an energy source for rapid growth. One of their evolutionary advantages is that they can outgrow many of their competitors when supplied with glucose. The dependence on glucose and the ability to respond to glucose starvation not only is common in microbes and unicellular organisms, but also pertains to multicellular eukaryotes. A large number of cancer cells, for example, rely mostly on glucose and glycolysis even when oxygen is present (i.e., the Warburg effect), which leads to outcompeting of noncancerous cells in terms of proliferation. Furthermore, starvation from glucose and other carbon sources is especially detrimental for cell types such as neurons, which depend on an uninterrupted carbon supply. Interestingly, energy deficits and low glucose levels have been correlated with neurodegenerative diseases such as Alzheimer’s (1–3). Thus, understanding how cells cope with starvation is crucial for elucidating both normal cellular processes as well as aberrant behaviors in disease.
In microbes such as the budding yeast Saccharomyces cerevisiae, starvation is especially ubiquitous (4) and must be counteracted very rapidly. Budding yeast have developed an intricate and rapid response to glucose starvation, including reduction of transcriptional and translational activity (5–7), autophagy of cytoplasmic components and lipids for energetic needs (8), and reduction of macromolecular diffusivity (9, 10). Starvation states can differ depending on the type of starvation or nutrient limitation (11).
Previous studies have predominantly focused on starvation-induced metabolic changes that occur over hours or longer (8, 12, 13). Yet, metabolic pools rapidly deplete and fill within seconds (14, 15), and any initial responses have likely been missed in these studies. Furthermore, metabolic changes can occur much faster than transcriptional and protein abundance changes (16), and metabolism is therefore likely among the first responders to changes in the environment. However, the rapid metabolic changes that occur at the onset of starvation have remained unclear, and the critical energy sources that are utilized upon acute glucose starvation—leading to a new energy equilibrium within minutes (9)—have not been well characterized. Additionally, it is poorly understood how metabolism communicates with the rest of the cell to ensure long-term survival.
To begin to address some of these questions, we performed a metabolomic analysis upon acute glucose starvation in budding yeast. Our results reveal that upper glycolysis metabolites deplete within seconds of acute glucose starvation while intracellular lipid pools increase or are maintained. In addition, we found that the metabolic flow through amino acid metabolites changes significantly. Based on these findings we assessed the effect of lipid digestion and autophagy on cellular energy status upon acute glucose starvation and found that lipid degradation and autophagy act together to ensure ATP maintenance and are important for cellular survival in acute glucose starvation.
Results
Respiration Is Crucial for Survival and Energy Maintenance upon Acute Glucose Starvation.
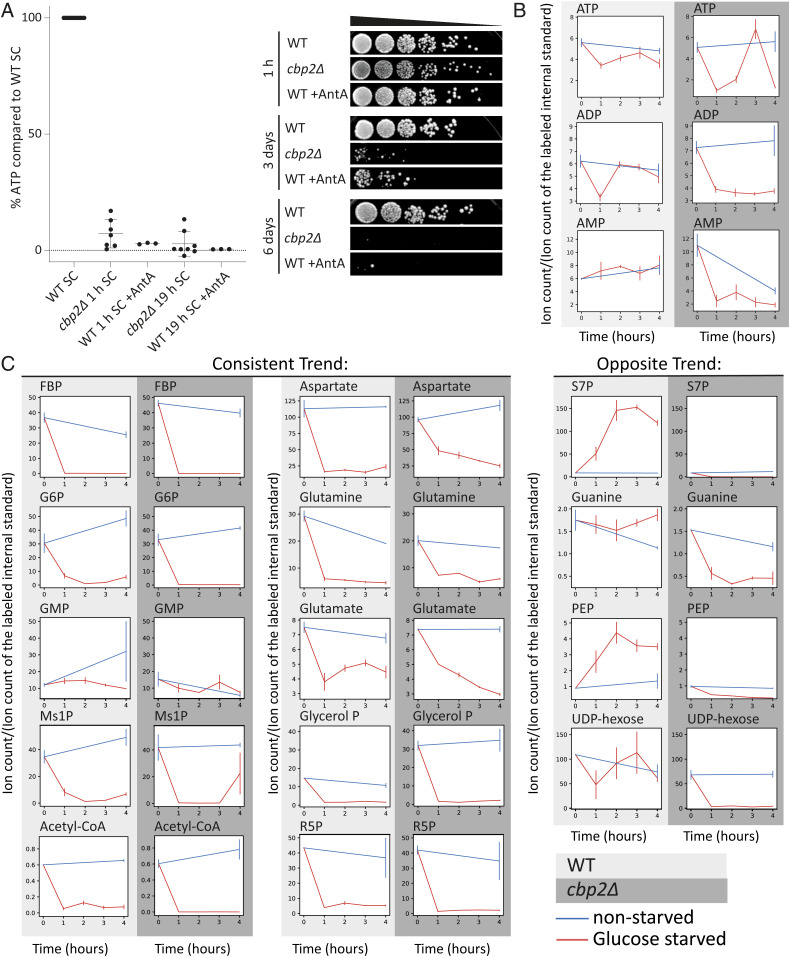
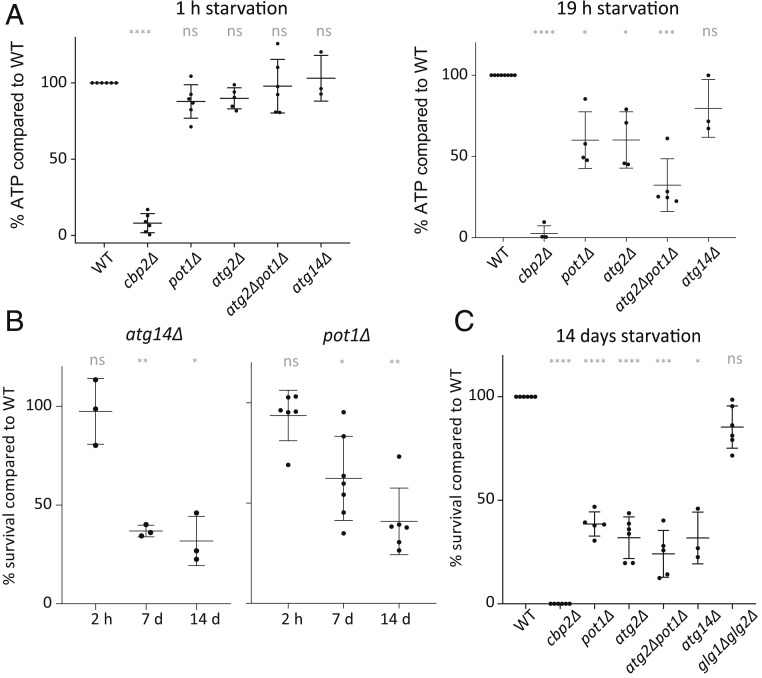
Our previous results suggested that respiration is critical for energy production upon acute glucose withdrawal (9). To address the question of how important respiration activity is for the acute glucose starvation response, we genetically deleted CBP2 in order to abolish aerobic respiration. The CBP2 gene product facilitates the splicing of the cytochrome B oxidase pre-RNA (17). Therefore, a knockout of CBP2 inhibits the ability of cells to respire. In cbp2Δ mutants, intracellular ATP levels dropped to nondetectable levels within 1 h of acute glucose starvation and remained comparably low for 19 h (one-way ANOVA test, 1- and 19-h comparison: P value = 0.12). Additionally, we used Antimycin A, which specifically and rapidly inhibits the electron transport chain in mitochondria, avoiding any potential adaptation effects that might have occurred in the long-term absence of respiration in cbp2Δ cells. Similar to the cbp2Δ mutant, ATP levels decreased strongly within 1 h of starvation and remained low (one-way ANOVA test, 1- and 19-h comparison: P value = 0.8). Furthermore, the survival rate during starvation in the absence of respiration was dramatically reduced (Fig. 1A). This showed that respiratory activity is critical to provide the necessary energy, which correlated with survival during starvation.
Fig. 1.
The ability to respire is crucial for survival and energy maintenance upon acute glucose starvation. (A) Relative ATP levels in the respiratory-deficient cbp2Δ mutant and wild-type cells treated with the respiration inhibitor Antimycin A, compared to wild type after 1 and 19 h of acute glucose starvation. Mean, SD, and biological replicates are shown. (Right) Survival after 1 h, 3 d, and 6 d of acute starvation for wild-type (WT), cbp2Δ mutants, and wild-type cells treated with Antimycin A (WT + AntA) during starvation. (B) Relative change in energy charges during starvation. Change in ion intensity for ATP, ADP, and AMP after 0, 1, 2, 3, and 4 h of acute glucose starvation (red, glucose starved), compared to nonstarved cells (blue, nonstarved). Comparison between WT and cbp2Δ cells. Average and SE (error bar) of two biological replicates are shown. (C) Change in ion intensity for metabolites after 0, 1, 2, 3, and 4 h of acute glucose starvation (red, glucose starved), compared to nonstarved cells (blue, nonstarved). Comparison between WT cells and cbp2Δ cells. FBP, fructose bisphosphate; G6P, glucose-6-phosphate; Glycerol P, Glycerol phosphate; GMP, guanosine monophosphate; Ms1P, mannose 1-phosphate; R5P, ribose-5-phosphate; S7P, sedoheptulose-7-guanine; PEP, phosphoenolpyruvate. Average and SE (error bar) of two biological replicates are shown.
Metabolite Pools Deplete Globally within 1 h of Starvation in Cells Unable to Respire.
Having established that respiration is required for energy maintenance and survival upon glucose starvation (Fig. 1A), we next aimed to identify the substrate(s) feeding respiration under these conditions. We hypothesized that respiration substrates cannot be efficiently metabolized in a respiration-deficient mutant and hence might accumulate in these cells. We therefore measured and compared central carbon metabolites between wild-type and cbp2Δ cells on a timescale of 1 to 4 h of starvation (Fig. 1B). A large swath of intracellular metabolites exhibited similar depletion patterns between the two strains, including many central metabolites in glycolysis (e.g., glycerol-phosphate, fructose-bisphosphate, and glucose-6-phosphate) and in the citric acid cycle (e.g., acetyl-CoA, glutamate, glutamine). Some select metabolites, such as glucose-6-phosphate, acetyl-CoA, ribose-5-phosphate, and glutamate, depleted more completely in the cbp2Δ mutants compared to wild type, and others, including sedoheptulose-7-guanine, phosphoenolpyruvate, and UDP-hexose, diminished entirely during glucose starvation in cbp2Δ but remained constant or even accumulated in wild-type cells. However, we could not detect any intracellular metabolite that specifically accumulated in cbp2Δ cells upon glucose starvation. We also noted the energy charges (ATP, ADP, and AMP) and observed varying patterns between the two strains; specifically, in wild type, the abundances equilibrated within 2 h (e.g., ADP and AMP) or depleted less severely (ATP). In cbp2Δ, the measured charges fluctuated wildly (e.g., ATP) or were more depleted compared to wild type (e.g., ADP, AMP), suggesting a dysregulation of energy homeostasis for respiratory mutants. We conclude that intracellular metabolite pools deplete rapidly in both wild-type and respiration-deficient cells and do so even more rapidly or more completely when cells are unable to respire.
Maintenance of ATP Levels and Increased Survival under Acute Glucose Starvation Are Explained neither by Extracellular Amino Acids nor by Intracellular Glycogen.
Our initial analyses of intracellular metabolites did not provide us with obvious candidates fueling respiration that became apparent through accumulation in a respiration-deficient mutant. We next focused our attention on candidate substrates during starvation, namely extracellular metabolites as well as intracellular glycogen reserves.
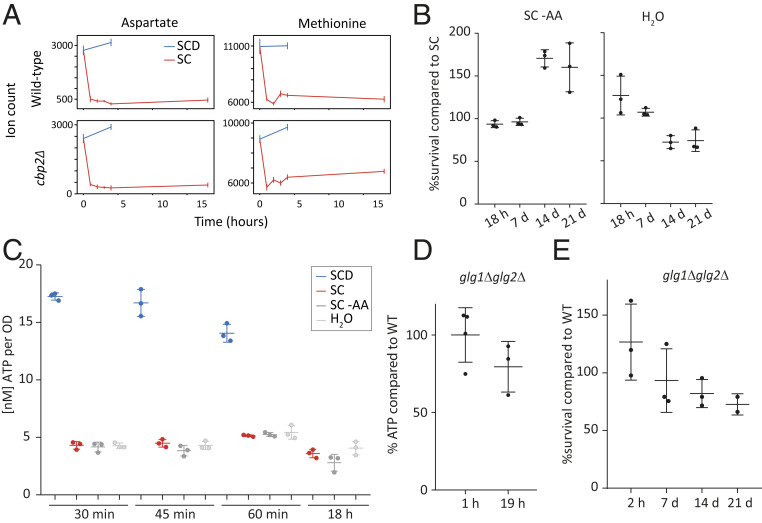
Extracellular metabolites either could be used as an external energy source or point to the activity of intracellular pathways based on secreted metabolites. To examine changes in extracellular metabolites, we grew cells to OD 0.5 to 0.8 in synthetic complete medium with dextrose (SCD), essential amino acids, and a yeast nitrogen source (see Materials and Methods for details). The cells were then acutely starved by washing in the same synthetic media lacking dextrose (glucose). The supernatant was sampled before and after the transition, and a control experiment was conducted by washing the cells with SCD to ensure that medium change itself did not lead to secondary metabolic effects. The strongest effect that we observed was a rapid depletion of extracellular amino acids within the first hour of acute glucose starvation—specifically, two exemplary amino acids, aspartate and methionine (Fig. 2A). Monitoring their abundances beyond this first hour, no additional appreciable changes could be observed for up to 19 h. Since our glucose starvation medium contains essential amino acids, this could suggest that extracellular amino acids are assimilated and catabolized during acute starvation. To test whether the extracellular amino acids serve as energy sources on a short timescale during glucose starvation, we measured intracellular ATP levels within the uptake period of 1 h or within longer starvation up to 19 h in cells starved of glucose (SC), and starved of glucose and amino acids (SC−AA). Furthermore, we starved cells by washing them in water (H2O) to assess the effect of any extracellular component (i.e., all nitrogen sources) present in the SC medium (synthetic complete medium without glucose) (Fig. 2C). The ATP levels did not drop much lower in water nor in the medium lacking glucose and amino acids compared to medium lacking only glucose, for neither rapid (30 min) nor short (19 h) starvation duration.
Fig. 2.
Extracellular amino acids and intracellular glycogen levels are not the main short-term energy source upon acute glucose starvation. (A) Extracellular aspartate and methionine deplete primarily within the first 1 h of starvation. Wild-type and cbp2Δ mutants were first grown in SCD (synthetic complete medium with glucose) and then switched to SCD or SC (synthetic complete medium without glucose), indicated by time point 0 h. Extracellular samples were taken and measured for relative abundance of ions corresponding to aspartate and methionine. Error bars indicate the SE of two biological replicates. (B) Survival of wild-type yeast cells after 18 h to 21 d of acute glucose and amino acid starvation (SC−AA) and acute starvation in water (H2O), compared to survival of cells only starved from glucose (SC). (C) ATP levels after hours and minutes of starvation in SC, SC–AA, or H2O. (D) ATP levels of glg1Δglg2Δ mutants compared to wild-type after 19 h of acute glucose starvation. (E) Quantified spotting assays after 2 h to 21 d of starvation. Mean and SD and values of biological replicates are shown.
To complement the ATP measurements with functional evidence, we also conducted spotting assays for cells starved in SC media, in SC media without amino acids (SC–AA), and in water (H2O) to assess survival and recovery after the starvation stress. Cells starved from both glucose and amino acids initially showed no survival difference compared to cells starved from glucose only (up to day 7 of starvation) and even seemed to have a survival advantage after 14 d of starvation (Fig. 2B and SI Appendix, Fig. S1). Survival of cells washed in water instead of SC or SC−AA was only slightly and insignificantly impaired after 14 to 21 d compared to the glucose-starved cells (Fig. 2B and SI Appendix, Fig. S1). Although extracellular amino acids were taken up within 1 h of starvation, we thus conclude that they neither affected ATP levels upon acute glucose starvation nor provided cells with a survival advantage long term.
Another potential candidate substrate during glucose starvation is intracellular glycogen. Under various starvation conditions, intracellular glycogen resources were proposed to build up or to be used for energy maintenance (18). Since glycogen was shown to decrease to nonmeasurable levels after glucose starvation (19), we tested whether intracellular glycogen resources sustain ATP maintenance and survival during short-term starvation. However, a mutant deficient in glycogen synthesis (glg1Δglg2Δ) did not adversely affect ATP levels after 1 to 19 h of acute glucose starvation and showed no significant survival deficiency even after 21 d (Fig. 2 D and E and SI Appendix, Fig. S5). Thus, glycogen stores in the cell are not a main energy source upon acute glucose starvation.
Central Carbon Metabolites Exhibit Subminute Changes to Glucose Deprivation.
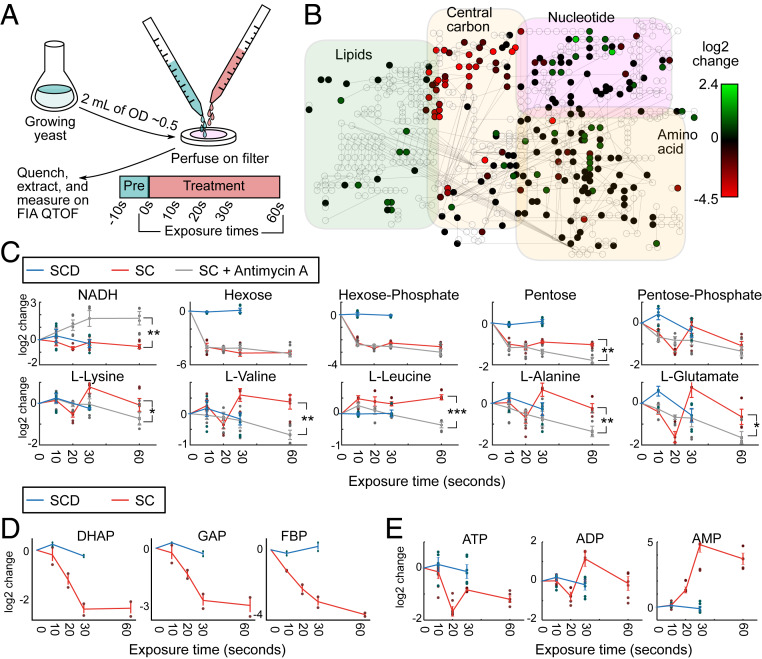
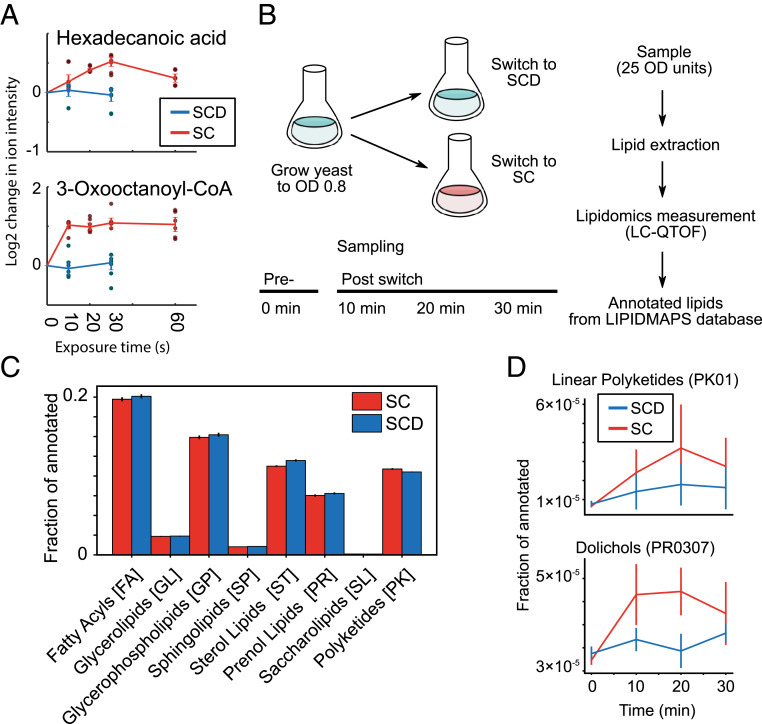
Our initial survey did not provide us with good candidate substrates that yeast cells utilize to ensure their survival upon acute glucose starvation, but suggests that many major metabolic changes occur on a timescale faster than 1 h. Hence, our experiments thus far might have missed initial responses that could be important for long-term survival. Therefore, we expanded our metabolomic analyses to earlier time points. Since metabolic pools can deplete and fill within seconds (14, 15), we aimed for measurements on a 10- to 60-s timescale. We employed a high-throughput, untargeted mass spectrometry approach (15, 20) to measure the global, starvation-induced metabolic changes that occur in budding yeast. Yeast cells were cultivated in SCD media, and using a rapid filtration setup (21, 22), we dispensed ∼1 OD unit of yeast on a filter, perfused with SCD media for at least 10 s followed by exposure to media without glucose (SC) for varying times (10, 20, 30, and 60 s) (Fig. 3A). The yeast-containing filters were rapidly quenched after the designated exposure time, and intracellular metabolites were extracted. Relative changes of intracellular metabolite concentrations were measured and annotated (20). This semiquantitative method allowed us to measure the response of ∼350 ions that can be associated with 650 metabolites known to the metabolome of Saccharomyces cerevisiae (23). Rapid fold changes occurred in many metabolites visualized on a metabolic map (Fig. 3B).
Fig. 3.
Central carbon metabolites exhibit subminute changes to glucose deprivation. (A) Fast filtration setup. Growing yeast (∼1 OD*mL) were deposited onto filters and quickly perfused with pretreatment media (SCD). At the 0-s time point, cells were perfused with either SCD or SC (starvation) medium, and after exposure for a given amount of time (10, 20, 30, and 60 s), the filters were rapidly quenched in extraction solution and measured on mass spectrometry for small metabolites (m/z < 1,000 g/mol). (B) A metabolic map of central carbon metabolism shows rapid depletion of metabolites in upper glycolysis and increase in lipid synthesis. The log2 change in ion intensity is shown between 60 s exposure of SC media versus the average of the 10- and 30-s exposure on SCD media. (C) Glycolytic metabolites deplete strongly within 60 s upon exposure to SC media. The log2-adjusted values of intensity for specific ions are shown and labeled according to the annotated compound. Measurement time indicates the exposure time for the given media for the cells on the filter (SCD, blue; SC, red; SC with Antimycin A, gray). Six dots are shown for each time point (three biological replicates and two technical measurement replicates). Time point 0 s is extrapolated from the average of the SCD condition. Error bars indicate the SE for three biological replicates. Significances are shown between the SC and SC with Antimycin A condition for time 60 s (* 0.01 < P < 0.05; **10−4 < P < 0.01, and ***P < 10−4) and calculated via Student’s t test. (D) Measurement of other metabolites (DHAP, dihydroxyacetone phosphate; GAP, glyceraldehyde 3-phosphate; FBP, fructose bisphosphate) with another mass spectrometer quantification method also corroborate the depletion of upper glycolysis metabolites. Two dots are shown for each time point (two biological replicates). Error bars indicate the SE for two biological replicates. (E) ATP, ADP, and AMP levels as indicators of cellular energy homeostasis rapidly change during starvation.
How does respiration affect the metabolism at these early time points? To answer this question, we treated cells with Antimycin A and measured them in parallel with untreated cells in our fast filtration setup. An increase of NADH in Antimycin A-treated cells confirmed the successful application of the drug, since the block of the electron transport chain inhibits NADH oxidation (24) (Fig. 3C). Glucose, as indicated by the hexose ion, decreased within the first 10 s under both starvation conditions, indicating that the cells use up internal glucose very rapidly upon starvation.
Furthermore, the Antimycin A treatment in starvation led to a stronger decrease of most amino acid pools compared to cells that were only starved (Fig. 3C and SI Appendix, Fig. S2), indicating that these metabolite pools are either degraded and potentially explored for energy generation much faster than in cells capable of respiration or that protein degradation slows down in Antimycin A-treated cells, leading to less generation of amino acids. These observations echoed our earlier observations about respiratory-deficient cells with generally lower metabolic pools over long timescales.
Glycolysis metabolites such as hexose phosphates decreased under both conditions within 10 s. Intermediates of the pentose phosphate pathway such as ribose phosphates (corresponding to the pentose phosphate ion) and potential precursors such as D-ribose (pentose) also decreased under both conditions, but were then maintained at lower levels in the untreated cells while they depleted even more under the respiratory-deficient condition (Fig. 3C), correlating with the decreased phosphoenolpyruvate levels observed in the cbp2Δ cells at later time points (Fig. 1B).
To further substantiate these observations in wild-type untreated cells, we obtained metabolite concentrations with a targeted mass-spectrometry method (25) and found other glycolytic and pentose phosphate pathway metabolites to exhibit a similar rapid depletion (Fig. 3D), specifically dihydroxyacetone phosphate, glyceraldehyde 3-phosphate, and fructose bisphosphate. The rapid depletion was comparable to an earlier study, examining Escherichia coli carbon starvation (14). These results suggest that, upon removal of the glucose input downstream, metabolic activity continues, leading to the successive depletion of metabolic pools. Such depletion is expected to start with upper glycolysis metabolites as the entry point for glucose.
The energy charges of the cells (e.g., ATP, ADP, and AMP) showed similar rapid changes (Fig. 3E). ATP depleted within seconds, and AMP increased in a near-equivalent time frame. Our data suggest that global metabolic changes occur within seconds of starvation.
Interestingly, we observed that both intracellular amino acid and lipid pools changed considerably within these initial time points. We therefore focused on these two classes for our further studies.
Autophagy Is Important for Energy Maintenance upon Acute Glucose Starvation and Ensures Long-Term Survival.
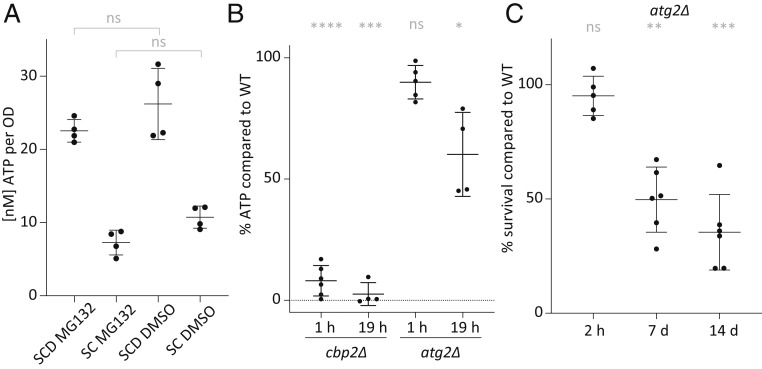
Since our map of starvation-induced metabolite changes revealed changes of amino acid metabolites, we examined whether amino acid digestion could provide a pathway for ATP production through respiration and whether intracellular proteins, which are synthesized prior to starvation, could be an amino acid source upon acute glucose removal. Since proteasomal degradation is one of the main specific protein degradation pathways, we explored whether proteasomal degradation contributed to ATP generation and maintenance within 1 h of glucose starvation. To test this, proteasomal activity was blocked using the small molecular inhibitor MG132. ATP concentrations in MG132-treated cells compared with the control cells showed no significant changes upon glucose starvation for 1 h (Fig. 4A). Due to its potential cellular toxicity, we did not test the effect of MG132 on long-term survival, but our results nevertheless suggest that proteasomal degradation is not a significant energy source during the first hours of acute starvation.
Fig. 4.
Unlike proteasomal activity, bulk autophagy contributes to survival and energy maintenance upon acute glucose starvation. (A) ATP levels after 1 h of pretreatment with MG132 or DMSO as a control, followed by 1 h in acute SC or SCD medium with MG132/DMSO only. (B) ATP levels in cbp2Δ and atg2Δ relative to WT cells after 1 and 19 h of acute glucose starvation. (C) Survival of atg2Δ cells after 2 h to 14 d of glucose starvation. Means, SDs, and biological replicate values are shown. Significances are shown between the wild-type and mutants (ns, indicates nonsignificant; *0.05 > P > 0.01; **0.01 > P > 0.001; ***0.001 > P > 0.0001; and ****P < 0.0001). Significance was calculated using a one-way ANOVA test followed by a Holm-Sidak test.
Another pathway that frees building blocks and carbon substrates is bulk autophagy, which removes cytosolic components, nonspecifically triggering their degradation in the vacuole. We therefore examined whether bulk autophagy is important for the cellular response to glucose starvation. First, we used GFP-Atg8 as a marker for autophagy (26). The appearance of free GFP in the vacuole reveals that autophagy is activated within 19 h of glucose starvation (SI Appendix, Fig. S4). We then generated a mutant incapable of organizing the preautophagosomal structure atg2Δ (27). Since bulk autophagy and the cytoplasm-to-vacuole transport (CvT) pathway rely largely on the same machinery, including ATG2, the atg2Δ mutant will affect additionally any specific process that relies on the CvT for shuttling to the vacuole and degradation therein. Importantly, the deletion of ATG2 did not affect the cells’ ability to respire and grow on nonfermentable carbon sources such as glycerol (SI Appendix, Fig. S3) but led to impaired autophagy levels both in glucose starvation and nitrogen starvation (SI Appendix, Fig. S4). ATP levels after 19 h of glucose starvation significantly decreased in the atg2Δ mutant compared to wild-type cells (Fig. 4B). In addition, fewer atg2Δ cells survived 7 d of glucose starvation in comparison to the isogenic wild-type control (Fig. 4C and SI Appendix, Fig. S5). Thus, bulk autophagy and specific autophagic processes involving the CvT pathway contributed to energy maintenance, correlating with survival in acute glucose starvation.
Our data further implied that there must be alternative pathways to generate ATP in the short term as autophagy deficiency did not decrease ATP levels to the same levels as seen in the complete absence of respiration (Fig. 4B) and did also not fully impair long-term survival.
β-Oxidation Is Required for Energy Maintenance and Survival upon Acute Glucose Starvation Together with Autophagy.
In addition to amino acids, lipid metabolite levels changed on these very rapid timescales, and we observed a rapid increase in some lipids in wild-type untreated cells, such as hexadecanoic acid and 3-oxooctanoyl-CoA (Fig. 5A). There are two potential nonexclusive models that could explain the changes that we see. On one hand, since a majority of lipids in the cell originates from membranes, the observed changes could be caused by global membrane remodeling during starvation. On the other hand, the cells might liberate lipids to use them as an energy/carbon source during starvation, leading to an increase of free fatty acyls. To test the first model, we utilized a lipid extraction method designed for yeast (28) and measured the lipidome over time (Fig. 5 B and C), sampling cells before glucose withdrawal and every 10 min after. We could annotate over 10,000 ions by exact mass matching to over 400 classes in the eight categories within the LIPID MAPS scheme (29) and classified the different classes as increasing or decreasing (Fig. 5B). In our dataset, the three main membrane lipid classes (sphingolipids, phospholipids, and sterol lipids) were enriched consistent with the fact that the majority of lipids in cells originates from membranes (30). No major differences appeared between the lipids measured under control versus glucose starvation conditions. Nonetheless, a few traces were found to change, and particularly, lipids annotated to dolichols and linear polyketides exhibited an upward trend during starvation (Fig. 5D). While this suggests that specific lipids and lipid classes may be synthesized or depleted in direct response to starvation, the overall lipid composition and therefore cellular membranes did not seem to appreciably change within rapid timescales (30 min or less). This argues that yeast cells do not undergo a major membrane remodeling during acute glucose starvation.
Fig. 5.
The lipidome during rapid starvation. (A) Hexadecanoid acid and 3-oxooctanoyl-CoA as examples of lipid-related metabolites that accumulate within 10 to 60 s. The log2-adjusted values of intensity for specific ions are shown and labeled according to the annotated compound. Measurement time indicates the exposure time for the given media for the cells on the filter (SCD, blue; SC, red). Six dots are shown for each time point (three biological replicates and two technical measurement replicates). Time point 0 s is extrapolated from the average of the SCD condition. Error bars indicate the SE for three biological replicates. (B) Experimental setup for measuring yeast lipidomics upon starvation entry. Exponentially growing yeast cells (OD 0.8 in SCD media) were sampled (time point 0 min) and then resuspended in fresh SCD or SC media. Samples were taken every 10 min thereafter. All samples were processed (see Methods) and measured. Putative lipids were annotated based on m/z and correspondence to the LIPID MAPS database. (C) The normalized distribution of annotated lipids using the LIPID MAPS identifiers. The eight major lipid categories are shown. Error bars indicate the SE between two biological replicates. (D) The lipid classes linear polyketides (PK01) and dolichols (PR0307) were identified as accumulating in glucose starvation compared to nutrient-rich conditions. Error bars indicate the SE between two biological replicates.
Since our results did not support the hypothesis that the changes that we saw in lipid metabolites were caused by a major reorganization of cellular membranes, we hypothesized and examined whether the liberated lipids were used as an energy source to fuel respiration during starvation. µ-Lipophagy was recently shown to play a role during long-term glucose restriction from 2% (wt/vol) to 0.4% (wt/vol) glucose (8). The study suggests that lipid droplets are digested through microlipophagy mediated by Atg14 to ensure survival in limiting glucose conditions. We therefore tested whether µ-lipophagy is also necessary for ATP level maintenance upon acute complete glucose starvation. Within 19 h of starvation, the ATP levels in atg14Δ mutants deviated only slightly from values measured in wild-type cells (Fig. 6A). However, consistent with the results obtained by Seo et al. (8), acute complete glucose starvation led to survival deficiency after 7 d (Fig. 6B). We conclude that µ-lipophagy mediated by Atg14 did not contribute significantly to energy maintenance within the first 19 h of acute glucose starvation, but had long-term effects on survival of the cells after 7 d.
Fig. 6.
Lipid degradation and autophagy ensure survival and energy maintenance upon acute glucose starvation. (A) ATP levels in mutants compared to WT after 1 or 19 h of acute glucose starvation. (B) Survival of atg14Δ and pot1Δ cells after 2 h to 14 d of acute glucose starvation. (C) Survival of mutant cells compared to WT cells after 14 d of acute glucose starvation. Significances are shown between the wild-type and mutants (ns, nonsignificant; *0.05 > P > 0.01; **0.01 > P > 0.001; ***0.001 > P > 0.0001; and ****P < 0.0001). Significance was calculated using a one-way ANOVA test followed by a Holm-Sidak test.
While µ-lipophagy specifically may contribute only to energy maintenance after the first day of starvation, general lipid digestion in peroxisomes could play a role early on. β-Oxidation occurs exclusively in peroxisomes in yeast (31). To attenuate global lipid degradation in peroxisomes, we deleted the gene coding for Pot1. Pot1 is the only 3-ketoacyl-CoA thiolase in yeast that catalyzes the last step of β-oxidation, producing acetyl-CoA to feed into the citric acid cycle (32). Indeed, pot1Δ cells showed decreased ATP levels within 19 h of glucose starvation, demonstrating that β-oxidation of fatty acids contributes to ATP maintenance upon glucose starvation (Fig. 6A). Furthermore, survival of pot1Δ cells decreased after 7 to 14 d in the absence of glucose (Fig. 6 B and C and SI Appendix, Fig. S5).
Together, our data suggest that lipid consumption by β-oxidation contributes to intracellular ATP levels upon acute glucose starvation within several hours of stress and correlates with a benefit for long-term survival. By contrast, µ-lipophagy, as a more specific way to consume lipids, does not seem to contribute to short-term ATP maintenance, but is important for long-term survival.
Similar to the results for the autophagy-deficient mutant, the ATP and survival levels were not completely abolished in the pot1Δ mutant and did not reach the levels of the respiratory-deficient cbp2Δ mutant. We therefore wondered whether bulk autophagy and β-oxidation might complement each other and generated a pot1Δatg2Δ double-deletion strain. Consistent with the hypothesis that the two pathways contribute in an additive manner to energy generation upon short-term glucose starvation, we observed that after 19 h of starvation the double-deletion strain showed lower levels of intracellular ATP than each of the single-deletion mutants (Fig. 6 A and C). While we observed in some experiments also a survival deficiency in the double compared to the single mutants, this difference was not statistically significant. We conclude that lipid degradation and autophagy work in parallel to ensure energy maintenance upon glucose starvation.
Discussion
Cells commonly experience sudden changes in nutrient availability; therefore, strategies to overcome nutrient scarcity are critical to ensure survival in periods of starvation. However, the immediate metabolic response to starvation remains poorly characterized. Here, we aimed to identify the main immediate energy resources of budding yeast upon acute glucose starvation. We showed that respiration is needed for survival and energy maintenance upon glucose starvation, and we examined the metabolic resources that are fed into the respiratory chain. We found that, within seconds of glucose starvation, upper glycolysis metabolites decrease drastically. Furthermore, we show that autophagy and β-oxidation are critical energy providers as early as within the first day and play a central role for survival of yeast during long-term starvation.
The role of autophagy in starvation for glucose-grown cells is controversial. A few studies conclude that autophagy is neither activated nor necessary for short-term survival during starvation (12, 33), whereas other studies suggest the opposite (8, 34). We observed that autophagy is essential for cell survival after glucose starvation within 7 d and that ATP levels depend on autophagy within the first 24 h of starvation. While Adachi et al. (33) suggest that autophagy is not induced within a few hours of glucose starvation, they also show that within days autophagy becomes important for survival of these cells. Our data agree with the latter, show that autophagy is induced within the first 19 h of starvation, and additionally suggest that energy levels, as measured by ATP, decrease in autophagy-deficient cells already within 19 h.
In the respiratory-deficient cbp2Δ mutant, we found sedoheptulose-7-guanine (S7P), guanine, and phosphoenolpyruvate (PEP) to deplete during starvation in direct contrast to wild-type cells. S7P, guanine, and PEP are all potentially connected through gluconeogenesis, the pentose phosphate pathway, and nucleotide synthesis. Low PEP concentration would suggest that the cbp2Δ mutants have lower gluconeogenic flux (35), which also correlates with the decreased ribose levels observed for rapid timescales (10 to 60 s) in the Antimycin A-treated starved cells. High concentration of PEP is needed in order to drive the pathway to glucose-6-phosphate and consequently through the pentose phosphate pathway (where S7P resides), eventually becoming nucleotides (e.g., guanine). Gluconeogenic synthesis of PEP is primarily catalyzed by PEP carboxykinase. In yeast this reaction is driven by GTP hydrolysis. Given the lower levels of energy cofactors and presumably of energy fluxes in cbp2Δ cells (Fig. 1A), the production of PEP by carboxykinase is likely down-regulated (36).
An earlier study concluded that µ-lipophagy is triggered by glucose deprivation via the global energy regulator AMPK (8) within several days of starvation. Yeast AMPK activity is known to directly correlate with increased AMP/ATP ratio (37). We had observed changes in the energy charges of the cells (e.g., ATP, ADP, and AMP) (Fig. 3E) congruent with the observations of Seo et al. (8). Strikingly, we measured ATP to deplete within seconds while AMP increased in a near-equivalent time frame. This activity may suggest that AMPK potentially activates earlier and conveys the intracellular signaling to manage starvation within seconds. While AMPK signaling has been long studied, the kinetics of induction have not been entirely revealed (38). Our data open up the possibility that AMPK signaling may be activated within seconds of starvation.
One of the expected responses from AMPK signaling is an increase in β-oxidation activity. While a direct inhibition of Atg14 dependent µ-lipophagy did not affect the general energy status within the first 19 h of starvation, we found that the ability to perform general β-oxidation is important for ATP maintenance as well as survival in acute glucose starvation and that lipid intermediates increase within seconds after glucose starvation. Our lipidomics dataset suggests that these immediate changes are not due to large-scale membrane remodeling since we find that only a few lipid traces increase upon glucose starvation, in particular polyketides and dolichols (Fig. 5D). Polyketides are a structurally very heterogeneous class of compounds of which many have been attributed to antimicrobial function (39). The cells might manufacture the polyketides as an antimicrobial measure during starvation. Less microbial competition for remaining nutrients as well as nutrient freeing by eliminating potential competitors could be a rational strategy to ensure survival during starvation.
The other lipid that increased during 30 min of starvation was dolichol, which is required for protein glycosylation in the endoplasmic reticulum (Fig. 5D). Here dolichol functions together with UDP-glucose as a carrier to deliver substrates for glycosylation (40). Interestingly, we also observed the depletion of UDP-hexose (presumably UDP-glucose) in cbp2Δ cells within 1 to 4 h (Fig. 1B). In cbp2Δ cells, all potential intracellular energy sources, including UDP-hexoses, might be drained within hours of starvation due to the lack of efficient energy generation via respiration, while wild-type cells might refrain from degrading metabolites needed for rapid regrowth after starvation. The increase of dolichol in wild-type cells within 30 min of starvation, on the other hand, could point to an inhibition of glycosylation upon carbon starvation. Indeed, previous evidence suggests that the dolichol pathway is transcriptionally down-regulated in response to glucose starvation (41, 42).
In this paper, we sought to better characterize the strategies that yeast cells employ as they enter starvation. Our results show that yeast starvation is multifaceted and entails responses at many levels (e.g., metabolites, energy level) and pathways (β-oxidation, autophagy). We conclude that multiple metabolic responses are needed to ensure a sufficient supply of substrates for respiration as the cells maximize their survivability.
Methods
Strains and Growth.
All strains used in this study have a W303 background, and precise genotypes are listed in the SI Appendix, Table S1. Yeast strains were grown in liquid SCD medium (synthetic complete with 2% [wt/vol] glucose, containing 6.7 g/L yeast nitrogen base [Difco] and amino acids according to SI Appendix, Table S2) at pH 5.0 (titrated with HCl/KOH) at 30 °C rotating. YPD (Yeast extract–Peptone–Dextrose) plates (2% glucose [wt/vol, Sigma], 2% Bacto peptone [wt/vol, BD], 1% yeast extract [wt/vol, BD], 0.01% Adenine Hemisulfate [wt/vol, Sigma], and 2% Bacto agar [wt/vol, Chemie Brunschwig]) or YPGly plates (same as YPD plates, but with 2% glycerol [wt/vol, Sigma] substituting the glucose and without 0.01% Adenine Hemisulfate) were used for spotting assays. Gene deletions were performed by homologous recombination of a PCR-amplified cassette encoding antibiotic resistance, functional amino acid-encoding genes, or functional nucleotide-encoding genes according to SI Appendix, Table S1 (43).
Acute Starvation and Drug Treatments.
For acute glucose starvation, cells were washed three times in SC medium by repeating the following steps three times: 1) centrifuging for 1 min to pellet the cells, 2) removing supernatant, and 3) resuspending the cells in SC medium. Control cells in SCD were treated in the same way by washing three times in SCD medium. For SC–AA or H2O treatment, cells were washed three times either in SC medium lacking the added amino acids (SC−AA) (SI Appendix, Table S2) or in sterile water (H2O), both with a pH of 5.0 as in the SCD and SC medium. For nitrogen starvation, cells were washed three times in a 1.7 g/L yeast nitrogen base supplemented with 2% glucose. For MG132 treatment, strains with a deletion of PDR5 were used. Cells were treated with 100 µM MG132 (Sigma Fluka Aldrich) for 60 min at 30 °C. For combinations of MG132 treatment and glucose starvation, cells were pretreated as described with MG132 and then washed three times in SC medium containing MG132. For Antimycin A treatment, cells were treated with 10 µM Antimycin A (Sigma Fluka Aldrich) in starvation medium. Antimycin A was added to the medium used for washing of the samples as well.
ATP Measurements.
ATP measurements were done according to ref. 8 with minor modifications. Cells were pelleted and resuspended in 750 μL 90% acetone. Normalization was done according to OD. The cells were then incubated at 90 °C for ∼10 min to evaporate the acetone, when about 50 μL of solution remained. A total of 450 μL of buffer (10 mM Tris pH 8.0, 1 mM ethylenediaminetetraacetic acid) was added to the solution, and ATP was measured with an ATP Determination Kit (Thermo Scientific) on a CLARIOstar microplate reader (BMG).
Survival Assays.
Cells were acutely starved and incubated at 30 °C rotating. Cells were normalized by the OD measured within the first hour of starvation and spotted onto YPD plates, starting at an OD of 0.2, and in a 6 to 8× dilution series, diluting 5× at every step. The spotting plates were incubated at 30 °C for 1 to 2 d. Percentages of survival compared to wild type were assessed by counting colony forming units (CFUs) per dilution after the indicated days of starvation, compared with the CFUs counted after the same number of days in starvation of the wild type.
Autophagy Levels during Glucose Starvation.
Wild-type and Δatg2 cells expressing GFP-Atg8 were starved from glucose or nitrogen for 19 h, and thereafter the cells were imaged using an inverted Ti microscope (Nikon) to check for localization of GFP signal to the vacuole of cells.
Fast Filtration Wash, Sampling, and Extraction.
For each measurement, ∼1 OD unit of cells (OD 600*mL) was captured on filter paper using a fast filtration setup (21, 22). Immediately, the cells were suffused to flowing pretreatment media (SCD media) for at least 10 s. Upon media switch, the flow of pretreatment media was stopped, and the posttreatment media was followed for a given amount of time (SC media for 10, 20, 30, and 60 s or SCD for 0 and 20 s). After the posttreatment media, the cells and filter paper were immediately quenched in 4 mL of extraction solution of 40:40:20% acetonitrile:methanol:water at −20 °C. The extraction solution with cells was incubated at −20 °C overnight, and the extraction mix was subsequently transferred to 15 mL tubes. The extraction solvent was evaporated at 0.12 mbar to complete dryness in a SpeedVac setup (Martin Christ), and the samples were dissolved in 100 µL of water and transferred to a 96-well plate for measurement. Samples were stored at −20 °C until measurement.
Metabolomics Measurement and Annotation.
For untargeted analysis (Figs. 2A, 3 A–C and E, and 5A), extracts were measured using flow-injection analysis quadrupole time-of-flight in negative ionization mode and annotated to metabolites as described in a previous study (20). For targeted analysis (Figs. 1B and 3D), measurement and analysis is described in previous work (25). For all targeted analysis measurements, an internal standard of fully labeled C13 extract was used to normalize all data (44).
Lipidomics Extraction.
For each sampling, ∼25 OD units of cells (OD600*mL) were captured on filter paper using a fast filtration setup. The cells with filter paper were immediately quenched in 10 mL of yeast lipid extraction solution (15:15:5:1:0.018% of ethanol, water, diethyl ether, pyridine, and 4.2 N ammonium hydroxide, respectively) at −20 °C. The extraction solution with cells was incubated at −20 °C overnight, and the extraction mix was subsequently transferred to 15 mL tubes. The extraction solvent was evaporated using a SpeedVac setup (Martin Christ), and the samples were dissolved in 100 µL of 45:45:10% of isopropanol, methanol, and water, respectively, and transferred to glass vials with inserts. Samples were stored at −20 °C until measurement.
Lipidomics Measurement.
Chromatographic separation and analysis by mass spectrometry was done using a 1200 series HPLC system with a Phenomenex Kinetex column (1.7 µL × 100 mm × 2.1 mm) with a SecurityGuard Ultra (Part No: AJ-9000) coupled to an Agilent Technologies 6550 Accurate-Mass Q-Tof. Solvent A: H2O, 10 mM formic acid; Solvent B: acetonitrile, 10 mM formic acid. Ten microliters of extract were injected, and the column (C18) was eluted at 1.125 mL/min. Initial conditions were 60% solvent B: 0 to 2 min; 95% B: 2 to 4 min; and 60% B: 4 to 5 min under initial conditions. Spectra were collected in negative ionization mode from 50 to 3,200 mz with high resolution at 4 GHz. Continuous infusion of calibrants (Agilent compounds HP-321, HP-921, HP-1821) ensured exact masses over the whole mass range. We converted the raw data files to the mzML format using msConvert and processed them in R using the XCMS (ver. 3.0.2) and CAMERA (ver. 1.34.0). M-H and M+FA-H ions were annotated using LIPID MAPS (vers. March 2017) with a mass tolerance of 0.005 amu (29).
Data Availability Statement.
All data discussed in the paper along with custom scripts used to generate figures can be found in SI Appendix or on GitHub at https://github.com/karsekar/yeast-starvation.
Supplementary Material
Acknowledgments
We thank the U.S. and K.W. laboratory members for feedback and advice; Elisa Dultz, Stephanie Heinrich, and Ruchika Sachdev for critical reading of the manuscript; John Peter Arun Thomas for advice on the lipid extraction; Marieke F. Buffing and Brendan Ryback for fast filtration help; and Marieke F. Buffing and Tobias Fuhrer for targeted mass spectrometry help. This work was supported by Swiss National Science Foundation Grant SNF 31003A_179275 (to K.W.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: All ion data as well as custom scripts for the respective figures are provided via GitHub (https://github.com/karsekar/yeast-starvation).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913370117/-/DCSupplemental.
References
- 1.Lauretti E., Li J.-G., Di Meco A., Praticò D., Glucose deficit triggers tau pathology and synaptic dysfunction in a tauopathy mouse model. Transl. Psychiatry 7, e1020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Leon M. J., et al. , Positron emission tomographic studies of aging and Alzheimer disease. AJNR Am. J. Neuroradiol. 4, 568–571 (1983). [PMC free article] [PubMed] [Google Scholar]
- 3.O’Connor T., et al. , Phosphorylation of the translation initiation factor eIF2α increases BACE1 levels and promotes amyloidogenesis. Neuron 60, 988–1009 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Virgilio C., The essence of yeast quiescence. FEMS Microbiol. Rev. 36, 306–339 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Ashe M. P., De Long S. K., Sachs A. B., Glucose depletion rapidly inhibits translation initiation in yeast. Mol. Biol. Cell 11, 833–848 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jona G., Choder M., Gileadi O., Glucose starvation induces a drastic reduction in the rates of both transcription and degradation of mRNA in yeast. Biochim. Biophys. Acta 1491, 37–48 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Metzl-Raz E., et al. , Principles of cellular resource allocation revealed by condition-dependent proteome profiling. eLife 6, e28034 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo A. Y., et al. , AMPK and vacuole-associated Atg14p orchestrate μ-lipophagy for energy production and long-term survival under glucose starvation. eLife 6, e21690 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyner R. P., et al. , A glucose-starvation response regulates the diffusion of macromolecules. eLife 5, e09376 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munder M. C., et al. , A pH-driven transition of the cytoplasm from a fluid- to a solid-like state promotes entry into dormancy. eLife 5, e09347 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klosinska M. M., Crutchfield C. A., Bradley P. H., Rabinowitz J. D., Broach J. R., Yeast cells can access distinct quiescent states. Genes Dev. 25, 336–349 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang M. J., et al. , Glucose starvation inhibits autophagy via vacuolar hydrolysis and induces plasma membrane internalization by down-regulating recycling. J. Biol. Chem. 289, 16736–16747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefevre S. D., van Roermund C. W., Wanders R. J. A., Veenhuis M., van der Klei I. J., The significance of peroxisome function in chronological aging of Saccharomyces cerevisiae. Aging Cell 12, 784–793 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Link H., Fuhrer T., Gerosa L., Zamboni N., Sauer U., Real-time metabolome profiling of the metabolic switch between starvation and growth. Nat. Methods 12, 1091–1097 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Sekar K. et al., Synthesis and degradation of FtsZ quantitatively predict the first cell division in starved bacteria. Mol. Syst. Biol. 14, e8623 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milo R., Jorgensen P., Moran U., Weber G., Springer M., BioNumbers: The database of key numbers in molecular and cell biology. Nucleic Acids Res. 38, D750–D753 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw L. C., Lewin A. S., The Cbp2 protein stimulates the splicing of the omega intron of yeast mitochondria. Nucleic Acids Res. 25, 1597–1604 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson W. A., et al. , Regulation of glycogen metabolism in yeast and bacteria. FEMS Microbiol. Rev. 34, 952–985 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomsson E., et al. , Carbon starvation can induce energy deprivation and loss of fermentative capacity in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 69, 3251–3257 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuhrer T., Heer D., Begemann B., Zamboni N., High-throughput, accurate mass metabolome profiling of cellular extracts by flow injection-time-of-flight mass spectrometry. Anal. Chem. 83, 7074–7080 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Link H., Kochanowski K., Sauer U., Systematic identification of allosteric protein-metabolite interactions that control enzyme activity in vivo. Nat. Biotechnol. 31, 357–361 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Rabinowitz J. D., Kimball E., Acidic acetonitrile for cellular metabolome extraction from Escherichia coli. Anal. Chem. 79, 6167–6173 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Kanehisa M., et al. , Data, information, knowledge and principle: Back to metabolism in KEGG. Nucleic Acids Res. 42, D199–D205 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kormančíková V., Kováč L., Vidová M., Oxidative phosphorylation in yeast. V. Phosphorylation efficiencies in growing cells determined from molar growth yields. Biochim. Biophys. Acta 180, 9–17 (1969). [DOI] [PubMed] [Google Scholar]
- 25.Buescher J. M., Moco S., Sauer U., Zamboni N., Ultrahigh performance liquid chromatography-tandem mass spectrometry method for fast and robust quantification of anionic and aromatic metabolites. Anal. Chem. 82, 4403–4412 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Cheong H., Klionsky D. J., “Biochemical methods to monitor autophagy-related processes in yeast” in Autophagy: Lower Eukaryotes and Non-Mammalian Systems, Part A, Klionsky D. J., Ed. (Methods in Enzymology, Academic Press, 2008), pp. 1–26. [DOI] [PubMed] [Google Scholar]
- 27.Barth H., Meiling-Wesse K., Epple U. D., Thumm M., Autophagy and the cytoplasm to vacuole targeting pathway both require Aut10p. FEBS Lett. 508, 23–28 (2001). [DOI] [PubMed] [Google Scholar]
- 28.da Silveira Dos Santos A. X., et al. , Systematic lipidomic analysis of yeast protein kinase and phosphatase mutants reveals novel insights into regulation of lipid homeostasis. Mol. Biol. Cell 25, 3234–3246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fahy E., et al. , Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 50 (suppl.), S9–S14 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei D., et al. , High-resolution three-dimensional reconstruction of a whole yeast cell using focused-ion beam scanning electron microscopy. Biotechniques 53, 41–48 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Kunau W.-H., et al. , “β-oxidation systems in eukaryotic microorganisms” in Peroxisomes in Biology and Medicine, Fahimi H. D., Sies H., Eds. (Proceedings in Life Sciences, Springer, Berlin, Heidelberg, 1987), pp. 128–140. [Google Scholar]
- 32.Igual J. C., Matallaná E., Gonzalez-Bosch C., Franco L., Pérez-Ortin J. E., A new glucose-repressible gene identified from the analysis of chromatin structure in deletion mutants of yeast SUC2 locus. Yeast 7, 379–389 (1991). [DOI] [PubMed] [Google Scholar]
- 33.Adachi A., Koizumi M., Ohsumi Y., Autophagy induction under carbon starvation conditions is negatively regulated by carbon catabolite repression. J. Biol. Chem. 292, 19905–19918 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi C., et al. , Formation of a Snf1-Mec1-Atg1 Module on mitochondria governs energy deprivation-induced autophagy by regulating mitochondrial respiration. Dev. Cell 41, 59–71.e4 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Sauer U., Eikmanns B. J., The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol. Rev. 29, 765–794 (2005). [DOI] [PubMed] [Google Scholar]
- 36.De Torrontegui G., Palacián E., Losada M., Phosphoenolpyruvate carboxykinase in gluconeogenesis and its repression by hexoses. Biochem. Biophys. Res. Commun. 22, 227–231 (1966). [DOI] [PubMed] [Google Scholar]
- 37.Wilson W. A., Hawley S. A., Hardie D. G., Glucose repression/derepression in budding yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP:ATP ratio. Curr. Biol. 6, 1426–1434 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Hedbacker K., Carlson M., SNF1/AMPK pathways in yeast. Front. Biosci. 13, 2408–2420 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herbst D. A., Townsend C. A., Maier T., The architectures of iterative type I PKS and FAS. Nat. Prod. Rep. 35, 1046–1069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kornfeld R., Kornfeld S., Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 54, 631–664 (1985). [DOI] [PubMed] [Google Scholar]
- 41.Harada Y., et al. , Metabolically programmed quality control system for dolichol-linked oligosaccharides. Proc. Natl. Acad. Sci. U.S.A. 110, 19366–19371 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kukuruzinska M. A., Lennon K., Growth-related coordinate regulation of the early N-glycosylation genes in yeast. Glycobiology 4, 437–443 (1994). [DOI] [PubMed] [Google Scholar]
- 43.Baudin A., Ozier-Kalogeropoulos O., Denouel A., Lacroute F., Cullin C., A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 21, 3329–3330 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett B. D., Yuan J., Kimball E. H., Rabinowitz J. D., Absolute quantitation of intracellular metabolite concentrations by an isotope ratio-based approach. Nat. Protoc. 3, 1299–1311 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in the paper along with custom scripts used to generate figures can be found in SI Appendix or on GitHub at https://github.com/karsekar/yeast-starvation.