Abstract
The syndrome of multiple morphological abnormalities of the sperm flagella (MMAF) is a specific kind of asthenoteratozoospermia with a mosaic of flagellar morphological abnormalities (absent, short, bent, coiled, and irregular flagella). MMAF was proposed in 2014 and has attracted increasing attention; however, it has not been clearly understood. In this review, we elucidate the definition of MMAF from a systematical view, the difference between MMAF and other conditions with asthenoteratozoospermia or asthenozoospermia (such as primary mitochondrial sheath defects and primary ciliary dyskinesia), the knowledge regarding its etiological mechanism and related genetic findings, and the clinical significance of MMAF for intracytoplasmic sperm injection and genetic counseling. This review provides the basic knowledge for MMAF and puts forward some suggestions for further investigations.
Keywords: asthenoteratozoospermia, disease-causing genes, intracytoplasmic sperm injection, male infertility, multiple morphological abnormalities of the sperm flagella
INTRODUCTION
Infertility is a worldwide medical and social problem, with physical and psychosocial consequences. Asthenozoospermia refers to the decrease or lack of motile sperm in one ejaculate and is frequently combined with oligo- or teratozoospermia, occurring in approximately 19% of infertile men.1,2,3 The syndrome of multiple morphological abnormalities of the sperm flagella (MMAF) is a specific kind of asthenoteratozoospermia,4 which features a combination of aberrant flagellar phenotypes (absent, short, bent, coiled, and irregular flagella) and seriously impaired sperm motility. It is a new concept, being first defined in 2014.5 Previous terms included “dysplasia of the fibrous sheath,” “short tails” or “stump tails”.4,6 Another similarly noteworthy kind of asthenozoospermia is observed in men with primary ciliary dyskinesia (PCD). PCD is a hereditary disorder caused by motility defects in the cilia and flagella and is manifested by multisystemic dysfunctions such as rhinitis, sinusitis, recurrent respiratory infections, situs inversus (approximately 50%), and infertility.7 In humans, motile cilia and sperm flagella have a common axoneme structure that is evolutionarily conserved. The structure comprises nine peripheral double-microtubules (DMT) plus two central pairs (CP), which is termed a 9+2 structure. An emerging issue is whether MMAF is a phenotypic variation of the classical PCD or merely another category of defects.6,8,9
Based on the current studies,4,10 a genetic origin is highly suspected for MMAF and the search for the responsible genes has made great progress. Unlike other teratozoospermia where approximately 80% of the patients are affected by mutations in a single gene, such as DPY19L2 in globozoospermiaand AURKC in macrozoospermia,6 the etiology of MMAF is more heterogeneous.11 Whole-exome sequencing analysis has uncovered high frequencies of mutations in DNAH1, CFAP44, and CFAP43 that are responsible for approximately one-third of all MMAF cases.12,13 Other studies involving AK7, CFAP69, CEP135, AKAP3, or AKAP4 have also provided strong genetic evidence for MMAF.12,14,15,16 These studies have revealed different molecular mechanisms for the occurrence of MMAF. However, a systematic view for these mechanisms, as well as the knowledge of the genetic link or difference between MMAF and PCD, has been lacking.
No spontaneous pregnancy has been reported in MMAF patients.10 Intracytoplasmic sperm injection (ICSI) is the only way for these patients to conceive with female partners. Although successful fertilization or pregnancies were reported through ICSI, multiple studies have shown a frequent aneuploidy and low quality sperm nucleus related to sperm flagellar defects.4 Therefore, close attention should be paid to the overall outcome of ICSI and the clinical management for patients with MMAF. Additionally, genetic counseling is highly significant in helping evaluate and avoid the risk of transmission of genetic defects by ICSI in these patients.
In this review, we focus on MMAF, a specific kind of asthenoteratozoospermia. We elucidate the definition of MMAF, provide an update of the genetic etiology of the phenotype, detail the potential molecular mechanisms, and summarize the clinical significance arising from new findings that will inform future investigations.
MMAF, A TYPE OF ASTHENOTERATOZOOSPERMIA
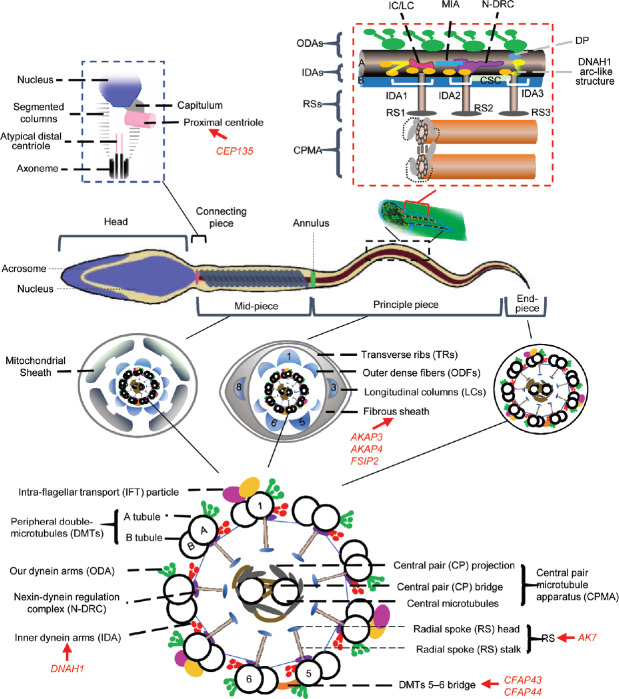
Eukaryotic flagella are classified along with most eukaryotic motile cilia as undulipodia (9+2 structure)17 to emphasize the distinctive wavy appendage role in cellular function or motility. MMAF is a developmental disturbance of the sperm flagella. Human sperm flagella can be divided into three parts – the mid-piece, principal piece, and end-piece – based on the distribution of accessory structures uniquely expressed in flagella and not in mobile cilia.18 Outer dense fibers (ODFs) are present in both the mid- and principal pieces surrounding the axoneme. The mitochondria are arranged helically to the mid-piece, which are replaced by the fibrous sheath (FS) in the principal piece, while no peri-axonemal structures exist in the end piece.14
Definition of MMAF
MMAF is not a recent discovery.8,9,19,20,21,22 The first case was reported over 40 years ago. However, the incidence of MMAF in infertile males has been unclear. One important reason might be that the category of sperm defect by standardized morphological analysis is underappreciated and may require electron microscopy examination in some cases. Moreover, a diagnosis achieved through electron microscopy is mainly performed for those with reduced sperm motility. Flagellar abnormities, such as those seen in MMAF, are rarely identified during routine semen analyses.21 This has been changed with the recent advent of standardized criteria for the assessment of the abnormal morphology of sperm flagella, including absent, short, bent, coiled, and irregular tail, according to the 5th Edition of the World Health Organization Standards for the Evaluation of Human Semen (Table 1).23
Table 1.
The defined morphology of sperm tail
| Term | Standards according to the 5th edition of the WHO standards (2010) |
|---|---|
| No tail | Isolated head, no tail observed |
| Short tail | A tail length (mid-piece plus principal piece) no more than five times the major axis length of the head |
| Bent/ misaligned tail |
May include neck anomalies, misalignment of the head major axis and midpiece axis or an acute angle (≤120°) between the head major axis and midpiece axis or an acute angle (≤120°) between the midpiece and the principal piece axes |
| Coiled tail | Completely or partially coiled tail, with the coil close to or around the head (flat coiling or coiling at the extremity of the tail reminiscent of hypo-osmotic coiling aspects should not be included in this category) |
| Irregularly shaped tail |
Irregular width of the midpiece or changing calibre of the principle piece; or the principle piece is not thinner than the midpiece |
The typical abnormities of sperm flagella lead us to identify MMAF. However, the closer we get to the definition, the more we believe that it is far from being arbitrary. Since the sperm head is generally normal, MMAF damage ought to occur at the latest stages of spermiogenesis during flagellum elongation in spermatids, producing an effect on flagellar assembly.19 The defects, at the very least, need to involve the principal piece of the flagellum which constitutes approximately 75% of the entire length of the flagellum, to produce a phenotype with various tail morphologies.24 In other words, most of the defects may be ascribed to the dysfunction of axonemal or FS located at this piece. Furthermore, it is not only a mosaic of morphological abnormalities presenting absent, short, bent, coiled, and irregular tail, but also a mosaic of ultrastructural flagellar defects including absent CP, dysplasia of fibrous sheath (DFS), absence of dynein arms or disorganized DMT, suggesting that heterogeneously genetic factors are involved in the causes of MMAF.
Difference between MMAF and other types of asthenozoospermia
MMAF should be differentiated from other types of asthenozoospermia, including primarily mitochondrial sheath (MS) defects, PCD-related defects, annulus dysfunction and defects of ion channels. For example, the primarily MS defects are confined to the mid-piece that constantly shows mitochondrial changes in the ultrastructure, or the absence or reduction of mitochondria,25,26,27 while MMAF affects many other axonemal or peri-axonemal structures only partially accompanied by MS disorganization (sometimes with a differently thickened MS). Besides, the annulus, a ring-like structure demarcating the mid-piece and principal piece of the sperm tail (Figure 1), is generally missing in sperm with primarily MS defects, while in MMAF, the annulus is often retained just below the connecting piece and does not migrate caudally.28 In contrast to the reduced mitochondrial membrane potential in primarily MS defect sperm, the mitochondria in MMAF can present a high membrane potential suggesting a functionally active condition in a large number of cells.26
Figure 1.
Structure of a mature human sperm. Sperm flagellum is structurally divided into three parts: mid-piece, principal piece and end-piece; the cross-section of each part is shown. Annulus is a ring structure demarcating the mid-piece and the principal piece of the sperm tail. The axoneme is a highly evolutionarily conserved structure present in the whole flagellum. The schematic structure of the axoneme from the transverse view illustrates the approximate localizations of each component. The mid-piece consists of a helical MS surrounding the axoneme, which is replaced by the FS in the principal piece; the terminal piece is devoid of any peri-axonemal structures. The red box at top right is a longitudinal view illustrating the outer doublet A-tubule of IADs, OADs and regulatory structures within a single 96 nm axonemal repeat. DNAH1 may form an arc-like structure directly connected to the RS3.5 The black box at top left is the schematic structure of the connecting piece. The suggested locations of DNAH1, CFAP43, CFAP44, AK7, AKAP3, AKAP4, CEP135 are also marked in the drawing with red fonts and arrows. RSs: radial spokes; CPMA: central pair microtubule apparatus; IC/LC: intermediate chain/light chain; N-DRC: nexin-dynein regulatory complex; MIA: modifier of inner arms complex; CSC: calmodulin- and spoke associated complex; DP: distal protrusion; MS: mitochondrial sheath; FS: fibrous sheath; IADs: inner arm dyneins; OADs: outer arm dyneins.
The spermatozoon of infertile male patients with PCD resembles the presentation of MMAF to some extent.29,30,31,32,33 However, this pathophysiological aspect of PCD has not been investigated systematically, and it should be noted that sperm parameters are not even recorded in most PCD patients.4 Indeed, the etiology of PCD patients and MMAF may overlap, due to the similarities between cilia and flagella in the axonemal structure. However, there are situations when patients with PCD are not infertile. Based on current studies in MMAF, we suggest MMAF is a specific cause of asthenoteratozoospermia, independent of PCD. There are several reasons for this suggestion. First, there are still phenotypic discordances between sperm flagella and cilia, indicating distinctions in the management of axonemes between cilia and flagella.34 Second, mutations in more than 40 genes responsible for various ultrastructural defects have so far been identified as accounting for the genetic etiology of 70% of PCD affected individuals, but few of these genes appear to result in morphologically heterogeneous phenotypes of sperm flagella.4,33 This suggests different genetic origins, corresponding to the comprehensive proteome analysis where over 700 specific flagellar proteins were identified including many axonemal proteins,35 indicating that many genes could be exclusively connected to flagellar biogenesis.36
Other types of asthenozoospermia caused by annulus dysfunction or defects of ion channels located at the sperm plasma membrane are also different from MMAF according to phenotypes and biogenesis. The former may result from a septin complex consisting of SEPT1, 4, 6, and 7 and coordinated by SEPT12 (Figure 2).6,28 The latter is attributed to three main proteins. CATSPER1/2 may function in the Ca2+ channel regulating Ca2+ fluxes during sperm movement and capacitation,37 while another protein SLC26A8 localized to the annulus may act as an anion transporter6,38 (Figure 2).
Figure 2.
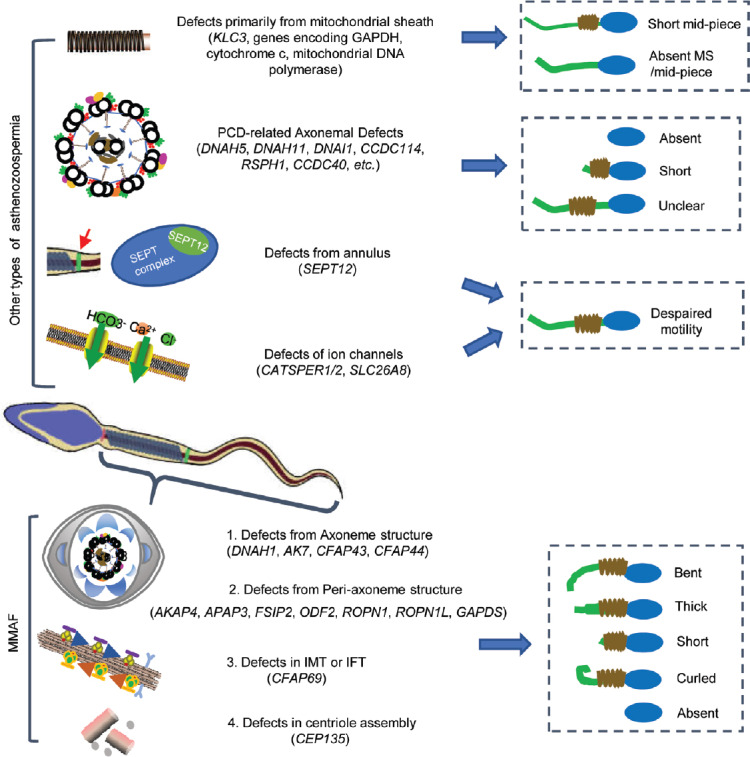
Representation of the genes linked to different kinds of asthenozoospermia and the typical phenotype of each kind. In other types of asthenozoospermia, the typical phenotype of four types of asthenozoospermia and related genes are shown, including PCD-related axonemal defects, primary defects from mitochondrial sheath, defects from annulus, and defects of ion channels. In MMAF, four main mechanisms are given, including defects from axoneme structure, defects from peri-axoneme structure, defects in IMT or IFT, and defects in centriole assembly. PCD: primary ciliary dyskinesia; MMAF: multiple morphological abnormalities of the sperm flagella; IMT: intra-manchette transport; IFT: intra-flagellar transport.
MECHANISMS FOR MMAF AND RELATED DISEASE-CAUSING GENES
The pathogenic mechanisms of MMAF are enigmatic. In this section, we summarize the current findings on the disease-causing genes and their potential mechanisms, with the hope of providing some insight for future investigations.
Structure defects of the axoneme and related disease-causing genes
The typical axoneme consists of nine DMTs (tubule A and B) and two central singlet microtubules. Other structures that are highly organized on the DMTs include outer dynein arms (ODAs), inner dynein arms (IDAs), nexin-dynein regulation complex and radial spokes (RSs) (Figure 1).34 Seven subunits are arranged in the IDAs; they comprise three different groups (IDA1 to IDA3) with a 3-2-2 pattern.5 More importantly, there are three different RS (RS1, RS2, and RS3) that are respectively anchored to the inner arm bases of IDA1, IDA2, and IDA3, which allow a connection between external DMTs and the two CPs. Nexin-dynein regulation complex, which localize closely to the RSs and IDAs, connect the inside bases of tubule A to the adjacent tubule B.39 Ultrastructure studies revealed that the two CP microtubules are different, with two distinct sets of projections with 16 and 32 nm repeats.40,41 These function as a central pair microtubule apparatus.42,43 Owing to a particular position of CP, any destabilization caused by IDAs, ODAs, or RS might induce the disappearance or disorganization of central microtubules.34 The 9+2 ultrastructure of the axonemes appears equivalent to cilia and flagella. However, molecular differences may still exist, such as in dynein stability and extractability as demonstrated in the phylum Mollusca, genera Mytilus and Spisula.44,45
The variable configurations of sperm axonemes in different species (such as Asphondylia ruebsaameni, Anguilla anguilla and American horseshoe crabs) allow us to illustrate the functions of axonemal components.44 The periodical wave propagation (including the amplitude and waveform) is achieved in the presence of IDAs, while the planar movement of the flagellum seems to originate from other structures like the RS and CP. ODAs seem only to regulate the speed of wave propagation, and the DMTs are preserved in positions where dynein molecule slide past each other.34,44 The CP of microtubules may regulate DMT sliding and possibly taxis. They may not be crucial for motility in all species, since CP is frequently absent or replaced by a nonmicrotubule core in some species.34,46,47
DNAH1
Dynein axonemal heavy chain 1 (DNAH1), which is an IDA gene, is the first and so far only gene universally acknowledged to cause MMAF when mutated. The estimated rate of MMAF caused by DNAH1 mutations is 24.6% (34/138), from five convincing studies.5,13,48,49 In Dnah1 knockout mice, transmission electron microscopy has revealed a missing globule of the IDA3 head, resulting in a 3-2-1 arrangement.5 The observation suggests that DNAH1 may be an IDA3 element that provides an anchoring site for RS3. The finding of missing ODAs suggests that DNAH1 may localize between RS3 and ODAs on microtubules (Figure 1). The absence of CP in a reported 41.5% of patients harboring DNAH1 mutation is mostly secondary to the IDAs defects in MMAF. Another study also reported that the occurrence of sperm head anomalies was 21.8% in MMAF with DNAH1 mutation.49 However, the effect of DNAH1 on the sperm head remains to be investigated.
The progressive motility of sperm with DNAH1 mutation is comparatively higher than in other genetic causes for MMAF (Table 2), ranging from 0% to 13%. This is strange considering the important role of IDAs for sperm motility. Other heavy chain dynein may compensate for DNAH1 function in flagella. What echoes this speculation is that among all the dynein chains the identified heavy dynein chains are the most abundant; with up to 15 kinds (their functions are mostly unclear). It has been suggested that DNAH12 is one potential candidate for DNAH1 replacement or compensation in cilia.5 In addition, the role of DNAH1 in cilia may not be as crucial as it is in flagella, which is reflected by its decreased expression in the trachea compared to the testis and could also explain the elimination of other PCD symptoms in DNAH1 mutant MMAF patients.
Table 2.
The characteristics of multiple morphological abnormalities of the sperm flagella with mutations in current-identified pathogenetic genes
| Gene name | Protein location | MMAF morphology (%) | Patients, % (n) | PM (%) | Sperm phenotype (human) | KO mice | Phenotypes of the KO mice | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absent flagella | Short flagella | Coiled flagella | Bent flagella | Irregular caliber | ||||||||
| DNAH1 | IDA | 28.5 | 42 | 18 | 5 | 42 | 38.9% (7/18) | 6.25 | Lack IDA; disorganized 9+2; absent CP (47%); disorganized FS (90%); supernumerary dense fibers with absence of mitochondrion |
No | No structural defects of the axoneme were observed; a decrease in the beat frequency of approximately 50% |
5 |
| 16.4 | 55 | 4 | 11.8 | – | 57.1% (12/21) | 1.8 | Disordered MS; missing sperm tail, except for the partial midsection; missing or disarranged 9+2; missing ODA |
No | NA | 48 | ||
| 25.6 | 49.8 | 9 | 6 | 35 | 7.7% (6/78) | 2.6 | NA | No | NA | 13 | ||
| 10.7 | 82.2 | 9.1 | 7.9 | 45.3 | 44.4% (4/9) | 0 | NA | No | NA | 48 | ||
| 2.6 | 63.8 | 5.4 | NA | NA | 41.7% (5/12) | 13 | Anomalies of the head (21.8%) | No | NA | 49 | ||
| CFAP43 | Probably located next to DMTs 5–6 | 12.5 | 40 | 24.5 | 6.7 | 6 | 10.0% (3/30) | 0 | Most spermatozoa lacked CP and had hyperplasia of FS |
Yes | MMAF | 50 |
| 21.8 | 65.3 | 8.2 | 10.3 | 20.2 | 12.8% (10/78) | 0 | 9+0 (82%); absent CP+disorganized DMT (13.6%); short tails with unorganized cytoplasmic mass; CPC is not aligned with DMTs 3 and 8 |
Yes | MMAF; short tail with a cytoplasmic mass containing flagellum components; uneven DMTs; absent CP |
13 | ||
| 10 | 78 | 4 | 8 | NA | 3.7% (1/27) | 0 | NA | No | NA | 51 | ||
| CFAP44 | Probably located next to DMTs 5–6 | 42.5 | 40.5 | 5.0 | 4.0 | 7.5 | 3.3% (1/30) | 0 | Thickened FS and an absent CP |
Yes | MMAF | 50 |
| 36.8 | 52.2 | 14.4 | 9 | 28.4 | 7.7% (6/78) | 0 | 9+0 (67%); 9+1 (10%); absent CP+disorganized DMT (19%) |
Yes | Normal length, but irregular caliber of midpiece (85%), absence or irregular distribution of DMTs; a third LC increases asymmetry |
13 | ||
| 17.6 | 55.8 | 12 | 4.4 | NA | 18.5% (5/27) | 0.2 | NA | No | NA | 51 | ||
| AK7 | Sperm flagellum | NA | NA | NA | NA | NA | 100% (2/2) | 0-1 | Abnormal and incomplete MS, DFS, and disorganization of axonemal structure with lack of CP |
Yes | Severer than usual MMAF; (testes) presence of sperm head without elongated flagella; (epididymis) very few sperm |
14 |
| AKAP4 | FS | NA | NA | NA | NA | NA | 100% (1/1) | NA | High XY disomy; disorganized FS and altered axonemal structure, FS remnants embedded in a cytoplasmic residue; some lacking DAs, DMTs and CP |
No | NA | 67 |
| FSIP2 | FS | 7.5 | 57.5 | 64 | NA | 44.8 | 100% (4/4) | NA | Completely DFS; ODFs remained present; totally absent MS; hypertrophic FS extended up to the sperm neck; abnormal axoneme |
No | NA | 71 |
| CFAP69 | Mid-piece | 6.5 | 46 | 4 | 2.5 | 4 | 2.6% (2/78) | NA | Absent or disorganized CP and DMT; high-rate head malformations, in particular thin heads and an abnormal acrosomal region |
Yes | MMAF and a few head defects; dramatic cytoplasm with mitochondria; Disorganized ODFs; Disorganized and largely absent FS; Manchette and flagellum components accumulated |
12 |
| CEP135 | Next to PC | 40 | 45 | 12 | 3 | 46 | 100% (1/1) | <1.8 | NA | No | NA | 15 |
DFS: dysplasia of the fibrous sheath; PM: progressive motility; KO: knock-out; FS: fibrous sheath; MS: mitochondrial sheath; CP: central pair of microtubules; CPC: central pair complex; DA: dynein arm; ODA: outer dynein arm; DMT: double microtubules; PC: proximal centriole; NA: not available; MMAF: multiple morphological abnormalities of the sperm flagella; DNAH1: dynein axonemal heavy chain 1; IDA: inner dynein arm; CFAP43: cilia- and flagella-associated protein 43; AK7: adenylate kinase 7; AKAP4: A-kinase anchoring protein 4; FSIP2: fibrous sheath interacting protein 2; CFAP69: cilia and flagella associated protein 69; CEP135: centrosomal protein 135; ODFs: Outer dense fibers
CFAP43 and CFAP44
Three studies reported that mutations in two genes encoding cilia- and flagella-associated protein 43(CFAP43) and CFAP44 could lead to MMAF, with an estimated occurrence of 10.4% (14/135) and 8.9% (12/135), respectively (Table 2).13,50,51 These two proteins may be specifically located next to DMT 5–6 bridge (Figure 1).52 The two-part 5–6 bridge is a doublet-specific structures that links DMTs 5 and 6 in the axonemes of many animal cilia and flagella.13,52,53,54,55,56 This bridge limits the inter-doublet sliding against its neighbor, offering a firm plane that is vertical to the bending plane of the flagella. CFAP43 and CFAP44 might share physical interactions with the DMT 5–6 bridge and the absence of one could destabilize the entire complex, leading to both peri-axonemal and axonemal defects.
Coutton et al.13 reported that the rate of the (9+0) conformation is higher in CFAP43-mutated than in CFAP44-mutated patients (81.8% vs 66.7%). While the (9+1) conformation was observed only for the CFAP44 patients (approximately 10% of these patients), the residual CP in CFAP43 patients was ubiquitous misoriented. Disorganized DMT and hyperplasia of the FS are common. Overall, the CFAP43 mutant seems to be associated with a much severer behavior than the CFAP44 mutant in humans and in mice. Since the absence of CFAP43 or CFAP44 has no visible impact on motile cilia in humans and mice, they may interact with the axoneme with flagella specific extra-axonemal structures.
AK7
Another axonemal gene suspected of being related with MMAF is adenylate kinase 7 (AK7), which encodes a phosphotransferase. Lores et al.14 used whole-exome sequencing to reveal an AK7 homozygous mutation c.2018T>G (p. Leu673Pro) in MMAF siblings who did not display any PCD features. Although the AK7 protein is absent in mature spermatozoa, protein expression in airway epithelial cells is not defective. Only the mutant transcripts can be detected in sperm cells and airway epithelial cells, which eliminate the possibility of an alternative transcript. It is strange that a structurally defective protein could function normally in one organ, while the same defective protein can be absent and lead to dysfunction in another organ. Lores et al.14 argued that a structural defect in the AK7 protein has different functional properties in sperm cells and in airway epithelial cells, or a sperm-specific protein quality control/proteasome machinery (such as the ubiquitylation pathway) to degrade the mutated AK7 protein.
AK7 is the only adenylate kinase that contains a Dpy-30 domain at the C-terminus (Table 3) which may be a dimerization motif analogous binding to the A-kinase anchoring protein domain. Other genes containing the Dpy-30 domain are related to the RSs of sperm flagella. Considering the discovery of the ubiquitin domain in Ciona radial spoke, AK7 may be involved in the proteasome-dependent regulation of protein kinase a localized at the RS. Before the Lores et al.14 study, a few studies had investigated the role of AK7 in PCD.57,58,59 The evidence indicates that AK7 has a crucial role in cilia and flagella. Therefore, it remains possible mutations causing absent AK7 protein in humans may also lead to PCD.
Table 3.
Characteristics of the pathogenetic genes in multiple morphological abnormalities of the sperm flagella
| Gene | Protein structural domains | Summary of overall gene expression pattern | Organs with enhanced expression at different levels |
|---|---|---|---|
| DNAH1 | Pfam: DHC-N2; AAA_6; AAA_5; AAA_7; AAA_8; MT; AAA_9; Dynein heavy |
Distinct expression in cilia of respiratory epithelium and fallopian tube |
Protein- or RNA-level: nasopharynx; fallopian tube; testis |
| CFAP43 | SMART: WD40*5 | Tissue enhanced in reproductive organ | RNA-level: testis; fallopian tube; epididymis |
| CFAP44 | SMART: WD40*6 | Cytoplasmic expression in Spleen, cerebral cortex and skin | Protein-level: spleen, cerebral cortex and skin RNA-level: testis; fallopian tube |
| AK7 | Pfam: ADK; DPY-30 | Distinct expression in ciliated cells, accompanied with cytoplasmic expression in several tissues |
Protein-level: fallopian tube; endometrium; nasopharynx RNA-level: fallopian tube; testis |
| AKAP3 | SMART: AKAP_110 | Cytoplasmic expression in spermatids and epididymis |
Protein- or RNA-level: testis; epididymis |
| AKAP4 | SMART: AKAP_110 | Selective expression in spermatids of seminiferous ducts |
Protein- or RNA-level: testis |
| FSIP2 | No | Tissue enriched (testis) | Protein- or RNA-level: testis |
| CFAP69 | No | Luminal membranous expression in fallopian tube, respiratory epithelia and small intestines |
Protein-level: nasopharynx; lung; intestine; fallopian tube RNA-level: prostate; fallopian tube; epididymis; testis |
| CEP135 | No | Expressed in all | RNA-level: lymph node; testis; endometrium; thymus |
DNAH1: dynein axonemal heavy chain 1; CFAP43: cilia- and flagella-associated protein 43; AK7: adenylate kinase 7; AKAP4: A-kinase anchoring protein 4; FSIP2: fibrous sheath interacting protein 2; CFAP69: cilia and flagella associated protein 69; CEP135: centrosomal protein 135
Structural defects of peri-axoneme and related disease-causing genes
In MMAF, the abnormalities in sperm flagella are not present or apparent in cilia, leading us to wonder if the unique peri-axonemal structures possessed by flagella could be the reason.34 The mid-piece is formed by a helical MS encircled by nine ODFs which link to one specific axonemal DMT (Figure 1). The principal piece that comprises the main length of the flagellum,24,60 consists of ribs of the FS under the plasma membrane surrounding the ODFs, and two longitudinal columns of the FS instead of ODF 3 and 8 (Figure 1). The MS is formed by a helix of approximately 13 gyri, with two mitochondria per gyrus.26,61,62 Anchoring of the MS is sustained by filament complexes,63 where kinesin light chain 3 (KLC3) may bridge ODF1 with a mitochondrial porin at the outer membrane (Figure 1).61
Of the differential sperm flagellar peri-axonemal components, the FS and ODFs appear responsible for dampening the wave amplitude of flagella in sticky environments but not for active movement.64 Moreover, the central pair microtubule apparatus, FS and ODFs (especially the thick ODF1, 5, and 6) collectively restrict the beat of sperm in the plane vertical to the CP line and FS column (Figure 1). The FS can also serve as a scaffold to anchor glycolytic enzymes and signal transduction proteins.65 Therefore, defects of the peri-axonemal structures may lead to sperm immobility and male infertility, among which DFS (the old term for MMAF) is the most concerning.66 Some of the DFS is directly due to mutations in genes encoding FS or ODFs. Others would result from scaffold elements in the axoneme for the assembly of FS or ODFs. Next we will focus on the former.
AKAP3 and AKAP4
AKAP3 and AKAP4 are structural proteins of FS required for anchoring protein kinase A to FS (Figure 1).16 AKAP3 may play a role in organizing the basic FS structure, whereas AKAP4 may act in completing FS assembly.67,68 Previous studies have found that mutations in AKAP3 and AKAP4 may be related to DFS. However, no whole genome level study has validated the relationship between these gene mutations and DFS.67,69 In addition, the phenotype observed in AKAP4 mutant sperm features absent or extremely short tails with disorganized or altered axonemal and peri-axonemal structures. The description of high XY disomy is presented in an infertile man with AKAP4 gene deletion and DFS,67 suggesting that AKAP4 may play a role both in the sperm centrosome, and flagella. Overall, the phenotype appears severer than that in other MMAF cases, because the AKAPs are involved in signal transduction and glycolysis regulation, which may affect cascades of potentially functional proteins.70 Another study did not find gene mutations in either of the two genes responsible for DFS in humans, underlying other potential causative genes for DFS.16
Other peri-axoneme related disease-causing genes
More than 15 proteins have been associated with the FS, but how they assemble to produce this exclusive cytoskeletal structure is unclear.24 Other genes reported to cause DFS include FSIP2, ODF2, ROPN1, ROPN1L and GAPDS. FS interacting protein 2 (FSIP2) localizes to the FS and directly interact with AKAP4. Whole-exome sequencing revealed that FSIP2 causes MMAF.71 The sperm flagella in FSIP2-mutated patients showed aberrant expression or absence of components of CPC, IDA and ODA. These phenotypes indicate that DFS may alter the direct interaction of DMTs 3 and 8 with FS in the principal piece and completely destabilize of the axoneme. The absence of FSIP2 may also disrupt the anchoring of cAMP-dependent protein kinase A to AKAP4 and impair the maintenance of sperm function.72
XL169 (Odf2+/-) chimeric mice may produce more elongated spermatids with missing ODFs.73 The ROPN1 (Ropporin) and ROPN1L (Rhophilin) proteins bind AKAP3.74 These proteins compensate for each other, possibly maintaining the incorporation of AKAP3 into FS and affecting the protein kinase A-dependent signaling processes. Furthermore, enzymatic activity of GAPDS was investigated in semen from seven DFS patients.75 The enzymatic deficiency could be mainly ascribed to a GAPDS expression disorder resulting from the mutation in the intron region of GAPDS. The mechanisms of these genes in DFS or MMAF need to be investigated further.
Defects in protein transport and related disease-causing genes
The last phase of spermatogenesis is spermatid elongation, where protein transport is active.76 Two bi-directional transport platforms – the intra-manchette transport (IMT) and intra-flagellar transport (IFT) – deliver proteins to the growing tail and deforming head.77 It is reasonable to assume that impairment of either platform contributes to MMAF. An ultrastructural study of testis also revealed that flagellar anomalies in MMAF occur during spermatid elongation, implying an internal relationship between MMAF and protein-transport mechanisms.5,9,19,48,78 Both IFT and IMT are based on microtubular tracks and use motors for the trafficking of cargo-related transport complexes.76 IFT particles contain the IFT-A and -B complexes. IFT-B contains 16 subunits and mediates anterograde trafficking motored by kinesin-2. IFT-A contains six subunits plus TULP3, and mediates retrograde trafficking powered by dynein-2 (Figure 3).79 The manchette transiently appears around the elongating head during late spermatid elongation.80
Figure 3.
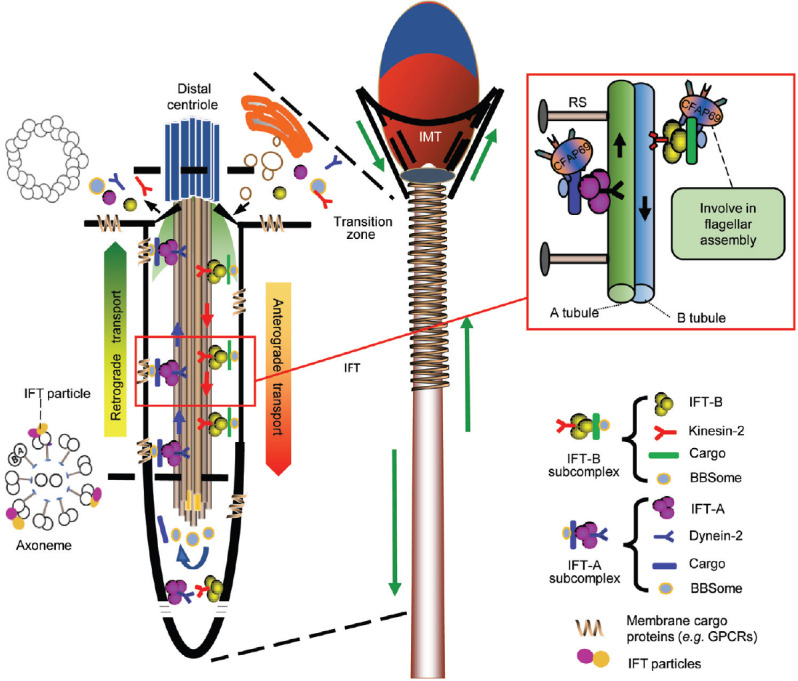
Two microtubular-based bi-directional delivery platforms in sperm. The IMT and IFT. The manchette is a transient skirt-like structure surrounding the elongating spermatid head and is only present during spermatid elongation. The IFT process is shown at the left. The IFT-B subcomplex comprises of IFT-B (16 IFT subunits), dynein-2, BBSome and cargos mediating anterograde trafficking from the flagellar base to the tip, while the IFT-A subcomplex assembles with IFT-A (6 IFT subunits plus TULP3), kinesin-2, BBSome and cargos mediating retrograde trafficking. CFAP69 may participate in flagellar assembly through the transport mechanism and its possible involvement in the IFT is depicted in the right box. IMT: intra-manchette transport; IFT: intra-flagellar transport.
CFAP69
The cilia and flagella associated protein 69 gene (CFAP69) has been investigated (Table 2).12 The first functional investigation by Talaga et al.81 found a regulatory role of CFAP69 in olfactory transduction kinetics, followed closely by the discovery of deleterious mutations in CFAP69 causing MMAF in humans. While the olfactory cilia lacking CFAP69 in mice appeared morphologically normal, mutations in CFAP69 caused widespread and severe defects throughout the MS, ODFs and axoneme (Table 2) indicating a distinct biophysiological role of CFAP69 in cilia and flagella. Sperm head malformations were also observed and appeared more common in humans, suggesting that CFAP69 may be involved in both head and flagellum development.
CFAP69 is located in the mid-piece of the sperm flagellum and also in the olfactory cilia of mice containing no mitochondria or ODFs. A recent mass spectrometry analysis revealed that SPEF2 might interact with CFAP69.82 SPEF2 may participate in sperm head shaping and CPC assembly through the IFT and IMT pathway during spermiogenesis with a similar sperm phenotype as CFAP69 when mutated,82,83,84 except that loss of SPEF2 function also results in PCD. The further observations of abundant split microtubule doublets for manchette axoneme and singlet microtubules in testicular sperm collectively indicate that CFAP69 may participate in the transport of microtubule or other cargos required for flagellar assembly (Figure 3). Similar to other disease-causing genes for MMAF, CFAP69 mutations in humans cause flagellar defects without other clinical features. This observation again implies some functions of sperm flagellum that differ from other cilia. Further investigations in a larger population are needed.
Abnormal centriole assembly and related disease-causing genes
The connecting piece is a connection between the head and flagellum, constituting the most cranial segment of the sperm tail.85 During spermiogenesis, the two centrioles move towards one side of the nucleus and transform to constitute the connecting piece, which is essential for the biogenesis of capitulum and segmented columns, and for axoneme assembly (Figure 1).85,86 The atypical distal centriole is the template of the axonemal DMTs. The proximal centriole contacts the nucleus as a concavity, where the nuclear membrane forms a basal plate with dense electron on the cytoplasmic side (Figure 1).87 Most alternations involving the connecting piece are headless motile tails, and bent and tailless heads. However, an MMAF-like phenotype and DFS with isolated short/thick tails have also been reported.15,88
CEP135
Sha et al.15 recently reported that a homozygous mutationin centrosomal protein 135 (CEP135) was associated with MMAF through whole-exome sequencing. Previous studies suggested that CEP135 functions in centriole biogenesis and especially in CP assembly.89,90
In cultured human cells, CEP135 localizes near the proximal end of basal bodies (Figure 1),91 and CEP135 upregulation leads to the accumulation of fibrous polymers in the centrosome and cytoplasm.92 In the study by Sha et al.,15 CEP135 was expressed ectopically at lower levels in the flagellum near the centriole with protein aggregates frequently seen in the centrosome and flagella. The authors did not provide a detailed description of sperm flagella or functional validation. Further investigations are needed concerning the role of CEP135 during spermiogenesis and the interacting proteins.
Since functional explorations of genes in humans are difficult, it is crucial to use animal models to elucidate the function of related disease-causing genes in MMAF and identify possible candidate genes. However, three limitations are apparent. First, many antibodies for the proteins of interest are unavailable or are of low quality for immunohistochemistry, immunofluorescence, or western blotting. The resulting circuitous detection of other marker proteins may wrongly assign a location or function to the target protein. Second, the lack of homology between humans and animals means that homologous genes may have different functions and produce diverse phenotypes in human and animal models. Third, many of the identified genes involved in MMAF lack validation in different unrelated families. Further studies are necessary, especially with larger population.
CLINICAL CONSIDERATION FOR MMAF
Since almost all MMAF patients have impaired sperm motility, ICSI is currently the only treatment option4 due to lack of empirically medicinal therapy available to improve semen parameters. Reports of at least 24 males having DFS/MMAF have been identified with ICSI outcomes.93,94,95,96,97,98,99 The immotile spermatozoa showed a viability of 15%–57%, and the fertilization rate varied from 38.9% to 75%. Clinical pregnancy occurred in 54.2% (13/24) of the couples, and 11 offspring were delivered. Unsuccessful pregnancies have been reported, and males with MMAF resulting from abnormal centriole assembly seem more likely to experience a disappointing ICSI outcome.15 Overall, fertilization and pregnancy achieved by ICSI are effective in MMAF patients, regardless of severe flagellar defects, although the fertilization rate is variable. Testicular sperm is better than immotile ejaculated sperm and has been recommended for use rather than completely immotile ejaculated sperm.100
There is a risk of genetic defects apart from the sperm morphological defects; therefore, genetic counseling is suggested for MMAF patients when ICSI is utilized. According to mutations identified in four known MMAF-related genes (DNAH1, CFAP43, CFAP44 and CFAP69) in eight main studies (Table 2), the diagnosis efficiency of MMAF phenotype by whole-exome sequencing is 30.7% (60/195). This is very promising and is comparable to that obtained in other genetic diseases with high genetic heterogeneity,101,102 supporting the routine use of whole-exome sequencing in genetic studies of MMAF males. Moreover, to improve the diagnosis rate and discover novel gene mutations with lower frequency, whole-exome sequencing-based genetic studies should be performed in larger cohorts of MMAF individuals. Comparative proteomic analysis can also facilitate the discovery of MMAF biomarkers and potential pathogenesis of MMAF.103 Thus, with a broadened list of causative genes, we can expect that improved treatments will become available for more patients.
SUMMARY
This decade has witnessed great progress in the field of the reproductive genetics with the aid of next-generation sequencing and CRISPR-cas9. Here, we attempt to identify the distinct characteristics of MMAF in comparison of other causes leading to asthenozoospermia, and to elucidate the etiology of MMAF based on flagellum assembly and the updated genetic findings (including DNAH1, CFAP43, CFAP44, AK7, AKAP3, AKAP4, CFAP69 and CEP135). We hope these efforts will propel further exploration in this field, and facilitate adequate genetic counseling and the expected treatment outcome for patients.
AUTHOR CONTRIBUTIONS
WLW and CFT conceived and drafted the manuscript. YQT designed and helped to draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (81771645 and 81471432 to YQT), and Graduate Research and Innovation Projects of Central South University (Grant 2017zzts071 to CFT).
REFERENCES
- 1.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curi SM, Ariagno JI, Chenlo PH, Mendeluk GR, Pugliese MN, et al. Asthenozoospermia: analysis of a large population. Arch Androl. 2003;49:343–9. doi: 10.1080/01485010390219656. [DOI] [PubMed] [Google Scholar]
- 3.Krausz C, Riera-Escamilla A. Genetics of male infertility. Nat Rev Urol. 2018;15:369–84. doi: 10.1038/s41585-018-0003-3. [DOI] [PubMed] [Google Scholar]
- 4.Coutton C, Escoffier J, Martinez G, Arnoult C, Ray PF. Teratozoospermia: spotlight on the main genetic actors in the human. Hum Reprod Update. 2015;21:455–85. doi: 10.1093/humupd/dmv020. [DOI] [PubMed] [Google Scholar]
- 5.Ben Khelifa M, Coutton C, Zouari R, Karaouzene T, Rendu J, et al. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am J Hum Genet. 2014;94:95–104. doi: 10.1016/j.ajhg.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray PF, Toure A, Metzler-Guillemain C, Mitchell MJ, Arnoult C, et al. Genetic abnormalities leading to qualitative defects of sperm morphology or function. Clin Genet. 2017;91:217–32. doi: 10.1111/cge.12905. [DOI] [PubMed] [Google Scholar]
- 7.Knowles MR, Zariwala M, Leigh M. Primary ciliary dyskinesia. Clin Chest Med. 2016;37:449–61. doi: 10.1016/j.ccm.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afzelius BA, Eliasson R. Flagellar mutants in man: on the heterogeneity of the immotile-cilia syndrome. J Ultrastruct Res. 1979;69:43–52. doi: 10.1016/s0022-5320(79)80041-6. [DOI] [PubMed] [Google Scholar]
- 9.Ross A, Christie S, Edmond P. Ultrastructural tail defects in the spermatozoa from two men attending a subfertility clinic. J Reprod Fertil. 1973;32:243–51. doi: 10.1530/jrf.0.0320243. [DOI] [PubMed] [Google Scholar]
- 10.Chemes HE, Alvarez Sedo C. Tales of the tail and sperm head aches: changing concepts on the prognostic significance of sperm pathologies affecting the head, neck and tail. Asian J Androl. 2012;14:14–23. doi: 10.1038/aja.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bracke A, Peeters K, Punjabi U, Hoogewijs D, Dewilde S. A search for molecular mechanisms underlying male idiopathic infertility. Reprod Biomed Online. 2018;36:327–39. doi: 10.1016/j.rbmo.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Dong FN, Amiri-Yekta A, Martinez G, Saut A, Tek J, et al. Absence of CFAP69 causes male infertility due to multiple morphological abnormalities of the flagella in human and mouse. Am J Hum Genet. 2018;102:636–48. doi: 10.1016/j.ajhg.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coutton C, Vargas AS, Amiri-Yekta A, Kherraf ZE, Ben Mustapha SF, et al. Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat Commun. 2018;9:686. doi: 10.1038/s41467-017-02792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lores P, Coutton C, El Khouri E, Stouvenel L, Givelet M, et al. Homozygous missense mutation L673P in adenylate kinase 7 (AK7) leads to primary male infertility and multiple morphological anomalies of the flagella but not to primary ciliary dyskinesia. Hum Mol Genet. 2018;27:1196–211. doi: 10.1093/hmg/ddy034. [DOI] [PubMed] [Google Scholar]
- 15.Sha YW, Xu X, Mei LB, Li P, Su ZY, et al. A homozygous CEP135 mutation is associated with multiple morphological abnormalities of the sperm flagella (MMAF) Gene. 2017;633:48–53. doi: 10.1016/j.gene.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 16.Turner RM, Musse MP, Mandal A, Klotz K, Jayes FC, et al. Molecular genetic analysis of two human sperm fibrous sheath proteins, AKAP4 and AKAP3, in men with dysplasia of the fibrous sheath. J Androl. 2001;22:302–15. [PubMed] [Google Scholar]
- 17.Rohatgi R, Snell WJ. The ciliary membrane. Curr Opin Cell Biol. 2010;22:541–6. doi: 10.1016/j.ceb.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaba K. Sperm flagella: comparative and phylogenetic perspectives of protein components. Mol Hum Reprod. 2011;17:524–38. doi: 10.1093/molehr/gar034. [DOI] [PubMed] [Google Scholar]
- 19.Barthelemy C, Tharanne MJ, Lebos C, Lecomte P, Lansac J. Tail stump spermatozoa: morphogenesis of the defect. An ultrastructural study of sperm and testicular biopsy. Andrologia. 1990;22:417–25. doi: 10.1111/j.1439-0272.1990.tb02020.x. [DOI] [PubMed] [Google Scholar]
- 20.Baccetti B, Burrini AG, Capitani S, Collodel G, Moretti E, et al. Notulae seminologicae 2 The 'short tail' and 'stump' defect in human spermatozoa. Andrologia. 1993;25:331–5. [PubMed] [Google Scholar]
- 21.Marmor D, Grob-Menendez F. Male infertility due to asthenozoospermia and flagellar anomaly: detection in routine semen analysis. Int J Androl. 1991;14:108–16. doi: 10.1111/j.1365-2605.1991.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 22.Gatimel N, Moreau J, Parinaud J, Leandri RD. Sperm morphology: assessment, pathophysiology, clinical relevance, and state of the art in 2017. Andrology. 2017;5:845–62. doi: 10.1111/andr.12389. [DOI] [PubMed] [Google Scholar]
- 23.Auger J, Jouannet P, Eustache F. Another look at human sperm morphology. Hum Reprod. 2016;31:10–23. doi: 10.1093/humrep/dev251. [DOI] [PubMed] [Google Scholar]
- 24.Eddy EM, Toshimori K, O’Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61:103–15. doi: 10.1002/jemt.10320. [DOI] [PubMed] [Google Scholar]
- 25.Folgero T, Bertheussen K, Lindal S, Torbergsen T, Oian P. Mitochondrial disease and reduced sperm motility. Hum Reprod. 1993;8:1863–8. doi: 10.1093/oxfordjournals.humrep.a137950. [DOI] [PubMed] [Google Scholar]
- 26.Piomboni P, Focarelli R, Stendardi A, Ferramosca A, Zara V. The role of mitochondria in energy production for human sperm motility. Int J Androl. 2012;35:109–24. doi: 10.1111/j.1365-2605.2011.01218.x. [DOI] [PubMed] [Google Scholar]
- 27.Chemes HE. Phenotypic varieties of sperm pathology: genetic abnormalities or environmental influences can result in different patterns of abnormal spermatozoa. Anim Reprod Sci. 2018;194:41–56. doi: 10.1016/j.anireprosci.2018.04.074. [DOI] [PubMed] [Google Scholar]
- 28.Kuo YC, Lin YH, Chen HI, Wang YY, Chiou YW, et al. SEPT12 mutations cause male infertility with defective sperm annulus. Hum Mutat. 2012;33:710–9. doi: 10.1002/humu.22028. [DOI] [PubMed] [Google Scholar]
- 29.Hjeij R, Onoufriadis A, Watson CM, Slagle CE, Klena NT, et al. CCDC151 mutations cause primary ciliary dyskinesia by disruption of the outer dynein arm docking complex formation. Am J Hum Genet. 2014;95:257–74. doi: 10.1016/j.ajhg.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loges NT, Olbrich H, Becker-Heck A, Haffner K, Heer A, et al. Deletions and point mutations of LRRC50 cause primary ciliary dyskinesia due to dynein arm defects. Am J Hum Genet. 2009;85:883–9. doi: 10.1016/j.ajhg.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho KJ, Noh SH, Han SM, Choi WI, Kim HY, et al. ZMYND10 stabilizes intermediate chain proteins in the cytoplasmic pre-assembly of dynein arms. PLoS Genet. 2018;14:e1007316. doi: 10.1371/journal.pgen.1007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raidt J, Wallmeier J, Hjeij R, Onnebrink JG, Pennekamp P, et al. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur Respir J. 2014;44:1579–88. doi: 10.1183/09031936.00052014. [DOI] [PubMed] [Google Scholar]
- 33.Fassad MR, Shoemark A, le Borgne P, Koll F, Patel M, et al. C11orf70 mutations disrupting the intraflagellar transport-dependent assembly of multiple axonemal dyneins cause primary ciliary dyskinesia. Am J Hum Genet. 2018;102:956–72. doi: 10.1016/j.ajhg.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linck RW, Chemes H, Albertini DF. The axoneme: the propulsive engine of spermatozoa and cilia and associated ciliopathies leading to infertility. J Assist Reprod Genet. 2016;33:141–56. doi: 10.1007/s10815-016-0652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker MA, Naumovski N, Hetherington L, Weinberg A, Velkov T, et al. Head and flagella subcompartmental proteomic analysis of human spermatozoa. Proteomics. 2013;13:61–74. doi: 10.1002/pmic.201200350. [DOI] [PubMed] [Google Scholar]
- 36.Amaral A, Castillo J, Estanyol JM, Ballesca JL, Ramalho-Santos J, et al. Human sperm tail proteome suggests new endogenous metabolic pathways. Mol Cell Proteomics. 2013;12:330–42. doi: 10.1074/mcp.M112.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hildebrand MS, Avenarius MR, Fellous M, Zhang Y, Meyer NC, et al. Genetic male infertility and mutation of CATSPER ion channels. Eur J Hum Genet. 2010;18:1178–84. doi: 10.1038/ejhg.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dirami T, Rode B, Jollivet M, Da Silva N, Escalier D, et al. Missense mutations in SLC26A8, encoding a sperm-specific activator of CFTR, are associated with human asthenozoospermia. Am J Hum Genet. 2013;92:760–6. doi: 10.1016/j.ajhg.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oda T, Yanagisawa H, Kikkawa M. Detailed structural and biochemical characterization of the nexin-dynein regulatory complex. Mol Biol Cell. 2015;26:294–304. doi: 10.1091/mbc.E14-09-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linck RW, Olson GE, Langevin GL. Arrangement of tubulin subunits and microtubule-associated proteins in the central-pair microtubule apparatus of squid (Loligo pealei) sperm flagella. J Cell Biol. 1981;89:309–22. doi: 10.1083/jcb.89.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson GE, Linck RW. Observations of the structural components of flagellar axonemes and central pair microtubules from rat sperm. J Ultrastruct Res. 1977;61:21–43. doi: 10.1016/s0022-5320(77)90004-1. [DOI] [PubMed] [Google Scholar]
- 42.Carbajal-Gonzalez BI, Heuser T, Fu X, Lin J, Smith BW, et al. Conserved structural motifs in the central pair complex of eukaryotic flagella. Cytoskeleton (Hoboken) 2013;70:101–20. doi: 10.1002/cm.21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oda T, Yanagisawa H, Yagi T, Kikkawa M. Mechanosignaling between central apparatus and radial spokes controls axonemal dynein activity. J Cell Biol. 2014;204:807–19. doi: 10.1083/jcb.201312014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inaba K. Molecular basis of sperm flagellar axonemes: structural and evolutionary aspects. Ann N Y Acad Sci. 2007;1101:506–26. doi: 10.1196/annals.1389.017. [DOI] [PubMed] [Google Scholar]
- 45.Tang WJ, Bell CW, Sale WS, Gibbons IR. Structure of the dynein-1 outer arm in sea urchin sperm flagella I Analysis by separation of subunits. J Biol Chem. 1982;257:508–15. [PubMed] [Google Scholar]
- 46.Henley C, Costello DP, Thomas MB, Newton WD. The “9+1” pattern of microtubules in spermatozoa of Mesostoma (Platyhelminthes, Turbellaria) Proc Natl Acad Sci U S A. 1969;64:849–56. doi: 10.1073/pnas.64.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baccetti B, Afzelius BA. The biology of the sperm cell. Monogr Dev Biol. 1976:1–254. [PubMed] [Google Scholar]
- 48.Sha Y, Yang X, Mei L, Ji Z, Wang X, et al. DNAH1 gene mutations and their potential association with dysplasia of the sperm fibrous sheath and infertility in the Han Chinese population. Fertil Steril. 2017;107:1312–8e2. doi: 10.1016/j.fertnstert.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Amiri-Yekta A, Coutton C, Kherraf ZE, Karaouzene T, Le Tanno P, et al. Whole-exome sequencing of familial cases of multiple morphological abnormalities of the sperm flagella (MMAF) reveals new DNAH1 mutations. Hum Reprod. 2016;31:2872–80. doi: 10.1093/humrep/dew262. [DOI] [PubMed] [Google Scholar]
- 50.Tang S, Wang X, Li W, Yang X, Li Z, et al. Biallelic mutations in CFAP43 and CFAP44 cause male infertility with multiple morphological abnormalities of the sperm flagella. Am J Hum Genet. 2017;100:854–64. doi: 10.1016/j.ajhg.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sha YW, Wang X, Xu X, Su ZY, Cui Y, et al. Novel mutations in CFAP44 and CFAP43 cause multiple morphological abnormalities of the sperm flagella (MMAF) Reprod Sci. 2017;26:26–34. doi: 10.1177/1933719117749756. [DOI] [PubMed] [Google Scholar]
- 52.Lin J, Heuser T, Song K, Fu X, Nicastro D. One of the nine doublet microtubules of eukaryotic flagella exhibits unique and partially conserved structures. PLoS One. 2012;7:e46494. doi: 10.1371/journal.pone.0046494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Afzelius B. Electron microscopy of the sperm tail; results obtained with a new fixative. J Biophys Biochem Cytol. 1959;5:269–78. doi: 10.1083/jcb.5.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satir P. Studies on cilia: II. examination of the distal region of the ciliary shaft and the role of the filaments in motility. J Cell Biol. 1965;26:805–34. doi: 10.1083/jcb.26.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deiner M, Tamm SL, Tamm S. Mechanical properties of ciliary axonemes and membranes as shown by paddle cilia. J Cell Sci. 1993;104(Pt 4):1251–62. doi: 10.1242/jcs.104.4.1251. [DOI] [PubMed] [Google Scholar]
- 56.Gibbons IR. The relationship between the fine structure and direction of beat in gill cilia of a lamellibranch mollusc. J Biophys Biochem Cytol. 1961;11:179–205. doi: 10.1083/jcb.11.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernandez-Gonzalez A, Kourembanas S, Wyatt TA, Mitsialis SA. Mutation of murine adenylate kinase 7 underlies a primary ciliary dyskinesia phenotype. Am J Respir Cell Mol Biol. 2009;40:305–13. doi: 10.1165/rcmb.2008-0102OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mata M, Lluch-Estelles J, Armengot M, Sarrion I, Carda C, et al. New adenylate kinase 7 (AK7) mutation in primary ciliary dyskinesia. Am J Rhinol Allergy. 2012;26:260–4. doi: 10.2500/ajra.2012.26.3784. [DOI] [PubMed] [Google Scholar]
- 59.Milara J, Armengot M, Mata M, Morcillo EJ, Cortijo J. Role of adenylate kinase type 7 expression on cilia motility: possible link in primary ciliary dyskinesia. Am J Rhinol Allergy. 2010;24:181–5. doi: 10.2500/ajra.2010.24.3468. [DOI] [PubMed] [Google Scholar]
- 60.Escalier D. New insights into the assembly of the periaxonemal structures in mammalian spermatozoa. Biol Reprod. 2003;69:373–8. doi: 10.1095/biolreprod.103.015719. [DOI] [PubMed] [Google Scholar]
- 61.Amaral A, Lourenco B, Marques M, Ramalho-Santos J. Mitochondria functionality and sperm quality. Reproduction. 2013;146:R163–74. doi: 10.1530/REP-13-0178. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Ou Y, Cheng M, Saadi HS, Thundathil JC, et al. KLC3 is involved in sperm tail midpiece formation and sperm function. Dev Biol. 2012;366:101–10. doi: 10.1016/j.ydbio.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 63.Olson GE, Winfrey VP. Mitochondria-cytoskeleton interactions in the sperm midpiece. J Struct Biol. 1990;103:13–22. doi: 10.1016/1047-8477(90)90081-m. [DOI] [PubMed] [Google Scholar]
- 64.Lindemann CB. Functional significance of the outer dense fibers of mammalian sperm examined by computer simulations with the geometric clutch model. Cell Motil Cytoskeleton. 1996;34:258–70. doi: 10.1002/(SICI)1097-0169(1996)34:4<258::AID-CM1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 65.Lehti MS, Sironen A. Formation and function of sperm tail structures in association with sperm motility defects. Biol Reprod. 2017;97:522–36. doi: 10.1093/biolre/iox096. [DOI] [PubMed] [Google Scholar]
- 66.Chemes HE, Brugo S, Zanchetti F, Carrere C, Lavieri JC. Dysplasia of the fibrous sheath: an ultrastructural defect of human spermatozoa associated with sperm immotility and primary sterility. Fertil Steril. 1987;48:664–9. doi: 10.1016/s0015-0282(16)59482-5. [DOI] [PubMed] [Google Scholar]
- 67.Baccetti B, Collodel G, Estenoz M, Manca D, Moretti E, et al. Gene deletions in an infertile man with sperm fibrous sheath dysplasia. Hum Reprod. 2005;20:2790–4. doi: 10.1093/humrep/dei126. [DOI] [PubMed] [Google Scholar]
- 68.Visser L, Westerveld GH, Xie F, van Daalen SK, van der Veen F, et al. A comprehensive gene mutation screen in men with asthenozoospermia. Fertil Steril. 2011;95:1020–4e1–9. doi: 10.1016/j.fertnstert.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 69.Baccetti B, Collodel G, Gambera L, Moretti E, Serafini F, et al. Fluorescence in situ hybridization and molecular studies in infertile men with dysplasia of the fibrous sheath. Fertil Steril. 2005;84:123–9. doi: 10.1016/j.fertnstert.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 70.Turner RM, Johnson LR, Haig-Ladewig L, Gerton GL, Moss SB. An X-linked gene encodes a major human sperm fibrous sheath protein, hAKAP82. Genomic organization, protein kinase A-RII binding, and distribution of the precursor in the sperm tail. J Biol Chem. 1998;273:32135–41. doi: 10.1074/jbc.273.48.32135. [DOI] [PubMed] [Google Scholar]
- 71.Martinez G, Kherraf ZE, Zouari R, Fourati Ben Mustapha S, Saut A, et al. Whole-exome sequencing identifies mutations in FSIP2 as a recurrent cause of multiple morphological abnormalities of the sperm flagella. Hum Reprod. 2018;33:1973–84. doi: 10.1093/humrep/dey264. [DOI] [PubMed] [Google Scholar]
- 72.Welch EJ, Jones BW, Scott JD. Networking with AKAPs: context-dependent regulation of anchored enzymes. Mol Interv. 2010;10:86–97. doi: 10.1124/mi.10.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tarnasky H, Cheng M, Ou Y, Thundathil JC, Oko R, et al. Gene trap mutation of murine outer dense fiber protein-2 gene can result in sperm tail abnormalities in mice with high percentage chimaerism. BMC Dev Biol. 2010;10:67. doi: 10.1186/1471-213X-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fiedler SE, Dudiki T, Vijayaraghavan S, Carr DW. Loss of R2D2 proteins ROPN1 and ROPN1L causes defects in murine sperm motility, phosphorylation, and fibrous sheath integrity. Biol Reprod. 2013;88:41. doi: 10.1095/biolreprod.112.105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elkina YL, Kuravsky ML, Bragina EE, Kurilo LF, Khayat SS, et al. Detection of a mutation in the intron of sperm-specific glyceraldehyde-3-phosphate dehydrogenase gene in patients with fibrous sheath dysplasia of the sperm flagellum. Andrologia. 2017;49 doi: 10.1111/and.12606. doi: 101111/and12606 [Epub 2016 May 2] [DOI] [PubMed] [Google Scholar]
- 76.Lehti MS, Sironen A. Formation and function of the manchette and flagellum during spermatogenesis. Reproduction. 2016;151:R43–54. doi: 10.1530/REP-15-0310. [DOI] [PubMed] [Google Scholar]
- 77.Yang SM, Li HB, Wang JX, Shi YC, Cheng HB, et al. Morphological characteristics and initial genetic study of multiple morphological anomalies of the flagella in China. Asian J Androl. 2015;17:513–5. doi: 10.4103/1008-682X.146100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rawe VY, Galaverna GD, Acosta AA, Olmedo SB, Chemes HE. Incidence of tail structure distortions associated with dysplasia of the fibrous sheath in human spermatozoa. Hum Reprod. 2001;16:879–86. doi: 10.1093/humrep/16.5.879. [DOI] [PubMed] [Google Scholar]
- 79.San Agustin JT, Pazour GJ, Witman GB. Intraflagellar transport is essential for mammalian spermiogenesis but is absent in mature sperm. Mol Biol Cell. 2015;26:4358–72. doi: 10.1091/mbc.E15-08-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen SR, Batool A, Wang YQ, Hao XX, Chang CS, et al. The control of male fertility by spermatid-specific factors: searching for contraceptive targets from spermatozoon's head to tail. Cell Death Dis. 2016;7:e2472. doi: 10.1038/cddis.2016.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Talaga AK, Dong FN, Reisert J, Zhao H. Cilia- and flagella-associated protein 69 regulates olfactory transduction kinetics in mice. J Neurosci. 2017;37:5699–710. doi: 10.1523/JNEUROSCI.0392-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lehti MS, Zhang FP, Kotaja N, Sironen A. SPEF2 functions in microtubule-mediated transport in elongating spermatids to ensure proper male germ cell differentiation. Development. 2017;144:2683–93. doi: 10.1242/dev.152108. [DOI] [PubMed] [Google Scholar]
- 83.Sironen A, Kotaja N, Mulhern H, Wyatt TA, Sisson JH, et al. Loss of SPEF2 function in mice results in spermatogenesis defects and primary ciliary dyskinesia. Biol Reprod. 2011;85:690–701. doi: 10.1095/biolreprod.111.091132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sironen A, Hansen J, Thomsen B, Andersson M, Vilkki J, et al. Expression of SPEF2 during mouse spermatogenesis and identification of IFT20 as an interacting protein. Biol Reprod. 2010;82:580–90. doi: 10.1095/biolreprod.108.074971. [DOI] [PubMed] [Google Scholar]
- 85.Fawcett DW, Phillips DM. The fine structure and development of the neck region of the mammalian spermatozoon. Anat Rec. 1969;165:153–64. doi: 10.1002/ar.1091650204. [DOI] [PubMed] [Google Scholar]
- 86.Zamboni L, Stefanini M. The fine structure of the neck of mammalian spermatozoa. Anat Rec. 1971;169:155–72. doi: 10.1002/ar.1091690203. [DOI] [PubMed] [Google Scholar]
- 87.Fishman EL, Jo K, Nguyen QP, Kong D, Royfman R, et al. A novel atypical sperm centriole is functional during human fertilization. Nat Commun. 2018;9:2210. doi: 10.1038/s41467-018-04678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moretti E, Pascarelli NA, Belmonte G, Renieri T, Collodel G. Sperm with fibrous sheath dysplasia and anomalies in head-neck junction: focus on centriole and centrin 1. Andrologia. 2017;49 doi: 10.1111/and.12701. doi: 101111/and12701 [Epub 2016 Sep 5] [DOI] [PubMed] [Google Scholar]
- 89.Mottier-Pavie V, Megraw TL. Drosophila bld10 is a centriolar protein that regulates centriole, basal body, and motile cilium assembly. Mol Biol Cell. 2009;20:2605–14. doi: 10.1091/mbc.E08-11-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsuura K, Lefebvre PA, Kamiya R, Hirono M. Bld10p, a novel protein essential for basal body assembly in Chlamydomonas: localization to the cartwheel, the first ninefold symmetrical structure appearing during assembly. J Cell Biol. 2004;165:663–71. doi: 10.1083/jcb.200402022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, et al. Plk4-induced centriole biogenesis in human cells. Dev Cell. 2007;13:190–202. doi: 10.1016/j.devcel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 92.Ohta T, Essner R, Ryu JH, Palazzo RE, Uetake Y, et al. Characterization of Cep135, a novel coiled-coil centrosomal protein involved in microtubule organization in mammalian cells. J Cell Biol. 2002;156:87–99. doi: 10.1083/jcb.200108088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stalf T, Sanchez R, Kohn FM, Schalles U, Kleinstein J, et al. Pregnancy and birth after intracytoplasmic sperm injection with spermatozoa from a patient with tail stump syndrome. Hum Reprod. 1995;10:2112–4. doi: 10.1093/oxfordjournals.humrep.a136244. [DOI] [PubMed] [Google Scholar]
- 94.McLachlan RI, Ishikawa T, Osianlis T, Robinson P, Merriner DJ, et al. Normal live birth after testicular sperm extraction and intracytoplasmic sperm injection in variant primary ciliary dyskinesia with completely immotile sperm and structurally abnormal sperm tails. Fertil Steril. 2012;97:313–8. doi: 10.1016/j.fertnstert.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 95.Olmedo SB, Nodar F, Chillik C, Chemes HE. Successful intracytoplasmic sperm injection with spermatozoa from a patient with dysplasia of the fibrous sheath and chronic respiratory disease. Hum Reprod. 1997;12:1497–9. doi: 10.1093/humrep/12.7.1497. [DOI] [PubMed] [Google Scholar]
- 96.Favero R, Rizzo F, Baccetti B, Piomboni P. Embryo development, pregnancy and twin delivery after microinjection of 'stump' spermatozoa. Andrologia. 1999;31:335–8. doi: 10.1046/j.1439-0272.1999.00288.x. [DOI] [PubMed] [Google Scholar]
- 97.Olmedo SB, Rawe VY, Nodar FN, Galaverna GD, Acosta AA, et al. Pregnancies established through intracytoplasmic sperm injection (ICSI) using spermatozoa with dysplasia of fibrous sheath. Asian J Androl. 2000;2:125–30. [PubMed] [Google Scholar]
- 98.Ravel C, Chantot-Bastaraud S, Siffroi JP, Escalier D, Antoine JM, et al. Tail stump syndrome associated with chromosomal translocation in two brothers attempting intracytoplasmic sperm injection. Fertil Steril. 2006;86:719e1–7. doi: 10.1016/j.fertnstert.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 99.Mitchell V, Rives N, Albert M, Peers MC, Selva J, et al. Outcome of ICSI with ejaculated spermatozoa in a series of men with distinct ultrastructural flagellar abnormalities. Hum Reprod. 2006;21:2065–74. doi: 10.1093/humrep/del130. [DOI] [PubMed] [Google Scholar]
- 100.Yang S, Gao L, Wang W, Ding J, Xu Y, et al. Successful intracytoplasmic sperm injection with testicular spermatozoa from a man with multiple morphological abnormalities of the sperm flagella: a case report. J Assist Reprod Genet. 2018;35:247–50. doi: 10.1007/s10815-017-1057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Farwell KD, Shahmirzadi L, El-Khechen D, Powis Z, Chao EC, et al. Enhanced utility of family-centered diagnostic exome sequencing with inheritance model-based analysis: results from 500 unselected families with undiagnosed genetic conditions. Genet Med. 2015;17:578–86. doi: 10.1038/gim.2014.154. [DOI] [PubMed] [Google Scholar]
- 102.Fogel BL, Lee H, Strom SP, Deignan JL, Nelson SF. Clinical exome sequencing in neurogenetic and neuropsychiatric disorders. Ann N Y Acad Sci. 2016;1366:49–60. doi: 10.1111/nyas.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cao X, Cui Y, Zhang X, Lou J, Zhou J, et al. Proteomic profile of human spermatozoa in healthy and asthenozoospermic individuals. Reprod Biol Endocrinol. 2018;16:1–16. doi: 10.1186/s12958-018-0334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]