Introduction
In order to ensure animal welfare during the course of scientific enquiry, there is a strong framework of animal welfare standards for the use of animals in biomedical research [1]. Within the United states much of animal research in the public sector is covered under the Public Health Service Policy on Humane Care and Use of Laboratory Animals (PHS Policy), which is under the provision of the Health Research Extension Act (HREA, 1985) (Public Law 99-158) [2].
The PHS Policy requires that all institutions using live vertebrate animals in PHS supported research must have an institutional animal care and use committee (IACUC) to oversee the care and use of its animals. These institutions are required to use the Guide for the Care and Use of Laboratory Animals (Guide), as a primary standard for implementing their animal care and use programs. Compliance with the Animal Welfare Act Regulations (AWARs) is also an important requirement of the PHS Policy.
Per the PHS policy, the IACUC must have at least five members including the chairperson, a veterinarian with direct or delegated program authority, a practicing scientist, a member whose primary concerns are in a nonscientific area, and a member who is not affiliated with the institution other than as a member of the IACUC. The AWAR requirements for IACUC composition are that it consists of at least three members including a veterinarian and a member not affiliated with the institution.
In addition to ensuring ethical and humane use of animals, the IACUC, due to its structure and function, is uniquely positioned to contribute to the quality of scientific work performed at an institution [3]. Quality of scientific research output is supported by sound experimental design and strategy, rigorous and comprehensive evaluation criteria, responsible research practices, and adequate oversight and training. Additionally, many of the same factors that affect the quality of animal welfare may also impact the quality of scientific research. It is a well-known fact that healthy animals housed in optimal conditions yield the most reliable data, whereas compromised welfare negatively impacts physiology, immunology, and behavior of animals leading to skewed and misrepresented results [3,4]. Additionally, variables in animal care and health can affect repeatability and reproducibility of experiments, and standardization of practices within an animal care program can help reduce variability [4]. Factors that can be standardized to a certain extent include housing practices (lighting, temperature, food, bedding, noise levels, etc.), genetic background, animal source, and health status (disease status, gut microflora, etc.) [4].
While at an institutional level, responsible research is a broad concept encompassing everything from conflict of interest to reproducibility to data management, the conduct of day-to-day research practices in a reliable manner is what constitutes responsible research. The central role of IACUCs in research animal use and oversight helps it safeguard responsible animal research by ensuring ethical, scientifically sound, standardized practices in animal research. The various roles of the IACUC in responsible animal research are outlined in the chart below (Figure 1) and will be discussed further in this review.
Figure 1.
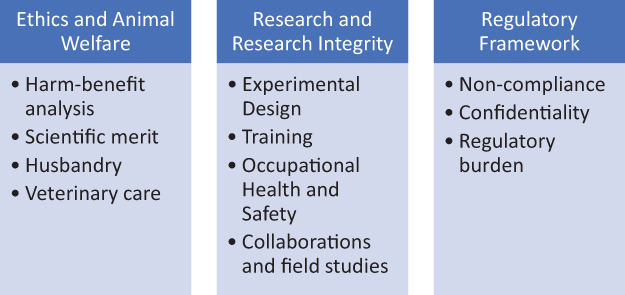
Broad categorization of IACUC functions that contribute to responsible conduct of animal research. Some of these domains may overlap however, the cumulative effect of these roles serves to promote animal welfare, facilitate good research practices, satisfy regulatory requirements, and ensure research quality.
Harm-benefit analysis and the 3Rs
IACUC reviews are intended to not only protect animal welfare, but also to ensure that animals are used in a way that is scientifically meaningful [5]. The Guide states that “the IACUC is obliged to weigh the benefits of the study against potential animal welfare concerns.” The concept of balancing benefits of the science against the potential harm to the animals, more commonly referred to as harm-benefit analysis, is included in most systems of animal research oversight. Potential harm can be assessed by considering likely adverse effects (type, frequency, extent), predicted impact (number of animals involved, species, severity of impact), and methods established to limit harm (minimization of pain, distress, and injury) [6].
Balancing these risks against the potential benefits is more challenging. Determining the immediate benefits of a study only covers a small aspect of the overall impact of the science. Benefits are built incrementally over time, with each set of results adding to the cumulative knowledge in the research field [7]. Addressing the end goals of the research (conservation, chemotherapy, calculating the environmental impact) can provide some insight into the long-term benefits offered by the animal study. Assessing the potential benefits of a study also ties into the scientific merit of the research. While there are no explicit requirements in the federal regulations for the IACUC to do a scientific merit review, US Government Principle II states that “procedures involving animals should be designed and performed with due consideration of their relevance to human or animal health, the advancement of knowledge, or the good of society” [8, 9].
Because there are no regulatory guidelines for conducting a harm-benefit analysis, most IACUCs use the 3Rs defined in the 1959 paper by Russell and Burch, as a framework for analyses [10]. Implementation of the 3Rs when conducting harm-benefit analyses serves to limit the harm by defining and setting acceptable limits prior to conduct of research [16]. Refinement (of procedures, techniques, study design, husbandry methods) can help to reduce the severity of effects and help minimize the pain, distress, suffering, or lasting harm on animals. Reduction helps to not only limit the number of animals used for a study, but also to maximize the information gathered per animal, through measures such as tissue and data sharing. Replacement, the third “R” refers to the use of biological models such as tissues, cells, organoids, or non-biological models such as computer and mathematical models, or the use of invertebrates or another species that is less sentient.
Using current literature to search for alternatives is a requirement of the USDA/APHIS [12], and an implied requirement in the US Government Principles and the Guide. This literature search for alternatives incorporates the tenets of the 3Rs into protocol review. Investigators are asked to consider the use of alternatives in the planning and experimental design phase of their investigations and to procedures that cause more than slight or momentary pain or distress. Therefore, alternatives are not just confined to replacement of animal models with non-animal models and computer simulations, but techniques for minimization of pain and distress, use of less invasive procedures, reduction of total numbers of animals used, use of enrichment, and establishment of humane endpoints can all be considered alternatives.
Experimental design and strategy
When selecting and optimizing animal models, the primary aim is to use a model system that provides reliable and valid data with minimal confounding variables. Here the IACUC takes into account not only the existing body of knowledge in the field, but also the experience of the investigators, the resources available at the institution, and the institutional experience of handling those specific animal models. For accurately assessing the data needs of the experiment, the IACUC requires a thorough understanding of the research plan and therefore, the plan description should contain the experimental variables being tested, the parameters of testing, and methods to be employed in the study [13]. The research plan and objectives should be understandable by all IACUC members, including non-scientists and outside members. Procedures on live animals must be described in detail in the order that they will be conducted. If a procedure or process must be validated before collecting data (such as placement of recording electrodes), then the validation criteria and tests applied should be described (for example pilot studies).
Next is determination of experimental groups and group sizes. Group sizes can be based on practical considerations such as litter sizes or the maximum number that can be housed together. The most commonly used measure for determining sample size is the a priori power analysis and takes into consideration factors such as effect size (the minimum difference between test and control that the study would detect), population standard deviation of the effect, desired power value, and significance level [14]. A well-designed experiment would be able to show adequately powered studies with the minimum number of animals required to achieve scientific validity consistent with the aims of the study [11, 15].
Study outcomes can be affected by several variables such as environmental changes, genetic factors, gender, and age differences [14]. In order to minimize the impact of variation, it is crucial to use control groups in a study. Positive controls may be the use of experimental protocols with known, previously standardized effects. Negative controls may be untreated animals or administration of the vehicle without active ingredients. For surgery, negative controls can be sham surgeries without the final surgical treatment.
Experimental Reproducibility and Reporting
Recently, the research community has raised concerns about experimental reproducibility. Failure to adequately prepare and report experimental procedures involving animals can result in potential scientific, ethical, and economic implications for the entire research process and the reputation of those involved in it [17]. The IACUC can play a vital role in assisting investigators by ensuring research studies are planned and conducted appropriately, and in doing so, investigators will report high quality, comprehensive experimental design and data.
Appropriate planning of research studies is the most important start for ensuring their quality, reproducibility, and translatability. PREPARE (Planning Research and Experimental Procedures on Animals: Recommendations for Excellence) Guidelines and Checklist are a dynamic aid that can be modified at the institution level to ensure all aspects of the study can be addressed before it is begun. PREPARE guidelines cover three broad areas which determine the quality for the preparation of animal studies: Formulation of the Study; Dialogue between Scientists and the Animal Facility; and Quality Control of the Components of the Study [18]. The PREPARE guidelines and checklist are available for free in a number of languages.
The ARRIVE guidelines consist of a checklist of 20 items describing the minimum information that all scientific publications reporting research using animals should include, such as the number and specific characteristics of animals used (including species, strain, sex, and genetic background); details of housing and husbandry; and the experimental, statistical, and analytical methods (including details of methods used to reduce bias such as randomization and blinding). All the items in the checklist have been included to promote high-quality, comprehensive reporting to allow an accurate critical review of what was done and what was found [17].
In designing IACUC questionnaires and conducting protocol reviews, the IACUC can refer to the PREPARE and ARRVE checklists to ensure that the researcher has considered the essential criteria for designing, conducting and reporting quality animal research.
Review of proposed and ongoing procedures
The review of the actual procedures follows very specific criteria. Aspects specific to the study such as administration of materials, appropriate evaluation points, effect on daily functions such as feeding, and any specialized equipment required for the study are discussed in detail. All procedures proposed to be conducted on live animals such as surgical procedures, behavioral testing, imaging, irradiation procedures, and blood collection are described in the order executed. Additionally, any training given to animals, such as performing a task for rewards, must be described. Nonstandard husbandry practices may be required for some genotypes. For example, diabetic animals may require frequent change of bedding due to increased urination [19].
Any procedures that involve surgery should include the appropriate pre- and post-procedural care and describe the short- and long-terms effects of the surgery on the animal. All procedures that may result in more than momentary pain, distress, or discomfort must be identified. The IACUC then assesses the interventions described to alleviate the pain and discomfort. Measures to relieve unavoidable pain and distress such as appropriate anesthesia, analgesia, and palliative care are described and reviewed by the IACUC, as well as special care or housing following surgery or manipulations. If necessary, the IACUC can coordinate a discussion with the PI and consult with subject matter experts on specific outcomes that may be affected by use of analgesics, and suggest pilot studies to analyze extraneous variability introduced by analgesics [20]. If the study has reasons for withholding pain alleviation measures, then those reasons must be described and scientifically justified. Additionally, non-pharmacologic strategies for partial pain relief can also be explored. In order to show that they are following professionally acceptable standards of care, the AWAR requires institutions to report the number of animals in experiments in specific pain categories designated by the USDA [21]. While this requirement is for regulated species only, many institutions use these categories to classify all research animals. This helps with adoption of performance standards that encourages individual assessment, scoring, and interventional regimes and helps IACUCs provide a structured program of pain management, and clearly delineate and predict outcomes.
As per the AWAR, “Animals that would otherwise experience severe or chronic pain or distress that cannot be relieved will be painlessly euthanized at the end of the procedure or, if appropriate, during the procedure” (section 2.31 [d][1][v]). In the case of unrelieved pain and distress, criteria established prior to the start of the study serve as a basis for terminating animal use. These are called humane endpoints as they reduce the duration of animal pain and distress to the minimum possible time while still attaining study objectives [22]. Strategies to establish these criteria include listing of clinical signs that indicate pain and distress, defining moribundity, quantifying pain (eg, with pain scales) [23]. The IACUC’s understanding of the overall study objectives, species specific requirements, and best practices help it ensure that pain and distress to animals is confined to the minimal levels that are unavoidable for the conduct of beneficial research.
In addition to a review of proposed research, an IACUC evaluates all components of the animal program including the ongoing research activities. The Animal Welfare Act Regulations require institutions to conduct continuing review of animal activities at least once per year [24]. The IACUC has the authority to suspend previously approved activities if they are not being conducted as per the descriptions submitted to and approved by the committee. The Guide includes methods of ongoing review such as:
continuing protocol review including review of unexpected outcomes and safety issues,
laboratory inspections and examination of procedure rooms, including review of surgical and anesthesia records and observation of procedures,
veterinary observations of certain procedures such as appropriate aseptic techniques,
IACUC observation of laboratory practices and approved procedures,
regular and frequent observations of animals by animal care staff, and
inspections for regulatory purposes such as storage of controlled substances.
Husbandry and veterinary care
Appropriate animal housing and environment, with adequate enrichment and opportunity for species-appropriate behaviors, are essential contributors to animal welfare [25]. They allow animals to grow, interact, and reproduce normally while allowing the research study to meet its objectives. Housing strategy is a constantly evolving system developed and implemented by the animal program in consultation with experts and the veterinarian, overseen and reviewed by the IACUC. Considerations that go into the design and management of housing systems include space allocations, ambient temperatures and their effect on core body temperatures, humidity, air quality (or water quality in the case of aquatic species), illumination, noise and vibrations, and species-specific behaviors (such as perches, substrates for burrowing). Variations in these factors in both the immediate physical environment of the animals and the larger secondary enclosure should be monitored regularly as they may contribute to physiologic alteration and disease susceptibility. The materials used in the enclosures should be safe, durable, nontoxic, appropriate for frequent cleaning and sanitization, and be consistent with the objectives of animal use [26]. Sources of potable, uncontaminated drinking water and palatable, uncontaminated, nutritionally balanced, and consistent diets also reduce experimental variation. Additional considerations include environmental enrichment and social enrichment to promote physical and psychological wellbeing. All enrichment strategies are regularly reviewed, assessed, and modified by the IACUC to ensure maximum benefit to the animals and to monitor their effect on experimental outcome.
Occupational health and safety
Federal regulations require all institutions have an occupational health and safety program (OHSP) to establish standards that help minimize health and safety risks to institutional employees [27]. Management of an OHSP requires coordination with the IACUC, researchers, environmental health and safety, and other institutional programs. Components of a comprehensive OHSP include risk assessment and hazard identification, prevention strategies, control measures, personnel training, medical evaluation, and personal protection. The IACUC serves as an integral part of an institution’s OHSP, providing links between many functions related to health and safety such as:
review of proposed research (can help with risk assessment and identification of potential hazards, prioritizing potential hazards),
monitoring of animal use (can help in compliance with, and periodic review of established safety procedures),
occupational health management (work closely with OHS to identify new at-risk employees, provide information regarding the specific nature of occupational hazards), and
administration (ensure appropriate training to personnel, provide advice and information for budget planning and resource allocation) [26].
Training of research personnel
The AWARs and PHS Policy require institutions to provide appropriate training to for all animal users. There is great diversity in the roles and responsibilities of members of the animal care and use community at institutions [28] and because of this the development and implementation of an effective training program that accommodates the needs of the various members can be challenging. As the central body linking research, veterinary, and animal care staff, the IACUC is involved in ensuring that personnel are appropriately trained to perform animal research and to provide care. The IACUC also helps to assess the effectiveness of the various training programs as part of its review of the animal program [29]. Any recommendations for enhancement are submitted to the institutional official in the semiannual reports.
Outside collaborations and contracts
An increasing number of institutions are participating in collaborative studies or outsourcing in vivo work, with all or part of the animal work being conducted outside the premises of the institution. Collaborations serve to maximize the use of animals in research by allowing sharing of resources and thereby minimizing the number of animals that may be required for scientific research. Sponsoring institutions and their IACUCs are mainly responsible for oversight and management of such studies, to maintain an acceptable standard of animal care and use. Clear, well-defined agreements at each stage of the approval process ensure a smooth conduct of animal activities. For collaborations, it is advisable to have memoranda of understanding (MOUs) or other formal written agreements, detailing the responsibilities of each institution’s IACUCs with regard to ownership, transport, tissue use, training of personnel, and data collection [30]. For contract studies, these agreements should cover confidentiality, designated work sites, ownership of animals, and assurance that the contractor will comply with applicable regulations and guidelines. To ensure the quality of research data, it is important to harmonize the standards of veterinary care and animal husbandry between the collaborating institutions. The extent of coordination can vary depending on their individual USDA registration, AAALAC accreditation, and PHS Assurance status [31]. International collaborations at foreign performance sites must also be reviewed by an ethics board or IACUC-like body depending on the laws in the country.
International collaborations and harmonization
While there is considerable variation in legal oversight, animal welfare standards, and their implementation across the world, there are some core principles that animal welfare programs share, such as the tenets of the Three Rs [32], the five freedoms of animal welfare, and the International Guiding Principles [33]. Development and implementation of standards can occur at the national, state (or province/region), and institutional levels [32]. In the US, the two major laws in research animal welfare, the Animal Welfare Act and the Health Research Extension Act, place the central role of oversight on the IACUC. The Canadian oversight system is decentralized, with self-monitoring and oversight provided by the Canadian Council on Animal Care. The European Directive (Directive 2010/63/EU) applies to all members of the European Union, though there is some variation in how they choose to exercise the directive. In Australia laboratory animal legislation is mainly overseen by governments specific to each province or state, in compliance with a central Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, while in New Zealand, primary oversight is provided by the National Animal Ethics Advisory Committee [34]. In recent years, with increasing global collaborative research, there is a trend towards harmonization of standards and practices of research animal welfare. Global organizations such as AAALAS contribute to this harmonization by standardization of best practices, training, and accreditation programs.
Field studies
IACUC oversight of field studies, whether in the country or in foreign locations, presents unique challenges, such as local and federal regulations regarding wildlife, health and safety of professionals, and review of animal procedures [35]. Because field studies differ from procedures in controlled laboratory environments, it may be appropriate for the IACUC to bring in consultants or outside experts such as wildlife biologists or environmentalists to ensure humane handling of animals. The study may require one or more permits depending upon the species studied, the location of the study site, and the type of study. The IACUCs may ensure that appropriate permits are obtained by the PI or institution prior to the start of the study. The IACUC should be aware of occupational health concerns for personnel involved in field studies, and work with the OHSP to minimize risks, and ensure that personnel have been adequately trained for the field work [36].
Noncompliance and animal welfare concerns
While overseeing animal studies, the IACUC may encounter noncompliance issues that may be detrimental to animal welfare and human health. The IACUC investigation into the noncompliance then aims to answer questions that will help identify the faults in the system, minimizing their impact, and preventing reoccurrences. Questions that need to be asked include: How many animals were affected? What measures were in place to monitor unapproved procedures? What training measures can be implemented to address the requirements of the staff and to prevent similar occurrences in the future? Based on the results of the investigation, the IACUC can introduce any appropriate changes to the animal program to address the deficiencies identified. Further, IACUCs are responsible for reporting to OLAW through the Institutional Official (IO), any noncompliance cases detected in the animal program [37]. For USDA-regulated species, items such as change of operations, protocol suspensions, or uncorrected significant deficiencies from a semi-annual inspection (9 CFR §2.31[c][3]) must be reported to the USDA. IACUCs should have appropriate mechanisms that ensure ease of reporting observed non-compliance, prompt investigation of concerns, and the protection of individuals from reprisal for a fast and accurate resolution. The IACUCs role in investigation of allegations is complicated one, and may involve conflict resolution and communication with public relations and legal offices [36].
Confidentiality
The IACUC must rigorously maintain the confidentiality of personal information and proprietary information submitted to it by investigators. Inappropriate disclosure of data can negatively impact scientific research and make the research community mistrustful of the IACUC. In the case of animal research, there is the additional risk of being targeted by people opposed to the use of animals in research. However, information disclosed to an IACUC or to a government agency may become available to the public through FOIA requests (Freedom of Information Act) or state-specific open records laws. This includes information submitted as well as the noncompliance reports mentioned previously. Exemptions to disclosure include personally identifiable information or data that can impact on an individual level, trade secrets, privileged, commercial or financial information, or any other information that can harm the competitive position or government interests such as program effectiveness or compliance [38].
Regulatory Burden
A good animal care and use program incorporates several components that, while not directly implemented by the IACUC, depend on the IACUC’s role in ensuring that performance standards in all these component areas are established and operational (eg, Occupational Health and Safety, facilities management, environment health and safety, etc.). However, this can sometimes make it difficult to balance compliance with responsible research practices and the burden this compliance may pose to the IACUC and researchers. When this burden becomes disproportionately high, it can affect productivity in research and add to institutional expenses [39].
Developing performance standards tailored to institutional program, that satisfy regulatory requirements while keeping burden and cost at a minimum can be quite challenging. But when done effectively, can help to achieve the “intersection point” of least burden and minimal risk [40]. This in turn, ensures a balance between effective compliance and research success. There has been extensive research [40–43] and multiple efforts on this front including the latest Draft Report from the 21st Century Cures Act Section 2034(d) Working Group Reducing Administrative Burden for Researchers: Animal Care and Use in Research.
Conclusions
Primary oversight responsibilities of an institution’s animal program rests with its IACUC, which supports the balance of good science practices with good animal welfare. The IACUC, along with husbandry care staff, veterinarians, and research personnel develop the appropriate animal care program to meet these standards of care, while taking into account the research objectives and resources available. A well-run IACUC will enable researchers to navigate the regulatory environment through a science-based, flexible program while still placing the humane care of animals as its number one priority and can assist the PI in developing and reporting high quality, reproducible studies
Oversight and support provided by the IACUC is at the local level, enabling institutions to tailor the process to their unique circumstances as long as certain criteria are met [44]. This process, however, places the heavy responsibility of oversight on the institution. And in an effort to maintain compliance to federal standards, it is not unusual for institutions to place excessive (though well-intentioned) regulations on its animal use community. This regulatory burden can be minimized by implementing a comprehensive program of animal care and use based on scientifically determined standards of animal welfare and research quality [45]. In order for the IACUC to maintain a culture of care and responsibility, it should be empowered by institutional commitment through availability of adequate resources, clear lines of authority and communication among the research community, veterinary staff, and other personnel, and joint effort from the entire animal research community.
References
- 1. Bradfield JF, Bennett BT, Gillett CS. (2014). Oversight of Research Animal Welfare in the United States Laboratory Animals Regulations and Recommendations for Global Collaborative Research. Ch. 2. Academic Press, Waltham, MA: p 5-59. [Google Scholar]
- 2. National Academies (US) Committee on Measuring, Economic and Other Returns on Federal Research Investments Measuring the Impacts of Federal Investments in Research: A Workshop Summary. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 3. Everitt JI, Berridge BR. The role of the IACUC in the design and conduct of animal experiments that contribute to translational success. ILAR J 2017;58(1):129–34. [DOI] [PubMed] [Google Scholar]
- 4. Pritt SL, Hammer RE. The Interplay of Ethics, Animal Welfare, and IACUC Oversight on the Reproducibility of Animal Studies. Comp Med. 2017;67(2):101–5. [PMC free article] [PubMed] [Google Scholar]
- 5. Varga O Critical analysis of assessment studies of the animal ethics review process. Animals (Basel) 2013;3(3):907–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bronstad A, Newcomer CE, Decelle Tet al. Current concepts of harm-benefit analysis of animal experiments - report from the AALAS-FELASA Working Group on Harm-Benefit Analysis - part 1. Lab Anim 2016;50:1 Suppl):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Griffin G, Clark JM, Zurlo Jet al. Scientific uses of animals: harm-benefit analysis and complementary approaches to implementing the three Rs. Rev Sci Tech 2014;33(1):265–72. [DOI] [PubMed] [Google Scholar]
- 8. Prentice ED, Crouse DA, Mann MD. Scientific merit review: the role of the IACUC. Ilar News 1992;34(1-2):15–9. [DOI] [PubMed] [Google Scholar]
- 9. Public Health Service policy on humane care and use of laboratory animals Fed Regist. 1985;50(90):19584–5. [PubMed] [Google Scholar]
- 10. Burch RL The progress of humane experimental technique since 1959: a personal view. Altern Lab Anim 2009;37(3):269–75. [DOI] [PubMed] [Google Scholar]
- 11. NC3Rs Experimental design and statistics. Retrieved fromhttps://www.nc3rs.org.uk/experimental-designstatistics. Accessed May 21, 2018.
- 12. NRC Definition of Pain and Distress and Reporting Requirements for Laboratory Animals: Proceedings of the Workshop Washington, DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- 13. Johnson PD, Besselsen DG. Practical aspects of experimental design in animal research. ILAR J 2002;43(4):202–6. [DOI] [PubMed] [Google Scholar]
- 14. Festing MF, Altman DG. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J 2002;43(4):244–58. [DOI] [PubMed] [Google Scholar]
- 15. Fitts DA Ethics and animal numbers: informal analyses, uncertain sample sizes, inefficient replications, and type I errors. J Am Assoc Lab Anim Sci. 2011;50(4):445–53. [PMC free article] [PubMed] [Google Scholar]
- 16. NC3Rs The 3Rs. Retrieved fromhttps://www.nc3rs.org.uk/the-3rs. Accessed May 21, 2018.
- 17. Kilkenny C, Browne WJ, Cuthill ICet al. Improving bioscience research reporting: the ARRIVE Guidelines for Reporting Animal Research. PLoS Biology 2010;8(6):e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith AJ, Clutton RE, Lilley Eet al. PREPARE: Guidelines for Planning Animal Research and Testing. Lab Anim. 2018;52(2):135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Good DJ Using obese mouse models in research: special considerations for IACUC members, animal care technicians, and researchers. Lab Anim 2005;34:30. [DOI] [PubMed] [Google Scholar]
- 20. Carbone L Pain Management Standards in the Eighth Edition of the Guide for the Care and Use of Laboratory Animals. J Am Assoc Lab Anim Sci. 2012;51(3):322–8. [PMC free article] [PubMed] [Google Scholar]
- 21. Carbone L Justification for the use of animals In: Jerald Silverman MAS, Murthy S, eds. The IACUC Handbook. Boca Raton: CRC Press; 2014. [Google Scholar]
- 22. Stokes WS Humane endpoints for laboratory animals used in regulatory testing. ILAR J 2002;43(Suppl_1):S31–8. [PubMed] [Google Scholar]
- 23. Toth LA The moribund state as an experimental endpoint. ILAR J 2000;41(2):72–9. [DOI] [PubMed] [Google Scholar]
- 24. Oki GS, Prentice ED, Garnett NLet al. Model for performing institutional animal care and use committee: continuing review of animal research. Contemp Top Lab Anim Sci 1996;35(5):53–6. [PubMed] [Google Scholar]
- 25. Baumans V Baumans, V. Science-based assessment of animal welfare: laboratory animals. Rev Sci Tech. 2005;24:503–13. [PubMed] [Google Scholar]
- 26. NRC Guide for the Care and Use of Laboratory Animals In The National Academies Collection: Reports funded by National Institutes of Health (8th ed.). Washington, DC: National Academies Press (US); 2011. [Google Scholar]
- 27. Wald PH, Stave GM. Occupational medicine programs for animal research facilities. ILAR J 2003;44(1):57–71. [DOI] [PubMed] [Google Scholar]
- 28. Benoit JN, Bayne KA. Training the trainee: the institution’s responsibility to the often forgotten. Lab Anim 2005;34:46. [DOI] [PubMed] [Google Scholar]
- 29. Anderson LC Institutional and IACUC responsibilities for animal care and use education and training programs. ILAR J 2007;48(2):90–5. [DOI] [PubMed] [Google Scholar]
- 30. Silverman J Too many IACUCs in the kitchen. Lab Anim 2016;45(4):137. [DOI] [PubMed] [Google Scholar]
- 31. Underwood WJ Contracting in vivo research: what are the issues? J Am Assoc Lab Anim Sci. 2007;46(4):16–9. [PubMed] [Google Scholar]
- 32. Bayne K, Bayvel ACD, Williams V. Chapter 6 - Laboratory Animal Welfare: International Issues In: Bayne K, Turner PV, eds. Laboratory Animal Welfare. Boston: Academic Press; 2013. [Google Scholar]
- 33. Guillén J, Vergara P (2017) Global guiding principles: a tool for harmonization In: Guillen J (ed)Laboratory animals: regulations and recommendations for the care and use of animals in research. Academic Press, Cambridge: pp 1--13. [Google Scholar]
- 34. Vasbinder MA, Locke P. Introduction: global laws, regulations, and standards for animals in research. ILAR J 2017;57(3):261–5. [DOI] [PubMed] [Google Scholar]
- 35. Laber K, Kennedy BW, Young L. Field studies and the IACUC: protocol review, oversight, and occupational health and safety considerations. Lab Anim 2007;36(1):27–33. [DOI] [PubMed] [Google Scholar]
- 36. Brovarney LN. IACUCs from an academic perspective In Petrie WK, Wallace, S.L. (eds.), The Care and Feeding of an IACUC (2nd ed.). Boca Raton: CRC Press; 2015. [Google Scholar]
- 37. NOT-OD-13-044 (2005). Guidance on Prompt Reporting to OLAW under the PHS Policy on Humane Care and Use of Laboratory Animals.
- 38. Chimes M, Sankar P. Confidential and proprietary information In: Jerald Silverman MAS, Murthy S, eds. The IACUC Handbook. Boca Raton: CRC Press; 2014. [Google Scholar]
- 39. Thulin JD, Bergdall VK, Bradfield JF.. Facilitating the research process: limiting regulatory burden and leveraging performance standards In Management of Animal Care and Use Programs in Research, Education, and Testing, 2nd ed CRC Press/Taylor & Francis, Boca Raton (FL); 2018. [PubMed] [Google Scholar]
- 40. Thulin JD, Bradfield JF, Bergdall VKet al. The cost of self-imposed regulatory burden in animal research. FASEB J 2014;28(8):3297–300. [DOI] [PubMed] [Google Scholar]
- 41. Haywood JR, Greene M. Avoiding an overzealous approach: a perspective on regulatory burden. ILAR J 2008;49(4):426–34. [DOI] [PubMed] [Google Scholar]
- 42. Plante A, James ML. Program Oversight Enhancements (POE): the big PAM. ILAR J. 2008;49(4):419–25. [DOI] [PubMed] [Google Scholar]
- 43. Pritt S, McNulty JA, Greene Met al. Decreasing institutionally imposed regulatory burden for animal research. Lab Anim. 2016;45(8):297–300. [DOI] [PubMed] [Google Scholar]
- 44. Collins J. Role of the IACUC, bioethics and scientific review, essential environment for IACUC success In Petrie WK, Wallace SL. (eds.), The Care and Feeding of an IACUC (2nd ed.). Boca Raton: CRC Press; 2015. [Google Scholar]
- 45. Klein HJ, Bayne KA. Establishing a culture of care, conscience, and responsibility: addressing the improvement of scientific discovery and animal welfare through science-based performance standards. ILAR J. 2007;48(1):3–11. [DOI] [PubMed] [Google Scholar]
- 46. Council for International Organizations of Medical Sciences-International Council of Laboratory Animal Science (CIOMS-ICLAS) (2012). International guiding principles for biomedical research involving animals. https://grants.nih.gov/grants/olaw/Guiding_Principles_2012.pdf. Accessed June 14, 2018.
- 47. European Commission (2010). Directive 2010/63/EU of the European Parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union L276/33.
- 48. USDA Animal Welfare Act and animal welfare regulations. Washington, DC: U.S. Dept. of Agriculture, Animal and Plant Health Inspection Service; 2005. [Google Scholar]


