Abstract
Recombinant AgB8/1 as the most evaluated antigen for serological diagnosis of Cystic Echinococcosis (CE) can provide early and accurate diagnosis for proper management and treatment of the disease. Thus, the secretory production of this recombinant protein is the main goal and the application of signal peptides at the N terminus of the desired protein can help to achieve this goal. The present study applied few bioinformatics tools to evaluate several signal peptides to offer the best candidate for extracellular production of AgB8/1 of Echinococcus granulosus in Escherichia coli. The sequences related to signal peptides were obtained from “Signal Peptide Website” and were checked by “UniProt”. In addition, UniProt was employed to retrieve the sequence of AgB8/1. Then, the probable signal peptide sequences and their cleavage site locations were determined by SignalP 4.1 followed by evaluation of their physicochemical features, using ProtParam. The solubility of the target recombinant proteins was accessed by SOLpro. Finally, PRED-TAT and ProtCompB were implemented to predict protein secretion pathways and final destinations. Among the 39 candidate signal peptides, ENTC2_STAAU and ENTC1_STAAU are the best ones which are stable and soluble in connection with AgB8/1 and can secrete target protein through Sec pathway. The signal peptides recommended in this investigation are valuable for rational designing of secretory stable and soluble AgB8/1. Such information is useful for future experimental production of the mentioned antigen.
Key Words: Antigen B, Echinococcus granulosus, Signal Peptide, In Silico
INTRODUCTION
Antigen B (AgB) is a major antigen of Echinococcus granulosus cyst fluid, which has been extensively evaluated for the diagnosis of Cystic Echinococcosis (CE) or hydatid cyst [1]. Hydatid cyst affects human health and welfare, consisting of both direct and indirect costs, calculated around 3 billion USD in the endemic areas [2]. On the other hand, since most of the patients at the early stages of the disease are asymptomatic, the physical imaging methods cannot be used for routine screening of CE infection. This calls for creation of an easy to use and cost-effective methods such as serological tests [3, 4]. A serological test based on AgB subunits can be used as an effective diagnostic tool for patient’s follow-up after surgical or pharmacological treatment. AgB as a highly immunogenic antigen has shown high specificity and sensitivity in the serological diagnosis of CE. The antigen is a multimeric protein consisting of 8 kDa subunits, including 8-12, 16 and 24 kDa antigens [5-7]. It was confirmed that the 8 kDa subunit is the most suitable antigen for the serological diagnosis of CE. 8 kDa a subunit of AgB, called antigen B8/1, has exhibited the highest diagnostic sensitivity and specificity in comparison with other antigens that were applied for the serodiagnosis of CE [8]. In this regard, another investigation found specific antibodies against these two antigens, using western blotting [9]. The production of recombinant antigen B8/1 expressed in a heterologous system have several advantages such as ease of purification and reduction of cross-reactivity [10, 11]. The recombinant antigen could be produced in prokaryotic systems such as different strains of E. coli, recognized as the most common and alluring prokaryotic host to obtain recombinant proteins [12, 13].
E.coli as a desirable host for recombinant protein expression has several advantages including short generation time, an engineered genome and low-cost maintenance [14]. With the advent of recombinant DNA technology, increasing the solubility of a heterologous protein can be considered for large-scale bio-manufacturing, which can lead to serious problems such as misfolding and accumulation of protein that results in the formation of inclusion bodies. Inclusion body is a misfolded, insoluble aggregation of denatured proteins that reduce the yield of correctly folded proteins [15-17]. To overcome this difficulty, the target protein should be transferred to the oxidizing situation that exist in the sub-compartment of E.coli called periplasm [18]. Production of the secretory type of recombinant proteins using E coli offers a solution to this problem. Proteins transportation to the periplasm has some advantages, since the process of protein purification can be facilitated if the desired protein exist in this area that has fewer proteins, and its content can be selectively secreted by osmotic pressure or other approaches This approach provides several benefits including ease of purification, prevention of protease attack and N-terminal Met extension as well as having a more properly folded protein. An optimal secretion procedure consists of different stages depending on several factors.
Signal peptide is one of the most significant elements affecting different stages of secretion process and the yield of protein. Therefore, the bioinformatics assessment of the signal peptide sequence is recommended to determine the potential signal peptide A signal peptide is a short sequence consisting of 15-30 specific amino acids added to the N-terminus of proteins to permit their exportation to the outside of cytoplasm [17, 19-21]. Signal peptides structure consists of three parts including a positively-charged amino-terminal (n-region), a central hydrophobic core (h-region) and a polar carboxyl-terminal domain (c-region) [22]. The selection and connection of a signal peptide, appropriate to the target protein, is a critical step [23]. To this end, some bioinformatics tools are available to predict the suitable signal peptides based on their specific characteristics [24].
To the best of our knowledge, there is no published data with respect to the secretory production of AgB8/1, using appropriate signal peptides. Consequently, in the present study an in silico approach assessing different signal peptides was used in order to suggest the best choice for secretory production of AgB8/1 in E.coli.
MATERIALS AND METHODS
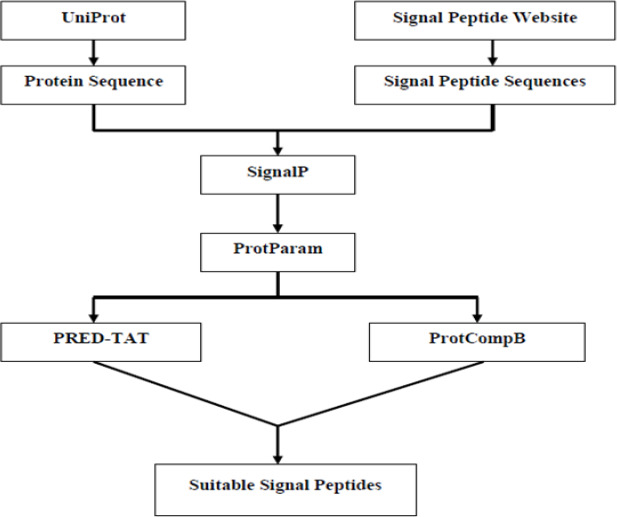
Data Collection: The amino acid sequence related to AgB8/1 was obtained using UniProt server at http://www.uniprot.org/. The signal peptide sequences and their different parts including n, h and c-regions were achieved from Signal Peptide Website at http://www.signal peptide.de/. All signal peptides were confirmed experimentally. The selected signal peptides were fused to AgB8/1 for further analysis. The total procedure of study is shown in Figure 1.
Figure 1.
Flowchart of the procedure
Prediction of the existence of signal peptide and cleavage site position: Several bioinformatics tools are employed to predict the presence of signal sequences and location of their cleavage sites. The SignalP has the most accuracy and reliability among signal peptide identifying tools. Therefore, for the mentioned purposes the SignalP 4.1 web server (http://www.cbs.dtu.dk/services/SignalP/) which is based on a hidden Markov model (HMM) was applied [25]. SignalP predicts signal peptides probability for target protein and defines cleavage sites.
Evaluation of Physicochemical Parameters and Solubility: The ProtParam software related to the ExPASy server at http://web.expasy.org/protparam/ was implemented for assessment of physicochemical properties of the signal peptides, including aliphatic index, GRAVY (grand average of hydropathicity), instability index, positive charge and theoretical pI [26]. SOLpro, an online server at http://scratch.proteomics.ics.uci.edu/ was utilized to predict the solubility of the recombinant protein expressed in E.coli. The submitted protein sequences are categorized as soluble or insoluble determined by a probability score. This server employs a sequence-based technique for predicting protein solubility in E. coli. Finally, this server emails the results and their related probability score [27].
Prediction of Secretion Pathway and Sub-cellular location: “PRED-TAT” online server (http://www.compgen.org/tools/PRED-TAT) was employed to predict secretion pathway of B8/1 fused signal peptides. PRED-TAT defines secretion pathway based on Hidden Markov Models (HMMs). The Sec pathway is the most desirable pathway for protein secretion in E. coli. Then, “ProtCompB” online server (http://www.softberry.com) was implemented to predict localization of signal peptide fused B8/1 dependent on neural networks. The most favorable result for secretion destination is “secreted” state [28, 29].
RESULTS
The AgB8/1 sequence was retrieved from UniProt (UniProt ID: Q2EN83). Next, the information related to 39 signal peptides of different organisms are shown in Table 1. The collected data consist of signal peptides separated from secretory proteins which are specific for eubacteria. The signal peptide related scores (C, S, Y, S-mean and D), various regions of signal peptides consist of n, h and, c regions and their cleavage site positions are shown in Table 2.
Table 1.
Collected amino acid sequences dataset
| No. | Accession | Signal Peptide | Source | Amino Acid Sequence |
|---|---|---|---|---|
| 1 | P0A910 | OMPA_ECOLI | E. coli (strain K12) | MKKTAIAIAVALAGFATVAQA |
| 2 | P00634 | PPB_ECOLI | E. coli (strain K12) | MKQSTIALALLPLLFTPVTKA |
| 3 | P06996 | OMPC_ECOLI | E. coli (strain K12) | MKVKVLSLLVPALLVAGAANA |
| 4 | P09169 | OMPT_ECOLI | E. coli (strain K12) | MRAKLLGIVLTTPIAISSFA |
| 5 | P02931 | OMPF_ECOLI | E. coli (strain K12) | MMKRNILAVIVPALLVAGTANA |
| 6 | P0C1C1 | PEL2_ERWCA | Erwinia carotovora | MKYLLPTAAAGLLLLAAQPAMA |
| 7 | P22542 | HSTI_ECOLX | Escherichia coli | MKKNIAFLLASMFVFSIATNAYA |
| 8 | P02932 | PHOE_ECOLI | E. coli (strain K12) | MKKSTLALVVMGIVASASVQA |
| 9 | P0AEX9 | MALE_ECOLI | E. coli (strain K12) | MKIKTGARILALSALTTMMFSASALA |
| 10 | P69776 | LPP_ECOLI | E. coli (strain K12) | MKATKLVLGAVILGSTLLAG |
| 11 | P02943 | LAMB_ECOLI | E. coli (strain K12) | MMITLRKLPLAVAVAAGVMSAQAMA |
| 12 | P32890 | ELBP_ECOLX | Escherichia coli | MNKVKCYVLFTALLSSLYAHG |
| 13 | P31746 | CDGT_BACS2 | Bacillus sp. (strain 1-1) | MNDLNDFLKTILLSFIFFLLLSLPTVAEA |
| 14 | P0A618 | MPT53_MYCTU | Mycobacterium tuberculosis | MSLRLVSPIKAFADGIVAVAIAVVLMFGLANTPRAVAA |
| 15 | Q50906 | APA_MYCTU | Mycobacterium tuberculosis | MHQVDPNLTRRKGRLAALAIAAMASASLVTVAVPATANA |
| 16 | Q9XD84 | TIBA_ECOLX | Escherichia coli | MNKVYNTVWNESTGTWVVTSELTRKGGLRPRQIKRTVLAGLIAGLLMPSMPALA |
| 17 | P06717 | ELAP_ECOLX | Escherichia coli | MKNITFIFFILLASPLYA |
| 18 | Q8FDW4 | SAT_ECOL6 | Escherichia coli O6 | MNKIYSLKYSAATGGLIAVSELAKRVSGKTNRKLVATMLSLAVAGTVNA |
| 19 | P06608 | ASPG_ERWCH | Erwinia chrysanthemi | MERWFKSLFVLVLFFVFTASA |
| 20 | Q05044 | SLAP_LACBR | Lactobacillus brevis | MQSSLKKSLYLGLAALSFAGVAAVSTTASA |
| 21 | P34071 | ENTC2_STAAU | Staphylococcus aureus | MNKSRFISCVILIFALILVLFTPNVLA |
| 22 | Q47692 | TSH_ECOLX | Escherichia coli | MNRIYSLRYSAVARGFIAVSEFARKCVHKSVRRLCFPVLLLIPVLFSAGSLA |
| 23 | P07965 | HST3_ECOLX | Escherichia coli | MKKSILFIFLSVLSFSPFA |
| 24 | P39180 | AG43_ECOLI | E. coli (strain K12)) | MKRHLNTCYRLVWNHMTGAFVVASELARARGKRGGVAVALSLAAVTSLPVLA |
| 25 | P13811 | ELBH_ECOLX | Escherichia coli | MNKVKFYVLFTALLSSLCAHG |
| 26 | P13423 | PAG_BACAN | Bacillus anthracis | MKKRKVLIPLMALSTILVSSTGNLEVIQA |
| 27 | Q0Z8B6 | HJM79_ENTHR | Enterococcus hirae | MKKKVLKHCVILGILGTCLAGIGTGIKVDA |
| 28 | P01553 | ENTC1_STAAU | Staphylococcus aureus | MNKSRFISCVILIFALILVLFTPNVLA |
| 29 | P15917 | LEF_BACAN | Bacillus anthracis | MNIKKEFIKVISMSCLVTAITLSGPVFIPLVQG |
| 30 | P24093 | DRAA_ECOLX | Escherichia coli | MKKLAIMAAASMVFAVSSAHA |
| 31 | P62605 | FIM1C_ECOLX | Escherichia coli | MKLKFISMAVFSALTLGVATNAS |
| 32 | A2TJI4 | CEXE_ECOLX | Escherichia coli | MKKYILGVILAMGSLSAIA |
| 33 | P20862 | FANH_ECOLX | Escherichia coli | MIKKVPVLLFFMASISITHA |
| 34 | O88093 | HBP_ECOLX | Escherichia coli | MNRIYSLRYSAVARGFIAVSEFARKCVHKSVRRLCFPVLLLIPVLFSAGSLA |
| 35 | O68900 | PET_ECOLX | Escherichia coli | MNKIYSIKYSAATGGLIAVSELAKKVICKTNRKISAALLSLAVISYTNIIYA |
| 36 | Q03155 | AIDA_ECOLX | Escherichia coli | MNKAYSIIWSHSRQAWIVASELARGHGFVLAKNTLLVLAVVSTIGNAFA |
| 37 | P25394 | FMF7_ECOLX | Escherichia coli | MKRLVFISFVALSMTAGSAMA |
| 38 | P13720 | PAPG_ECOLX | Escherichia coli | MKKWFPAFLFLSLSGGNDALA |
| 39 | O32591 | ESPP_ECOLX | Escherichia coli | MNKIYSLKYSHITGGLIAVSELSGRVSSRATGKKKHKRILALCFLGLLQSSYSFA |
Table 2.
In silico evaluation of signal peptides for AgB8/1
| Protein Name | n-region | h-region | c-region | Cleavage Site | C-score | Y-score | S-score | S-mean | D-score |
|---|---|---|---|---|---|---|---|---|---|
| OMPA_ECOLI | 1-4(4) | 5-13(9) | 14-21(8) | AQA | 0.710 | 0.838 | 0.997 | 0.963 | 0.897 |
| PPB_ECOLI | 1-4(4) | 5-16(12) | 17-21(5) | TKA | 0.386 | 0.594 | 0.992 | 0.928 | 0.751 |
| OMPC_ECOLI | 1-4(4) | 5-16(12) | 17-21(5) | ANA | 0.754 | 0.865 | 0.997 | 0.976 | 0.917 |
| OMPT_ECOLI | 1-4(4) | 5-12(8) | 13-20(8) | AMT | 0.436 | 0.590 | 0.994 | 0.887 | 0.730 |
| OMPF_ECOLI | 1-4(4) | 5-17(13) | 18-22(5) | ANA | 0.776 | 0.877 | 0.996 | 0.969 | 0.920 |
| PEL2_ERWCA | 1-3(3) | 4-18(15) | 19-22(4) | AMA | 0.835 | 0.910 | 0.996 | 0.966 | 0.936 |
| HSTI_ECOLX | 1-3(3) | 4-18(15) | 19-23(5) | AYA | 0.803 | 0.892 | 0.998 | 0.972 | 0.930 |
| PHOE_ECOLI | 1-4(4) | 5-15(11) | 16-21(6) | VQA | 0.656 | 0.805 | 0.998 | 0.950 | 0.873 |
| MALE_ECOLI | 1-5(5) | 6-20(15) | 21-26(6) | ALA | 0.593 | 0.768 | 0.999 | 0.968 | 0.862 |
| LPP_ECOLI | 1-5(5) | 6-16(11) | 17-20(4) | TQA | 0.452 | 0.592 | 0.996 | 0.891 | 0.733 |
| LAMB_ECOLI | 1-7(7) | 8-16(9) | 17-25(9) | AMA | 0.691 | 0.826 | 0.998 | 0.965 | 0.891 |
| ELBP_ECOLX | 1-5(5) | 6-15(10) | 16-21(6) | AHG | 0.731 | 0.849 | 0.998 | 0.945 | 0.894 |
| CDGT_BACS2 | 1-10(10) | 11-21(11) | 22-29(8) | AEA | 0.616 | 0.762 | 0.990 | 0.821 | 0.789 |
| MPT53_MYCTU | 1-15(15) | 15-25(11) | 25-38(14) | AVA | 0.611 | 0.711 | 0.926 | 0.726 | 0.718 |
| APA_MYCTU | 1-14(14) | 14-24(11) | 25-39(15) | ANA | 0.293 | 0.504 | 0.995 | 0.845 | 0.664 |
| TIBA_ECOLX | 1-36(36) | 37-46(10) | 47-54(8) | ALA | 0.640 | 0.776 | 0.989 | 0.463 | 0.629 |
| ELAP_ECOLX | 1-2(2) | 2-13(12) | 14-18(5) | LYA | 0.596 | 0.767 | 0.998 | 0.957 | 0.856 |
| SAT_ECOL6 | 1-33(33) | 34-44(11) | 45-49(5) | VNA | 0.567 | 0.713 | 0.960 | 0.474 | 0.601 |
| ASPG_ERWCH | 1-6(6) | 7-17(11) | 18-21(5) | ASA | 0.746 | 0.860 | 0.999 | 0.964 | 0.909 |
| SLAP_LACBR | 1-7(7) | 8-24(17) | 24-30(7) | ASA | 0.555 | 0.715 | 0.990 | 0.934 | 0.818 |
| ENTC2_STAAU | 1-9(9) | 10-20(11) | 21-27(7) | VLA | 0.793 | 0.888 | 0.998 | 0.973 | 0.928 |
| TSH_ECOLX | * | 0.235 | 0.474 | 0.995 | 0.598 | 0.532 | |||
| HST3_ECOLX | 1-3(3) | 4-13(10) | 14-19(6) | PFA | 0.526 | 0.723 | 0.998 | 0.970 | 0.839 |
| AG43_ECOLI | 1-35(35) | 36-45(10) | 46-52(7) | VLA | 0.502 | 0.660 | 0.946 | 0.516 | 0.592 |
| ELBH_ECOLX | 1-7(7) | 8-14(7) | 15-21(7) | AHG | 0.504 | 0.706 | 0.998 | 0.964 | 0.828 |
| PAG_BACAN | * | 0.310 | 0.424 | 0.934 | 0.700 | 0.554 | |||
| HJM79_ENTHR | 1-9(9) | 10-20(11) | 21-30(10) | CLA | 0.322 | 0.509 | 0.957 | 0.815 | 0.653 |
| ENTC1_STAAU | 1-5(5) | 6-21(16) | 22-32(11) | VLA | 0.793 | 0.888 | 0.998 | 0.973 | 0.928 |
| LEF_BACAN | 1-9(9) | 10-22(13) | 23-33(11) | TQA | 0.324 | 0.459 | 0.860 | 0.705 | 0.575 |
| DRAA_ECOLX | 1-3(3) | 4-16(13) | 17-21(5) | AHA | 0.482 | 0.622 | 0.891 | 0.827 | 0.698 |
| FIM1C_ECOLX | * | 0.258 | 0.295 | 0.867 | 0.541 | 0.386 | |||
| CEXE_ECOLX | * | 0.454 | 0.422 | 0.564 | 0.437 | 0.428 | |||
| FANH_ECOLX | * | 0.407 | 0.436 | 0.695 | 0.511 | 0.464 | |||
| HBP_ECOLX | * | 0.147 | 0.156 | 0.217 | 0.168 | 0.160 | |||
| PET_ECOLX | * | 0.158 | 0.161 | 0.349 | 0.259 | 0.197 | |||
| AIDA_ECOLX | * | 0.240 | 0.165 | 0.214 | 0.121 | 0.149 | |||
| FMF7_ECOLX | 1-2(2) | 3-14(12) | 15-21(7) | AMA | 0.497 | 0.569 | 0.852 | 0.719 | 0.625 |
| PAPG_ECOLX | * | 0.335 | 0.372 | 0.762 | 0.504 | 0.421 | |||
| ESPP_ECOLX | * | 0.473 | 0.233 | 0.262 | 0.170 | 0.210 |
The signal peptides named as TSH_ECOLX, PAG_BACAN, FIM1C_ECOLX, CEXE_ECOLX, FANH_ECOLX, HBP_ECOLX, PET_ECOLX, AIDA_ECOLX, PAPG_ECOLX and ESPP_ECOLX were reported with D-score values under cut-off; hence, they are not considered as appropriate signal peptides for the secretion of AgB8/1 and discarded for next steps of the study.
The physicochemical parameters are shown in Table 3. As expected, the results obtained from ProtParam showed that the net positive charges of all the target signal peptides were between +1 to +8, since these sequences were related to native signal peptides of E. coli or other living hosts.
Table 3.
Prediction of signal peptides physico-chemical properties and solubility
| Signal Peptides |
Amino Acid
Length |
Net Positive Charge | Aliphatic Index | GRAVY |
Instability Index
(alone) |
Instability index
(fused to B8/1) |
Solubility |
|---|---|---|---|---|---|---|---|
| OMPA_ECOLI | 21 | 2 | 121.43 | 1.295 | Stable (9.52) | Stable (25.67) | Soluble (0.58) |
| PPB_ECOLI | 21 | 2 | 139.52 | 0.971 | Unstable (56.02) | Stable (35.24) | Soluble (0.60) |
| OMPC_ECOLI | 21 | 2 | 171.90 | 1.552 | Stable (14.37) | Stable (26.66) | Soluble (0.52) |
| OMPT_ECOLI | 20 | 2 | 146.50 | 1.290 | Stable (2.62) | Stable (24.46) | Soluble (0.55) |
| OMPF_ECOLI | 22 | 2 | 150.91 | 1.259 | Unstable (67.18) | Stable (37.83) | Soluble (0.55) |
| PEL2_ERWCA | 22 | 1 | 138.18 | 1.191 | Unstable (41.42) | Stable (32.32) | Soluble (0.56) |
| HSTI_ECOLX | 23 | 2 | 102.17 | 1.026 | Stable (32.43) | Stable (30.42) | Insoluble (0.75) |
| PHOE_ECOLI | 21 | 2 | 130.00 | 1.195 | Stable (1.44) | Stable (24.00) | Soluble (0.66) |
| MALE_ECOLI | 26 | 3 | 113.08 | 1.012 | Stable (2.85) | Stable (23.29) | Soluble (0.75) |
| LPP_ECOLI | 20 | 2 | 161.00 | 1.400 | Stable (10.64) | Stable (26.05) | Soluble (0.63) |
| LAMB_ECOLI | 25 | 2 | 125.20 | 1.332 | Unstable (42.97) | Stable (32.95) | Soluble (0.61) |
| ELBP_ECOLX | 21 | 2 | 111.43 | 0.695 | Stable (26.85) | Stable (29.24) | Soluble (0.54) |
| CDGT_BACS2 | 29 | 1 | 151.38 | 1.183 | Stable (17.41) | Stable (26.57) | Insoluble (0.52) |
| MPT53_MYCTU | 38 | 3 | 141.32 | 1.403 | Stable (24.78) | Stable (28.23) | Insoluble (0.55) |
| APA_MYCTU | 39 | 4 | 107.95 | 0.467 | Unstable (42.02) | Stable (33.81) | Soluble (0.78) |
| TIBA_ECOLX | 54 | 7 | 99.26 | 0.043 | Unstable (47.89) | Stable (37.07) | Soluble (0.71) |
| ELAP_ECOLX | 18 | 1 | 141.11 | 1.500 | Unstable (88.98) | Stable (40.60) | Insoluble (0.65) |
| SAT_ECOL6 | 49 | 7 | 109.59 | 0.357 | Stable (14.27) | Stable (23.98) | Soluble (0.50) |
| ASPG_ERWCH | 21 | 2 | 106.67 | 1.352 | Stable (29.64) | Stable (29.81) | Insoluble (0.76) |
| SLAP_LACBR | 30 | 2 | 107.67 | 0.837 | Stable (25.39) | Stable (28.65) | Insoluble (0.60) |
| ENTC2_STAAU | 27 | 2 | 169.63 | 1.730 | Unstable (49.08) | Stable (34.66) | Soluble (0.53) |
| HST3_ECOLX | 19 | 2 | 123.16 | 1.416 | Unstable (52.87) | Stable (34.23) | Insoluble (0.82) |
| AG43_ECOLI | 52 | 7 | 108.85 | 0.465 | Stable (26.67) | Stable (28.61) | Soluble (0.65) |
| AGAR_ALTAT | 23 | 1 | 165.22 | 1.361 | Stable (13.84) | Stable (26.31) | Insoluble (0.69) |
| ELBH_ECOLX | 21 | 2 | 111.43 | 0.890 | Stable (31.10) | Stable (30.11) | Soluble (0.54) |
| HJM79_ENTHR | 30 | 5 | 139.67 | 0.890 | Stable (-6.90) | Stable (19.92) | Soluble (0.64) |
| ENTC1_STAAU | 27 | 2 | 169.63 | 1.730 | Unstable (49.08) | Stable (34.66) | Soluble (0.53) |
| LEF_BACAN | 33 | 3 | 132.73 | 1.042 | Unstable (46.72) | Stable (34.73) | Insoluble (0.81) |
| DRAA_ECOLX | 21 | 2 | 98.10 | 1.162 | Stable (16.49) | Stable (27.10) | Soluble (0.76) |
| FMF7_ECOLX | 21 | 2 | 102.38 | 1.290 | Stable (29.55) | Stable (29.79) | Insoluble (0.63) |
Based on the results, OMPC_ECOLI, ENTC1_STAAU, ENTC2_STAAU, AGAR_ALTAT and, LPP_ECOLI had the highest aliphatic indexes. Additionally, the information indicated that ENTC1_STAAU, OMPC_ECOLI and ELAP_ECOLX had the highest GRAVYs, sequentially. The least instability index belonged to HJM79_ENTHR, PHOE_ECOLI, OMPT_ECOLI and MALE_ECOLI, respectively. PPB_ECOLI, OMPF_ECOLI, PEL2_ERWCA, LAMB_ECOLI, APA_MYCTU, TIBA_ECOLX, ELAP_ECOLX, ENTC2_STAAU, HST3_ECOLX, ENTC1_STAAU and, LEF_BACAN had instability index over 40, which meant that they are unstable; hence, were excluded from the study in the next step. Based on the results of SOLpro, the AgB8/1 connected to HSTI_ECOLX, ELBP_ECOLX, CDGT_BACS2, MPT53_MYCTU, ASPG_ERWCH, SLAP_LACBR, TSH_ECOLX, AGAR_ALTAT and, FMF7_ECOLX will play a role as an insoluble protein. Additionally, the AgB8/1 linked to MALE_ECOLI had the maximum solubility.
According to PRED-TAT results, all fused proteins can be secreted via Sec pathway, except APA_MYCTU, TIBA_ECOLX and AG43_ECOLI. Also, ProtCompB results showed that only PPB_ECOLI, APA_MYCTU, ENTC2_STAAU and ENTC1_STAAU could target soluble B8/1 out of the cytoplasm. Therefore, it can be predicted that TIBA_ECOLX and AG43_ECOLI direct the protein into transmembrane segments.
DISCUSSION
In our recent investigation we reported a successful expression of AgB8/1 in E.coli but easy purification can be accelerated by secretory production of a protein [30]. Since there are no suggested signal peptides for secretory production of AgB8/1 in E.coli; hence, in the present study several bioinformatics tools were used to suggest appropriate signal peptides to achieve secretory production of Echinococcus granulosus B8/1 antigen. For this purpose, 39 prokaryotic signal peptides were assessed computationally.
SignalP (version 4.1) was implemented to predict the presence of signal peptides and cleavage site locations. This server has two capabilities including differentiation between signal peptides and other sequences and also it can determine cleavage site locations. The SignalP uses an artificial neural network algorithm to calculate some scores, such as C, S, Y, and D score. The C-score (raw cleavage site score) plays a role in discriminating signal peptide cleavage sites from every other position. The S-score (signal peptide score) separates the signal peptide sequences from the mature area of proteins by defining the presence or absence of signal peptide. The Y-score (combined cleavage site score) is defined as a combination (geometric average) of the C-score and the slope of the S-score, which can lead to a better cleavage site estimation in comparison with the raw C-score alone. The Y-score is used to differentiate between C-score peaks through the selection of signal peptide where the slope of the S-score is sharp. The mean S is the average S-score, belonging to a possible signal peptide. D-score (discrimination score) is a weighted average of the mean S and the max Y scores, which can be used to distinguish signal peptides from non-signal peptide sequences. The cut-off for all scores was set on 0.570 [25].
The combination of several signal peptides including OMPA_ECOLI, OMPC_ECOLI, OMPF_ECOLI, PEL2_ERWCA, HSTI_ECOLX, PHOE_ECOLI, MALE_ECOLI, LAMB_ECOLI, ELBP_ECOLX, ELAP_ECOLX, ASPG_ERWCH, SLAP_LACBR, ENTC2_STAAU, HST3_ECOLX, ELBH_ ECOLX, ENTC1_STAAU and AgB8/1 exhibited high D-scores using SignalP 4.0. Therefore, they can be regarded as appropriate signal peptides for AgB8/1.
The results of some other in silico investigations were in accordance with our results because they reported that the signal peptides called OMPC_ECOLI, OMPA_ECOLI, OMPF_ECOLI, PHOE_ECOLI, MALE_ECOLI and PEL2_ERWCA had the highest D-scores, too [6, 7].
The physico-chemical characteristics of the signal peptides play a significant role in the protein secretion. After evaluation by the SignalP server, the signal peptides that were reported with a D-score lower than the cut-off were discarded and the remaining were analyzed using ProtParam software to investigate their physico-chemical characteristics and stability. Proteins with the instability index<40 were regarded as stable and the instability index>40 meant that the signal peptide might be unstable [26]. Among the fusion proteins evaluated in this study, those connected to OMPT_ECOLI, MALE_ECOLI and HJM79_ENTHR were the most stable.
If the positive net charge of the n-region turns to zero or to a negative value, the transportation rate of the desired protein decreases significantly. These positive charges facilitate the interaction between signal peptide, the phospholipids and the translocation machinery located in the membrane. Therefore, the existence of one or more basic amino acids in the n-region permits the evolution of a useful signal peptide [19]. In this study, the net positive charge was 2 for most of the signal peptides. The CDGT_BACS2 with a net charge of -2 was the lowest one, and in contrast, AG43_ECOLI had the highest net positive charge of 7.
The reduction of hydrophobicity of the h-region has an inhibitory effect on the protein processing and translocation, which requires a minimal length and a minimum hydrophobic density of the h-region. Hence, disturbing this region by polar or charged amino acid residues can reduce or even completely terminate membrane transportation [31].
The hydrophobicity levels of the signal peptides were estimated by considering the aliphatic index and GRAVY (Table 3). The aliphatic index of a protein shows the relative volume filled by aliphatic side chains (alanine, valine, isoleucine, and leucine). The grand average of hydropathy (GRAVY) value for a peptide or protein is introduced as the sum of hydropathy values of all the amino acids, divided by the number of residues in the sequence. A lesser hydrophobicity results in a higher solubility [26]. Based on the obtained GRAVY and aliphatic index amount, some signal peptides including LPP_ECOLI, MPT53_MYCTU, ELAP_ECOLX, ENTC2_STAAU, AGAR_ALTAT and ENTC1_STAAU showed the highest hydrophobicity levels among all the remaining signal peptides.
The feature of cleavage efficiency has a great influence on the protein secretion level since the cleavage step is the rate-limiting factor in the protein secretion process. The determinative positions of C-region are considered as 1 and 3 prior to the cleavage site displayed as the (-3,-1) rule or AXA motif [19]. These positions are usually occupied by alanine, constructing the so-called Ala-X-Ala box, which is identified and cut by signal peptidase. Almost half of the signal peptides in this study had AXA motif in their cleavage sites as shown in Table 1.
The solubility of the passenger proteins identified by amino acid sequences can be considered as a key factor for secretion [32]. Therefore, the above mentioned stable signal peptides were accessed by SOLpro to define their solubility. SOLpro was used to predict the susceptibility of a protein to be soluble during overexpression in E.coli. The total accuracy of the SOLpro is 74.15% with a threshold of 0.5. SOLpro accurately labels 68.1% of the soluble proteins and 80.3% of the insoluble proteins [28]. In the midst of various signal peptides, HSTI_ECOLX, CDGT_BACS2, ASPG_ERWCH, SLAP_LACBR, and AGAR_ALTAT were supposed to construct insoluble proteins while it was reported that the target protein with HSTI_ECOLX was insoluble, but the combination of the other four signal peptides (CDGT_BACS2, ASPG_ERWCH, SLAP_LACBR, and AGAR_ALTAT) and the desired protein was soluble [20]. On the other hand, the fusion of OMPA_ECOLI, OMPC_ECOLI, PHOE_ECOLI and MALE_ECOLI with our desired proteins were suggested to be soluble whereas they were considered as insoluble in Zamani et al., study [33].
As shown in Table 4, most signal peptides can secret B8/1 through Sec pathway. According to PRED-TAT, TIBA_ECOLX and AG43_ECOLI direct target protein into transmembrane section and the results were confirmed with ProtCompB. E.coli excretes 90% of its secretory proteins through Sec system, which can secret unfolded proteins while Tat system secrets fully folded proteins. Protein folding in cytoplasm is time-consuming and might result in protein accumulation and aggregation in cytoplasm. Hence, Sec pathway is more desirable to avoid inclusion bodies formation [34-36]. APA_MYCTU, ENTC2_STAAU and ENTC1_STAAU can secrete B8/1 to medium which APA_MYCTU can secrete through Tat pathway while ENTC2_STAAU and ENTC1_STAAU use Sec pathway to excrete the protein out of bacteria.
Table 4.
Secretion sorting and sub-cellular location of SPs
|
Secretion pathway
|
Sub-cellular Localization
|
||||||
|---|---|---|---|---|---|---|---|
| Signal peptides | Type of SP |
Reliability
Score (%) |
Cytoplasmic | Membrane |
Secreted
(extracellular) |
Periplasmic | Final prediction site |
| OMPA_ECOLI | Sec | 0.973 | 0.85 | 5.65 | 1.38 | 2.12 | Inner Membrane |
| PPB_ECOLI | Sec | 0.949 | 0.13 | 4.11 | 0.13 | 5.64 | Periplasmic |
| OMPC_ECOLI | Sec | 0.949 | 0.18 | 7.75 | 0.08 | 1.99 | Inner Membrane |
| OMPT_ECOLI | Sec | 0.913 | 0.21 | 9.79 | 0.00 | 0.00 | Inner Membrane |
| OMPF_ECOLI | Sec | 0.908 | 0.08 | 9.92 | 0.00 | 0.00 | Inner Membrane |
| PEL2_ERWCA | Sec | 0.988 | 0.35 | 3.79 | 0.00 | 5.86 | membrane bound Periplasmic |
| PHOE_ECOLI | Sec | 0.966 | 0.00 | 9.86 | 0.14 | 0.00 | Inner Membrane |
| MALE_ECOLI | Sec | 0.979 | 0.00 | 0.00 | 0.00 | 10.00 | membrane bound Periplasmic |
| LPP_ECOLI | Sec | 0.926 | 0.00 | 7.25 | 2.75 | 0.00 | Inner Membrane |
| LAMB_ECOLI | Sec | 0.974 | 0.00 | 6.65 | 0.02 | 3.33 | Inner Membrane |
| ELBP_ECOLX | Sec | 0.899 | 0.35 | 6.81 | 1.72 | 1.72 | Inner Membrane |
| APA_MYCTU | Tat | 0.829 | 0.00 | 0.42 | 9.53 | 0.05 | Secreted |
| TIBA_ECOLX | TM segment | 0.841 | 0.00 | 9.34 | 0.50 | 0.15 | Inner Membrane |
| SAT_ECOL6 | Sec | 0.897 | 0.00 | 7.54 | 2.46 | 0.00 | Inner Membrane |
| ENTC2_STAAU | Sec | 0.973 | 0.00 | 2.25 | 7.73 | 0.02 | Secreted |
| AG43_ECOLI | TM segment | 0.928 | 0.00 | 8.39 | 1.38 | 0.22 | Inner Membrane |
| ELBH_ECOLX | Sec | 0.917 | 0.51 | 5.31 | 1.29 | 2.89 | Inner Membrane |
| HJM79_ENTHR | Sec | 0.926 | 0.00 | 10.00 | 0.00 | 0.00 | Inner Membrane |
| ENTC1_STAAU | Sec | 0.973 | 0.00 | 2.25 | 7.73 | 0.02 | Secreted |
| DRAA_ECOLX | Sec | 0.987 | 0.08 | 2.99 | 0.01 | 6.92 | membrane bound Periplasmic |
The variation in the results of the aforementioned investigations in comparison with ours might be due to differences in the targeted proteins, since the combination of different proteins with the same signal peptides can lead to the different solubility of the proteins.
Acknowledgements:
This study was financially supported by the office of vice-chancellor for research of Shiraz University of Medical Sciences (Grant No. 1396-01-01-15941). The results described in this paper were part of MSc student thesis of Seyyed Hossein Khatami. The authors wish to thank Mr.H.Argasi at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript.
Conflict of Interest:
There is no conflict of interests.
References
- 1.Vishwakarma VK, Upadhyay PK, Gupta JK, et al. Pathophysiologic role of ischemia reperfusion injury: A review. J Indian Coll Cardiol. 2017;7(3):97–104. [Google Scholar]
- 2.Matern C. Acupuncture for dogs and cats: A pocket atlas. 1st ed. New York, USA: Thieme ; 2011. p. p2. [Google Scholar]
- 3.Zhao JX, Tian YX, Xiao HL, et al. Effects of electro-acupuncture on hippocampal and cortical apoptosis in a mouse model of cerebral ischemia-reperfusion injury. J Tradit Chin Med. 2011;31(4):349–355. doi: 10.1016/s0254-6272(12)60017-x. [DOI] [PubMed] [Google Scholar]
- 4.Zhao JX, Xu H, Tian YX, et al. Effect of electro-acupuncture on brain-derived neurotrophic factor mRNA expression in mouse hippocampus following cerebral ischemia-reperfusion injury. J Tradit Chin Med. 2013;33(2):253–257. doi: 10.1016/s0254-6272(13)60135-1. [DOI] [PubMed] [Google Scholar]
- 5.Yu L, Liao Y, Wu H, et al. Effects of electroacupuncture and Chinese kidney-nourishing medicine on polycystic ovary syndrome in obese patients. JTradit Chin Med. 2013;33(3):287–293. doi: 10.1016/s0254-6272(13)60166-1. [DOI] [PubMed] [Google Scholar]
- 6.Yili W, Yuanxiang T, Jianxin Z, et al. Effect of electroacupuncture on gene expression in calcium signaling pathway in hippocampal cells in mice with cerebral ischemia reperfusion. J Tradit Chin Med. 2017;37(2):252–260. doi: 10.1016/s0254-6272(17)30052-3. [DOI] [PubMed] [Google Scholar]
- 7.Radovsky A, Katz L, Ebmeyer U, et al. Ischemic neurons in rat brains after 6, 8, or 10 minutes of transient hypoxic ischemia. Toxicol Pathol. 1997;25(5):500–505. doi: 10.1177/019262339702500512. [DOI] [PubMed] [Google Scholar]
- 8.Frias Neto CA, Koike MK, Saad KR, et al. Effects of ischemic preconditioning and cilostazol on muscle ischemia-reperfusion injury in rats. Acta Cir Bras2014. 29(Suppl 3):17–21. doi: 10.1590/s0102-86502014001700004. [DOI] [PubMed] [Google Scholar]
- 9.Bulkley GB. Free radical-mediated reperfusion injury: A selective review. Br J Cancer Suppl. 1987;8:66–73. [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgartner WA, Williams GM, Fraser CD Jr, et al. Cardiopulmonary bypass with profound hypothermia An optimal preservation method for multiorgan procurement. Transplantation. 1989;47(1):123–127. doi: 10.1097/00007890-198901000-00027. [DOI] [PubMed] [Google Scholar]
- 11.Vishwakarma VK, Qureshi SS, Agrawal V, et al. Role of atrial natriuretic peptides in various conditions. Int J Pharm Biol Sci. 2016;7(3):20–27. [Google Scholar]
- 12.Adibhatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2010;12(1):125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- 13.Han J, Wang D, Yu B, et al. Cardioprotection against ischemia-reperfusion by licochalcone B in isolated rat hearts. Oxid Med Cell Longev. 2014;2014:134862. doi: 10.1155/2014/134862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Li H, Yang SJ. Tribulosin protects rat hearts from ischemia/reperfusion injury. Acta Pharmacol Sin. 2010;31(6):671–678. doi: 10.1038/aps.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maxwell SR, Lip GY. Reperfusion injury: A review of the pathophysiology, clinical manifestations and therapeutic options. Int J Cardiol. 1997;58(2):95–117. doi: 10.1016/s0167-5273(96)02854-9. [DOI] [PubMed] [Google Scholar]
- 16.Park JL, Lucchesi BR. Mechanisms of myocardial reperfusion injury. Ann Thorac Surg. 1998;68(5):1905–1912. doi: 10.1016/s0003-4975(99)01073-5. [DOI] [PubMed] [Google Scholar]
- 17.Chandrashekhar VM, Ranpariya VL, Ganapaty S, et al. Neuroprotective activity of Matricaria recutita Linn against global model of ischemia in rats. J Ethnopharmacol. 2010;127(3):645–651. doi: 10.1016/j.jep.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Garcia JH, Kalimo H, Kamijyo Y, et al. Cellular events during partial cerebral ischemia Electron microscopy of feline cerebral cortex after middle-cerebral-artery occlusion. Virchows Arch B cell Pathol. 1977;25(3):191–206. doi: 10.1007/BF02889433. [DOI] [PubMed] [Google Scholar]
- 19.Petito CK, Babiak T. Early proliferative changes in astrocytes in postischemic noninfarcted rat brain. Ann Neurol. 1982;11(5):510–518. doi: 10.1002/ana.410110511. [DOI] [PubMed] [Google Scholar]
- 20.Lukaszevicz AC, Sampaïo N, Guégan C, et al. High sensitivity of protoplasmic cortical astroglia to focal ischemia. J Cereb Blood Flow Metab. 2002;22(3):289–298. doi: 10.1097/00004647-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Pantino L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27(9):1641–1647. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- 22.Inchauspe AA. Traditional Chinese medical criteria about the use of Yongquan as a life support maneuver. In: Kuang H, editor. Recent advances in theories and practice of Chinese medicine. Shanghai, China: InTech ; 2012. pp. 361–368. [Google Scholar]
- 23.Yu YP, Ju WP, Li ZG, et al. Acupuncture inhibits oxidative stress and rotational behavior in 6-hydroxydopamine lesioned rat. Brain Res. 2010;1336:58–65. doi: 10.1016/j.brainres.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Wu B, Nie K, et al. Effects of acupuncture on declined cerebral blood flow, impaired mitochondrial respiratory function and oxidative stress in multi-infarct dementia rats. Neurochem Int. 2014;65:23–29. doi: 10.1016/j.neuint.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Qi YC, Xiao XJ, Duan RS, et al. Effect of acupuncture on inflammatory cytokines expression of spastic cerebral palsy rats. Asian Pac J Trop Med. 2014;7(6):492–495. doi: 10.1016/S1995-7645(14)60081-X. [DOI] [PubMed] [Google Scholar]
- 26.Abe K, Hayashi N, Terada H. Effect of endogenous nitric oxide on energy metabolism of rat heart mitochondria during ischemia and reperfusion. Free Radic Biol Med. 1999;26(3-4):379–387. doi: 10.1016/s0891-5849(98)00222-6. [DOI] [PubMed] [Google Scholar]
- 27.Akhlaghi M, Bandy B. Mechanisms of flavonoid protection against myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 2009;46(3):309–317. doi: 10.1016/j.yjmcc.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Özbal S, Erbil G, Koçdor H, et al. The effects of selenium against cerebral ischemia-reperfusion injury in rats. Neurosci Lett. 2008;438(3):265–269. doi: 10.1016/j.neulet.2008.03.091. [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi K, Nakano Y, Imai T, et al. A novel nuclear factor erythroid 2-related factor 2 (Nrf2) activator RS9 attenuates brain injury after ischemia-reperfusion in mice. Neuroscience. 2016;333:302–310. doi: 10.1016/j.neuroscience.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 30.Savardashtaki A, Sarkari B, Arianfar F, Mostafavi-Pour Z. Immunodiagnostic value of Echinococcus granulosus recombinant B8/1 subunit of antigen B. Iran J Immunol. 2017;14:111–122. [PubMed] [Google Scholar]
- 31.Negahdaripour M, Nezafat N, Hajighahramani N, Soheil Rahmatabadi S, Hossein Morowvat M, Ghasemi Y. In silico study of different signal peptides for secretory production of interleukin-11 in Escherichia coli. Curr Proteomics. 2017;14:112–121. [Google Scholar]
- 32.Jia B, Jeon CO. High-throughput recombinant protein expression in Escherichia coli: current status and future perspectives. Open Biol. 2016;6:160196. doi: 10.1098/rsob.160196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zamani M, Nezafat N, Negahdaripour M, Dabbagh F, Ghasemi Y. In silico evaluation of different signal peptides for the secretory production of human growth hormone in E. coli. Int J Pept Res Ther. 2015;21:261–268. [Google Scholar]
- 34.Reed B, Chen R. Biotechnological applications of bacterial protein secretion: from therapeutics to biofuel production. Res Microbiol. 2013;164:675–682. doi: 10.1016/j.resmic.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Rusch SL, Kendall DA. Interactions that drive Sec-dependent bacterial protein transport. Biochemistry. 2007;46:9665–9673. doi: 10.1021/bi7010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green ER, Mecsas J. Bacterial secretion systems:an overview. Microbiol Spectr. 2016:4. doi: 10.1128/microbiolspec.VMBF-0012-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]