Abstract
Background:
It is estimated that one third of the world's population is infected with Mycobacterium tuberculosis (Mtb), the causative agent of Tuberculosis (TB). The BCG vaccine is widely used to fight against TB; however, many question its ability to provide complete protection from Mtb. Recently, the "Region of Difference 1" (RD1) set of genes were shown to be involved in the pathogenesis of Mtb. Downstream of RD1 transcription region, two proteins are encoded, known as EspB and EspC, which were found to contribute to Mtb virulence.In this study these two proteins are targeted as potential vaccine candidates against TB.
Methods:
The EspB and EspC Mtb genes were codon-optimized for expression and synthesis in Escherichia coli (E. coli). The amplicons were cloned into a pET21a expression vector and transformed into E. coli BL21(DE3). The expression and purity of the expressed proteins (i.e. rEspC, rEspB and rEspC/EspB) were confirmed by SDS-PAGE and Western blotting. Moreover, BALB/c mice were immunized against Mtb using the recombinant proteins. Finally, the mice sera were analyzed via Western blotting.
Results:
EspC, EspB, and EspC/EspB fusion genes were cloned and expressed in E. coli. Both SDS-PAGE and Western blots confirmed the presence and successful purification of the desired proteins. Moreover, antisera produced against the purified recombinant proteins reacted with Mtb proteins.
Conclusion:
rEspC, rEspB, and rEspC/EspB could be expressed and purified using an E. coli expression system. The recombinant proteins induced the production of antibodies in BALB/c mice that reacted with Mtb proteins.
Key Words: EspB, EspC, ESX-1, Mycobacterium tuberculosis
Introduction
Tuberculosis (TB) ranks alongside HIV/AIDS as a major cause of mortality by infectious diseases worldwide (1). As much as one-third of the world’s population is latently infected with Mycobacterium tuberculosis (Mtb), the causative agent of TB (2). Furthermore, the situation has been complicated with the advent of Mtb/HIV co-infection and the emergence of drug resistance against these infections (2). Bacille Calmette-Guérin (BCG) is an attenuated strain of Mycobacterium bovis and is the only available vaccine against TB; however, it displays highly variable efficacy against adult pulmonary TB (3). Strategies to improve TB vaccines include the development of subunit and live-attenuated vaccines (2, 4). These strategies would include administration of BCG or recombinant BCG for priming, followed by a booster inoculation with a subunit vaccine containing DNA or proteins/peptides (4-6). Rather than be used as a replacement for BCG, the subunit vaccines are currently being evaluated as the potential boosters (2, 4). The subunit TB vaccines tested to date are made of culture filtrates, single proteins, protein mixtures and fusion proteins (7).
The proteins secreted by virulent Mycobacteria species are found to play an important role in the modulation of the host immune responses (8, 9). Mycobacteria contain specific secretion systems known as ESX systems (8, 10). The proteins encoded by the region of difference 1 (RD1) gene are involved in the ESX-1is system and are absent in all vaccine strains of BCG (8, 11). The production of immunogenic proteins contained in Mtb culture filtrate, namely ESAT-6 and CFP-10, are mediated by the ESX-1 system (12). Furthermore, there are two additional ESX-1 substrates known as EspB and EspC. These proteins are encoded by genes located outside of the RD-1 region; however, they require ESX-1 components for their production. Interestingly, the substrates of the ESX-1 system are mutually dependent upon each other for their production (8, 13). EspB, encoded by Rv3881c gene, is a 61- kDa protein that after secretion, through ESX-1, becomes cleaved to produce a 51-kDa protein that plays an important role in Mtb virulence (14). Previously, it has been shown that EspB is required for cytolysis, bacterial spreading, and ESAT-6 production (15, 16). EspC (11 kDa) is encoded by Rv3615c gene of Mtb H37Rv, and similar to EspB, is involved in Mtb virulence and can be recognized by T cells isolated from TB-infected individuals (11, 17).
There are many technical problems which hamper DNA amplification, recombinant expression, and purification of mycobacterial proteins expressed in Escherichia coli (E. coli). Conversely, it is necessary to have access to full-length Mtb-specific proteins to study their immunoreactivity in vivo and to improve the diagnostic testing (11, 18).
Considering that fusion proteins contain multiple epitopes, and ensure broad coverage of a genetically heterogeneous population, and the production of one recombinant fusion protein would be more cost-effective than producing two separate proteins (19), we used synthetically-prepared gene fragments that were codon-optimized for E. coli to be used for production of single and fusion EspB, EspC, and EspC/EspB recombinant proteins. The immunoreactivities of these recombinant proteins were then assessed in vivo, and the resultant antisera after the infection was analyzed via Western blotting.
Materials and methods
Gene synthesis and cloning
The nucleotide sequences encoding EspB and EspC were selected from the Mtb genome (Mtb H37Rv, complete genome; NCBI nucleotide accession # NC_000962; BioProject PRJNA57777; Assembly: GCF_000195955.2), based on their corresponding protein sequences found using NCBI protein accession numbers NP_218398 and NP_218132, respectively. A 1722-bp DNA fragment containing genes that encode EspC and EspB proteins, which are separated by 9 nucleotides, were drafted and codon-optimized for expression in E. coli and synthesized by a service provider (Biomatic, Canada). Using primers which incorporated the appropriate restriction sites (Table 1) and the synthesized fragment as a template, amplicons encoding EspC (flanked by BamHI and HindIII restriction sites) or EspB alone (flanked by HindIII and XhoI restriction sites) were prepared and TA-cloned by ligation into pTZ57R/T cloning vectors (InsTAclone ™ PCR Cloning Kit, Thermo Scientific, USA). Following colony-touch PCR screening using the above-mentioned primer sets, plasmids were prepared from positive clones using PrimePrep™ Plasmid DNA Isolation Kit (GeNet Bio, S. Korea). The integrities of the extracted plasmids were verified by restriction digestion analysis.
Table 1.
PCR primers. The underlined segments depict the incorporated restriction sites. The bold nucleotides in EspBF were added.to correct the ORF.
| Primer | Restriction site | Sequence (5’-3’) |
|---|---|---|
| EspBfuF | HindIII | GTAAGCTTACCCAGTCTCAGACCG |
| EspBF | HindIII | GTAAGCTTGTACCCAGTCTCAGACC |
| EspBR | XhoI | GACTCGAGTTTAGATTCTTTAGAGTCC |
| EspCF | BamHI | GTGGATCCACCGAAAACCTGAC |
| EspCR | HindIII | GTAAGCTTGGTGAACAGACCGTC |
The confirmed TA-cloned plasmids were double-digested by their corresponding flanking restriction enzymes and the inserts were purified using a Gel Purification Kit (GeNet Bio), and cloned in-frame into appropriately double-digested pET21a expression vectors (Novagen, USA) to produce recombinant-EspC (rEspC), and -EspB (rEspB) proteins. The recombinant plasmids were subsequently transformed into E. coli BL21 (DE3) and plated on LB agar containing 50 μg/ml ampicillin.
To construct the recombinant EspC/EspB fusion sequence, an amplicon encoding EspB-fu was prepared using EspBfuF and EspBR primers and ligated to a pTZ57R/T cloning vector (pTZ-B). Then, rEspC (originating from a pET21a vector) and pTZ-B plasmids were digested by the restriction enzymes, HindIII and XhoI, and the purified fragments (i.e. EspB-fu insert) and the rEspC plasmids were purified using the Gel Purification Kit and PrimePrep™ PCR Purification Kit (GeNet Bio), respectively and ligated by DNA T4 ligase (Vivantis, Malaysia). After screening via colony-touch PCR, the absence of mutations and the presence of a correct open reading frame (ORF) in the recombinant construct was confirmed by restriction digestion analysis and DNA sequencing (Gen Fanavaran Co. Iran).
Expression and purification of the recombinant proteins
To express the recombinant proteins, single colonies from each construct were grown (37 °C, 200 RPM) in LB broth containing 50 µg/ml ampicillin. The cultures were induced with isopropyl β-D-1-thiogalactopyranoside (IPTG) at concentrations of 0.5, 1, 1.5 and 2 mM when an A600 of ∼ 0.5 was reached. To determine an optimal time, the induced cells were incubated for 1, 2, 3 or 4 h. Protein purification was achieved using Ni-NTA resin (The QIAexpressionist™, Qiagen, Germany) and performed according to the manufacturer’s instructions under denaturing conditions with 8 M urea. The proteins were dialyzed overnight against several PBS changes and the concentrations were subsequently quantified using a Bradford assay (20).
SDS-PAGE and Western blot analyses of the recombinant proteins
The production and purification of each recombinant protein was confirmed by SDS-PAGE and Western blotting. The proteins were separated with 12% SDS-PAGE, according to Laemmli (21) and transferred onto PVDF membranes (Millipore, USA) in a Semi-Dry Transfer Cell (Bio-Rad Laboratories Inc., USA) at 90 V for 1 h in Tris-Glycine buffer with 10% methanol. The membranes were blocked overnight with PBS containing 1%BSA at 4 °C. Monoclonal anti-His antibodies (Abcam, UK) were added to the membranes and incubated for 1 h at room temperature (RT). Horseradish peroxidase-labeled goat anti-mouse IgG antibodies (Sigma, Germany) were used as the secondary antibody. The membranes were washed and the bands were visualized with DAB (3, 3’-Diaminobenzidine).
To confirm that the antibodies raised against the recombinant proteins reacted with the bacterial epitopes, antisera were collected and their reaction to Mtb proteins was analyzed using Western blotting. Mtb was provided by Tuberculosis and Pulmonary Department of Pasteur Institute of Iran. Pelleted Mtb cells were lysed by ultra-sonication with three 40 s-pulses at maximum speed and incubations on ice. They were subsequently centrifuged at 3000 xg for 2 min at 4 °C to remove the unbroken cells. The resulting supernatant underwent SDS-PAGE and Western blotting as described above.
Production and precipitation of dextran
Six female BALB/c (6-8-week-old) mice were purchased from Pasteur Institute of Iran (Production Complex, Karaj, Iran). The protocols for all animal experiments were approved by the Animal Ethics Committee of Pasteur Institute of Iran. Mice were immunized with rEspC, rEspB, and rEspC/EspB proteins (15 µg/ml) using Quil A® (InvivoGen, USA) as an adjuvant. All mice were injected subcutaneously with 3 doses on day 0, 15 and 30. Serum samples were extracted from all mice 15 days following the last immunization.
Results
Gene Cloning
EspC/EspB, EspC and EspB genes were cloned into pET-21a expression vectors and transformed into E. coli BL21. To verify successful cloning events, direct PCR amplifications (colony-touch PCR) of the EspC/EspB, EspC, and EspB genes were conducted using the recombinant bacteria as a template. The amplicons were subjected to 1% agarose gel electrophoresis which indicated the presence of the genes in their related constructs (Fig.1). The integrity of each recombinant expression vector was further tested by restriction enzyme digestion analysis. Expected fragments corresponding to ∼5400 bp for pET-21a, 1722 bp, 312 bp, and 1400 bp for EspC/EspB, EspC, and EspB, respectively, were obtained (Fig.2). The correct ORF insertion of all fragments in the vector, and the absence of alterations in the coding nucleotides were confirmed by nucleotide sequencing (data not shown).
Fig. 1.
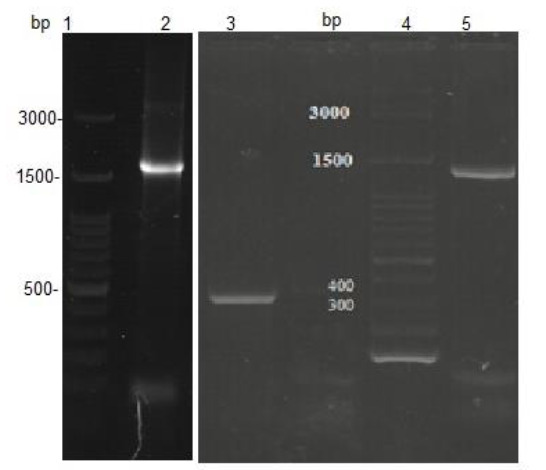
Agarose gel electrophoresis of the colony-touch PCR products. Lanes 1, 4: DNA 100 bp ladder; lane 2: EspC/EspB amplicon; lane 3: EspC amplicon; lane 5: EspB amplicon
Fig. 2.
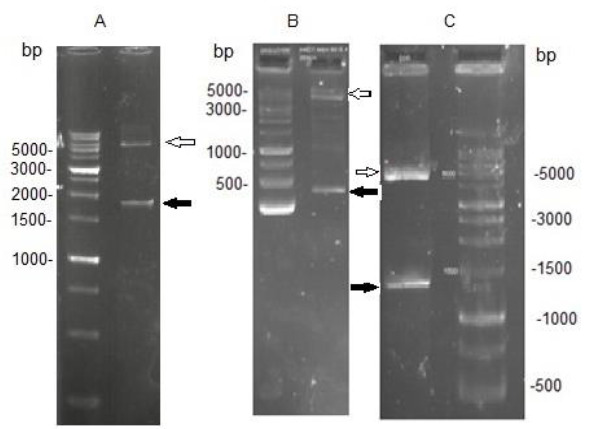
Double digestion by restriction enzymes confirmed correct cloning of EspC/EspB (A), EspC (B), and EspB (C) genes. White arrows indicate the vectors and the black arrows indicate the inserts.
Genes expressions
E. coli BL21 cells harboring pET-21a-EspC/EspB, pET-21a-rEspC, and pET-21a-EspB were used for IPTG-controlled expressions of the recombinant proteins. The bacteria were induced with different concentrations of IPTG at different time points. Induction with 0.5 mM of IPTG for 3 h was determined as the optimal condition for expression of the three proteins. The recombinant proteins were purified by Ni-NTA resin and their purities were verified by SDS-PAGE. A single band with an approximate Mw of 69 kDa for rEspC/EspB, 13 kDa for rEspC, and 61 kDa for rEspB were indicatives of obtaining pure recombinant proteins. The expressions of the recombinant proteins were further verified by Western blotting (Fig.3).
Fig. 3.
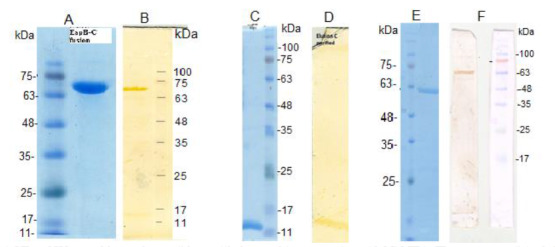
SDS-PAGE and Western blot analyses of the purified recombinant proteins with Ni-NTA. The presence of single bands in SDS-PAGE were indicatives of purified rEspC/EspB fusion protein (A), rEspC (C), and rEspB (E) proteins. Western blotting with anti-His antibodies confirmed the total expressions of the above-mentioned recombinant proteins (B, D, F), respectively.
The conservation of epitopes on the recombinant proteins was verified by the binding of anti-EspC/B,-EspC, and -EspB antisera to soluble Mtb proteins in an immunoblot analysis. In Fig. 4, the antisera reacted with two proteins that had a Mw of ∼ 61 and ∼13 kDa, corresponding to Mtb natural EspB and EspC proteins, respectively. These results indicate that the recombinant proteins could induce the production of antibodies capable of interacting with EspC and EspB proteins in their natural forms.
Fig. 4.
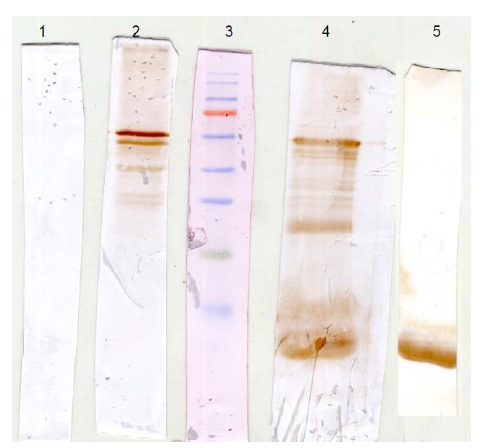
Verification of identical epitopes between rEspC/B, rEspC, and rEspB and natural EspC, and EspB. The supernatant of a whole-cell lysate of Mtb was examined by an immunoblot analysis using normal mouse serum (1), anti- rEspC/B antiserum (2), anti- rEspB antiserum (4), and anti-rEspC antiserum (5), along with a pre-stained Mw marker (3).
Discussion
TB poses a serious challenge to public health across the world. The disease has been successfully controlled by the BCG vaccine during the 20th century; however, the variable efficacies of this vaccine calls for the development of alternate prophylactics to further reduce morbidity and mortality rates of the disease (22, 23). Replacing the BCG vaccine is challenging, yet desirable due to its drawbacks such as increased safety concerns (especially when administered to immunocompromised individuals), the inability to induce a long-term T-cell memory response and most importantly, its variable efficacy in TB-endemic regions (3). Currently, the most promising prophylactic strategy against TB are subunit vaccines, since they are likely to remain unaffected by exposure to environmental mycobacteria and can be used as boosters for the BCG (24).
The observation that only live but not dead bacilli elicit substantial protection against TB indicates that the secreted proteins and active export of antigenic proteins are critical to maximizing protective immune responses (16, 19). The ESX-1 secretion system, which is involved in the virulence, is a mycobacteria-specific secretion system that is absent in BCG. Therefore, the proteins involved in this system could be considered as Mtb specific antigens and would not be affected by previous BCG vaccinations. Several substrates, components, and regulatory molecules are involved in the ESX-1 secretory system. It has been suggested that ESX-1 and its secreted proteins are involved in the escape of Mtb from the phagosomal compartments and modulation of the immune responses. Two well-known secretory proteins of this system, ESAT-6 and CFP-10, are needed for full virulence of Mtb and have been widely used in the diagnosis of Mtb infection. EspC and EspB are other secretion substrates of the ESX-1 system which have been less studied. EspB is involved with the maintenance of intracellular ESAT6 levels and the secretion of ESAT6 and CFP10. This protein plays a role in preventing phagosome/lysosome fusion as well as intracellular growth of Mtb in macrophages (25, 26). The EspC gene is encoded outside of the RD1 locus; however, its secretion is dependent on the RD1 encoded Esx-1 system (27). This protein is highly immunodominant in humans and mice (11, 17, 28). The important roles of EspC and EspB in TB pathogenesis and the absence of these proteins in BCG, make them promising candidates for the vaccines and the diagnostic tools. Moreover, since an effective subunit vaccine comprising multiple epitopes ensures broad coverage of a genetically heterogeneous population, a fusion protein composed of both EspC and EspB was designed (29).
The present study aimed to clone and express EspC, EspB and the fusion EspC/EspB proteins in E. coli and to evaluate their immunogenicity in vivo. The proteins were successfully expressed in the host and could be identified by an anti-His antibody. However, the SDS-PAGE displayed heavier Mws than their predicted sizes. This is not unusual since several other investigations have also reported similar findings and linked this result to a high content of acidic amino acids and/or proline in the protein (30-32). The Mws of rEspc and rEspB, as predicted based on their amino acid composition https://web.expasy.org/protparam/>, were approximately 13 and 51 kDa, respectively. Both EspC and EspB are acidic proteins with a predicted isoelectric point (pI) of 6.1 and 5.02, respectively, whereas rEspB contains ∼ 13% acidic residues (Asp + Glu). This discrepancy could also be attributed to the high percentage of proline (∼ 7.1%). The high percentage of proline results in increased rigidity of the molecules and, therefore, a higher Mw as determined by SDS- PAGE. Due to a lower pI and percentage of proline (∼ 1.6 %) compared to rEspB, the Mw of EspC was detected as the expected value. The calculated Mw and the pI of the fusion protein were ∼ 61 and ∼ 5.1, respectively. However, the determined Mw by SDS-PAGE was approximately 69 kDa. This could also be explained by a high percentage of acidic residues and proline (10.2 % and 6.3 %; respectively) in the EspC/EspB fusion protein.
We immunized BALB/c mice with the recombinant proteins to obtain antisera. All of the recombinant proteins were immunogenic and induced antibody responses. Moreover, our data indicated that Mtb proteins were recognized by anti-EspC, -EspB and the fusion protein -EspC/EspB IgG. These findings suggest the conservation of several epitopes of the native proteins found in Mtb which could also be detected by our recombinant proteins.
In conclusion, the results of this study indicate that EspC, EspB, and the fusion of EspC and EspB proteins can be expressed in E. coli. These recombinant proteins were immunogenic and the antibodies raised against them were able to recognize Mtb proteins.
Acknowledgements
This project was funded and performed at Pasteur Institute of Iran. Authors declare no conflicts of interest.
References
- 1.Geldmacher C, Ngwenyama N, Schuetz A, Petrovas C, Reither K, Heeregrave EJ, et al. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med medicine. 2010;207(13):2869–81. doi: 10.1084/jem.20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen P, Kaufmann SH. Novel vaccination strategies against tuberculosis. Cold Spring Harb Perspect Med. 2014;4(6) doi: 10.1101/cshperspect.a018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewer TF. Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin Infect Dis. 2000;31 Suppl 3:S64–7. doi: 10.1086/314072. [DOI] [PubMed] [Google Scholar]
- 4.Skeiky YA, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol. . 2006;4(6):469–76. doi: 10.1038/nrmicro1419. [DOI] [PubMed] [Google Scholar]
- 5.Ahn SK, Tran V, Leung A, Ng M, Li M, Liu J. Recombinant BCG Overexpressing phoP-phoR Confers Enhanced Protection against Tuberculosis. . Mol Ther. 2018;26(12):2863–2874. doi: 10.1016/j.ymthe.2018.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufmann SH. Fact and fiction in tuberculosis vaccine research: 10 years later. Lancet Infect Dis. 2011;11(8):633–40. doi: 10.1016/S1473-3099(11)70146-3. [DOI] [PubMed] [Google Scholar]
- 7.Barker LF, Brennan MJ, Rosenstein PK, Sadoff JC. Tuberculosis vaccine research: the impact of immunology. Curr Opin Immunol. 2009;21(3):331–8. doi: 10.1016/j.coi.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Forrellad MA, Klepp LI, Gioffre A, Sabio y, Garcia J, Morbidoni HR, de la Paz Santangelo M, et al. Virulence factors of the Mycobacterium tuberculosis complex. Virulence. 2013;4(1):3–66. doi: 10.4161/viru.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Champion PA, Stanley SA, Champion MM, Brown EJ, Cox JS. C-terminal signal sequence promotes virulence factor secretion in Mycobacterium tuberculosis. Science. 2006;313(5793):1632–6. doi: 10.1126/science.1131167. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann SH, Weiner J, von Reyn CF. Novel approaches to tuberculosis vaccine development. Int J Infect Dis. 2017;56:263–267. doi: 10.1016/j.ijid.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Millington KA, Fortune SM, Low J, Garces A, Hingley-Wilson SM, Wickremasinghe M, et al. Rv3615c is a highly immunodominant RD1 (Region of Difference 1)-dependent secreted antigen specific for Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 2011;108(14):5730–5. doi: 10.1073/pnas.1015153108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raghavan S, Manzanillo P, Chan K, Dovey C, Cox JS. Secreted transcription factor controls Mycobacterium tuberculosis virulence. Nature. 2008;454(7205):717–21. doi: 10.1038/nature07219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortune S, Jaeger A, Sarracino D, Chase M, Sassetti C, Sherman D, et al. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc Natl Acad Sci U S A. 2005;102(30):10676–81. doi: 10.1073/pnas.0504922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin B, Chon JS, MacGurn JA, Carlsson F, Cheng TL, Cox JS, et al. A mycobacterium ESX-1-secreted virulence factor with unique requirements for export. . PLoS Pathog. 2007;3(8):e105. doi: 10.1371/journal.ppat.0030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korotkova N, Piton J, Wagner JM, Boy-Rottger S, Japaridze A, Evans TJ, et al. Structure of EspB, a secreted substrate of the ESX-1 secretion system of Mycobacterium tuberculosis. . J Struct Biol. 2015;191(2):236–44. doi: 10.1016/j.jsb.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong KW . The Role of ESX-1 in Mycobacterium tuberculosis Pathogenesis. Microbiol Spectr. 2017;5(3) doi: 10.1128/microbiolspec.TBTB2-0001-2015. [DOI] [PubMed] [Google Scholar]
- 17.Tan S, Lin N, Huang M, Wang Q, Tan Y, Li B, et al. CTL immunogenicity of Rv3615c antigen and diagnostic performances of an ESAT-6/CFP-10/Rv3615c antigen cocktail for Mycobacterium tuberculosis infection. . Tuberculosis (Edinb). 2017;107:5–12. doi: 10.1016/j.tube.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Pathak A, Goyal R, Sinha A, Sarkar D. Domain structure of virulence-associated response regulator PhoP of Mycobacterium tuberculosis: role of the linker region in regulator-promoter interaction(s). . J Biol Chem. 2010;285(45):34309–18. doi: 10.1074/jbc.M110.135822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang ZH, Sun RF, Lin C, Chen FZ, Mai JT, Liu YX, et al. Immunogenicity and Protective Efficacy of a Fusion Protein Tuberculosis Vaccine Combining Five Esx Family Proteins. . Front Cell Infect Microbiol. 2017;7:226. doi: 10.3389/fcimb.2017.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 ;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Gowthaman U, Mushtaq K, Tan AC, Rai PK, Jackson DC, Agrewala JN. Challenges and solutions for a rational vaccine design for TB-endemic regions. Crit Rev Microbiol. 2015;41(3):389–98. doi: 10.3109/1040841X.2013.859125. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann SH, Hussey G, Lambert PH. New vaccines for tuberculosis. Lancet (London, England). . 2010;375(9731):2110–9. doi: 10.1016/S0140-6736(10)60393-5. [DOI] [PubMed] [Google Scholar]
- 24.Brandt L, Feino Cunha J, Weinreich Olsen A, Chilima B, Hirsch P, Appelberg R. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. . Infection and immunity. 2002 ;70(2):672–8. doi: 10.1128/iai.70.2.672-678.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen JM, Zhang M, Rybniker J, Boy-Rottger S, Dhar N, Pojer F, et al. Mycobacterium tuberculosis EspB binds phospholipids and mediates EsxA-independent virulence. Mol Microbiol. 2013;89(6):1154–66. doi: 10.1111/mmi.12336. [DOI] [PubMed] [Google Scholar]
- 26.Huang D, Bao L. Mycobacterium tuberculosis EspB protein suppresses interferon-gamma-induced autophagy in murine macrophages. J Microbiol Immunol Infect. 2016;49(6):859–865. doi: 10.1016/j.jmii.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. . J Bacteriol. 1996;178(5):1274–82. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Su Z, Zhang X, Hu C, Yu J, Gao Q, et al. Generation of Mycobacterium tuberculosis-specific recombinant antigens and evaluation of the clinical value of antibody detection for serological diagnosis of pulmonary tuberculosis. Int J Mol Med. 2013 ;31(3):751–7. doi: 10.3892/ijmm.2013.1254. [DOI] [PubMed] [Google Scholar]
- 29.Davila J, McNamara LA, Yang Z. Comparison of the predicted population coverage of tuberculosis vaccine candidates Ag85B-ESAT-6, Ag85B-TB10.4, and Mtb72f via a bioinformatics approach. . PloS one. 2012;7(7):e40882. doi: 10.1371/journal.pone.0040882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proft T, Hilbert H, Layh-Schmitt G, Herrmann R. The proline-rich P65 protein of Mycoplasma pneumoniae is a component of the Triton X-100-insoluble fraction and exhibits size polymorphism in the strains M129 and FH. . J Bacteriol. 1995;177(12):3370–8. doi: 10.1128/jb.177.12.3370-3378.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poetsch A, Molday LL, Molday RS. The cGMP-gated channel and related glutamic acid-rich proteins interact with peripherin-2 at the rim region of rod photoreceptor disc membranes. J Biol Chem. . 2001;276(51):48009–16. doi: 10.1074/jbc.M108941200. [DOI] [PubMed] [Google Scholar]
- 32.Korschen HG, Beyermann M, Muller F, Heck M, Vantler M, Koch KW. Interaction of glutamic-acid-rich proteins with the cGMP signalling pathway in rod photoreceptors. . Nature. . 1999;400(6746):761–6. doi: 10.1038/23468. [DOI] [PubMed] [Google Scholar]


