Abstract
Background:
The current study aims to investigate the relationship of miR-24 expression with plasma methotrexate (MTX) levels, therapy-related toxicities, and event-free survival (EFS) in Iranian pediatric acute lymphoblastic leukemia (ALL) patients.
Methods:
The study included 74 ALL patients in consolidation phase and 41 healthy children. RNA was extracted from plasma, polyadenylated, and reverse transcribed. miR-24 expression was determined by quantitative polymerase chain reaction (qPCR). Plasma MTX concentrations were measured by high performance liquid chromatography (HPLC) 48 h after high-dose methotrexate (HD-MTX) injection. The diagnosis of ALL was further subclassified as B-ALL or T-ALL via flow cytometry.
Results
miR-24 expression was less in pediatric ALL patients than in the control group (p = 0.0038). Furthermore, downregulation of miR-24 was correlated with intermediate- to high-grade HD-MTX therapy toxicities (p = 0.025). Nevertheless, no statistically significant associations were seen between miR-24 levels and plasma MTX levels 48 h after HD-MTX administration (p > 0.05) or EFS in pediatric ALL patients (p > 0.05).
Conclusion:
miR-24 expression may contribute to interindividual variability in response to intermediate- to highgrade HD-MTX therapy toxicities under Berlin Frankfurt Munster (BFM) treatment.
Key Words: Acute Lymphoblastic Leukemia, Event Free Survival, Methotrexate, Mir, Toxicity
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy in children, representing almost 75% of leukemic cases in patients < 15 years old. The disease is characterized by high remission (up to 95%) and survival rates (8090%); nevertheless, clinical concerns remain. Most of different protocols for the treatment of ALL have three phases: induction, consolidation and maintenance. Many cases of resistance to treatment and disease relapse occur in maintenance and consolidation therapies but rarely occur during induction therapy. Some patients experience adverse outcomes during chemotherapy; in particular, highdose methotrexate (HD-MTX) toxicity is a major challenge (1, 2). High MTX, (doses > 1000 mg/m2) are intravenously administered in the consolidation phase of ALL treatment. Some patients do not respond appropriately HD-MTX therapy; some develop therapy-related toxicities, interrupting and delaying treatment. Identifying these patients in advance is necessary for effective treatment (2, 3).
MicroRNAs (miRNAs) are short non-coding RNA molecules that play a role in many cellular processes, from proliferation and growth to differentiation and apoptosis. The biological roles of most microRNAs are not yet fully understood. MicroRNAs are implicated in regulating approximately 30% of human genes by targeting sequences in the genes’ 3' untranslated regions (UTRs). The 3' UTR targeting of mRNAs can result in decreased levels of corresponding transcripts. Research has shown that unique miRNA expression patterns are associated with the cytogenetic and clinical outcome of leukemia types, such as chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML) (4-10).
MiR-24, a ubiquitously expressed miRNA, participates in erythropoiesis (11), is involved in apoptosis and proliferation regulation, and contributes to hematopoietic stem cell (HSC) survival (12, 13). Increased miR-24 expression has been observed in adult AML with t(8;21) (14), Hodgkin lymphoma (HL) (15), breast cancer (16), and pediatric acute leukemia (17). One recent study showed that miR-24 overexpression is associated with shorter prognosis in acute leukemia. In contrast, decreased miR-24 expression was reported in ALL in several studies (17-18).
It has been shown that plasma miR-24, miR-320, and miR-420 are potential biomarkers for colorectal carcinoma (CRC), especially in the early stage (19). Mishra et al. reported that the 829 C>T polymorphism results in DHFR overexpression and MTX resistance. This polymorphism is located near the miR-24 binding site in the 3' UTR of the human dihydrofolate reductase (DHFR) gene (20).
However, the role of miR-24 in HD-MTX pharmacokinetics, therapy-related toxicities, and outcomes in pediatric ALL is not yet fully understood. Here, we explored the association between miR-24 expression and plasma MTX levels, therapy-related toxicities, and pediatric ALL patient outcomes.
Materials and Methods
This collaborative study was performed at the Iran University of Medical Sciences (IUMS) and Mahak Hospital, both of Tehran, Iran, between September 2015 and February 2019. Seventy-four ALL patients and 41 children with no hematologic or malignant disorders as controls were included in this study. Informed consent forms were signed by study subjects’ parents.
Peripheral blood specimens were collected from all study subjects. The ALL diagnoses were performed according to World Health Organization (WHO) 2016 criteria based on morphological findings and immunophenotyping. The diagnoses were further subclassified as B-cell ALL (CD22+, CD19+, CD20+, CytoCD79α, HLA-DR+, CD38, CD10+) or T-cell ALL (CD3+, CD7+, CD1a, CD2+, CD4, CD5+, CD8, CytoCD3+) (Becton Dickinson, CA, USA). The Cellquest program was used to analyze the results. The eligible criteria were age ≤ 16-years, complete cytomorphologic remission, and absence of other active malignancies.
The initial treatment was performed according to the Berlin Frankfurt Munster (BFM) 2009 protocol. In this protocol, the consolidation regime consisted of administering cyclophosphamide, mercaptopurine, HD-MTX (2000-4000 mg/m2/day), cytarabin, and leucovorin. All patients received four courses of HDMTX every two weeks during the chemotherapy consolidation phase. HD-MTX is used for central nervous system (CNS) prophylaxis in ALL patients. Doses were 2000 mg/m2/day for low-risk patients and, 4000 mg/m2/day for high-risk and T-cell ALL patients. Plasma MTX levels were measured by high performance liquid chromatography (HPLC) (Knaure, Berlin, Germany).
The plasma MTX levels at 48 h (with normal level < 1 µmol/L) were independent of the treatment protocol and patient ages. Changes in MTX levels were seen only after 48 h. Based on this result and previous studies, the plasma MTX levels were measured 48 h after the start of the first HD-MTX infusion. Leucovorin at 15 mg/m2 was administered 42 h after the start of the HDMTX injection (2, 21, 22).
The toxic effects of HD-MTX on hematopoietic and liver tissue were investigated.Therapy-related toxicities were graded according to the Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0) (23). miR-24 expression was measured 48 h after the HD-MTX injection. miR-24 was measured during the last course of intravenous HD-MTX injection in the consolidation phase. The highest toxicity grade was measured in each patient during the consolidation phase. HD-MTX toxicities were reported according to a six-step scoring system (0-V).
Total RNA was isolated from peripheral blood using RNX-Plus BD (SinaClon Corporation, Tehran, Iran) according to the manufacturer’s protocol. The total RNA concentration and purity were determined spectrophotometrically at 260 and 280 nm. RNA integrity was visually confirmed by agarose gel electrophoresis. Then, 2.0 µg of total RNA was polyadenylated in a 20 µl reaction containing 1 µl of rATP (10 mM), 0.2 µl of Escherichia coli poly A polymerase, 2 µl of poly-A polymerase buffer, and RNase-free water (BONmiR Detection kits, Tehran, Iran) at 37 °C for 30 min. The reaction was then incubated at 65 °C for 20 min to inactivate the enzyme.
The polyadenylated RNA was reverse transcribed at 25 °C for 10 min and then at 45 °C for 60 min in a 20 µl reaction containing 0.7 µl of reverse transcriptase (RT) enzyme, 0.2 µl of dNTP mix (100 mM), and 4 µl of RT buffer (BONmiR Detection kits, Tehran, Iran). This reaction was then incubated at 70 °C for 10 min to inactivate the enzyme.
miR-24 was quantified using a SYBR-Green fluorescent-based quantitative polymerase chain reaction (qPCR) assay with gene-specific forward and universal reverse primers (BONmiR Detection kits, Tehran, Iran).
The qPCR was performed in a LightCycler® 96 Real-Time PCR System (Roche, Germany). The 13 µl reaction mixture contained 1 µl of cDNA (25 ng), 6.5 µl of 2X miRNA qPCR master mix, 1 µl each of miRNAspecific forward and universal reverse primers, and nuclease-free PCR-grade H2O. All samples were amplified in duplicate. The thermal cycling conditions consisted of a 2 min polymerase activation step at 95 °C and 40 cycles of denaturation at 95 °C for 10 sec and primer annealing and extension at 60 °C for 30 sec. The reaction specificity was confirmed by melting curve analysis. The 2−ΔΔCT method was applied for the relative quantification of miR-24 levels, using U6 small nuclear RNA (snRNA) as the reference control for normalization (BONmiR Detection kits, Tehran, Iran).
Statistical analysis
miRNA level differences between leukemic and healthy individuals were determined using the nonparametric Mann-Whitney U test. Receiver operating characteristics (ROCs) were analyzed to evaluate the discriminatory ability of miR-24 for pediatric ALL. The association between miR-24 level differences of triple groups were evaluated by non-parametric Kruskal– Wallis test. Event-free survival probabilities were determined by Kaplan-Meier survival curves using the log-rank test. All statistical tests were two-sided. P values < 0.05 were considered statistically significant. SPSS software (version 25.0) and Graph Pad Prism software (version 8.0.1) were used to analyze statistics.
Results
Of the 74 1-16-year-old ALL patients, 46 (62.2%) were boys and 28 (37.8%) were girls (Table 1). Their mean and median ages were 6.5 ± 4.5 and 5, respectively. M2M3 response (≥ 5% blasts) was seen in 14 (18.9%) patients. Most of the patients were B-ALL (74.3%). Each patient in this study received HD-MTX. Twentynine patients (39.2%) had plasma MTX levels > 1 µmol/L after 48 h. More patients had hematopoietic than had hepatic toxicity (58.1% vs. 35.1%). No patient developed Grade V toxicity.
Table 1.
Clinicopathological characteristics and MTX toxicities experienced in the 74 ALL patients.
| Variables | N (%) |
|---|---|
| Age | |
| 1-9 years | 53 (71.6) |
| <1 or ≥10 years | 21 (28.4) |
| Gender | |
| Male | 46 (62.2) |
| Female | 28 (37.8) |
| WBC count | |
| < 50000 | 58 (78.4) |
| ≥50000 | 16 (21.6) |
| Immunophenotype | |
| B-lineage | 55 (74.3) |
| T-lineage | 19 (25.7) |
| BM on day 15 | |
| <5% blasts | 60 (81.1) |
| ≥5% blasts | 14 (18.9) |
| High-dose MTX | |
| 4 g/m2/day | 26 (35.1) |
| 2 g/m2/day | 48 (64.9) |
| MTX plasma levels at 48 h | |
| > 1 µmol/L | 29 (39.2) |
| ≤ 1 µmol/L | 45 (60.8) |
| Toxicity | |
| Hematopoietic | |
| - Grade 0 | 31 (41.9) |
| - Grade I-IV | 43 (58.1) |
| Hepatic | |
| - Grade 0 | 48 (64.9) |
| - Grade I-IV | 26 (35.1) |
| Interventions indicated | |
| With | 34 (45.9) |
| Without | 40 (54.1) |
| Status | |
| With event | 22 (29.7) |
| Without event | 52 (70.3) |
ALL: Acute lymphoblastic leukemia, BM: bone marrow, MTX: Methotrexate, SD: Standard deviation, WBC: white blood cell
Of the 41 1-16-year-old healthy controls, 24 (58.53%) were male and 17 (41.47%) were female. Their mean and median ages were 9.6 ± 4.1 and 10 years, respectively. Their leukocyte count was normal (4.1-10.3×103 leukocytes/mm3).
We examined miR-24 levels in all the subjects. The miR-24 levels were significantly less in the ALL patients than in the healthy controls (p = 0.0038, Fig. 1A). The ability of miR-24 to discriminate between the ALL patients and the healthy controls was demonstrated by ROC analysis (AUC: 0.662; 95% CI 0.550–0.775; p = 0.004; Fig. 1B) and univariate logistic regression analysis (odds ratio (OR) = 0.374; 95% CI: 0.169-0.827; p = 0.009). Furthermore, multivariate logistic regression analysis demonstrated the reduced expression of miR-24 (OR = 0.430; 95% CI: 0.1900.972; p = 0.039) could differentiate between leukemic and normal specimens independently of patients’ ages and genders.
Fig. 1.
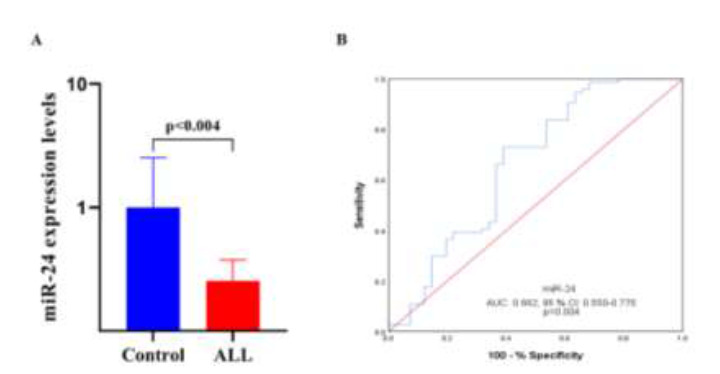
miR-24 expression in healthy controls (n = 41) and pediatric ALL patients (n = 74). Peripheral blood was obtained from all study subjects and total RNA was extracted from plasma. The RNA was polyadenylated and reverse transcribed, and miR-24 expression was determined by quantitative polymerase chain reaction (qPCR). (A) miR-24 expression healthy controls and pediatric ALL patients. (B) ROC curve analysis of miR-24 expression to discriminate between pediatric ALL patients and healthy controls. AUC: area under the curve, CI: confidence interval.
These findings suggest that downregulation of miR24 expression may play a role in the pathogenesis and therapy response of pediatric ALL patients.
To explore the effect of miR-24 levels on pharmacokinetics, the relationship between miR-24 expression and plasma MTX levels at 48 h were assessed. Patients were divided into two groups; those with plasma MTX levels ≤ 1 µmol/L (the not increased group), and those with plasma MTX levels > 1 µmol/L (the increased group). No statistically significant difference in miR-24 expression was found between the two groups (p = 0.998); however, miR-24 expression was significantly less in both MTX groups than in the controls (p = 0.004 for the not increased, and 0.015 for the increased, groups, Fig. 2A).
Fig. 2.
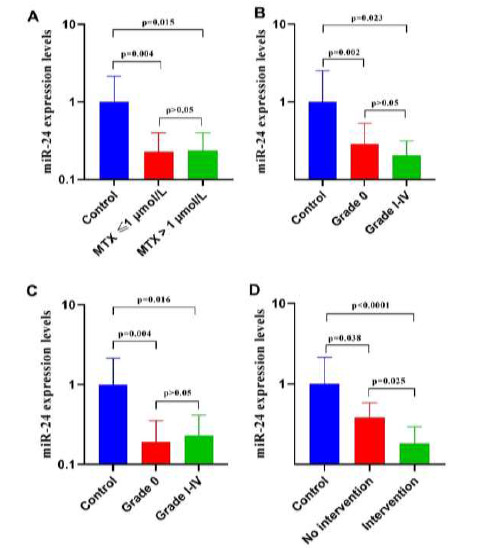
miR-24 expression in experimental and control groups. Peripheral blood was obtained from all study subjects and total RNA was extracted from plasma. The RNA was polyadenylated and reverse transcribed, and miR-24 expression was determined by quantitative polymerase chain reaction (qPCR). miR-24 expression from the groups described was compared. (A) Not increased plasma MTX levels (45 patients), increased plasma MTX levels (29 patients), and control groups (41 healthy controls); (B) With hematologic toxicity (43 patients), without hematologic toxicity (31 patients), and control groups (41 healthy controls); (C) With hepatic toxicity (26 patients), without hepatic toxicity (48 patients), and control groups (41 healthy controls). (D) With intervention (34 patients), without intervention (40 patients), and control groups (41 healthy controls).
We also measured miR-24 levels in patients with grades 0-IV therapy-related toxicities. Patients were again divided into two groups; those without (grade 0), and those with (grades I-IV), therapy-related toxicities. No statistically significant difference in miR-24 expression was found between the two groups; however, miR-24 expression was significantly less in both groups than in controls (p = 0.002 for grade 0, and 0.023 for grade I-IV, groups, Fig. 2B)
In current study, miR-24 levels in patients with grades 0-IV hepatic toxicity were also measured. Patients were divided into two groups; those with no (grade 0), and those with (grades I-IV), hepatic toxicity. No significant difference in miR-24 expression was found between the two groups (p = 0.999); however, miR-24 expression was significantly less in both groups than in healthy controls (p = 0.004 for grade 0, and 0.016 for grade 1-IV, groups, Fig. 2C).
We also investigated whether interventions indicated affected miR-24 expression. ˝Interventions indicated˝ are defined as lowering the administered MTX dose, discontinuing MTX, or hospitalization for hematopoietic or hepatic toxicities. Patients were divided into two groups; those with no interventions indicated (Grades 0 and I), and those with interventions indicated (grades II-IV). miR expression was significantly less in the intervention than in the no intervention group (p = 0.025). miR-24 expression was also significantly less in both groups than in controls (p = 0.038 for the no intervention, and p < 0.0001 for the interventions indicated, groups, Fig. 2D). Overall, we conclude that reduced miR-24 expression is significantly associated with intermediate- to high-grade HD-MTX therapy toxicities.
To investigate the association between the miR-24 levels and EFS, patients were followed for 40 months. Initially, patients were divided in two groups; those with reduced, and those with increased, miR-24 expression. The median miR-24 value was used as the cut-off to divide the 74 ALL patients with ALL into the two groups (5). Those with miR-24 levels less than the cut-off value were included in the downregulation group (n = 37), and those with miR-24 levels greater than the cut-off value were included in the upregulation group (n = 37).
A Kaplan-Meier survival curve using the log-rank test found no statistically significant difference in survival rates between patients with low and high miR-24 expression (p = 0.628, Fig. 3A). Bone marrow response on day 15 was significantly correlated with decreased EFS in patients with pediatric ALL (p = 0.027, Fig. 3B). This result was confirmed by univariate Cox regression analysis (OR= 2.629; 95% CI: 1.070-6.464; p = 0.035).
Fig. 3.
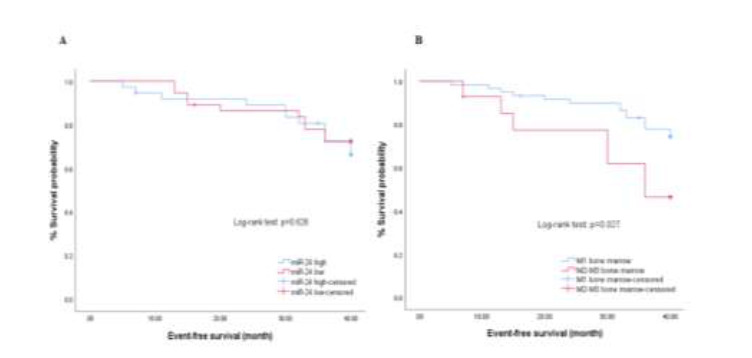
miR-24 expression levels in ALL patients (74 patients). (A) Kaplan – Meier survival curves for the EFS according to expression levels of miR-24 and (B) bone marrow response (day 15).
Discussion
Toxicities from HD-MTX therapy are a notable concern and are associated with poor prognosis (2). Despite the applied clinical considerations, patients often do not respond appropriately HD-MTX therapy, with some being intrinsically resistant (3). Therefore, the discovery of novel markers that help predict patient responses to HD-MTX therapy is critical. It has been demonstrated that microRNAs are valuable tumor markers and are often implicated in pathogenesis, response to therapy, prognosis, and outcomes (24, 25).
miR-24 has been reported to regulate normal erythropoiesis via targeting of human activin receptor type 1, ALK4 (11). Numerous studies have revealed roles for miR-24 in a variety of malignancies including AML, ALL, CLL, breast cancer, and Hodgkin lymphoma (HL) (17, 26, 27). As discussed, miR-24 expression varies depending on the cancer type.
miR-24 plays an important role in breast cancer progression (16) and is also associated with resistance to apoptosis (15) and resistance to a variety of chemotherapy drugs (20, 26, 27); for example, Han X. et al. reported that miR-24 overexpression led to tamoxifen resistance in breast cancer (26).
Consistent with our study, Mi et al. reported decreased miR-24 expression in pediatric ALL patients (18). Moreover, Organista-Nava. et al. reported lower miR-24 expression in pediatric ALL patients than in healthy controls or pediatric AML patients. Although, recent study showed ALL patients with high miR-24 expression had lower overall survival (OS) rates than those with low miR-24 (17). This finding was contrary to our results; however, in our study, all the pediatric ALL patients were in consolidation phase.
Another study found that miR-24 could cause MTX resistance (20). More recent studies indicated that miR-24 expression affects treatment outcomes and toxicity (15-17, 27). Numerous studies examined the role of miR-24 in cancers; however, none of these studies demonstrated a clear association between miR24 and MTX pharmacokinetics and experienced toxicities. Therefore, this study was designed to investigate the relationship of miR-24 expression with plasma MTX levels and therapy-related toxicities during HD-MTX therapy.
In brief, our data showed that miR-24 expression was lower in pediatric ALL patients than in healthy children. ROC curve and logistic regression analysis verified the ability of miR-24 to discriminate normal from leukemic peripheral blood specimens. In addition, low miR-24 expression was significantly associated with intermediate- to high-grade HD-MTX therapy toxicities. This finding demonstrated that miR24 is associated with numerous manifestations of HDMTX therapy. However, no significant correlation was found between miR-24 expression and plasma MTX levels or patient outcomes in pediatric ALL.
In conclusion, our results indicated the miR-24 expression is associated with HD-MTX therapy during the consolidation phase of pediatric ALL treatment. No significant association was found between miR-24 expression and plasma MTX levels or pediatric ALL patient outcomes. Our results indicate that miR-24 expression may contribute to interindividual variability in response to HD-MTX therapy; however, further investigations are required to clarify these findings.
Acknowledgements
This work was supported by the Iran University of Medical Sciences under Grant number 1395.9221534203. We gratefully thank those who help in this study in Mahak Hospital. All authors declare they have no conflicts of interest.
References
- 1.Piatopoulou D, Avgeris M, Drakaki I, Marmarinos A, Xagorari M, Baka M, et al. Clinical utility of miR143/miR-182 levels in prognosis and risk stratification specificity of BFM-treated childhood acute lymphoblastic leukemia. Ann Hematol. 2018;97(7):1169–82. doi: 10.1007/s00277-018-3292-y. [DOI] [PubMed] [Google Scholar]
- 2.Suthandiram S, Gan GG, Zain SM, Bee PC, Lian LH, Chang KM, et al. Effect of polymorphisms within methotrexate pathway genes on methotrexate toxicity and plasma levels in adults with hematological malignancies. Pharmacogenomics. 2014;15(11):1479–94. doi: 10.2217/pgs.14.97. [DOI] [PubMed] [Google Scholar]
- 3.Kodidela S, Suresh Chandra P, Dubashi B. Pharmacogenetics of methotrexate in acute lymphoblastic leukaemia: why still at the bench level? Eur J Clin Pharmacol. 2014;70(3):253–60. doi: 10.1007/s00228-013-1623-4. [DOI] [PubMed] [Google Scholar]
- 4.Piatopoulou D, Avgeris M, Marmarinos A, Xagorari M, Baka M, Doganis D, et al. miR-125b predicts childhood acute lymphoblastic leukaemia poor response to BFM chemotherapy treatment. Br J Cancer. 2017;117(6):801–12. doi: 10.1038/bjc.2017.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng Z, Chen X, Zhu D, Luo Z, Yang M. Low Expression of Circulating MicroRNA-34c is Associated with Poor Prognosis in Triple-Negative Breast Cancer. Yonsei Med J. 2017;58(4):697–702. doi: 10.3349/ymj.2017.58.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353(17):1793–801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 7.Garzon R, Volinia S, Liu CG, FernandezCymering C, Palumbo T, Pichiorri F, et al. MicroRNA signatures associated with cytogenetics and prognosis in acute myeloid leukemia. Blood. 2008;111(6):31839. doi: 10.1182/blood-2007-07-098749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jongen-Lavrencic M, Sun SM, Dijkstra MK, Valk PJ, Lowenberg B. MicroRNA expression profiling in relation to the genetic heterogeneity of acute myeloid leukemia. Blood. 2008;111(10):5078–85. doi: 10.1182/blood-2008-01-133355. [DOI] [PubMed] [Google Scholar]
- 9.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 10.Dorantes-Acosta E, Pelayo R. Lineage switching in acute leukemias: a consequence of stem cell plasticity? Bone Marrow Res. 2012;2012:406796. doi: 10.1155/2012/406796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Huang Z, Xue H, Jin C, Ju XL, Han JD, et al. MicroRNA miR-24 inhibits erythropoiesis by targeting activin type I receptor ALK4. Blood. 2008;111(2):588–95. doi: 10.1182/blood-2007-05-092718. [DOI] [PubMed] [Google Scholar]
- 12.Georgantas RW 3rd, Hildreth R, Morisot S, Alder J, Liu CG, Heimfeld S, et al. CD34+ hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proc Natl Acad Sci U S A. 2007;104(8):2750–5. doi: 10.1073/pnas.0610983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen T, Rich A, Dahl R. MiR-24 promotes the survival of hematopoietic cells. PLoS One. 2013;8(1):e55406. doi: 10.1371/journal.pone.0055406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin JY, Tang Q, Qian W, Qian J, Lin J, Wen XM, et al. Increased expression of miR-24 is associated with acute myeloid leukemia with t(8;21) Int J Clin Exp Pathol. 2014;7(11):8032–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan Y, Kluiver J, Koerts J, de Jong D, Rutgers B, Abdul Razak FR, et al. miR-24-3p Is Overexpressed in Hodgkin Lymphoma and Protects Hodgkin and Reed-Sternberg Cells from Apoptosis. Am J Pathol. 2017;187(6):1343–55. doi: 10.1016/j.ajpath.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Khodadadi-Jamayran A, Akgol-Oksuz B, Afanasyeva Y, Heguy A, Thompson M, Ray K, et al. Prognostic role of elevated mir-24-3p in breast cancer and its association with the metastatic process. Oncotarget. 2018;9(16):12868–78. doi: 10.18632/oncotarget.24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Organista-Nava J, Gomez-Gomez Y, IlladesAguiar B, Del Carmen Alarcon-Romero L, SaavedraHerrera MV, Rivera-Ramirez AB, et al. High miR-24 expression is associated with risk of relapse and poor survival in acute leukemia. Oncol Rep. 2015;33(4):1639–49. doi: 10.3892/or.2015.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mi S, Lu J, Sun M, Li Z, Zhang H, Neilly MB, et al. MicroRNA expression signatures accurately discriminate acute lymphoblastic leukemia from acute myeloid leukemia. Proc Natl Acad Sci U S A. 2007;104(50):19971–6. doi: 10.1073/pnas.0709313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang Z, Tang J, Bai Y, Lin H, You H, Jin H, et al. Plasma levels of microRNA-24, microRNA-320a, and microRNA-423-5p are potential biomarkers for colorectal carcinoma. J Exp Clin Cancer Res. 2015;34:86. doi: 10.1186/s13046-015-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishr PJ, Humeniuk R, Mishra PJ, LongoSorbello GS, Banerjee D, Bertino JR. A miR-24 microRNA binding-site polymorphism in dihydrofolate reductase gene leads to methotrexate resistance. Proc Natl Acad Sci USA. 2007;104(33):13513-8;104(33):13513–8. doi: 10.1073/pnas.0706217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez C, Wang YM, Sutow WW, Herson J. Significance of the 48-hour plasma level in high-dose methotrexate regimens. Cancer Clin Trials. 1978;1(2):107-11;1(2):107–11. [PubMed] [Google Scholar]
- 22.Liu SG, Li ZG, Cui L, Gao C, Li WJ, Zhao XX. Effects of methylenetetrahydrofolate reductase gene polymorphisms on toxicities during consolidation therapy in pediatric acute lymphoblastic leukemia in a Chinese population. Leuk Lymphoma. 2011;52(6):1030–40. doi: 10.3109/10428194.2011.563883. [DOI] [PubMed] [Google Scholar]
- 23.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–87. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 24.de Oliveira JC, Brassesco MS, Scrideli CA, Tone LG, Narendran A. MicroRNA expression and activity in pediatric acute lymphoblastic leukemia (ALL) Pediatr Blood Cancer. 2012;59(4):599–604. doi: 10.1002/pbc.24167. [DOI] [PubMed] [Google Scholar]
- 25.Zhu XL, Ren LF, Wang HP, Bai ZT, Zhang L, Meng WB, et al. Plasma microRNAs as potential new biomarkers for early detection of early gastric cancer. World J Gastroenterol. 2019;25(13):1580–91. doi: 10.3748/wjg.v25.i13.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han X, Li Q, Liu C, Wang C, Li Y. Overexpression miR-24-3p repressed Bim expression to confer tamoxifen resistance in breast cancer. J Cell Biochem. 2019;120(8):12966–76. doi: 10.1002/jcb.28568. [DOI] [PubMed] [Google Scholar]
- 27.Roscigno G, Puoti I, Giordano I, Donnarumma E, Russo V, Affinito A, et al. MiR-24 induces chemotherapy resistance and hypoxic advantage in breast cancer. Oncotarget. 2017;8(12):19507–21. doi: 10.18632/oncotarget.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]


