Abstract
The evidence showing the involvement of microglial activation in the development of drug addiction remain scarce as microglia have not been systematically investigated in self-administered mice, a gold standard rodent model for drug addiction. Here we established the stable cocaine self-administration mice to examine microglial activation levels in various brain regions related to reward circuitry. Immunostaining for Iba1 showed a significant upregulation of intensity in the striatum but not in the medial prefrontal cortex (mPFc), hippocampus or thalamus. Further validation experiments showed that cocaine self-administered mice had significantly increased mRNA expression of ccl2 and IL1β in the striatum but not the mPFc compared to saline controls. Consistently, we found elevated protein levels of Iba1, CCL2, TLR4 and mature IL1β in the striatum, not in the mPFc of cocaine-receiving mice. In addition, cocaine-stimulated microglia had modified morphology including a reduced number of intersections, a shortened length and number of processes in the NAc. In summary, our results demonstrated that cocaine mediated microglial activation in a region-specific manner in vivo. These findings indicate that microglia could be activated in the early stage of cocaine addiction directly supporting the rationale that dysregulation on neuroimmune signaling is inherently involved in the development of drug addiction.
Keywords: cocaine, self-administration, microglia, neuroinflammation
Introduction
Microglia, the resident brain macrophage, account for 10 – 15% of all brain cells and constitute the first line of defense against various types of internal and external insults including abused drugs. Accumulating evidence indicates that microglial activation is involved in the development of the addictive properties induced by multiple abused drugs including cocaine. Dysregulation of neuroimmune signaling may be responsible for substance use disorder [1–3] and glial modulators have been suggested as a potential treatment for psychostimulant abuse [4].
Our previous findings demonstrated that cocaine activated microglia in vitro and in mice with passive injection and various mechanisms were involved in cocaine-mediated microglial activation [5–7]. Findings from other groups also showed that cocaine acted on (toll like receptor) TLR4-mediated signaling to enhance the production and release of IL1β in the ventral tegmental area (VTA), which ultimately, increased the synaptic dopamine levels in the striatum contributing to cocaine addiction [8]. On the other hand, mitigation of microglial activation showed blunted cocaine-mediated behavioral changes. TLR4 knockout (KO) mice exhibited abnormal in low-frequency stimulation-induced neuronal plasticity and an attenuation on cocaine-mediated hyperlocomotion and reward learning [9]. Similarly, TLR3 inhibition through both genetic and pharmacological approaches significantly attenuated cocaine-induced conditional place preference (CPP), locomotor activity and self-administration in mice [10]. In addition to rodent models, non-human primate rhesus macaques with cocaine exposure also showed increased neuroinflammation. In the nucleus accumbens (NAc) brain region of rhesus monkeys with cocaine self-administration, a cluster of upregulated immune response and inflammation related genes was identified and associated predominately with microglial activation [11]. Consistently, elevations in microglial activation were found in the frontal white matter of these monkeys following prolonged cocaine self-administration [12].
Previous studies demonstrating the association of neuroimmune dysregulation with drug addiction were mostly performed in in vitro cell line models or in rodents with passive abused-drug injections. In this study, we examined the extent of microglial activation in various brain regions related to reward circuitry in cocaine self-administered mice. Our results indicated that cocaine significantly increased microglial activation primarily in the striatum but not in other regions in the early phase of cocaine addiction implying microglia in the striatum are the most susceptible to cocaine exposure and may play a critical role in the induction stage of drug addiction.
Materials and Method
Animals and reagents
Wild type C57BL/6J mice (male, 10 – 12 weeks) were purchased from Jackson lab (Maine, USA). All the animals were housed under conditions of constant temperature and humidity on a 12-h light, 12-h dark cycle, with lights on at 0700 h. Food and water were available ad libitum. All animal procedures were performed according to the protocols approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and the National Institutes of Health. A total number of 18 mice was used for this study (control: 8, cocaine: 10). Mice receiving either sham operation or saline self-administration served as controls. Cocaine hydrochloride (Sigma, C5776) was freshly dissolved into 0.9% saline prior to use.
Antibodies
Iba1 (Novus Biological(NB)-100-1028), CCL2 (Abcam, ab25124), IL1β (Abcam, ab9722), Caspase-1 (Adipogen, AG-20B-0042-C100), TLR4 (NB100-56566), β-Actin (Sigma, A5441), anti-rabbit Ig/anti-mouse conjugated with HRP (Jackson immunoresearch, 111-035-003, 115-035-003), Iba1 (WAKO, 019-197441), goat anti-rabbit Ig conjugated with Alexa Flour 594 (Invitrogen, A11012).
Surgery procedure
WT mice were anesthetized with the combination of intraperitoneal injection of ketamine hydrochloride and xylazine (94 and 12.5 mg/kg, respectively) and permanent indwelling catheters (Silastic, 0.012 in. I.D. × 0.025 in. O.D.) were implanted into the right jugular vein. The catheter was tied to the jugular vein with surgical silk and then passed subcutaneously to the back of the mouse where it was affixed to a pedestal consisting of metal tubing and circular mesh (Plastics One, 26GA, 315BM-8-5UP). After the procedure, mice were allowed 7 days of recovery in their home cages, whereas the catheters were flushed daily with 0.9% sodium chloride and 20 U/ml of heparin to avoid clogging of the catheter.
Cocaine self-administration
All equipment was purchased from Coulborn Instruments Company. Each unit consists of a sound-mitigation cubicle and a cage for self-administration equipped with right and left nosepokes, where the active (right) nosepoke is cue-paired with a house light. The mice once placed in the cage were securely attached to the drug infusion pump through a system consisting of tubing, a metal protective spring and a balance arm, allowing for the free roaming of the animal. Mice were trained to self-administer cocaine during one daily session according a fixed ratio 1 (FR1) schedule (one active nosepoke was equal to the one injection of cocaine). Mice were rewarded for the nose-poking of the cue-paired active sensor by the infusion of intravenous cocaine (1.0 mg/kg) delivered in a volume of 20 μl for 5 seconds while the nose-poking of the inactive sensor resulted in no consequence. A 15 seconds timeout followed each drug injection to prevent the cocaine overdose. Each session lasted for a maximum of 2 hours or until the mouse received 30 cocaine infusions. All responses were recorded automatically using the computer software Graphic State Notation 4 (Coulbourne Instruments). After the training period of 5 – 7 days, mice established a stable cocaine self-intake according to the following criteria: (1) the ratio of active poke vs. inactive poke exceeds 3:1; (2) the minimum number of cocaine infusions is 10; and (3) the variation in cocaine intake over three consecutive days is below 20%. After the establishment of cocaine self-intake, mice proceeded to receive cocaine infusions under an FR1 schedule for an additional two weeks (daily 2 h sessions, 1.0 mg/kg) with the schedule of 6 days on cocaine and the 1 days off. Mice were sacrificed one day after the last cocaine self-administration session; the brains were removed and stored for later use.
Western blots
The brain tissues were lysed in radio-immunoprecipitation assay buffer with the addition of protease and phosphatase inhibitors, clarified by centrifugation and the protein concentrations were determined using a BCA Protein Assay. Equal amounts of protein (15 μg) were loaded and run on SDS-PAGE gels. The proteins were subsequently transferred onto PVDF membrane, blocked with 3% milk in PBS, followed by the incubation with the primary antibodies in 3% milk overnight at 4 0C. The next day, blots were incubated with the corresponding peroxidase-conjugated secondary antibodies for 1 h at room temperature. Anti-β-actin antibody was used for reprobing each membrane to ensure equal loading. Signals were detected using chemiluminescent substrate for peroxidase (Thermoscientific, 34578). Images were created using the FlourChem R FR1038 Imaging System (Protein Simple) and analyzed using ImageJ software (NIH).
RNA extraction, reverse transcription, and quantitative polymerase chain reaction (qPCR)
Total RNA was extracted using Trizol reagent (Invitrogen, 15596-018) and was dissolved in DEPC-treated H2O and quantified. Reverse transcription reactions were performed using a Verso cDNA kit (Invitrogen, AB-1453/B). The RNA extraction and RT were performed according to the recommended protocols. QPCRs were performed by using taqman qPCR mastermix (Fisher Scientific, 4444557). Reaction systems were set up as follows: 10 μl mastermix, 1 μl primer, and 9 μl distilled H2O. 96-well plates were placed into a 7500 fast real-time PCR system (Applied Biosystems, Grand Island, NY) for program running. Mouse primers for Tnf, Il6, Il1b, and Ccl2 were purchased from (Invitrogen, Mm00443258, Mm00446190, Mm00434200, and Mm00441242).
Microglial Iba1 staining
Brain sections (50 μm) were cut and co-incubated with primary Iba1 antibody (Fujifilm Wako, 019-19741) overnight at 4 °C. Secondary Alexa Fluor 488 goat anti-rabbit IgG was added for 2 h followed by mounting of sections with DAPI (Invitrogen, 36935). Fluorescent images were acquired on a Zeiss Observer and images were processed using AxioVs 40 4.8.0.0 software (Carl Zeiss). Photographs were acquired using an AxioCam MRm digital camera. The analysis of the microglial cells was performed utilizing AxioVision, imageJ and Fiji software. For the calculation of the Iba1 intensity the images were processed using several imageJ functions and the resulting image was saved and re-opened in AxioVision software, where AxioVision Wizard macros was applied to calculate the area and the intensity of the Iba1 staining. For the evaluation of the Iba1 expression, the area of the staining was multiplied by the staining intensity resulting in the total staining parameter (Area X Intensity = Total staining) expressed in artificial units (AU).
Morphology analysis
For the microglial morphology analysis, the z-stack images were opened in Fiji. Microglial cells were traced using Simple Neurite tracer macros [13]. Usually three microglial cells were traced from each image. The tracings of the microglial cells were analyzed using Sholl analysis plugin, with the radius step size of 5 μm. The area under the curve (AUC) was calculated separately for each cell with the formula: x=(B1+B2)/2*5, where B1 and B2 are the number of intersections on the two neighboring radiuses and the number five represents the radius step size selected for the Sholl analysis of microglia. Representative images of the microglial cells were obtained with Simple neurite tracer function of volume filling, following the extraction function. To determine if microglial cells from the mice receiving cocaine are re-arranging their processes and more asymmetric, we employed half-Sholl analysis function. We divided each microglial cell into two halves on condition that the halves should have the most possible inequity in the process length and/or numbers, i.e. one half of the cell will contain more branches then another half. The Sholl analysis was run separately for each hemisphere and later AUC was calculated. To determine the microglia asymmetry index we divided the AUC from the larger half into the AUC of the smaller half of the cell. This index will represent the asymmetry of the branches within the single cell.
Statistical analysis
The results are presented as means ± SEM. For comparisons between two groups an unpaired two-tailed student’s T-test was used; for comparisons among multiple groups, one/two-way ANOVA analysis was used. All statistical tests were performed with GraphPad Prism (La Jolla, CA, USA). Probability levels of < 0.05 were considered statistically significant.
Results
Establishment of the cocaine self-administration mouse model
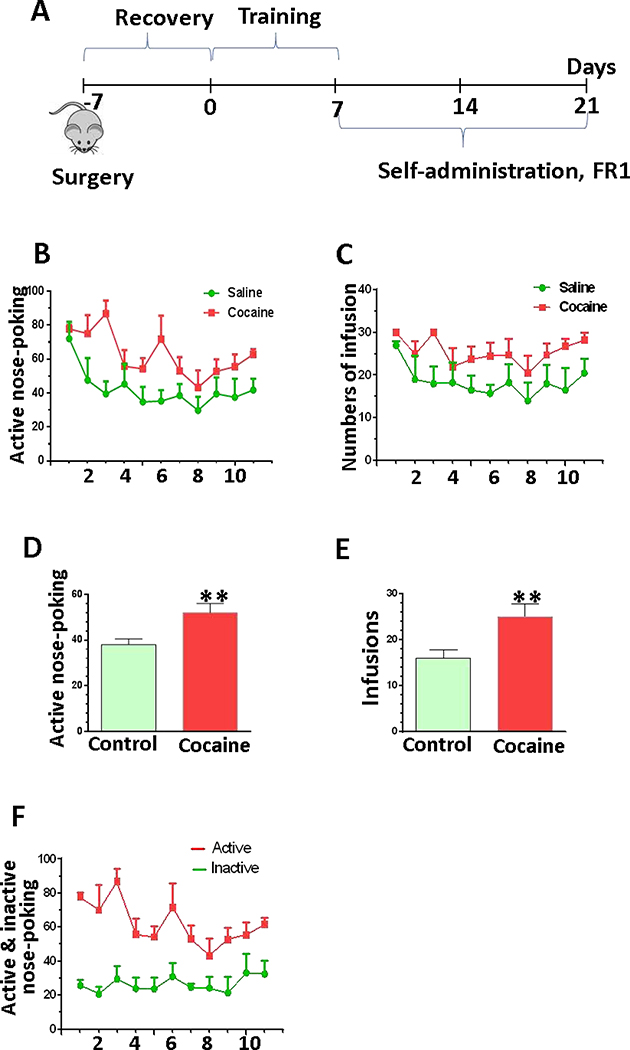
To better understand the involvement of microglia in the development of drug addiction, we first established cocaine self-administered mice. Mice received the implant surgery in jugular vein and recovered for one week. Then mice were put into a self-administration box receiving cocaine infusions (1 mg/kg/fusion) at FR = 1 schedule for 2 h daily sessions. Another group of mice received saline infusions using the same settings as control group. Once the mice reached the criteria for stable cocaine self-administration, they were exposed to cocaine for another two weeks. Twenty-four hours after the last session, mice were sacrificed, and the brains were removed and stored for later experiments (Fig. 1A). Our results showed that the number of active nose-pokes and the number of infusions from cocaine-exposed mice were significantly higher than those from saline mice (Fig. 1D, P = 0.03; Fig. 1E, P = 0.01). In cocaine self-administered mice, the numbers of active nose-poking were significantly higher than the number of inactive nose-poking during this two-week time period (Fig. 1F, P< 0.001). Taken together, these results clearly demonstrated the successful establishment of cocaine self-administration mouse model.
Figure 1.
The difference between saline and cocaine uptake in mouse self-administration model. (A) Schematic for WT mice were receiving cocaine (n=4) or saline (n=4) during the three weeks of self-administration. (B) The average number of the active nosepokes during the first 11 sessions of the cocaine/saline self-administration. (C) The average number of infusions for the first 11 sessions of the cocaine/saline self-administration. (D and E) The mean number of active nosepokes and the mean number of infusions obtained for the last 5 cocaine/saline self-administration sessions, when mice became trained to routinely self-administer the cocaine. (F) The average number of the active nosepokes and inactive nosepokes during the first 11 sessions of the cocaine self-administration.
Region-specific microglial activation in cocaine self-administered mice
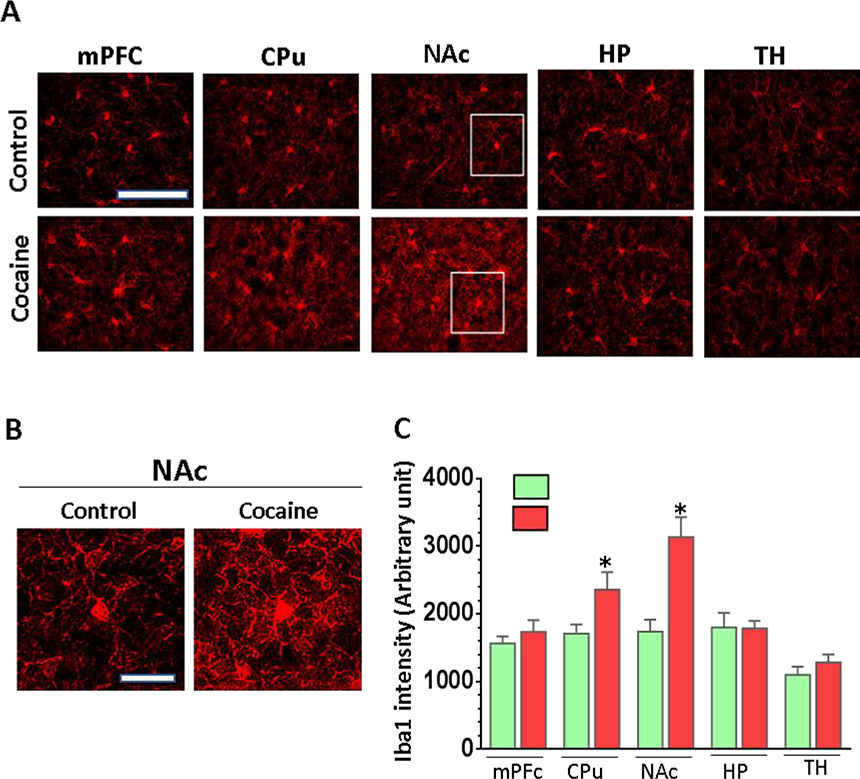
Brain sections representative of specific brain regions from cocaine self-administered and control mice were prepared and stained with Iba1 and the intensity of Iba1 was compared between groups. We selected five reward-circuitry related regions including the medial frontal cortex (mPFC), the dorsal striatum (CPu), the ventral striatum (NAc), the hippocampal stratum radiatum layer in CA1 area (HP) and the ventral lateral thalamic nuclei (TH) in this comparison. As shown in Fig. 2A, microglia significantly increased the expression of Iba1 in the CPu (1.43 ± 0.05 folds, P = 0.02) and NAc (1.81 ± 0.11 folds, P = 0.01) in the brains of the mice with cocaine self-administration compared to control mice. However, we did not observe a significant difference in expression of Iba1 in the mPFc (P = 0.23), HP (P = 0.37), or TH (P = 0.56) areas between these two groups of mice (Fig. 2C). These results suggested that in the initial stage of drug addiction, microglia in the brain striatum, particularly the NAc area, were most susceptible to activation compared to other regions.
Figure 2.
Microglial Iba1 immunostaining in various brain regions in cocaine self-administered mice. Tissue sections containing various brain regions (mPFc, CPu, NAc, HP, TH) from cocaine or control were stained with Iba1. (A) Representative images of Iba1 staining in PFC, CPu, NAc, HP and TH areas of the brains (scale = 100 μM. (B) The representative pictures of Iba1-positive microglia in NAc region from control and cocaine-receiving mice (scale = 20 μM). (C) Quantitative analysis of Iba1 staining in different brain regions showed microglial activation in CPu and NAc areas but not in mPFC, HP, and TH of cocaine self-administered mice.
Cocaine differentially upregulated the levels of pro-inflammatory mediators in the brain
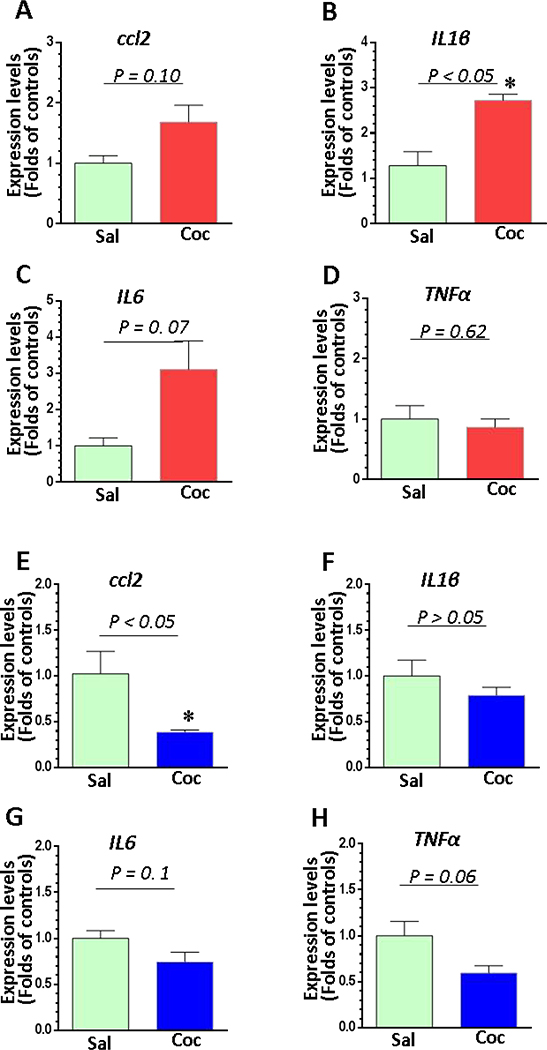
We chose the striatum and the mPFc to examine the mRNA expression of several neuroinflammatory mediators including ccl2, il1β, il6, and tnfα. As shown in Figs. 3A–3D, in the striatum, we found a significant increase in abundance of IL1β mRNA in cocaine self-administered mice compared to saline controls (P = 0.04); there was also a strong trend towards elevated mRNA levels of il6 (P = 0.07) and ccl2 (P = 0.1). There was no significant difference in TNFα mRNA between these two groups (P = 0.62). However, in the mPFc the magnitude of ccl2 mRNA was significantly decreased in cocaine-exposed brains compared to controls (P = 0.01), while the mRNA levels of il1β (P = 0.17), il6 (P = 0.1) and tnfα (P = 0.06) revealed a decreasing trend in cocaine self-administered mice (Figs. 3E–3H). In summary, our findings showed that cocaine enhanced neuroinflammation in the NAc but not in the mPFc suggesting microglia in these two regions were differentially regulated by cocaine.
Figure 3.
The mRNA expression of pro-inflammatory cytokines and a chemokine in the striatum and mPFC in cocaine self-administered and control mice (n = 4). Brain tissues containing striatum or PFC from mice receiving saline (n = 4) or cocaine (n = 4) were examined for the expression of the pro-inflammatory cytokines il1β, il6, tnfα, and ccl2. (A - D) The expression levels of il1β, il6, tnfα, and ccl2 in NAc of cocaine self-administered and control mice. (E- H) The expression levels of il1β, il6, tnfα, and ccl2 in mPFc of cocaine self-administered and control mice.
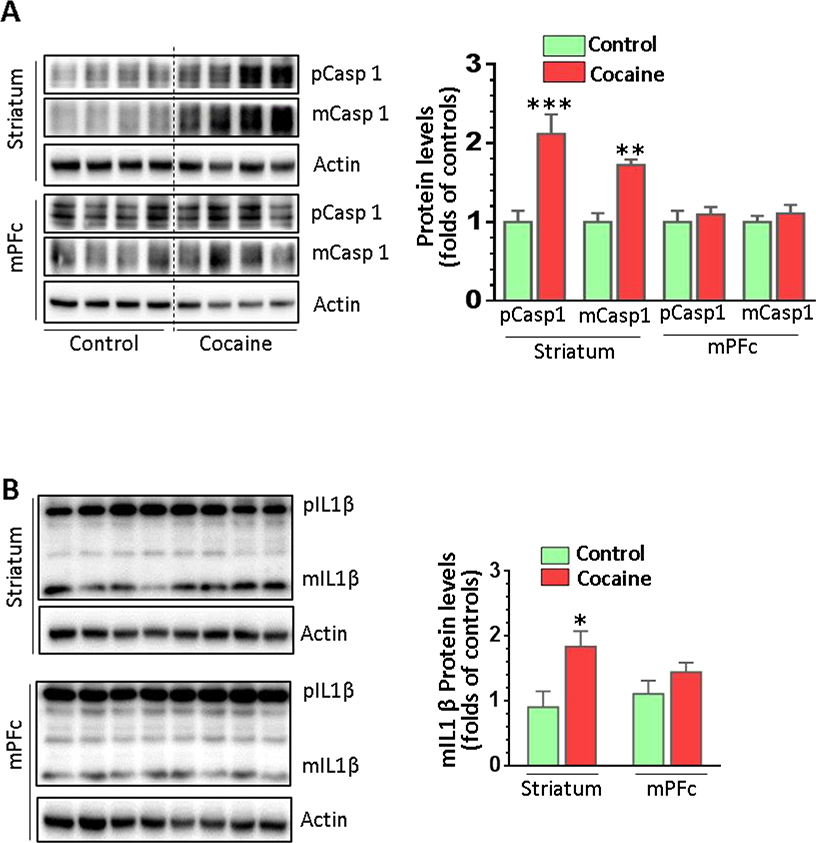
Cocaine differentially modulated the levels of various neuroimmune proteins in the brain
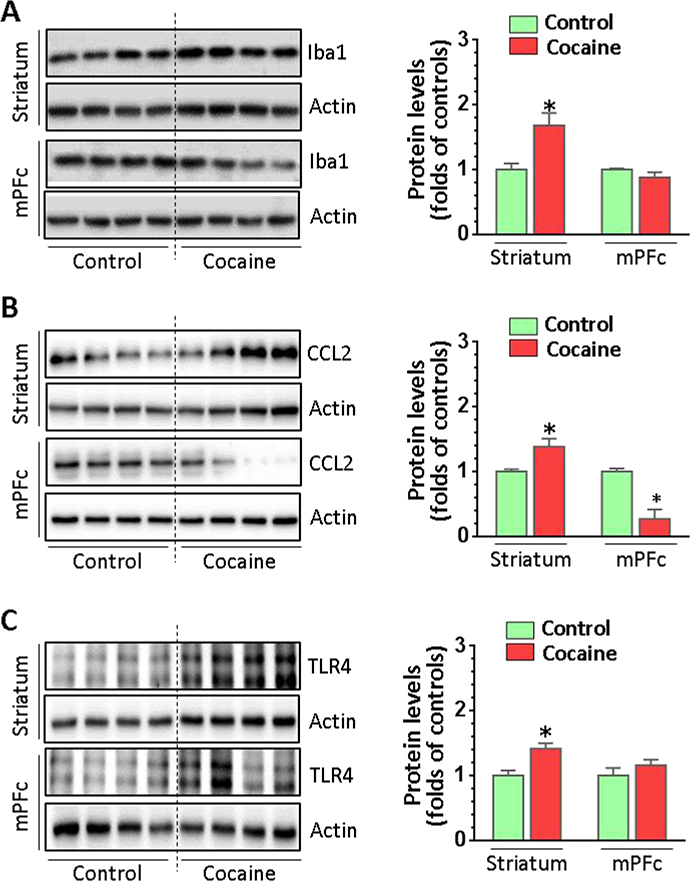
We further investigated whether cocaine could regulate microglial markers at the protein level in vivo. Protein homogenates from both the striatum and the mPFc of cocaine-exposed mice and controls were prepared to examine the expression of Iba1. Cocaine significantly increased Iba1 in the striatum but not in the mPFc (Fig. 4A, P = 0.03). Interestingly, we found cocaine-increased CCL2 (P = 0.01) and cocaine-decreased CCL2 levels (P = 0.02) in the striatum and mPFc, respectively (Fig. 4B). For TLR4, there was a significant increase in the striatum (P = 0.01) but no change in the mPFc comparing cocaine self-administered mice to controls (Fig. 4C). We also examined the levels of caspase 1 and IL1β. Similar expression patterns for pro-caspase 1 (pCasp1), mature caspase 1 (mCasp1) and mature IL1β (mIL1β) were identified; pCasp1 (P = 0.001), mCasp1 (P = 0.005), and mIL1β (P = 0.02) significantly increased their levels in the striatum but not in the mPFc in cocaine-administered mice (Figs. 5A and 5B). Overall, these results strongly suggested that cocaine activated microglia in vivo in a region-specific manner.
Figure 4.
The protein expression of the Iba1, CCL2, and TLR4 in cocaine self-administered and control mice. Brain tissues containing the striatum or mPFc from cocaine self-administered (n = 6) or control mice (n = 4) were assessed for the microglial activation. (A) Iba1 expression was upregulated in the striatum but not in mPFc of cocaine self-administered mice. (B) CCL2 was differentially altered in the striatum and mPFc in cocaine self-administered mice. (C) TLR4 was differentially altered in the striatum and mPFc in cocaine self-administered mice.
Figure 5.
The protein expression of caspase 1 and IL1β in cocaine self-administered and control mice. Brain tissues containing the striatum or mPFc from mice with cocaine self-administration (n = 6) or control animals (n = 4) were analyzed for the expression of the inflammatory proteins Caspl and IL1β. (A) Both pro-Casp1 and mature Casp1 isoforms were upregulated in the striatum but not in the mPFc in the mice with cocaine self-administration. (B) The expression of mature IL-1β was significantly increased in the striatum but not in the mPFc in mice with cocaine self-administration.
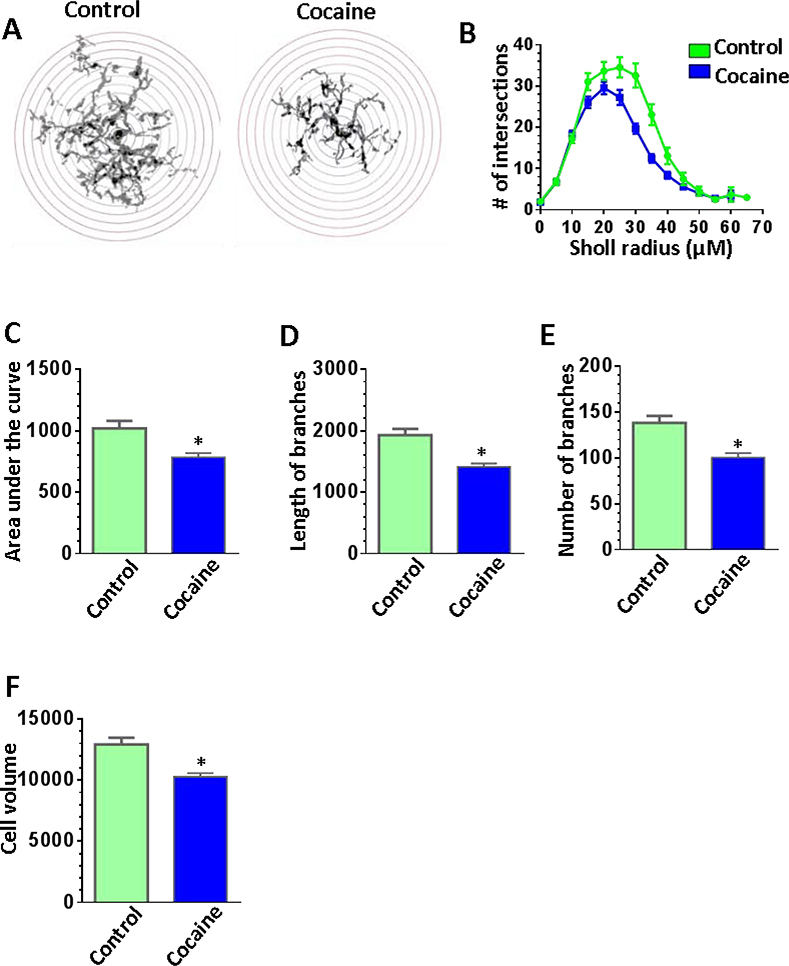
Alteration of microglial morphology in the NAc of mice with cocaine self-administration
Brain sections containing the NAc were prepared and immunostained with Iba1 to produce multiple z-stack images which were then processed and analyzed by ImageJ software and a simple neurite tracer plugin (32 cells from four control mice and 48 cells from 6 cocaine self-administered mice). Sholl analysis of the microglia revealed that the highly ramified microglia of the NAc shell region became less branched under cocaine treatment (Fig. 6A) as indicated by the lower number of intersections at the distance of 20 to 40 μm from the cell body (Fig. 6B) and the reduced area under the curve (Fig. 6C, P = 0.01). The length of processes and the number of branches were significantly reduced in the microglia from the NAc of cocaine self-administered mice compared to control mice (Figs. 6D and 6E). Surprisingly the volume of the cocaine activated microglia was also decreused (Fig. 6F, P = 0.04) probably due to the reduced microglial branching.
Figure 6.
Morphological analysis of microglia in the NAc from cocaine self-administered and control mice. Iba1 positive microglial cells in the NAc from mice with cocaine self-administration (n = 6) or from controlled animals (n = 4) were traced and analyzed using ImageJ software. A total number of 32 cells from control mice and 48 cells from cocaine self-administered mice was included into the analysis. (A) Representative images of the traced microglial cells from the NAc of controlled and cocaine self-administered mice, enclosed in the range of radiuses for the Sholl analysis. The radius step size is 5 μm. (B) Sholl analysis curves displayed the reduced number of intersections for cocaine-exposed microglia in the NAc. (C) The area under curve (AUC) calculated from Sholl analysis curves was significantly decreased in cocaine treated microglia comparing with the control group. (D, E, and F) Cocaine-exposed microglia have shorter branches, reduced number of branches, and less cell volume relatively to the untreated microglia.
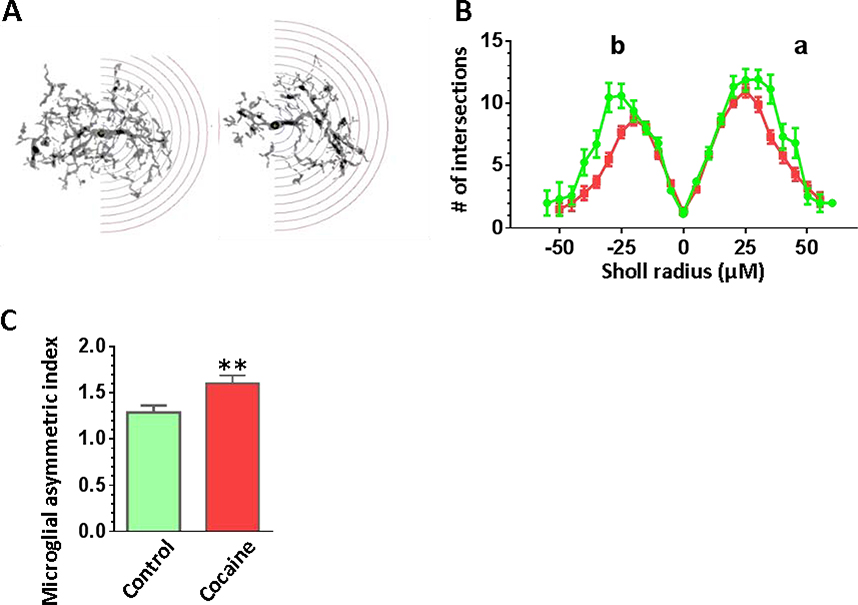
We next investigated whether cocaine could rearrange microglial processes and branches indicating their high mobility in vivo. Briefly, each microglial cell was conditionally divided into halves where one half contained the maximal number of the branches and the opposite half contained the least number. The representative pictures of the NAc microglia subjected to the hemi-sholl analysis were shown in Fig. 7A. The results from the hemi-sholl analysis were graphed separately for each hemisphere in Fig. 7B, where the data for the more branched hemisphere is indicated as curve “a” and the data for the least ramified side is specified as curve “b”. The areas under each curve were obtained and we calculated the index of microglial asymmetry as the ratio of curve a/curve b for each cell separately. We found that microglia in the NAc of the cocaine self-administered mice became more asymmetric compared to microglia from control mice indicating enhanced microglial mobility in cocaine-exposed mice as shown in Fig. 7C (P = 0.03).
Figure 7.
Morphological analysis revealed the increased asymmetry of the cocaine-treated microglia in the NAc. Iba1 positive microglia in the NAc were traced and analyzed using hemi-Sholl analysis. (A) The representative images of NAc microglial cells from control or cocaine-exposed groups. The hemisphere with the higher branching is enclosed into the range of radiuses for the hemi-Sholl analysis, the radius step is 5 μm. (B) Sholl analysis curves calculated separately for the hemisphere with larger number of branches (a) and for the hemisphere with lesser number or branches (b). (C) Cocaine-treated microglial branches are more asymmetrical as shown by the asymmetry index calculated as a ratio between the AUCs of the hemi-Sholl analysis analyzed separately from sides a and b.
Discussion
In this study, we established the cocaine self-administration mouse model and demonstrated that cocaine mediates microglial activation in a region-specific manner in vivo through immunostaining, biochemical approaches and morphological analysis. Our findings are consistent with previous findings and strongly implicate that dysregulation of neuroimmune signaling is inherently involved in the development of cocaine addiction.
Emerging evidence showed that microglial activation/neuroinflammation is involved in cocaine addiction [1–3]. We have previously demonstrated that cocaine is capable of activating microglia [5–7] suggesting the potential contribution of neuroimmune signaling on cocaine-mediated biochemical and behavioral changes. However, such findings were mostly derived from in vitro cell culture models and from mice with passive injections. In fact, the route of cocaine administration (passive vs. active injection), the dose of usage, the regimen of administration, and the detection time all influence the experimental outcome and could lead to differential outcomes. For example, there were huge differences in immune signaling between passively injected mice and cocaine self-administered mice [14]. Till now, the functional status of microglia has not been systematically investigated in self-administered mice which is gold standard model for drug addiction.
Our results demonstrated that in cocaine self-administered mice, microglia are activated in a region-specific manner. Increased Iba1 immunostaining was found in the striatum but not in the other brain regions including the mPFc, HP, and TH indicating that striatal microglia were most susceptible to cocaine exposure in vivo. One previous study reported that increased microglial activation was only found in frontal white matter of monkey brains following prolonged cocaine self-administration [12]. Such inconsistency on the regions of microglial activation may due to the species, cocaine exposure time and amount, etc. Previous investigations have demonstrated that the mPFc, HP, and TH were also involved in the cycle of cocaine addiction [15, 16]. The fact that we did not observe microglial activation in these brain regions may be due to several reasons. One possibility is that microglia in various areas are a heterogeneous population [17, 18], therefore, it is possible that microglia in various regions have different sensitivity to cocaine exposure. Our results indicated that microglia in the striatum were more susceptible to cocaine which is in agreement with data that the striatum (both dorsal and ventral parts) is acutely affected by the drugs of abuse [19, 20]. Additionally, we exposed these mice to cocaine for the relatively short time-period of three weeks, which corresponds to the early recreational stage of drug addiction. Previous studies have indicated that the hippocampus and thalamus are deeply involved during the later stages of cocaine abuse, such as withdrawal and relapse [16, 21]. Thus, it is possible that microglial activation could be observed in the other brain regions as well at the stages of withdrawal and relapse, however further investigation is needed.
Our study indicated that microglial activation could be an earlier event for cocaine addiction. The elevated neuroinflammation may enhance the sensitivity of cocaine-mediated changes on neurotransmitters such as synaptic dopamine or glutamate concentrations to promote the development of drug addiction. Findings from other groups support this hypothesis. Cocaine could enhance the production and release of IL1β in the ventral tegmental area (VTA) which mediates elevated synaptic dopamine levels in the striatum [8]. So targeting neuroimmune signaling could be a potential therapeutic approach to ameliorate cocaine addiction.
Various pathways have been implicated in cocaine-mediated microglial activation including ER/autophagy dysregulation, TLR4 signaling, and miRs dysregulation in different models [5–7]. Previous studies demonstrated that IL1β was significantly elevated in serum obtained from cocaine self-administered mice but not from experimenter-administered ones [14]; IL1β was also upregulated in the VTA brain region of cocaine self-administered mice [8]. Since IL1β is the main product of NLRP3 inflammasome recruitment in microglia, the involvement of the TLR4/NLRP3/Casp1/IL1β axis might underlie microglial activation in cocaine self-administered mice. Indeed, we also found increased mRNA and protein of IL1β in the striatum of cocaine self-administered mice. Additionally, we reported that the upstream regulators for mIL1β production, TLR4 receptor and mCasp1, were also elevated in the striatum of mice receiving cocaine. These results implied that the TLR4/NLRP3/Casp1/ IL1β axis may underlie cocaine-mediate microglial activation; however more work is needed to determine if this is the main pathway for cocaine-mediated microglial activation.
Highlights.
We established cocaine self-administered mice.
We observed microglial activation the brain striatum.
Cocaine differentially modulates microglial functional status in vivo.
NLRP3/IL1β may play critical roles in cocaine-mediated microglial activation.
Acknowledgement
This work was supported by the pilot funding from NE Center for Substance Abuse Research and NIH NIDA grants DA047156, DA044586. Thanks for Shannon E. Callen for critical reading. Thanks for Dr. Klause Miczek (Tufts University) for technical support on self-administration.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Catale C, et al. , Microglial alterations induced by psychoactive drugs: A possible mechanism in substance use disorder? Semin Cell Dev Biol, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Bachtell RK, et al. , Glial and neuroinflammatory targets for treating substance use disorders. Drug Alcohol Depend, 2017. 180: p. 156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui C, Shurtleff D, and Harris RA, Neuroimmune mechanisms of alcohol and drug addiction. Int Rev Neurobiol, 2014. 118: p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beardsley PM and Hauser KF, Glial modulators as potential treatments of psychostimulant abuse. Adv Pharmacol, 2014. 69: p. 1–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao K, et al. , Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J Neuroinflammation, 2016. 13: p. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Periyasamy P, et al. , Cocaine-Mediated Downregulation of miR-124 Activates Microglia by Targeting KLF4 and TLR4Signaling. Mol Neurobiol, 2018. 55(4): p. 3196–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo ML, et al. , Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy, 2015. 11(7): p. 995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Northcutt AL, et al. , DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry, 2015. 20(12): p. 1525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashima DT and Grueter BA, Toll-like receptor 4 deficiency alters nucleus accumbens synaptic physiology and drug reward behavior. Proc Natl Acad Sci U S A, 2017. 114(33): p. 8865–8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu R, et al. , Toll-like receptor 3 modulates the behavioral effects of cocaine in mice. J Jeuroinflammation, 2018. 15(1): p. 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallender EJ, et al. , Transcriptomic profiling of the ventral tegmental area and nucleus accumbens in rhesus macaques following long-term cocaine self-administration. Drug Alcohol Depend, 2017. 175: p. 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith HR, et al. , Regional elevations in microglial activation and cerebral glucose utilization in frontal white matter tracts of rhesus monkeys following prolonged cocaine self-administration. Brain Struct Funct, 2019. 224(4): p. 1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longair MH, Baker DA, and Armstrong JD, Simple Neurite Tracer: open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics, 2011. 27(17): p. 2453–4. [DOI] [PubMed] [Google Scholar]
- 14.Calipari ES, et al. , Granulocyte-colony stimulating factor controls neural and behavioral plasticity in response to cocaine. Nat Commun, 2018. 9(1): p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castilla-Ortega E, et al. , A place for the hippocampus in the cocaine addiction circuit: Potential roles for adult hippocampal neurogenesis. Neurosci Biobehav Rev, 2016. 66: p. 15–32. [DOI] [PubMed] [Google Scholar]
- 16.Matzeu A and Martin-Fardon R, Drug Seeking and Relapse: New Evidence of a Role for Orexin and Dynorphin Co-transmission in the Paraventricular Nucleus of the Thalamus. Front Neurol, 2018. 9: p. 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayata P, et al. , Epigenetic regulation of brain region-specific microglia clearance activity. Nat Neurosci, 2018. 21(8): p. 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Biase LM, et al. , Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia. Neuron, 2017. 95(2): p. 341–356 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bobadilla AC, et al. , Corticostriatal plasticity, neuronal ensembles, and regulation of drug-seeking behavior. Prog Brain Res, 2017. 235: p. 93–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchta WC and Riegel AC, Chronic cocaine disrupts mesocortical learning mechanisms. Brain Res, 2015. 1628(Pt A): p. 88–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGlinchey EM and Aston-Jones G, Dorsal Hippocampus Drives Context-Induced Cocaine Seeking via Inputs to Lateral Septum Neuropsychopharmacology, 2018. 43(5): p. 987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]