Abstract
Candida species, including C. albicans in particular, can cause superficial or invasive disease, often in patients with known acquired immunodeficiencies or iatrogenic conditions. The molecular and cellular basis of these infections in patients with such risk factors remained largely elusive, until the study of inborn errors of immunity clarified the basis of the corresponding inherited and “idiopathic” infections. Superficial candidiasis, also known as chronic mucocutaneous candidiasis (CMC), can be caused by inborn errors of IL-17 immunity. Invasive candidiasis can be caused by inborn errors of CARD9 immunity. In this chapter, we review both groups of inborn errors of immunity, and discuss the contribution of these studies to the deciphering of the critical mechanisms of anti-Candida immunity in patients with other conditions.
Introduction
The genus Candida, which contains about 200 different species, belongs to the phylum Ascomycota. Candida spp. are the most common cause of fungal infection in humans1–2, but only a few species (approximately 20) can cause disease. Candida albicans, C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei account for about 90% of these diseases, and their prevalence depends on the geographic location, patient populations, and clinical settings3. C. albicans remains the major cause of invasive candidiasis, but C. glabrata (in northern Europe, USA, Canada) and C. parapsilosis (in southern Europe, Asia, Latin America) have emerged as important or even major pathogens4–7. Candida spp. have been reported to be the fourth most common nosocomial pathogen in the bloodstream, or at least within the top ten of such pathogens1, 8. These Candida spp. are resident commensal yeasts in the oro-gastrointestinal and genitourinary tracts in healthy individuals. However, they can also act as pathogens in humans, causing superficial infections of the skin, scalp, nails, or oral and genital mucosae, or invasive, often life-threatening, systemic infections (candidemia) that may be disseminated to internal organs (leading to meningoencephalitis, brain abscesses, endophthalmitis, endocarditis, peritonitis, osteomyelitis, intra-abdominal abscesses, lung infections, etc.)9–11. The infections they cause are a serious public health problem, with mortality often exceeding 40% (partly due to late diagnosis, the late initiation of antifungal therapies, and the emergence of resistance to antifungal drugs), and substantial costs associated with patient care and long hospital stays11–18.
Various risk factors, iatrogenic or acquired, are known, such as HIV infection, mainly associated with oro-pharyngeal candidiasis19. Invasive candidiasis is mostly associated with organ transplantation, hemodialysis, parenteral nutrition, intravenous catheters, abdominal surgery, extensive burns, long-term stay in intensive care units, or the administration of broad-spectrum antibiotics or of immunosuppressive agents such as chemotherapy1, 20. In this context, invasive candidiasis is an increasing problem in elderly patients, with significantly higher mortality rates as compared to younger patients21–22. Neonates are also at risk of invasive forms of candidiasis, such as the central nervous system (CNS) candidiasis reported in low-birth weight or preterm neonates23–26. These fungal diseases frequently strike individuals with many risk factors. As a result, their pathogenesis remains poorly understood at the molecular and cellular levels. The study of primary immunodeficiencies (PID) with “syndromic” candidiasis, whether superficial or invasive, and, more recently, that of inborn errors of immunity in otherwise healthy patients with “isolated” candidiasis, whether superficial or invasive, has progressively shed light on the mechanisms conferring protective immunity to Candida spp.27–36. The elucidation of the pathogenesis of these fungal diseases in patients with inherited immunodeficiencies (ID) has important clinical implications for the patients and their families, with the possibility of genetic diagnoses and counseling, but should also facilitate the development of novel prophylactic or curative treatments with a rational basis, for PID patients and patients with other more common conditions (e.g. acquired ID). Research into the genetic basis of Candida diseases is important, given the high mortality associated with Candida diseases, despite the availability of antifungal drugs, and the increasing frequency of antifungal drug-resistant strains37.
Chronic mucocutaneous candidiasis and inborn errors of IL-17 immunity
Mucocutaneous candidiasis is characterized by Candida spp. infections of the nails, skin, scalp, and/or oral and genital mucosae35, 38–43. Mucosal candidiasis, such as oral thrush, is relatively frequent in individuals on steroid or antibiotic treatments. Up to 75% of women present at least one episode of vulvovaginal candidiasis during their lifetime, and recurrent (> 1 episode) vulvovaginal candidiasis has been estimated to have a global annual prevalence of 3,871 per 100,000 women44. Chronic mucocutaneous candidiasis (CMC) is characterized by severe, persistent or recurrent (relapse upon discontinuation of treatment) disease43. CMC, present as severe oropharyngeal candidiasis, is very common in AIDS patients19, 45. Similarly, in the context of PID, CMC is frequent in patients with broad T-cell defects, such as combined or severe combined immunodeficiencies (CID or SCID, respectively)30–31, 46–47. This inherited form of CMC, usually referred to as CMC disease (CMCD), is rare, affecting approximately one in every 50,000 individuals. The CMC in affected patients is syndromic, as it is associated with many other clinical manifestations, mostly infectious and/or autoimmune. Syndromic CMC is also common in some PIDs without major global apparent T-cell deficiencies, albeit with milder clinical features. These PIDs include autosomal dominant (AD) STAT1 gain-of-function (GOF), a complex and heterogeneous PID in which CMC is one of the first features observed and is common to most patients, and often severe33–34, 48–53. It is frequently associated with other infectious diseases, typically mucocutaneous bacterial, viral, or fungal diseases, and less frequently with invasive infectious diseases, autoimmune manifestations, and oro-esophageal squamous cell carcinoma28, 30, 34–35, 43, 49–51, 53–54. Another such PID is hyper-IgE syndrome (HIES), another complex PID characterized by severe skin and pulmonary staphylococcal disease, severe eczema, high serum IgE levels, and some developmental abnormalities48, 55. It may be AD due to heterozygous dominant negative mutations of the gene encoding the transcription factor STAT356–57, or autosomal recessive (AR) due to biallelic loss-of-function (LOF) mutations of the gene encoding another transcription factor, Zinc Finger Protein (ZNF)34158–59. About 80% of patients develop oral thrush, onychomycosis, and/or vaginal candidiasis. CMC is also frequent and the only infectious disease common to most patients with AR autoimmune polyendocrine syndrome type 1 (APS-1, also called APECED, autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy). This syndrome is characterized by multi-organ autoimmunity due to bi-allelic mutations of the gene encoding the transcription factor AIRE60–62. Other PIDs in which CMC is milder or less frequent (25% to 35%) include AR ROR / T deficiency, characterized by disseminated BCG-diseases63, AR IL-12p40 and IL-12R 1 deficiencies, characterized by a selective predisposition to mycobacterial and Salmonella diseases64–65, and AR CARD9 deficiency, generally characterized by invasive fungal diseases32, 66–68. A final group of patients displays early CMCD in an otherwise healthy context, with the exception of mucocutaneous staphylococcal disease in some patients. This condition is often referred to as isolated CMCD (see below).
Investigations of the molecular and cellular bases of PID with syndromic CMCD suggested that IL-17A/F-mediated immunity might protect against mucocutaneous candidiasis and that CMCD might result from inborn errors of IL-17A/F immunity34–35, 69–71. Indeed, all of them are characterized by impaired IL-17A/F immunity, due to abnormally low levels of circulating IL-17A/F-producing T (Th17) cells, or to the presence of autoantibodies directed against IL-17 cytokines. Indeed, patients with AD STAT1 GOF have very low proportions of Th17 cells, both ex vivo or and after differentiation in vitro50, 53–54, 72–74. This Th17 cell deficiency may result from enhanced/overt STAT1 signaling downstream from the STAT3-dependent IL-6, IL-21, and IL-23 cytokines, which is critical for the development and maintenance of Th17 cells70–71, 75, enhanced STAT1 signaling downstream from IFN-α/β, IFN-γ, and IL-27, which has been shown to inhibit the development of Th17 cells via STAT176–78, or both these mechanisms53, 79. Patients with AD HIES also have very low proportions of ex vivo and in vitro-differentiated Th17 cells80–83, due to an impairment of STAT3-dependent signaling downstream from IL-6R, IL-21R, and IL-23R70. Similarly, patients with AR HIES and ZNF341 deficiency also have abnormally low proportions of ex vivo and in vitro-differentiated Th17 cells, due to the disruption of ZNF341-dependent STAT3 transcription and activity55, 58–59. As expected, patients with AR deficiencies of ROR / T, a master transcription factor of Th17 cells84–87, also have barely detectable levels of Th17 cells63, and patients with IL-12p40 and IL-12R 1 deficiencies, in whom the production of and response to IL-23 and IL-12 are abolished, also have low levels of circulating Th17 cells74, 83. Most, but not all of the tested patients with deficiencies of CARD9, an adaptor transducing signals downstream from C-type lectin receptors following the recognition of fungal cell wall components, have low circulating levels of Th17 cells, probably due an impairment of the induction of pro-Th17 cytokines (e.g. IL-6, IL-23) by phagocytes after activation by fungal ligands32, 66–67, 88 (Figure). Finally, APS1 patients, who suffer from multiple autoimmune endocrinopathies due to LOF mutations of the gene encoding AIRE, a transcription factor involved in the removal of self-reactive T cells89–90, frequently harbor high levels of antibodies against various self-antigens91, including neutralizing autoantibodies directed against cytokines such as IFN-α and IFN-ω62, 92 in particular, but also against Th17 cytokines, such as IL-17A, IL-17F, and IL-2269, 93–94. These studies have paved the way for the identification of inborn errors of IL-17-mediated immunity conferring CMC in otherwise healthy individuals, or in individuals with mucocutaneous Staphylococcus aureus infections33–35.
Figure. Inborn errors of IL-17 immunity in patients with isolated or syndromic CMCD.
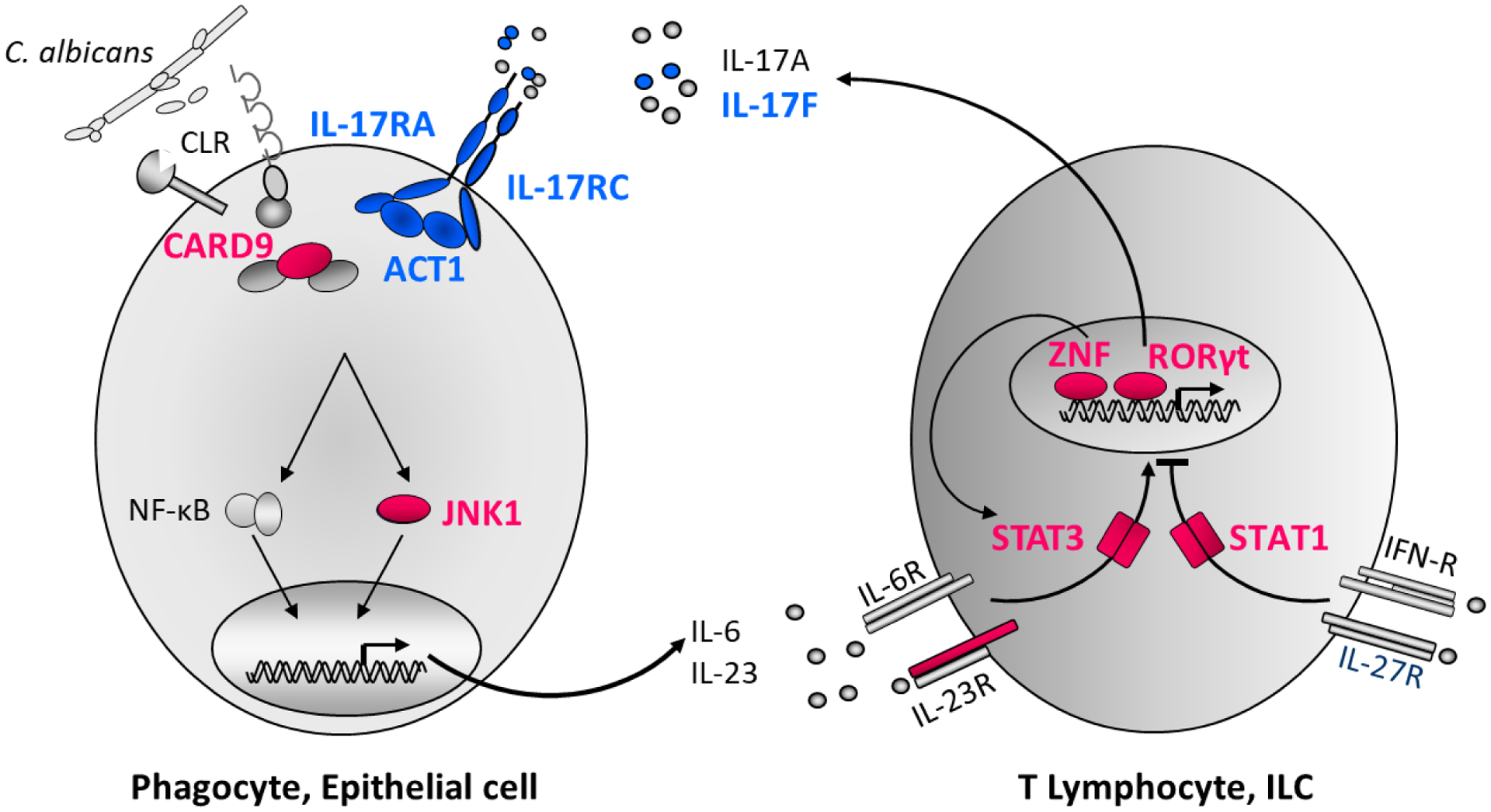
Schematic representation of IL-17A/F immunity and cooperation between cells recognizing C. albicans and responding to IL-17A/F (phagocytes and epithelial cells), and cells producing IL-17A/F (T and innate lymphocytes). Human IL-17A/F immunity is crucial for protective mucocutaneous immunity against C. albicans. Proteins for which mutations in the corresponding genes underlie CMCD are shown in blue or red. Monoallelic LOF mutations of IL17F and of MAPK8, and bi-allelic LOF mutations of IL17RA, IL17RC and ACT1 impair IL-17A and IL-17F immunity (via IL-17RA/IL-17RC). Bi-allelic LOF mutations of IL12RB1, RORC, ZNF341, monoallelic LOF mutations of STAT3 and monoallelic GOF mutations of STAT1 impair IL-17A/F production. Mutations of IL17F, IL17RA, IL17RC and ACT1 underlie isolated CMCD (blue), whereas mutations of IL12RB1, STAT1, STAT3, ZNF341 and RORC underlie syndromic CMCD (in red).
A candidate approach identified AD IL-17F and AR IL-17RA deficiencies, each in a single family, in 2011, as the first genetic etiologies of isolated CMCD28, 95. Indeed, a heterozygous private missense variation of IL17F, predicted to be deleterious, was identified in five relatives from an Argentinian multiplex family with early-onset CMC. The index patient had also had recurrent upper respiratory tract infections, asthma, and recurrent episodes of furunculosis since infancy. In these patients, the proportions of ex vivo IL-17A- and IL-22-producing T cells were within the control ranges, but IL-17F levels were not evaluated. The mutation was shown to impair the binding of IL-17F to its receptor, which consists of IL-17RA/IL-17RC, on the surface of control fibroblasts. Studies with control fibroblasts and keratinocytes revealed an impairment of the responses to mutant IL-17F homodimers, but also of that to heterodimers containing the mutant protein (IL-17A/mutant IL-17F, wild type IL-17F/mutant IL-17F), showing that the mutant IL-17F was hypomorphic and exerted a dominant-negative effect on IL-17A- or wild-type IL-17F-mediated responses95. A second family of Tunisian-German origin has since been reported, in which a woman and her son carrying a heterozygous mutation of IL17F both presented CMC with an onset in early childhood, with no other infectious phenotype; the causal effect of the variant in this family has yet to be characterized96. In parallel, AR complete IL-17RA was reported in a patient born to consanguineous Moroccan parents. This patient suffered from early-onset CMC and cutaneous S. aureus infection, and was homozygous for a nonsense mutation affecting the extracellular part of IL-17RA. Additional homozygous nonsense, missense, frameshift, splice site, and large deletion mutations have since been found in a total of 23 patients with AR IL-17RA deficiency, from 13 unrelated kindreds originating from Morocco, Turkey, Japan, Saudi Arabia, Algeria, Argentina, and Sri Lanka28, 97–98. All patients displayed early-onset CMC. About 70% of these patients also presented staphylococcal skin diseases, and 40% developed recurrent bacterial infections of the respiratory tract28, 95, 97–98. AR complete IL-17RC deficiency was subsequently identified by whole-exome sequencing in three unrelated patients with early-onset CMC in the absence of any other infectious phenotype, including staphylococcal disease in particular; these patients were born to consanguineous families originating from Turkey and Argentina99. AR ACT1 deficiency is the fourth genetic defect responsible for isolated CMCD. It was identified in two siblings, born to consanguineous Algerian parents, with early-onset CMC and recurrent skin and scalp S. aureus disease. Both patients were found to carry a homozygous missense mutation of TRAF3IP2 encoding ACT1, which is a key downstream adapter in the IL-17 response pathway100–102. The fibroblasts of all IL-17RA-, IL-17RC-, and ACT1-deficient patients failed to respond to IL-17A and IL-17F homodimers and heterodimers95, 97–99, 103. In addition, IL-17RA-and ACT1-deficient PBMCs, unlike PBMCs from patients with AR IL-17RC deficiency, failed to respond to IL17E/IL-2598, 103, which signals through IL-17RA/IL-17RB in an ACT1 dependent manner104. An AD deficiency of JNK1, a component of the MAPK signaling pathway105–106, was recently identified in a multiplex family originating from France with syndromic CMCD, in which three individuals from three generations presented early-onset CMC, mucocutaneous S. aureus infections, and a complex connective tissue disorder107. In vitro studies showed that the private MAPK8 c.311+1G>A identified in the three patients was a loss-of-expression variant. JNK1 is involved in various signaling pathways, including the IL-17 pathway in particular108–110. Accordingly, the fibroblasts of heterozygous patients displayed impaired cellular responses to IL-17A and IL-17F. JNK1 also acts downstream from TGFβ1, which has been shown to participate in human Th17 differentiation in vitro84, 111–112. The proportions of ex vivo and in vitro-differentiated Th17 cells were indeed low for these patients. This study reported a fifth genetic disorder in the IL-17 response pathway, underlying AD CMCD by haploinsufficiency, with impaired cellular responses to IL-17A/F and impaired IL-17A/F production (Figure). Altogether, these five human genetic disorders demonstrate the essential role of IL-17A- and IL-17F-mediated immunity in mucocutaneous protection against Candida and, to a lesser extent, as found in IL-17RA-, ACT1- and JNK1-deficient patients, against disease caused by S. aureus. They also suggest that IL-17A- and IL-17F-dependent immunity is otherwise redundant for protection against fungi other than Candida, bacteria other than S. aureus, viruses, or even against invasive candidiasis or staphylococcal disease.
Invasive candidiasis and inborn errors of CARD9 immunity
Invasive candidiasis (IC) is defined as infections of the bloodstream (candidemia) or deep-seated infections caused by Candida spp. and it ranks among the most frequent healthcare-associated bloodstream infections1, 113. Unlike CMC, patients with broad T-cell disorders are not particularly prone to IC. Furthermore, very few patients with CMC display IC, and vice versa. This suggests that T-cell dependent immunity is not essential for protection against IC and that different mechanisms are involved in immunity to superficial and invasive candidiasis. In IC, phagocytes, neutrophils in particular, but probably also, to a lesser extent, based on mouse studies, monocytes, macrophages and dendritic cells, are essential for protective immunity11. Indeed, IC is classically described in patients with acquired profound qualitative or quantitative disorders of neutrophils and monocytes/macrophages8. IC is relatively rare among patients with PID, and has been reported only occasionally in this context. Patients with severe congenital neutropenia (SCN), and mutations of ELA2, HAX1, or other genes, may develop syndromic IC. For example, IC was reported in 2% (n=486) of the patients from the French SCN registry30. A few patients with AR leukocyte adhesion disorder type-1 (LAD-1), due to CD18 deficiency resulting from biallelic mutations of ITGB2 and leading to the impaired endothelial adhesion and transmigration of neutrophils into infected tissues, may display IC114–116. In both cases, IC probably results from impaired neutrophil accumulation at the site of infection. Some patients with complete AR myeloperoxidase (MPO) deficiency and concomitant diabetes or with X-linked or AR chronic granulomatous disease (CGD) caused by mutations of genes encoding NADPH oxidase subunits and impaired oxidative burst-dependent Candida killing by phagocytes may also display syndromic IC117–118. Cases of deep-seated organ candidiasis have been reported in CGD patients, with central nervous system (CNS), soft tissue, lymph node, or liver diseases, and enhanced susceptibility to C. lusitaniae, a Candida spp. rarely disease-causing in non-CGD patients119–121. Finally, in a large international study of patients with STAT1 GOF (n=274), 3.6% were reported to have syndromic IC50.
Since its discovery in 2009, in a large multiplex consanguineous family from Iran with CMC and possible brain disease caused by Candida spp.66, CARD9 (C-type lectin receptor adaptor caspase recruitment domain-containing protein 9) deficiency has emerged as the only known inborn error of immunity conferring a selective susceptibility to fungal diseases in otherwise healthy individuals, with no other infectious or noninfectious manifestations32, 36, 122. Over 70% of patients with CARD9 deficiency have developed IC, with a strong tropism for the CNS. Indeed, about 80% of patients with probable or proven IC have CNS diseases, such as meningoencephalitis, brain abscesses, masses mimicking metastasis, or a combination of these manifestations32, 66, 68, 123–130. Strikingly, these patients present no concomitant diseases of the kidney, liver, or spleen, as typically seen in CARD9-expressing infected patients, probably as a result of CARD9-independent mechanisms of protective immunity131. Gastrointestinal tract, bone, eye, intra-abdominal organ (liver and mesenteric LNs), or mucocutaneous surface involvement may also occur, as some patients have been reported to have severe colitis, osteomyelitis, endophthalmitis, intra-abdominal candidiasis, or CMC66–68, 125, 127–128, 132–134. The onset of invasive disease is particularly variable, with a substantial proportion of CARD9-deficient patients presenting with IC as adults, with a mean age of 21.9 years (median age: 17.5 years; range [3.5–58.0 years]). CARD9-deficient patients with CMC and/or IC have been identified in nine countries around the world (Algeria, Morocco, Iran, Turkey, Pakistan, Canada, Italy, El Salvador, and South Korea), and one patient was of mixed European origin. Of all Candida spp., C. albicans is the most frequently involved in infections. It has been detected in 93% of patients, the other Candida spp. detected, C. glabrata and C. dubliniensis, each being found in a single patient32.
CARD9, which is part of the CARD9/BCL10/MALT1 (CBM) complex, is mainly expressed in phagocytic cells, and transduces signals downstream from C-type lectin receptors (CLRs), including Dectin-1 (CLEC7A), Dectin-2 (CLEC6A), Dectin-3 (CLEC4D), and Mincle (CLEC4E), which are specific for β-glucans (Dectin-1), α-mannans (Dectin-2 and Dectin-3), and glycolipids (Mincle) from the fungal cell walls135–136. In humans, upon receptor stimulation and SYK activation, the CBM complex activates the NF-κB, MAPK, and ERK pathways, thereby stimulating the transcription of genes encoding pro-inflammatory cytokines and chemokines, such as IL-2, IL-10, IL-12, tumor necrosis factor (TNF)-α, pro-Th17 cytokines (IL-1β, IL-6, IL-23), granulocyte-macrophage colony-stimulating factor (GMCSF), and CXCL1 or CXCL2137.
CARD9-deficient patients have no overt immunological phenotype: they have normal leukocyte counts; when tested, T-cell proliferation in response to mitogen or antigens is mostly normal, and the phagocyte oxidative burst, tested in vitro in the dihydrorhodamine (DHR) assay, is also normal. However, high eosinophil counts, high serum IgE levels, or both, have been observed in several CARD9-deficient patients, and the reasons for this remain unknown32. CSF samples were analyzed in some patients and revealed hyperproteinorrhachia, and hypoglycorrhachia and pleocytosis, mostly with mononuclear cells (lymphocytes and/or monocytes) and eosinophils, but, remarkably, no neutrophils128 (by contrast to patients with Candida meningitis wild-type for CARD9, for whom neutrophils generally predominate in the CSF128). IL-17-mediated immunity was evaluated in about half the CARD9-deficient patients (with candidiasis or other fungal diseases), and was impaired in two-thirds of those tested, with no clear correlation between impaired or normal IL-17 immunity and the presence or absence of CMC32. PBMCs, monocytes, and in vitro monocyte-derived macrophages or DCs tested in vitro upon stimulation with heat-killed C. albicans displayed impaired responses in terms of pro-inflammatory cytokine or chemokine production32. A selective defect of the killing of unopsonized (but not opsonized) C. albicans yeasts but not hyphae by neutrophils has been reported in vitro and has been suggested to contribute to Candida CNS disease, due to the lower levels of opsonization in the CNS32. However, a lack of neutrophil recruitment to infection sites (e.g. CNS), consistent with the absence of neutrophils from the CSF fluids of CARD9-deficient patients with Candida CNS infections, contrasting with their blood neutrophil counts within the normal range, appears to be the major CARD9-dependent mechanism underlying IC in these patients27, 122, 128. Recently, based on Card9−/− mouse studies, abnormally low levels of IL-1β-dependent CXCL1 production by microglial cells following stimulation with the fungal toxin candidalysin were proposed as an explanation for the defective recruitment of neutrophils to the Candida-infected CNS, and impaired CNS Candida clearance128, 138.
Conclusion
The investigation of patients with PID and syndromic CMC or IC, or of otherwise healthy patients with CMC or IC provides us with a unique opportunity to elucidate the molecular and cellular bases of these diseases and to gain insight into the pathophysiological mechanisms underlying them. The knowledge gained in the context of PID can be applied to other settings, such as hematological malignancies or AIDS, for example. The spectrum of inborn errors underlying CMC and, to a lesser extent, IC, is expanding and has already provided important insight into the role of specific immune pathways in anti-Candida host defense. Indeed, IL-17-mediated immunity has emerged over the last 10 years as crucial against CMC and, to a lesser extent, mucocutaneous S. aureus diseases. However, it seems to be redundant against IC, invasive staphylococcal diseases, and other common microbes (including fungi and bacteria). Inherited CARD9 deficiency is a genetic etiology of CMC and IC. Remarkably, Candida diseases can occur at any age, from early childhood to late adulthood. The adult onset seen in several CARD9-deficient patients is an uncommon feature of inborn errors of immunity and should lead clinicians to consider CARD9 deficiency in adults presenting with unexplained Candida diseases. Next-generation sequencing in patients with CMC or IC without inborn errors of the IL-17 pathway or CARD9 will probably reveal new genetic defects that may further elucidate the pathogenesis of Candida infections in patients with inborn errors of IL-17 immunity or CARD9. The comprehensive genetic dissection of Candida diseases (in patients with syndromic PID or in otherwise healthy individuals) should shed new light on the molecular and cellular mechanisms conferring protective immunity to Candida spp., and should pave the way for more rational therapies based on a better understanding of the underlying pathophysiological mechanisms. The clinical implications extend well beyond patients with fungal diseases due to inborn errors of immunity, to patients with fungal diseases due to other causes.
Acknowledgments
I warmly thank Jean-Laurent Casanova and the members of the HGID laboratory for helpful discussions. This work was supported by the St. Giles Foundation, the Rockefeller University, INSERM, Paris Descartes University, HHMI, and the French National Research Agency (ANR) under the “Investissement d’avenir” program (grant number ANR-10-IAHU-01), the “LTh-MSMD-CMCD” project (Grant ANR-18-CE93-0008-01), the “HGDIFD” project (grant number ANR-14-CE15-0006-01) and the “EURO-CMC” project (grant number ANR-14-RARE-0005-02).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Bibliography
- 1.Kullberg BJ; Arendrup MC, Invasive Candidiasis. N Engl J Med 2016, 374 (8), 794–5. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA; Diekema DJ, Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007, 20 (1), 133–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner SA; Butler G, The Candida pathogenic species complex. Cold Spring Harb Perspect Med 2014, 4 (9), a019778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horn DL; Neofytos D; Anaissie EJ; Fishman JA; Steinbach WJ; Olyaei AJ; Marr KA; Pfaller MA; Chang CH; Webster KM, Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 2009, 48 (12), 1695–703. [DOI] [PubMed] [Google Scholar]
- 5.Pfaller MA; Diekema DJ; Turnidge JD; Castanheira M; Jones RN, Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infect Dis 2019, 6 (Suppl 1), S79–S94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klingspor L; Tortorano AM; Peman J; Willinger B; Hamal P; Sendid B; Velegraki A; Kibbler C; Meis JF; Sabino R; Ruhnke M; Arikan-Akdagli S; Salonen J; Doczi I, Invasive Candida infections in surgical patients in intensive care units: a prospective, multicentre survey initiated by the European Confederation of Medical Mycology (ECMM) (2006–2008). Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2015, 21 (1), 87 e1–87 e10. [DOI] [PubMed] [Google Scholar]
- 7.Leroy O; Gangneux JP; Montravers P; Mira JP; Gouin F; Sollet JP; Carlet J; Reynes J; Rosenheim M; Regnier B; Lortholary O; AmarCand Study G, Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med 2009, 37 (5), 1612–8. [DOI] [PubMed] [Google Scholar]
- 8.Wisplinghoff H; Bischoff T; Tallent SM; Seifert H; Wenzel RP; Edmond MB, Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004, 39 (3), 309–17. [DOI] [PubMed] [Google Scholar]
- 9.Kim J; Sudbery P, Candida albicans, a major human fungal pathogen. J Microbiol 2011, 49 (2), 171–7. [DOI] [PubMed] [Google Scholar]
- 10.Eggimann P; Bille J; Marchetti O, Diagnosis of invasive candidiasis in the ICU. Ann Intensive Care 2011, 1, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas PG; Lionakis MS; Arendrup MC; Ostrosky-Zeichner L; Kullberg BJ, Invasive candidiasis. Nat Rev Dis Primers 2018, 4, 18026. [DOI] [PubMed] [Google Scholar]
- 12.Chakrabarti A; Singh R, The emerging epidemiology of mould infections in developing countries. Curr Opin Infect Dis 2011, 24 (6), 521–6. [DOI] [PubMed] [Google Scholar]
- 13.Lass-Florl C, The changing face of epidemiology of invasive fungal disease in Europe. Mycoses 2009, 52 (3), 197–205. [DOI] [PubMed] [Google Scholar]
- 14.Casadevall A, Global Catastrophic Threats from the Fungal Kingdom : Fungal Catastrophic Threats. Curr Top Microbiol Immunol 2019. [DOI] [PubMed] [Google Scholar]
- 15.Jeffery-Smith A; Taori SK; Schelenz S; Jeffery K; Johnson EM; Borman A; Candida auris Incident Management, T.; Manuel R; Brown CS, Candida auris: a Review of the Literature. Clin Microbiol Rev 2018, 31 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng MF; Yang YL; Yao TJ; Lin CY; Liu JS; Tang RB; Yu KW; Fan YH; Hsieh KS; Ho M; Lo HJ, Risk factors for fatal candidemia caused by Candida albicans and non-albicans Candida species. BMC Infect Dis 2005, 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almirante B; Rodriguez D; Park BJ; Cuenca-Estrella M; Planes AM; Almela M; Mensa J; Sanchez F; Ayats J; Gimenez M; Saballs P; Fridkin SK; Morgan J; Rodriguez-Tudela JL; Warnock DW; Pahissa A; Barcelona Candidemia Project Study, G., Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, barcelona, Spain, from 2002 to 2003. J Clin Microbiol 2005, 43 (4), 1829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudlaugsson O; Gillespie S; Lee K; Vande Berg J; Hu J; Messer S; Herwaldt L; Pfaller M; Diekema D, Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis 2003, 37 (9), 1172–7. [DOI] [PubMed] [Google Scholar]
- 19.de Repentigny L; Lewandowski D; Jolicoeur P, Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin Microbiol Rev 2004, 17 (4), 729–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortega M; Marco F; Soriano A; Almela M; Martinez JA; Lopez J; Pitart C; Mensa J, Candida species bloodstream infection: epidemiology and outcome in a single institution from 1991 to 2008. J Hosp Infect 2011, 77 (2), 157–61. [DOI] [PubMed] [Google Scholar]
- 21.Barchiesi F; Orsetti E; Mazzanti S; Trave F; Salvi A; Nitti C; Manso E, Candidemia in the elderly: What does it change? PLoS One 2017, 12 (5), e0176576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H; Liu N; Yin M; Han H; Yue J; Zhang F; Shan T; Guo H; Wu D, The epidemiology, antifungal use and risk factors of death in elderly patients with candidemia: a multicentre retrospective study. BMC Infect Dis 2014, 14, 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barton M; O’Brien K; Robinson JL; Davies DH; Simpson K; Asztalos E; Langley JM; Le Saux N; Sauve R; Synnes A; Tan B; de Repentigny L; Rubin E; Hui C; Kovacs L; Richardson SE, Invasive candidiasis in low birth weight preterm infants: risk factors, clinical course and outcome in a prospective multicenter study of cases and their matched controls. BMC Infect Dis 2014, 14, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez M; Moylett EH; Noyola DE; Baker CJ, Candidal meningitis in neonates: a 10-year review. Clin Infect Dis 2000, 31 (2), 458–63. [DOI] [PubMed] [Google Scholar]
- 25.Huang CC; Chen CY; Yang HB; Wang SM; Chang YC; Liu CC, Central nervous system candidiasis in very low-birth-weight premature neonates and infants: US characteristics and histopathologic and MR imaging correlates in five patients. Radiology 1998, 209 (1), 49–56. [DOI] [PubMed] [Google Scholar]
- 26.Pahud BA; Greenhow TL; Piecuch B; Weintrub PS, Preterm neonates with candidal brain microabscesses: a case series. J Perinatol 2009, 29 (4), 323–6. [DOI] [PubMed] [Google Scholar]
- 27.Drummond RA; Lionakis MS, Candidiasis of the Central Nervous System in Neonates and Children with Primary Immunodeficiencies. Curr Fungal Infect Rep 2018, 12 (2), 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J; Vinh DC; Casanova JL; Puel A, Inborn errors of immunity underlying fungal diseases in otherwise healthy individuals. Curr Opin Microbiol 2017, 40, 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilmis B; Puel A; Lortholary O; Lanternier F, New clinical phenotypes of fungal infections in special hosts. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2016, 22 (8), 681–7. [DOI] [PubMed] [Google Scholar]
- 30.Lanternier F; Cypowyj S; Picard C; Bustamante J; Lortholary O; Casanova JL; Puel A, Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr 2013, 25 (6), 736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antachopoulos C; Walsh TJ; Roilides E, Fungal infections in primary immunodeficiencies. Eur J Pediatr 2007, 166 (11), 1099–117. [DOI] [PubMed] [Google Scholar]
- 32.Corvilain E; Casanova JL; Puel A, Inherited CARD9 Deficiency: Invasive Disease Caused by Ascomycete Fungi in Previously Healthy Children and Adults. J Clin Immunol 2018, 38 (6), 656–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada S; Puel A; Casanova JL; Kobayashi M, Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. Clinical & translational immunology 2016, 5 (12), e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puel A; Cypowyj S; Marodi L; Abel L; Picard C; Casanova JL, Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol 2012, 12 (6), 616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puel A; Picard C; Cypowyj S; Lilic D; Abel L; Casanova JL, Inborn errors of mucocutaneous immunity to Candida albicans in humans: a role for IL-17 cytokines? Curr Opin Immunol 2010, 22 (4), 467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drummond RA; Franco LM; Lionakis MS, Human CARD9: A Critical Molecule of Fungal Immune Surveillance. Front Immunol 2018, 9, 1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perfect JR, The antifungal pipeline: a reality check. Nat Rev Drug Discov 2017, 16 (9), 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glocker E; Grimbacher B, Chronic mucocutaneous candidiasis and congenital susceptibility to Candida. Curr Opin Allergy Clin Immunol 2010, 10 (6), 542–50. [DOI] [PubMed] [Google Scholar]
- 39.Vazquez JA; Sobel JD, Mucosal candidiasis. Infect Dis Clin North Am 2002, 16 (4), 793–820, v. [DOI] [PubMed] [Google Scholar]
- 40.Eyerich K; Foerster S; Rombold S; Seidl HP; Behrendt H; Hofmann H; Ring J; Traidl-Hoffmann C, Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J Invest Dermatol 2008, 128 (11), 2640–5. [DOI] [PubMed] [Google Scholar]
- 41.Kirkpatrick CH, Chronic mucocutaneous candidiasis. Pediatr Infect Dis J 2001, 20 (2), 197–206. [DOI] [PubMed] [Google Scholar]
- 42.Lilic D, New perspectives on the immunology of chronic mucocutaneous candidiasis. Curr Opin Infect Dis 2002, 15 (2), 143–7. [DOI] [PubMed] [Google Scholar]
- 43.Eyerich K; Eyerich S; Hiller J; Behrendt H; Traidl-Hoffmann C, Chronic mucocutaneous candidiasis, from bench to bedside. Eur J Dermatol 2010, 20 (3), 260–5. [DOI] [PubMed] [Google Scholar]
- 44.Denning DW; Kneale M; Sobel JD; Rautemaa-Richardson R, Global burden of recurrent vulvovaginal candidiasis: a systematic review. The Lancet infectious diseases 2018, 18 (11), e339–e347. [DOI] [PubMed] [Google Scholar]
- 45.Pirofski LA; Casadevall A, Rethinking T cell immunity in oropharyngeal candidiasis. J Exp Med 2009, 206 (2), 269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vinh DC, Insights into human antifungal immunity from primary immunodeficiencies. The Lancet infectious diseases 2011, 11 (10), 780–792. [DOI] [PubMed] [Google Scholar]
- 47.Picard C; Bobby Gaspar H; Al-Herz W; Bousfiha A; Casanova JL; Chatila T; Crow YJ; Cunningham-Rundles C; Etzioni A; Franco JL; Holland SM; Klein C; Morio T; Ochs HD; Oksenhendler E; Puck J; Tang MLK; Tangye SG; Torgerson TR; Sullivan KE, International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J Clin Immunol 2018, 38 (1), 96–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olbrich P; Freeman AF, STAT1 and STAT3 mutations: important lessons for clinical immunologists. Expert Rev Clin Immunol 2018, 14 (12), 1029–1041. [DOI] [PubMed] [Google Scholar]
- 49.Depner M; Fuchs S; Raabe J; Frede N; Glocker C; Doffinger R; Gkrania-Klotsas E; Kumararatne D; Atkinson TP; Schroeder HW Jr.; Niehues T; Duckers G; Stray-Pedersen A; Baumann U; Schmidt R; Franco JL; Orrego J; Ben-Shoshan M; McCusker C; Jacob CM; Carneiro-Sampaio M; Devlin LA; Edgar JD; Henderson P; Russell RK; Skytte AB; Seneviratne SL; Wanders J; Stauss H; Meyts I; Moens L; Jesenak M; Kobbe R; Borte S; Borte M; Wright DA; Hagin D; Torgerson TR; Grimbacher B, The Extended Clinical Phenotype of 26 Patients with Chronic Mucocutaneous Candidiasis due to Gain-of-Function Mutations in STAT1. J Clin Immunol 2016, 36 (1), 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toubiana J; Okada S; Hiller J; Oleastro M; Lagos Gomez M; Aldave Becerra JC; Ouachee-Chardin M; Fouyssac F; Girisha KM; Etzioni A; Van Montfrans J; Camcioglu Y; Kerns LA; Belohradsky B; Blanche S; Bousfiha A; Rodriguez-Gallego C; Meyts I; Kisand K; Reichenbach J; Renner ED; Rosenzweig S; Grimbacher B; van de Veerdonk FL; Traidl-Hoffmann C; Picard C; Marodi L; Morio T; Kobayashi M; Lilic D; Milner JD; Holland S; Casanova JL; Puel A; International S. G.-o.-F. S. G., Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood 2016, 127 (25), 3154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boisson-Dupuis S; Kong XF; Okada S; Cypowyj S; Puel A; Abel L; Casanova JL, Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes. Curr Opin Immunol 2012, 24 (4), 364–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engelhardt KR; Grimbacher B, Mendelian traits causing susceptibility to mucocutaneous fungal infections in human subjects. J Allergy Clin Immunol 2012, 129 (2), 294–305; quiz 306–7. [DOI] [PubMed] [Google Scholar]
- 53.Liu L; Okada S; Kong XF; Kreins AY; Cypowyj S; Abhyankar A; Toubiana J; Itan Y; Audry M; Nitschke P; Masson C; Toth B; Flatot J; Migaud M; Chrabieh M; Kochetkov T; Bolze A; Borghesi A; Toulon A; Hiller J; Eyerich S; Eyerich K; Gulacsy V; Chernyshova L; Chernyshov V; Bondarenko A; Grimaldo RM; Blancas-Galicia L; Beas IM; Roesler J; Magdorf K; Engelhard D; Thumerelle C; Burgel PR; Hoernes M; Drexel B; Seger R; Kusuma T; Jansson AF; Sawalle-Belohradsky J; Belohradsky B; Jouanguy E; Bustamante J; Bue M; Karin N; Wildbaum G; Bodemer C; Lortholary O; Fischer A; Blanche S, et al. , Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med 2011, 208 (8), 1635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van de Veerdonk FL; Plantinga TS; Hoischen A; Smeekens SP; Joosten LA; Gilissen C; Arts P; Rosentul DC; Carmichael AJ; Smits-van der Graaf CA; Kullberg BJ; van der Meer JW; Lilic D; Veltman JA; Netea MG, STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med 2011, 365 (1), 54–61. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Q; Boisson B; Beziat V; Puel A; Casanova JL, Human hyper-IgE syndrome: singular or plural? Mamm Genome 2018, 29 (7–8), 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paulson ML; Freeman AF; Holland SM, Hyper IgE syndrome: an update on clinical aspects and the role of signal transducer and activator of transcription 3. Curr Opin Allergy Clin Immunol 2008, 8 (6), 527–33. [DOI] [PubMed] [Google Scholar]
- 57.Minegishi Y, Hyper-IgE syndrome. Curr Opin Immunol 2009, 21 (5), 487–92. [DOI] [PubMed] [Google Scholar]
- 58.Frey-Jakobs S; Hartberger JM; Fliegauf M; Bossen C; Wehmeyer ML; Neubauer JC; Bulashevska A; Proietti M; Frobel P; Noltner C; Yang L; Rojas-Restrepo J; Langer N; Winzer S; Engelhardt KR; Glocker C; Pfeifer D; Klein A; Schaffer AA; Lagovsky I; Lachover-Roth I; Beziat V; Puel A; Casanova JL; Fleckenstein B; Weidinger S; Kilic SS; Garty BZ; Etzioni A; Grimbacher B, ZNF341 controls STAT3 expression and thereby immunocompetence. Science immunology 2018, 3 (24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beziat V; Li J; Lin JX; Ma CS; Li P; Bousfiha A; Pellier I; Zoghi S; Baris S; Keles S; Gray P; Du N; Wang Y; Zerbib Y; Levy R; Leclercq T; About F; Lim AI; Rao G; Payne K; Pelham SJ; Avery DT; Deenick EK; Pillay B; Chou J; Guery R; Belkadi A; Guerin A; Migaud M; Rattina V; Ailal F; Benhsaien I; Bouaziz M; Habib T; Chaussabel D; Marr N; El-Benna J; Grimbacher B; Wargon O; Bustamante J; Boisson B; Muller-Fleckenstein I; Fleckenstein B; Chandesris MO; Titeux M; Fraitag S; Alyanakian MA; Leruez-Ville M; Picard C; Meyts I, et al. , A recessive form of hyper-IgE syndrome by disruption of ZNF341-dependent STAT3 transcription and activity. Science immunology 2018, 3 (24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Husebye ES; Perheentupa J; Rautemaa R; Kampe O, Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I. J Intern Med 2009, 265 (5), 514–29. [DOI] [PubMed] [Google Scholar]
- 61.Constantine GM; Lionakis MS, Lessons from primary immunodeficiencies: Autoimmune regulator and autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Immunol Rev 2019, 287 (1), 103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferre EM; Rose SR; Rosenzweig SD; Burbelo PD; Romito KR; Niemela JE; Rosen LB; Break TJ; Gu W; Hunsberger S; Browne SK; Hsu AP; Rampertaap S; Swamydas M; Collar AL; Kong HH; Lee CR; Chascsa D; Simcox T; Pham A; Bondici A; Natarajan M; Monsale J; Kleiner DE; Quezado M; Alevizos I; Moutsopoulos NM; Yockey L; Frein C; Soldatos A; Calvo KR; Adjemian J; Similuk MN; Lang DM; Stone KD; Uzel G; Kopp JB; Bishop RJ; Holland SM; Olivier KN; Fleisher TA; Heller T; Winer KK; Lionakis MS, Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI insight 2016, 1 (13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okada S; Markle JG; Deenick EK; Mele F; Averbuch D; Lagos M; Alzahrani M; Al-Muhsen S; Halwani R; Ma CS; Wong N; Soudais C; Henderson LA; Marzouqa H; Shamma J; Gonzalez M; Martinez-Barricarte R; Okada C; Avery DT; Latorre D; Deswarte C; Jabot-Hanin F; Torrado E; Fountain J; Belkadi A; Itan Y; Boisson B; Migaud M; Arlehamn CSL; Sette A; Breton S; McCluskey J; Rossjohn J; de Villartay JP; Moshous D; Hambleton S; Latour S; Arkwright PD; Picard C; Lantz O; Engelhard D; Kobayashi M; Abel L; Cooper AM; Notarangelo LD; Boisson-Dupuis S; Puel A; Sallusto F; Bustamante J; Tangye SG, et al. , IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with biallelic RORC mutations. Science 2015, 349 (6248), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosain J; Kong XF; Martinez-Barricarte R; Oleaga-Quintas C; Ramirez-Alejo N; Markle J; Okada S; Boisson-Dupuis S; Casanova JL; Bustamante J, Mendelian susceptibility to mycobacterial disease: 2014–2018 update. Immunol Cell Biol 2019, 97 (4), 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bustamante J; Boisson-Dupuis S; Abel L; Casanova JL, Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Seminars in immunology 2014, 26 (6), 454–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glocker EO; Hennigs A; Nabavi M; Schaffer AA; Woellner C; Salzer U; Pfeifer D; Veelken H; Warnatz K; Tahami F; Jamal S; Manguiat A; Rezaei N; Amirzargar AA; Plebani A; Hannesschlager N; Gross O; Ruland J; Grimbacher B, A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med 2009, 361 (18), 1727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lanternier F; Pathan S; Vincent QB; Liu L; Cypowyj S; Prando C; Migaud M; Taibi L; Ammar-Khodja A; Stambouli OB; Guellil B; Jacobs F; Goffard JC; Schepers K; Del Marmol V; Boussofara L; Denguezli M; Larif M; Bachelez H; Michel L; Lefranc G; Hay R; Jouvion G; Chretien F; Fraitag S; Bougnoux ME; Boudia M; Abel L; Lortholary O; Casanova JL; Picard C; Grimbacher B; Puel A, Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med 2013, 369 (18), 1704–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alves de Medeiros AK; Lodewick E; Bogaert DJ; Haerynck F; Van Daele S; Lambrecht B; Bosma S; Vanderdonckt L; Lortholary O; Migaud M; Casanova JL; Puel A; Lanternier F; Lambert J; Brochez L; Dullaers M, Chronic and Invasive Fungal Infections in a Family with CARD9 Deficiency. J Clin Immunol 2016, 36 (3), 204–9. [DOI] [PubMed] [Google Scholar]
- 69.Kisand K; Lilic D; Casanova JL; Peterson P; Meager A; Willcox N, Mucocutaneous candidiasis and autoimmunity against cytokines in APECED and thymoma patients: clinical and pathogenetic implications. Eur J Immunol 2011, 41 (6), 1517–27. [DOI] [PubMed] [Google Scholar]
- 70.Korn T; Bettelli E; Oukka M; Kuchroo VK, IL-17 and Th17 Cells. Annu Rev Immunol 2009, 27, 485–517. [DOI] [PubMed] [Google Scholar]
- 71.Miossec P; Korn T; Kuchroo VK, Interleukin-17 and type 17 helper T cells. N Engl J Med 2009, 361 (9), 888–98. [DOI] [PubMed] [Google Scholar]
- 72.Hiller J; Hagl B; Effner R; Puel A; Schaller M; Mascher B; Eyerich S; Eyerich K; Jansson AF; Ring J; Casanova JL; Renner ED; Traidl-Hoffmann C, STAT1 Gain-of-Function and Dominant Negative STAT3 Mutations Impair IL-17 and IL-22 Immunity Associated with CMC. J Invest Dermatol 2018, 138 (3), 711–714. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y; Ma CA; Lawrence MG; Break TJ; O’Connell MP; Lyons JJ; Lopez DB; Barber JS; Zhao Y; Barber DL; Freeman AF; Holland SM; Lionakis MS; Milner JD, PD-L1 up-regulation restrains Th17 cell differentiation in STAT3 loss- and STAT1 gain-of-function patients. J Exp Med 2017, 214 (9), 2523–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma CS; Wong N; Rao G; Nguyen A; Avery DT; Payne K; Torpy J; O’Young P; Deenick E; Bustamante J; Puel A; Okada S; Kobayashi M; Martinez-Barricarte R; Elliott M; Sebnem Kilic S; El Baghdadi J; Minegishi Y; Bousfiha A; Robertson N; Hambleton S; Arkwright PD; French M; Blincoe AK; Hsu P; Campbell DE; Stormon MO; Wong M; Adelstein S; Fulcher DA; Cook MC; Stepensky P; Boztug K; Beier R; Ikinciogullari A; Ziegler JB; Gray P; Picard C; Boisson-Dupuis S; Phan TG; Grimbacher B; Warnatz K; Holland SM; Uzel G; Casanova JL; Tangye SG, Unique and shared signaling pathways cooperate to regulate the differentiation of human CD4+ T cells into distinct effector subsets. J Exp Med 2016, 213 (8), 1589–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Louten J; Boniface K; de Waal Malefyt R, Development and function of TH17 cells in health and disease. J Allergy Clin Immunol 2009, 123 (5), 1004–11. [DOI] [PubMed] [Google Scholar]
- 76.Guo B; Chang EY; Cheng G, The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest 2008, 118 (5), 1680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diveu C; McGeachy MJ; Boniface K; Stumhofer JS; Sathe M; Joyce-Shaikh B; Chen Y; Tato CM; McClanahan TK; de Waal Malefyt R; Hunter CA; Cua DJ; Kastelein RA, IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol 2009, 182 (9), 5748–56. [DOI] [PubMed] [Google Scholar]
- 78.El-behi M; Ciric B; Yu S; Zhang GX; Fitzgerald DC; Rostami A, Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol 2009, 183 (8), 4957–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirahara K; Ghoreschi K; Laurence A; Yang XP; Kanno Y; O’Shea JJ, Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine Growth Factor Rev 2010, 21 (6), 425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minegishi Y; Saito M; Nagasawa M; Takada H; Hara T; Tsuchiya S; Agematsu K; Yamada M; Kawamura N; Ariga T; Tsuge I; Karasuyama H, Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med 2009, 206 (6), 1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Milner JD; Brenchley JM; Laurence A; Freeman AF; Hill BJ; Elias KM; Kanno Y; Spalding C; Elloumi HZ; Paulson ML; Davis J; Hsu A; Asher AI; O’Shea J; Holland SM; Paul WE; Douek DC, Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 2008, 452 (7188), 773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma CS; Chew GY; Simpson N; Priyadarshi A; Wong M; Grimbacher B; Fulcher DA; Tangye SG; Cook MC, Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med 2008, 205 (7), 1551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Beaucoudrey L; Puel A; Filipe-Santos O; Cobat A; Ghandil P; Chrabieh M; Feinberg J; von Bernuth H; Samarina A; Janniere L; Fieschi C; Stephan JL; Boileau C; Lyonnet S; Jondeau G; Cormier-Daire V; Le Merrer M; Hoarau C; Lebranchu Y; Lortholary O; Chandesris MO; Tron F; Gambineri E; Bianchi L; Rodriguez-Gallego C; Zitnik SE; Vasconcelos J; Guedes M; Vitor AB; Marodi L; Chapel H; Reid B; Roifman C; Nadal D; Reichenbach J; Caragol I; Garty BZ; Dogu F; Camcioglu Y; Gulle S; Sanal O; Fischer A; Abel L; Stockinger B; Picard C; Casanova JL, Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med 2008, 205 (7), 1543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manel N; Unutmaz D; Littman DR, The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol 2008, 9 (6), 641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ivanov II; McKenzie BS; Zhou L; Tadokoro CE; Lepelley A; Lafaille JJ; Cua DJ; Littman DR, The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126 (6), 1121–33. [DOI] [PubMed] [Google Scholar]
- 86.Yang XO; Pappu BP; Nurieva R; Akimzhanov A; Kang HS; Chung Y; Ma L; Shah B; Panopoulos AD; Schluns KS; Watowich SS; Tian Q; Jetten AM; Dong C, T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 2008, 28 (1), 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu J; Yamane H; Paul WE, Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 2010, 28, 445–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X; Wang W; Lin Z; Wang X; Li T; Yu J; Liu W; Tong Z; Xu Y; Zhang J; Guan L; Dai L; Yang Y; Han W; Li R, CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J Allergy Clin Immunol 2014, 133 (3), 905–8 e3. [DOI] [PubMed] [Google Scholar]
- 89.Finnish-German AC, An autoimmune disease APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet 1997, 17 (4), 399–403. [DOI] [PubMed] [Google Scholar]
- 90.Nagamine K; Peterson P; Scott HS; Kudoh J; Minoshima S; Heino M; Krohn KJ; Lalioti MD; Mullis PE; Antonarakis SE; Kawasaki K; Asakawa S; Ito F; Shimizu N, Positional cloning of the APECED gene. Nat Genet 1997, 17 (4), 393–8. [DOI] [PubMed] [Google Scholar]
- 91.Meyer S; Woodward M; Hertel C; Vlaicu P; Haque Y; Karner J; Macagno A; Onuoha SC; Fishman D; Peterson H; Metskula K; Uibo R; Jantti K; Hokynar K; Wolff ASB; collaborative A. p.; Krohn K; Ranki A; Peterson P; Kisand K; Hayday A, AIRE-Deficient Patients Harbor Unique High-Affinity Disease-Ameliorating Autoantibodies. Cell 2016, 166 (3), 582–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meager A; Visvalingam K; Peterson P; Moll K; Murumagi A; Krohn K; Eskelin P; Perheentupa J; Husebye E; Kadota Y; Willcox N, Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med 2006, 3 (7), e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kisand K; Boe Wolff AS; Podkrajsek KT; Tserel L; Link M; Kisand KV; Ersvaer E; Perheentupa J; Erichsen MM; Bratanic N; Meloni A; Cetani F; Perniola R; Ergun-Longmire B; Maclaren N; Krohn KJ; Pura M; Schalke B; Strobel P; Leite MI; Battelino T; Husebye ES; Peterson P; Willcox N; Meager A, Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 2010, 207 (2), 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Puel A; Doffinger R; Natividad A; Chrabieh M; Barcenas-Morales G; Picard C; Cobat A; Ouachee-Chardin M; Toulon A; Bustamante J; Al-Muhsen S; Al-Owain M; Arkwright PD; Costigan C; McConnell V; Cant AJ; Abinun M; Polak M; Bougneres PF; Kumararatne D; Marodi L; Nahum A; Roifman C; Blanche S; Fischer A; Bodemer C; Abel L; Lilic D; Casanova JL, Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med 2010, 207 (2), 291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Puel A; Cypowyj S; Bustamante J; Wright JF; Liu L; Lim HK; Migaud M; Israel L; Chrabieh M; Audry M; Gumbleton M; Toulon A; Bodemer C; El-Baghdadi J; Whitters M; Paradis T; Brooks J; Collins M; Wolfman NM; Al-Muhsen S; Galicchio M; Abel L; Picard C; Casanova JL, Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 2011, 332 (6025), 65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bader O; Weig MS; Gross U; Schon MP; Mempel M; Buhl T, Photo quiz. A 32-year-old man with ulcerative mucositis, skin lesions, and nail dystrophy. Chronic mucocutaneous candidiasis by multidrug-resistant Candida albicans. Clin Infect Dis 2012, 54 (7), 972, 1035–6. [DOI] [PubMed] [Google Scholar]
- 97.Fellmann F; Angelini F; Wassenberg J; Perreau M; Arenas Ramirez N; Simon G; Boyman O; Demaria O; Christen-Zaech S; Hohl D; Belfiore M; von Scheven-Gete A; Gilliet M; Bochud PY; Perrin Y; Beck Popovic M; Bart PA; Beckmann JS; Martinet D; Hofer M, IL-17 receptor A and adenosine deaminase 2 deficiency in siblings with recurrent infections and chronic inflammation. J Allergy Clin Immunol 2016, 137 (4), 1189–1196 e2. [DOI] [PubMed] [Google Scholar]
- 98.Levy R; Okada S; Beziat V; Moriya K; Liu C; Chai LY; Migaud M; Hauck F; Al Ali A; Cyrus C; Vatte C; Patiroglu T; Unal E; Ferneiny M; Hyakuna N; Nepesov S; Oleastro M; Ikinciogullari A; Dogu F; Asano T; Ohara O; Yun L; Della Mina E; Bronnimann D; Itan Y; Gothe F; Bustamante J; Boisson-Dupuis S; Tahuil N; Aytekin C; Salhi A; Al Muhsen S; Kobayashi M; Toubiana J; Abel L; Li X; Camcioglu Y; Celmeli F; Klein C; AlKhater SA; Casanova JL; Puel A, Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL-17RA deficiency. Proc Natl Acad Sci USA 2016, 113 (51), E8277–E8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ling Y; Cypowyj S; Aytekin C; Galicchio M; Camcioglu Y; Nepesov S; Ikinciogullari A; Dogu F; Belkadi A; Levy R; Migaud M; Boisson B; Bolze A; Itan Y; Goudin N; Cottineau J; Picard C; Abel L; Bustamante J; Casanova JL; Puel A, Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med 2015, 212 (5), 619–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qian Y; Liu C; Hartupee J; Altuntas CZ; Gulen MF; Jane-Wit D; Xiao J; Lu Y; Giltiay N; Liu J; Kordula T; Zhang QW; Vallance B; Swaidani S; Aronica M; Tuohy VK; Hamilton T; Li X, The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol 2007, 8 (3), 247–56. [DOI] [PubMed] [Google Scholar]
- 101.Chang SH; Park H; Dong C, Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem 2006, 281 (47), 35603–7. [DOI] [PubMed] [Google Scholar]
- 102.Linden A, A role for the cytoplasmic adaptor protein Act1 in mediating IL-17 signaling. Sci STKE 2007, 2007 (398), re4. [DOI] [PubMed] [Google Scholar]
- 103.Boisson B; Wang C; Pedergnana V; Wu L; Cypowyj S; Rybojad M; Belkadi A; Picard C; Abel L; Fieschi C; Puel A; Li X; Casanova JL, An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity 2013, 39 (4), 676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gaffen SL, Structure and signalling in the IL-17 receptor family. Nature reviews. Immunology 2009, 9 (8), 556–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnson GL; Nakamura K, The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta 2007, 1773 (8), 1341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hotamisligil GS; Davis RJ, Cell Signaling and Stress Responses. Cold Spring Harb Perspect Biol 2016, 8 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li J; Ritelli M; Ma CS; Rao G; Habib T; Corvilain E; Bougarn S; Cypowyj S; Grodecka L; Levy R; Beziat V; Shang L; Payne K; Avery DT; Migaud M; Boucherit S; Boughorbel S; Guennoun A; Chrabieh M; Rapaport F; Bigio B; Itan Y; Boisson B; Cormier-Daire V; Syx D; Malfait F; Zoppi N; Abel L; Freiberger T; Dietz HC; Marr N; Tangye SG; Colombi M; Casanova JL; Puel A, Chronic mucocutaneous candidiasis and connective tissue disorder in humans with impaired JNK1-dependent responses to IL-17A/F and TGF-beta. Science immunology 2019, 4 (41). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van der Velden J; Janssen-Heininger YM; Mandalapu S; Scheller EV; Kolls JK; Alcorn JF, Differential requirement for c-Jun N-terminal kinase 1 in lung inflammation and host defense. PLoS One 2012, 7 (4), e34638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chang SH; Dong C, Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal 2011, 23 (7), 1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gaffen SL; Jain R; Garg AV; Cua DJ, The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nature reviews. Immunology 2014, 14 (9), 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Volpe E; Servant N; Zollinger R; Bogiatzi SI; Hupe P; Barillot E; Soumelis V, A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol 2008, 9 (6), 650–7. [DOI] [PubMed] [Google Scholar]
- 112.Yang L; Anderson DE; Baecher-Allan C; Hastings WD; Bettelli E; Oukka M; Kuchroo VK; Hafler DA, IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 2008, 454 (7202), 350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McCarty TP; Pappas PG, Invasive Candidiasis. Infect Dis Clin North Am 2016, 30 (1), 103–24. [DOI] [PubMed] [Google Scholar]
- 114.Kuijpers TW; van Bruggen R; Kamerbeek N; Tool AT; Hicsonmez G; Gurgey A; Karow A; Verhoeven AJ; Seeger K; Sanal O; Niemeyer C; Roos D, Natural history and early diagnosis of LAD-1/variant syndrome. Blood 2007, 109 (8), 3529–37. [DOI] [PubMed] [Google Scholar]
- 115.Fischer A; Lisowska-Grospierre B; Anderson DC; Springer TA, Leukocyte adhesion deficiency: molecular basis and functional consequences. Immunodefic Rev 1988, 1 (1), 39–54. [PubMed] [Google Scholar]
- 116.Mellouli F; Ksouri H; Barbouche R; Maamer M; Hamed LB; Hmida S; Hassen AB; Bejaoui M, Successful treatment of Fusarium solani ecthyma gangrenosum in a patient affected by leukocyte adhesion deficiency type 1 with granulocytes transfusions. BMC Dermatol 2010, 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Winkelstein JA; Marino MC; Johnston RB Jr.; Boyle J; Curnutte J; Gallin JI; Malech HL; Holland SM; Ochs H; Quie P; Buckley RH; Foster CB; Chanock SJ; Dickler H, Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000, 79 (3), 155–69. [DOI] [PubMed] [Google Scholar]
- 118.Lehrer RI; Cline MJ, Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest 1969, 48 (8), 1478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Estrada B; Mancao MY; Polski JM; Figarola MS, Candida lusitaniae and chronic granulomatous disease. Pediatr Infect Dis J 2006, 25 (8), 758–9. [DOI] [PubMed] [Google Scholar]
- 120.Levy O; Bourquin JP; McQueen A; Cantor AB; Lachenauer C; Malley R, Fatal disseminated Candida lusitaniae infection in an infant with chronic granulomatous disease. Pediatr Infect Dis J 2002, 21 (3), 262–4. [DOI] [PubMed] [Google Scholar]
- 121.Segal BH; Leto TL; Gallin JI; Malech HL; Holland SM, Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000, 79 (3), 170–200. [DOI] [PubMed] [Google Scholar]
- 122.Drummond RA; Lionakis MS, Mechanistic Insights into the Role of C-Type Lectin Receptor/CARD9 Signaling in Human Antifungal Immunity. Frontiers in cellular and infection microbiology 2016, 6, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Drewniak A; Gazendam RP; Tool AT; van Houdt M; Jansen MH; van Hamme JL; van Leeuwen EM; Roos D; Scalais E; de Beaufort C; Janssen H; van den Berg TK; Kuijpers TW, Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood 2013, 121 (13), 2385–92. [DOI] [PubMed] [Google Scholar]
- 124.Gavino C; Cotter A; Lichtenstein D; Lejtenyi D; Fortin C; Legault C; Alirezaie N; Majewski J; Sheppard DC; Behr MA; Foulkes WD; Vinh DC, CARD9 deficiency and spontaneous central nervous system candidiasis: complete clinical remission with GM-CSF therapy. Clin Infect Dis 2014, 59 (1), 81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lanternier F; Mahdaviani SA; Barbati E; Chaussade H; Koumar Y; Levy R; Denis B; Brunel AS; Martin S; Loop M; Peeters J; de Selys A; Vanclaire J; Vermylen C; Nassogne MC; Chatzis O; Liu L; Migaud M; Pedergnana V; Desoubeaux G; Jouvion G; Chretien F; Darazam IA; Schaffer AA; Netea MG; De Bruycker JJ; Bernard L; Reynes J; Amazrine N; Abel L; Van der Linden D; Harrison T; Picard C; Lortholary O; Mansouri D; Casanova JL; Puel A, Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species-induced meningoencephalitis, colitis, or both. J Allergy Clin Immunol 2015, 135 (6), 1558–68 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Herbst M; Gazendam R; Reimnitz D; Sawalle-Belohradsky J; Groll A; Schlegel PG; Belohradsky B; Renner E; Klepper J; Grimbacher B; Kuijpers T; Liese J, Chronic Candida albicans Meningitis in a 4-Year-Old Girl with a Homozygous Mutation in the CARD9 Gene (Q295X). Pediatr Infect Dis J 2015, 34 (9), 999–1002. [DOI] [PubMed] [Google Scholar]
- 127.Gavino C; Hamel N; Zeng JB; Legault C; Guiot MC; Chankowsky J; Lejtenyi D; Lemire M; Alarie I; Dufresne S; Boursiquot JN; McIntosh F; Langelier M; Behr MA; Sheppard DC; Foulkes WD; Vinh DC, Impaired RASGRF1/ERK-mediated GM-CSF response characterizes CARD9 deficiency in French-Canadians. J Allergy Clin Immunol 2016, 137 (4), 1178–1188 e7. [DOI] [PubMed] [Google Scholar]
- 128.Drummond RA; Collar AL; Swamydas M; Rodriguez CA; Lim JK; Mendez LM; Fink DL; Hsu AP; Zhai B; Karauzum H; Mikelis CM; Rose SR; Ferre EM; Yockey L; Lemberg K; Kuehn HS; Rosenzweig SD; Lin X; Chittiboina P; Datta SK; Belhorn TH; Weimer ET; Hernandez ML; Hohl TM; Kuhns DB; Lionakis MS, CARD9-Dependent Neutrophil Recruitment Protects against Fungal Invasion of the Central Nervous System. PLoS Pathog 2015, 11 (12), e1005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Celmeli F; Oztoprak N; Turkkahraman D; Seyman D; Mutlu E; Frede N; Koksoy S; Grimbacher B, Successful Granulocyte Colony-stimulating Factor Treatment of Relapsing Candida albicans Meningoencephalitis Caused by CARD9 Deficiency. Pediatr Infect Dis J 2016, 35 (4), 428–31. [DOI] [PubMed] [Google Scholar]
- 130.Cetinkaya PG; Ayvaz DC; Karaatmaca B; Gocmen R; Soylemezoglu F; Bainter W; Chou J; Chatila TA; Tezcan I, A young girl with severe cerebral fungal infection due to card 9 deficiency. Clin Immunol 2018, 191, 21–26. [DOI] [PubMed] [Google Scholar]
- 131.Primary immunodeficiency diseases. Report of an IUIS Scientific Committee. International Union of Immunological Societies. Clin Exp Immunol 1999, 118 Suppl 1, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rieber N; Gazendam RP; Freeman AF; Hsu AP; Collar AL; Sugui JA; Drummond RA; Rongkavilit C; Hoffman K; Henderson C; Clark L; Mezger M; Swamydas M; Engeholm M; Schule R; Neumayer B; Ebel F; Mikelis CM; Pittaluga S; Prasad VK; Singh A; Milner JD; Williams KW; Lim JK; Kwon-Chung KJ; Holland SM; Hartl D; Kuijpers TW; Lionakis MS, Extrapulmonary Aspergillus infection in patients with CARD9 deficiency. JCI insight 2016, 1 (17), e89890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jones N; Garcez T; Newman W; Denning D, Endogenous Candida endophthalmitis and osteomyelitis associated with CARD9 deficiency. BMJ Case Rep 2016, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gavino C; Mellinghoff S; Cornely OA; Landekic M; Le C; Langelier M; Golizeh M; Proske S; Vinh DC, Novel bi-allelic splice mutations in CARD9 causing adult-onset Candida endophthalmitis. Mycoses 2018, 61 (1), 61–65. [DOI] [PubMed] [Google Scholar]
- 135.Shiokawa M; Yamasaki S; Saijo S, C-type lectin receptors in anti-fungal immunity. Curr Opin Microbiol 2017, 40, 123–130. [DOI] [PubMed] [Google Scholar]
- 136.Perez de Diego R; Sanchez-Ramon S; Lopez-Collazo E; Martinez-Barricarte R; Cubillos-Zapata C; Ferreira Cerdan A; Casanova JL; Puel A, Genetic errors of the human caspase recruitment domain-B-cell lymphoma 10-mucosa-associated lymphoid tissue lymphoma-translocation gene 1 (CBM) complex: Molecular, immunologic, and clinical heterogeneity. J Allergy Clin Immunol 2015, 136 (5), 1139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roth S; Ruland J, Caspase recruitment domain-containing protein 9 signaling in innate immunity and inflammation. Trends Immunol 2013, 34 (6), 243–50. [DOI] [PubMed] [Google Scholar]
- 138.Drummond RA; Swamydas M; Oikonomou V; Zhai B; Dambuza IM; Schaefer BC; Bohrer AC; Mayer-Barber KD; Lira SA; Iwakura Y; Filler SG; Brown GD; Hube B; Naglik JR; Hohl TM; Lionakis MS, CARD9(+) microglia promote antifungal immunity via IL-1beta- and CXCL1-mediated neutrophil recruitment. Nat Immunol 2019, 20 (5), 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]


