Abstract
It remains unknown whether the comparative effectiveness of direct oral anticoagulants (DOACs) and warfarin differs between atrial fibrillation (AF) patients with and without a history of stroke or transient ischemic attack (TIA). Using 2012–2014 Medicare claims data, we identified patients newly diagnosed with AF in 2013–2014 who initiated apixaban, dabigatran, rivaroxaban or warfarin. We categorized patients based on a history of stroke or TIA. We constructed Cox proportional hazard models that included indicator variables for treatment groups, a history of stroke or TIA, and the interaction between them, and controlled for demographics and clinical characteristics. DOACs were generally more effective than warfarin in stroke prevention; however, there were important differences between subgroups defined by a history of ischemic stroke. In particular, the superiority of dabigatran compared to warfarin in ischemic stroke prevention was more pronounced in patients with a history of stroke or TIA [hazard ratio (HR) 0.64; 95%CI 0.48–0.85] than in patients with no history of stroke or TIA (HR 0.94; 95%CI 0.75–1.16; p-value for interaction=0.034). There was no difference in the risk of stroke between apixaban, dabigatran, and rivaroxaban in patients with no history of stroke or TIA. However, among patients with a history of stroke or TIA, the risk of stroke was lower with dabigatran (HR 0.64;95%CI 0.48–0.85) and rivaroxaban (HR 0.70;95%CI 0.56–0.87), compared to apixaban (p-value for both interactions<0.05). In conclusion, the comparative effectiveness of DOACs differs substantially between patients with and without a history of stroke or TIA; specifically, apixaban is less effective in patients with a history of stroke or TIA.
Keywords: Anticoagulants, History of Stroke or TIA, Atrial Fibrillation
Introduction
Since 2010, the Food and Drug Administration (FDA) has approved four direct oral anticoagulants (DOACs) for stroke prevention in atrial fibrillation (AF), including the direct thrombin inhibitor dabigatran, and the direct factor Xa inhibitors rivaroxaban, apixaban and edoxaban. Although no clinical trials have compared head-to-head the effectiveness and safety of DOACs, numerous observational studies have directly compared DOACs. In these studies, rivaroxaban was generally associated with a lower effectiveness in stroke prevention and a higher bleeding risk than apixaban and dabigatran1–6. However, no studies have evaluated whether the comparative effectiveness and safety of DOACs differ between patients with and without a history of stroke or TIA. This is relevant because first, patients with previous stroke or TIA have a particularly high risk of recurrent stroke7, and second, prior studies have reported important differences in the effectiveness and safety of DOACs across patient subgroups defined by age and renal function8–11. To address this evidence gap, we conducted a retrospective cohort study using 2012–2014 Medicare claims data. We hypothesized that the comparative effectiveness but not the comparative safety of DOACs would differ between subgroups defined by history of stroke or TIA.
Methods
Using 2012–2014 Medicare Part D claims data from a 5% random sample, we first identified patients who were newly diagnosed with AF in 2013–2014 and who had continuous Part D enrollment (Figure 1). According to the Center for Medicare and Medicaid Services (CMS) Chronic Condition Data Warehouse (CCW), AF was defined as having one inpatient or two outpatient claims with primary or secondary International Classification of Disease, Ninth Revision (ICD-9) code 427.31. After excluding patients who did not fill a prescription for anticoagulation agents after the first diagnosis, our sample included 21,265 patients. We categorized them into four treatment groups, according to the oral anticoagulant agent first initiated after AF diagnosis: apixaban (n=2358), dabigatran (n=1415), rivaroxaban (n=5139), and warfarin (n=12353). Low dose was defined as initiating apixaban 2.5mg, dabigatran 75mg, or rivaroxaban 10 mg or 15mg. High dose included initiating apixaban 5mg, dabigatran 150mg, and rivaroxaban 20 mg. Low dose was not defined for warfarin users since the dosage of warfarin is based on INR monitoring. The index date was defined as the date of the first prescription filled for an oral anticoagulant drug after the first diagnosis of AF.
Figure 1.
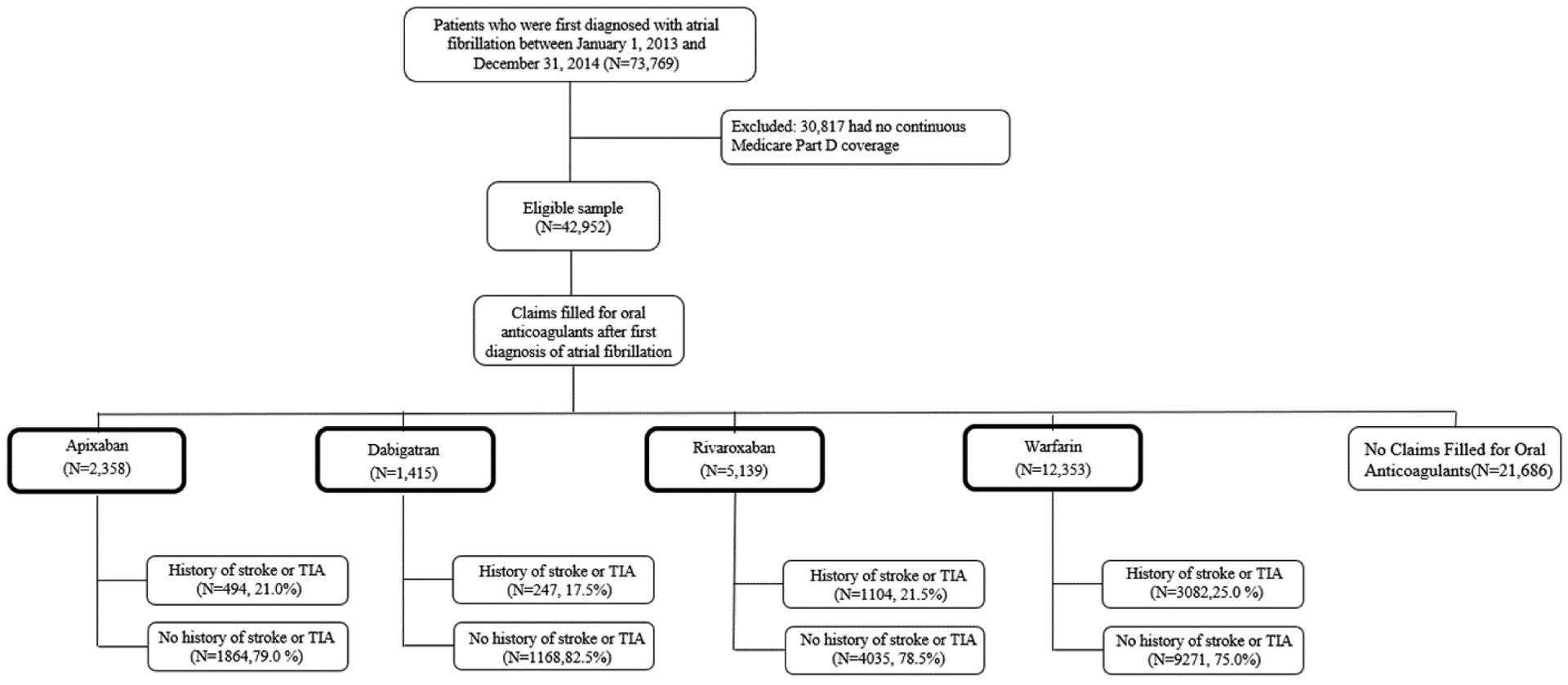
Selection of the Study Sample
Abbreviations: TIA=Transient Ischemic Attack
Using Medicare Part D data from 2012 to 2014, we identified patients who were newly diagnosed with atrial fibrillation between January 1, 2013 and December 31, 2014 and excluded those who had no continuous Part D enrollment. Patients were categorized by anticoagulant drug first used and history of stroke or TIA
All study participants were categorized into two subgroups based on the presence or absence of a previous occurrence of a stroke or TIA. History of stroke or TIA was defined following the CMS CCW definition which traces back the first diagnosis of stroke or TIA to the first month of Medicare eligibility.12All patients were followed from the index date until switch of anticoagulation therapy, therapy discontinuation, death or end of the study (December 31, 2014). Discontinuation was defined as having a gap of therapy of at least 60 days.11 Our study was approved by the institutional review board at the University of Pittsburgh as exempt.
The primary effectiveness outcomes were the risk of ischemic stroke and of other thromboembolic (TE) event. The primary safety outcomes were the risk of any bleeding event and of gastrointestinal (GI) bleeding. Secondary effectiveness and safety outcomes included the composite risk of stroke, other TE events, and death, risk of death, and risk of intracranial (IC) bleeding. The list of codes used to define outcomes is provided in Supplemental Table 1.1,13,14
Demographic and clinical characteristics were assessed on the index date. Demographic characteristics included age, gender, race and Medicaid eligibility. Clinical characteristics included CHA2DS2-VASc score, HAS-BLED score, CMS priority comorbidities, liver disease, vascular disease, a history of alcohol or drug use, a history of bleeding, antiplatelet use, and nonsteroidal anti-inflammatory drug (NSAIDS) use. CHA2DS2-VASc score is a stroke risk stratification tool for patients with AF.15HAS-BLED score is a prediction measure of the risk of major bleeding on anticoagulation.16 Because claims data do not include INR information, we calculated HAS-BLED score as the sum of all factors except for labile INR, as previously done in the literature.1,11 CMS priority comorbidities included chronic kidney disease, hypertension, acute myocardial infarction, diabetes mellitus, congestive heart failure (CHF), and number of other CMS priority comorbidities.
Baseline patient characteristic were compared across the four treatment groups using the Kruskal-Wallis test for continuous variables and the chi-square or Fisher exact tests for categorical variables. Kaplan-Meier curves were created to estimate the unadjusted cumulative incidence rates of effectiveness and safety outcomes. Cox proportional hazard models were constructed to compare time-to-event across treatment groups. Cox models included indicator variables for treatment group, for a history of stroke or TIA, and the interaction-term between the treatment group and the subgroup variable. Cox models controlled for all covariates listed above except CHA2DS2-VASc and HAS-BLED scores. CHA2DS2-VASc and HAS-BLED scores were not included in the models because all the individual factors used in the calculation of these scores were included. Since there are six possible treatment comparisons, we applied Bonferroni correction and adjusted the significance level to 0.0083(=0.05/6). All statistical analyses were conducted using statistical software SAS (version 9.4).
Results
The proportion of patients with a history of stroke or TIA was highest for the warfarin group, followed by rivaroxaban, apixaban and lowest for dabigatran (Figure 1). Apixaban users were the oldest on average, and warfarin users had the highest percentage of Medicaid eligibility (Table 1).
Table 1.
Baseline Patient Characteristics, Stratified by History of Stroke or Transient Ischemic Attack, by Treatment Group
| No History of Stroke or TIA | |||||
|---|---|---|---|---|---|
| Variable | Apixaban (n=1864) | Dabigatran (n=1168) | Rivaroxaban (n=4035) | Warfarin (n=9271) | P-Value |
| Follow-up(days) | 187±140 | 300±192 | 256±181 | 277±187 | <0.001 |
| Age(year) | 76.7±8.5 | 74.2±8.6 | 75.7±8.5 | 75.4±10.3 | <0.001 |
| <65 | 79(4.2%) | 73(6.3%) | 214(5.3%) | 959(10.3%) | |
| 65–74 | 716(38.4%) | 582(49.8%) | 1730(42.9%) | 3346(36.1%) | |
| ≥ 75 | 1069(57.4%) | 513(43.9%) | 2091(51.8%) | 4966(53.6%) | |
| Men | 829(44.5%) | 557(47.7%) | 1833(45.4%) | 4115(44.4%) | 0.153 |
| White | 1636(87.8%) | 1014(86.8%) | 3549(88.0%) | 7829(84.5%) | <0.001 |
| Black | 89(4.8%) | 56(4.8%) | 188(4.7%) | 746(8.0%) | <0.001 |
| Hispanic | 66(3.5%) | 43(3.7%) | 166(4.1%) | 414(4.5%) | 0.216 |
| Other races | 73(3.9%) | 55(4.7%) | 132(3.3%) | 282(3.0%) | 0.010 |
| Medicaid eligibility | 341(18.3%) | 273(23.4%) | 781(19.4%) | 2616(28.2%) | <0.001 |
| Low dose* | 446(23.9%) | 207(17.7%) | 1191(29.5%) | 0(0%) | <0.001 |
| CHA2DS2-VASc score† | 4.12±1.35 | 3.77±1.40 | 3.96±1.38 | 4.14±1.44 | <0.001 |
| HAS-BLED score‡ | 3.43±0.74 | 3.24±0.76 | 3.36±0.78 | 3.38±0.83 | <0.001 |
| Chronic Kidney Disease§ | 573(30.7%) | 267(22.9%) | 1118(27.7%) | 3645(39.3%) | <0.001 |
| Hypertension § | 1730(92.8%) | 1022(87.5%) | 3647(90.4%) | 8212(88.6%) | <0.001 |
| Acute Myocardial Infraction§ | 12, 6.8%) | 55(4.7%) | 246(6.1%) | 792(8.5%) | <0.001 |
| Diabetes § | 811(43.5%) | 472(40.4%) | 1618(40.1%) | 4289(46.3%) | <0.001 |
| Congest Heart Failure§ | 824(44.2%) | 458(39.2%) | 1694(42.0%) | 4920(53.1%) | <0.001 |
| No. of other Center for Medicare and Medicaid Service priority comorbidities‖ | 5.42±2.47 | 4.56±2.62 | 5.29±2.57 | 5.31 ±2.73 | <0.001 |
| Liver disease# | 23(1.2%) | 10(0.9%) | 54(1.3%) | 113(1.2%) | 0.627 |
| Vascular disease** | 427(22.9%) | 234(20.03%) | 902(22.4%) | 2545(27.5%) | <0.001 |
| Alcohol or drug use†† | 25(1.34%) | 15(1.28%) | 57(1.41%) | 125(1.35%) | 0.986 |
| History of bleeding‡‡ | 263(14.1%) | 139(11.9%) | 573(14.2%) | 1513(16.3%) | <0.001 |
| Use of antiplatelet agents§§ | 191(10.3%) | 92(7.9%) | 370(9.2%) | 810(8.7%) | 0.100 |
| Use of Nonsteroidal Anti-inflammatory Drug‖‖ | 216(11.6%) | 138(11.8%) | 515(12.8%) | 896(9.7%) | <0.001 |
| History of Stroke or TIA | |||||
| Variable | Apixaban (n=494) | Dabigatran (n=247) | Rivaroxaban (n=1104) | Warfarin (n=3082) | P-Value |
| Follow-up(days) | 178±139 | 271±192 | 256±178 | 267±183 | <0.001 |
| Age(year) | 80.3±8.3 | 78.3±8.4 | 78.8±8.6 | 77.9±10.0 | <0.001 |
| <65 | 13(2.6%) | 8(3.2%) | 57(5.2%) | 274(8.9%) | |
| 65–74 | 110(22.3%) | 75(30.4%) | 283(25.6%) | 785(25.5%) | |
| ≥ 75 | 371(75.1%) | 164(66.4%) | 764(69.2%) | 2023(65.6%) | |
| Men | 173(35.0%) | 108(43.7%) | 412(37.3%) | 1211(39.3%) | 0.079 |
| White | 430(00.7%) | 201(81.4%) | 904(81.9%) | 2467(80.1%) | <0.001 |
| Black | 23(4.7%) | 18(7.3%) | 90(8.2%) | 371(12.0%) | <0.001 |
| Hispanic | 16(3.2%) | 14(5.7%) | 67(6.1%) | 144(4.7%) | 0.078 |
| Other races | 17(3.4%) | 14(5.7%) | 43(3.9%) | 100(3.24%) | 0.213 |
| Medicaid eligibility | 122(24.7%) | 70(28.3%) | 301(27.3%) | 1129(36.6%) | <0.001 |
| Low dose* | 179(36.2%) | 66(26.7%) | 446(40.4%) | 0(0%) | <0.001 |
| CHA2DS2-VASc score† | 6.81±1.26 | 6.56±1.26 | 6.73±1.31 | 6.79±1.34 | 0.013 |
| HAS-BLED score‡ | 4.76±0.79 | 4.64±0.75 | 4.68±0.79 | 4.70±0.80 | 0.136 |
| Chronic Kidney Disease § | 230(46.6%) | 93(37.7%) | 444(40.2%) | 1601(52.0%) | <0.001 |
| Hypertension § | 476(93.4%) | 236(95.6%) | 1069(96.8%) | 2977(96.6%) | 0.782 |
| Acute Myocardial Infraction§ | 47(9.5%) | 24(9.7%) | 126(11.4%) | 422(13.7%) | 0.012 |
| Diabetes § | 244(49.4%) | 125(50.6%) | 558(50.5%) | 1696(55.0%) | 0.013 |
| Congestive Heart Failure § | 259(52.4%) | 135(54.7%) | 596(54.0%) | 1924(62.4%) | <0.001 |
| No. of other Center for Medicare and Medicaid Service priority comorbidities‖ | 6.62±2.45 | 6.31±2.56 | 6.80±2.53 | 6.76±2.58 | 0.017 |
| Liver disease# | 5(1.0%) | 1(0.4%) | 11(1.0%) | 35(1.1%) | 0.864 |
| Vascular disease** | 225(45.6%) | 91(36.8%) | 502(45.5%) | 1460(47.4%) | 0.013 |
| Alcohol or drug use†† | 2(0.4%) | 1(0.4 %) | 13(1.2%) | 42(1.4%) | 0.221 |
| History of bleeding‡‡ | 95(19.2%) | 48(19.4%) | 196(17.8%) | 701(22.7%) | 0.003 |
| Use of antiplatelet agents§§ | 126(25.5%) | 60(24.3%) | 270(24.5%) | 624(20.3%) | 0.003 |
| Use of Nonsteroidal Anti-inflammatory Drug‖‖ | 57(11.5%) | 29(11.7%) | 137(12.4%) | 297(9.6%) | 0.054 |
Abbreviations: TIA=Transient Ischemic Attack.
Continuous variables are expressed as mean and standard deviation (square brackets). Categorical variables are expressed as frequency and percentage (square brackets). P-value were calculated by Kruskal-Wallis test for continuous variables and Chi-square test for categorical variables.
Low-dose was defined as initiating apixaban 2.5mg, dabigatran 75mg, rivaroxaban 15mg or 10mg. Low-dose was only defined for DOAC users because warfarin dosing is based on international normalized ratio (INR) monitoring.
CHADS2-VASc score is a prediction measure of the risk of stroke in patients with atrial fibrillation. In the calculation of CHASDs-VASc score, age of 65–74 years, CHF, hypertension, diabetes mellitus, vascular disease, and sex category (i.e. female sex) are assigned one point, and age of ≥75 years, a history of stroke or TIA are assigned two points; CHASD2-VAS score is calculated as the sum of all points.
HAS-BLED score is a prediction measure of the risk of bleeding. It was calculated as the sum of the following factors: age of > 65 years, hypertension, renal disease, liver disease, using antiplatelet agents or NSAIDs, a history of stroke, major bleeding and alcohol or drug use, and labile INR. Because INR levels are not included in claims data, HAS-BLED score was calculated as the sum of all factors except for labile INR.
Center for Medicare and Medicaid Service (CMS) priority comorbidities were calculated using the CMS Chronic Condition Warehouse definitions.
Other CMS priority conditions included Alzheimer’s disease, related disorders or senile dementia, anemia, asthma, benign prostatic hyperplasia, cataract, chronic obstructive pulmonary disease, depression, ischemic heart disease, hip or pelvic fracture, glaucoma, hyperlipidemia, osteoporosis, rheumatoid arthritis or osteoarthritis, breast cancer, colorectal cancer, prostate cancer, lung cancer and endometrial cancer.
Liver disease was defined as having at least one inpatient or outpatient claim with primary or secondary ICD-9 code 571.xx in the year before index date.
Vascular disease was defined as having one inpatient or outpatient claim with primary or secondary ICD-9 codes 440.0x, 440.2x, 440.9x, 441.3x, 441.4x, 441.5x, 441.9x, 443.9x, 444.22, 444.81, 447.1x, 443.81, 250.70, 433.10, 433.11, 433.30 in the year before the index date.
Alcohol and drug use were defined as having at least one inpatient or outpatient claim with primary or secondary ICD-9 codes 303.xx, 304.xx, 305.xx in the year before the index date.
A history of bleeding was defined as having a claim with ICD-9 codes for any bleeding event in the year before the index date.
Antiplatelet drug use was defined as filling at least one prescription for aspirin, clopidogrel, prasugrel, dipyridamole, ticlopidine or ticagrelor in the six months before the index date.
Nonsteroidal Anti-inflammatory Drug use was defined as filling at least one prescription for diclofenac, ibuprofen, naproxen, ketoprofen, fenoprofen, flurbiprofen, piroxicam, meloxicam, mefenamic acid or indomethacin in the six months before the index date.
Table 2 shows the unadjusted cumulative incidence rates of primary outcomes and Supplemental Table 1 of secondary outcomes. Figure 2 shows Kaplan-Meier survival curves for primary outcomes.
Table 2.
Unadjusted Cumulative Incidence Rates of Primary Effectiveness and Safety Outcomes at One-year Follow-up, Stratified by History of Stroke or Transient Ischemic Attack, by Treatment Group
| No History of Stroke or TIA | ||||
|---|---|---|---|---|
| Apixaban (n=1864) | Dabigatran (n=1168) | Rivaroxaban (n=4035) | Warfarin (n=9271) | |
| Effectiveness Outcomes | ||||
| Ischemic Stroke | ||||
| Number of events | 106(5.69%) | 95(8.13%) | 281(6.96%) | 787(8.49%) |
| Cumulative incidence at 1 year | 0.10(0.08,0.12) | 0.10(0.08,0.13) | 0.10(0.09,0.11) | 0.11(0.11,0.12) |
| Other Thromboembolic Event | ||||
| Number of events | 36(1.93%) | 37(3.17%) | 133(3.3%) | 723(7.80%) |
| Cumulative incidence at 1 year | 0.03(0.02,0.04) | 0.04(0.02,0.05) | 0.04(0.03,0.05) | 0.09(0.09,0.10) |
| Safety Outcomes | ||||
| Any Bleeding Event | ||||
| Number of events | 219(11.75%) | 203 (17.38%) | 814 (20.17%) | 1868(20.15%) |
| Cumulative incidence at 1 year | 0.20(0.17,0.23) | 0.21(0.18,0.24) | 0.27(0.25,0.28) | 0.26(0.25,0.27) |
| GI Bleeding | ||||
| Number of events | 64(3.43%) | 58(4.97%) | 314(7.78%) | 617(6.66%) |
| Cumulative incidence at 1 year | 0.06(0.04,0.07) | 0.06(0.05,0.08) | 0.10(0.09,0.12) | 0.09(0.08,0.10) |
| History of Stroke or TIA | ||||
| Apixaban (n=494) | Dabigatran (n=247) | Rivaroxaban (n=1104) | Warfarin (n=3082) | |
| Effectiveness Outcomes | ||||
| Ischemic Stroke | ||||
| Number of events | 124(25.10%) | 52(21.05%) | 265(24.00%) | 942(30.56%) |
| Cumulative incidence at 1 year | 0.37(0.30,0.44) | 0.27(0.20,0.34) | 0.32(0.28,0.35) | 0.38(0.35,0.40) |
| Other Thromboembolic Event | ||||
| Number of events | 32(6.48%) | 19(7.69%) | 107(9.69%) | 385(12.49%) |
| Cumulative incidence at 1 year | 0.12(0.07,0.16) | 0.10(0.05,0.15) | 0.12(0.10,0.15) | 0.16(0.14,0.17) |
| Safety Outcomes | ||||
| Any Bleeding Event | ||||
| Number of events | 65(13.16%) | 55(22.27%) | 230 (20.83%) | 719(23.33%) |
| Cumulative incidence at 1 year | 0.21(0.16,0.27) | 0.27(0.20,0.34) | 0.29(0.25,0.33) | 0.29(0.27,0.31) |
| GI Bleeding | ||||
| Number of events | 19(3.85%) | 24(9.72%) | 93(8.42%) | 252(8.18%) |
| Cumulative incidence at 1 year | 0.05(0.03,0.08) | 0.13(0.08,0.18) | 0.12(0.10,0.15) | 0.11(0.09,0.12) |
Abbreviations: TIA=Transient Ischemic Attack; GI= gastrointestinal.
Cumulative incidence of effectiveness and safety outcomes were calculated from Kaplan-Meier curves. Number of events are presented as frequency and percentage of the respective treatment group. Cumulative incidence at 1 year are presented as cumulative incidence and 95% confidence interval
Figure 2.
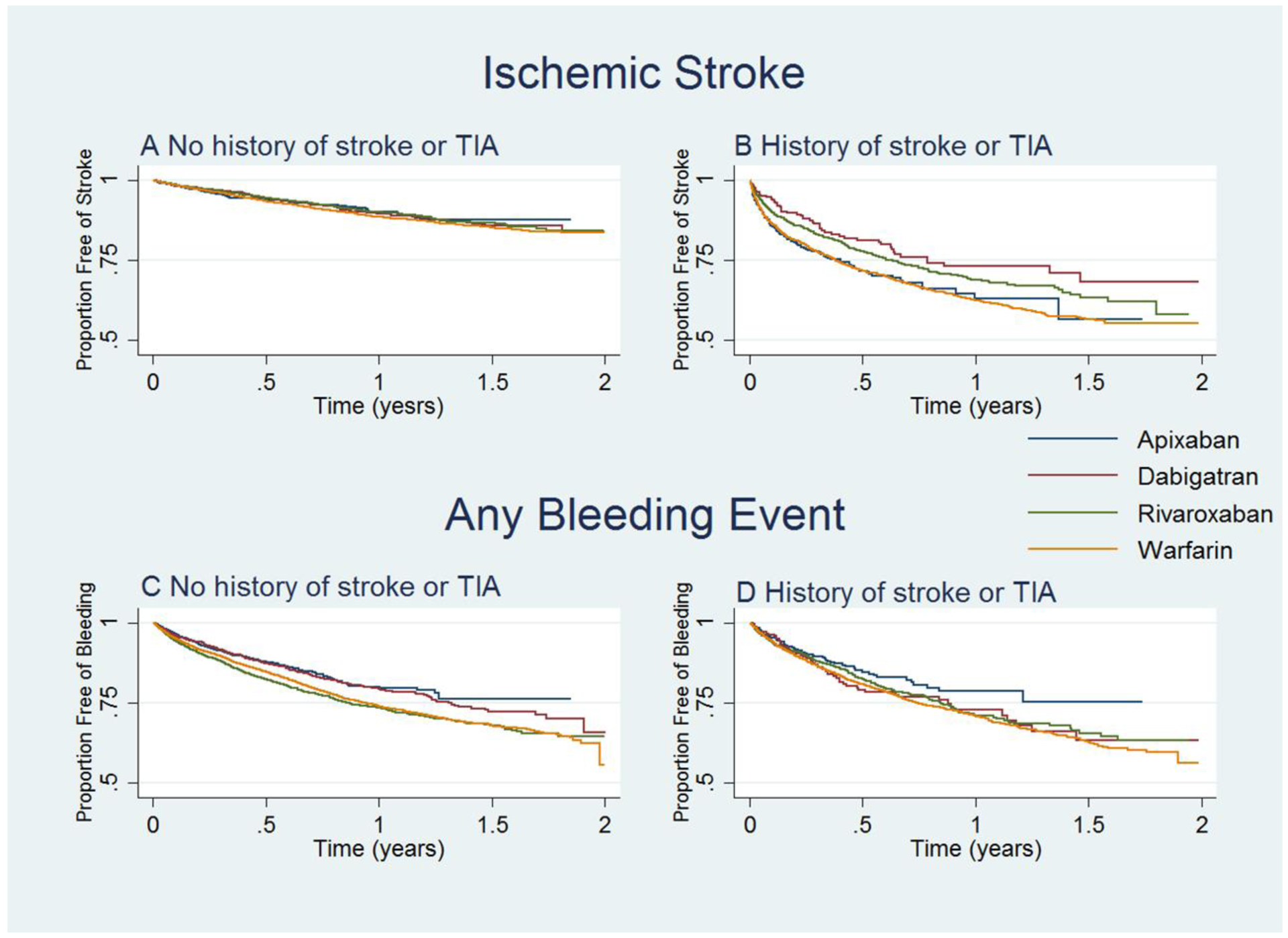
Survival Curves for Ischemic Stroke and Any Bleeding Event, by Subgroup
Abbreviation: TIA= transient ischemic attack
A: Kaplan-Meier curve for ischemic stroke for patients with no history of stroke or TIA
B: Kaplan-Meier curve for ischemic stroke for patients with history of stroke or TIA
C: Kaplan-Meier curve for any bleeding event for patients with no history of stroke or TIA
D: Kaplan-Meier curve for any bleeding event for patients with history of stroke or TIA
The comparative risk of stroke between DOACs and warfarin was consistent across the two subgroups defined by a history of stroke or TIA except for the comparison between dabigatran and warfarin (Figure 3). The risk of stroke was lower with dabigatran than warfarin, and this superiority of dabigatran was more pronounced in patients with a history of stroke or TIA. DOACs were also associated with a lower risk of other TE events compared to warfarin, and this superiority in TE prevention was more marked in the subgroup without a history of stroke or TIA. Similar findings were observed for the composite risk of stroke, other TE events, and death: DOACs were associated with lower risk than warfarin in both subgroups with the exception of the comparison between apixaban and warfarin among patients with a history of stroke or TIA (Supplemental Table 2).
Figure 3.
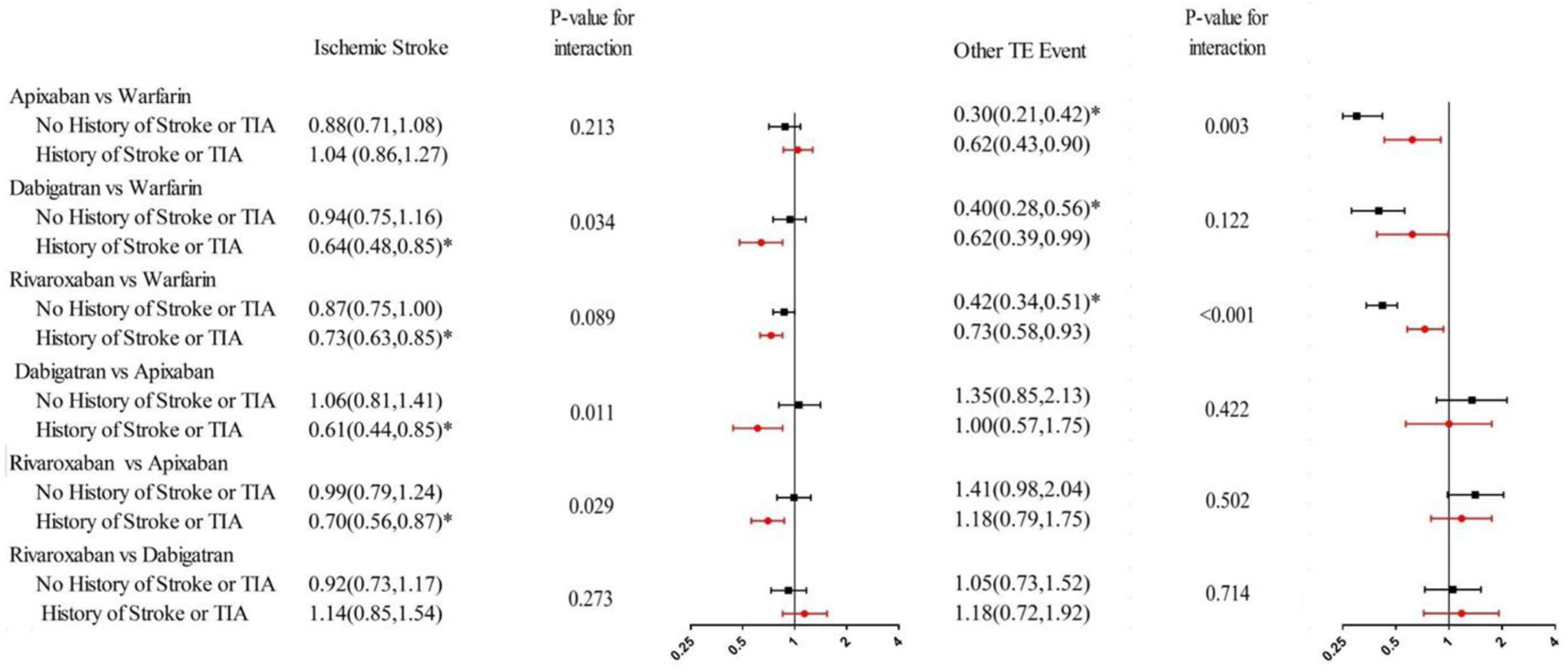
Adjusted Hazard Ratio for Effectiveness Outcomes, by Subgroup.
Abbreviation: TIA= transient ischemic attack; TE= Thromboembolic. *p value< 0.0083.
Adjusted hazard ratio were estimated with Cox proportional hazard models controlled for demographic and clinical characteristics including age, gender, race, eligibility for Medicaid coverage, chronic kidney disease, diabetes, hypertension, congestive heart failure, liver disease, vascular disease, number of other CMS priority conditions, a history of bleeding, use of NSAIDs, use of antiplatelet drug, use of drug or alcohol. We used Bonferroni correction to adjust the significance level to 0.0083(0.05/6) since we performed six pairwise comparisons.
There was no difference in the risk of stroke between apixaban and dabigatran or between apixaban and rivaroxaban for patients with no history of stroke or TIA (Figure 4). However, for patients with a history of stroke or TIA, the risk of ischemic stroke was lower with dabigatran and rivaroxaban, when compared to apixaban.
Figure 4.
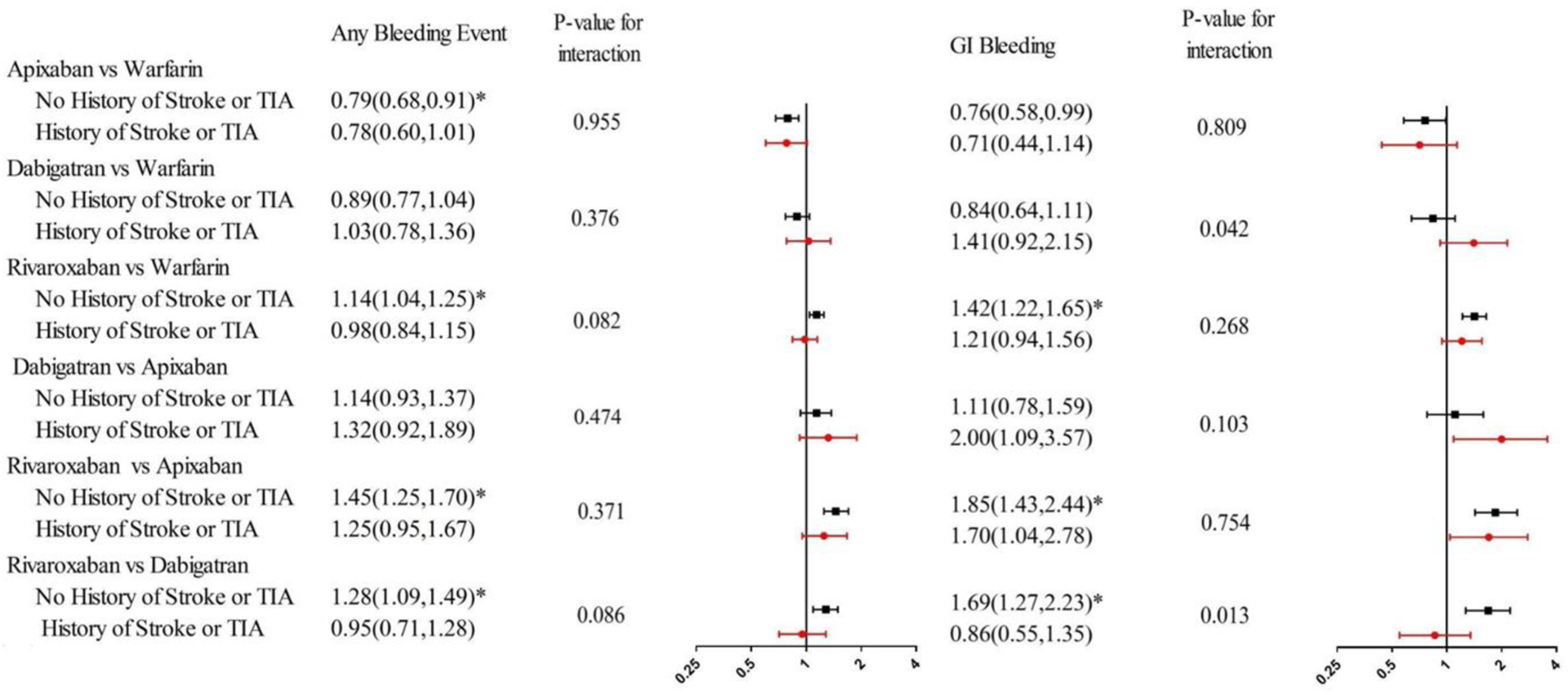
Adjusted Hazard Ratio for Any Bleeding Event and Gastrointestinal Bleeding, by Subgroup.
Abbreviation: TIA= transient ischemic attack; GI= gastrointestinal. *p value <0.0083.
Adjusted hazard ratio were estimated with Cox proportional hazard models controlled for demographic and clinical characteristics including age, gender, race, eligibility for Medicaid coverage, chronic kidney disease, diabetes, hypertension, congestive heart failure, liver disease, vascular disease, number of other CMS priority conditions, a history of bleeding, use of NSAIDs, use of antiplatelet drug, use of drug or alcohol. We used Bonferroni correction to adjust the significance level to 0.0083(0.05/6) since we performed six pairwise comparisons
The cumulative incidence of bleeding was higher for warfarin and rivaroxaban compared to apixaban and dabigatran (Table 2). There was no difference in the risk of any bleeding for DOAC versus warfarin across subgroups defined by history of stroke or TIA (Figure 4). There was a significant interaction between treatment group and a history of stroke or TIA for the comparison of GI bleeding between rivaroxaban and dabigatran: while the risk of GI bleeding did not differ between two DOACs for patients with a history of stroke or TIA, the risk of GI bleeding was higher with rivaroxaban than dabigatran among patients without a history of stroke or TIA.
Discussion
To our best knowledge, our study was the first to test how the comparative effectiveness and safety of DOACs versus warfarin differs in subgroups defined by history of stroke or TIA. Our study yielded three main findings: First, the superiority of dabigatran and rivaroxaban in stroke prevention as compared to warfarin was more pronounced in patients with a history of stroke or TIA. Second, although there was no difference in stroke prevention between apixaban and warfarin, apixaban was less effective compared to dabigatran and rivaroxaban for patients with a history of stroke or TIA. Third, there was no difference in the comparative safety of each DOAC and warfarin between patients with and without a history of stroke or TIA.
Our results for the comparative effectiveness of DOACs versus warfarin by history of stroke or TIA differ from subgroup analyses of the RE-LY, ARISTOTLE, and ROCKET clinical trials.17–19 For example, in a subgroup analysis of the RE-LY trial for patients with previous stroke or TIA, the risk of stroke or systemic embolism did not significantly differ between dabigatran 150mg and warfarin (RR 0.75, 95% CI 0.52–1.08).17 Additionally, our results for the comparison between rivaroxaban and warfarin are different from those by Coleman et al., who found that rivaroxaban significantly reduced the risk of stroke and IC bleeding among patients who had a previous stroke or TIA while there was no significant difference in stroke prevention for the comparison between dabigatran and warfarin.20The divergent results could be due to several reasons, including different characteristics of the study populations, and of patterns of DOAC prescribing in the real-world clinical practice.
Our study has important clinical implications for the management of oral anticoagulation in AF patients. The superiority of dabigatran and rivaroxaban over warfarin was more pronounced in patients with a history of ischemic stroke and TIA. Moreover, the commonly used apixaban was inferior to dabigatran and rivaroxaban in stroke prevention among patients with a history of stroke or TIA. Combined, these results suggest that dabigatran may be the preferred DOAC in patients with AF and a history of stroke and TIA, while apixaban and dabigatran would both be favorable in patients without a history of stroke and TIA. Unfortunately, our claims data analyses do not allow us to explore the mechanism underlying these differences. Further research is needed in order to validate these differences in other patient cohorts, preferably using data sources that contain clinical information, in order to minimize residual confounding due to unobserved effects. In any case, our results reinforce the need to tailor the choice of anticoagulation therapy to patients’ characteristics, and to weigh both risks of bleeding and stroke prevention. This is especially true for high risk patients, as it is the case of those with previous stroke or TIA.
Our study is subject to some limitations. First, claims data lack laboratory results such as INR levels. Additionally, claims data do not include information about drug adherence. Second, the mean follow-up period was around 300 days, so we are not able to observe long-term outcomes associated with anticoagulation treatment. Third, patients on the dabigatran group were younger, had lower CHA2DS2-Vasc score, and a lower prevalence of chronic conditions. Although we controlled for these variables in our analyses, it is possible that our findings are affected by residual confounding21. Likewise, for patients without a history of stroke or TIA, use of antiplatelets and NSAIDs was higher on the rivaroxaban group than the dabigatran group. Although we adjusted for the use of these medications, our results for the comparative risk of bleeding could have been affected by residual confounding, overestimating differences in bleeding risk between two agents. Nevertheless, these differences are not specific concerning because we aim to test if there are differences in the comparative effectiveness and safety of DOACs, and warfarin between subgroups defined by history of stroke or TIA. Fourth, the different approval dates of DOACs might have had an impact on our results, because patterns of prescribing could have changed over time
In conclusion, using Medicare data, we found differences in the comparative effectiveness of DOACs and warfarin between patients with and without a history of stroke or TIA. Although our results need to be validated in other patient cohorts, our findings reinforce the need to tailor anticoagulation to clinical characteristics of AF patients.
Supplementary Material
Sources of Funding:
Hernandez is funded by the National Heart, Lung and Blood Institute (grant number K01HL142847).
Disclosures:
Saba has received research support from Boston Scientific. Hernandez has received scientific advisory board consulting fees from Pfizer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hernandez I, Zhang Y, Saba S. Comparison of the Effectiveness and Safety of Apixaban, Dabigatran, Rivaroxaban, and Warfarin in Newly Diagnosed Atrial Fibrillation. Am J Cardiol 2017;120:1813–1819. [DOI] [PubMed] [Google Scholar]
- 2.Mantha S, Ansell J. An indirect comparison of dabigatran, rivaroxaban and apixaban for atrial fibrillation. Thromb Haemost 2012;108:476–484. [DOI] [PubMed] [Google Scholar]
- 3.Lip GY, Larsen TB, Skjoth F, Rasmussen LH. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2012;60:738–746. [DOI] [PubMed] [Google Scholar]
- 4.Harenberg J, Marx S, Diener HC, Lip GY, Marder VJ, Wehling M, Weiss C. Comparison of efficacy and safety of dabigatran, rivaroxaban and apixaban in patients with atrial fibrillation using network meta-analysis. Int Angiol 2012;31:330–339. [PubMed] [Google Scholar]
- 5.Bai Y, Deng H, Shantsila A, Lip GY. Rivaroxaban Versus Dabigatran or Warfarin in Real-World Studies of Stroke Prevention in Atrial Fibrillation: Systematic Review and Meta-Analysis. Stroke 2017;48:970–976. [DOI] [PubMed] [Google Scholar]
- 6.Staerk L, Gerds TA, Lip GYH, Ozenne B, Bonde AN, Lamberts M, Fosbol EL, Torp-Pedersen C, Gislason GH, Olesen JB. Standard and reduced doses of dabigatran, rivaroxaban and apixaban for stroke prevention in atrial fibrillation: a nationwide cohort study. J Intern Med 2018;283:45–55. [DOI] [PubMed] [Google Scholar]
- 7.Hankey GJ. Secondary stroke prevention. Lancet Neurol 2014;13:178–194. [DOI] [PubMed] [Google Scholar]
- 8.Deitelzweig S, Keshishian A, Li X, Kang A, Dhamane AD, Luo X, Balachander N, Rosenblatt L, Mardekian J, Pan X, Nadkarni A, Di Fusco M, Garcia Reeves AB, Yuce H, Lip GYH. Comparisons between Oral Anticoagulants among Older Nonvalvular Atrial Fibrillation Patients. J Am Geriatr Soc 2019;67:1662–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lip GY, Mitchell SA, Liu X, Liu LZ, Phatak H, Kachroo S, Batson S. Relative efficacy and safety of non-Vitamin K oral anticoagulants for non-valvular atrial fibrillation: Network meta-analysis comparing apixaban, dabigatran, rivaroxaban and edoxaban in three patient subgroups. Int J Cardiol 2016;204:88–94. [DOI] [PubMed] [Google Scholar]
- 10.Lega JC, Bertoletti L, Gremillet C, Chapelle C, Mismetti P, Cucherat M, Vital-Durand D, Laporte S. Consistency of safety and efficacy of new oral anticoagulants across subgroups of patients with atrial fibrillation. PLoS One 2014;9:e91398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez I, Zhang Y, Saba S. Effectiveness and Safety of Direct Oral Anticoagulants and Warfarin, Stratified by Stroke Risk in Patients With Atrial Fibrillation. Am J Cardiol 2018. [DOI] [PubMed] [Google Scholar]
- 12.Chronic Conditions Data Warehouse:Condition Category 2019.
- 13.Hernandez I, Baik SH, Pinera A, Zhang Y. Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med 2015;175:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez I, Zhang Y. Comparing Stroke and Bleeding with Rivaroxaban and Dabigatran in Atrial Fibrillation: Analysis of the US Medicare Part D Data. Am J Cardiovasc Drugs 2017;17:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY. Assessment of the CHA2DS2-VASc Score in Predicting Ischemic Stroke, Thromboembolism, and Death in Patients With Heart Failure With and Without Atrial Fibrillation. Jama 2015;314:1030–1038. [DOI] [PubMed] [Google Scholar]
- 16.Zhu W, He W, Guo L, Wang X, Hong K. The HAS-BLED Score for Predicting Major Bleeding Risk in Anticoagulated Patients With Atrial Fibrillation: A Systematic Review and Meta-analysis. Clin Cardiol 2015;38:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diener HC, Connolly SJ, Ezekowitz MD, Wallentin L, Reilly PA, Yang S, Xavier D, Di Pasquale G, Yusuf S. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol 2010;9:1157–1163. [DOI] [PubMed] [Google Scholar]
- 18.Easton JD, Lopes RD, Bahit MC, Wojdyla DM, Granger CB, Wallentin L, Alings M, Goto S, Lewis BS, Rosenqvist M, Hanna M, Mohan P, Alexander JH, Diener HC. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol 2012;11:503–511. [DOI] [PubMed] [Google Scholar]
- 19.Hankey GJ, Patel MR, Stevens SR, Becker RC, Breithardt G, Carolei A, Diener HC, Donnan GA, Halperin JL, Mahaffey KW, Mas JL, Massaro A, Norrving B, Nessel CC, Paolini JF, Roine RO, Singer DE, Wong L, Califf RM, Fox KA, Hacke W. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol 2012;11:315–322. [DOI] [PubMed] [Google Scholar]
- 20.Coleman CI, Peacock WF, Bunz TJ, Alberts MJ. Effectiveness and Safety of Apixaban, Dabigatran, and Rivaroxaban Versus Warfarin in Patients With Nonvalvular Atrial Fibrillation and Previous Stroke or Transient Ischemic Attack. Stroke 2017;48:2142–2149. [DOI] [PubMed] [Google Scholar]
- 21.Brown JD, Shewale AR, Talbert JC. Adherence to Rivaroxaban, Dabigatran, and Apixaban for Stroke Prevention for Newly Diagnosed and Treatment-Naive Atrial Fibrillation Patients: An Update Using 2013–2014 Data. J Manag Care Spec Pharm 2017;23:958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


