Abstract
Background:
Diabetes is associated with increased prevalence of TB infection in the US. We assessed associations between diabetes and interferon-gamma (IFN-γ) TB antigen response among adults with TB infection using US representative data.
Methods:
National Health and Nutrition Examination (NHANES) participants >19 years from 2011–2012 with positive QuantiFERON®-TB Gold-In-Tube (QFT) results were eligible. Diabetes was defined by combination of self-report and glycated hemoglobin (HbA1c). Quantitative IFN-γ TB antigen was classified as high (≥10 IU/mL), intermediate (1.01–9.99 IU/mL), or low (0.35–1.00 IU/mL). Analyses accounted for NHANES weighted design.
Results:
Among NHANES participants >19 years, n=513 had positive QFT (5.9%). Among those with positive QFT, diabetes prevalence was 22.2% and pre-diabetes was 25.9%. Overall, 16.7% of positive QFT participants had high IFN-γ TB antigen levels including 21.7% among those with diabetes, 20.8% among those with pre-diabetes, and 12.6% among euglycemic participants. In adjusted analyses, high IFN-γ TB antigen response was more common among those with pre-diabetes (aOR 1.9, 95%CI 1.0, 3.6) compared to euglycemic participants.
Conclusion:
Higher antigen responses may reflect immunopathy consistent with an exaggerated inflammatory but ineffectual response to TB or a reflection of more Mtb replication in participants with pre-diabetes or diabetes.
Keywords: tuberculosis infection, diabetes mellitus, interferon-gamma
1. INTRODUCTION
Patients with diabetes mellitus have two to three times the risk of developing active tuberculosis (TB) disease.1, 2 Further, patients with TB disease and diabetes comorbidity have an increased risk of poor clinical outcomes including death and relapse.3, 4 Emerging evidence suggests that patients with diabetes also have higher prevalence of latent TB infection (LTBI).5 Data from the National Health and Nutrition Examination Survey (NHANES) show that patients with diabetes had twice the prevalence of LTBI as compared to euglycemic participants.6,7 However, there is currently limited epidemiologic evidence to explain how diabetes may contribute to increased prevalence of LTBI.8
Immunopathy due to diabetes or pre-diabetes is characterized in part by chronic inflammation driven by persistent hyperglycemia and results in several complications. Murine and other animal models of diabetes demonstrate impaired innate responses in resident alveolar macrophages after exposure to aerosolized Mycobacterium tuberculosis (Mtb).9 The suboptimal initial immune responses also result in reduced early production of pulmonary IFN-γ, a key cytokine in human response to TB, that likely leads to higher bacterial burden.10
Clinical data are largely concordant with animal models, wherein active TB patients with diabetes comorbidity have higher bacterial burden and higher levels of proinflammatory cytokines, compared to patients without diabetes.11, 12 Generally, data from clinical TB studies indicate patients with diabetes demonstrate a robust TB antigen-specific immune response,10, 13, 14 but diabetes is paradoxically associated with worse TB clinical outcomes including delayed culture conversion, increased risk of mortality, and higher rates of TB relapse.3, 15, 16 While immune mechanisms in TB disease and diabetes have been characterized, the impact of diabetes and pre-diabetes on human immune responses in the context of LTBI are not understood. Although diabetes is an established risk factor for TB disease, additional information to identify individuals with diabetes and LTBI at greatest risk of progression to TB disease is urgently needed.
Previous studies among children, adolescents, and adults who converted from a negative to positive LTBI test reported that increased quantitative QFT IFN-γ values were predictive of progression TB disease.17–19 However only two small cross-sectional studies from India have examined QFT IFN-γ values among patients with co-occurring LTBI and diabetes.20, 21 They reported diminished plasma levels of circulating type 1 cytokines (including IFN-γ) and flow cytometry measured CD4+ (Th1, Th2 and Th17) cells in patients with diabetes or pre-diabetes and LTBI compared to patients with LTBI only.20, 21 Given the limited understanding about the immune responses in people with both diabetes and LTBI, we aimed to determine whether diabetes or pre-diabetes were associated with increased TB antigen responses among adults with LTBI using NHANES data representative of the US adult population.
2. METHODS
We performed cross-sectional analyses of 2011–2012 NHANES data. We used data collected from NHANES in-person interviews, health examination, and laboratory measurements. All participants gave informed consent, details of NHANES methodology has been published previously.22 Eligibility criteria included participants >19 years who completed NHANES measures, were positive for LTBI by QuantiFERON®-TB Gold-In-Tube (QFT), and had a valid measure of diabetes status recorded (n=513). According to manufacturer guidelines, a QFT result was positive if the IFN-γ TB antigen response was ≥0.35 IFN-γ IU/ml above the Nil value.23
2.1. Measures and definitions
Participants’ diabetes status was defined based on a combination of self-reported diabetes mellitus and plasma glycated hemoglobin (HbA1c) measurement. Participants who self-reported previous diabetes diagnosis by a healthcare professional were defined as having diabetes regardless of HbA1c results. In the absence of self-reported diabetes, participants were classified by HbA1c as euglycemic (≤5.6%), pre-diabetes (5.7–6.4%), and diabetes (≥6.5%). Among participants with diabetes, additional analyses were conducted with diabetes further classified as poorly controlled (HbA1c ≥7%) or controlled (HbA1c <7%), and based on whether it was a previously known diagnosis (e.g., self-reported by the participant) or a new diagnosis (no self-reported diabetes diagnosis and HbA1c ≥6.5%).
Among NHANES participants with a positive QFT result, we categorized the continuous IFN-γ TB antigen response level into three groups as low (0.35–1.0 IU/mL), medium (1.01–9.9 IU/mL), and high (≥10 IU/mL) response.24, 25 In secondary analyses we categorized the continuous IFN-γ TB antigen, Nil control, and mitogen control results into three groups based on interquartile ranges.
Covariates of interest were abstracted from NHANES interview, health examination, and laboratory modules. Tuberculin skin test (TST) was performed with 0.1 ml of tuberculin antigen (Tubersol), read by NHANES staff 46–74 hours after placement, and results were categorized by induration size as positive (≥10 mm), negative (<10 mm), and missing. Current smokers were defined as those who self-reported currently smoking every day or some days and having smoked ≥100 cigarettes in their lifetime. Former smokers were defined as those who self-reported not currently smoking at all but having smoked ≥100 cigarettes in their lifetime and those who self-reported having smoked <100 cigarettes in their lifetime were defined as never smokers. Plasma vitamin D levels were categorized as sufficient (≥40 nmol/L) and insufficient (<40 nmol/L). Participants were defined as foreign born if they self-reported being born outside the United States. Anti-HCV test was performed using VITROS Anti-HCV assay; those with repeated positive reactions to anti-HCV assay were confirmed as positive using the Chiron RIBA HCV 3.0 strip.
2.2. Statistical Analysis
We compared the unadjusted relationship between diabetes status and antigen response by reporting the proportion (prevalence differences [PD] and 95% confidence intervals [CI]) of participants with high (≥10 IU/mL) IFN-γ TB antigen across categories of diabetes and assessed significance with Rao Chi-square tests. Logistic regression was used to estimate adjusted odds ratios (aOR) of high (≥10 IU/mL) IFN-γ TB antigen comparing those with diabetes and pre-diabetes to euglycemic participants. To estimate the causal effect of diabetes on high TB antigen response, we used a purposeful selection of covariates for regression models based on observed bivariate associations, previous literature, and directed acyclic graph theory.26, 27 Secondary analyses 1) determined whether the relationship between diabetes status and IFN-γ TB antigen response varied by place of birth (US/foreign born) or TST status; and 2) assessed the relationship between diabetes status with other outcomes including low IFN-γ TB antigen (0.35–1.00 IU/mL), Nil control (0.0–0.03 IU/mL), and mitogen responses (0.0–6.0 IU/mL). Sensitivity analyses were performed to assess the association between diabetes and IFN-γ TB antigen response using 1) different definitions of high IFN-γ TB antigen and 2) with alternate covariate specifications in regression models. Significance was defined based on 95% CIs that did not contain the null value (1.0 for odds ratios and 0.0 for prevalence differences). All analyses accounted for weighted designs of the NHANES study sample using SAS version 9.4 (Cary, NC) survey procedures.
3. RESULTS
In the 2011–2012 NHANES cycle, n=4,980 adults had valid results available for both diabetes status and QFT and of these, n=513 were QFT positive (Figure 1). A total of 0.4% (22/4980) of adult participants who received a QFT test had indeterminate results including 0.2% among euglycemic, 0.6% among participants with pre-diabetes, and 0.9% among those with diabetes (p=0.03). Among those with QFT positive results, most were male (55.7%), aged 35–64 years (58.8%), foreign born (51.6%), and HIV negative (58.0%) (Supplemental Table A). The estimated prevalence of diabetes among those with QFT positive results was 22.2% (95%CI 16.6, 27.8%) and pre-diabetes was 25.9% (95%CI 22.1, 29.7%). Among those with QFT positive results and diabetes, the mean HbA1c was 7.3% (95%CI 6.9–7.8%). Nearly half (42.5%, 95%CI 27.0, 58.0%) were poorly controlled (HbA1c ≥7.0%), and 24.4% (95%CI 15.6, 33.2%) were newly diagnosed.
Figure 1.
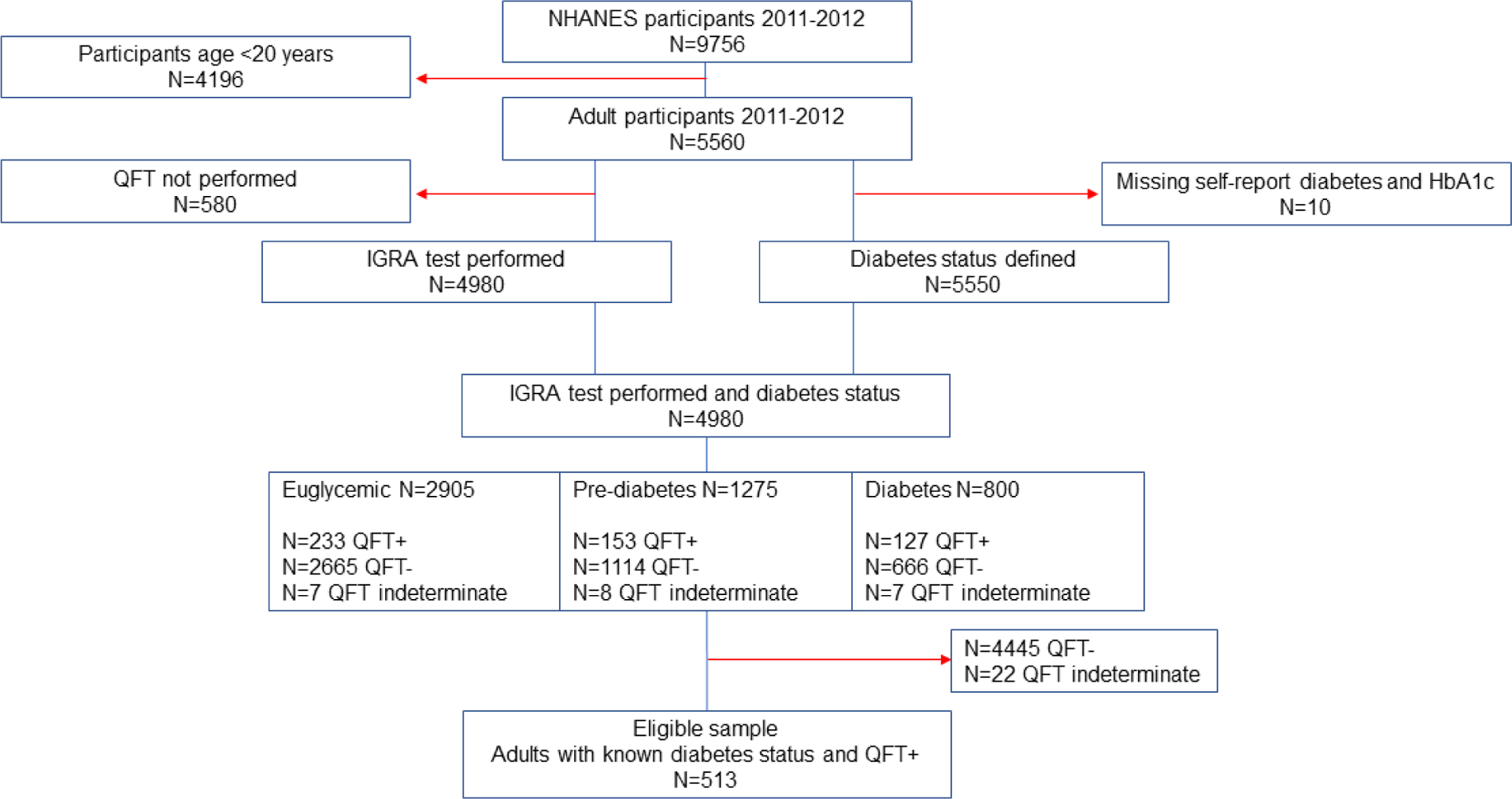
Flow diagram of eligible study participants from NHANES 2011–2012
Prevalence estimates cannot be obtained from the flow diagram because is does not account for weighted survey design
The prevalence of high IFN-γ TB antigen response (≥10 IU/mL) among QFT positive participants with diabetes was 21.7%, compared with 12.6% among euglycemic participants (prevalence difference [PD] 9.1% 95%CI −2.0, 20.2%) (Table 1). The prevalence of high IFN-γ TB antigen response was 20.8% among QFT positive participants with pre-diabetes (PD 8.2% 95%CI −2.1, 18.4% compared to euglycemic participants). Compared to euglycemic participants, the prevalence of high IFN-γ TB antigen was highest among participants with poorly controlled diabetes (31.3%, PD 18.7% 95%CI 0.0, 37.5%) and among patients with known diabetes (22.1%, PD 9.6% 95%CI −1.6, 20.7%).
Table 1.
Weighted prevalence of IFN-γ TB antigen levels among US adults classified as QFT positive (n=513), NHANES 2011–2012
| Categories of IFN-γ TB Antigen Response (IU/mL) | |||||
|---|---|---|---|---|---|
| 0.35–1.00 % (95%CI) | 1.01–9.9 % (95%CI) | ≥10 % (95%CI) | p-value * | Prevalence difference of ≥10 IU/mL % (95% CI) | |
| Diabetes status | |||||
| Diabetes | 37.4 (28.0, 46.7) | 41.0 (31.3, 50.7) | 21.7 (12.5, 30.9) | 0.30 | 9.1 (−2.0, 20.2) |
| Pre-diabetes | 35.6 (20.7, 50.6) | 43.6 (31.0, 56.1) | 20.8 (11.2, 30.4) | 8.2 (−2.1, 18.4) | |
| Euglycemic | 36.5 (26.6, 46.5) | 50.9 (41.8, 60.0) | 12.6 (7.6, 17.6) | REF | |
| Diabetes Status | |||||
| Poor control | 31.5 (18.5, 44.5) | 37.2 (20.8, 53.6) | 31.3 (15.2, 47.5) | 0.13 | 18.7 (0.0, 37.5) |
| Good control | 41.8 (28.0, 55.6) | 43.8 (32.7, 55.0) | 14.4 (5.9, 22.8) | 1.8 (−7.4, 11.0) | |
| Pre-diabetes | 35.6 (20.7, 50.6) | 43.6 (31.0, 56.1) | 20.8 (11.2, 30.4) | 8.2 (−2.1, 18.4) | |
| Euglycemic | 36.5 (26.6, 46.5) | 50.9 (41.8, 60.0) | 12.6 (7.6, 17.6) | REF | |
| Diabetes Status | |||||
| New diagnosis | 22.0 (7.1, 36.8) | 57.9 (38.5, 77.3) | 20.2 (6.2, 34.1) | 0.15 | 7.6 (−7.9, 23.0) |
| Known diabetes | 42.3 (31.0, 53.7) | 35.5 (23.9, 47.2) | 22.1 (12.8, 31.5) | 9.6 (−1.6, 20.7) | |
| Pre-diabetes | 35.6 (20.7, 50.6) | 43.6 (31.0, 56.1) | 20.8 (11.2, 30.4) | 8.2 (−2.1, 18.4) | |
| Euglycemic | 36.5 (26.6, 46.5) | 50.9 (41.8, 60.0) | 12.6 (7.6, 17.6) | REF | |
| Age (years) | |||||
| 20–34 | 29.3 (18.1, 40.5) | 50.7 (35.9, 65.5) | 20.0 (4.9, 35.2) | 0.68 | REF |
| 35–64 | 39.4 (28.8, 50.1) | 45.1 (35.7, 54.5) | 15.5 (11.7, 19.3) | −4.5 (−18.8, 9.7) | |
| ≥65 | 34.1 (23.1, 45.0) | 48.3 (37.5, 59.1) | 17.6 (12.0, 23.3) | −2.4 (−18.6, 13.8) | |
| Sex | |||||
| Female | 38.1 (25.6, 50.6) | 48.0 (38.2, 57.9) | 13.9 (9.6, 18.2) | 0.49 | REF |
| Male | 35.2 (26.6, 43.9) | 45.8 (35.9, 55.7) | 19.0 (14.4, 23.6) | 5.1 (0.3, 9.9) | |
| BMI | |||||
| <18.5 | 53.7 (18.8, 88.6) | 39.2 (9.5, 68.9) | 7.1 (0.0, 21.4) | 0.02 | −5.2 (−17.6, 7.1) |
| 18.5–24.9 | 42.2 (29.2, 55.2) | 45.5 (35.8, 55.2) | 12.3 (7.2, 17.4) | REF | |
| 25.0–29.9 | 27.3 (16.1, 38.6) | 56.8 (45.6, 67.9) | 15.9 (10.6, 21.2) | 3.6 (−5.1, 12.2) | |
| ≥30.0 | 39.3 (28.9, 49.7) | 38.8 (29.4, 48.1) | 21.9 (13.9, 30.0) | 9.6 (0.9, 18.3) | |
| TST† | |||||
| Positive | 18.7 (13.3, 24.2) | 47.0 (39.7, 54.4) | 34.2 (28.0, 40.4) | <0.01 | 27.2 (21.1, 33.2) |
| Negative | 50.1 (39.8, 60.4) | 42.9 (33.9, 51.9) | 7.0 (3.2, 10.9) | REF | |
| Missing | 30.9 (11.7, 50.2) | 56.2 (38.2, 74.3) | 12.8 (6.1, 19.5) | - | |
| Education | |||||
| <9tn grade | 24.9 (17.1, 32.7) | 53.3 (43.1, 63.6) | 21.8 (12.7, 30.8) | 0.04 | 12.7 (2.4, 23.0) |
| 9tn–12tn grade | 29.1 (19.4, 38.8) | 46.4 (34.1, 58.8) | 24.4 (16.0, 32.9) | 15.4 (4.4, 26.4) | |
| HS grad/GED | 38.3 (26.1, 50.5) | 47.4 (35.0, 59.8) | 14.3 (8.4, 20.2) | 5.3 (−0.6, 11.1) | |
| Some college | 44.5 (24.9, 64.0) | 35.2 (21.4, 48.9) | 20.4 (10.0, 30.8) | 11.3 (−0.1, 22.7) | |
| ≥College grad | 40.5 (24.9, 56.2) | 50.4 (35.2, 65.6) | 9.1 (4.0, 14.1) | REF | |
| Race/ethnicity | |||||
| Hispanic | 22.9 (18.1, 27.6) | 51.1 (43.3, 59.0) | 26.0 (20.8, 31.2) | <0.01 | 20.3 (12.9, 27.6) |
| NH White | 54.9 (36.7, 73.1) | 39.4 (22.1, 56.6) | 5.7 (0.7, 10.8) | REF | |
| NH Black | 32.0 (24.8, 39.2) | 38.1 (30.5, 45.6) | 29.9 (20.2, 39.7) | 24.2 (14.4, 34.0) | |
| NH Asian | 27.5 (20.0, 35.0) | 60.4 (51.6, 69.1) | 12.1 (8.5, 15.8) | 6.4 (−0.3, 13.1) | |
| Other‡ | 31.6 (2.7, 60.4) | 61.8 (29.0, 94.6) | 6.6 (0.0, 22.6) | 0.9 (−5.3, 7.0) | |
| Smoking Status | |||||
| Current | 37.5 (24.6, 50.5) | 46.4 (35.6, 57.3) | 16.0 (7.6, 24.5) | 0.35 | −0.9 (−10.5, 8.8) |
| Former | 42.9 (30.8, 55.0) | 40.2 (27.9, 52.4) | 16.9 (10.1, 23.8) | 0.1 (−7.2, 7.1) | |
| Never | 32.2 (23.9, 40.6) | 50.9 (42.4, 59.3) | 16.9 (13.3, 20.5) | REF | |
| Vitamin D (nmol/L) | |||||
| Insufficient (<40) | 33.5 (20.0, 47.0) | 39.0 (26.9, 51.2) | 27.5 (19.2, 35.8) | 0.01 | 12.7 (4.0, 21.5) |
| Sufficient (≥40) | 35.9 (28.1, 43.8) | 49.3 (41.8, 56.8) | 14.8 (10.7, 18.8) | REF | |
| Missing | 80.6 (49.5, 100.0) | 15.4 (0.0, 42.6) | 4.0 (0.0, 13.1) | - | |
| Previous active TB§ | |||||
| Yes | 14.0 (0.0, 30.3) | 62.9 (34.7, 91.1) | 23.1 (0.0, 48.4) | 0.15 | 6.6 (−19.4, 32.6) |
| No | 37.1 (29.8, 44.5) | 46.3 (40.0, 52.6) | 16.5 (12.5, 20.6) | REF | |
| Foreign born∥ | |||||
| No | 48.3 (35.5, 61.1) | 41.6 (29.9, 53.2) | 10.2 (6.0, 14.3) | <0.01 | REF |
| Yes | 25.4 (21.0, 29.8) | 51.7 (45.3, 58.1) | 22.9 (18.5, 27.3) | 12.7 (6.7, 18.7) | |
| Time in US among foreign born | |||||
| <5 years | 37.8 (7.9, 67.8) | 44.0 (29.3, 58.8) | 18.1 (0.0, 37.7) | 0.44 | REF |
| ≥5 years | 23.7 (18.7, 28.8) | 53.1 (46.4, 59.7) | 23.2 (18.2, 28.2) | 5.1 (−16.4, 26.5) | |
| Missing | 32.4 (2.3, 62.6) | 41.4 (19.5, 63.4) | 26.1 (13.1, 39.1) | - | |
| HIV antibody test¶ | |||||
| Positive | 55.0 (0.0, 100.0) | 45.0 (0.0, 100.0) | - | N/A | N/A |
| Negative | 30.6 (23.1, 38.0) | 51.4 (43.0, 59.9) | 18.0 (12.6, 23.4) | - | |
| Missing | 44.6 (34.2, 55.0) | 40.4 (31.9, 48.9) | 15.0 (10.5, 19.5) | - | |
| Anti-HCV test | |||||
| Positive | 15.5 (0.0, 42.0) | 41.4 (0.1, 82.8) | 43.1 (14.5, 71.7) | 0.08 | 26.5 (−2.0, 55.0) |
| Negative | 35.8 (28.3, 43.3) | 47.5 (40.5, 54.5) | 16.6 (12.9, 20.4) | REF | |
| Missing | 77.0 (42.7, 100.0) | 19.2 (0.0, 50.0) | 3.8 (0.0, 12.4) | - | |
All p-values obtained using Rao-Scott chi-square test. Missing values excluded from Rao-Scott chi- square test.
Positive TST classified as an induration ≥10 mm.
Includes multi-racial.
Classified based on participant’s response to “Were you ever told that you had active tuberculosis or TB?”.
Foreign born individuals include participants who reported being born in one of the five United States territories.
N=2 participants were HIV positive therefore p-value and prevalence difference estimates were unavailable.
Bold indicates 95% confidence interval estimate did not include null value
After adjusting for age, sex, vitamin D level, and BMI, the adjusted odds of high IFN-γ TB antigen response among participants with diabetes was 1.8 (95%CI 0.8, 4.3) times the odds among euglycemic participants (Table 2). The aOR for high IFN-γ TB antigen response comparing those with pre-diabetes to euglycemic participants was 1.9 (95%CI 1.0, 3.6). In adjusted analysis, patients with diabetes or pre-diabetes combined were more likely to have high IFN-γ TB antigen response compared to euglycemic participants (aOR 1.9, 95%CI 1.0, 3.4).
Table 2.
Odds of high IFN-gamma TB antigen response (≥10 IU/mL) among NHANES 2011–2012 participants with latent TB infection, N=513
| Diabetes classification | Odds ratio (95%CI) | Adjusted odds ratio (95%CI) * | Adjusted odds ratio (95%CI) † |
|---|---|---|---|
| Diabetes | 1.9 (0.9, 4.1) | 2.2 (1.0, 4.9) | 1.8 (0.8, 4.3) |
| Pre-diabetes | 1.8 (0.9, 3.6) | 2.0 (1.0, 4.1) | 1.9 (1.0, 3.6) |
| Euglycemic | REF | REF | REF |
| Diabetes-poor control | 3.2 (1.1, 8.8) | 3.3 (1.1, 9.9) | 2.7 (0.9, 8.3) |
| Diabetes- good control | 1.2 (0.5, 2.5) | 1.4 (0.7, 3.0) | 1.1 (0.5, 2.7) |
| Pre-diabetes | 1.8 (0.9, 3.6) | 2.0 (1.0, 4.1) | 1.9 (1.0, 3.6) |
| Euglycemic | REF | REF | REF |
| Diabetes-new | 1.7 (0.6, 4.9) | 2.0 (0.7, 5.4) | 1.5 (0.5, 4.1) |
| Diabetes-known | 2.0 (1.0, 4.2) | 2.3 (1.0, 5.2) | 2.0 (0.8, 4.8) |
| Pre-diabetes | 1.8 (0.9, 3.6) | 2.0 (1.0, 4.1) | 1.9 (1.0, 3.6) |
| Euglycemic | REF | REF | REF |
| Pre-diabetes/diabetes | 1.9 (1.1, 3.3) | 2.1 (1.1, 3.9) | 1.9 (1.0, 3.4) |
| Euglycemic | REF | REF | REF |
Adjusted for age and sex
Adjusted for age, sex, vitamin D, and BMI
Bold indicates 95% confidence interval estimate did not include null value
The relationship between diabetes status and high IFN-γ TB antigen response varied by place of birth. Among participants born in the US, the relative association between diabetes (aOR 7.5 95%CI 1.5, 37.9) and pre-diabetes (aOR 7.5 95%CI 3.1, 18.0) with high TB antigen was strong. Among foreign born participants the odds of high TB antigen response was similar by diabetes status (aOR diabetes 1.0, 95%CI 0.5,2.4; aOR prediabetes 1.0, 95%CI 0.4, 2.3).
3.1. Diabetes status and IFN-γ TB antigen response stratified by TST status
Among participants with positive QFT, n=410 (79.9%) also had valid TST results (Table 3). Among participants with concordant positive QFT and TST results (n=208) the proportion with high IFN-γ TB antigen response among those with diabetes (34.1%) and pre-diabetes (41.2%) was non-significantly greater than euglycemic (27.5%) participants. The adjusted relative association between diabetes (aOR 1.4, 95%CI 0.5, 3.5) and pre-diabetes (aOR 1.7, 95%CI 0.7, 4.1) with high IFN-γ TB antigen response among TST-QFT concordant participants was modest. Among those who were discordant (QFT positive and TST negative, n=202), the proportion with high IFN-γ TB antigen response was greater in those with diabetes (14.0%) and pre-diabetes (6.9%) compared to euglycemic participants (4.3%). The adjusted odds of having a high IFN-γ TB antigen response in those with diabetes (aOR 4.8, 95%CI 1.0, 22.0) was significantly greater than euglycemic participants.
Table 3.
Effect modification between diabetes and pre-diabetes with high IFN-gamma TB antigen (≥10 IU/mL) among strata of tuberculin skin test results, N=513
| Effect of diabetes status on TB antigen within levels of TST** | Mean antigen, lU/mL (95%CI) | Mean TST induration, mm (95%CI) | TB antigen ≥10 IU/mL % (95%CI) | Crude odds ratio ≥10 IU/mL (95%CI) | Adjusted odds ratio* ≥10 IU/mL (95%CI) |
|---|---|---|---|---|---|
| TST positive, N=208 (induration ≥10 mm) | |||||
| Diabetes | 5.3 (3.6, 7.0) | 15.2 (14.1, 16.2) | 34.1 (17.1, 51.1) | 1.4 (0.5, 3.7) | 1.4 (0.5, 3.5) |
| Pre-diabetes | 5.9 (4.6, 7.1) | 15.7 (15.1, 16.2) | 41.2 (26.9, 55.4) | 1.8 (0.8, 4.0) | 1.7 (0.7, 4.1) |
| Euglycemic | 5.1 (4.1, 6.1) | 16.2 (15.5, 17.0) | 27.5 (18.1, 37.0) | REF | REF |
| TST negative, N=202 (induration <10mm) | |||||
| Diabetes | 3.3 (2.0, 4.6) | 1.9 (0.8, 2.9) | 14.0 (0.7, 27.2) | 3.6 (0.8, 16.4) | 4.8 (1.0, 22.0) |
| Pre-diabetes | 2.2 (1.3, 3.1) | 2.0 (0.9, 3.2) | 6.9 (0.0, 14.0) | 1.6 (0.4, 7.2) | 1.7 (0.4, 7.0) |
| Euglycemic | 1.7 (1.2, 2.2) | 1.5 (0.6, 2.3) | 4.3 (0.2, 8.5) | REF | REF |
| TST not done, N=103 (QFT positive) | |||||
| Diabetes | 3.9 (1.9, 5.8) | NA | 20.6 (2.2, 39.0) | 2.9 (0.4, 20.0) | 4.0 (0.6, 29.1) |
| Pre-diabetes | 5.0 (3.6, 6.4) | NA | 18.7 (7.7, 29.8) | 2.6 (0.6, 10.9) | 2.2 (0.4, 10.9) |
| Euglycemic | 3.1 (1.4, 4.7) | NA | 8.2 (0.0, 18.0) | REF | REF |
Table 3 Abbreviations TST: tuberculin skin test; QFT: QuantiFERON®-TB Gold-In-Tube
Adjusted for age, sex, history of active TB
Likelihood ratio p-value for interaction term between TST status and diabetes status =0.63
Bold indicates 95% confidence interval estimate did not include null value
3.2. Relationship between diabetes and low IFN-γ TB antigen, Nil control, and mitogen responses
The prevalence of low IFN-γ TB antigen, Nil control, and mitogen responses did not meaningfully differ by diabetes status (Supplemental Table B). The prevalence of low IFN-γ TB antigen response (0.35, 1.0 IU/mL) was similar in those with diabetes (37.4%, 95%CI 28.0, 46.7%), pre-diabetes (35.6%, 95%CI 20.7, 50.6%), and euglycemic participants (36.5%, 95%CI 26.6, 46.5%). The mean Nil (IU/mL) results were similar across categories of diabetes status (diabetes 0.07, prediabetes 0.06, euglycemic 0.06) as were the mean mitogen (IU/mL) results (diabetes 5.08, pre-diabetes 5.68, euglycemic 5.97).
3.3. Sensitivity Analyses
To assess the potential misclassification of high IFN-γ TB antigen, we performed additional analyses using ≥75th percentile of antigen response (>6.57 IU/mL) as the cut-point for high IFN-γ TB antigen response. Using the alternate cut-point, the odds of high IFN-γ TB antigen was higher in those with diabetes (aOR 1.7, 95%CI 0.8, 3.7) and pre-diabetes (aOR 1.9, 95%CI 1.1, 3.3) compared to euglycemic participants in a model adjusted for age, sex, vitamin D level, and BMI (data not shown). To assess systematic error due to covariate specification in adjusted models, we performed eight additional analyses to assess the relationship between diabetes status and high IFN-γ TB antigen using alternate sets of independent variables (Supplemental Table C). Compared to euglycemic participants, the range of estimated aOR for high IFN-γ TB antigen among those with diabetes was 1.5 to 2.2 and for pre-diabetes the aOR range was 1.4 to 2.0.
4. DISCUSSION
In this cross-sectional study of NHANES 2011–2012 adults with LTBI, we found that one in five participants with diabetes or pre-diabetes had high IFN-γ TB antigen response measured by QFT, more than 1.5 times the proportion among euglycemic participants. After adjusting for likely confounding factors, we reported that participants with pre-diabetes had almost double the odds of high IFN-γ TB antigen response compared to euglycemic participants. Importantly, we observed the greatest proportion of high IFN-γ TB antigen, nearly one-third, among participants with poorly controlled diabetes. In preliminary assessment of interaction, we also found evidence to suggest that the relationship between diabetes status and high IFN-γ TB antigen response may vary by place of birth (US vs. foreign born) and TST status. Overall these findings suggest that in the context of diabetes and pre-diabetes, TB antigen response to Mtb infection may be similar to IFN-γ responses among patients’ with TB disease and concurrent diabetes, wherein antigen-specific immune responses are exaggerated compared to euglycemic patients.28–30
Previous in vitro studies have reported both increased and reduced IFN-γ TB antigen responses among patients with LTBI and diabetes.21, 28, 31–33 Kumar et. al. reported lower pro-inflammatory cytokine expression, including IFN-γ TB antigen stimulated responses, in Indian adults with LTBI (QFT and TST concordantly positive) and diabetes compared to non-diabetic controls with LTBI (IFN-γ 2.4 pg/mL vs 7.3 pg/mL).21 In contrast, we observed higher IFN-γ TB antigen responses in NHANES participants with diabetes and pre-diabetes; our divergent findings are likely attributed to differences in the study sample populations. Unlike the small samples sizes in the study from India (n=22 per group), we included all 2011–2012 NHANES participants with QFT results (n=513), regardless of TST results. Another difference compared to the study from India was that our model estimates for the relationship between diabetes status and TB antigen response were adjusted for BMI. Diabetes and pre-diabetes occur at relatively lower BMI levels in Southeast Asians compared to in US, therefore varying environmental risk factors and pathogenesis of diabetes may partially explain the different observed cytokine effects in patients from India compared to the US.34 In addition, in the Indian study participants may have been exposed to TB at an earlier age and at repeated times. Nonetheless, we did report that the association between diabetes status and high TB antigen response among NHANES participants was observed mainly among US born. If this finding is true, our results would be consistent with the Kumar et al findings. The life-course timing and frequency of exposure to TB infection is likely different in high-burden TB disease settings which could also contribute to different observed IFN-γ responses by place of birth in our study.35
More studies have evaluated IFN-γ TB antigen expression in patients with TB disease and diabetes. A 2011 cross-sectional study conducted near the Texas-Mexico border that included patients with microbiologically confirmed TB disease (n=169) reported that diabetes and higher hbA1c were associated with double the odds of being QFT positive.32 The same study among patients with TB disease also reported a significantly higher median IFN-γ TB antigen response to ESAT-6 and CFP-10 antigens in patients with diabetes and among those with higher hbA1c levels.32 Another cross-sectional study from Singapore evaluated QFT responses among n=275 patients with pulmonary TB.36 The Singapore study reported a non-significant increased quantitative IFN-γ TB antigen response in patients with diabetes compared to those without diabetes (median 2.5 IU/mL vs. 2.0 IU/mL, p-value 0.47).36 A third study from Tanzania among patients with TB disease reported a higher proportion of those with diabetes (~32%) to be QFT negative compared to no diabetes (~8%).37 However, the study from Tanzania only included n=16 patients with TB and diabetes (n~5 QFT negative). Our results are not directly comparable to the Texas-Mexico and Singapore studies because we only included adults with LTBI and additionally evaluated the relationship between both diabetes and pre-diabetes with IFN-γ TB antigen response. Nonetheless, our findings were more consistent with the studies from Texas-Mexico and Singapore, which reported an overall trend toward increased IFN-γ TB antigen response associated with diabetes.
Clinical and diagnostic implications of a higher IFN-γ TB antigen response among those with LTBI and pre-diabetes requires additional consideration. Our data are consistent with established findings that in the context of TB disease, diabetes and pre-diabetes are associated with an increased pro-inflammatory cytokine response typically associated with successful host defense against TB.12, 30 In addition, if persons with diabetes or pre-diabetes exposed to Mtb have impaired activation of innate immune responses, as observed in humans with diabetes and TB disease38, 39, higher initial Mtb bacterial burden may partially explain the increased prevalence of LTBI and exaggerated IFN-γ TB antigen observed in adults with diabetes and pre-diabetes. It is also plausible that the relative increase in IFN-γ TB antigen responses among patients with diabetes and positive QFT but negative TST reflects an underlying immunopathy related to diabetes that is currently unknown. A previous cohort study of patients with confirmed active TB disease from the U.S. state of Georgia reported high mortality rates among TST negative diabetes patients (15.4% vs. 5.6% overall mortality), thus negative TST in the context of TB may indicate clinically relevant immunopathy.40 The same study reported 40% of those with concurrent diabetes had a negative TST result compared to 29% (p <0.05) among patients without diabetes.40 Whether there is a relationship between increased IFN-γ TB antigen response and immunopathy in patients with hyperglycemia will require prospective studies to assess longitudinal clinical outcomes for both TB disease and TB infection.
Our study was subject to limitations. First, we used IFN-γ TB antigen response values available from the commercial QFT test and therefore were not able to differentiate between antigen values >10 IU/mL. Consequently, we were unable to perform a linear regression to estimate the correlation between diabetes status or hbA1c with the continuous IFN-γ TB antigen response. Second, our study did not measure any other cytokine or immune responses. Third, NHANES was a cross-sectional design and therefore we were unable to determine whether pre-diabetes was associated with increased IFN-γ TB antigen response or whether the increased antigen response could have increased the risk of pre-diabetes. Fourth, residual bias from unmeasured confounders or misclassification of known confounders like HIV status may have resulted in systematic error of our estimated aOR. Despite limitations, we analyzed data from NHANES, a large population-based study designed to be representative of the US adult population. Also lending credibility to our findings, we used validated study definitions of diabetes, pre-diabetes, and LTBI which were measured using well-established laboratory procedures.
4.1. Conclusion
Global diabetes and pre-diabetes epidemics continue to expand rapidly. The link between LTBI and hyperglycemia, including diabetes and pre-diabetes, remains poorly understood. An improved understanding of why adults with diabetes and pre-diabetes have higher prevalence of LTBI will contribute greatly to global efforts to improve TB disease control and prevention. We demonstrated an association between pre-diabetes and increased IFN-γ TB antigen response among adults with LTBI who participated in 2011–2012 NHANES. In US adults, the higher prevalence of LTBI in patients with diabetes and pre-diabetes is therefore unlikely to be explained by inhibited IFN-γ TB antigen responses in those with hyperglycemia.
Supplementary Material
HIGHLIGHTS.
Latent tuberculosis infection prevalence is higher in patients with pre-diabetes
Adults with LTBI and diabetes had higher quantitative QFT TB antigen responses
LTBI-diabetes link unlikely due to hyperglycemia inhibited IFN-γ TB antigen responses
FUNDING
This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [grant numbers R03AI133172 to M.J.M. and K24AI114444 to N.R.G.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008;5:e152. doi: 08-PLME-RA-0743 [pii] 10.1371/journal.pmed.0050152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayashi S, Chandramohan D. Risk of active tuberculosis among people with diabetes mellitus: systematic review and meta-analysis. Trop Med Int Health 2018;23:1058–1070. doi: 10.1111/tmi.13133 [DOI] [PubMed] [Google Scholar]
- 3.Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lonnroth K, Ottmani SE, Goonesekera SD, Murray MB. The impact of diabetes on tuberculosis treatment outcomes: A systematic review. BMC Med 2011;9:81. doi: 1741-7015-9-81 [pii] 10.1186/1741-7015-9-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riza AL, Pearson F, Ugarte-Gil C, Alisjahbana B, van de Vijver S, Panduru NM, Hill PC, Ruslami R, Moore D, Aarnoutse R, Critchley JA, van Crevel R. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diabetes Endocrinol 2014;2:740–753. doi: 10.1016/S2213-8587(14)70110-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MR, Huang YP, Kuo YT, Luo CH, Shih YJ, Shu CC, Wang JY, Ko JC, Yu CJ, Lin HH. Diabetes Mellitus and Latent Tuberculosis Infection: A Systemic Review and Metaanalysis. Clin Infect Dis 2017;64:719–727. doi: 10.1093/cid/ciw836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez L, Zhu L, Castellanos ME, Liu Q, Chen C, Hallowell BD, Whalen CC. Glycemic Control and the Prevalence of Tuberculosis Infection: A Population-based Observational Study. Clin Infect Dis 2017;65:2060–2068. doi: 10.1093/cid/cix632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barron MM, Shaw KM, Bullard KM, Ali MK, Magee MJ. Diabetes is associated with increased prevalence of latent tuberculosis infection: Findings from the National Health and Nutrition Examination Survey, 2011–2012. Diabetes Res Clin Pract 2018;139:366–379. doi: 10.1016/j.diabres.2018.03.022 [DOI] [PubMed] [Google Scholar]
- 8.Magee MJ, Salindri AD, Gujral UP, Auld SC, Bao J, Haw JS, Lin HH, Kornfeld H. Convergence of non-communicable diseases and tuberculosis: a two-way street? Int J Tuberc Lung Dis 2018;22:1258–1268. doi: 10.5588/ijtld.18.0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez N, Ketheesan N, West K, Vallerskog T, Kornfeld H. Impaired Recognition of Mycobacterium tuberculosis by Alveolar Macrophages From Diabetic Mice. J Infect Dis 2016;214:1629–1637. doi: 10.1093/infdis/jiw436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol 2014;44:617–626. doi: 10.1002/eji.201344301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol 2007;37:518–524. doi: 2006-0478OC [pii] 10.1165/rcmb.2006-0478OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez N, Kornfeld H. Tuberculosis and diabetes: from bench to bedside and back. Int J Tuberc Lung Dis 2019;23:669–677. doi: 10.5588/ijtld.18.0805 [DOI] [PubMed] [Google Scholar]
- 13.Kumar Nathella P, Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology 2017;152:13–24. doi: 10.1111/imm.12762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar NP, Moideen K, Viswanathan V, Sivakumar S, Menon PA, Kornfeld H, Babu S. Heightened circulating levels of antimicrobial peptides in tuberculosis-Diabetes co-morbidity and reversal upon treatment. PLoS One 2017;12:e0184753. doi: 10.1371/journal.pone.0184753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jimenez-Corona ME, Cruz-Hervert LP, Garcia-Garcia L, Ferreyra-Reyes L, Delgado-Sanchez G, Bobadilla-Del-Valle M, Canizales-Quintero S, Ferreira-Guerrero E, Baez-Saldana R, Tellez-Vazquez N, Montero-Campos R, Mongua-Rodriguez N, Martinez-Gamboa RA, Sifuentes-Osornio J, Ponce-de-Leon A. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax 2013;68:214–220. doi: 10.1136/thoraxjnl-2012-201756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huangfu P, Ugarte-Gil C, Golub J, Pearson F, Critchley J. The effects of diabetes on tuberculosis treatment outcomes: an updated systematic review and meta-analysis. Int J Tuberc Lung Dis 2019;23:783–796. doi: 10.5588/ijtld.18.0433 [DOI] [PubMed] [Google Scholar]
- 17.Nemes E, Rozot V, Geldenhuys H, Bilek N, Mabwe S, Abrahams D, Makhethe L, Erasmus M, Keyser A, Toefy A, Cloete Y, Ratangee F, Blauenfeldt T, Ruhwald M, Walzl G, Smith B, Loxton AG, Hanekom WA, Andrews JR, Lempicki MD, Ellis R, Ginsberg AM, Hatherill M, Scriba TJ, Team CS, the Adolescent Cohort Study T. Optimization and Interpretation of Serial QuantiFERON Testing to Measure Acquisition of Mycobacterium tuberculosis Infection. Am J Respir Crit Care Med 2017;196:638–648. doi: 10.1164/rccm.201704-0817OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machingaidze S, Verver S, Mulenga H, Abrahams DA, Hatherill M, Hanekom W, Hussey GD, Mahomed H. Predictive value of recent QuantiFERON conversion for tuberculosis disease in adolescents. Am J Respir Crit Care Med 2012;186:1051–1056. doi: 10.1164/rccm.201206-1134OC [DOI] [PubMed] [Google Scholar]
- 19.Andrews JR, Nemes E, Tameris M, Landry BS, Mahomed H, McClain JB, Fletcher HA, Hanekom WA, Wood R, McShane H, Scriba TJ, Hatherill M. Serial QuantiFERON testing and tuberculosis disease risk among young children: an observational cohort study. Lancet Respir Med 2017;5:282–290. doi: 10.1016/S2213-2600(17)30060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar NP, Moideen K, Dolla C, Kumaran P, Babu S. Prediabetes is associated with the modulation of antigen-specific Th1/Tc1 and Th17/Tc17 responses in latent Mycobacterium tuberculosis infection. PLoS One 2017;12:e0178000. doi: 10.1371/journal.pone.0178000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar NP, George PJ, Kumaran P, Dolla CK, Nutman TB, Babu S. Diminished systemic and antigen-specific type 1, type 17, and other proinflammatory cytokines in diabetic and prediabetic individuals with latent Mycobacterium tuberculosis infection. J Infect Dis 2014;210:1670–1678. doi: 10.1093/infdis/jiu329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National health and nutrition examination survey: sample design, 2011–2014. Vital Health Stat 2 2014:1–33. [PubMed] [Google Scholar]
- 23.National Health and Nutrition Examination Survey NCfHS. National Health and Nutrition Examination Survey 2011–2012 Tuberculosis Quantiferon In Gold Data Documentation, Codebook, and Frequencies. US Centers for Disease Control and Prevention, National Center for Health Statistics. [Google Scholar]
- 24.Woo KS, Choi JL, Kim BR, Han JY, Kim JM, Kim KH. Repeatability of QuantiFERONTB gold in-tube assay results near cut-off points. Ann Lab Med 2016;36:76–78. doi: 10.3343/alm.2016.36.1.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, Committee IE, Centers for Disease C, Prevention. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep 2010;59:1–25. [PubMed] [Google Scholar]
- 26.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48. doi: 00001648-199901000-00005 [pii] [PubMed] [Google Scholar]
- 27.Kleinbaum D, Klein M. Logistic Regression: A Self-Learning Text, Third Edition. New York, NY: Springer Science, 2010. [Google Scholar]
- 28.Restrepo BI, Fisher-Hoch SP, Pino PA, Salinas A, Rahbar MH, Mora F, Cortes-Penfield N, McCormick JB. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis 2008;47:634–641. doi: 10.1086/590565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, Babu S. Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J Infect Dis 2013;208:739–748. doi: 10.1093/infdis/jit241jit241 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prada-Medina CA, Fukutani KF, Pavan Kumar N, Gil-Santana L, Babu S, Lichtenstein F, West K, Sivakumar S, Menon PA, Viswanathan V, Andrade BB, Nakaya HI, Kornfeld H. Systems Immunology of Diabetes-Tuberculosis Comorbidity Reveals Signatures of Disease Complications. Sci Rep 2017;7:1999. doi: 10.1038/s41598-017-01767-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stalenhoef JE, Alisjahbana B, Nelwan EJ, van der Ven-Jongekrijg J, Ottenhoff TH, van der Meer JW, Nelwan RH, Netea MG, van Crevel R. The role of interferon-gamma in the increased tuberculosis risk in type 2 diabetes mellitus. Eur J Clin Microbiol Infect Dis 2008;27:97–103. doi: 10.1007/s10096-007-0395-0 [DOI] [PubMed] [Google Scholar]
- 32.Walsh MC, Camerlin AJ, Miles R, Pino P, Martinez P, Mora-Guzman F, Crespo-Solis JG, Fisher-Hoch SP, McCormick JB, Restrepo BI. The sensitivity of interferon-gamma release assays is not compromised in tuberculosis patients with diabetes. Int J Tuberc Lung Dis 2011;15:179–184, i-iii. [PMC free article] [PubMed] [Google Scholar]
- 33.Chee CB, Gan SH, Khinmar KW, Barkham TM, Koh CK, Liang S, Wang YT. Comparison of sensitivities of two commercial gamma interferon release assays for pulmonary tuberculosis. J Clin Microbiol 2008;46:1935–1940. doi: 10.1128/JCM.02403-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol 2014;2:56–64. doi: 10.1016/S2213-8587(13)70112-8 [DOI] [PubMed] [Google Scholar]
- 35.Lienhardt C From exposure to disease: the role of environmental factors in susceptibility to and development of tuberculosis. Epidemiol Rev 2001;23:288–301. [DOI] [PubMed] [Google Scholar]
- 36.Gan SH, KhinMar KW, Barkham TM, Koh CK, Shen L, Wang YT, Chee CB. Interferon-gamma responses to Mycobacterium tuberculosis-specific antigens in diabetes mellitus. Eur Respir J 2014;44:805–808. doi: 10.1183/09031936.00226613 [DOI] [PubMed] [Google Scholar]
- 37.Faurholt-Jepsen D, Aabye MG, Jensen AV, Range N, Praygod G, Jeremiah K, Changalucha J, Faurholt-Jepsen M, Jensen L, Jensen SM, Krarup H, Ravn P, Friis H, Andersen AB. Diabetes is associated with lower tuberculosis antigen-specific interferon gamma release in Tanzanian tuberculosis patients and non-tuberculosis controls. Scand J Infect Dis 2014;46:384–391. doi: 10.3109/00365548.2014.885657 [DOI] [PubMed] [Google Scholar]
- 38.Kumar NP, Moideen K, Bhootra Y, Nancy A, Viswanathan V, Shruthi BS, Sivakumar S, Natarajan M, Kornfeld H, Babu S. Elevated circulating levels of monocyte activation markers among tuberculosis patients with diabetes co-morbidity. Immunology 2019;156:249–258. doi: 10.1111/imm.13023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 2015;144:171–185. doi: 10.1111/imm.12394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salindri AD, Auld SC, Schechter MC, Gandhi NR, Magee MJ. Negative tuberculin skin test result predicts all-cause mortality among tuberculosis patients with HIV and diabetes comorbidity. Ann Epidemiol 2019;doi.org/ 10.1016/j.annepidem.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


