Abstract
OBJECTIVE:
We aimed to assess the impact of the burden and patterns of multi-morbidity on disability domains.
DESIGN:
In a cross-sectional study of 425 older adults from the Boston Rehabilitative Impairment Study of the Elderly, participants self-reported 13 chronic conditions and underwent assessment of body function (leg strength, velocity, and power, trunk extensor endurance, leg range of motion, foot sensation), activities (400 m walk test, Short Physical Performance Battery, Late Life Function and Disability Instrument (LLFDI) function scores) and participation (LLFDI-participation scores). We tested the association between multi-morbidity patterns (identified by Latent Class Analysis, LCA) and disablement measures, as well as multi-morbidity burden (captured by a multi-morbidity score) and disablement measures.
RESULTS:
LCA identified three classes - low multi-morbidity, high multi-morbidity and predominantly musculoskeletal conditions. Class membership (multi-morbidity pattern) was not associated with disablement measures, but multi-morbidity score was associated with poor performance in all domains. A 1-point higher multi-morbidity score was associated with lower scores in body functions (by 0.06 Standard Deviation (SD) unit), activities (0.07 - 0.10 SD units) as well as the participation (0.07 - 0.09 units).
CONCLUSION:
Multi-morbidity counts may be an excellent tool for risk stratification and identification of persons in need of rehabilitation.
Keywords: multi-morbidity, disablement, older adults, mobility, function
INTRODUCTION
Multi-morbidity, defined as the co-occurrence of at least two chronic conditions,1 is present in about 77% of older adults in the United States2. Despite this high prevalence, and the considerable implications for individuals as well as society3, research on the incremental impact of multi-morbidity (as opposed to individual chronic conditions) on health outcomes has lagged behind.4 One of the most important effects of multi-morbidity is its impact on disability, with current literature suggesting that specific patterns5,6 or the overall burden7,8 may be responsible for the accelerated disablement in multi-morbid individuals. However, the causal pathway from multi-morbidity to disability and the relative impact of disease burden on various domains of disablement is unclear. It is possible that chronic disease primarily affects a set of peripheral neuromuscular attributes important for mobility. It is also possible that the effect is at least partly mediated by brain pathology and cognitive changes, which may lead to a greater effect on participation in life activities than what is expected from peripheral effects. Unraveling this is important for developing effective rehabilitation prescriptions for older adults at risk for disability.
The World Health Organization’s International Classification of Functioning (ICF), the primary conceptual model for clinical practice and research on disability across the world, defines three disablement domains - body functions and structure, activity and participation.9 The Boston Rehabilitative Impairment Study of the Elderly (Boston RISE) , which has measures in all these domains in a population of older adults with mobility limitations, provides a unique opportunity to examine the influence of multi-morbidity on the disablement process. In this study, we aimed to examine the effect of the burden and patterns of multi-morbidity on different disablement domains among Boston RISE participants. We hypothesized that participants with greater burden as well as specific patterns of multi-morbidity identified from the data, would have greater deficits in body functions and structure, worse activity limitations and poorer participation.
MATERIALS AND METHODS
The Boston RISE is a longitudinal cohort study of 430 primary care patients aged 65 years and older from 9 large primary care practices located across the greater Boston area, recruited between December 2009 and January 2012. The primary aim of the study was to identify rehabilitative impairments (neuromuscular impairments targeted in rehabilitative care) that are most responsible for mobility decline and disability progression among older adults. Study methods have been published.10 Targeted recruitment was used to approximate ethnic/racial representation of older adults residing within a 10-mile radius of the study clinic. Methods were approved by the Spaulding Rehabilitation Hospital Institutional Review Board and written consent was obtained from all participants. Eligibility included difficulty or task modification with walking one-half mile and/or climbing one flight of stairs and the absence of moderate or severe dementia (Mini-Mental State Examination score <18) or severe mobility limitation (Short Physical Performance Battery score <4). Baseline assessments were conducted over two visits; the second was conducted within 2 weeks of the first. This study was a secondary analysis of cross-sectional data from Boston RISE participants at their baseline and conforms to all STROBE guidelines and reports the required information accordingly (see Supplementary Checklist).
Multi-morbidity
Participants were asked: “Has a doctor or health professional ever told you that you have this condition?” and responded, “yes”, “no”, “don’t know” to a list of 13 chronic conditions - diabetes, high blood pressure, heart disease, cancer, lung disease, kidney disease, stomach or liver disease, anemia or other blood disease, arthritis (osteoarthritis or rheumatoid arthritis), depression, neurological disorders, osteoporosis and back pain. Back pain, a patient reported condition, was included in this list to identify persons with chronic back pain that had warranted clinical consultation. Boston RISE participants are likely to have consulted their primary care provider for chronic back pain as all of them were recruited from primary care practices and had access to healthcare if needed. Participants were considered to have the condition if they responded “yes” to the item and not to have the condition if they said “no”. Participants who said “don’t know” were considered to have missing data. Multi-morbidity burden was described using a multi-morbidity score, a simple count of the number of conditions that the participant reported out of the 13 listed conditions. Multi-morbidity patterns were identified using Latent Class Analysis.
Disablement domains
Body functions and structures:
Leg strength was measured in each leg by determining the one repetition maximum (1RM) for each leg with a Keiser pneumatic leg press machine using a previously published protocol.11 The maximum value observed on either side was recorded as the peak leg strength. This was normalized for body weight. Peak power was recorded as the highest recorded power out of five trials performed with each leg at 40% and 70% of the 1 RM. Peak leg velocity was calculated by dividing peak power by the graphically displayed force at peak power recorded during the testing. Trunk extensor muscle endurance was measured with the participant lying prone on a specialized plinth positioned 45° from vertical with feet fixed in position on a footplate and the body supported below the waist by the table.12 The participant maintained their trunk in a neutral position within the sagittal plane in line with their pelvis and legs for as long as possible up to 2.5 minutes with arms across the chest. The time for which the position was maintained was recorded in seconds. Knee and ankle ROM were measured with a goniometer.13 Ankle range of motion (ROM) was considered to be impaired if there was inability to dorsiflex past 90° or plantar flex past 110° in either leg. Foot sensation was measured over the dorsum of the big toes using the Semmes-Weinstein monofilament test employing the standard clinical 10 g and 1.4g monofilaments.14 Foot sensation was considered to be impaired if the participant could not perceive 3 out of 4 touches from both the monofilaments on both sides. Both impaired ankle ROM and sensory loss were dichotomized as being present or absent.
Activities:
We used performance measures as well as self-report to assess the activities domain. The Short Physical Performance Battery (SPPB) includes three performance measures: progressive standing balance, usual pace walking speed, and a five-repetition chair stand test. For the standing balance test, participants were asked to maintain their balance for 10 seconds with their legs in side by side, semi-tandem and full tandem positions. For walking speed, participants walked at their usual pace over a 4-meter walking course; time was recorded and the best performance of two walks was considered for calculation of walk speed. In the chair stand test, participants were asked to stand up and down five times, as quickly as they could with their hands folded across their chest. Time to complete the test was recorded. Using established cut points, scores for each component (0 to 4) were summed to create a total score between 0 and 12, with higher scores indicating better performance. Gait speed was also considered as a distinct outcome variable, apart from the SPPB. Walking ability was measured using the 400 meters walk test, during which participants walked as fast as they could to cover the distance. We also considered scores in 2 subdomains of the Late Life Function Instrument (LLFI) - Advanced Lower Extremity Function (activities that involve a high level of physical ability and endurance, such as walking several blocks) and Basic Lower Extremity Function (activities primarily involving standing, stooping, and short walking activities).
Participation:
Participation was assessed using the Late-Life Disability Instrument (LLDI) that measures both frequency of participation and limitations in capability to perform 16 life tasks. Frequency dimension questions ask, “How often do you” do a specific task while limitation dimension questions ask “To what extent do you feel limited” in doing the task. Scores in the two dimensions were considered as distinct measures.
Confounders
Age, gender and race were self-reported. Weight and height were measured using standardized techniques and body mass index (BMI) was calculated. Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE) questionnaire. 15 Pain was assessed using 2 subscales of the Brief Pain Inventory (BPI).16 The 4-item BPI pain severity subscale measures global chronic pain severity (pain lasting more than 2 weeks), and the 7-item pain interference subscale measures pain interference with daily activities. The Mini Mental Status Examination (MMSE) scores were used as a measure of global cognition.17
Statistical Analysis
For this analysis, we excluded 5 participants who did not have complete data on chronic conditions (as they responded that they did not know whether they have a certain condition), resulting in a sample size of 425. We described multi-morbidity among participants in terms of burden (multi-morbidity score) as well as patterns. Predominant patterns of multi-morbidity in this population were identified using latent class analysis (LCA). Latent class analysis enables the grouping of individuals into mutually exclusive groups or latent classes based on their answers to a set of categorical indicator variables. In LCA models, variation of the observed indicator variables (presence or absence of 13 chronic conditions in this case) is modeled as a function of membership in unobserved latent classes (chronic disease patterns, in this scenario). Increasingly complex models (with more latent classes) were developed and compared using sample size adjusted Bayesian information criteria (BIC). A final model was selected based on low BIC and clinical interpretability of the latent classes. Subsequently, class membership probabilities were computed, and participants were assigned class membership based on highest computed probability. Characteristics of the sample in terms of multi-morbidity score and multi-morbidity patterns were described using means and percentages. Linear and logistic regression models examined relationship between multi-morbidity burden (score) and disablement variables, as well as latent class membership (patterns) and disablement variables. To allow for comparison of effect sizes, we also built regression models using standardized outcome measures. Disablement measures that were continuous were standardized by subtracting the mean and dividing by the standard deviation. To allow for fair comparison between multi-morbidity score and multi-morbidity LCA class, we categorized the multi-morbidity score into 3 groups (0-2, 3-5, ≥6) and repeated the regression analyses. All regression models adjusted for age, gender, race, BMI, PASE scores, pain scores and MMSE scores. As there were missing values in some of the disablement domain measures, we performed a sensitivity analysis using multiple imputed datasets and compared the results with complete case analysis. LCA was performed using MPlus and other analyses were conducted using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
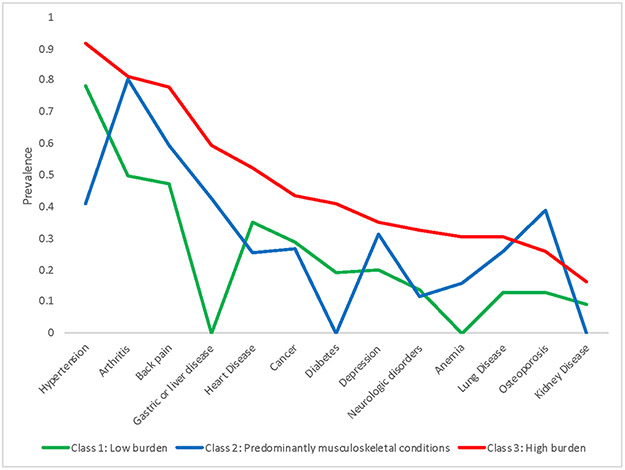
The overall means and percentages of baseline measures among Boston RISE participants, as well as distribution of measures based on multi-morbidity burden and multi-morbidity classes are shown in Table 1. The mean age of the participants in this study was 76.5 years, 67.5% were women and 17.4% were non-white. LCA identified three different multi-morbidity classes in this sample, with each class representing a general pattern in comorbid conditions at the individual level in this population. The prevalence of each chronic condition in the three groups is shown in Figure 1. Class 1 was a low multi-morbidity group with a low prevalence of all chronic conditions compared to Class 2 and Class 3. A majority of Class 1 participants had hypertension (78%) and an average 3.2 chronic conditions. Class 2 participants had an average of 4 chronic conditions, with a predominance of musculoskeletal conditions (arthritis - 80%, back pain - 60%, osteoporosis - 39%) compared to Class 1 and Class 3, but vascular disease burden in this class was lower than the other classes. Class 3 was a high multi-morbidity group with an average of 6.6 conditions. This group had a high prevalence of multiple chronic conditions including vascular, musculoskeletal and metabolic [(hypertension (91%), arthritis (81%), backpain (78%), heart disease (52%), diabetes (41%) and cancer (43%)] when compared to the other two classes.
Table 1.
Baseline characteristics [Mean (Standard Deviation), unless indicated] according to Multi-morbidity Pattern (Latent Class) and Multi-morbidity Score, 425 older adults, Boston RISE, 2009-2012.
| Characteristic | Multi-morbidity Latent Class | Multi-morbidity Score | ||||
|---|---|---|---|---|---|---|
| Low burden | Musculoskeletal | High burden | 0-2 | 3-5 | >=6 | |
| n=162 | n=149 | n=114 | n=85 | n=221 | n=119 | |
| Age | 76.7 (7.3) | 76.3 (6.7) | 76.6 (7.0) | 77.5 (7.4) | 75.9 (6.7) | 76.9 (7.2) |
| Women* | 98 (60.5) | 112 (75.2) | 77 (67.5) | 53 (62.4) | 150 (67.9) | 84 (70.6) |
| Non-white* | 30 (18.5) | 21 (14.1) | 23 (20.2) | 69 (18.8) | 38 (17.2) | 20 (16.8) |
| BMI (kg/m2) | 30.6 (7.0) | 27.8 (4.9) | 30.4 (6.1) | 29.1 (5.8) | 29.7 (6.4) | 29.7 (6.1) |
| PASE Score | 177.5 (66.9) | 177.5 (71.0) | 169.0 (70.6) | 182.6 (67.0) | 175.5 (69.4) | 169.5 (70.7) |
| Pain Score | 2.1 (1.7) | 2.7 (2.1) | 3.0 (1.8) | 1.7 (1.5) | 2.6 (1.9) | 3.0 (2.0) |
| MMSE score | 27.1 (2.5) | 27.6 (2.2) | 27.6 (2.2) | 27.2 (2.4) | 27.4 (2.4) | 27.6 (2.3) |
| Co-morbidity score | 3.2 (1.3) | 4.0 (1.6) | 6.6 (1.4) | 1.7 (0.5) | 4.1 (0.8) | 6.9 (1.1) |
| Leg Strength (N/kg) | 9.7 (2.6) | 9.5 (2.7) | 9.0 (2.2) | 10.3 (2.6) | 9.4 (2.7) | 9.0 (2.1) |
| Leg Velocity (m/s) | 1.0 (0.3) | 1.0 (0.2) | 1.0 (0.3) | 1.0 (0.3) | 1.0 (0.3) | 1.0 (0.3) |
| Leg Power (Nm/s) | 366.8 (168.4) | 325.1 (159.8) | 331.3 (162.4) | 390.0 (188.2) | 335.8 (155.3) | 318.4 (155.4) |
| Trunk Ext. endurance (s) | 100.6 (58.4) | 94.4 (59.0) | 89.3 (57.8) | 105.5 (56.0) | 97.5 (59.1) | 84.1 (57.9) |
| Ankle ROM impairment, N (%) | 86 (20.2) | 58 (13.7) | 61 (14.4) | 43 (50.6) | 101 (45.7) | 61 (51.3) |
| Foot sensory loss, N (%) | 51 (32.3) | 36 (24.7) | 34 (30.1) | 26 (30.6) | 61 (28.4) | 34 (29.1) |
| 400 m walk time (min) | 6.1 (2.1) | 6.1 (2.0) | 6.3 (2.0) | 5.9 (1.6) | 6.1 (2.1) | 6.4 (2.0) |
| SPPB score | 8.6 (2.3) | 9.1 (2.3) | 8.4 (2.1) | 9.3 (2.2) | 8.7 (2.2) | 8.3 (2.2) |
| Gait Speed (m/s) | 0.9 (0.2) | 0.9 (0.2) | 0.9 (0.2) | 1.0 (0.2) | 0.9 (0.2) | 0.9 (0.2) |
| LLFDI - BLF | 67.8 (11.3) | 66.2 (13.1) | 63.5 (11.5) | 70.2 (12.9) | 66.5 (11.5) | 62.4 (11.7) |
| LLFDI - ALF | 42.8 (12.3) | 43.6 (14.8) | 38.6 (14.5) | 47.5 (13.5) | 41.9 (14.1) | 38.1 (15.3) |
| LLFDI - PL | 71.6 (12.8) | 68.2 (11.6) | 66.2 (9.7) | 73.8 (14.1) | 68.7 (11.0) | 66.1 (10.5) |
| LLFDI - PF | 52.4 (5.8) | 52.9 (5.7) | 51.6 (5.1) | 54.1 (6.0) | 52.2 (5.7) | 51.4 (4.9) |
Abbreviations: SD –Standard Deviation, N- number, BMI-Body Mass Index, PASE-Physical Activity Score of Elderly, MMSE- Mini Mental Status Examination, Ext. - extensor, ROM-Range of Movement, SPPB – Short Physical Performance Battery, LLFDI-Late Life Function and Disability Instrument, BLF – Basic Leg Function, ALF – Advanced Leg Function, PL- Participation Limitation, PF -Participation frequency
Figure 1.
Prevalence of chronic conditions across multimorbidity latent classes among Boston RISE participants
Results of multi-variable regression testing association between multi-morbidity scores and disablement measures are displayed in Table 2. Results of regression after imputation of missing data (leg strength,10.1%, leg velocity,11.5%, leg power,11.5%, trunk extensor endurance, 5.9%, knee flexion ROM,1.2%, and ankle ROM impairment,1.8%) were not different from results of complete case analysis, hence results of complete case analysis are presented. Results using multi-morbidity score as a categorical variable (3 categories: 0-2, 3-5, ≥6) produced results similar to that of the continuous measure; we present result for the continuous variable here. For every one-point higher multi-morbidity score, participants had significantly lower values of leg strength, leg power, trunk extensor endurance, SPPB score, gait speed, and all four LLFDI sub-scores. Multi-morbidity score was not independently associated with leg velocity, range of motion, sensory loss, or 400 m walk time. The standardized coefficients in Table 2 demonstrate some differences in the strength of relationships between multi-morbidity burden and different disablement domains. A one-point higher multi-morbidity score was associated with a decrease of 0.06 standard deviation (SD) units in the body structures and functions domain, 0.07 to 0.10 SD unit decrease in the activities domain score and a 0.07 to 0.09 units decrease in the participation domain score. There were no significant associations between multi-morbidity patterns (latent class membership) and disablement outcomes (Tables 3 and 4).
Table 2.
Association between multi-morbidity score and disability domains in Boston RISE participants
| Disability Outcome measures |
Beta Coefficient |
Odds Ratio |
95%CI | Standardized Beta Coefficient |
95%CI | P value |
|---|---|---|---|---|---|---|
| Body function/structures | ||||||
| Leg Strength (N/kg) | −0.15 | −0.26, −0.03 | −0.06 | −0.10, −0.01 | 0.01 | |
| Leg Velocity (m/s) | −0.01 | −0.02, 0.002 | −0.04 | −0.09, 0.01 | 0.10 | |
| Leg Power (Nm/s) | −9.78 | −16.18, −3.38 | −0.06 | −0.10, −0.02 | 0.003 | |
| Trunk Extensor Endurance (s) | −3.76 | −6.61, −0.92 | −0.06 | −0.11, −0.02 | 0.01 | |
| Ankle ROM Impairment | 1.01 | 0.91, 1.13 | - | - | 0.81 | |
| Foot Sensory loss | 1.00 | 0.89, 1.12 | - | - | 0.99 | |
| Activity | ||||||
| 400-minute walk time | 0.08 | −0.02, 0.18 | 0.04 | −0.01, 0.09 | 0.10 | |
| SPPB score | −0.17 | −0.27, 0.07 | −0.07 | −0.12, −0.03 | 0.001 | |
| Gait Speed | −0.02 | −0.03, 0.01 | −0.08 | −0.12, - 0.04 | 0.0002 | |
| LLFDI -BLF | −0.99 | −1.52, −0.46 | −0.08 | −0.13, −0.04 | 0.0003 | |
| LLFDI - ALF | −1.41 | −2.03, −0.79 | −0.10 | −0.14, −0.05 | <0.0001 | |
| Participation | ||||||
| LLFDI - PL | −1.08 | −1.63, −0.53 | −0.09 | −0.14, −0.04 | 0.0002 | |
| LLFDI - PF | −0.39 | −0.64, −0.14 | −0.07 | −0.11, −0.03 | 0.002 |
Multivariable linear and logistic regression models with disability measures as the dependent variables and the multi-morbidity score as the main predictor variable, with each model adjusted for age, race, gender, Body Mass Index, Physical activity score, pain score, and Mini Mental State Examination Score. The estimate represents the number of units of change in outcome associated with an increase of 1 point in the multi-morbidity score except for Range of Motion impairment and sensory loss where the estimates are the Odds Ratios. Abbreviations: CI -Confidence Interval, ROM-Range of Movement, SPPB – Short Physical Performance Battery, LLFDI-Late Life Function and Disability Instrument, BLF – Basic Leg Function, ALF – Advanced Leg Function, PL- Participation Limitation, PF -Participation frequency
Table 3.
Association between multi-morbidity pattern (latent class) and body functions domain in Boston RISE participants
| Disablement Outcomes | Beta Coefficient | Odds Ratio | 95% CI | P value |
|---|---|---|---|---|
| Leg Strength (N/kg) | ||||
| Low burden | Reference | - | - | |
| Musculoskeletal | −0.32 | −0.84, 0.21 | 0.23 | |
| High burden | −0.57 | −1.14, −0.01 | 0.05 | |
| Leg Velocity (m/s) | ||||
| Low burden | Reference | - | - | |
| Musculoskeletal | 0.02 | −0.07, 0.03 | 0.47 | |
| High burden | −0.02 | −0.07, 0.04 | 0.59 | |
| Leg Power | ||||
| Low burden | Reference | - | - | |
| Musculoskeletal | −15.58 | −45.16, 14.01 | 0.30 | |
| High burden | −25.23 | −56.89, 6.43 | 0.12 | |
| Trunk Extensor Endurance | ||||
| Low burden | Reference | - | - | |
| Musculoskeletal | −7.75 | −21.19, 5.68 | 0.26 | |
| High burden | −6.88 | −20.99, 7.22 | 0.34 | |
| Ankle ROM Impairment | ||||
| Low burden | Reference | - | - | |
| Musculoskeletal | 0.67 | 0.41, 1.09 | 0.09 | |
| High burden | 0.95 | 0.57, 1.59 | 0.53 | |
| Foot Sensory loss | ||||
| Low burden | Reference | - | - | |
| Musculoskeletal | 0.76 | 0.44, 1.32 | 0.43 | |
| High burden | 0.86 | 0.49, 1.53 | 0.97 |
Multivariable linear regression models with disability measures as the dependent variables and the multi-morbidity pattern as the main predictor variable, with each model adjusted for age, race, gender, Body Mass Index, Physical activity score, pain score, and Mini Mental State Examination score. The estimate represents the number of units of change in the disablement outcome associated with the musculoskeletal and high burden multimorbidity pattern compared to the low burden pattern except for binary dependent variables of Range of Motion impairment and sensory loss where the estimates are the Odds Ratios.
Abbreviations: CI -Confidence Interval, ROM –Range of Motion
Table 4.
Association between multi-morbidity pattern (latent class) and activities/participation domains in Boston RISE participants
| Disablement Outcomes | Beta Coefficient | 95% CI | P value |
|---|---|---|---|
| 400-meter walk time | |||
| Low burden | Reference | - | - |
| Musculoskeletal | −0.05 | −0.51, 0.40 | 0.82 |
| High burden | 0.17 | −0.32, 0.65 | 0.50 |
| SPPB score | |||
| Low burden | Reference | - | - |
| Musculoskeletal | 0.43 | −0.04, 0.90 | 0.07 |
| High burden | −0.09 | −0.58, 0.41 | 0.73 |
| Gait Speed | |||
| Low burden | Reference | - | - |
| Musculoskeletal | 0.02 | −0.03, 0.06 | 0.47 |
| High burden | −0.02 | −0.07, 0.02 | 0.36 |
| LLFDI - BLF | |||
| Low burden | Reference | - | - |
| Musculoskeletal | −0.84 | −3.35, 1.67 | 0.51 |
| High burden | −2.04 | −4.68, 0.60 | 0.13 |
| LLFDI - ALF | |||
| Low burden | Reference | - | - |
| Musculoskeletal | 0.51 | −2.45, 3.46 | 0.74 |
| High burden | −2.41 | −5.52, 0.70 | 0.13 |
| LLFDI - PL | |||
| Low burden | Reference | - | - |
| Musculoskeletal | −2.59 | −5.20, 0.02 | 0.05 |
| High burden | −4.04 | −6.78, −1.29 | 0.004 |
| LLFDI - PF | |||
| Low burden | Reference | - | - |
| Musculoskeletal | −0.06 | −1.23, 1.11 | 0.92 |
| High burden | −0.65 | −1.88, 0.58 | 0.30 |
Multivariable linear regression models with disability measures as the dependent variables and the multi-morbidity pattern as the main predictor variable, with each model adjusted for age, race, gender, Body Mass Index, Physical activity score, pain score, and Mini Mental Status Examination score. The estimate represents the number of units of change in outcome associated with an increase of 1 in the multi-morbidity score.
Abbreviations: CI -Confidence Interval, ROM-Range of Movement
DISCUSSION
In this study of the association between multi-morbidity and ICF disablement domains, we found that multi-morbidity burden was a significant predictor of worse performance in all three disablement domains. However, we did not find any association between multi-morbidity patterns and disability measures. This indicates that a simple count of major chronic conditions may be more reflective of an individual’s disablement status than the spectrum of conditions that are present. This has important implications to clinicians providing care for older adults with multi-morbidity – it identifies the chronic disease count as a simple tool that could be used to identify those at risk for impending mobility disability. Such a tool could be easily implemented in geriatric specialty clinics and primary care practices, helping to streamline the implementation of universal preventive rehabilitation for older adults at risk for mobility disability.
Our findings also provide fresh insight regarding how chronic disease burden may affect different aspects of the disablement process. While previous research has examined the role of individual diseases or multi-morbidity on specific measures like muscle strength,18 gait speed,19 or ADL disability20, we have compared the relative impact of disease on multiple measures in the three different disablement domains. This is important because, while profoundly disabling neurological conditions like stroke and dementia are known to affect all domains, it has been less evident whether the burden of multiple chronic conditions among ambulatory community living older adults as in Boston RISE can have a significant impact on certain domains like the participation domain. While their effects on structure and function, and even activities, are known to an extent, their role in participation could be limited, given the potential for adaptation. However, our findings indicate that disease burden has a similar impact on all three domains with a possible stronger impact on the participation domain. This aligns with findings that older adults with chronic diseases exhibit reduced social participation, beyond what could be caused by locomotor activity limitation.21 This phenomenon could be due to the effects of disease on the brain, communication and sensory limitations caused by disease or environmental factors perpetuated by the disease conditions.
Further research is needed to confirm the pathways in which disease burden may be leading to participation restrictions. However, the strong impact of disease on participation, greater than what was anticipated from its effect on function, provides evidence for our hypothesis that chronic diseases contribute to disablement through both central and peripheral disease processes. The peripheral processes may contribute to impairments, functional limitations and participation restriction, but participation may be particularly influenced by brain changes as well, especially those that affect cognitive processes. We did adjust for cognitive status using MMSE scores, which was largely within normal limits across groups. However, the MMSE, a global measure of cognitive function, may not be sensitive enough to detect subtle differences in executive function that is important for planning, organizing, and initiating community participation.
Several researchers have attempted to develop latent classes of multi-morbidity among older adults but there has been limited consensus among their findings. Olaya et. al, identified three latent classes – healthy, metabolic/stroke and cardiorespiratory/mental/arthritis whereas our three classes distinguished participants into high multi-morbidity, low multi-morbidity and a group with a high prevalence of musculoskeletal conditions and less vascular disease. 22 Larsen et. al identified seven classes of multi-morbidity 23 whereas Whitson et. al identified six.24 Some identified classes are common between studies; the wide variety in classes identified by different studies indicate that multi-morbidity among older adults is very heterogeneous and defies strict classification into groups. The concept of multi-morbidity latent class is intuitively attractive in both clinical care and research because of the possibility of using class-membership as a measure of global health status and aligning research outcomes as well as clinical care to specific disease patterns. Our findings confirm that although it is possible to derive clinically plausible multi-morbidity patterns using latent class analyses, these may not be useful at the individual level for detecting risk for poor physical function and mobility. However, a simple count of chronic conditions, irrespective of the pattern, may be a good index for disability risk assessment. This principle is the basis of the cumulative deficit theory of frailty25 and has been utilized effectively in the development of frailty indices that count symptoms, signs, diseases and disabilities, the sheer number of conditions thereby carrying more weight than the actual nature of the deficits.26
Our findings are important for the clinical care of the aging population. Disability and poor physical function have a substantial impact on the quality of life of the individual, increase the risk of hospitalization, prolong hospital stay and increase healthcare costs. It is important that care providers identify multi-morbidity as a strong risk factor for functional loss and institute preventive interventions and rehabilitative care. Our findings indicate that interventions should address neuromuscular impairments and, focus on individuals with a disproportionate burden of comorbidities. Other factors that may be influencing social participation need to be identified and addressed.
Strengths of our study include extensive neuromuscular attribute measures and functional measures, and a relatively large sample of older primary care patients. However, disease conditions were self-reported, and duration and treatment of illness were not considered. Our design was cross-sectional, so we cannot confirm the temporal relationship of the observed associations. The study sample included only older patients with known mobility problems and was not population based. Hence, these findings may not be generalizable to diverse populations.
CONCLUSION
Multi-morbidity burden, rather than specific patterns of multi-morbidity, is associated with poor performance in all three disablement domains – body structure and function, activities and participation – among older adults at risk for disability. This suggests that multi-morbidity counts may be an excellent tool for risk stratification and identification of persons in need of rehabilitation.
Supplementary Material
Summary Text:
What is known: Older adults with multi-morbidity have a higher burden of disability
What s new: Disability burden is related to the number of chronic conditions, rather than any specific pattern of multimorbidity that is common among older adults
ACKNOWLEDGEMENTS
Funding sources:
This study was supported by the National Institute on Aging (R01 AG032052-03); the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1K24HD070966-01 to J.B.); and the National Center for Research Resources in a grant to the Harvard Clinical and Translational Science Center (1 UL1 RR025758-01). EL was supported by NINDS R01 NS086882. MEJ was supported by the National Institute on Disability, Independent Living and Rehabilitation Research through its Advanced Rehabilitation Research and Training Fellowship program.
Footnotes
Conflict of Interest: None
References
- 1.Salive ME. Multimorbidity in older adults. Epidemiologic reviews. 2013;35:75–83. [DOI] [PubMed] [Google Scholar]
- 2.Rocca WA, Boyd CM, Grossardt BR, et al. Prevalence of multimorbidity in a geographically defined American population: patterns by age, sex, and race/ethnicity. Mayo Clinic proceedings. 2014;89(10):1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzuto D, Melis RJF, Angleman S, Qiu C, Marengoni A. Effect of Chronic Diseases and Multimorbidity on Survival and Functioning in Elderly Adults. Journal of the American Geriatrics Society. 2017;65(5):1056–1060. [DOI] [PubMed] [Google Scholar]
- 4.Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing research reviews. 2011;10(4):430–439. [DOI] [PubMed] [Google Scholar]
- 5.Quinones AR, Markwardt S, Botoseneanu A. Multimorbidity Combinations and Disability in Older Adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2016;71(6):823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson CA, Jones M, Tooth L, Mishra GD, Byles J, Dobson A. Multimorbidity patterns are differentially associated with functional ability and decline in a longitudinal cohort of older women. Age and ageing. 2015;44(5):810–816. [DOI] [PubMed] [Google Scholar]
- 7.Marengoni A, von Strauss E, Rizzuto D, Winblad B, Fratiglioni L. The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. Journal of internal medicine. 2009;265(2):288–295. [DOI] [PubMed] [Google Scholar]
- 8.Jindai K, Nielson CM, Vorderstrasse BA, Quinones AR. Multimorbidity and Functional Limitations Among Adults 65 or Older, NHANES 2005-2012. Preventing chronic disease. 2016;13:E151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jette AM. Toward a common language for function, disability, and health. Physical therapy. 2006;86(5):726–734. [PubMed] [Google Scholar]
- 10.Holt NE, Percac-Lima S, Kurlinski LA, et al. The Boston Rehabilitative Impairment Study of the Elderly: a description of methods. Archives of physical medicine and rehabilitation. 2013;94(2):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callahan D, Phillips E, Carabello R, Frontera WR, Fielding RA. Assessment of lower extremity muscle power in functionally-limited elders. Aging clinical and experimental research. 2007;19(3):194–199. [DOI] [PubMed] [Google Scholar]
- 12.Suri P, Kiely DK, Leveille SG, Frontera WR, Bean JF. Trunk muscle attributes are associated with balance and mobility in older adults: a pilot study. PM & R : the journal of injury, function, and rehabilitation. 2009;1(10):916–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins MA, Riddle DL, Lamb RL, Personius WJ. Reliability of goniometric measurements and visual estimates of knee range of motion obtained in a clinical setting. Physical therapy. 1991;71(2):90–96; discussion 96-97. [DOI] [PubMed] [Google Scholar]
- 14.Olaleye D, Perkins BA, Bril V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinic. Diabetes research and clinical practice. 2001;54(2):115–128. [DOI] [PubMed] [Google Scholar]
- 15.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. Journal of clinical epidemiology. 1993;46(2):153–162. [DOI] [PubMed] [Google Scholar]
- 16.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–318. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 18.Cheung C-L, Nguyen U-SDT, Au E, Tan KCB, Kung AWC. Association of handgrip strength with chronic diseases and multimorbidity: A cross-sectional study. Age. 2013;35(3):929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortiz PJ, Tello T, Aliaga EG, et al. Effect of multimorbidity on gait speed in well-functioning older people: A population-based study in Peru. Geriatrics & gerontology international. 2018;18(2):293–300. [DOI] [PubMed] [Google Scholar]
- 20.Su P, Ding H, Zhang W, et al. The association of multimorbidity and disability in a community-based sample of elderly aged 80 or older in Shanghai, China. BMC geriatrics. 2016;16(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adamson J, Lawlor DA, Ebrahim S. Chronic diseases, locomotor activity limitation and social participation in older women: cross sectional survey of British Women’s Heart and Health Study. Age and ageing. 2004;33(3):293–298. [DOI] [PubMed] [Google Scholar]
- 22.Olaya B, Moneta MV, Caballero FF, et al. Latent class analysis of multimorbidity patterns and associated outcomes in Spanish older adults: a prospective cohort study. BMC geriatrics. 2017;17(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen FB, Pedersen MH, Friis K, Glumer C, Lasgaard M. A Latent Class Analysis of Multimorbidity and the Relationship to Socio-Demographic Factors and Health-Related Quality of Life. A National Population-Based Study of 162,283 Danish Adults. PloS one. 2017;12(1):e0169426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitson HE, Johnson KS, Sloane R, et al. Identifying Patterns of Multimorbidity in Older Americans: Application of Latent Class Analysis. Journal of the American Geriatrics Society. 2016;64(8):1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. The journals of gerontology Series A, Biological sciences and medical sciences. 2007;62(7):722–727. [DOI] [PubMed] [Google Scholar]
- 26.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC geriatrics. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.