Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is characterized by an overwhelming cytokine response. Various treatment strategies have been attempted.
Methods and Results
A 61-year-old man with heart transplantation in 2017 presented with fever, cough, and dyspnea, and was confirmed positive for coronavirus disease 2019 (COVID-19). Laboratory tests showed significant elevations in C-reactive protein and interleukin-6 (IL-6). Echocardiogram showed left ventricular ejection fraction 58% (with ejection fraction 57% 6 months prior). Given the lack of clear management guidelines, the patient was initially managed symptomatically. However, the patient subsequently had a rapid respiratory deterioration with worsening inflammatory markers on day 5 of admission. Tocilizumab (anti-IL-6R) was in low supply in the hospital. The patient was offered clazakizumab (anti-IL-6) for compassionate use. Patient received 25 mg intravenously × 1 dose. Within 24 hours, he showed significant improvement in symptoms, oxygen requirements, radiological findings, and inflammatory markers. There was a transient leukopenia that improved in 4 days. He was discharged home on day 11, with negative nasopharyngeal SARS-CoV-2 PCR as an outpatient on day 35, development of positive serum COVID-19 IgG antibody, and he continued to do well on day 60, with no heart-related symptoms.
Conclusion
Clazakizumab is a monoclonal antibody against human IL-6, which may be helpful in inhibiting the cytokine response to SARS-CoV-2 in COVID-19. Although not yet FDA approved, it is being investigated for treatment of renal antibody-mediated rejection. Clinical trials of clazakizumab for treatment of COVID-19 are underway worldwide.
Highlights
-
•
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is characterized by an overwhelming cytokine response.
-
•
A 61-year-old man with heart transplantation in 2017 presented with fever, cough, and dyspnea, and was confirmed positive for coronavirus disease 2019 (COVID-19). Laboratory tests showed significant elevations in C-reactive protein, interleukin-6 (IL-6), and other inflammatory markers. Echocardiography showed normal LV function.
-
•
The patient received 25 mg of clazakizumab (anti-IL-6 monoclonal antibody) as a single dose, with significant improvement in symptoms, oxygen requirements, radiological findings, and inflammatory markers within 24 hours, and discharge to home on day 11. He had a negative nasopharyngeal SARS-CoV-2 polymerase chain reaction as an outpatient on day 35, developed a positive serum COVID-19 IgG antibody, and was continuing to do well clinically on day 60.
-
•
Clazakizumab is a monoclonal antibody against human IL-6, which may be helpful in inhibiting the cytokine response to SARS-CoV-2 in COVID-19.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is characterized by an overwhelming inflammatory state with massive dysregulation of cytokines, contributing to widespread organ damage. Inhibition of the cytokine pathway could theoretically stop this cascade and interleukin-6 (IL-6) is one such target. Data from the literature [1,2] and from our own center (S. Jordan, unpublished data, 2020) indicate that IL-6 is the predominant cytokine seen with SARS-CoV-2 pneumonia and substantiates the use of therapies against IL-6 or IL-6 receptor to dampen the cytokine storm in these patients. The present case highlights the potential utility of clazakizumab as an IL-6 inhibitor in reducing respiratory morbidity of COVID-19 in a patient infected with SARS-CoV-2 after heart transplant (HTx).
Report
A 61-year-old man who had orthotopic HTx in May 2017 with normal postoperative graft function presented with 1 week of dyspnea on exertion and nonproductive cough. He had a sick contact (his wife) who was later diagnosed with COVID-19. His medications included tacrolimus 4 mg each morning and 3 mg each evening, and mycophenolate mofetil 1000 mg twice a day. He was also on chronic prednisone 5 mg daily for rheumatoid arthritis and lisinopril 10 mg daily for hypertension. His medical history included diabetes mellitus and a remote history of bladder cancer in remission.
On presentation, his temperature was 38°C, heart rate 92 beats/min, and blood pressure 130/93 mm Hg. His oxygen saturation was 98% on room air. He had mild acute renal injury with serum creatinine 1.4 mg/dL. He had normal white blood cell count of 4.8 × 103 cells/μL with a normal differential of 71% neutrophils, 18% lymphocytes, and 0.4% eosinophils. He had mild anemia (hemoglobin 12 g/dL) and mild thrombocytopenia (platelets 121 × 103 cells/μL). Liver function tests, serum troponin, serum glucose, electrocardiogram and echocardiogram (normal graft function with left ventricular ejection fraction 58%; prior ejection fraction 57% 6 months before) were unremarkable. Chest radiography showed new bilateral lung infiltrates consistent with pneumonia.
SARS-CoV-2 polymerase chain reaction (PCR) testing done on admission through a nasopharyngeal swab was positive and a repeat test done the next day confirmed the results. Subsequent blood tests included elevated erythrocyte sedimentation rate (50 mm/h, reference <20 mm/h), C-reactive protein (CRP, 133 mg/L, reference <5 mg/L), myoglobin (78 ng/mL, reference <72 ng/mL), ferritin (1172 ng/mL, reference <275 ng/mL), D-dimer (1.31 μg/mL, reference <0.7 μg/mL), and lactate dehydrogenase (257 U/L, reference <220 U/L). Serum tacrolimus level was 11.7 ng/mL (goal 5-10 ng/mL), and the extent of immunosuppression using the T-cell immune function assay (Cylex test) showed an ATP level of 39 ng/mL (reference for low immune cell response ≤225 ng/mL, indicating over-immunosuppression).
Given the initial clinical stability, he was initially managed by supportive measures. Tacrolimus dose was decreased to 2 mg each morning and 1 mg each evening, and mycophenolate mofetil to 750 mg twice a day, to reduce over-immunosuppression.
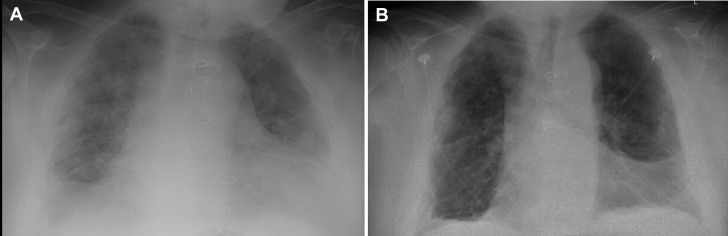
On day 5 of admission, he had worsening of oxygen saturation and rapid escalation of oxygen therapy to 7 L through a facemask and there was a discussion regarding invasive ventilation. He was hypotensive at 96/67 mm Hg and tachycardic (111 beats/min). Chest radiography showed interval worsening of underlying pneumonia (Fig 1 A). His CRP increased to 158 mg/L, with an elevated IL-6 level of 34 pg/mL (reference <7 pg/mL).
Fig 1.
(A) Interval worsening of bilateral pulmonary infiltrates. (B) Improvement in parenchymal infiltrates within 4 days of clazakizumab therapy.
Given the patient's deteriorating condition and his immunosuppressed state, a multidisciplinary discussion took place involving the infectious disease, pulmonary, medicine, and HTx teams. A decision was made to treat with an IL-6 inhibitor. Tocilizumab was reserved in the hospital for intubated, critically ill patients. Compassionate use of clazakizumab (Vitaeris) was approved by the FDA after obtaining the patient's consent and Institutional Review Board (IRB) approval. Tuberculosis was ruled out. Cytomegalovirus PCR was negative. Subsequently, the patient received a one-time dose of clazakizumab 25 mg in 50 mL normal saline, given over 30 minutes. No immediate side effects were noted.
On the following day, the patient showed significant symptomatic improvement and the oxygen requirement dropped to 2 L/min by nasal cannula. CRP decreased to 139 mg/L and continued to decrease to 8 mg/L 6 days after the clazakizumab dose. Serum ferritin levels dropped from a peak of 1172 to 673 ng/mL. White blood cell count decreased to 2.9 × 103 cells/μL, following which mycophenolate mofetil was discontinued and tacrolimus dose was reduced further to 1 mg twice a day. The serum tacrolimus levels predominantly remained within goal (5-10 ng/mL) for the rest of the hospital stay. He was continued on his home prednisone dose; no intravenous steroid dose was administered. White blood cell count subsequently increased to 4.5 × 103 cells/μL in 4 days, and no worsening was noted in the other cell counts. No other relevant abnormalities were noted in other blood tests; IL-6 levels were not repeated. His chest radiograph showed interval improvement in the parenchymal infiltrates (Fig 1B), and he was discharged home on day 11 of admission with the above changes in his medications. The repeat nasopharyngeal SARS-CoV-2 PCR was negative as an outpatient on day 35, and the serum COVID-19 IgG antibody was positive, consistent with prior infection. The patient continued to do well as an outpatient at day 60, with no ongoing heart-related symptoms.
Discussion
Herein we report a case of an immunosuppressed HTx recipient with SARS-CoV-2 infection and manifestations of COVID-19. Given the sudden clinical deterioration and signs of overwhelming inflammation in the form of cytokine storm, he was treated with an anti-IL-6 monoclonal antibody, clazakizumab. This was associated with a rapid and significant clinical recovery and an improvement in inflammatory markers within a few days without any untoward adverse event. The case highlights a possible treatment strategy for patients with COVID-19 to aid resolution of pulmonary infiltrates in an immunocompromised transplant recipient.
The syndrome of COVID-19 after infection with SARS-CoV-2 is characterized by an overwhelming inflammatory state with massive dysregulation of cytokines, called cytokine storm. Excessive cytokine production may trigger secondary hemophagocytic lymphohistiocytosis with widespread tissue and organ damage. IL-6 is a pleiotropic cytokine within the cascade of host defense contributing to acute phase reaction, innate immune response activation, and other defensive activities [3]. A pathologically excessive production of IL-6 can result in chronic inflammation, characteristic of multiple systemic inflammatory conditions [3] associated with severe infections, including the previous H1N1 influenza pandemic [4].
In an observational study of 69 patients with COVID-19, most patients had elevated lactate dehydrogenase, CRP, ferritin, and D-dimer [1]. In association with these, the study also noted an increase in the baseline IL-6 levels, especially in severe infections. The authors suggested a potential use of IL-6 as a biomarker and also a target for future treatment of the disease [1]. Consequently, an attempt has been made to identify a role for IL-6 inhibitors in the treatment of patients with COVID-19. Tocilizumab is a humanized monoclonal antibody against the human IL-6 receptor. Xu et al [5] reported 21 hospitalized patients with COVID-19 treated with tocilizumab (400 mg single dose). The patients had significant improvement in their oxygen requirements the next day, associated with a drop in CRP levels. Including previously critically ill patients, 91% of patients were subsequently discharged home within the study period and no adverse events were reported. A multicenter randomized, double-blinded, placebo-controlled phase III clinical trial is currently underway to establish the safety and efficacy of tocilizumab in the treatment of severe COVID-19 pneumonia (NCT04320615) [6].
Clazakizumab is a humanized IgG1 monoclonal antibody directed against human IL-6 instead of the IL-6 receptor [7]. Clazakizumab’s efficacy and safety have been reported in rheumatoid arthritis and are being studied in late antibody-mediated rejection of kidney allografts [7]. Randomized placebo-controlled studies examining the benefits of clazakizumab in prevention and treatment of SARS-CoV-2 pneumonia are now underway at Cedars-Sinai Medical Center and NYU Langone Medical Center (NCT04348500 and NCT04343989) [8,9].
Owing to the current lack of proven therapies and clear guidelines, various other options also were considered in our patient. Hydroxychloroquine was not used, given the possibility of cardiac toxicity, and lack of randomized trial results in COVID-19. Tocilizumab has been restricted in our medical center for use in critically ill patients only. Clazakizumab was available on a compassionate-use basis and approved by the FDA and local IRB, following administration of which the patient had significant reduction in inflammatory markers, and was discharged within 6 days of therapy. The high tacrolimus level at presentation and low T-cell immune function (Cylex) level suggested over-immunosuppression, which can be a risk factor for SARS-CoV-2 infection. Subsequently, the immunosuppression was reduced. Despite the continued immunosuppression, he was able to mount a strong cytokine response. It is possible that the reduction in immunosuppression may have contributed to the cytokine response.
Previous reports suggest that clazakizumab is significantly more potent (3-120 times) in in vitro and ex vivo assays [10] when compared with tocilizumab. Shin et al [11] reported a trial comparing the IL-6 signaling inhibition among kidney transplant patients with chronic antibody mediated rejection treated with clazakizumab (n = 8) or tocilizumab (n = 11). The study noted a significant increase in soluble IL-6 receptor and IL-6 after tocilizumab therapy (P = .03), which may contribute to an increased risk of rejection following cessation of therapy. No such increase was noted with clazakizumab therapy (P = .7). However, robust comparative data between these 2 agents is missing. Nevertheless, clazakizumab provides a viable alternative to tocilizumab, especially when tocilizumab is in short supply, as is the case at many centers. Another concern with tocilizumab is the potential for high levels of circulating IL-6R, negating tocilizumab through early saturation of the monoclonal before inhibition of cellular signaling can be achieved [12]. This is often manifested as a slow decline or no decline in inflammatory markers (ie, CRP). As a result, patients may need additional doses to achieve inhibition of the IL-6/IL-6R signaling pathway. This has been noted in patients with the capillary leak syndrome where additional doses of tocilizumab are required 12 hours post-initial dose to fully inhibit IL-6/IL-6R signaling [13]. In this regard, the distribution and volume of the ligand (IL-6) should be much easier to inhibit.
Clazakizumab is a well-tolerated agent with adverse effects that are dose-dependent, including mild infections, injection-site reaction, increase in aminotransferase and cholesterol levels, decreased neutrophil and platelet counts, while serious and opportunistic infections, malignancies, and autoimmune disorders are uncommon (5%-9% incidence) [7,14]. No adverse effects were noted in the current patient. The patient had normal graft function through the infection and remains currently asymptomatic on outpatient follow-up.
With regard to the heart transplant program, because of the risk to both donors and recipients from SARS-CoV-2 and COVID-19 during this time (March to May 2020), only urgent (status 1, 2, or 3) recipients were transplanted, and both donors and recipients were tested for SARS-CoV-2 by nasopharyngeal swab or bronchoalveolar lavage before transplant. After mid-May 2020, all status designations (1 through 6) for recipients were considered for transplant, but testing for SARS-CoV-2 was continued for all donors and recipients before transplant.
In conclusion, clazakizumab can be a safe and efficacious option for management of the cytokine storm characteristic of severe COVID-19 pneumonia, including transplant recipients. Further trials are underway to establish this hypothesis.
References
- 1.Liu T., Zhang J., Yang Y. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020 doi: 10.15252/emmm.202012421. accessed June 15,2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toniati P., Piva S., Cattalini M. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020:102568. doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimoto N., Kishimoto T., Yoshizaki K. Anti-interleukin 6 receptor antibody treatment in rheumatic disease. Ann Rheum Dis. 2000;59(Suppl. 1):i21–i27. doi: 10.1136/ard.59.suppl_1.i21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paquette S.G., Banner D., Zhao Z. Interleukin-6 is a potential biomarker for severe pandemic H1N1 influenza A infection. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X., Han M., Li T. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv. 2020;202003:V1. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roche H.-L. A study to evaluate the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia 2020. https://clinicaltrials.gov/ct2/show/record/NCT04320615 [accessed 08.04.20]
- 7.Eskandary F., Durr M., Budde K. Clazakizumab in late antibody-mediated rejection: study protocol of a randomized controlled pilot trial. Trials. 2019;20:37. doi: 10.1186/s13063-018-3158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan S.C. A phase II trial to evaluate the safety and tolerability of Clazakizumab® (anti-IL- 6 monoclonal) compared to placebo for the treatment of COVID-19 infection 2020. https://clinicaltrials.gov/ct2/show/study/NCT04348500 [accessed 18.04.20]
- 9.Lonze B. A randomized placebo-controlled safety and dose-finding study for the use of the IL-6 inhibitor clazakizumab in patients with life-threatening COVID-19 infection 2020. https://clinicaltrials.gov/ct2/show/record/NCT04343989 [accessed 18.04.20]
- 10.Zhao Q, Pang J, Shuster D. Anti-IL-6 antibody clazakizumab is more potent than tocilizumab in blocking in vitro and ex vivo IL-6-induced functions. Abstract presented at: ACR/ARHP Annual Meeting. October 25-26, 2013; San Diego, CA.
- 11.Shin B, Ge S, Petrosyan A. Clazakizumab (CLZ, anti-IL-6 antibody) and tocilizumab (TCZ, anti-IL-6 receptor [r] antibody) treatments differentially affect IL-6/IL-6R signaling by modulating soluble IL-6R (sIL-6R) and gp130 (sgp130) in kidney transplant patients (KTx Pts) treated for chronic antibody-mediated rejection (cABMR). Paper presented at: ATC Annual Meeting. June 3, 2019; Boston, MA.
- 12.Jordan S.C., Choi J., Kim I. Interleukin-6, a cytokine critical to mediation of inflammation, autoimmunity and allograft rejection: therapeutic implications of IL-6 receptor blockade. Transplantation. 2017;101:32–44. doi: 10.1097/TP.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 13.Chen F., Teachey D.T., Pequignot E. Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J Immunol Methods. 2016;434:1–8. doi: 10.1016/j.jim.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mease P.J., Gottlieb A.B., Berman A. The efficacy and safety of clazakizumab, an anti-interleukin-6 monoclonal antibody, in a phase IIb study of adults with active psoriatic arthritis. Arthritis Rheumatol. 2016;68:2163–2173. doi: 10.1002/art.39700. [DOI] [PubMed] [Google Scholar]