Abstract
Aims
To determine how long SARS-CoV-2 virus RNA persists in fecal specimens in children with COVID-19.
Methods
Retrospectively, ten children with confirmed COVID-19 in the Jinan Infectious Disease Hospital Affiliated to Shandong University were enrolled between January 23, 2020 to March 9, 2020. Epidemiological, clinical, laboratory, and radiological characteristics of the children were analyzed. RT-PCR assays were performed to detect the SARS-CoV-2 virus RNA in the respiratory tract and fecal specimens in the follow-up after discharge.
Results
Among ten patients, five (50%) were asymptomatic and five (50%) showed mild symptoms of respiratory illness. The average age of asymptomatic children was younger than that of symptomatic children (p = 0.03). The decreases in white blood cell (WBC) (p = 0.03) and lymphocyte (p = 0.03) counts were more severe in symptomatic patients than those in asymptomatic patients. During the follow-up examination after discharge, seven out of ten patients contained SARS-CoV-2 virus RNA in their fecal specimens, despite all patients showed negative results in respiratory tract specimens. One out of those seven patients relapsed. The median time from onset to being negative results in respiratory tract and fecal specimens was 9 days and 34.43 days, respectively.
Conclusions
SARS-CoV-2 virus RNA persists much longer in the gastrointestinal (GI) tract than that in respiratory tract.
Keywords: SARS-CoV-2, COVID-19, Children, Fecal specimen
Introduction
Since the first atypical pneumonia case was reported in Wuhan, China on December 31, 2019 [1], the viral pathogen was quickly identified as a novel coronavirus by deep sequencing of viral genome (http://virological.org/ and https://www.gisaid.org/) [2]. On February 11, 2020, the World Health Organization officially named the new virus as SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus2) and the disease caused by SARS-CoV-2 infection as COVID-19 (Coronavirus Disease 2019) [3]. The COVID-19 pandemic quickly spread in China and other countries and represents a severe global health threat.
According to the Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th version) [4], discharge criteria are defined as: (1) temperature returned to a normal level for more than 3 days; (2) respiratory symptoms significantly improved; (3) pulmonary imaging showed significant improvement in acute exudative lesions; (4) two consecutive virus RNA tests in sputum, nose swab and other respiratory tract specimens showing negative results (sampling should be at least 24 h apart). In this retrospective study, we performed follow-up virus RNA tests on ten children with COVID-19 within 2 weeks after discharge and obtained negative results in the respiratory tract specimens and urine samples from all of them. In sharp contrast, we discovered that seven of them contained SARS-CoV-2 virus RNA in fecal specimens. One male patient with a positive result in fecal specimen test relapsed after discharge and showed progressive clinical symptoms including fever, cough, nausea, vomiting and diarrhea. In sum, these findings indicate that for children with COVID-19, SARS-CoV-2 virus RNA persists longer in GI tract than that in respiratory tract and urine.
Methods
Human subjects
The present study is a retrospective descriptive clinical study. A total of ten children with confirmed COVID-19 in the Jinan Infectious Disease Hospital Affiliated to Shandong University were enrolled in this study between January 23, 2020 to March 9, 2020. This study has been approved by the ethics board of the Jinan Infectious Diseases Hospital Affiliated to Shandong University (No.20200203). Informed consent was obtained from each patient or their guardians. Diagnosis of patients was based on the Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th version) [4]. Diagnostic criteria for asymptomatic cases include: individuals infected by SARS-CoV-2 who remain asymptomatic throughout the course of the infection with or without abnormal chest computed tomography (CT) findings. Diagnostic criteria for mild cases: mild clinical symptoms, no radiographic findings of pneumonia; for regular cases: fever, respiratory symptoms, and radiographic manifestations of pneumonia; for severe cases: (1) respiratory distress, respiratory frequency ≥30 times/min, (2) hypoxemia with resting oxygen saturation ≤93%, and (3) arterial partial oxygen pressure (PaO2)/oxygen absorption concentration (FiO2) ≤300 mmHg (1 mmHg = 0.133 kPa); for critical cases: (1) respiratory failure and mechanical ventilation is required, (2) shock, and (3) complication with other organ failure requiring ICU care.
Data collection
The medical records of patients were collected and analyzed, including demographic data, exposure history, symptoms, signs, laboratory findings, and chest CT scanning. Data collection forms from electronic medical records were used to collect data. Disease onset was defined as the day when the symptom was noticed.
RTPCR
Respiratory tract and fecal specimens were collected from each patient. SARS-CoV-2 virus RNA was detected by RT-PCR assay using the Dual-target Detection Kit from the Shanghai Jienuo Company. For RT-PCR assay, the cut-off value was 40. A cycle threshold (Ct) value less than 37 was defined as positive.
Statistical analysis
Collected data were recorded into Microsoft Excel for Macintosh (version 16.30).Statistical analysis was carried out with Statistical Package for Social Sciences (SPSS) (version 16.0) and Prism (version 5.0) for Macintosh. Measurement data were described as mean ± standard deviation. Background factors were compared using the Student’s t-test (numerical data) or the Chi-square test (categorical data). In all tests, p values of less than 0.05 were considered statistically significant.
Results
Retrospective analysis of patients with COVID-19
A total of ten children (three male and seven female) were enrolled in this study, with a median age of 5.08 years (range, 9 month-14 years). All the children were in familial clusters. One child (10%) was diagnosed as mild case and nine (90%) as regular cases. Clinical symptoms were mild or even absent, four (40%) children showed fever, two children (20%) experienced dry cough, and one child (10%) had phlegm. Among these ten patients, five (50%) were asymptomatic and five (50%) showed mild symptoms of respiratory illness, including two asymptomatic females (40%) with a median age of 2.36 years and five symptomatic females(100%) with a median age of 7.80 years. The average age of asymptomatic children was younger than that of symptomatic children (p = 0.03) (Table 1 ). Laboratory tests showed that the decreases in white blood cell (WBC) (p = 0.03) and lymphocyte (p = 0.03) counts were more severe in symptomatic patients than those in asymptomatic patients (Table1). Chest CT scanning revealed that among five children with lung lesions, three (60%) of them showing bilateral involvement in symptomatic children with no difference compared with asymptomatic children (p = 0.29, p = 0.64) (Table1). All patients received a combination therapy of interferon atomized inhalation with ribavirin, with a median hospital stays of 19.8 days. Discharge criteria were based on the Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th version).
Table 1.
Clinical features, Laboratory tests, and CT scan of COVID-19 in symptomatic and asymptomatic children.
| Median (IQR) |
|||||
|---|---|---|---|---|---|
| Normal range | Total (N = 10) | Symptomatic (5,50%) | Asymptomatic (5,50%) |
P | |
| Age, median, y | 5.08 | 7.80 | 2.36 | 0.03 | |
| Gender | M (3,30%) | M (0,0%) | M (3,60%) | 0.04 | |
| F (7,70%) | F (5,100%) | F (2,40%) | |||
| Clinical type | 0.29 | ||||
| Mild | 1,10% | 0,0% | 1,20% | ||
| Conventional | 9,90% | 5,100% | 4,80% | ||
| Time, days | 3.75 ± 5.91 | 6.60 ± 7.64 | 0.90 ± 0.22 | 0.17 | |
| Hospital stays | 19.80 ± 6.70 | 21.60 ± 6.88 | 18.00 ± 6.75 | 0.43 | |
| WBC × 109/L | 4−10 | 6.35 ± 2.77 | 4.55 ± 2.01 | 8.15 ± 2.28 | 0.03 |
| N × 109/L | 2−7 | 1.85 ± 0.60 | 1.56 ± 0.38 | 2.14 ± 0.68 | 0.13 |
| L × 109/L | 0.8−4 | 4.03 ± 2.26 | 2.56 ± 1.89 | 5.50 ± 1.59 | 0.03 |
| M × 109/L | 0.12−0.8 | 0.35 ± 0.12 | 0.34 ± 0.15 | 0.36 ± 0.09 | 0.81 |
| HBG | 110−150 | 125.90 ± 7.85 | 125.40 ± 9.89 | 126.40 ± 6.35 | 0.45 |
| PLT × 109/L | 100−300 | 244.20 ± 89.69 | 221.00 ± 47.19 | 267.40 ± 120.53 | 0.72 |
| ALT | 0−40 | 24.80 ± 34.32 | 13.00 ± 2.55 | 36.60 ± 47.92 | 0.33 |
| AST | 0−40 | 31.30 ± 7.09 | 25.80 ± 4.76 | 36.80 ± 3.83 | 0.00 |
| CRP | 0.068−8.2 | 1.38 ± 3.34 | 2.39 ± 4.74 | 0.37 ± 0.35 | 0.40 |
| PCT | 0−0.05 | 0.05 ± 0.02 | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.71 |
| LDH | 109−245 | 307.90 ± 92.47 | 243.60 ± 23.11 | 372.20 ± 91.49 | 0.03 |
| CK | 26−140 | 99.80 ± 45.54 | 92.64 ± 29.07 | 106.96 ± 60.76 | 0.65 |
| Myo | 10−46 | 11.67 ± 3.99 | 12.47 ± 5.57 | 10.87 ± 1.79 | 0.56 |
| Ctn | 0−0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.010.00 | |
| BUN | 2.9−8.2 | 4.25 ± 1.31 | 3.96 ± 0.91 | 4.54 ± 1.68 | 0.52 |
| Cr | 50.4−98.1 | 44.61 ± 5.83 | 47.64 ± 7.24 | 41.58 ± 1.09 | 0.10 |
| PT | 8.8−13.8 | 12.01 ± 0.50 | 11.94 ± 0.67 | 12.09 ± 0.33 | 0.68 |
| D-dimer | 0−0.5 | 0.34 ± 0.20 | 0..39 ± 0.28 | 0.30 ± 0.06 | 0.54 |
| IL-6 | 0−7 | 2.50 ± 2.49 | 3.30 ± 3.49 | 1.70 ± 0.45 | 0.34 |
| Pneumonia | 9,90% | 5,100% | 4,80% | 0.29 | |
| CT (bilateral) | 6,66.7% | 3,60% | 3,75% | 0.64 | |
| CT (unilateral) | 3,33.3% | 2,40% | 1,40% | ||
Note: Time: from onset to diagnosis.
Persistence of SARS-CoV-2 virus RNA in feces after discharge
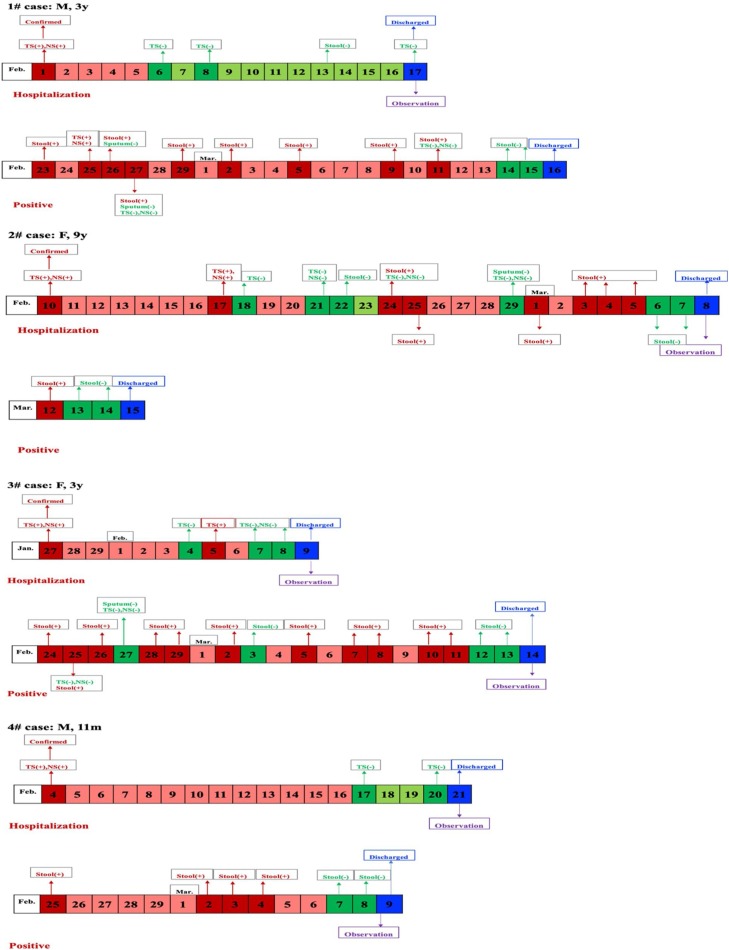
All children returned to the Hospital for follow-up tests 2 weeks after discharge. Respiratory tract specimens, including throat swab, nose swab and sputum, and fecal specimens were examined for virus RNA. Some of the patients were also tested for virus RNA in urine samples. RTPCR assay revealed negative results in the respiratory tract specimens and urine samples from all patients. In sharp contrast, seven of them contained SARS-CoV-2 virus RNA in fecal specimens. As a precaution, those seven patients, included three males (42.9%) with a median age of 4.69 years, were re-admitted for isolation and therapy. Were viewed the clinical data from the first hospitalization and found no difference in clinical features, laboratory findings, and chest CT findings between children showing positive and negative results in feces virus RNA tests (Table 2 ). Among those seven patients containing virus RNA in feces, six children showed no clinical symptoms, with normal laboratory tests and lung chest CT scanning. All seven patients received traditional Chinese medicine therapy in the 2nd hospitalization. The median time from onset to being negative results in respiratory tract and fecal specimens was 9 days and 34.43 days, respectively. The changing course in virus RNA detection of those seven patients is shown in Fig. 1 .
Table 2.
Clinical features, Laboratory tests, and CT scan of COVID-19 in children with positive and negative results in stool specimens.
| Median (IQR) |
|||||
|---|---|---|---|---|---|
| Normal range | Total (N = 10) | Positive (7,70%) | Negative (3,30%) | p | |
| Age, median, y | 5.08 | 4.69 | 6.00 | 0.68 | |
| Gender | M (3,30%) | M (3,42.9%) | M (0,0%) | 0.18 | |
| F (7,70%) | F (4,57.1%) | F (3,100%) | |||
| Clinical type | 0.49 | ||||
| Mild | 1,10% | 1,14.3% | 0,0% | ||
| Conventional | 9,90% | 6,85.7% | 3,100% | ||
| symptomatic | 5,50% | 3,42.9% | 2,66.7% | 0.49 | |
| asymptomatic | 5,50% | 4,57.1% | 1,33.3% | ||
| Time, days | 3.75 ± 5.91 | 2.21 ± 1.78 | 7.33 ± 10.97 | 0.50 | |
| Hospital stays | 19.80 ± 6.70 | 17.71 ± 4.68 | 24.67 ± 9.24 | 0.14 | |
| Signs and symptoms | |||||
| Fever | 4,40% | 3,42.9% | 1,33.3% | 0.78 | |
| Dry cough | 2,20% | 0,0% | 2,66.7% | 0.02 | |
| Phlegm | 1,10% | 0.0% | 1,33.3% | 0.11 | |
| WBC × 109/L | 4−10 | 6.35 ± 2.77 | 6.68 ± 2.77 | 5.60 ± 3.23 | 0.60 |
| N × 109/L | 2−7 | 1.85 ± 0.60 | 1.89 ± 0.60 | 1.77 ± 0.74 | 0.79 |
| L × 109/L | 0.8−4 | 4.03 ± 2.26 | 4.34 ± 2.27 | 3.30 ± 2.52 | 0.54 |
| M × 109/L | 0.12−0.8 | 0.35 ± 0.12 | 0.31 ± 0.12 | 0.43 ± 0.06 | 0.15 |
| HBG | 110−150 | 125.90 ± 7.85 | 125.57 ± 8.68 | 126.67 ± 7.09 | 0.85 |
| PLT × 109/L | 100−300 | 244.2 ± 89.69 | 237.0 ± 106.04 | 261.00 ± 43.09 | 0.72 |
| ALT | 0−40 | 24.80 ± 34.32 | 29.29 ± 41.09 | 14.33 ± 1.15 | 0.56 |
| AST | 0−40 | 31.30 ± 7.09 | 32.29 ± 7.57 | 29.00 ± 6.58 | 0.53 |
| CRP | 0.068−8.2 | 1.38 ± 3.34 | 1.86 ± 3.98 | 0.25 ± 0.11 | 0.52 |
| PCT | 0−0.05 | 0.05 ± 0.02 | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.57 |
| LDH | 109−245 | 307.9 ± 92.47 | 302.14 ± 65.99 | 321.33 ± 158.21 | 0.86 |
| CK | 26−140 | 99.80 ± 45.54 | 101.97 ± 55.35 | 94.73 ± 9.21 | 0.75 |
| Myo | 10−46 | 11.67 ± 3.99 | 12.38 ± 4.42 | 10.00 ± 2.65 | 0.42 |
| Ctn | 0−0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.010.00 | |
| BUN | 2.9−8.2 | 4.25 ± 1.31 | 4.53 ± 1.42 | 3.60 ± 0.87 | 0.33 |
| Cr | 50.4−98.1 | 44.61 ± 5.83 | 45.47 ± 6.94 | 42.60 ± 0.36 | 0.51 |
| PT | 8.8−13.8 | 12.01 ± 0.50 | 12.02 ± 0.36 | 12.00 ± 0.85 | 0.96 |
| D-dimer | 0−0.5 | 0.34 ± 0.20 | 0..40 ± 0.20 | 0.21 ± 0.12 | 0.17 |
| IL-6 | 0−7 | 2.50 ± 2.49 | 2.93 ± 2.94 | 1.50 ± 0.00 | 0.44 |
| Pneumonia | 9,90% | 6,85.7% | 3,100% | 0.49 | |
| CT (bilateral) | 6,66.7% | 5,83.3% | 1,33.3% | 0.13 | |
| CT (unilateral) | 3,33.3% | 1,16.7% | 2,66.7% | ||
Note: Time; from onset to diagnosis.
Fig. 1.
The change course of 7 cases with positive results for COVID-19 in stool specimen.
Relapse of a patient with persistent SARS-CoV-2 virus RNA in feces after discharge
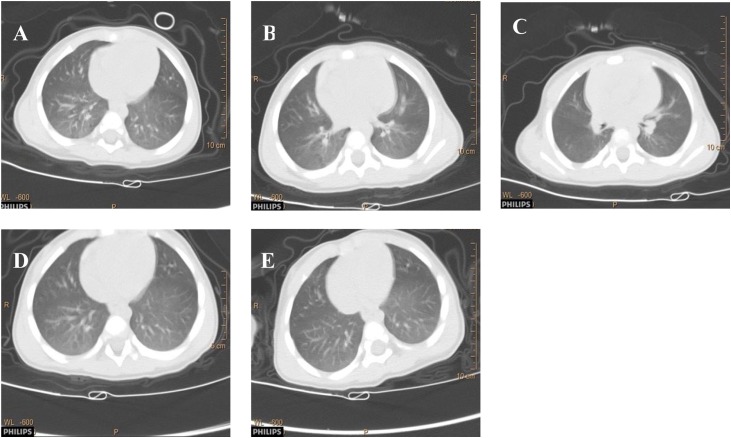
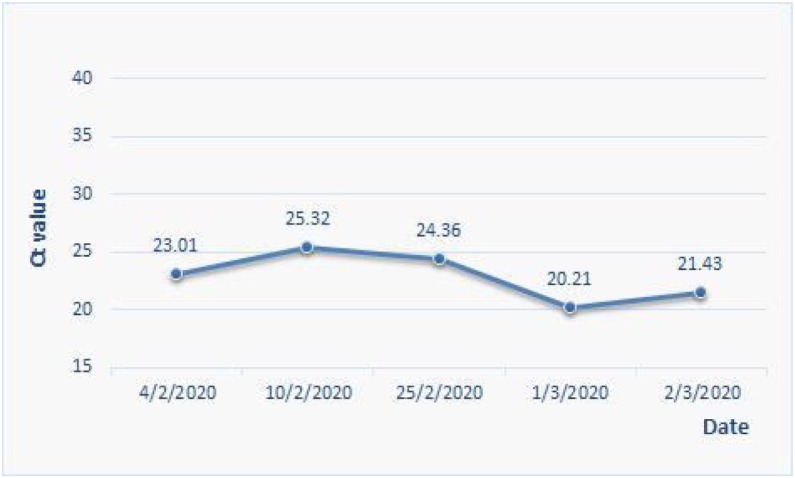
Among seven patients containing virus RNA in feces, one asymptomatic 11-month-old boy returned to the Hospital with fever, cough, nausea, vomiting, and diarrhea nine days after discharge. His clinical conditions were reassessed, as shown in Fig. 2 and Table 3. Virus RNA tests showed negative results in respiratory tract specimens (throat swab and sputum) but positive results in fecal specimens. Potential infection of other viruses such as influenza virus (A, B, H7 and N9 subtypes), EB virus, cytomegalovirus, enterovirus-71, coxsackie virus and enterovirus, or bacteria such as mycoplasma pneumoniae and chlamydia pneumoniae, were all considered and ruled out. The patient showed clinical symptoms of respiratory illness including fever, cough and mucus accumulation in the lung. CT scanning revealed the progression of lung inflammatory lesions compared to those during the 1st hospitalization (Fig. 2). During virus RNA detection by RT-PCR assays, the Ct value maintained in a similar level in readmittance compared with that previously confirmed (Fig. 3 ). There were also gastrointestinal symptoms such as nausea, vomiting, and diarrhea. The patient was readmitted for treatment with interferon atomization inhalation combined with cephalosporin antibiotics. Three days later, his temperature returned to a normal level, and he was discharged after 7-day hospital stay. Discharge criteria were based on the Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th version). The changing course in virus RNA detection is shown in Fig. 1 (#6 patient).
Fig. 2.
Transverse chest CT images of case 6# with time.
Table 3.
Clinical data of an 11 month old boy.
| Normal range | Result | |
|---|---|---|
| Temperature | 38.7 °C | |
| Heart rate | 145 | |
| Respiratory rate | 22 | |
| WBC × 109/L | 4−10 | 4.46 |
| N × 109/L | 2−7 | 2.50 |
| L × 109/L | 0.8−4 | 1.50 |
| M × 109/L | 0.12−0.8 | 0.50 |
| HBG | 110−150 g/L | 109 |
| PLT × 109/L | 100−300 | 229 |
| ALT | 0−40 U/L | 20 |
| AST | 0−40 U/L | 37 |
| CRP | 0.068−8.2 mg/L | 1.08 |
| PCT | 0−0.05 μg/L | 0.113 |
| LDH | 109−245 U/L | 281 |
| CK | 26−140 U/L | 376 |
| BUN | 2.9−8.2 mmol/L | 6.0 |
| Cr | 50.4−98.1 μmmol/L | 42.4 |
| IL-6 | 0−7 pg/mL | 12.78 |
| Pneumonia | Yes | |
| CT (bilateral) | Yes | |
| Stool | Positive | |
| Throat swab | Negative | |
| Sputum | Negative | |
| EBV-DNA | Negative | |
| CMV-DNA | Negative | |
| H7 ifu | Negative | |
| Ifu A | Negative | |
| Ifu B | Negative | |
| N9 ifu | Negative | |
| Mpn | Negative | |
| Cpn | Negative | |
| EV-71 | Negative | |
| EV | Negative | |
| CA16 | Negative | |
Note: H7 ifu: H7 influenza virus; ifu A: influenza A; ifu B: influenza B; N9 ifu: N9 influenza virus; CMV: cytomegalovirus; EV-71: enterovirus-71; EV: enterovirus; CA-16: coxsackievirus-16.
Fig. 3.
The change of Ct value of RT-PCR for COVID-19 in the stool specimen of case 6# with time.
Discussion
COVID-19 has been spreading worldwide and the pandemic is ongoing. According to the experience of China, the country with the earliest outbreak, all people are susceptible to SARS-CoV-2 [5]. By 10 March 2020, a total of ten children were admitted to the Jinan Infectious Disease Hospital Affiliated to Shandong University. Clinical and follow-up data of these patients were retrospectively analyzed. We observed that seven out of these ten patients were positive for SARS-CoV-2 virus RNA in fecal specimens within 2 weeks after discharge but were negative for respiratory tract specimens and urine samples. This suggests that the shedding time of the virus is longer in GI tract than that in respiratory tract of children. Several recent studies reported persistent fecal viral shedding in both adults and children. For example, 39 out of 73 patients with COVID-19 tested positive for SARS-CoV-2 virus RNA in stool samples and 17 patients remained positive for SARS-CoV-2 virus RNA in stool after becoming negative in respiratory samples [6]. Another study with the cohort of COVID-19 patients in Hong Kong reported that the pooled prevalence of positive SARS-CoV-2 virus RNA in stool samples was 48%, 70% of which still maintained positive after loss of virus in respiratory specimens [7]. A clinical study of pediatric patients with COVID-19 reported that eight of ten children persistently tested positive on rectal swabs after respiratory testing was negative [8]. A similar study also detected SARS-CoV-2 virus RNA in fecal specimens from an asymptomatic child when the nasopharyngeal test showed negative result [9], Experimental studies with animals infected with SARS-CoV-2 also detected viral RNA in feces of golden hamsters [10] and ferrets [11], suggesting that fecal viral shedding is a common feature of SARS-CoV-2 virus infection. The prolonged persistence of SARS-CoV-2 virus RNA in fecal specimens suggests a possibility of the existence of infectious virus in GI tract. Indeed, one recent study isolated alive virus, which could induce cytotoxicity effect in Vero E 6 cells, in stool sample of patients with COVID-19. However, it is worth noting that there is still insufficient evidence to support a transmission through the fecal-oral route in patients.
ACE2 was confirmed to be a functional receptor for coronavirus [12], and is distributed in the lungs with age [[13], [14], [15]]. However, the relationship between distribution and aging in the small intestine and colon remains unclear. It is speculated that the number of ACE2 may be specifically distributed in the small intestine and colon of children. In addition, all children belonged to family clusters. Because of multi-member household infections, there is a high risk of viruses in household equipment. A previous study reported that samples from the toilet bowl, sink, and door handle were positive for nucleic acid even after cleaning [16]. Children have a characteristic of hyperactivity and not timely cleaning, and the likelihood of ingesting viral fragments is high. Finally, since children cannot effectively cough out airway secretions, there is also the possibility of a positive result in fecal specimens after swallowing.
During the follow-up, six of the seven patients with a positive fecal test had no clinical symptoms, laboratory abnormalities, or abnormal lung CT finding. One child developed respiratory and GI symptoms and was re-admitted 9 days after discharge. As winter and spring have a high incidence of respiratory diseases, potential infection with virus such as influenza virus, EB virus, cytomegalovirus, enterovirus-71, coxsackievirus and enterovirus, or bacteria such as mycoplasma pneumoniae and chlamydia pneumoniae, were all considered. However, laboratory tests did not support these potential infections, but the Ct value of RT-PCR for COVID-19 in stool specimens of the case remained at a high level. Although positive RT-PCR result in fecal specimens cannot confirm the presence of live viruses, the possibility that the SARS-CoV-2 exists in the gut and induces a delayed inflammatory response cannot be ruled out.
Contributors
J.Y. took responsibility for the integrity of the data and the accuracy of the data analysis. J.Y. and X.L. had full access to all data in the study. H.C. contributed to RT-PCR assay. L.L. contributed to analysis on chest CT scanning. W.D. and Q.L. conceived the study and wrote the manuscript.
Funding
This study supported by Jinan Science and Technology Bureau (No.202001005).
Conflicts of interest
None declared.
Ethical approval
The project was approved by the ethics board of the Jinan Infectious Diseases Hospital (No.20200203).
Acknowledgement
Thank Dr. Edward C. Mignot (Shandong University) for linguistic advice.
References
- 1.World Health Organization. WHO statement regarding cluster of pneumonia cases in Wuhan, China. Available: https://www.who.int/china/news/detail/09-01-2020-who-statement-regarding-cluster-of-pneumonia-cases-in-wuhan-china. [Accessed 11 January 2020].
- 2.National Health Commission of The People’s Republic of China. Interim protocol of diagnosis and treatment of 2019 novel coronavirus-associated pneumonia (the second version). Available: http://www.nhc.gov.cn/jkj/s3577/202001/c67cfe29ecf1470e8c7fc47d3b751e88.shtml [Accessed 18 January 2020].
- 3.WHO. Coronavirus. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. [Accessed 11 February 2020].
- 4.National Health Commission of People’s Republic of China. Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment(7th version) http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.
- 5.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, China CDC The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases in China. Zhong hua Liu Xing Bing Xue Za Zhi. 2020;41(February (2)):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6) doi: 10.1053/j.gastro.2020.02.055. 1831–1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung K.S., Hung I.F., Chan P.P., Lung K.C., Tso E., Liu R. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis [published online ahead of print, 2020 Apr 3] Gastroenterology. 2020;S0016-5085(20) doi: 10.1053/j.gastro.2020.03.065. 30448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang A., Tong Z.D., Wang H.L., Dai Y.X., Li K.F., Liu J.N. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis. 2020;26(6):1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sia S.F., Yan L.M., Chin A.W.H., Fung K., Choy K.T., Wong A. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters [published online ahead of print, 2020 May 14] Nature. 2020 doi: 10.1038/s41586-020-2342-5. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y.I., Kim S.G., Kim S.G.M., Kim E.H., Park S.J., Yu K.M. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27(5) doi: 10.1016/j.chom.2020.03.023. 704–709.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher T.M., Buchmeier M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279(2):371–374. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6(February (24)):11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamming I., Timens W., Bulthuis M.L. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardi S.I.I., Zennaro C., Palmisano S. Characterization and significance of ACE2 and Mas receptor in human colon adenocarcinoma. J Renin Angiotensin Aldosterone Syst. 2012;13(March (1)):202–209. doi: 10.1177/1470320311426023. [DOI] [PubMed] [Google Scholar]
- 16.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610––1612. doi: 10.1001/jama.2020.322. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]