Abstract
Coronavirus disease 19 (COVID-19), an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been associated with acute kidney injury, presumably due to acute tubular injury. However, this does not explain proteinuria, sometimes severe, and hematuria often observed. We present 2 African American patients with glomerulopathy demonstrated by kidney biopsy in the setting of acute kidney injury and COVID-19 infection. Kidney biopsy specimens showed a collapsing variant of focal segmental glomerulosclerosis in addition to acute tubular injury. Both patients were homozygous for apolipoprotein L1 (APOL1). COVID-19 infection likely caused the interferon surge as a second hit causing podocyte injury leading to collapsing focal segmental glomerulosclerosis. APOL1 testing should be strongly considered in African American patients with nephrotic-range proteinuria. More data from future kidney biopsies will further elucidate the pathology of kidney injury and glomerular involvement from COVID-19 infections.
Index Words: COVID, collapsing, glomerulopathy, FSGS, SARS, proteinuria, AKI
Introduction
During the last 5 months, coronavirus disease 19 (COVID-19), an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been spreading across the globe, with the total number of cases exceeding 5 million and the number of deaths at more than 300,000 and counting. The United States is currently the epicenter of COVID-19 infection. The interaction between SARS viruses and angiotensin-converting enzyme 2 (ACE2) has been proposed as the potential factor in their infectivity. Consequently, the lungs are the most common organs involved due to the abundance of ACE2. Involvement of other organs is not uncommon and acute kidney injury (AKI) was initially described in 3% to 9% of patients. Further reports suggested kidney involvement in ∼15% of patients, and proteinuria and hematuria have been observed in 44% and 26% of patients, respectively.1 Acute tubular injury was presumed to be the most common cause of AKI, but it did not explain proteinuria, sometimes severe, and hematuria, making a case for kidney biopsy to understand the underlying cause, when feasible. Studies have also shown that baseline chronic kidney disease or AKI is associated with increased mortality.1
We present 2 patients with kidney biopsy–demonstrated glomerulopathy as a cause of AKI during COVID-19 infection.
Case Reports
Case 1
A 28-year-old African American woman with a history of asthma presented to the emergency department reporting 5 days of fever, fatigue, cough, and shortness of breath. Physical examination was remarkable for temperature of 103.9°F, heart rate of 99 beats/min, respiratory rate of 22 breaths/min, lung auscultation with generalized wheezing, and right infra-axillary and basal crepitations.
Laboratory results are detailed in Tables 1 and 2.
Table 1.
Case 1 Blood Work on Admission and Day 4
| Laboratory Test | Reference | Day 0 | Day 4 |
|---|---|---|---|
| Hemoglobin, g/dL | 12.0-16.0 | 15.3 | 13.1 |
| WBC count, K/μL | 4.6-13.2 | 3,100 | 7.4 |
| Platelet count, K/μL | 135-420 | 143 | 147 |
| Sodium, mmol/L | 136-145 | 136 | 129 |
| Potassium, mmol/L | 3.5-5.5 | 3.6 | 4.1 |
| Carbon dioxide, mmol/L | 21-32 | 25 | 15 |
| SUN, mg/dL | 7.0-18 | 11 | 58 |
| Creatinine, mg/dL | 0.6-1.3 | 0.99 | 6.5 |
| eGFR, mL/min | >60 | >60 | 9 |
| Albumin, g/dL | 3.4-5.0 | 3.2 | 1.6 |
Note: Conversion factors for units: creatinine in mg/dL to μmol/L, ×88.4.
Abbreviations: eGFR, estimated glomerular filtration rate; SUN, serum urea nitrogen; WBC, white blood cell.
Table 2.
Case 1 Urinalysis on Admission and Past Results From 2017
| Reference | Admission | 2017 | |
|---|---|---|---|
| Proteins | Negative | 300 | Negative |
| Blood | Negative | Small | Negative |
| RBCs, /HPF | 0-5 | 0-2 | None |
| WBCs, /HPF | 0-5 | 0-2 | 9 |
Abbreviations: HPF, high-power field; RBCs, red blood cells; WBCs, white blood cells.
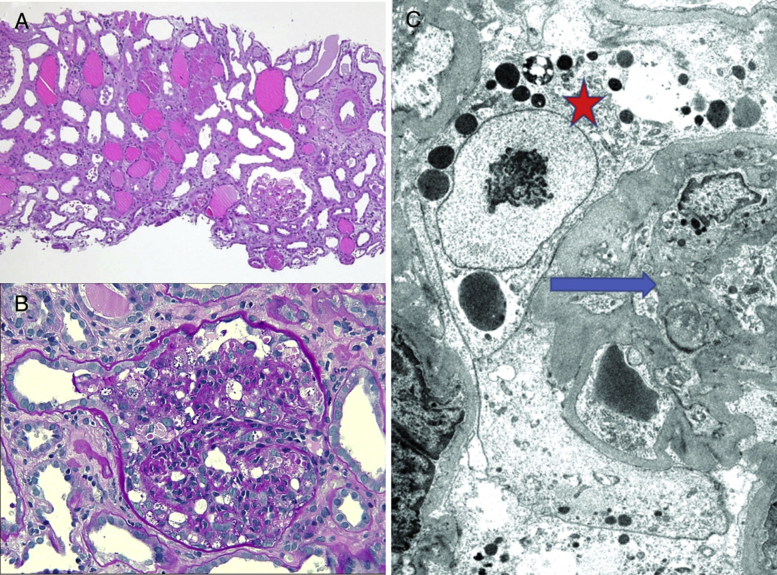
Figure 1.
(A). Low-power view demonstrates diffuse acute tubular injury, interstitial edema, and microcystic dilatation of proximal tubules. All 3 glomeruli were involved by a collapsing variant of focal segmental glomerulosclerosis (FSGS). (B). Higher-power image of a glomerulus shows characteristic features of collapsing FSGS. There is collapse of the glomerular tuft with marked activation/hypertrophy of the overlying podocytes, many of which contain prominent protein resorption droplets in their cytoplasm (periodic acid–Schiff stain; original magnification, ×200). (C) Electron photomicrograph shows collapsed glomerular basement membranes (blue arrow) with activated podocytes containing prominent protein resorption droplets in their cytoplasm (red star). Foot processes are globally effaced (uranyl acetate stain; original magnification, ×4,000).
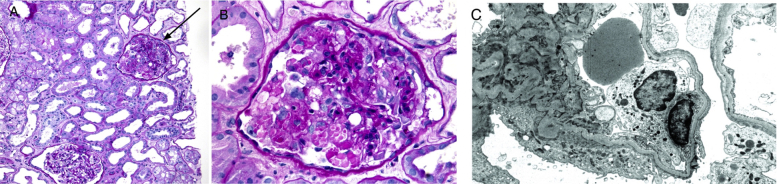
Figure 2.
(A) Low-power view demonstrates diffuse acute tubular injury and interstitial edema. One glomerulus (arrow) demonstrates a collapsing variant of focal segmental glomerulosclerosis (FSGS) (periodic acid–Schiff stain; original magnification, ×100). (B). Higher-power image of a glomerulus shows characteristic features of collapsing FSGS. There is collapse of the glomerular tuft with marked activation/hypertrophy of the overlying podocytes, many of which contain prominent protein resorption droplets in their cytoplasm (periodic acid–Schiff stain; original magnification, ×200). (C). Electron photomicrograph shows global podocyte foot-process effacement (uranyl acetate stain; original magnification, ×8,000),
The patient had a normal creatinine level (0.9 mg/dL) on admission and urinalysis showed significant proteinuria, which was new compared with the last available urinalysis from 2017. Spot urine protein-creatinine ratio was 2 g/g. Creatinine level increased to 6.5 mg/dL over 4 days. An x-ray of the chest showed mild linear densities at the right middle and lower lung zones. Kidney ultrasound showed enlarged kidneys with maintained corticomedullary differentiation. Serologic workup results, including antinuclear antibody and antineutrophil cytoplasmic antibody, were normal. Human immunodeficiency virus (HIV) and hepatitis B and C virus serologic test results were also negative. Complement levels were normal. She also had worsening hypoxia but did not require ventilation and was managed supportively. The COVID-19 reverse-transcriptase polymerase chain reaction assay later came back positive. Due to progressive worsening of kidney function, uremia, and metabolic acidosis, she was started on hemodialysis on day 7 of hospitalization.
Kidney biopsy was performed to determine the cause of AKI with proteinuria. Kidney biopsy results (Fig 1) revealed light microscopy with 31 glomeruli with 2 globally sclerosed and ∼50% with focal segmental glomerulosclerosis (FSGS), with collapsing features demonstrating variable collapse of the glomerular tuft with overlying podocyte activation/hypertrophy. Prominent protein resorption droplets were noted within the cytoplasm of activated podocytes. No endocapillary hypercellularity, tuft fibrinoid necrosis, or crescent formation were seen. There was mild interstitial fibrosis and tubular atrophy with superimposed interstitial edema and diffuse moderate to severe acute tubular injury. Patchy mononuclear inflammation was seen in the interstitium. Arterioles were without diagnostic abnormalities. Arteries revealed mild intimal fibroelastosis. Immunofixation was negative for diagnostic glomerular or extraglomerular deposits.
Electron microscopy demonstrated 2 glomeruli with FSGS with collapsing features similar to those seen by light microscopy. Marked interstitial edema and acute tubular injury were noted. Ultrastructural examination revealed glomerular basement membranes with extensive wrinkling and areas of collapse with global effacement of the overlying foot processes and marked activation of the overlying podocytes. No discrete immune complex–type electron-dense deposits were identified. No characteristic and diagnostic viral inclusions were identified within the glomerular podocytes or proximal tubular epithelial cells.
Dialysis was required for 34 days until kidney function improved. As of the publication of this report, serum creatinine level had not returned to baseline. apolipoprotein L1 (APOL1) genotyping was homozygous for G1 risk allele. Other COVID-19 symptoms have resolved.
Case 2
A 56-year-old African American man with a history of long-standing uncontrolled hypertension, chronic kidney disease stage 3 with baseline creatinine level of 2 mg/dL, and recent travel to New York 2 weeks previously presented to the emergency department with fever, chills, and dry cough for 7 days, along with nausea, vomiting, and diarrhea for 2 days. The patient was tachycardic to a heart rate of 114 beats/min, tachypneic with a respiratory rate of 24 breaths/min, and febrile with a temperature of 100.3°F. Physical examination was remarkable for left more than right bibasal crepitations on chest auscultation.
Laboratory results including urinalysis are outlined in Tables 3 and 4.
Table 3.
Case 2 Blood Work on Admission and Day 5
| Laboratory Test | Reference | Day 0 | Day 5 |
|---|---|---|---|
| Hemoglobin, g/dL | 12.0-16.0 | 14.9 | 12.9 |
| WBC count, K/μL | 4.6 – 13.2 | 7.6 | 8.8 |
| Platelet count, K/μL | 135-420 | 178 | 341 |
| Sodium, mmol/L | 136-145 | 137 | 135 |
| Potassium, mmol/L | 3.5-5.5 | 4.2 | 4.2 |
| Carbon dioxide, mmol/L | 21-32 | 21 | 18 |
| SUN, mg/dL | 7.0-18 | 33 | 58 |
| Creatinine, mg/dL | 0.6-1.3 | 3.17 | 7.72 |
| eGFR, mL/min | >60 | 25 | 9 |
| Albumin, g/dL | 3.4-5.0 | 2.6 | 0.8 |
Note: Conversion factors for units: creatinine in mg/dL to μmol/L, ×88.4.
Abbreviations: eGFR, estimated glomerular filtration rate; SUN, serum urea nitrogen; WBC, white blood cell.
Table 4.
Case 2 Urinalysis on Admission and Past Results From 2019
| Reference | On Admission | June 2019 | |
|---|---|---|---|
| Proteins | Negative | >1,000 | 100 |
| Blood | Negative | Moderate | Negative |
| RBCs, /HPF | 0-5 | 1-3 | None |
| WBCs, /HPF | 0-5 | 1-3 | 0-1 |
Abbreviations: HPF, high-power field; RBCs, red blood cells; WBCs, white blood cells.
Spot urinary protein-creatinine ratio was >21 g/g (urinary protein excretion > 2,500 mg/dL and urinary creatinine excretion of 118 mg/dL). An x-ray of the chest on admission showed hazy patchy opacity at the peripheral left middle to lower lung field. Kidney ultrasound revealed normal-size kidneys with some loss of corticomedullary differentiation. The patient developed hypoxia with worsening lung infiltrates bilaterally on chest x-ray. COVID-19 reverse-transcriptase polymerase chain reaction assay was positive. Creatinine levels progressively increased (3.17 to 7.72 mg/dL) ,and on day 5, hemodialysis was initiated for oliguric AKI and uremia. Antinuclear antibody, antineutrophil cytoplasmic antibody, complement levels, HIV, and hepatitis B and C virus test results were negative, as was workup for monoclonal gammopathy/myeloma.
Kidney biopsy was performed for severe AKI with nephrotic-range proteinuria. The patient was treated empirically with antibiotics, and oxygen requirement improved during the hospitalization. Kidney biopsy (Fig 2) showed 36 glomeruli on light microscopy with 11 globally sclerosed, and 5 others with FSGS with focal collapsing features demonstrating variable collapse of the glomerular tuft with overlying podocyte activation/hypertrophy. Prominent protein resorption droplets were noted within the cytoplasm of activated podocytes. Intracapillary foam cells were seen. No endocapillary hypercellularity, tuft fibrinoid necrosis, or crescent formation was seen. There was diffuse moderate to marked acute tubular injury with background mild interstitial fibrosis and tubular atrophy. Mild mononuclear inflammation was seen in the scarred portions of the interstitium. Arterioles demonstrated severe sclerosis and arteries revealed severe focally stenosing intimal fibroelastosis. Immunofixation results were unremarkable. Electron microscopy demonstrated 5 glomeruli, of which 1 revealed segmental tuft sclerosis with focal intracapillary foam cells. Acute tubular injury was noted. Ultrastructural examination demonstrated glomerular basement membranes of predominant normal thickness and architecture, with global effacement of the overlying foot processes and associated microvillus transformation. No discrete immune complex–type electron-dense deposits were identified. No characteristic and diagnostic viral inclusions were seen within podocyte and proximal tubular epithelial cell cytoplasm.
Dialysis was required for 23 days until kidney function improved. As of the publication of this report, serum creatinine level has not returned to baseline. APOL1 genotyping on the plasma was heterozygous for G1 and G2 alleles. Other COVID-19 symptoms have resolved.
Discussion
The exact mechanism of kidney involvement by COVID-19 has not been fully elucidated yet, but more data have been emerging. Possible causes considered are tubular injury due to cytokine storm, a direct cytopathic effect, and immune-mediated glomerulonephritis. Kidney histopathologic findings in 26 patients on postmortem analysis2 showed light microscopy findings of diffuse proximal tubule injury and erythrocyte aggregates obstructing capillary lumens. Immunofixation staining was nonspecific and minimal, which did not favor immune-mediated injury. No vasculitis was observed. Electron microscopy demonstrated viral particles in the cytoplasm, mostly in the proximal tubular epithelium and less in distal tubules. Viral particles were also demonstrated in podocytes. Focal segmental effacement has been seen in only 2 cases and both had a history of diabetes and hypertension. It is presumed that SARS Cov-2 enters the kidney through its interaction with the ACE2-dependent pathway, and RNA sequencing data for humans have shown significant expression of ACE2 in the kidneys. Larsen et al3 and Peleg et al4 each reported a case of collapsing glomerulopathy recently with COVID-19 infection.
Both patients had collapsing FSGS in addition to tubular injury, suggesting injury to the podocytes. Both our patients were African American. Viral particles were not seen in the biopsy specimens of either patient, and hence a direct cytopathic effect was not considered to be the mechanism of kidney injury, although viral levels below the detection threshold cannot be excluded. In situ hybridization was not done. It is also possible that collapsing glomerulopathy is a bystander effect of a virus-driven inflammatory response or due to circulating viral gene products.
Collapsing FSGS has been seen with other viral infections, including parvovirus, cytomegalovirus, and HIV infection. Variants of the APOL1 gene in African Americans have been shown to be associated with FSGS.5 These 2 patients had genetic susceptibility due to APOL1, and COVID-19 infection was a second hit. This could explain the collapsing FSGS in our patients, which has not been reported in any patient from China.
Current treatment of collapsing FSGS secondary to (other) infections includes treatment of the underlying infection and nonimmunosuppressive therapy with renin-angiotensin-aldosterone system blockers and statins. The role of steroids has been controversial. Regarding our cases, it is difficult to make specific treatment recommendations because currently there is no specific proven treatment for COVID-19 infection and use of renin-angiotensin-aldosterone system blockers during COVID-29 infection is controversial.6 Kidney biopsy was important for both prognostic and diagnostic reasons. On the 50-day follow-up, both our patients showed reasonable recovery of kidney function following recovery from COVID-19 infection.
It is reasonable to proceed with kidney biopsy in COVID-19–positive patients who have AKI and have additional clinical features that suggest glomerular injury. APOL1 testing should be strongly considered in African American patients with nephrotic-range proteinuria. More data from future kidney biopsies will further elucidate the pathology of kidney injury and glomerular involvement from COVID-19 infections. As we make our way through this pandemic and hopefully out of it, the data from such kidney biopsies may help us understand the disease process better, identify potential risk factors, and intervene early to improve outcomes.
Article Information
Authors’ Full Names and Academic Degrees
Sandeep Magoon, MD, Prasad Bichu, MD, Varun Malhotra, MD, Fatema Alhashimi, MD, Yanglin Hu, MD, Siddharth Khanna, MD, and Kabaye Berhanu, MD.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Patient Consent
The authors declare that they have obtained consent from the patients discussed in the report.
Peer Review
Received April 29, 2020. Evaluated by 1 external peer reviewer, with direct editorial input from the Editor-in-Chief. Accepted in revised form May 29, 2020.
Footnotes
Complete author and article information provided before references.
References
- 1.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen C.P., Bourne T.D., Wilson J.D., Saqqa O., Sharshir M.A. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19) Kidney Int Rep. 2020;5(6):935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peleg Y., Kudose S., D’Agati V. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;5(6):940–945. doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopp J.B., Winkler C.A., Zhao X. Clinical features and histology of apolipoprotein L1-associated nephropathy in the FSGS clinical trial. J Am Soc Nephrol. 2015;26(6):1443–1448. doi: 10.1681/ASN.2013111242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with COVID-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]