Abstract
Background
Minimally invasive surgery (MIS) is a common treatment option for paravertebral or psoas abscesses (PAs) in patients with spinal tuberculosis (ST). However, its efficacy remains controversial. The aim of the study was to evaluate the efficacy of MIS for PA with ST combined with anti-tuberculous chemotherapy.
Methods
A total of 106 consecutive patients who underwent MIS for ST with PA from January 2002 to Oct 2012 were reviewed. The MIS involved computed tomography (CT)-guided percutaneous catheter drainage and percutaneous catheter infusion chemotherapy. Clinical outcomes were evaluated based on the changes observed on preoperative and postoperative physical examination, inflammatory marker testing, and magnetic resonance imaging (MRI).
Results
The mean follow-up period was 7.21 ± 3.15 years. All surgeries were successfully completed under CT-guidance without intraoperative complications and all patients experienced immediate relief of their symptoms, which included fever and back pain. The preoperatively elevated erythrocyte sedimentation rate and C-reactive protein values returned to normal at a mean period of 3 months postoperatively. Solid bony union was observed in 106 patients and no abscesses were found on MRI examination.
Conclusion
MIS carries advantages in terms of less invasiveness, precise drainage, and enhanced local drug concentration. While the technique has not been fully characterized and clinically prove, its use in addition to conservative chemotherapy and open debridement and instrumental fixation may be recommended for patients with ST and PA.
Keywords: Minimally invasive surgery, Paravertebral abscess, Psoas abscess, Spinal tuberculosis
Background
According to the World Health Organization’s (WHO) 2015 Global Tuberculosis (TB) Report, newly emerging multidrug-resistant TB and TB alongside HIV have become a leading cause of death worldwide [1]. It was reported that China possesses the third highest prevalence of TB worldwide. Tb, which is mentioned in the historical literature, is caused by Mycobacterium tuberculosis, remains an immense public health concern both in China and globally [2].
Spinal tuberculosis (ST), which was first described in the European population by Percival Pott in 1779 [3], is found in half of all cases of extrapulmonary TB, with a predilection for the thoracic and lumbar regions [4].
Psoas or paravertebral abscesses (PAs), which were first reported by Mynter in 1881 [5], is a common complication associated with ST [6]. Tuberculous spondylitis-induced PA is not a rare entity in China, especially in developing areas [7]. The objective of treatment for PA with thoracolumbar or lumbar TB is the complete drainage of abscess with regular anti-TB chemotherapy. We have employed a minimally invasive approach involving PA elimination by percutaneous catheter drainage (PCD) and intervertebral percutaneous catheter infusion (PCI) chemotherapy for treating the TB lesions. This study aimed to review the outcomes achieved by our minimally invasive approach to PA with TS.
Methods
The study was approved by the ethics committee of the PLA General Hospital (Beijing, China) and carried out in accordance with the principles stated in the Declaration of Helsinki. In addition, written informed consent was obtained from all patients and their legal guardians to use and publish their medical images. No identifying information is shown in the images.
ST patients with PA treated at our center using our minimally invasive surgery (MIS) technique from January 2002 to October 2012 were retrospectively reviewed. The general inclusion criteria were significant cavity formation of paravertebral abscess or PA secondary to spinal TB involving the T8-L5 vertebrae without active pulmonary TB. Patients with any of the following exclusion criteria did not undergo MIS: (1) severe coagulation disorders; (2) concomitant obvious nerve or spinal cord compression symptoms with a Frankel classification level above grade C (not including grade C); (3) severe destruction of the vertebral body and obvious kyphosis or spinal instability requiring correction observed on the radiological examinations; (4) and co-infection with HIV/AIDS or other immune-compromising. Accordingly, 671 consecutive patients with ST were identified and 106 were included in this study (Fig. 1). The clinical data of the patients are shown in Table 1.
Fig. 1.
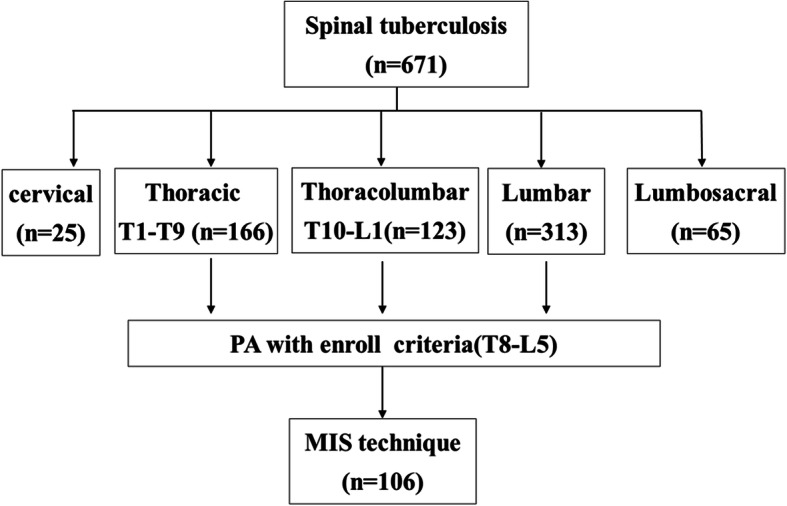
From January 2002 to October 2012, 671 consecutive spinal tuberculosis cases were retrospectively analyzed. 106 cases with PA treated by MIS technique were enrolled in the study
Table 1.
Patient characteristics
| Characteristic | No. (%) |
|---|---|
| Involved vertebra | |
| Thoracic vertebrae (T8–T10) | 22 (15.4) |
| Thoracolumbar vertebrae (T11–L2) | 63 (51.3) |
| Lumbar vertebrae (L3-L5) | 21 (33.3) |
| Abscess location | |
| Paravertebral abscess | 90 (51.3) |
| Psoas abscess | 47 (41.0) |
| Epidural abscess | 47 (41.0) |
| Complications | |
| Kyphosis | 7 (35.9) |
| Recurrent abscess | 4 (64.1) |
Diagnosis
The diagnosis of PA associated with tuberculous thoracolumbar or lumbar spondylitis was preoperatively based on the clinical symptoms, such as mild fever, night-sweats, back pain, and fatigue [8]; laboratory examinations such C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), purified protein derivative, and the interferon gamma release assay [9];, and imaging examinations such as X-ray, computed tomography (CT), and magnetic resonance imaging (MRI) [6]. All patients were administered experimental anti-TB therapy for 2–4 weeks preoperatively [10]. The diagnoses of tuberculosis-induced PA was confirmed by pathological examination, acid-fast staining and/or culture of mycobacterium TB [2].
MIS technique
All operations were performed under local anesthesia. PCI chemotherapy infusion via an epidural tube was directed towards the intervertebral TB lesions and PCD via a double-lumen was performed for drainage of the abscess cavity based on preoperative CT and MRI findings and intraoperative CT guidance. Disposable AS-E/S II anesthesia puncture kits were provided by Shandong Weigao Group Medical Polymer Co., Ltd. (Weihai, P. R. China).
For thoracic vertebral lesions, the epidural tubes were placed above the transverse process and into the intervertebral space, whereas for lumbar lesions they were placed through the Kambin triangle into the intervertebral space. Considering the need for an adequate volume to cover vertebral infectious lesions, double PCI chemotherapy was administered from both sides via 2 epidural tubes to increase drug concentration. For psoas abscesses or PAs, placement of the double-lumen was performed vertically from the dorsal side into abscess cavity (Fig. 2).
Fig. 2.
For thoracic vertebrae lesions, placement of epidural tubes were performed above the transverse process. For lumbar vertebrae, placement of abscess drainage tubes were performed from the Kambin triangle into the intervertebral space
The details of the surgical procedures are as follows. For PCI chemotherapy, once CT scanning confirmed placement of the epidural needle in the lesion’s center, the inner core was removed and the epidural tube placed. When the final position of the epidural tube was reconfirmed, the needle was removed and the tube was attached tightly to the skin with medical paste. When needed, the same procedure would be performed on the other side.
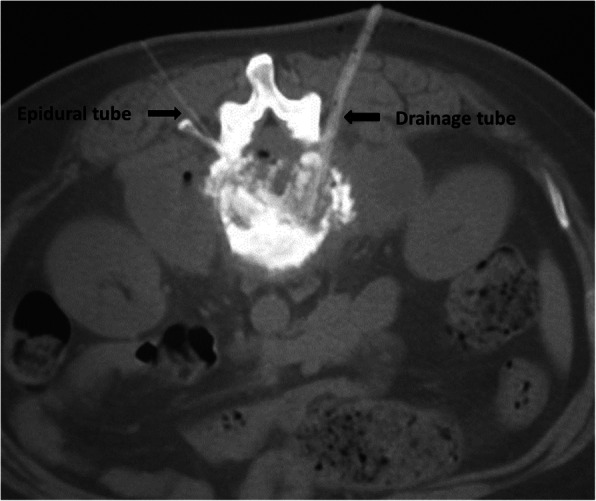
For PCD via a double-lumen tube, once puncture had been successfully accomplished, the inner core was replaced by a guidewire. Using the guidewire, a 5-mm OD expansion pipe with a tapered front was placed inside. A CT scan was performed to confirm proper placement of the 5-mm OD working tube. The double-lumen tube was them placed through the working tube in the PA cavity and CT confirmation was repeated, followed by careful removal of the working tube (Fig. 3).
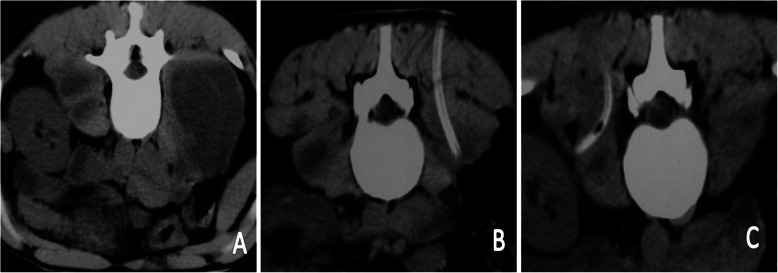
Fig. 3.
(a) The bilateral psoas abscess cavities were observed in the process of percutaneous puncture. (b) and (c) The abscess drainage tubes were placed in the middle of the abscess cavities under CT-guidance
Postoperative continuous drainage and local infusion chemotherapy
After the catheterization process was completed, the infusion tube was sealed with heparin saline and the drainage tube was connected to a negative-pressure sterile drainage bag. The abscess material obtained from the pretreatment puncture was subjected to TB and standard bacterial culture along with drug susceptibility testing [11, 12].
During local infusion of chemotherapy and abscess drainage, 24-h continuous lavage and drainage was performed using 500 ml of saline + 1 g isoniazid via the PCD tube over a period of 3–6 weeks. Chemotherapy (0.2 g isoniazid) was infused via the PCI tube over a period of 1 month [13]. If necessary, local chemotherapy infusion was extended to 3 months [14]. Extubation was not performed until the fluid drained from the abscess cavity was clear and without obvious necrotic tissue [15, 16]. After removal of the infusion and drainage tubes and patient discharge, oral anti-TB drugs were continued for 1 year, while functional rehabilitation and lumbar back muscle exercises were simultaneously performed under the protection of a thoracolumbar brace [17].
Efficacy evaluation
ESR and CRP levels were dynamically monitored preoperatively and post-operatively at regular intervals to evaluate the infection status. The visual analog scale (VAS) was used to evaluate symptom relief and the Oswestry disability index (ODI) was used to assess rehabilitation condition and patient activity. Follow-up MRI was performed to assess the PA status. Treatment success was defined as short- and long-term absence of symptoms and improvement in laboratory and findings (Fig. 4 and Fig. 5). Total recovery was defined as absence of recurrent infection or abscess, bony fusion, and relief from back pain.
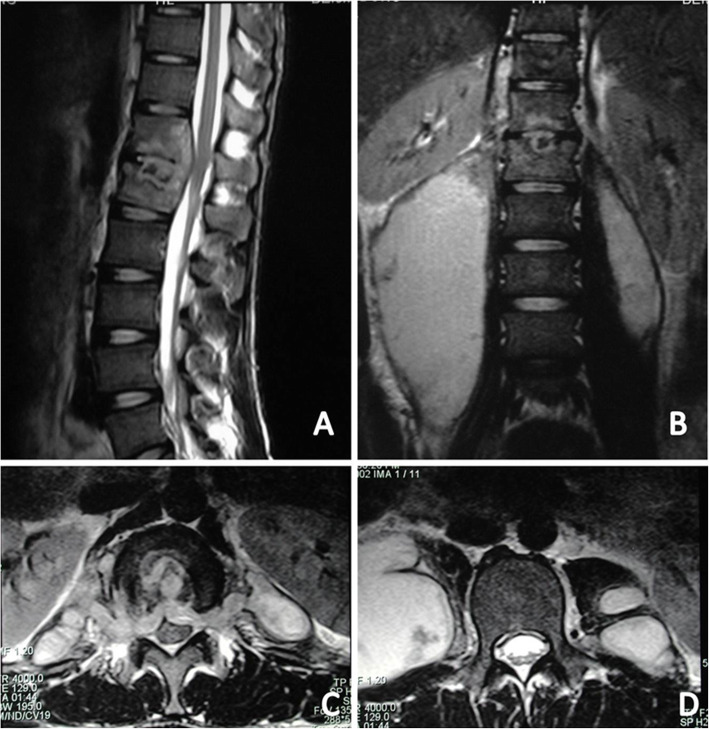
Fig. 4.
MRI showed that both sides of psoas abscess existed in a 22-year-old male patient with tuberculous spondylitis of T12-L1. a Sagittal T2-weighted MRI demonstrated a T12-L1 infection source and associated bilateral psoas muscle abscess. b Coronal T2-weighted magnetic resonance imaging (MRI) demonstrated that the right kidney was not in the right place because of the right huge psoas abscess. c and d showed the para-vertebral abscess and psoas abscess existed in the relative section of the axial section
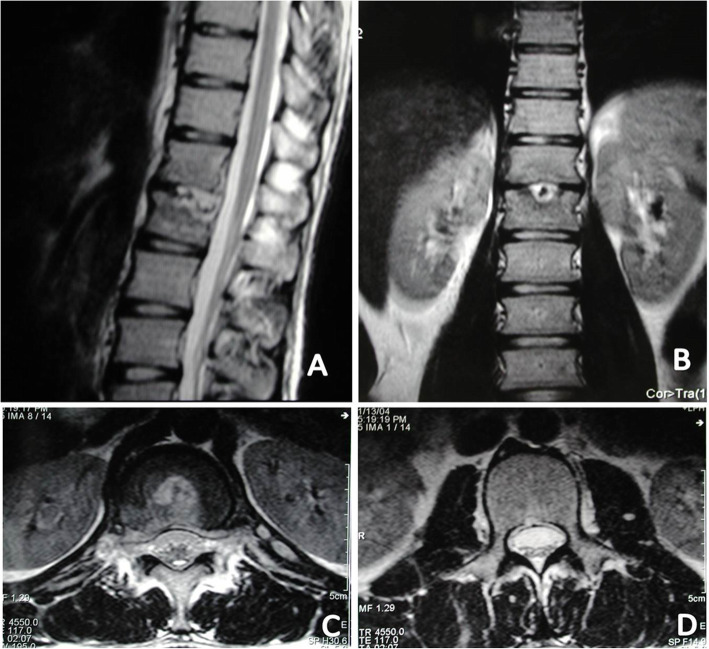
Fig. 5.
24 months after PCD and PCI chemotherapy, The MRI showed that intervertebral lesion were totally under control (a) with the right kidney was back to its right place and no abscess were observed in the bilateral psoas muscles in the coronal MRI (b) and in the axial MRI (d), and that the para-vertebral abscess also disappeared and the spinal cord were decompressed
Statistical analysis
SPSS 24.0 software (SPSS Inc., Chicago, IL) was used for the statistical analysis. The data were expressed as means ± standard deviations, and differences with a P-value < 0.05 were considered significant. The sample size was calculated using PASS 19.0.3 statistical software (NCSS Inc., USA). Sample size of cross-sectional study was calculated as below. Zα/2 is the parameter of significance testing(α = 0.05, Z0.05/2 = 1.96). P is the expected effective rate. According to the literatures reviewed [1, 3, 18], the effective rate is about 50% on the basis of preliminary estimation. Permissible error(δ) is in the range of 0–10%. To keep the accuracy of study, the Permissible error will be set as 10%. The design effect (deff) will be 1.5. According to the result of the calculation, the sample size of adequacy is 74. So the study is powered, since the sample size of the study is 106.
Results
The most common symptom of ST was back pain, which was observed in all 106 patients preoperatively. The preoperative and postoperative ODI and VAS was recorded and analyzed (Table 2).
Table 2.
VAS and ODI findings preoperatively and at 6 months
| Preoperative | Postoperative | P-value | |
|---|---|---|---|
| Back pain VAS | 6.5 ± 3.0 | 2.4 ± 2.3 | 0.006 |
| ODI | 33.6 ± 12.3 | 8.1 ± 4.6 | 0.011 |
VAS visual analog scale; ODI Oswestry disability index
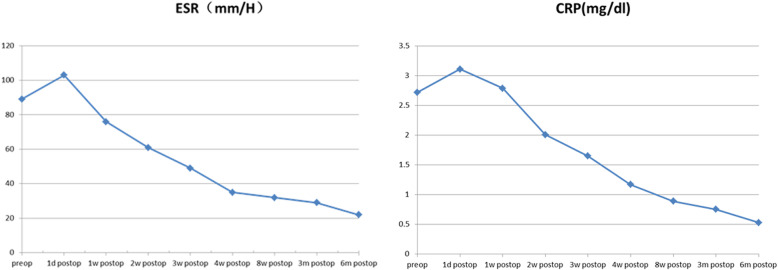
The elevated ESR and CRP levels returned to normal at a mean period of 3 months postoperatively. Statistical analysis demonstrated a significant difference in ESR and CRP levels preoperatively and at 8 weeks postoperatively (P < 0.05) (Fig. 6).
Fig. 6.
Statistical analysis demonstrated that there was significant difference between preoperative and eight weeks postoperative ESR and CRP (P < 0.05)
Four patients developed recurrent abscess during the follow-up period at 5, 9, 17, and 22 months, respectively. Among these 4 patients, 3 underwent repeated minimally invasive PCD and PCI and were subsequently cured. The remaining patient developed an iliac fossa abscess and underwent complete retroperitoneoscopic debridement [19].
During PCI chemotherapy, the infusion tubes of 14 patients were accidentally pulled out. The patients subsequently underwent re-placement and resumed chemotherapy.
The mean follow-up period was 7.21 ± 3.15 years. One patient died of pneumonia at 8 years postoperatively and another died of lung cancer at 11 years, with no recurrent infections or abscesses at the last follow-up examination in each. Seven patients underwent two-stage percutaneous pedicle screw fixation surgery to prevent severe kyphotic deformity (> 45°) after the MIS procedure [13]. At final follow-up, 97 patients were free from back pain and 7 had occasional back pain. Solid bony union was observed in 106 patients and no abscesses were found on MRI examinations. No other obvious complications were observed.
Discussion
The WHO estimates that there are about 10.4 million new cases and 1.8 million deaths from TB each year. One-third of these new cases (approximately 3 million) remain unknown to healthcare systems, and many patients are not receiving proper treatment [20]. TB is an infectious bacterial disease caused by Mycobacterium tuberculosis, which is transmitted between humans through the respiratory route. While it most commonly affects the lungs, it can damage all tissues [18]. About 10% of all TB cases present with musculoskeletal involvement, of which 50% involves the spine. The thoracic and lumbar spine are the most commonly involved sites (90% of cases) [3].
The treatment regimens for thoracic and lumbar TB reported in the English literature include anti-TB chemotherapy (usually combined treatment with isoniazid, rifampicin, pyrazinamide, and ethambutol) [2], traditional surgical treatment (anterior radical debridement with graft fusion or posterior debridement with fusion and fixation) [21–25], and minimally invasive approaches [19, 26–32].
The literature supports anti-TB chemotherapy as the gold standard treatment for ST [2, 18, 33]. Additional surgical intervention is performed if there is evident vertebral instability, failure of chemotherapy, progressive deformity, serious neurological impairment, or presence of extensive PA with or without epidural involvement [22, 24, 25, 29–32].
As to vertebral instability and progressive deformity, percutaneous pedicle screw fixation should be performed to prevent vertebral collapse and kyphotic deformity. In the presence of extensive PA, PCD [34–41] or retroperitoneoscopic drainage [19, 27, 28] are the recommended treatments.
Open surgery is required to confirm debridement of the lesion or abscess and decompression of the spinal cord or nerve roots if patients exhibit serious neurological impairment [42]. According to Hodgson et al. [43], paraplegia associated with ST can be classified into two groups: neurological impairment for less than 2 years or for more than 2 years. Impairment of shorter duration is associated with active disease and compression caused by the abscess and inflamed tissue. Longer duration of impairment is related to vertebral collapse, spinal deformity, and secondary compression [3].
Numerous studies on new and individualized treatment regimens for ST have been conducted [44–48]. For patients with thoracic or lumbar TB and PA, but no serious neurological impairment or angular kyphotic deformity, the management strategy should be focused on minimally invasive debridement and drainage of the abscess in addition to chemotherapy [19, 28]. PCD for ST with PA has been shown to be safe and effective [34, 36, 38, 40, 45, 48, 49]. In our study, we focused on the outcomes of MIS involving percutaneous catheter drainage PCD and PCI chemotherapy. Local chemotherapy is critical to ensuring an adequate drug concentration reaches the tubercular lesion [18, 33, 50].
The similarities and differences
The treatment regimens for thoracic and lumbar TB remained controversial. Individualized medical regimen need to be administrated according to the different situations of spinal tuberculosis. The suitable intervention method should be found out for the corresponding indications.
So the intervention regimens for thoracic and lumbar TB reported in the English literature include traditional open surgical treatment [25, 51] and minimally invasive approaches (percutaneous catheter drainage) [46, 48] on the basis of systematic anti-TB chemotherapy [21].
The similarities include alll treatment is administered on the basis of the systematic anti-TB chemotherapy [45].
The differences between our MIS method and traditional open surgical treatment were including indication and intervention method [24] as follow:
The indication for the MIS method including: (1) paravertebral abscess or posas abscess secondary to spinal tuberculosis involving the T8-L5 vertebrae, (2) without severe destruction of the vertebral body and obvious kyphosis or spinal instability, (3) without serious nerve or spinal cord compression symptoms. When the situations such as (2) or (3) occurs, open surgical operation is recommended instead of the MIS method [27, 28, 44].
The intervention method of the MIS method is percutaneous while the open surgery including anterior radical debridement with graft fusion or posterior debridement with fusion and fixation with huge invasiveness and more blood loss [22, 52].
The differences between our MIS method and the minimally invasive approach (percutaneous catheter drainage) were clarified below. Our MIS method involved PA elimination by percutaneous catheter drainage (PCD) and intervertebral percutaneous catheter infusion (PCI) chemotherapy for treating the TB lesions while percutaneous catheter drainage only focused on the drainage of abscess. Percutaneous catheter infusion (PCI) and irrigation with high concentrations of chemotherapy can eliminate remnant tubercle bacilli in the abscess cavity and reduce the rate of abscess recurrence [38, 41].
The principals of MIS
Considering the enrollment criteria, this study focused on MIS for treatment of the abscess and tuberculous vertebrae [28]. While CT-guided percutaneous drainage for ST had already been reported [15, 24, 34, 53–55], its outcomes have not been clearly and definitively elucidated. In addition, no large-scale, long-term studies have been conducted to investigate the efficacy of a minimally invasive approach involving PCD and PCI chemotherapy. The diagnostic and therapeutic value of PCD with PCI chemotherapy, owing to complete abscess drainage and adequate drug delivery to the infected vertebrae, has been previously reported [56–58]. Since infection of the T8–L5 vertebrae is common, PA and psoas abscess are not rare entities [15, 34, 55]. In fact, PAs, which include subdural and perivertebral abscesses, are one of the most common complications observed in the T8–L5 region [6]. In contrast, the anatomical location of the psoas abscess muscle means that psoas abscesses usually occur in the context of T12–L5 infection [4].
The advantages of PCD and PCI chemotherapy
The advantage of PCD is the continuous and complete drainage with postoperative negative-pressure Hemovac suction [57]. Furthermore, sticky abscesses can be washed out by pressurized irrigation with saline and isoniazid. Irrigation with high concentrations of isoniazid can eliminate remnant tubercle bacilli in the abscess cavity and reduce the rate of abscess recurrence [59].
The PCI approach enables precise and daily delivery of isoniazid to the tuberculous foci [60]. A high local drug concentration has been confirmed as the key factor for treatment of infection foci and avoiding recurrent infection [59, 61, 62]. Furthermore, debridement of the infected disc and adjacent vertebral endplates can be completed through the working tube during catheterization [24]. This minimally invasive technique leads to less morbidity than major open surgery, and provides effective relief of the patients’ back pain by reducing the intradiscal pressure and preserving spinal stability [13, 14, 28]. Therefore, our patients were able to start ambulating with brace protection as early as possible after PCD and PCI chemotherapy.
Limitations
This study has several limitations. First, the retrospective nature of this study lacks random assignment of patients and does not allow for comparison of the outcomes of different treatment methods. Second, due to the absence of a control group, it is difficult to definitively state that the MIS technique is superior to open surgery. Third, the feasibility and benefits of PCD and PCI chemotherapy in patients with extended indications, such as postoperative recurrent infection or multilevel involvement, need to be rigorously evaluated in controlled, prospective studies with large patient populations.
Conclusion
Based on the findings observed in this study, we propose that a minimally invasive approach involving PCD and PCI chemotherapy is an effective option for the treatment of thoracic and lumbar TB with PA and/or psoas abscess. Major open surgery is not always necessary in these cases.
Acknowledgements
Not Applicable.
Abbreviations
- MIS
Minimally invasive surgery
- PA
Psoas abscess
- ST
Spinal tuberculosis
- TB
Tuberculosis
- PCD
Percutaneous catheter drainage
- PCI
Percutaneous catheter infusion
- CRP
C-reactive protein
- ESR
Erythrocyte sedimentation rate
- PPD
Purified protein derivative
- IGRA
Interferon gamma release assay
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- VAS
Visual analog scale
- ODI
Oswestry disability index
Authors’ contributions
ZZ1, XZ2 and XZ3 conceptualized, collected and interpreted the clinical data, and wrote the manuscript. XZ1, YH, XW, CW, ZZ2 interpreted the clinical date and revised the manuscript critically for important content. All authors read and approved the manuscript.
Funding
No.
Availability of data and materials
Date that support the findings of this study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
We have obtained written consent from all patients and their legal guardians and the study was agreed by the ethics committee of the PLA General Hospital (Beijing, China).
Consent for publication
The written informed consents were obtained from all patients to use their data and accompanying images for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhifa Zhang, Yongyu Hao and Xiangyu Wang contributed equally to this work.
Contributor Information
Xifeng Zhang, Email: zhangxifeng301@sina.com.
Xuesong Zhang, Email: zhangxuesong301@outlook.com.
References
- 1.Dirlikov E, Raviglione M, Scano F. Global tuberculosis control: toward the 2015 targets and beyond. Ann Intern Med. 2015;163(1):52–58. doi: 10.7326/M14-2210. [DOI] [PubMed] [Google Scholar]
- 2.Raviglione M, Marais B, Floyd K, Lönnroth K, Getahun H, Migliori G, Harries A, Nunn P, Lienhardt C, Graham S, et al. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet. 2012;379(9829):1902–1913. doi: 10.1016/S0140-6736(12)60727-2. [DOI] [PubMed] [Google Scholar]
- 3.Laiho K, Kauppi M, Soini I. Tuberculosis and Pott's disease. N Engl J Med. 2003;348(15):1501. [PubMed] [Google Scholar]
- 4.de Nijs R. Spinal tuberculosis. Lancet. 2011;378(9807):e18. doi: 10.1016/S0140-6736(11)61482-7. [DOI] [PubMed] [Google Scholar]
- 5.Manzi A, Teodorani G. Acute primary psoitis. Archivio di ortopedia. 1954;67(3):253–267. [PubMed] [Google Scholar]
- 6.Martinez V, Rolland E, Bricaire F, Caumes E. Tuberculous paravertebral abscess. Lancet. 2004;363(9409):615. doi: 10.1016/S0140-6736(04)15593-1. [DOI] [PubMed] [Google Scholar]
- 7.Batirel A, Erdem H, Sengoz G, Pehlivanoglu F, Ramosaco E, Gülsün S, Tekin R, Mete B, Balkan I, Sevgi D, et al. The course of spinal tuberculosis (Pott disease): results of the multinational, multicentre Backbone-2 study. Clin Microbiol Infect. 2015;21(11):1008.e1009–1008.e1018. doi: 10.1016/j.cmi.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Diwakar L, Logan S, Ghaffar N, Hare A, Lynn W, Ash S. Low back pain: think of tuberculosis. BMJ. 2006;333(7560):201. doi: 10.1136/bmj.333.7560.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhagavat R, Kim H, Kim C, Terwilliger T, Mehta D, Srinivasan N, Chandra N. A genome-wide structure-based survey of nucleotide binding proteins in M. tuberculosis. Sci Rep. 2017;7(1):12489. doi: 10.1038/s41598-017-12471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yacoub W, Sohn H, Chan S, Petrosyan M, Vermaire H, Kelso R, Towfigh S, Mason R. Psoas abscess rarely requires surgical intervention. Am J Surg. 2008;196(2):223–227. doi: 10.1016/j.amjsurg.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Marais S, Roos I, Mitha A, Mabusha S, Patel V, Bhigjee A. Spinal tuberculosis: Clinicoradiological findings in 274 patients. Clin Infect Dis. 2018;67(1):89–98. 10.1093/cid/ciy020. [DOI] [PubMed]
- 12.Mandal N, Anand P, Gautam S, Das S, Hussain T. Diagnosis and treatment of paediatric tuberculosis: an insight review. Crit Rev Microbiol. 2017;43(4):466–480. doi: 10.1080/1040841X.2016.1262813. [DOI] [PubMed] [Google Scholar]
- 13.Liu T, Zhang X, Gao S, Jing F, Yang Y, Du L, Zheng G, Li P, Li C, Wang C. Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer. Oncotarget. 2016. [DOI] [PMC free article] [PubMed]
- 14.Kandwal P, Vijayaraghavan G, Jayaswal A. Management of Tuberculous Infection of the spine. Asian Spine J. 2016;10(4):792–800. doi: 10.4184/asj.2016.10.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colmenero J, Jiménez-Mejías M, Sánchez-Lora F, Reguera J, Palomino-Nicás J, Martos F, García de las Heras J, Pachón J. Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis. 1997;56(12):709–715. doi: 10.1136/ard.56.12.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staatz G, Adam G, Keulers P, Vorwerk D, Günther R. Spondylodiskitic abscesses: CT-guided percutaneous catheter drainage. Radiology. 1998;208(2):363–367. doi: 10.1148/radiology.208.2.9680560. [DOI] [PubMed] [Google Scholar]
- 17.HODGSON A. Ambulant treatment of spinal tuberculosis. Lancet. 1963;1(7272):110. doi: 10.1016/s0140-6736(63)91114-0. [DOI] [PubMed] [Google Scholar]
- 18.Bloom BR, Atun R, Cohen T, Dye C, Fraser H, Gomez GB, Knight G, Murray M, Nardell E, Rubin E, et al. Tuberculosis. In: Rd HKK, Bertozzi S, Bloom BR, Jha P, et al., editors. Major Infectious Diseases. Washington (DC): The International Bank for Reconstruction and Development / The World Bank (c) 2017 International Bank for Reconstruction and Development / The World Bank; 2017. [Google Scholar]
- 19.Zhang X, Zhang Z, Zhang Y, Wang J, Lu M, Hu W, Wang Y, Ma X, Wang Y. Minimally invasive retroperitoneoscopic surgery for psoas abscess with thoracolumbar tuberculosis. Surg Endosc. 2015;29(8):2451–2455. doi: 10.1007/s00464-014-3913-z. [DOI] [PubMed] [Google Scholar]
- 20.The Lancet Global H A new era for tuberculosis? Lancet Glob Health. 2018;6(10):e1045. doi: 10.1016/S2214-109X(18)30416-9. [DOI] [PubMed] [Google Scholar]
- 21.Wang ST, Ma HL, Lin CP, Chou PH, Liu CL, Yu WK, Chang MC. Anterior debridement may not be necessary in the treatment of tuberculous spondylitis of the thoracic and lumbar spine in adults: a retrospective study. Bone Joint J. 2016;98-b(6):834–839. doi: 10.1302/0301-620X.98B6.36472. [DOI] [PubMed] [Google Scholar]
- 22.Ukunda UNF, Lukhele MM. The posterior-only surgical approach in the treatment of tuberculosis of the spine: outcomes using cortical bone allografts. Bone Joint J. 2018;100-b(9):1208–1213. doi: 10.1302/0301-620X.100B9.BJJ-2017-1326.R2. [DOI] [PubMed] [Google Scholar]
- 23.Ansari S, Amanullah MF, Ahmad K, Rauniyar RK. Pott's spine: diagnostic imaging modalities and technology advancements. N Am J Med Sci. 2013;5(7):404–411. doi: 10.4103/1947-2714.115775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Li XL, Zhou XG, Zhou J, Dong J. Surgical treatment for spinal tuberculosis with bilateral paraspinal abscess or bilateral psoas abscess: one-stage surgery. J Spinal Disord Tech. 2014;27(8):E309–E314. doi: 10.1097/BSD.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 25.Soares Do Brito J, Tirado A, Fernandes P. Surgical treatment of spinal tuberculosis complicated with extensive abscess. Iowa Orthop J. 2014;34:129–136. [PMC free article] [PubMed] [Google Scholar]
- 26.Paley M, Sidhu PS, Evans RA, Karani JB. Retroperitoneal collections--aetiology and radiological implications. Clin Radiol. 1997;52(4):290–294. doi: 10.1016/s0009-9260(97)80056-6. [DOI] [PubMed] [Google Scholar]
- 27.Kodama K, Takase Y, Motoi I, Mizuno H, Goshima K, Sawaguchi T. Retroperitoneoscopic drainage of bilateral psoas abscesses under intraoperative laparoscopic ultrasound guidance. Asian J Endosc Surg. 2014;7(2):179–181. doi: 10.1111/ases.12091. [DOI] [PubMed] [Google Scholar]
- 28.Buyukbebeci O, Seckiner I, Karsli B, Karakurum G, Baskonus I, Bilge O, Kacira BK. Retroperitoneoscopic drainage of complicated psoas abscesses in patients with tuberculous lumbar spondylitis. Eur Spine J. 2012;21(3):470–473. doi: 10.1007/s00586-011-2049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lv GH, Wang B, Li J, Liu WD, Yin GH, Ma ZM. Thoracoscopy-assisted mini-open surgery for anterior column reconstruction in thoracic spinal tuberculosis. Orthop Surg. 2009;1(4):293–299. doi: 10.1111/j.1757-7861.2009.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh R, Gogna P, Parshad S, Karwasra RK, Karwasra PK, Kaur K. Video-assisted thoracic surgery for tubercular spondylitis. Minim Invasive Surg. 2014;2014:963497. doi: 10.1155/2014/963497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapoor SK, Agarwal PN, Jain BK, Jr, Kumar R. Video-assisted thoracoscopic decompression of tubercular spondylitis: clinical evaluation. Spine (Phila Pa 1976) 2005;30(20):E605–E610. doi: 10.1097/01.brs.0000182328.03082.e2. [DOI] [PubMed] [Google Scholar]
- 32.Detillon D, de Groot H, Hoebink E, Versteylen R, Veen E. Video-assisted thoracoscopic surgery as a diagnostic and therapeutic instrument in non-tubercular spondylodiscitis. Int J Spine Surg. 2015;9:55. doi: 10.14444/2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nene A, Bhojraj S. Results of nonsurgical treatment of thoracic spinal tuberculosis in adults. Spine J. 2005;5(1):79–84. doi: 10.1016/j.spinee.2004.05.255. [DOI] [PubMed] [Google Scholar]
- 34.Pombo F, Martin-Egana R, Cela A, Diaz JL, Linares-Mondejar P, Freire M. Percutaneous catheter drainage of tuberculous psoas abscesses. Acta Radiol. 1993;34(4):366–368. [PubMed] [Google Scholar]
- 35.Horvath G, Boda A. Repa I: [CT-guided percutaneous drainage in the management of psoas abscesses] Orv Hetil. 1994;135(47):2597–2602. [PubMed] [Google Scholar]
- 36.Dinc H, Onder C, Turhan AU, Sari A, Aydin A, Yulug G, Gumele HR. Percutaneous catheter drainage of tuberculous and nontuberculous psoas abscesses. Eur J Radiol. 1996;23(2):130–134. doi: 10.1016/0720-048x(96)01045-5. [DOI] [PubMed] [Google Scholar]
- 37.Gupta S, Suri S, Gulati M, Singh P. Ilio-psoas abscesses: percutaneous drainage under image guidance. Clin Radiol. 1997;52(9):704–707. doi: 10.1016/s0009-9260(97)80036-0. [DOI] [PubMed] [Google Scholar]
- 38.Dahniya MH, Hanna RM, Grexa E, Cherian MJ, Niazy MN, Badr S. Ibrahim F, al-Othman AN: percutaneous drainage of tuberculous iliopsoas abscesses under image guidance. Australas Radiol. 1999;43(4):444–447. doi: 10.1046/j.1440-1673.1999.00709.x. [DOI] [PubMed] [Google Scholar]
- 39.Dinc H, Ahmetoglu A, Baykal S, Sari A, Sayil O, Gumele HR. Image-guided percutaneous drainage of tuberculous iliopsoas and spondylodiskitic abscesses: midterm results. Radiology. 2002;225(2):353–358. doi: 10.1148/radiol.2252011443. [DOI] [PubMed] [Google Scholar]
- 40.Cantasdemir M, Kara B, Cebi D, Selcuk ND, Numan F. Computed tomography-guided percutaneous catheter drainage of primary and secondary iliopsoas abscesses. Clin Radiol. 2003;58(10):811–815. doi: 10.1016/s0009-9260(03)00274-5. [DOI] [PubMed] [Google Scholar]
- 41.Pieri S, Agresti P, Altieri AM, Ialongo P, Cortese A, Alma MG, de’ Medici L. Percutaneous management of complications of tuberculous spondylodiscitis: short- to medium-term results. Radiol Med. 2009;114(6):984–995. doi: 10.1007/s11547-009-0425-3. [DOI] [PubMed] [Google Scholar]
- 42.Varatharajah S, Charles YP, Buy X, Walter A, Steib JP. Update on the surgical management of Pott's disease. Orthop Traumatol Surg Res. 2014;100(2):229–235. doi: 10.1016/j.otsr.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, Donovan W, Graves D, Jha A, Jones L, et al. Reference for the 2011 revision of the international standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2011;34(6):547–554. doi: 10.1179/107902611X13186000420242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dave BR, Kurupati RB, Shah D, Degulamadi D, Borgohain N, Krishnan A. Outcome of percutaneous continuous drainage of psoas abscess: a clinically guided technique. Indian J Orthop. 2014;48(1):67–73. doi: 10.4103/0019-5413.125506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q, Hu M, Ma YZ. Luo XB: [case-control studies of two kinds of method for the treatment of lumbar tuberculosis with psoas abscess] Zhongguo Gu Shang. 2016;29(1):33–37. [PubMed] [Google Scholar]
- 46.Ye F, Zhou Q, Feng D. Comparison of the Anteroposterior and posterior approaches for percutaneous catheter drainage of Tuberculous psoas abscess. Med Sci Monit. 2017;23:5374–5381. doi: 10.12659/MSM.902848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lai Z, Shi S, Fei J, Han G, Hu S. A comparative study to evaluate the feasibility of preoperative percutaneous catheter drainage for the treatment of lumbar spinal tuberculosis with psoas abscess. J Orthop Surg Res. 2018;13(1):290. doi: 10.1186/s13018-018-0993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai Z, Shi SY, Fei J, Han GH, Hu SP. Feasibility study of preoperative percutaneous catheter drainage in the treatment of lumbar tuberculosis with psoas abscess. Zhongguo Gu Shang. 2018;31(11):998–1004. doi: 10.3969/j.issn.1003-0034.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Tsagouli P, Sotiropoulou E, Filippousis P, Sidiropoulou N, Georgiadi V, Thanos L. Contribution of computed tomography guided percutaneous drainage of tuberculous cold abscesses adjunctive to pharmaceutical anti-tubercular treatment. Eur J Radiol. 2012;81(3):562–565. doi: 10.1016/j.ejrad.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 50.Held M, Laubscher M, Zar HJ, Dunn RN. GeneXpert polymerase chain reaction for spinal tuberculosis: an accurate and rapid diagnostic test. Bone Joint J. 2014;96-b(10):1366–1369. doi: 10.1302/0301-620X.96B10.34048. [DOI] [PubMed] [Google Scholar]
- 51.Wong YW, Samartzis D, Cheung KMC, Luk K. Tuberculosis of the spine with severe angular kyphosis: mean 34-year post-operative follow-up shows that prevention is better than salvage. Bone Joint J. 2017;99-b(10):1381–1388. doi: 10.1302/0301-620X.99B10.BJJ-2017-0148.R1. [DOI] [PubMed] [Google Scholar]
- 52.Li W, Liu Z, Xiao X, Zhang Z, Wang X. Comparison of anterior transthoracic debridement and fusion with posterior transpedicular debridement and fusion in the treatment of mid-thoracic spinal tuberculosis in adults. BMC Musculoskelet Disord. 2019;20(1):570. doi: 10.1186/s12891-019-2945-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jutte P, Van Loenhout-Rooyackers J. Routine surgery in addition to chemotherapy for treating spinal tuberculosis. Cochrane Database Syst Rev. 2006;(1):CD004532. Published 2006 Jan 25. 10.1002/14651858.CD004532.pub2. [DOI] [PMC free article] [PubMed]
- 54.Sucu H, Ciçek C, Rezanko T, Bezircioğlu H, Erşahin Y, Tunakan M, Minoğlu M. Percutaneous computed-tomography-guided biopsy of the spine: 229 procedures. Joint Bone Spine. 2006;73(5):532–537. doi: 10.1016/j.jbspin.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Zou D, Zhou J, Zhou X, Jiang X. Clinical efficacy of CT-guided percutaneous huge Ilio-psoas abscesses drainage combined with posterior approach surgery for the management of dorsal and lumbar spinal tuberculosis in adults. Orthop Traumatol Surg Res. 2017;103(8):1251–1255. doi: 10.1016/j.otsr.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 56.Yang H, Hou K, Zhang L, Zhang X, Wang Y, Huang P, Xiao S. Minimally invasive surgery through the interlaminar approach in the treatment of spinal tuberculosis: a retrospective study of 31 patients. J Clin Neurosci. 2016;32:9–13. [DOI] [PubMed]
- 57.Hou X, Sun X, Zhang Z, Xie G, Zhang X. Computed tomography-guided percutaneous focal catheter infusion in the treatment of spinal tuberculosis. Acta Orthop Belg. 2014;80(4):501–507. [PubMed] [Google Scholar]
- 58.Zhang X, Ji J, Liu B. Management of spinal tuberculosis: a systematic review and meta-analysis. J Int Med Res. 2013;41(5):1395–1407. doi: 10.1177/0300060513498023. [DOI] [PubMed] [Google Scholar]
- 59.Ge Z, Wang Z, Wei M. Measurement of the concentration of three antituberculosis drugs in the focus of spinal tuberculosis. Eur Spine J. 2008;17(11):1482–1487. doi: 10.1007/s00586-008-0778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou K, Yang H, Zhang L, Zhang X, Xiao S, Lu N. Stepwise therapy for treating tuberculosis of the upper cervical spine: a retrospective study of 11 patients. Eur Neurol. 2015;74(1–2):100–106. doi: 10.1159/000437418. [DOI] [PubMed] [Google Scholar]
- 61.Halsey J, Reeback J, Barnes C. A decade of skeletal tuberculosis. Ann Rheum Dis. 1982;41(1):7–10. doi: 10.1136/ard.41.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johansen I, Nielsen S, Hove M, Kehrer M, Shakar S, Wøyen A, Andersen P, Bjerrum S, Wejse C, Andersen Å. Characteristics and clinical outcome of bone and joint tuberculosis from 1994 to 2011: a retrospective register-based study in Denmark. Clin Infect Dis. 2015;61(4):554–562. doi: 10.1093/cid/civ326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Date that support the findings of this study are available from the corresponding author on reasonable request.