Abstract
This Perspective focuses on RNA in biological and non-biological compartments resulting from liquid-liquid phase-separation (LLPS), with an emphasis on origins of life. In extant cells, intracellular liquid condensates, many of which are rich in RNAs and intrinsically disordered proteins, provide spatial regulation of biomolecular interactions that can result in altered gene expression. Given the diversity of biogenic and abiogenic molecules that undergo LLPS, such membrane-less compartments may have also played key roles in prebiotic chemistries relevant to the origins of life. The RNA World hypothesis posits that RNA may have served as both genetic information carrier and catalyst during the origin of life. Due to its polyanionic backbone, RNA can undergo LLPS by complex coacervation in the presence of polycations. Phase separation could provide a mechanism to concentrate monomers for RNA synthesis and selectively partition longer RNAs with enzymatic functions, thus driving prebiotic evolution. We introduce several types of LLPS that could lead to compartmentalization and discuss potential roles in template-mediated non-enzymatic polymerization of RNA and other related biomolecules, functions of ribozymes and aptamers, and benefits or penalties imparted by liquid-demixing. We conclude that tiny liquid droplets may have concentrated precious biomolecules and acted as bioreactors in the RNA World.
Graphical Abstract
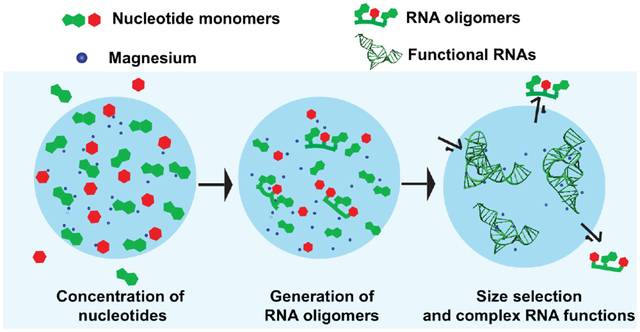
Introduction
Liquid-liquid phase coexistence in aqueous macromolecule solutions has been known for many years and its possible involvement in both living cells and prebiotic chemistry was considered early on. In 1929, Bungenberg de Jong and Kruyt coined the term “coacervate” to describe macromolecule-rich droplets that formed by associative phase separation. Commenting on the potential biological significance of these structures, they wrote, “On closer inspection of the ground mass of the protoplasm, it strikes one that this has some properties in common with the coacervates, so that there is a possibility that this ground mass may be considered as a coacervate or as a system of coacervates.”1 Oparin postulated that coacervates served an important role in the origin of life, ultimately evolving to form heterotropic microbes.2 As science progressed and roles for DNA as an informational molecule and lipid self-assembly as a means of providing compartmentalization became apparent, attention shifted away from coacervation.3 With the recent discovery that membraneless organelles including P granules and the nucleolus are in fact liquid-like phases,4–6 we now know that liquid-liquid phase separation (LLPS) is important in extant biology as a previously unappreciated strategy for subcellular compartmentalization. The prevalence and simplicity of phase separation in aqueous solutions of biogenic and abiogenic polymers,7 particularly as oligomer length, complexity, and concentration increase,8 suggests that these structures could have occurred early in prebiotic systems and cellular evolution.9 Indeed, intrinsically disordered proteins (IDPs), which are major components of extant membraneless organelles, are present in primitive organisms suggesting an early origin.10 Furthermore, disordered and conformationally dynamic regions of proteins have been hypothesized to have played key roles in evolution of functional proteins.11–12
In this Perspective, we consider the physical chemistry of liquid condensate formation and the roles of such membraneless compartments in extant biology and prebiotic chemistry as protocells (Figure 1, left). We examine formation of condensates comprised of IDPs and the biomolecules that partition into them. In extant biology, condensates can form in cytoplasm and the nucleolus where they partition mRNAs,13 lncRNAs14 and ribosomal RNAs15 among others, while in protocells, such membraneless compartments can form under diverse conditions and partition RNA oligonucleotides and functional RNAs. We then discuss possible roles of liquid condensates in chemistries that have been implicated in origin of life (Figure 1, right). Specifically, we explore advantages and limitations that are the result of LLPS on relevant prebiotic chemistries such as non-enzymatic polymerization of RNA and activities of RNA enzymes (ribozymes) and other functional RNAs. Overall, LLPS leads to the formation of membraneless organelles in extant cells and we hypothesize that it could have also provided compartmentalization on primordial Earth.
Figure 1:
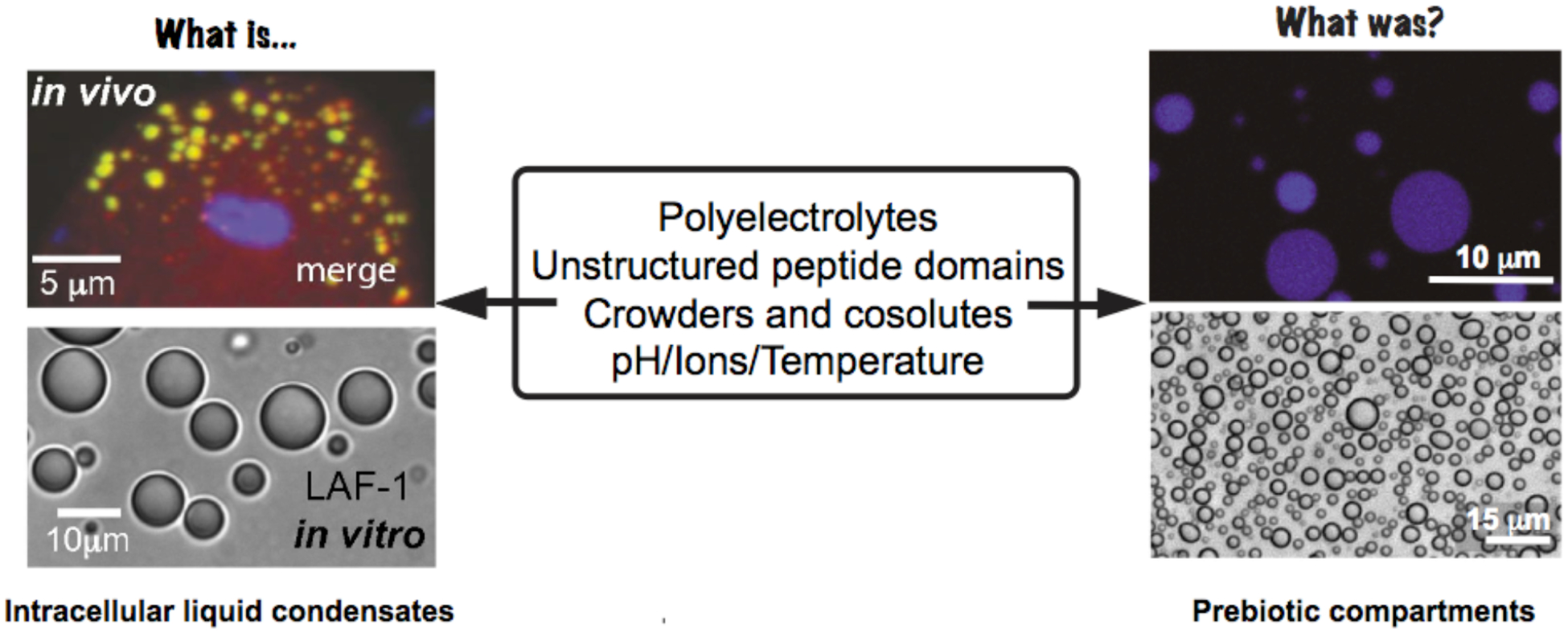
Phase separation in extant biology (left) and prebiotic chemistry (right). Low complexity regions of the LAF-1 helicase protein, charged peptides and RNA are involved in liquid-liquid phase separation inside cells (left)21. Similar interactions could have produced non-membranous compartments in the primordial earth that partition molecules (right). Spermine/polyU RNA coacervates (top)55 and PAH-ADP coacervates (bottom).45
PHYSICAL CHEMISTRY OF LIQUID-LIQUID PHASE SEPARATION
Classes of LLPS.
Many types of phase separation occur in macromolecule-containing aqueous systems. In considering the consequences of LLPS for extant biology and the RNA World, it is useful to distinguish between non-associative and associative phase separation, and to first consider simple, limiting cases (Figure 2). In non-associative, or segregative, phase separation, each phase is enriched in a different component.1,7 This is common in solutions containing two or more nonionic polymers such as polyethylene glycols (PEGs) mixed with polysaccharides such as dextran, and in solutions with one or more nonionic polymers and a high concentration of salt (e.g., ~0.5 M potassium phosphate).16 Various PEG/dextran and PEG/salt aqueous two-phase system have been used extensively for bioseparations.7,17–18 Due to their ability to generate coexisting phase compartments that provide macromolecular crowding and to localize biomolecules on the basis of their size and chemical structure, PEG/dextran aqueous two-phase systems (ATPS) with 5–10 weight percent of both PEG 8 kDa and dextran 10 or 500 kDa have also been used to mimic compartmentalization in extant biology.19,20 A possible limitation of non-associative phase separation as a model for prebiotic compartmentalization is that it can require relatively high total polymer concentrations.7 Therefore, it may best be considered for scenarios where organic concentrations are locally higher, for example due to wet/dry cycles.
Figure 2:
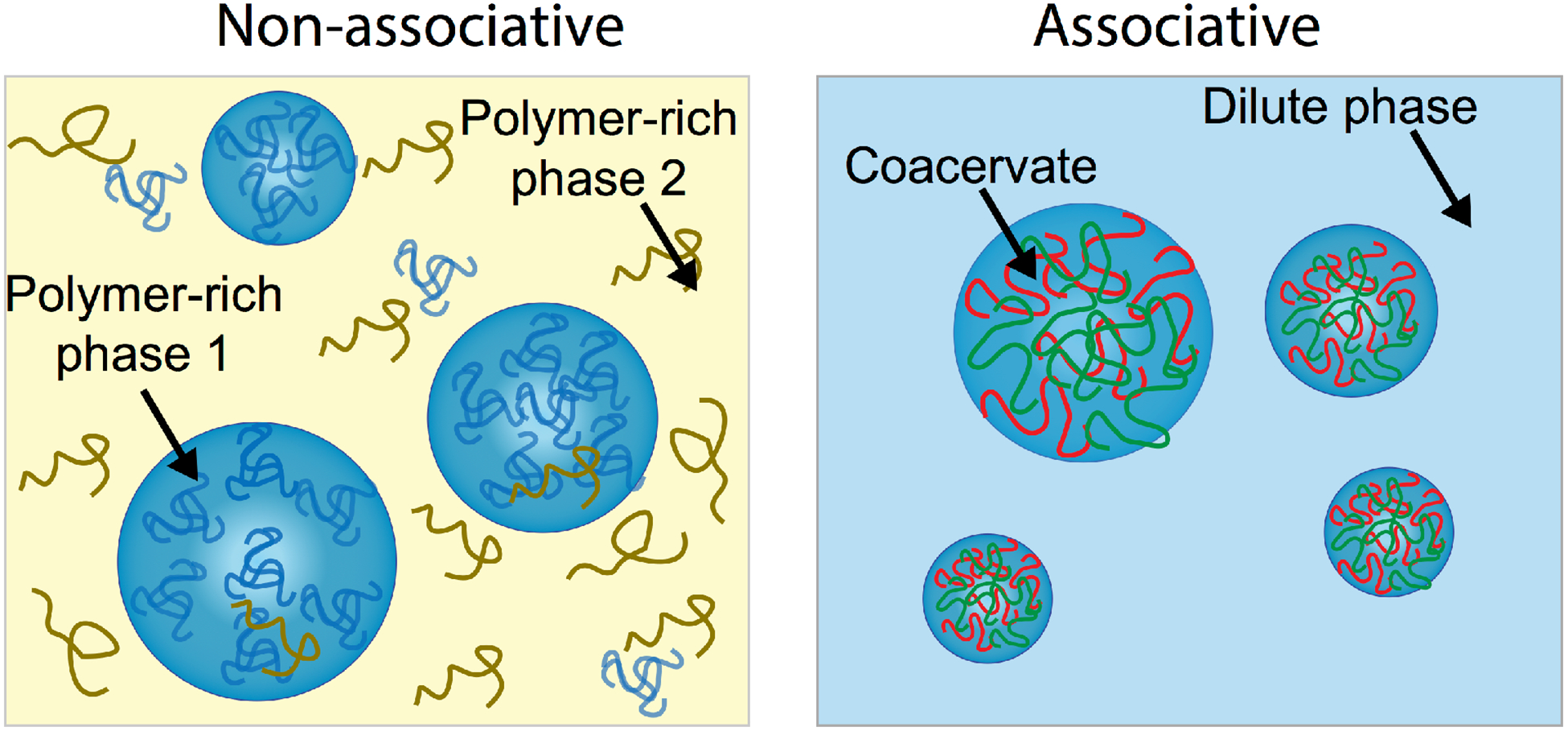
Non-associative and associative phase separation. In non-associative phase separation, solutions rich in two “incompatible” aqueous polymers form two distinct crowded phases (left). In associative phase separation, polymers interact and associate to form a very crowded polymer-rich phase and separate from the dilute bulk solution (right).
Associative LLPS, or coacervation, results in a macromolecule-dense phase termed the coacervate phase (Figure 2 right) along with a dilute supernatant phase.1 As an example, the IDP LAF-1 phase separates at concentrations above ~0.8 μM LAF-1 in vitro,21 generating dense protein-rich droplets related to intracellular membraneless organelles. Water is considered a poor solvent for many polypeptides, including IDPs with sequences rich in Gly and the polar uncharged residues Gln, Asn, and Ser.22–23 Such IDPs often exhibit concentration- and temperature-dependent phase transitions that can include coacervation as well as gelation or fibrillation.6 Associative LLPS for which ion pairing (“complexation”) is important is termed complex coacervation.1 With respect to prebiotic compartmentalization, this type of LLPS is of particular interest for concentrating nucleic acids due to their negatively-charged phosphodiester backbone. Several synthetic and biological polyelectrolytes that have been used to form complex coacervates are illustrated in Figure 3. Features that affect complex coacervation include the number, distribution, and strength of the ion pairing interactions, which can depend not only on functional group identity but also on polymer chain conformation and flexibility.24–25,26 Solution conditions can play a role as well, with pH determining the charge state of weak acid/base groups and ionic strength tuning the extent of polyelectrolyte/polyelectrolyte ion pairing for a given pH.27 These parameters also control coacervate water content and viscosity.28 Although multivalency drives polyelectrolyte association and is important for phase separation,8 complex coacervation can be achieved even for low molecular weight polyions, which is important in a prebiotic milieu where high molecular weight organic polymers may have been scarce. For example, Mann and coworkers demonstrated droplet formation in solutions of mononucleotides and oligolysine peptides.29
Figure 3:
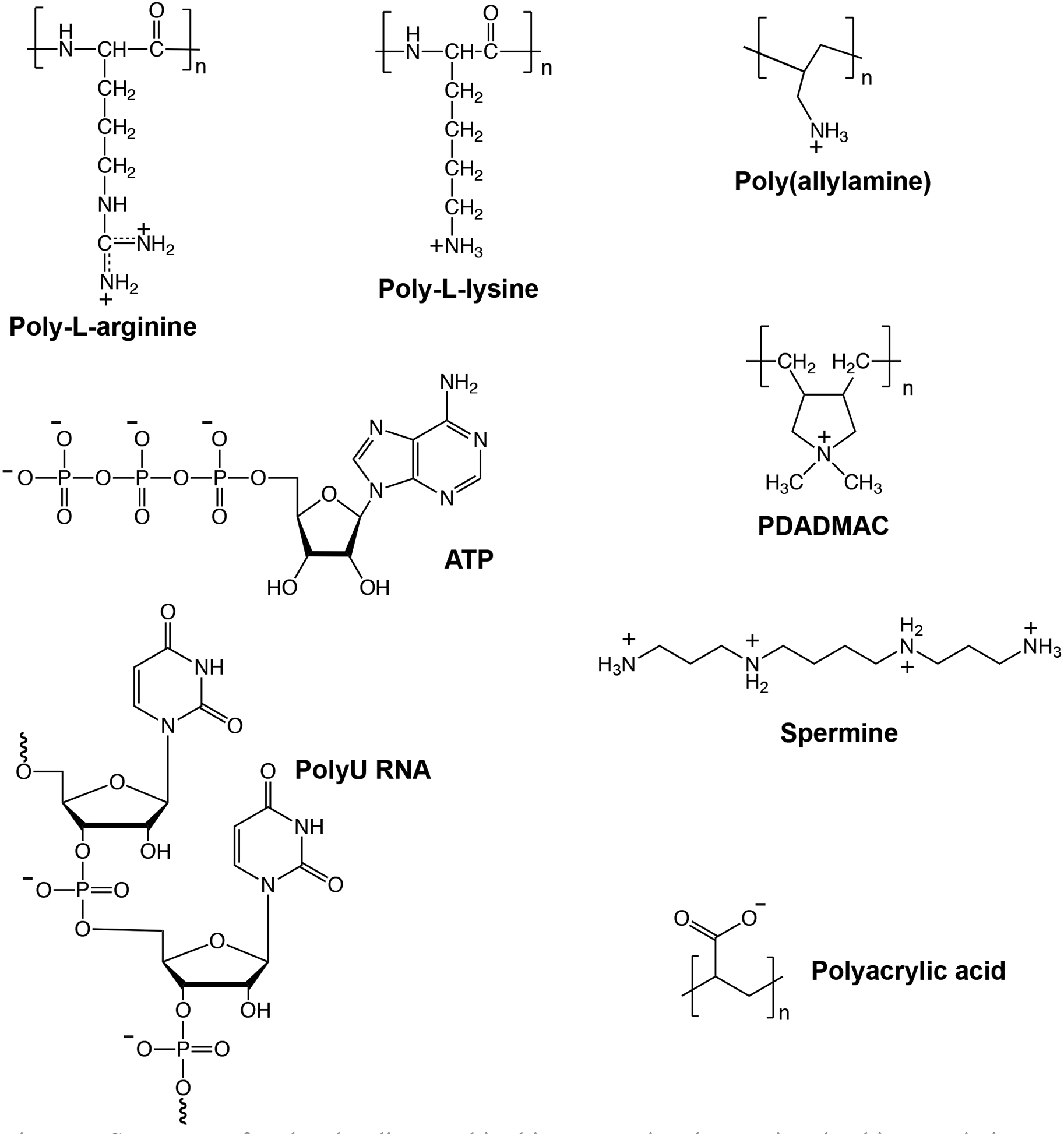
Structures of molecules discussed in this perspective that are involved in associative phase separation.
Due to the chemical complexity of biopolymers, multiple types of interaction can contribute to their phase behavior. In addition to simple charge-charge contacts, other polymer-polymer and polymer-solvent chemical interactions can be important for LLPS, including cation-p, dipole-dipole, and p - p stacking.23 In vitro LLPS of certain IDPs associated with membraneless organelles has shown sensitivity to increasing solution ionic strength. This observation, particularly coupled with the presence of structural domains rich in charged amino acid residues, suggests a complex coacervation mechanism.21,30–31 These systems also commonly exhibit temperature sensitivity; for example, formation of stress granules is favored at elevated temperature,32–33 while coacervate droplets rich in the disordered N-terminus of the protein Ddx4 are destabilized by both increased ionic strength and temperature.30 Specific biorecognition, for instance between multivalent protein-RNA or protein-protein binding partners, can also be important in phase separation and phase occupancy.34
The presence of additional molecules increases the complexity of biomolecular interactions and impacts phase behavior as well. RNA in particular can substantially impact LLPS of IDPs in vitro and is thought to be important in vivo due to the prevalence of RNAs in many membraneless organelles.35 Binding of RNA to RNA recognition motifs (RRMs) can drive phase separation of a number of proteins including hnRNPA1 and polypyrimidine tract-binding protein.36 Polymeric crowding agents such as PEG and ficoll have been shown to favor coacervation of some proteins, including IDPs37 and RNA/spermine systems.38 This is potentially relevant both for LLPS in the crowded interior of living cells, and for prebiotic coacervation under drying conditions where total polymer concentrations would be increased.39–40
Associative phase separation provides a potential mechanism for concentrating rare organic polymers into small-volume compartments in a prebiotic context. Although most studies have been conducted with relatively pure synthetic or biological polymers, the generality of the LLPS mechanisms indicates that polymer mixtures heterogeneous in length or composition can also be expected to undergo coacervation with different properties based on their compositions. Phase transitions in response to changes in temperature, pH or dilution/concentration could have provided means of cycling between compartmentalized and non-compartmentalized solutions. This would have not only allowed compartment-specific reactions and interactions to take place, but also diffusion of informational and small molecules during the non-compartmentalized state.
Physical chemistry of compartments formed by LLPS.
Characteristics of intracellular liquid organelles include surface tension-minimizing spherical shapes, coalescence upon contact, and often rapid exchange of biomolecules with the surrounding cytoplasm or nucleoplasm.13 These properties are consistent with formation by LLPS and in sharp contrast to membrane-bounded organelles that require specialized transport mechanisms to allow entry and egress of biomolecular solutes. Structures of liquid organelles provide a different type of intracellular compartment that can be more readily accessed and retains its major and minor components by equilibrium partitioning rather than a physical barrier such as a lipid bilayer membrane. In thinking about protocells, such an approach does not require energy input nor specialized transport mechanisms to collect and maintain locally higher concentrations of organic oligomers; hence its simplicity could offer advantages compared to lipid vesicles, particularly at the earliest stages of prebiotic evolution.
Coexisting aqueous phases provide distinct physicochemical environments that depend on phase composition. In addition to the obvious differences based on the distribution of phase-forming molecules (e.g., the dextran-rich phase of a PEG/dextran ATPS is enriched in dextran, or a complex coacervate is enriched in oppositely-charged polyelectrolytes), different phases can have a unique dielectric constant, viscosity, ionic strength, and water activity. This in turn affects the distribution of other solutes in the phase-separated medium and can alter reaction rates and equilibria.41–42 For example, in a 10 wt% PEG 8kDa / 10 wt% Dextran 10 kDa system, the dextran-rich phase has ~7x higher dextran concentration, nearly 5x lower PEG concentration, and 2-fold greater viscosity than the PEG-rich phase.43–44 Local concentrations of charged groups within complex coacervates can be in the molar range, and water content as low as ~40 wt %.8,24,45 Mann and coworkers have reported an apparent dielectric constant (ε) of ~60 inside peptide/nucleotide coacervate droplets (as compared with ε = 80 for water at 20 °C).29
Many membraneless organelles in extant Biology contain both RNA and proteins (ribonucleoprotein (RNP) bodies). A growing body of literature shows that the presence of RNA in IDP-based coacervates can alter the physical properties of these droplets; for example, resulting droplets have reduced local protein concentration and decreased viscosity.46 The physical properties of these compartments can also have important consequences for RNA structure, as will be discussed below.42
COMPARTMENTALIZATION BY LLPS
Increased local concentration of different biomolecules assists in association between them, ultimately allowing downstream reactions. For example, related genes of metabolic pathways in bacteria are often polycistronic wherein they are translated from the same mRNA.47 This strategy helps co-localize enzymes for downstream reactions and substrates are channeled efficiently. For instance, polyketide synthases consist of several distinct catalytic domains that are spatially organized through specific protein-protein interactions.48 Similar distinct interactions between “client” and “scaffold” proteins have been shown to effectively recruit proteins inside compartments formed by LLPS.34 Localization of biomolecules in ATPS, membraneless organelles, and complex coacervates can potentially assist in chemical reactions, as they can concentrate ions, small molecules, and large polymers.
In addition to LLPS, mineral surfaces,49 aerosols,50 and fatty-acid vesicles51 have been proposed as prebiotic compartmentalization strategies, and indeed multiple compartmentalization mechanisms likely coexisted, which could have facilitated prebiotic chemistry and chemical evolution. Compartmentalization through LLPS has several unique properties that would have been highly desirable in a prebiotic protocell. Specifically, LLPS allows uptake of freely diffusing solutes such as Mg2+ from the other phase since both phases coexist as liquid without the need of dedicated transporters that are common in modern biology. High concentrations of polymers and ions in LLPS also mimics biological intracellular environment which tends to be crowded.52 Furthermore, accumulation and concentration of larger molecules from a dilute external phase tends to be difficult in some fatty acid and lipid vesicles. While the absence of a physical barrier to solute entry/egress can be an advantage for compartmentalization, genetically distinct protocells require that informational molecules are not rapidly exchanged. Exchange rates for molecules encapsulated by LLPS can vary extensively with composition. For example, in ATP/oligolysine coacervates, an RNA oligomer was shown to exchange rapidly,53 while for PDDA/ATP coacervates generated microfluidically, a single-stranded DNA oligomer of similar length was shown not to exchange over 48 hr.54 In general, rates of RNA exchange between droplets will depend on coacervate composition, solution conditions (e.g., temperature, ionic strength), and RNA sequence and length, where longer RNAs are slower to exchange.53,55 Thus membraneless compartments can potentially serve as protocells, which have unique genetic identities depending on solution conditions. While a variety of molecules and ions can be compartmentalized by LLPS,9,18,29 in this Perspective we focus on partitioning of RNA and Mg2+ ions because of their potential roles in the origin of life on Earth in the context of RNA World hypothesis.
ROLES FOR MEMBRANELESS COMPARTMENTS IN ORIGINS OF LIFE CHEMISTRY
Protocells: Oligomerization of Monomers to Functional RNA Polymers.
The RNA World hypothesis has been investigated as a model for origin of functional biopolymers, prebiotic evolution, and subsequent origin of life on Earth (reviewed extensively).56–57 The origin of RNA polymers from non-enzymatic polymerization of monomers is still an active area of origins of life research. The anionic phosphoric acid moieties in both monomers and oligonucleotides of RNA and other alternative nucleic-acid molecules58 are ideal for complex coacervation driven by ion pairing interactions. Indeed, studies on ATP/polylysine coacervates53 revealed high partitioning of RNA oligonucleotides into the condensed phase. As shown in Figure 4, solutions of coacervates can be centrifuged to separate the condensed coacervate phase from the bulk solution. The concentration of analytes in the coacervate phase can be then determined by subtracting the amount of analyte in the bulk phase, which can be readily measured, from the known total amount of analyte. We used this method to study ATP- poly(allyl)amine (PAH) coacervates,45 where ATP and ADP concentrations within the condensed phase were found to be on the order of molar (Figure 4). Similar reports of enriched nucleotide concentrations have also been reported for coacervates comprised of ATP and polylysine.29
Figure 4:
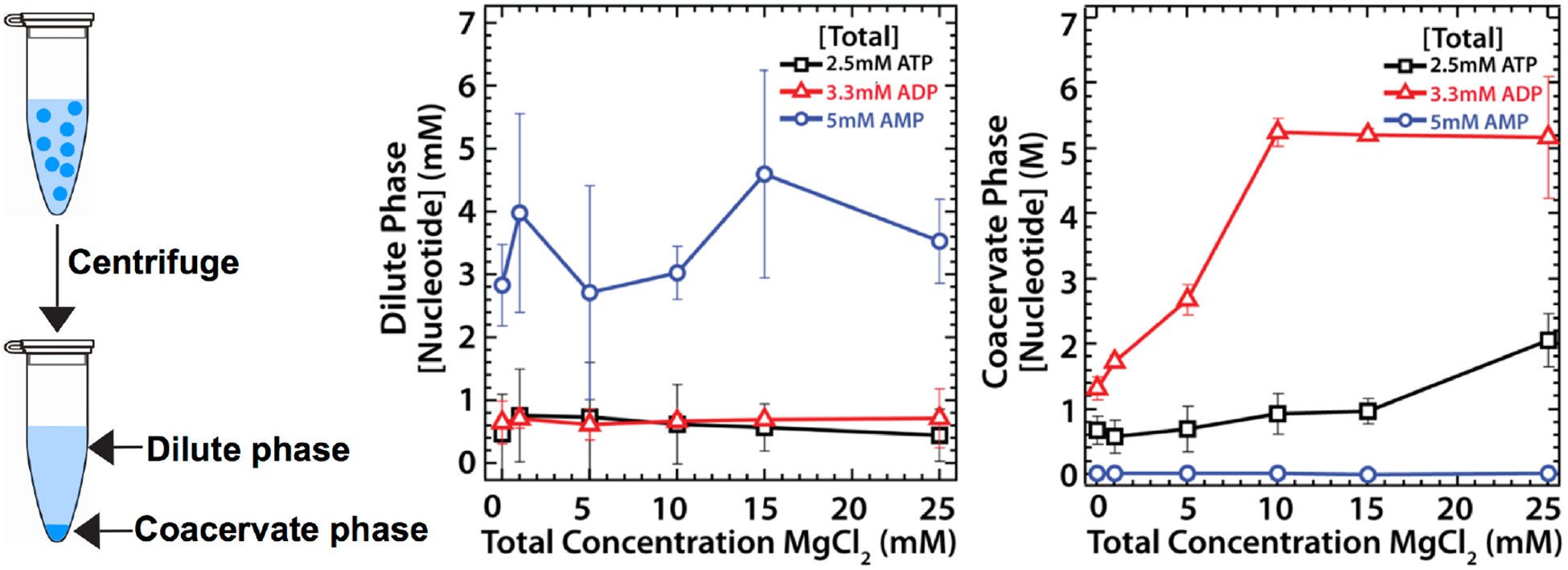
Coacervates concentrate monomers and polymers A) (left) Centrifugation separates condensed phase from the bulk solution. (middle) Concentration of nucleotides in dilute phase (mM). (right) Concentration of nucleotides in condensed phase (M).45
Increasing the concentration of reactants is one of the major ways that modern enzymes operate, through the so-called principle of approximation,59 and it is likely that coacervates can capture some of these same principles. For instance, non-enzymatic polymerization of RNA reactions are sensitive to concentrations of monomers, and experiments to synthesize functional RNA molecules via template-mediated non-enzymatic polymerization typically use very high concentrations (~50 mM) of activated nucleotide monomers.60–61 Mechanisms for attaining such high concentrations of monomers, which were presumably rare, outside of the laboratory setting have not been well explored. Furthermore, both experimental62–63 and recent in silico64 studies have shown that concentration and organization of amino acids along mineral surfaces can enhance polymerization under prebiotic conditions. Complex coacervates may have performed similar roles perhaps concentrating amino acids to provide early peptides to provide an exit from the RNA world.
Concentrating RNA by partitioning.
The first RNA polymers were likely generated by non-enzymatic polymerization of nucleotides,65 these reactions tend to proceed at timescales where degradation of the newly synthesized RNA and activated monomers becomes a major issue.65 Thus, compartmentalization of scarce and dilute RNA polymers would have been extremely beneficial to increase the local concentration of functional RNA molecules and protect them from destructive chemicals and radiation. Partitioning of solutes and polymers in ATPS is largely dependent on their respective chemical and physical properties. For example, in PEGDextran ATPS, hydrophobic peptides/denatured proteins tend to partition in the more hydrophobic PEG phase,9 whereas the anionic backbone of RNA makes it more favorable to partition in the dextran phase. For larger RNAs, the contact area with the dextran increases, thus they tend to show increased partitioning in dextran phase. Indeed, in PEG/dextran ATPS, increased-partitioning of RNA in the dextran phase strongly correlates with the size of the RNA.41
Although partitioning of RNAs inside complex coacervates is driven by ion pairing interactions, additional interactions can occur between the RNA and other molecules within the condensed phase. For example, in case of polyU RNA/spermine coacervates, A15 RNA partitions much strongly in the condensed phase compared to U15 or N15 RNAs.55 This is likely due to sequence-specific interactions between polyU RNA/spermine coacervates and A15 RNA.
It is helpful to consider how RNA partitions inside biological intracellular condensates and abiological complex coacervate. As mentioned earlier, intracellular condensates comprised of proteins have been found to contain RNA. While it is known that RNA functions are closely tied with their structures, detailed studies of RNA functions and structures inside condensed phases of coacervates are sparse. For example, in Ddx4-containing organelles, it was found that single-stranded RNA and DNA were partitioned in the interior of condensate irrespective of length.42 However, long double-stranded DNA and RNA were excluded, while short ones were only moderately absorbed (Figure 5 A). This partitioning behavior was rationalized in terms of the distortion produced by the nucleic acids on the underlying structure of the organelle interior (Figure 5A right). The authors posit that single-stranded nucleic acids, short double-stranded oligonucleotides, and compact RNA folds exert only minimum distortion on the “mesh-like weave” of the membraneless organelle, whereas long double-stranded nucleic acids distort the interior structure of the organelle and are thus excluded.42 Mechanisms for absorption nucleic acids are highly dependent on the constituents that make up the coacervate; therefore, this distortion of interior structures may not apply to other phase separated systems.
Figure 5:
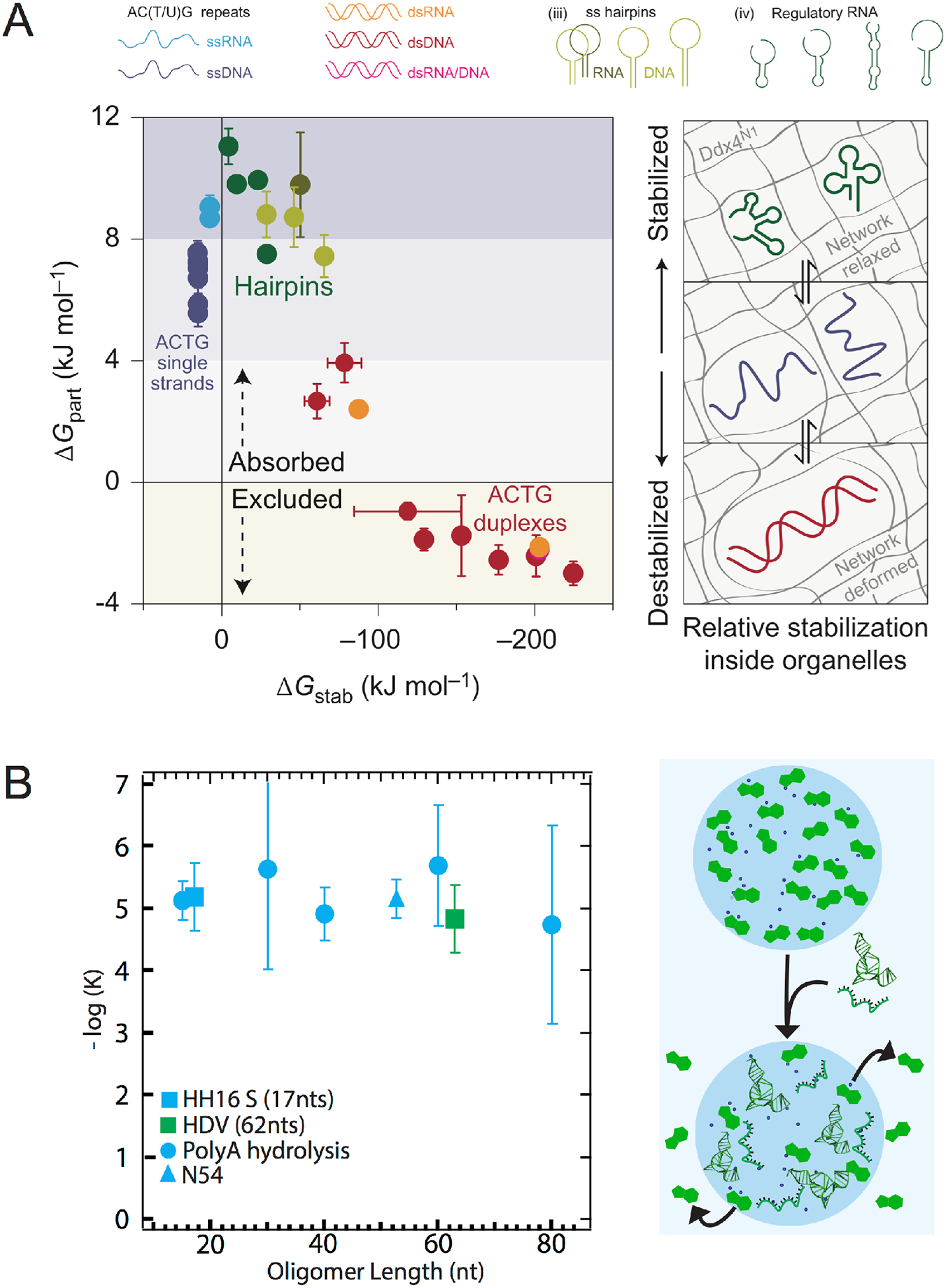
Multiple mechanisms for RNA partitioning inside membraneless compartments (A) Ddx4 protein condensates differentially exclude long double stranded DNA and RNA while absorbing single stranded nucleic acids and regulatory RNAs (left), ΔGpart= -RTln([in]/[out]), where [in] and [out] are concentrations of nucleotides inside and outside of droplets. Constraints in the interior structures of Ddx4 condensates allow a subset of nucleic acid sizes and structures to be absorbed.42 (B) PAH-ATP coacervates selectively partition RNAs irrespective of the structures and sizes (left)45. The mechanism for RNA partitioning is by displacement of ATP.
In case of the poly(allyl)amine hydrochloride (PAH)-ATP coacervate system,45 we found that different lengths of polyA RNA partition similarly (Figure 5B). Furthermore, RNAs containing 54 random nucleotides and the structured HDV ribozyme were absorbed in the condensed phase to similar extent as polyA RNA. Since the charge-charge interaction is the main driver of the PAH-ATP coacervate system, partitioning of RNAs is likely from the displacement of ATP (Figure 5B right), which has smaller charge valency. This mechanism also appears to serve as the basis for selection of longer RNA polymers in the coacervates.
There are large differences in partitioning coefficients for RNA in Ddx4 organelles and in PAH-ATP coacervates. For example, there is ~3000-fold smaller Keq values for RNAs of similar lengths in Ddx organelles. Since Ddx organelles are biological, there are likely other constraints such as structure and sequence that not only dictate partitioning, but also have biological functions. The PAH-ATP coacervates, on the other hand, are abiological, and the partitioning of RNAs are strictly based on the chemical interactions without any evolutionary history.
Effect of Compartmentalization on Ribozyme Reaction Rates.
Concentrating biomolecules by partitioning can enhance reaction rates. We investigated the effect of compartmentalization on single turnover kinetics for three different hammerhead ribozymes in PEG/dextran systems.41 Different volume ratios of dextran-rich to PEG-rich phase (VD:VP) allowed the effect of compartment size on ribozyme catalysis to be studied without altering the composition of the phases. Apart from the selective RNA partitioning in the dextran phase, we observed the highest reaction rate increase of ~70-fold at a 1:100 (VD:VP) volume ratio (Figure 6A), which occurs because substrate and enzyme are concentrated under kcat/KM conditions. Since ATPS increase polymer concentrations, they can additionally exert the crowding effects to help fold RNAs. Indeed, crowders such as PEG and dextran have been shown to increase cooperative folding in variants of tRNA.66 The mode for enhanced cooperative folding is RNA and crowder-specific, and can arise due to destabilization of secondary structures or stabilization of tertiary structures.67 Interestingly, viscous solvents have also been hypothesized to have alleviated strand inhibition during prebiotic RNA replication.68
Figure 6:
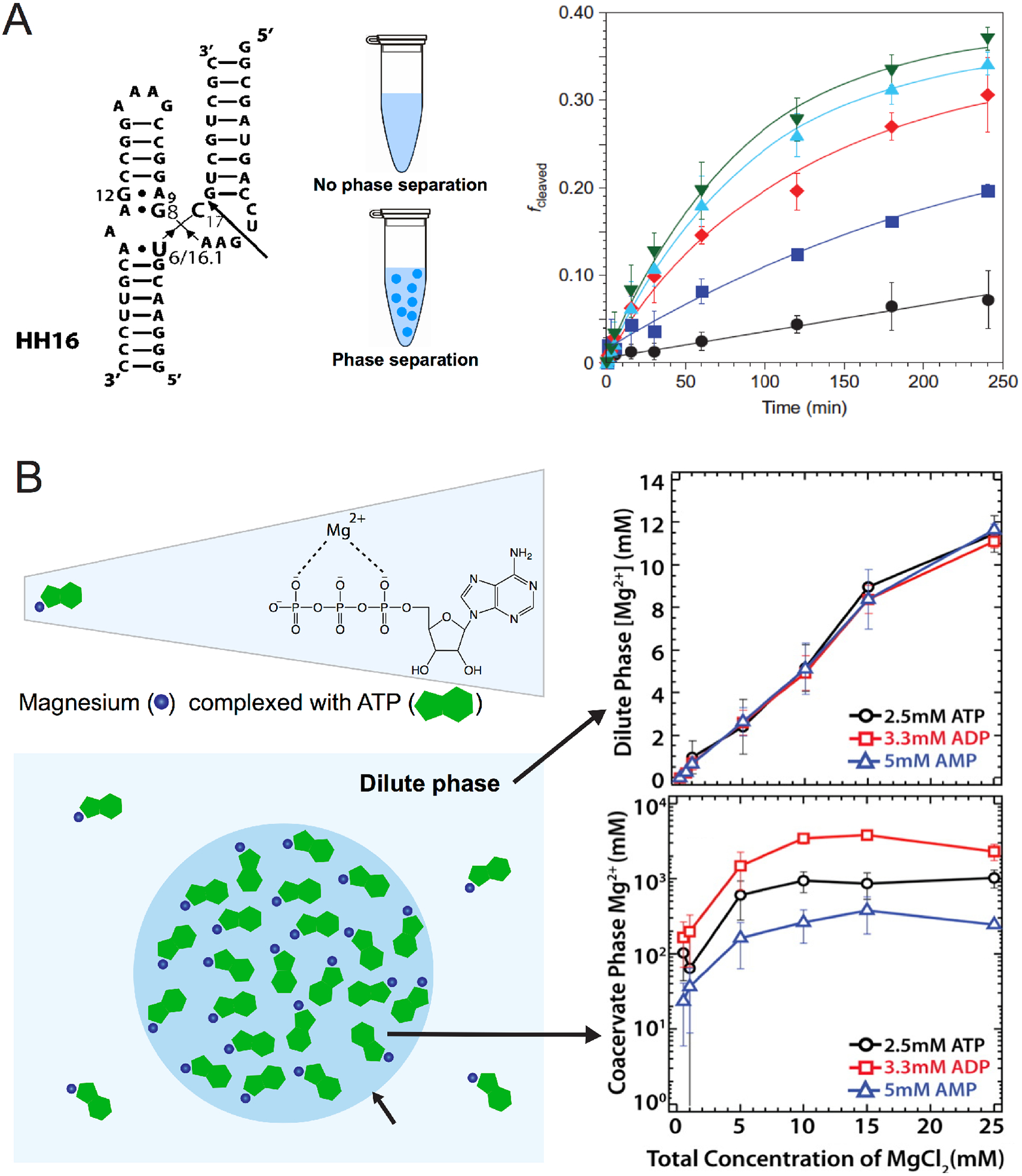
Ribozyme catalysis in non-associative phase separated system and Mg2+ partitioning in associative phase separated system. (A) Structure of the hammerhead ribozyme. Ribozyme catalysis was carried out in different dextran:PEG phase volumes. 1:0 (filled black circles), 1:5 (blue squares), 1:12.5 (red diamonds), 1:50 (blue triangles) and 1:100 (inverted green triangles).41 (B) Magnesium and other catalytic potentials inside complex coacervates. Magnesium associated with ATP is also partitioned inside the PAH-ATP coacervates.45
Mg2+ Partitioning Inside Coacervates.
It is well established that ribozymes and riboswitches often require Mg2+ or other metal ions for catalysis and folding.69–72 Interactions with components that form ATPS can concentrate metal ions,73 for example, iminodiacetic acid derivatized PEG has been used in PEG/Dextran ATPS to chelate copper and effectively extract heme proteins.74 Partitioning of Ca2+ ions in coacervates composed of elastin peptides have been known.75 Recent work has established that the condensed phase of PAH-ATP coacervate phase extensively partition Mg2+ ions.45 We found that Mg2+ ions in the condensed phase can reach concentrations in the order of ~1 M (Figure 6B). We reasoned that Mg2+ partitioned within the condensed phase while being coordinated by the phosphates of ATP.
Ribozymes and riboswitches often require Mg2+ ions for optimal activity.69–70,76 One of the most striking examples of the interplay between magnesium and ribozyme is the RNase P ribozyme, where both high (~0.2 mM) and low affinity (~3 mM) interactions are involved in catalysis.77 Magnesium ions are believed to stabilize the transition state and neutralize charges to assist in substrate association. While RNase P in bacteria and eukarya alike require associated proteins for function in vivo, bacterial RNase P RNA-alone is active at high magnesium concentrations in vitro78 with almost complete cleavage of precursor of bacterial ptRNAGly in 50 mM Mg2+.79 Increased Mg2+ concentration within the condensed phase of coacervates may have assisted the functional RNAs that would be non-functional in bulk solution with low concentration of required cations. Apart from the RNase P ribozyme, many other natural and artificial ribozymes show magnesium dependent increases in observed rate constants.80 Notably, high concentration of metals and salts in eutectic ice phase has been shown to assist in ribozyme catalyzed RNA polymerization.81
Apart from enhancement in ribozyme catalysis, hydrolysis of activated nucleotides used for non-enzymatic polymerization of RNA is also highly dependent on Mg2+ 82 and would disfavor function. It remains to be known how condensed phases rich in monomers, RNA primers and template, and Mg2+ conspire to affect the many rates and fidelity of non-enzymatic RNA polymerization.
Covalent and Non-covalent Control of Coacervation.
A key component of all of biology is regulation of function, turning on genes only when needed. The constituents of coacervates themselves can provide some level of control on formation and dissolution of these compartments. One of the most fundamental reactions in all domains of life is phosphoryl transfer, which is a common post-translational modification (PTM). Ribozymes with polynucleotide kinase activity have been discovered from multiple in vitro evolution experiments and they have been found to be dependent upon diverse metal ions.80,83–84 Furthermore, ribozyme-catalyzed covalent modification can selectively inactivate functional RNAs,85 analogous to differential protein regulation by PTMs. Notably, deoxyribozymes with kinase and phosphatase activities have also been isolated.86–87 Each phosphate group added to a substrate’s sidechains changes the charge density, thus affecting the propensity of phase separation.
While phase-separation through ribozyme/deoxyribozyme-mediated covalent modifications has not been explored yet, protein enzymes have been shown to actively induce or prevent phase separation through phosphoryl transfer. This has been demonstrated in coacervate systems made with polyU RNA and the cationic peptide RRASLRRASL.88 The phosphorylated form of this peptide, RRApSLRRApSL, cannot undergo coacervation with polyU RNA; coacervates form only after a phosphatase enzyme removes the two phosphates (Figure 7 A and B). This is because as the phosphate groups are lost from the peptide, there is a net increase in positive charge, which consequently increases interaction with the polyU RNA. Conversely, protein kinase A is able to transfer phosphoryl groups to unphosphorylated peptide RRASLRRASL causing a net reduction in positive charge, which weakens the polyelectrolyte interactions and results in dissolution of coacervates (Figure 7 C and D). Reversible coacervate systems of polylysine and ATP have also very recently been programmed by enzymatically converting between ATP, which could form coacervates with polylysine, and ADP, which could not.25 This system is notable in that both enzymes can remain active in the reaction mixture simultaneously, facilitating control over coacervate formation and dissolution.25
Figure 7:
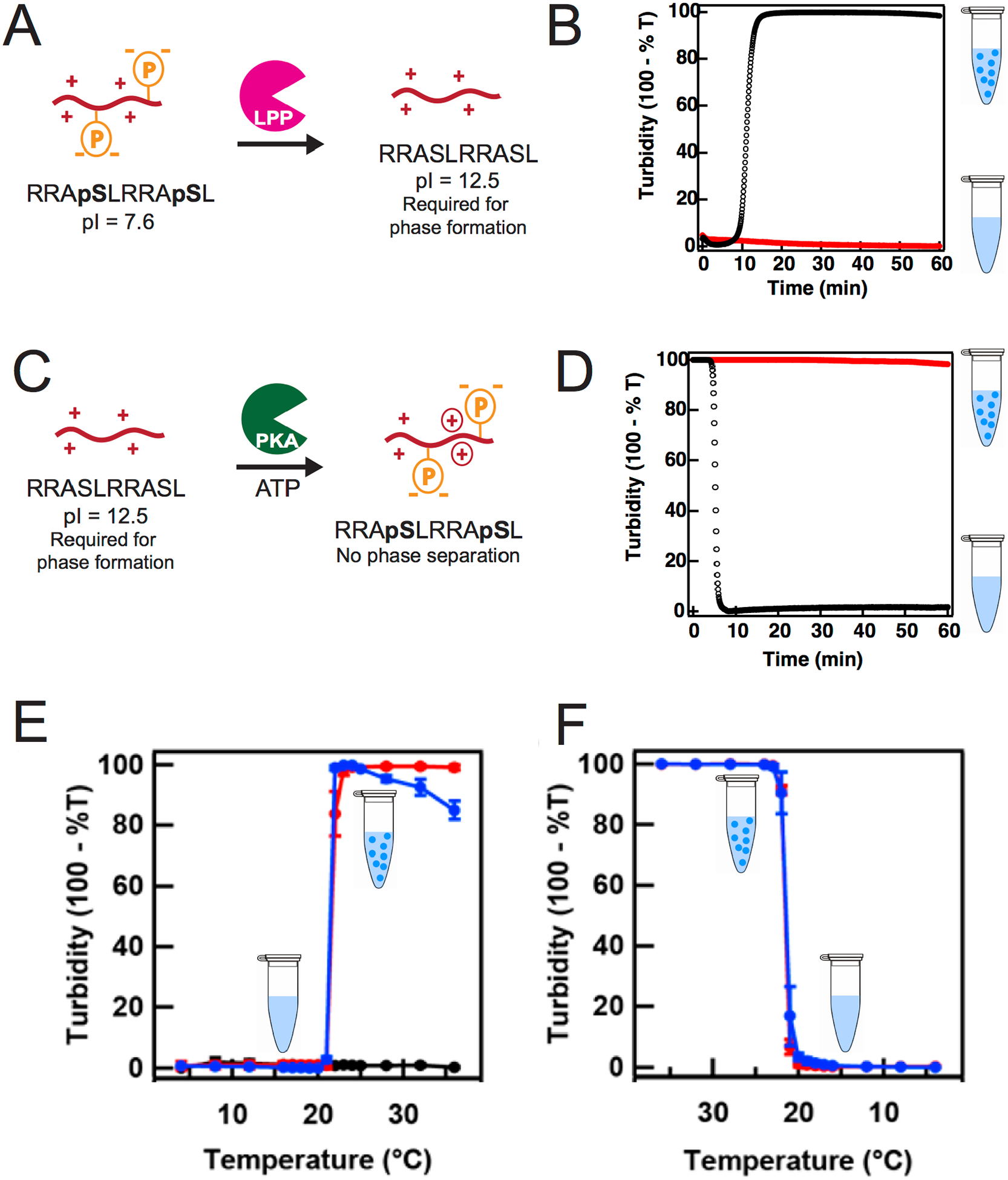
Molecular and environmental tuning of coacervates (A) and (B) Phosphatase enzyme increase the net positive charge by dephosphorylating RRASpLRRASpL peptide which forms coacervates with polyU RNA. (C) and (D) Kinase enzyme decrease the net positive charge by phosphorylating RRASLRRASL peptide and prevent coacervation with polyU RNA.88 For (B) and (D) appearance or disappearance of phase-separation is indicated by changes in turbidity measurements. Red trace indicates samples without any enzyme. (E) and (F) Turbidity plots indicating either the appearance or disappearance of PolyU RNA/ Spermine coacervates as a function of temperature.55
An important issue in protocells is protection of the biomolecules from degradation. Montmorillonite clay has been found to protect ribozymes from UV-irradiations.49 Interior of coacervates may also physically protect RNAs from factors that could destroy its integrity. This has been demonstrated for glycine and diglycine in coacervates made with partially sulfated and aminoacetylated polyvinyl alcohols.89 RNA molecules may be similarly protected from the insults in the bulk solution that would otherwise degrade or modify RNAs when inside coacervates.
While partitioning of RNA inside the condensed phase would have been advantageous for the RNA World, recycling and reshuffling of functional and non-functional RNA molecules is important too. Exchange of RNA53 and DNA90 molecules between coacervate droplets and dilute solutions have been shown. Dissolution of coacervates can readily release biomolecules into the bulk solution. This would allow specific functional RNAs to be taken up by other coacervates, where they can impart functions. Simple environmental changes such as temperature can significantly impact formation of coacervates. Recent example of this was demonstrated in the polyuridylic acid-spermine/spermidine coacervates,55 which showed a characteristic lower critical solution temperature (LCST) of ~20°C. Formation of coacervates were only observed at temperature greater than the LCST (Figure 7 E and F). Depending on the composition, coacervates can also have an upper critical solution temperature (UCST),91 above which they cannot phase separate. Dynamic coacervate systems92 that form or dissolve based on surrounding temperature could have been prevalent in early earth where temperature changes in diurnal cycle would have driven transient coacervation allowing coacervate-dependent functions of RNA such as ribozyme or aptamer functions, and then dissolve to allow these molecules to diffuse in the solution.
Coacervates and Catalytic Strategies.
All of the coacervate-forming molecules provided in Figure 3 have chemical moieties that can participate in one or several of the catalytic strategies described for naturally occurring self-cleaving ribozymes (Figure 8A)93. These catalytic strategies involve deprotonation of the O2′ nucleophile, ‘γ’; neutralization of the non-bridging oxygen (NBO) atoms of the scissile phosphate, ‘β’; protonation of the incipient oxyanion on the O5′, ‘δ’; and orientation of the in-line nucleophilic attack, ‘α’. Recently, we described two other catalytic strategies, which focus on activation of the O2′ nucleophile:94 a ‘γ′’ strategy that involves direct acidification of the O2′ by hydrogen bond donation, and a ‘γ″’ strategy that releases the O2′ nucleophile from inhibitory interactions (Figure 8A). These catalytic strategies can be facilitated by the components of the coacervates themselves. For example, arginine has the guanidinium group that resembles the N1-C2-N3 part of guanosine molecule that is involved in γ, γ′, and the γ″ catalytic strategies. On prebiotic earth, the nonenzymatic polymerization of RNA likely yielded some level of backbone heterogeneity, where some of the critical functional groups utilized for catalysis could have been mis-oriented; in such cases, components of the coacervates may have assisted in catalysis. These issues await further experimentation.
Figure 8:
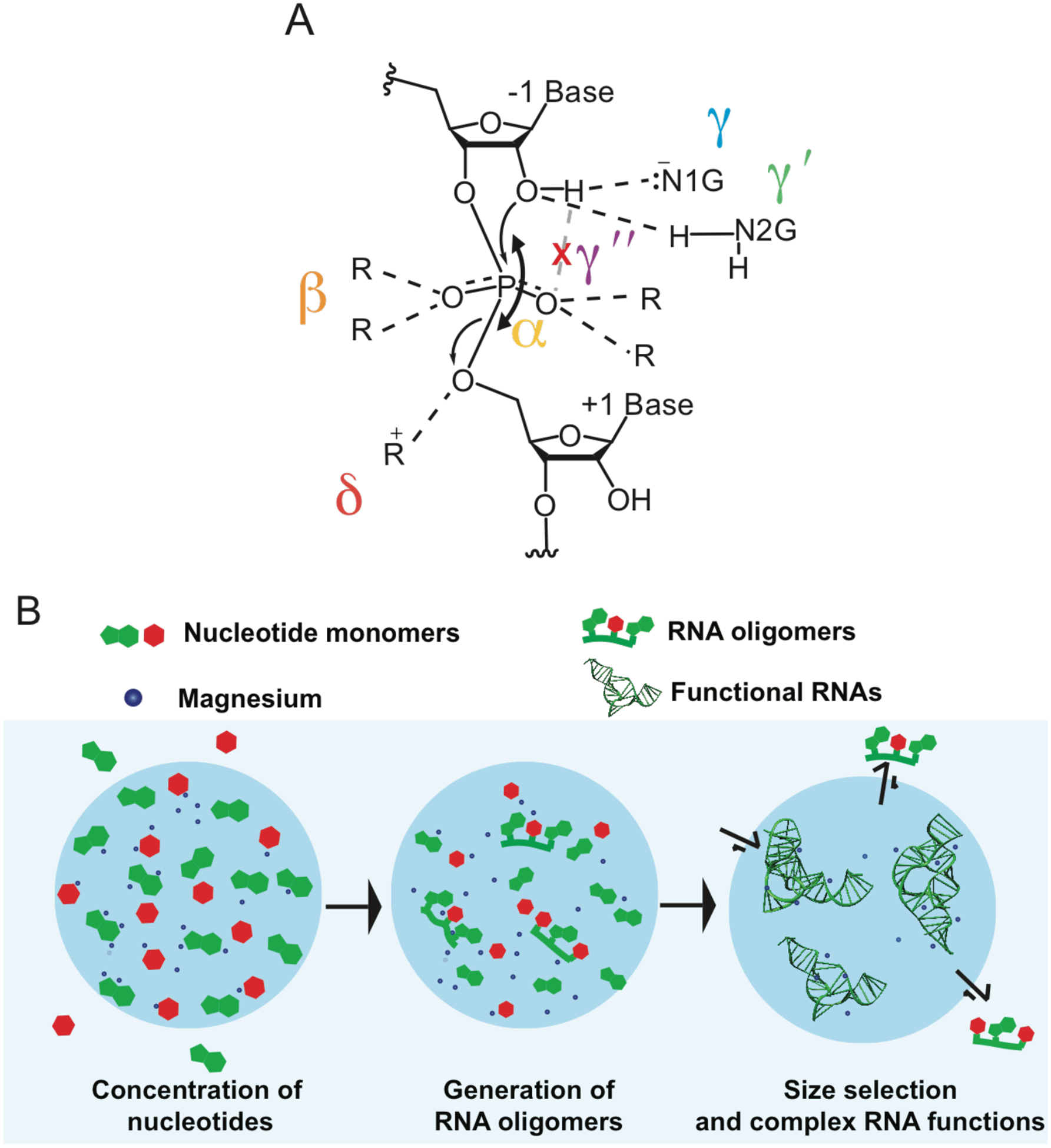
Potential contributions of coacervate components to prebiotic catalysis and evolution. (A) Several catalytic strategies used by naturally occurring self-cleaving ribozymes described in ref 94. (B) Scheme for coacervate-assisted RNA oligomerization and partitioning of larger RNAs.
Apart from coacervates enhancing catalytic potential of ribozymes, they could have also served as a basis for origin and selection of functional RNAs by taking advantage of the partitioning principles described above. A scheme for complex coacervate-based selection of longer and functional RNAs is shown in Figure 8B. As nucleotides concentrated within the coacervates, non-enzymatic polymerization of RNA would yield RNA oligonucleotides. Since the longer RNA molecules have more net charge, they would outcompete smaller oligonucleotides to remain inside complex coacervates. Because longer RNA molecules are more likely to fold into complex structures, functional RNA molecules such as aptamers and ribozymes would be more likely to originate within the condensed phase.
CONCLUSIONS
Phase-separated condensates are common in modern biology. While LLPS was implicated in origins of life on Earth by Oparin before the discovery of the genetic code and the RNA World hypothesis, relevance of such systems in prebiotic chemistry remains largely unexplored. Unique microenvironments that concentrate specific biomolecules are provided by LLPS. Both of these characteristics would have been beneficial during the origins of life on Earth where molecules would have been otherwise dilute and unfolded. As a later step to modern cells, which contain semi-permeable membranes, complex coacervates could have interacted with other prebiotic compartments, ultimately forming outer coatings at the liquid-liquid interface. For example, Mann and coworkers reported assembly of multilamellar fatty acid membranes around PDADMAC/ATP and oligolysine/RNA coacervates,95 and we have observed self-assembly of pre-formed liposomes at the surface of spermine/polyU RNA coacervate droplets.55
Since RNA molecules are highly polyanionic, they make excellent counterions for cationic polymers that are involved in coacervation. Strikingly, prebiotic syntheses of some RNA monomers has already been demonstrated;96–97 furthermore, oligonucleotides of RNA have been generated from mononucleotides in wet and dry cycles.98 Therefore, LLPS involving RNA molecules could have been highly relevant during the RNA World. Phase-separation-assisted synthesis of RNA oligonucleotides, selection of longer RNA molecules by partitioning, enhancement of ribozyme catalysis, and protection from a harsh and unfavorable external environment are all plausible roles for LLPS in the primordial Earth. Further investigations of RNA-containing LLPS systems in extant biology and simulated early Earth conditions promise to unravel new insights into the interplay between LLPS and RNA structure-functions.
Funding information
This work was supported by grants 393327 from Simons Foundation (R.R.P) and 80NSSC17K0034 from NASA Exobiology Program (F.P.C, C.D.K and P.C.B).
References
- 1.Bungenberg-de Jong HG; Kruyt HR (1929) Coacervation (partial miscibility in colloid systems). [Google Scholar]
- 2.Oparin AI, Origin of life. Macmillan: New York, 1938. [Google Scholar]
- 3.Lazcano A (2010) Historical development of origins research. Cold Spring Harb Perspect Biol 2, a002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brangwynne CP; Eckmann CR; Courson DS; Rybarska A; Hoege C; Gharakhani J; Julicher F; Hyman AA (2009) Germline p granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732. [DOI] [PubMed] [Google Scholar]
- 5.Brangwynne CP; Mitchison TJ; Hyman AA (2011) Active liquid-like behavior of nucleoli determines their size and shape in xenopus laevis oocytes. Proc Natl Acad Sci U S A 108, 4334–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin Y; Brangwynne CP (2017) Liquid phase condensation in cell physiology and disease. Science 357. [DOI] [PubMed] [Google Scholar]
- 7.(1962) Albertsson PA, partition of cell particles and macromolecules International publishers printers & booksellers, stockholm 1960. 231 s., preis sw.Kr. 35; —. Fette, Seifen, Anstrichmittel 64, 701–702. [Google Scholar]
- 8.Spruijt E; Westphal AH; Borst JW; Cohen Stuart MA; van der Gucht J (2010) Binodal compositions of polyelectrolyte complexes. Macromolecules 43, 6476–6484. [Google Scholar]
- 9.Keating CD (2012) Aqueous phase separation as a possible route to compartmentalization of biological molecules. Acc Chem Res 45, 2114–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costantini S; Sharma A; Raucci R; Costantini M; Autiero I; Colonna G (2013) Genealogy of an ancient protein family: The sirtuins, a family of disordered members. BMC Evol Biol 13, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pohorille A; Wilson MA; Shannon G (2017) Flexible proteins at the origin of life. Life (Basel) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokuriki N; Tawfik DS (2009) Protein dynamism and evolvability. Science 324, 203–207. [DOI] [PubMed] [Google Scholar]
- 13.Mitrea DM; Kriwacki RW (2016) Phase separation in biology; functional organization of a higher order. Cell Commun Signal 14, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engreitz JM; Ollikainen N; Guttman M (2016) Long non-coding rnas: Spatial amplifiers that control nuclear structure and gene expression. Nat Rev Mol Cell Biol 17, 756–770. [DOI] [PubMed] [Google Scholar]
- 15.Mitrea DM; Cika JA; Guy CS; Ban D; Banerjee PR; Stanley CB; Nourse A; Deniz AA; Kriwacki RW (2016) Nucleophosmin integrates within the nucleolus via multi-modal interactions with proteins displaying r-rich linear motifs and rrna. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(1995) Zaslavsky BY: Aqueous two-phase partitioning - physical chemistry and bioanalytical applications, marcel dekker, inc., new york, basel, oxford, isbn 0-8247-9461-3, 1995. Berichte der Bunsengesellschaft für physikalische Chemie 99, 694–694. [Google Scholar]
- 17.Wohlfarth C, Handbook of phase equilibria and thermodynamic data of aqueous polymer solutions 2012. [Google Scholar]
- 18.Aqueous two-phase systems. 1994; Vol. 228. [Google Scholar]
- 19.Long MS; Jones CD; Helfrich MR; Mangeney-Slavin LK; Keating CD (2005) Dynamic microcompartmentation in synthetic cells. Proc Natl Acad Sci U S A 102, 5920–5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long MS; Cans AS; Keating CD (2008) Budding and asymmetric protein microcompartmentation in giant vesicles containing two aqueous phases. J Am Chem Soc 130, 756–762. [DOI] [PubMed] [Google Scholar]
- 21.Elbaum-Garfinkle S; Kim Y; Szczepaniak K; Chen CC; Eckmann CR; Myong S; Brangwynne CP (2015) The disordered p granule protein laf-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc Natl Acad Sci U S A 112, 7189–7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das RK; Ruff KM; Pappu RV (2015) Relating sequence encoded information to form and function of intrinsically disordered proteins. Curr Opin Struct Biol 32, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brangwynne CP; Tompa P; Pappu RV (2015) Polymer physics of intracellular phase transitions. Nat Phys 11, 899–904. [Google Scholar]
- 24.Fu J; Fares HM; Schlenoff JB (2017) Ion-pairing strength in polyelectrolyte complexes. Macromolecules 50, 1066–1074. [Google Scholar]
- 25.Nakashima KK; Baaij JF; Spruijt E (2017) Reversible generation of coacervate droplets in an enzymatic network. Soft Matter. [DOI] [PubMed] [Google Scholar]
- 26.Chang LW; Lytle TK; Radhakrishna M; Madinya JJ; Velez J; Sing CE; Perry SL (2017) Sequence and entropy-based control of complex coacervates. Nat Commun 8, 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chollakup R; Smitthipong W; Eisenbach CD; Tirrell M (2010) Phase behavior and coacervation of aqueous poly(acrylic acid)−poly(allylamine) solutions. Macromolecules 43, 2518–2528. [Google Scholar]
- 28.Wang QF; Schlenoff JB (2014) The polyelectrolyte complex/coacervate continuum. Macromolecules 47, 3108–3116. [Google Scholar]
- 29.Koga S; Williams DS; Perriman AW; Mann S (2011) Peptide-nucleotide microdroplets as a step towards a membrane-free protocell model. Nat Chem 3, 720–724. [DOI] [PubMed] [Google Scholar]
- 30.Nott TJ; Petsalaki E; Farber P; Jervis D; Fussner E; Plochowietz A; Craggs TD; Bazett-Jones DP; Pawson T; Forman-Kay JD; Baldwin AJ (2015) Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol Cell 57, 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pak CW; Kosno M; Holehouse AS; Padrick SB; Mittal A; Ali R; Yunus AA; Liu DR; Pappu RV; Rosen MK (2016) Sequence determinants of intracellular phase separation by complex coacervation of a disordered protein. Mol Cell 63, 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wheeler JR; Matheny T; Jain S; Abrisch R; Parker R (2016) Distinct stages in stress granule assembly and disassembly. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel A; Lee HO; Jawerth L; Maharana S; Jahnel M; Hein MY; Stoynov S; Mahamid J; Saha S; Franzmann TM; Pozniakovski A; Poser I; Maghelli N; Royer LA; Weigert M; Myers EW; Grill S; Drechsel D; Hyman AA; Alberti S (2015) A liquid-to-solid phase transition of the als protein fus accelerated by disease mutation. Cell 162, 1066–1077. [DOI] [PubMed] [Google Scholar]
- 34.Banani SF; Rice AM; Peeples WB; Lin Y; Jain S; Parker R; Rosen MK (2016) Compositional control of phase-separated cellular bodies. Cell 166, 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonicka H; Shoubridge EA (2015) Mitochondrial RNA granules are centers for posttranscriptional RNA processing and ribosome biogenesis. Cell Rep. [DOI] [PubMed] [Google Scholar]
- 36.Molliex A; Temirov J; Lee J; Coughlin M; Kanagaraj AP; Kim HJ; Mittag T; Taylor JP (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Y; Protter DS; Rosen MK; Parker R (2015) Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol Cell 60, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marianelli AM; Miller BM; Keating CD (2017) Impact of macromolecular crowding on RNA/spermine complex coacervation and oligonucleotide compartmentalization. Soft Matter. [DOI] [PubMed] [Google Scholar]
- 39.Leamy KA; Assmann SM; Mathews DH; Bevilacqua PC (2016) Bridging the gap between in vitro and in vivo RNA folding. Q Rev Biophys 49, e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groen J; Foschepoth D; te Brinke E; Boersma AJ; Imamura H; Rivas G; Heus HA; Huck WT (2015) Associative interactions in crowded solutions of biopolymers counteract depletion effects. J Am Chem Soc 137, 13041–13048. [DOI] [PubMed] [Google Scholar]
- 41.Strulson CA; Molden RC; Keating CD; Bevilacqua PC (2012) RNA catalysis through compartmentalization. Nat Chem 4, 941–946. [DOI] [PubMed] [Google Scholar]
- 42.Nott TJ; Craggs TD; Baldwin AJ (2016) Membraneless organelles can melt nucleic acid duplexes and act as biomolecular filters. Nat Chem 8, 569–575. [DOI] [PubMed] [Google Scholar]
- 43.Davis BW; Aumiller WM Jr.; Hashemian N; An S; Armaou A; Keating CD (2015) Colocalization and sequential enzyme activity in aqueous biphasic systems: Experiments and modeling. Biophys J 109, 2182–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cacace DN; Keating CD (2013) Biocatalyzed mineralization in an aqueous two-phase system: Effect of background polymers and enzyme partitioning. J Mater Chem B 1, 1794–1803. [DOI] [PubMed] [Google Scholar]
- 45.Frankel EA; Bevilacqua PC; Keating CD (2016) Polyamine/nucleotide coacervates provide strong compartmentalization of mg2+, nucleotides, and RNA. Langmuir 32, 2041–2049. [DOI] [PubMed] [Google Scholar]
- 46.Wei MT; Elbaum-Garfinkle S; Holehouse AS; Chen CC; Feric M; Arnold CB; Priestley RD; Pappu RV; Brangwynne CP (2017) Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat Chem 9, 1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyers RA, Synthetic biology (current topics from the encyclopedia of molecular cell biology and molecular medicine). Wiley-VCH Verlag GmbH & Co. KGaA: 2015. [Google Scholar]
- 48.Wheeldon I; Minteer SD; Banta S; Barton SC; Atanassov P; Sigman M (2016) Substrate channelling as an approach to cascade reactions. Nat Chem 8, 299–309. [DOI] [PubMed] [Google Scholar]
- 49.Biondi E; Branciamore S; Maurel MC; Gallori E (2007) Montmorillonite protection of an uv-irradiated hairpin ribozyme: Evolution of the RNA world in a mineral environment. BMC Evol Biol 7 Suppl 2, S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffith EC; Carpenter BK; Shoemaker RK; Vaida V (2013) Photochemistry of aqueous pyruvic acid. Proc Natl Acad Sci U S A 110, 11714–11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adamala K; Szostak J (2013) Nonenzymatic template-directed RNA synthesis inside model protocells. Science 432, 1098–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou HX; Rivas G; Minton AP (2008) Macromolecular crowding and confinement: Biochemical, biophysical, and potential physiological consequences. Annu Rev Biophys 37, 375–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia TZ; Hentrich C; Szostak JW (2014) Rapid RNA exchange in aqueous two-phase system and coacervate droplets. Orig Life Evol Biosph 44, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Swaay D; Tang TY; Mann S; de Mello A (2015) Microfluidic formation of membrane-free aqueous coacervate droplets in water. Angew Chem Int Ed Engl 54, 8398–8401. [DOI] [PubMed] [Google Scholar]
- 55.Aumiller WM Jr.; Pir Cakmak F; Davis BW; Keating CD (2016) RNA-based coacervates as a model for membraneless organelles: Formation, properties, and interfacial liposome assembly. Langmuir 32, 10042–10053. [DOI] [PubMed] [Google Scholar]
- 56.Joyce GF (2002) The antiquity of RNA-based evolution. Nature 418, 212–221. [DOI] [PubMed] [Google Scholar]
- 57.Chen X; Li N; Ellington AD (2007) Ribozyme catalysis of metabolism in the RNA world. Chem Biodivers 4, 633–655. [DOI] [PubMed] [Google Scholar]
- 58.Krishnamurthy R (2017) Giving rise to life: Transition from prebiotic chemistry to protobiology. Acc Chem Res 50, 455–459. [DOI] [PubMed] [Google Scholar]
- 59.Jencks WP, Catalysis in chemistry and enzymology. Dover Publications Inc.: New York, 1969. [Google Scholar]
- 60.Adamala K; Engelhart AE; Szostak JW (2015) Generation of functional rnas from inactive oligonucleotide complexes by non-enzymatic primer extension. J Am Chem Soc 137, 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prywes N; Blain JC; Del Frate F; Szostak JW (2016) Nonenzymatic copying of RNA templates containing all four letters is catalyzed by activated oligonucleotides. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lahav N; White D; Chang S (1978) Peptide formation in the prebiotic era: Thermal condensation of glycine in fluctuating clay environments. Science 201, 67–69. [DOI] [PubMed] [Google Scholar]
- 63.Hazen RM; Sverjensky DA (2010) Mineral surfaces, geochemical complexities, and the origins of life. Cold Spring Harb Perspect Biol 2, a002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erastova V; Degiacomi MT; D GF; Greenwell HC (2017) Mineral surface chemistry control for origin of prebiotic peptides. Nat Commun 8, 2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szostak JW (2012) The eightfold path to non-enzymatic RNA replication. J Syst Chem 3, 2. [Google Scholar]
- 66.Strulson CA; Boyer JA; Whitman EE; Bevilacqua PC (2014) Molecular crowders and cosolutes promote folding cooperativity of RNA under physiological ionic conditions. RNA 20, 331–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leamy KA; Yennawar NH; Bevilacqua PC (2017) Cooperative RNA folding under cellular conditions arises from both tertiary structure stabilization and secondary structure destabilization. Biochemistry 56, 3422–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He C; Gallego I; Laughlin B; Grover MA; Hud NV (2017) A viscous solvent enables information transfer from gene-length nucleic acids in a model prebiotic replication cycle. Nat Chem 9, 318–324. [DOI] [PubMed] [Google Scholar]
- 69.Johnson-Buck AE; McDowell SE; Walter NG (2011) Metal ions: Supporting actors in the playbook of small ribozymes. Met Ions Life Sci 9, 175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schnabl J; Sigel RK (2010) Controlling ribozyme activity by metal ions. Curr Opin Chem Biol 14, 269–275. [DOI] [PubMed] [Google Scholar]
- 71.Nakano S.-i.; Proctor DJ; Bevilacqua PC (2001) Mechanistic characterization of the hdv genomic ribozyme: Assessing the catalytic and structural contributions of divalent metal ions within a multichannel reaction mechanism†. Biochemistry 40, 12022–12038. [DOI] [PubMed] [Google Scholar]
- 72.Denesyuk NA; Thirumalai D (2015) How do metal ions direct ribozyme folding? Nat Chem 7, 793–801. [DOI] [PubMed] [Google Scholar]
- 73.Rogers RD; Bond AH; Bauer CB; Zhang J; Griffin ST (1996) Metal ion separations in polyethylene glycol-based aqueous biphasic systems: Correlation of partitioning behavior with available thermodynamic hydration data. J Chromatogr B Biomed Appl 680, 221–229. [DOI] [PubMed] [Google Scholar]
- 74.Wuenschell GE; Naranjo E; Arnold FH (1990) Aqueous two-phase metal affinity extraction of heme proteins. Bioprocess Engineering 5, 199–202. [Google Scholar]
- 75.Urry DW (1976) On the molecular mechanisms of elastin coacervation and coacervate calcification. Faraday Discussions of the Chemical Society 61, 205. [DOI] [PubMed] [Google Scholar]
- 76.Bowman JC; Lenz TK; Hud NV; Williams LD (2012) Cations in charge: Magnesium ions in RNA folding and catalysis. Curr Opin Struct Biol 22, 262–272. [DOI] [PubMed] [Google Scholar]
- 77.Beebe JA; Kurz JC; Fierke CA (1996) Magnesium ions are required by bacillus subtilis ribonuclease p RNA for both binding and cleaving precursor trnaasp. Biochemistry 35, 10493–10505. [DOI] [PubMed] [Google Scholar]
- 78.Pace NR; Brown JW (1995) Evolutionary perspective on the structure and function of ribonuclease p, a ribozyme. J Bacteriol 177, 1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Warnecke JM; Held R; Busch S; Hartmann RK (1999) Role of metal ions in the hydrolysis reaction catalyzed by rnase p RNA from bacillus subtilis. J Mol Biol 290, 433–445. [DOI] [PubMed] [Google Scholar]
- 80.Biondi E; Poudyal RR; Forgy JC; Sawyer AW; Maxwell AW; Burke DH (2013) Lewis acid catalysis of phosphoryl transfer from a copper(ii)-ntp complex in a kinase ribozyme. Nucleic Acids Res 41, 3327–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Attwater J; Wochner A; Holliger P (2013) In-ice evolution of RNA polymerase ribozyme activity. Nat Chem 5, 1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kanavarioti A; Bernasconi CF; Doodokyan DL; Alberas DJ (1989) Magnesium ion catalyzed phosphorus-nitrogen bond hydrolysis in imidazolide-activated nucleotides. Relevance to template-directed synthesis of polynucleotides. J Am Chem Soc 111, 7247–7257. [DOI] [PubMed] [Google Scholar]
- 83.Lorsch JR; Szostak JW (1994) In vitro evolution of new ribozymes with polynucleotide kinase activity. Nature 371, 31–36. [DOI] [PubMed] [Google Scholar]
- 84.Poudyal RR; Nguyen PD; Lokugamage MP; Callaway MK; Gavette JV; Krishnamurthy R; Burke DH (2017) Nucleobase modification by an RNA enzyme. Nucleic Acids Res 45, 1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poudyal RR; Benslimane M; Lokugamage MP; Callaway MK; Staller S; Burke DH (2017) Selective inactivation of functional rnas by ribozyme-catalyzed covalent modification. ACS Synth Biol 6, 528–534. [DOI] [PubMed] [Google Scholar]
- 86.Chandrasekar J; Silverman SK (2013) Catalytic DNA with phosphatase activity. Proc Natl Acad Sci U S A 110, 5315–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walsh SM; Sachdeva A; Silverman SK (2013) DNA catalysts with tyrosine kinase activity. J Am Chem Soc 135, 14928–14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aumiller WM Jr.; Keating CD (2016) Phosphorylation-mediated RNA/peptide complex coacervation as a model for intracellular liquid organelles. Nat Chem 8, 129–137. [DOI] [PubMed] [Google Scholar]
- 89.Okihana H; Ponnamperuma C (1982) Functions of the coacervate droplets. Orig Life Evol Biosph 12, 347–353. [Google Scholar]
- 90.Vieregg JR; Lueckheide M; Marciel AB; Leon L; Bologna AJ; Rivera JR; Tirrell MV (2018) Oligonucleotide - peptide complexes: Phase control by hybridization. J Am Chem Soc. [DOI] [PubMed] [Google Scholar]
- 91.Noh M; Kang S; Mok Y; Choi SJ; Park J; Kingma J; Seo JH; Lee Y (2016) Upper critical solution temperature (ucst) phase transition of halide salts of branched polyethylenimine and methylated branched polyethylenimine in aqueous solutions. Chem Commun (Camb) 52, 509–512. [DOI] [PubMed] [Google Scholar]
- 92.Swanson JP; Monteleone LR; Haso F; Costanzo PJ; Liu T; Joy A (2015) A library of thermoresponsive, coacervate-forming biodegradable polyesters. Macromolecules 48, 3834–3842. [Google Scholar]
- 93.Breaker RR; Emilsson GM; Lazarev D; Nakamura S; Puskarz IJ; Roth A; Sudarsan N (2003) A common speed limit for RNA-cleaving ribozymes and deoxyribozymes. RNA 9, 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seith DD; Bingaman JL; Veenis AJ; Button AC; Bevilacqua PC (2017) Elucidation of catalytic strategies of small nucleolytic ribozymes from comparative analysis of active sites. ACS Catalysis 8, 314–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dora Tang TY; Rohaida Che Hak C; Thompson AJ; Kuimova MK; Williams DS; Perriman AW; Mann S (2014) Fatty acid membrane assembly on coacervate microdroplets as a step towards a hybrid protocell model. Nat Chem 6, 527–533. [DOI] [PubMed] [Google Scholar]
- 96.Powner MW; Gerland B; Sutherland JD (2009) Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459, 239–242. [DOI] [PubMed] [Google Scholar]
- 97.Stairs S; Nikmal A; Bucar DK; Zheng SL; Szostak JW; Powner MW (2017) Divergent prebiotic synthesis of pyrimidine and 8-oxo-purine ribonucleotides. Nat Commun 8, 15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Da Silva L; Maurel MC; Deamer D (2015) Salt-promoted synthesis of RNA-like molecules in simulated hydrothermal conditions. J Mol Evol 80, 86–97. [DOI] [PubMed] [Google Scholar]


