Clinical Implications.
-
•
Hydroxychloroquine is now commonly used off-label for the treatment of COVID-19 in combination with drugs, at doses and in populations where it is not typically used. We present a case that highlights that even in short course therapy acute generalized exanthematous pustulosis should be recognized as a potential adverse effect of hydroxychloroquine.
Acute generalized exanthematous pustulosis (AGEP) is a rare drug reaction characterized by acute, extensive formation of numerous nonfollicular sterile pustules on a background of edematous erythema.1 Hydroxychloroquine (HCQ) is widely used to treat rheumatic and dermatologic diseases, and is well known to cause AGEP.1 At the end of 2019, a novel coronavirus was identified as the cause of a cluster of pneumonia cases in Wuhan, city of China. It rapidly spread in China and outside, and on March 12, 2020, the World Health Organization declared a pandemic. HCQ has been reported to inhibit SARS-CoV-2 in vitro, but clinical data evaluating HCQ are limited, and its efficacy against SARS-CoV-2 is unknown. Nevertheless, given the lack of clearly effective interventions, HCQ is being used off-label in combination with drugs, at doses and in populations where it would not be traditionally used. Therefore, vigilance needs to be applied especially if this drug is not being used in clinical trial settings where adverse-event information and monitoring are more meticulous. Herein we report a case of AGEP induced by HCQ prescribed for COVID-19. We also reviewed literature about HCQ-induced AGEP and efficacy of HCQ in COVID-19.
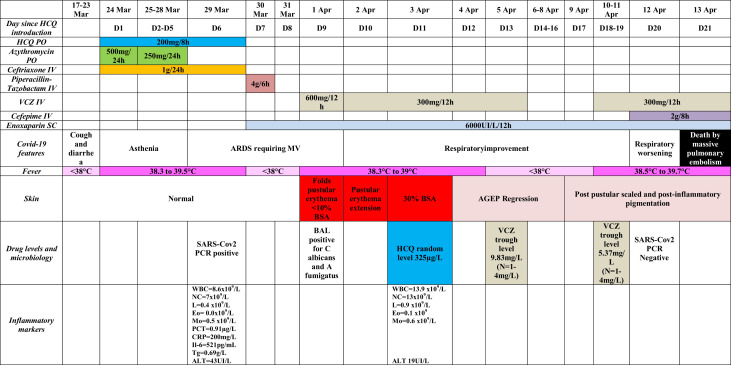
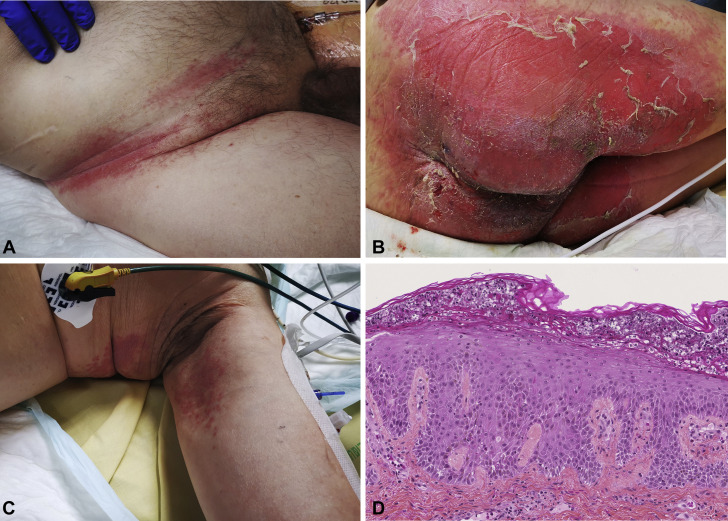
On March 23, a 76-year-old patient with a medical history of diabetes mellitus consulted the emergency department for cough and diarrhea since March 17. Chest computerized tomography scan revealed bilateral patchy ground glass opacities consistent with COVID-19 disease. He did not present with any severity criteria and returned home. The day after, clinical symptoms worsened with asthenia, fever, and dyspnea. Thus, on March 24, HCQ (200 mg 3 times daily) was introduced associated with azithromycin and ceftriaxone (Figure 1 ). On March 29, his condition worsened with acute respiratory distress syndrome (ARDS). He required invasive mechanical ventilation, and he was transferred to the intensive care unit. HCQ was stopped after a cumulative dose of HCQ of 3600 mg. The SARS-Cov-2 real-time polymerase chain reaction test from the nasopharynx was positive. He received bronchoscopy with bronchoalveolar lavage that identified Aspergillus fumigatus and Candida albicans. Screening for other respiratory microbes (bacteria, fungi, mycobacteria, and viruses) was negative (ARDS-infected patients with COVID-19 have frequent bacterial and fungal superinfection).2 The patient did not take any corticosteroid during his clinical course. Although COVID-19 improved with weaning of mechanical ventilation, the patient developed on April 3 a pustular eruption on a background of edematous erythema of 2 days' duration, which began on intertriginous areas (intergluteal, axillary, and inguinal) and rapidly affected 30% of body surface area (Figure 2 , A-C). Oral and genital mucosas were normal. Diagnosis of AGEP, symmetrical drug-related intertriginous and flexural exanthema (SDRIFE), and staphylococcal scaled skin syndrome (SSSS) was suggested. In parallel, fever was noticed and laboratory tests showed an increased leukocytosis with marked neutrophilia (from 7 × 109/L on March 29 to 13 × 109/L on April 3). HCQ dosage was 325 μg/L. Pustular smear and culture were negative for bacteria and fungus. Cultures from common sites of Staphylococcus aureus colonization and blood cultures were negative too, excluding SSSS diagnosis. A skin biopsy showed spongiform subcorneal and intracorneal pustules, some keratinocyte necrosis, and a dermal inflammatory infiltrate of neutrophils with perivascular accentuation, excluding SDRIFE diagnosis (Figure 2, C). Thus the diagnosis of AGEP was made, with a RegiSCAR score calculated to 11 (definite case >8),1 based on the rapid development of a febrile pustular eruption a few days after beginning a drug treatment, the clinical finding of pustules on a background of edematous erythema with flexural accentuation, a marked neutrophilia (>7 × 109/L), a pustular smear and culture negative for microbes, a resolution of the rash after drug discontinuation, and histologic features including intracorneal spongiform pustules and some necrotic keratinocytes. The rash was already present the day when voriconazole was started and 24 hours after the last dose of piperacillin-tazobactam (Figure 1), suggesting that these were less suggestive of the culprit drug compared with HCQ. The patient eventually died from massive pulmonary embolism 10 days after the AGEP diagnosis.
Figure 1.
Anamnestic, clinical, and biological features of the patient. AGEP, Acute generalized exanthematous pustulosis; ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; BAL, bronchoalveolar lavage; BSA, body surface area; CRP, C-reactive protein; Eo, eosinophil count; HCQ, hydroxychloroquine; IL-6, interleukin-6; IV, intravenous; L, lymphocyte count; Mo, monocyte count; MV, mechanical ventilation; N, normal range; NC, neutrophil count; PCR, polymerase chain reaction; PCT, procalcitonin; PO, per-os; SC, subcutaneous; Tg, triglycerides; VCZ, voriconazole; WBC, white blood cells.
Figure 2.
Clinical and pathological presentation of AGEP induced by HCQ. A-C, Several small pustules arising on a widespread erythema with typical flexural accentuation of AGEP. D, Histopathological features of the skin biopsy include spongiform subcorneal and intracorneal neutrophilic pustule, acanthosis, neutrophilic exocytosis, and rare necrotic keratinocytes (hematoxylin and eosin, ×180 magnification). AGEP, Acute generalized exanthematous pustulosis; HCQ, hydroxychloroquine.
HCQ has numerous skin side effects including maculopapular rash, cutaneous hyperpigmentation, pruritus, AGEP, Stevens-Johnson syndrome or toxic epidermal necrolysis, hair loss, and stomatitis, as previously reported.3, 4, 5 In AGEP, the average duration of drug exposure before onset of the symptoms depends on the causative drug. Antibiotics such as amoxicillin consistently have a short latency of 24 to 72 hours, whereas other medications, including HCQ, are often associated with latencies around 10 to 12 days or longer (16.2 days for HCQ in our review).6 The latency period of 9 days in our patient was shorter than described. The dysregulation of the Th17 pathway observed in cytokine storm induced by COVID-19 may explain a shorter delay of AGEP induced by HCQ. The PubMed database was searched for all peer-reviewed articles published until April 2020 using the following search terms: “hydroxychloroquine” and “acute generalized exanthematous pustulosis,” and found 35 cases (Table E1, available in this article's Online Repository at www.jaci-inpractice.org).
Use of HCQ is included in Chinese treatment guidelines and was reportedly associated with reduced disease progression. However, data supporting these claims are controversial. A randomized trial of 2 different doses of HCQ in 62 patients with COVID-19 reported a better outcome with higher doses.7 However, the endpoints specified in the protocol differed from those reported, and the trial seemed to have stopped prematurely. In an open-label study of 36 patients with COVID-19, treatment with azithromycin and HCQ was associated with a more rapid decline in viral RNA.8 However, there were methodological concerns about the control groups, and another observational study did not confirm these findings.9 In addition, in an observational study of nearly 1400 patients with COVID-19 admitted to a hospital in New York, HCQ use was reported in 811 patients and was associated with a higher risk of intubation or death (hazard ratio, 2.37).10 Despite these facts, some clinicians argued that HCQ is widely used and safe. Furthermore, amid the speculation regarding the beneficial roles of HCQ in COVID-19, shortages are feared. A shortage in HCQ would create serious problems for people with systemic lupus among others who are currently taking this drug. To conclude, AGEP should be included in the potential side effects of HCQ for the treatment of COVID-19.
Acknowledgments
Saint-Louis CORE (COvid REsearch) group: Archer G, Benattia A, Bergeron A, Bondeelle L, Bouaziz JD, Bouda D, Boutboul D, Brindel Berthon I, Bugnet E, Caillat Zucman S, Cassonnet S, Celli Lebras K, Chabert J, Chevret S, Clément M, Davoine C, De Castro N, De Kerviler E, De Margerie-Mellon C, Delaugerre C, Depret F, Denis B, Djaghout L, Dupin C, Farge-Bancel D, Fauvaux C, Feredj E, Feyeux D, Fontaine JP, Fremeaux-Bacchi V, Galicier L, Harel S, Jegu AL, Kozakiewicz E, Lebel M, Baye A, Le Goff J, Le Guen P, Lengline E, Liegeon G, Lorillon G, Madelaine Chambrin I, Martin de Frémont G, Meunier M, Molina JM, Morin F, Oksenhendler E, Peffault de la Tour R, Peyrony O, Plaud B, Salmona M, Saussereau J, Soret J.
Footnotes
No funding was received for this work.
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Contributor Information
Saint-Louis CORE (COvid REsearch) group:
G. Archer, A. Benattia, A. Bergeron, L. Bondeelle, J.D. Bouaziz, D. Bouda, D. Boutboul, Berthon I. Brindel, E. Bugnet, S. Caillat Zucman, S. Cassonnet, K. Celli Lebras, J. Chabert, S. Chevret, M. Clément, C. Davoine, N. De Castro, E. De Kerviler, C. De Margerie-Mellon, C. Delaugerre, F. Depret, B. Denis, L. Djaghout, C. Dupin, D. Farge-Bancel, C. Fauvaux, E. Feredj, D. Feyeux, J.P. Fontaine, V. Fremeaux-Bacchi, L. Galicier, S. Harel, Jegu AL, E. Kozakiewicz, M. Lebel, A. Baye, J. Le Goff, P. Le Guen, E. Lengline, G. Liegeon, G. Lorillon, I. Madelaine Chambrin, G. Martin de Frémont, M. Meunier, J.M. Molina, F. Morin, E. Oksenhendler, R. Peffault de la Tour, O. Peyrony, B. Plaud, M. Salmona, J. Saussereau, and J. Soret
Online Repository.
Table E1.
Reported cases of AGEP induced by hydroxychloroquine
| Year | First author | Study design | Sample size | Age | Sex | Underlying condition | Latency (d) | Approximative cumulative dose (mg) | Adverse reaction |
|---|---|---|---|---|---|---|---|---|---|
| 1996 | Assier-Bonnet | Case report | 1 | 36 | Female | Seronegative arthritis | 12 | 2400 | AGEP |
| 1996 | Vine | Case report | 1 | 38 | Female | Arthralgia | 21 | 4200 | Pustular psoriasis |
| 2004 | Evans | Case report | 1 | 28 | Female | SLE | 14 | 5600 | AGEP |
| 2004 | Welsch | Case report | 1 | MD | Female | Leukocytoclastic vasculitis | MD | MD | Pustular psoriasis |
| 2007 | Sidoroff | Retrospective case control study | 7 | 56 ± 21 | 6 Females, 1 Male | MD | Most cases between 10 and 12 | MD | AGEP |
| 2008 | Paradise | Case series | 3 | 36, 70, 79 | 2 Females, 1 Male | RA + SS, RA, PR | 21, 20, and 20 | 4200 | AGEP |
| 2009 | Di Lernia | Case report | 1 | 63 | Female | RA | 30 | 4000 | Recalcitrant AGEP |
| 2009 | Avram | Case report | 1 | 79 | Female | RA | 14 | MD | AGEP |
| 2009 | Lateef | Case report | 1 | 67 | Female | SLE | 21 | MD | Overlap TEN/AGEP |
| 2010 | Park | Case report | 1 | 38 | Female | DM | 21 | 4200 | AGEP |
| 2013 | Bailey | Case report | 1 | 48 | Female | SLE | 14 | 2800 | AGEP |
| 2015 | Charfi | Case report | 1 | 33 | Female | SLE | 17 | 3400 | AGEP |
| 2015 | Zhang | Case report | 1 | 60 | Female | SS | 25 | 4200 | AGEP |
| 2015 | Soria | Retrospective cohort | 7 | 60, 52, 48, 23, 45, 9, 66 | 5 Females, 2 Males | GA, facial dermatitis, photosensitivity, SLE, RA, CLE, mucinosis | 10, 3, 7, 18, 15, 15, and 8 | MD | 1 AGEP/DRESS 6 AGEP |
| 2015 | Pearson | Case report | 1 | 50 | Female | RA | 14 | 5600 | AGEP |
| 2017 | Duman | Case report | 1 | 21 | Female | RA | 21 | 4200 | AGEP |
| 2017 | Castner | Case report | 1 | 1 | Female | SS | 21 | MD | AGEP |
| 2018 | Mohaghegh | Case report | 1 | 44 | Female | Arthralgia | 5 | 1000 | Prolonged AGEP |
| 2018 | Mercogliano | Case report | 1 | 71 | Female | Seronegative arthritis | 14 | MD | Overlap TEN/AGEP |
| 2019 | Liccioli | Case report | 1 | 9 | Female | SS | 30 | 3000 | AGEP |
| 2019 | İslamoğlu | Case report | 1 | 64 | Female | SS | 20 | MD | Recalcitrant AGEP |
| 2020 | Our case | Case report | 1 | 76 | Male | COVID-19 | 9 | 3600 | AGEP |
CLE, Cutaneous lupus erythematous; DM, dermatomyositis; GA, granuloma annulare; MD, missing data; PR, polymyalgia rheumatic; RA, rheumatoid arthritis; SLE, systemic lupus erythematous; SS, Sjogren syndrome; TEN, toxic epidermal necrolysis.
References
- 1.Sidoroff A., Halevy S., Bavinck J.N., Vaillant L., Roujeau J.C. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113–119. doi: 10.1034/j.1600-0560.2001.028003113.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhou P., Liu Z., Chen Y., Xiao Y., Huang X., Fan X.-G. Bacterial and fungal infections in COVID-19 patients: a matter of concern [published online ahead of print April 22, 2020] Infect Control Hosp Epidemiol. [DOI] [PMC free article] [PubMed]
- 3.İslamoğlu Z.G.K., Karabağli P. A case of recalcitrant acute generalized exanthematous pustulosis with Sjogren’s syndrome: successfully treated with low-dose cyclosporine. Clin Case Rep. 2019;7:1721–1724. doi: 10.1002/ccr3.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohaghegh F., Jelvan M., Rajabi P. A case of prolonged generalized exanthematous pustulosis caused by hydroxychloroquine—literature review. Clin Case Rep. 2018;6:2391–2395. doi: 10.1002/ccr3.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma A.N., Mesinkovska N.A., Paravar T. Characterizing the adverse dermatologic effects of hydroxychloroquine: a systematic review. J Am Acad Dermatol. 2020;83:563–578. doi: 10.1016/j.jaad.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Sidoroff A., Dunant A., Viboud C., Halevy S., Bavinck J.N.B., Naldi L. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR) Br J Dermatol. 2007;157:989–996. doi: 10.1111/j.1365-2133.2007.08156.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. https://www.medrxiv.org/content/medrxiv/early/2020/03/30/2020.03.22.20040758.full.pdf Available from: Accessed April 10, 2020.
- 8.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Molina J.M., Delaugerre C., Goff J.L., Mela-Lima B., Ponscarme D., Goldwirt L. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]