Highlights
-
•
We demonstrate five consecutive cases of predominantly lobar COVID-19-associated intracerebral haemorrhage (ICH).
-
•
Patients were typically relatively young with a severe, prolonged inflammatory prodrome.
-
•
COVID-19-induced endotheliitis/endotheliopathy may underlie associated cerebrovascular events.
-
•
For the clinician, anticoagulation decisions must balance risk of thrombosis with risk of haemorrhage.
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and typically presents with fever and respiratory tract symptoms. However, cerebrovascular events are being increasingly recognised in association with COVID-19 (Klok et al., 2020, Mao et al., 2020, Markus and Brainin, 2020). A growing number of case reports and series have been published describing the clinical characteristics of patients with ischaemic strokes and COVID-19. Patterns are emerging in such reports including large vessel occlusion, multiterritory infarcts, venous thromboembolism, raised inflammatory markers, antiphospholipid antibody production, a younger age of presentation, and concurrent severe systemic inflammation with organ failure (Oxley et al., 2020, Avula et al., 2020 Apr 28, Morassi et al., 2020).
On the other hand, the clinical characteristics of intracerebral haemorrhage (ICH) in COVID-19 patients are much less well-described, with only isolated case reports published so far (Morassi et al., 2020, Vu et al., 2020, Muhammad et al., 2020 May 5, Sharifi-Razavi et al., 2020). Our aim was to provide a description of clinical, radiological and laboratory characteristics of consecutive patients presenting to King’s College Hospital (KCH) with ICH in association with COVID-19. We will discuss the possible pathophysiological mechanisms and clinical implications.
2. Methods
In this retrospective case series, we performed a hospital-wide search using the Radiology Information System looking at the reports of all Computed Tomography (CT) head examinations that had been performed from 1st February 2020 to 14th May 2020 at KCH. All scans were reported or reviewed by a consultant neuroradiologist. For all scans that had demonstrated ICH we identified the COVID-19 status of the patient using electronic patient records. SARS-CoV-2 nucleic acid tests were performed on nasopharyngeal swabs using reverse transcriptase-polymerase chain reaction (RT-PCR). Patients were included in the case series if they had tested positive for COVID-19 prior to their ICH and had continuing clinical features related to COVID-19. Informed consent was obtained for all patients.
3. Results
We identified five patients who presented with radiographic evidence of ICH and RT-PCR-confirmed COVID-19. We describe their clinical characteristics, imaging findings, laboratory data, and clinical course (Table 1 ).
Table 1.
Patient demographics, laboratory findings at time of ICH detection and clinical progression of the five cases.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Patient demographics | |||||
| Age (years) | 41 | 54 | 50 | 64 | 52 |
| Sex | Male | Female | Male | Female | Male |
| Vascular risk factors | HTN T2DM |
Warfarin DVT/PEs HTN T2DM |
HTN | Nil | HTN High cholesterol IHD |
| Laboratory findings at the time ICH diagnosed | |||||
| Haemoglobin (115 – 155 g/L) | 81 | 134 | 69 | 80 | 92 |
| White cell count (4.0 – 11.0 x 109/L) | 20.3 | 6.1 | 15.2 | 15.8 | 14.6 |
| Neutrophils (2.2 – 6.3 x 109/L) | 15.6 | 4.1 | 12.4 | 13.8 | 12.5 |
| Lymphocytes (1.3 – 4.0 x 109/L) | 3.2 | 1.4 | 1.4 | 0.9 | 0.9 |
| Monocytes (0.2–1.0 x 109/L) | 1.3 | 0.4 | 1.7 | 0.6 | 0.5 |
| Platelet count (150 – 450 x 109/L) | 510 | 270 | 72 | 221 | 313 |
| Aspartate Transaminase (10 – 50 IU/L) | 35 | 81 | 60 | 109 | 76 |
| Bilirubin (3 – 20 umol/L) | 3 | 12 | 47 | 3 | 5 |
| Creatinine (45 – 120 mmol/L) | 140 | 66 | 291 (on CVVHDF) | 453 (on PD) | 231 (on CVVHDF) |
| International Normalised Ratio (INR) (0.9 – 1.2 ratio) | 1.0 | 3.2 | 1.1 | 1.1 | 1.1 |
| Activated partial- thromboplastin time (APTT) (0.85 – 1.15 ratio) | 1.0 | 2.8 | 1.3 | 2.5 | 2.9 |
| C-reactive protein (<5 mg/L) | 1 | 7 | 138 | 330 | 77 |
| Fibrinogen (g/L) | 3.7 | – | 7.5 | 2.9 | – |
| D-Dimer (<500 ng/mL) | 1920 | 1400 | 8961 | 8000 | 7580 |
| Clinical progression of case | |||||
| Time between symptom onset and ICH identification (days) | 37 | 14 | 32 | 32 | 38 |
| Organ involvement prior to ICH | Respiratory Renal |
Nil | Respiratory Cardiac Renal Liver |
Respiratory Cardiac Renal |
Respiratory Cardiac Renal |
| Antithrombotic treatment at time of ICH | Prophylactic LMWH | Warfarin | Prophylactic LMWH | Intravenous heparin | Intravenous heparin |
| Location of ICH | Lobar (left frontal) | Lobar (right frontal) | Lobar (right frontal) | Basal ganglia (right posterior capsule) | Multi-lobar (right perirolandic, left frontal, left cingulate) |
| Modified Rankine Score at time of writing (0–6) | 4 | 5 | 5 | 5 | 5 |
Abbreviations: CVVHDF = Continuous venovenous haemodiafiltration, DVT = Deep vein thrombosis, HTN = Hypertension, IHD = Ischaemic heart disease, PD = Peritoneal dialysis, PE = Pulmonary embolism, T2DM = Type 2 diabetes mellitus.
3.1. Patient 1
A forty-one-year-old male bus driver with well-controlled hypertension and type 2 diabetes mellitus presented with an eight-day history of cough, fever and fatigue. CT examination of his chest demonstrated bilateral ground glass changes consistent with COVID-19, without evidence of pulmonary embolism (PE) on a CT pulmonary angiogram (CTPA).
The patient deteriorated over twenty-four hours requiring intubation and ventilation, and transfer to the intensive care unit (ICU). He remained in multiple organ failure (respiratory and renal) for one month before extubation and ward stepdown.
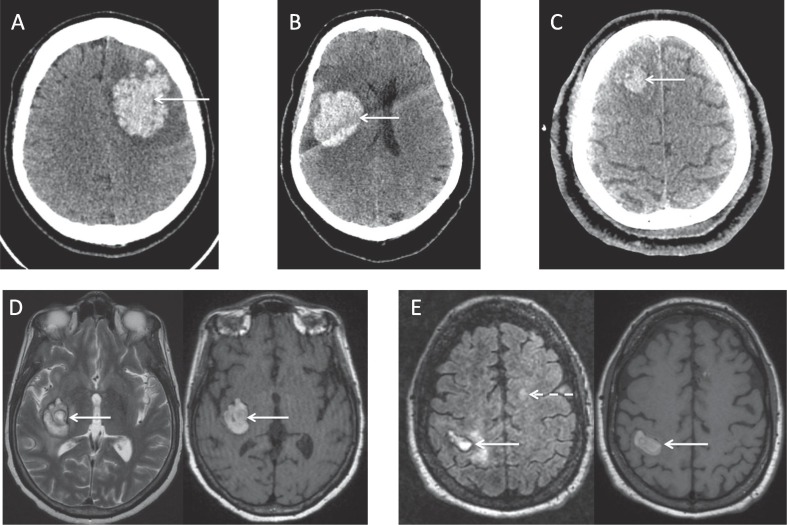
One day post-stepdown, the patient became drowsy with new right-sided hemiplegia. A CT head examination demonstrated a left frontal ICH requiring craniotomy and evacuation (Fig. 1 a.). An intracranial CT-angiogram (CT-A) was normal. At the time of ICH detection, clotting profile and platelets were normal, and the patient was receiving a prophylactic dose of low molecular weight heparin (LMWH). Three weeks post-ICH, he is rehabilitating on the stroke unit, requiring assistance of one with mobilising.
Fig. 1.
a Patient 1, Volumetric axial CT brain imaging demonstrates a large acute lobar intraparenchymal haematoma, centred on the left frontoparietal region (arrowed). There is surrounding hypo-attenuation and minimal localised mass effect. b. Patient 2, Volumetric axial CT brain imaging demonstrates an acute right frontoparietal intraparenchymal haematoma (arrowed). There is minimal effacement of the adjacent right lateral ventricle. c. Patient 3, Volumetric axial CT brain imaging demonstrates a subacute intraparenchymal haematoma, centred on the right superior frontal gyrus (arrowed). There is evidence of surrounding clot retraction. d. Patient 4, Axial T2-weighted (left) and volumetric non-contrast T1-weighted (right) MRI sequences of the brain demonstrate a subacute intraparenchymal haematoma, centred on the right posterior capsular region (arrowed). e. Patient 5, Axial FLAIR (left) and volumetric non-contrast T1-weighted (right) MRI sequences of the brain demonstrate a subacute intraparenchymal haematoma in the right perirolandic region (solid arrows). There is a left frontal white matter lesion visible (dashed arrow) on the FLAIR sequence, in keeping with the known history of demyelinating disease.
3.2. Patient 2
A fifty-four year old female who worked in housing presented with a two-week history of cough followed by a twelve-hour history of sudden onset dysarthria and left-sided hemiparesis. She had a history of multiple deep vein thromboses (DVTs) and PEs (for which she was on warfarin with a target INR of 3–4), well-controlled hypertension and type 2 diabetes mellitus.
On arrival, she had a Glasgow Coma Scale (GCS) score of 13 and a blood pressure of 145/99. Her International Normalised Ratio (INR) was 3.2 and platelet count was normal. A CT head examination showed a right frontal lobe ICH, which did not require surgical intervention (Fig. 1b.). An intracranial CT-A was normal. A chest radiograph showed typical COVID-19 changes. The patient was given prothrombin complex concentrate and vitamin K to reverse her INR, and was admitted to the hyperacute stroke unit (HASU) for further care. After a two-week admission, she was discharged to a rehabilitation centre for further therapy.
3.3. Patient 3
A fifty-year-old male taxi driver with well-controlled hypertension presented with a three-week history of cough, pleuritic chest pain and shortness of breath. Due to severe hypoxia he was immediately intubated and ventilated. A CT chest study demonstrated bilateral ground-glass opacities consistent with COVID-19 pneumonia, without evidence of PE on CTPA.
The patient was treated for multiple organ failure in ICU (lung, cardiac, renal and liver). Two weeks post-admission, he remained under heavy sedation and a CT head study was performed to look for cerebrovascular involvement as a potential complication of severe COVID-19. This demonstrated a right frontal lobe ICH (Fig. 1c.). An intracranial CT-A was normal. At the time of ICH detection, clotting profile was normal, platelets were normal, and he was on a prophylactic dose of LMWH. One week post-ICH, the patient remains in ICU requiring multiple organ support.
3.4. Patient 4
A sixty-four-year-old female administrator with no past medical history presented with a six-day history of cough, shortness of breath and fever. A CT chest examination revealed bilateral ground glass changes consistent with COVID-19 and multiple bilateral PEs on CTPA with evidence of right heart strain. Suffering from multiple organ failure (respiratory, cardiac and renal), the patient was immediately intubated and ventilated and moved to ICU, where she was commenced on a treatment dose of LMWH to treat PE.
Four weeks post-admission, she was noted to have a low GCS post-sedation wean. A CT head examination showed a subacute right-sided gangliocapsular ICH. An intracranial CT-A was normal. A subsequent magnetic resonance imaging (MRI) examination of the brain (Fig. 1d.) did not evidence any other abnormalities. At the time of ICH detection, activated partial thromboplastin time (APTT) was stable at 2.5, INR was 1 and platelets were normal. Three weeks post-ICH, the patient remains in ICU requiring multiple organ support.
3.5. Patient 5
A fifty-two-year-old male carer with a history of ischaemic heart disease, quiescent untreated multiple sclerosis, asthma, hypertension and hyperlipidaemia, presented with a two-week history of shortness of breath, cough, fevers and pleuritic chest pain. CT chest/CTPA showed bilateral consolidation and ground glass opacification without evidence of PE.
Three days post-admission, the patient’s respiratory function deteriorated and he was intubated, ventilated and moved to ICU where he required cardiac, respiratory, and renal support. He received ongoing intravenous heparin to enable dialysis. A CT head examination was performed three weeks later due to ongoing low GCS after sedation wean. This evidenced a subacute ICH in the right perirolandic region, with smaller petechial haemorrhages in the left paramedian frontal lobe and left cingulate sulcus. An intracranial CT-A was normal. At the time of ICH detection, APTT was stable at 2.9, INR was 1.1 and platelets were normal.
An MRI brain examination confirmed these findings, also demonstrating areas of surrounding ischaemia, alongside changes consistent with multiple sclerosis (Fig. 1e.). The patient was switched to a prophylactic dose of LMWH, which was returned to treatment dose nine days later. Seven weeks post-admission the patient remains on ICU, receiving multiple organ support
4. Discussion
4.1. Case features
This case series has highlighted a number of clinical characteristics of patients with ICH and COVID-19. Firstly, patients were relatively young with a mean age of 52.2 years (range 41 – 64 years), lower than expected for conventional ICH (D’Amore et al., 2013 Jun 1, Hansen et al., 2013 Oct 1). This is particularly interesting given COVID-19 morbidity and mortality generally increases with advancing age, which is not reflected in this cohort (Zhou et al., 2020). Moreover, a meta-analysis of pooled data from six studies looking at all subtypes of cerebrovascular events with COVID-19 identified the mean age range of patients to be 45 – 67 years, which is similar to this cohort (Aggarwal et al., 2020).
Secondly, it was noted that four out of five ICHs were located in lobar territories; all were within the anterior circulation, four within the frontal lobe, and one patient had multifocal haemorrhage in three locations. Lobar ICH occurs in 15 – 30% of conventional cases, and is predominantly associated with an underlying vascular abnormality (Aguilar and Brott, 2011). However, none of the patients were shown to have an underlying vascular abnormality on intracranial CT-A examination. Furthermore, all the lobar haemorrhages were in patients with pre-existing hypertension, classically associated with deep structure bleeds. In fact, only the single patient without a history of hypertension (patient 4) had an ICH in a deep structure. Additionally, there were no imaging markers to suggest that the ICHs in our series were haemorrhagic transformation of infarcts.
Thirdly, the delay between time of COVID-19 symptom onset and time of ICH diagnosis had a median of 32 days (range 14 – 38 days). During this period, all five patients had evidence of a period of prolonged inflammation, as demonstrated firstly by markedly raised D-Dimer values in all patients (Table 1), and secondly by severe end organ damage in four out of five patients requiring multiple organ support.
This leads to the question of whether a specific COVID-19-related pathogenic process might be occurring in the brain, culminating in a bleeding event. Alternatively, were the bleeding events simply stochastic, and related to general ICU risk factors? Clearly larger studies will be required to compare actual COVID-19-associated ICH incidence to its expected incidence. However, our case series seems to indicate a distinct clinical phenotype of COVID-19-associated ICH.
4.2. Proposed pathogenesis
SARS-CoV-2 has been reported to gain cellular entry via the surface angiotensin converting enzyme 2 (ACE2) receptor, which is known to be expressed in the vascular endothelium (Xia and Lazartigues, 2008). Endothelial cell infection and endotheliitis has been demonstrated in COVID-19 via histological identification of viral elements within endothelial cells and an accumulation of inflammatory cells and apoptosis (Varga et al., 2020). This is in keeping with a multitude of recent articles identifying endothelial injury as a focal point of COVID-19 pathogenesis (Magro et al., 2020, Su et al., 2020, Cao and Li, 2020).
We propose two possible mechanisms of ICH in COVID-19 deriving from endothelial injury. Firstly, we propose that both direct and indirect endothelial toxicity may be involved in COVID-19-associated ICH: the former via direct endothelial cell invasion; the latter through a combination of systemic factors including prothrombotic factors, inflammatory cytokine production, activation of coagulation cascades, and complement-mediated microvascular thrombosis (Ronaldson and Davis, 2017, Keep, et al., 2008). Regarding the latter, there is mounting evidence that COVID-19 infection leads to various types of thrombotic events (Klok et al., 2020, Levi et al., 2020, Bikdeli et al., 2020 Apr). Ultimately, disruption of tight junction protein complexes would occur, leading to blood brain barrier compromise and ICH (Ronaldson and Davis, 2017, Keep, et al., 2008).
Secondly, we suggest disruption of the renin-angiotensin system (RAS) may also play a role in COVID-19-mediated ICH. The RAS has distinct regulatory pathways in both the periphery and the brain, which could be impacted by SARS-CoV-2 via down regulation of endothelial ACE2 receptors, leading to cerebral blood flow dysautoregulation (Divani et al., 2020, Zhang et al., 2020 Apr 1). Interestingly, perfusion abnormalities have been described in patients with severe COVID-19: MR perfusion-weighted brain imaging conducted in patients with unexplained encephalopathy in ICU demonstrated bilateral frontotemporal hypoperfusion in eleven patients, three of whom had acute or subacute infarcts (Helms et al., 2020).
4.3. Conclusions and clinical learning points
Whilst it remains to be confirmed whether there is a causal relationship between ICH and COVID-19, this case series provides the first detailed characterisation of the COVID-19-related ICH phenotype. Bearing in mind the small sample size, this study provides the first evidence for a younger patient demographic, a lobar predominance, and a marked systemic inflammatory prodrome. The series also emphasises the clinical challenges implicit in treating the thrombotic consequences of COVID-19: three out of five patients in our series were on a treatment dose of anticoagulation prior to their ICH, and the remaining two were on a prophylactic dose of LMWH.
Added to this challenge is the difficulty of diagnosing ICH in heavily-sedated ICU patients who are most at risk of developing ICH. Clinicians should have a low threshold for suspicion and investigation in such patients, particularly those younger individuals receiving organ support beyond two weeks of their COVID-19 illness, who are also being treated with anticoagulants. Further research is required to determine the incidence and characteristics of ICH and COVID-19.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, L., Jin, H., Wang, M., Hu, Y., Chen, S., He, Q., et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol [Internet]. 2020 Apr 10 [cited 2020 May 20]; Available from: https://doi.org/10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed]
- Markus, H.S., Brainin, M., COVID-19 and stroke—A global World Stroke Organization perspective. Int J Stroke. 2020 Apr 20;1747493020923472. [DOI] [PubMed]
- Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl. J. Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avula A., Nalleballe K., Narula N., Sapozhnikov S., Dandu V., Toom S. COVID-19 presenting as stroke. Brain Behav. Immun. 2020 Apr 28;S0889–1591(20):30685–30691. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morassi, M., Bagatto, D., Cobelli, M., D’Agostini, S., Gigli, G.L., Bnà C, et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol [Internet]. 2020 May 20; Available from: https://doi.org/10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed]
- Vu D., Ruggiero M., Choi W.S., Masri D., Flyer M., Shyknevsky I. Three unsuspected CT diagnoses of COVID-19. Emerg. Radiol. 2020/04/13. 2020 Jun,;27(3):229–232. doi: 10.1007/s10140-020-01775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad S., Petridis A., Cornelius J.F., Hänggi D. Letter to editor: Severe brain haemorrhage and concomitant COVID-19 Infection: A neurovascular complication of COVID-19. Brain Behav. Immun. 2020 May 5;S0889–1591(20):30802–30803. doi: 10.1016/j.bbi.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Razavi, A., Karimi, N., Rouhani, N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect. 2020 Mar 27;35:100669–100669. [DOI] [PMC free article] [PubMed]
- D’Amore C., Paciaroni M., Silvestrelli G., Agnelli G., Santucci P., Lanari A. Severity of acute intracerebral haemorrhage, elderly age and atrial fibrillation: Independent predictors of poor outcome at three months. Eur. J. Intern. Med. 2013;24(4):310–313. doi: 10.1016/j.ejim.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Hansen B.M., Nilsson O.G., Anderson H., Norrving B., Säveland H., Lindgren A. Long term (13 years) prognosis after primary intracerebral haemorrhage: a prospective population based study of long term mortality, prognostic factors and causes of death. J. Neurol. Neurosurg. Amp Psychiatry. 2013;84(10):1150. doi: 10.1136/jnnp-2013-305200. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal, G., Lippi, G., Michael Henry, B. Cerebrovascular disease is associated with an increased disease severity in patients with Coronavirus Disease 2019 (COVID-19): A pooled analysis of published literature. Int J Stroke. 2020 Apr 20;1747493020921664. [DOI] [PubMed]
- Aguilar M.I., Brott T.G. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1(3):148–159. doi: 10.1177/1941875211409050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J. Neurochem. 2008;107(6):1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet Lond Engl. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020 doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, H., Yang, M., Wan, C., Yi, L.-X., Tang, F., Zhu, H.-Y., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int [Internet]. 2020 Apr 9; Available from: http://www.sciencedirect.com/science/article/pii/S0085253820303690. [DOI] [PMC free article] [PubMed]
- Cao W., Li T. COVID-19: towards understanding of pathogenesis. Cell Res. 2020;30(5):367–369. doi: 10.1038/s41422-020-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson, P.T., Davis, T.P. Mechanisms of Endothelial Injury and Blood-Brain Barrier Dysfunction in Stroke. In Primer on Cerebrovascular Diseases: Second Edition. Elsevier Inc. 2017. p. 220-226 https://doi.org/10.1016/B978-0-12-803058-5.00045-X.
- Keep, R.F. et al. (2008) Blood-brain barrier function in intracerebral hemorrhage. In: Zhou LF. et al. (eds) Cerebral Hemorrhage. Acta Neurochirurgica Supplementum, vol 105. Springer, Vienna. [DOI] [PubMed]
- Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;S0049–3848(20):30120–30121. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;S2352–3026(20):30145–30149. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up. J. Am. Coll Cardiol. 2020;17:27284. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divani A.A., Andalib S., Di Napoli M., Lattanzi S., Hussain M.S., Biller J. Coronavirus Disease 2019 and Stroke: Clinical Manifestations and Pathophysiological Insights. J. Stroke Cerebrovasc Dis. 2020;12 doi: 10.1016/j.jstrokecerebrovasdis.2020.104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020;15(NEJMc2008597) doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]