Abstract
Background
The novel severe acute respiratory syndrome coronavirus 2 virus that emerged in December 2019 causing coronavirus disease 2019 (COVID-19) has led to the sudden national reorganization of health care systems and changes in the delivery of health care globally. The purpose of our study was to use a survey to assess the global effects of COVID-19 on colorectal practice and surgery.
Materials and Methods
A panel of International Society of University Colon and Rectal Surgeons (ISUCRS) selected 22 questions, which were included in the questionnaire. The questionnaire was distributed electronically to ISUCRS fellows and other surgeons included in the ISUCRS database and was advertised on social media sites. The questionnaire remained open from April 16 to 28, 2020.
Results
A total of 287 surgeons completed the survey. Of the 287 respondents, 90% were colorectal specialists or general surgeons with an interest in colorectal disease. COVID-19 had affected the practice of 96% of the surgeons, and 52% were now using telemedicine. Also, 66% reported that elective colorectal cancer surgery could proceed but with perioperative precautions. Of the 287 respondents, 19.5% reported that the use of personal protective equipment was the most important perioperative precaution. However, personal protective equipment was only provided by 9.1% of hospitals. In addition, 64% of surgeons were offering minimally invasive surgery. However, 44% reported that enough information was not available regarding the safety of the loss of intra-abdominal carbon dioxide gas during the COVID-19 pandemic. Finally, 61% of the surgeons were prepared to defer elective colorectal cancer surgery, with 29% willing to defer for ≤ 8 weeks.
Conclusion
The results from our survey have demonstrated that, globally, COVID-19 has affected the ability of colorectal surgeons to offer care to their patients. We have also discussed suggestions for various practical adaptation strategies for use during the recovery period.
Keywords: Colorectal disease, Colorectal surgery, Guidance, SARS-CoV-2, Survey
Micro-Abstract
We have presented the results of a survey used to assess the global impact of coronavirus disease 2019 (COVID-19) on the delivery of colorectal surgery. Despite accessible guidance information, our results have demonstrated that COVID-19 has significantly affected the ability of colorectal surgeons to offer care to patients. We have also discussed practical adaptation strategies for use during the recovery phase.
Introduction
The novel coronavirus that emerged in Wuhan, China, in December 2019 rapidly crossed borders and spread worldwide owing to an interdependent and highly mobile global population. At present, with no specific therapeutic interventions or vaccines, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19) has continued to cause a human and economic tragedy affecting millions of people. COVID-19 has wreaked havoc on cancer care, because most health care systems have been required to reorganize their infrastructure and staffing to manage the pandemic. The COVIDSurg Collaborative study reported that during a 12-week period of peak COVID-19 disruption, 28.4 million elective surgeries worldwide will have been cancelled or postponed in 2020. The nonemergent procedure cancellation rate would be 72.3%, with 37.7% of cancer surgery cases affected.1
With the pace of viral spread and the lack of available clinical knowledge regarding the manifestations and natural history of those afflicted, many of our traditional methods of practicing surgery have been questioned or suspended. Critical questions for colorectal surgeons include the following:
How should we best offer care to patients, whether in the inpatient or outpatient setting?
How do we reassure our patients that coming to the hospital is safe, and how do we keep our staff and patients safe?
Is it safe to perform benign and malignant surgical procedures?
With some patients requiring emergency colorectal surgery, do we offer the same surgical or radiological interventions that we would have before COVID-19?
Other critically important colorectal clinical questions are more difficult to answer. These include the following:
How should we provide care for patients with early stage I/II colorectal cancer and locally advanced stage III colorectal malignancy?
Do we alter our neoadjuvant chemotherapy or chemoradiotherapy protocols?
Should we defer surgery for these patients and, if so, for how long would the deferral be appropriate?
What should the protocol be for those who have completed neoadjuvant therapy and should now undergo surgery?
What is the current perioperative risk for patients undergoing colorectal cancer surgery?
How concerned should we be about aerosolization of SARS-CoV-2 during colonoscopy or laparoscopic surgery?
How concerned should we be about aerosolization during intubation at the onset of a procedure?
Should we be offering alternative, noninvasive tests to exclude colorectal cancer for symptomatic patients?
If we offer surgery for benign and/or malignant colorectal disorders, what perioperative precautions and intraoperative precautions should be undertaken?
What comorbidities should be defined as conveying high perioperative risk and could lead to deferring surgery?
Should we modify our surgical approach for these disorders (eg, converting all procedures to open approaches and/or creating stomas or proximal diversionary stomas when a laparoscopic approach would have been preferable)?
Should our concerns of anastomotic leaks and reoperation during the pandemic lead to stoma formation?
How should we obtain written informed consent from patients?
Should we allow our trainees to operate?
To assess how these issues are affecting our global community of colorectal surgeons, the International Society of University Colon and Rectal Surgeons (ISUCRS) conducted a global survey of surgeons who manage colorectal disease to assess the effects of SARS-CoV-2 and to identify how colorectal practice has adapted to these sudden changes.
Materials and Methods
To understand the global effects of COVID-19 on colorectal practice and surgery, ISUCRS designed an online questionnaire that addressed the pertinent themes of outpatient services, inpatient care, elective surgery, and perioperative oncologic care. A panel of ISUCRS members were each asked to provide a few questions considered relevant to how COVID-19 had affected local practice. From the pooled questions, the panel agreed and selected 22 that were considered the most relevant (Figure 1 ). The questionnaire was distributed via the ISUCRS database and was advertised on social media sites. The questionnaire was open from April 16 to 28, 2020, and was anonymous unless respondents chose to include an e-mail address to allow the investigators to communicate with them and/or send the results of the survey directly to them.
Figure 1.
Coronavirus Disease 2019 (COVID-19) and the Global Effect on Colorectal Practice and Surgery Questionnaire
Abbreviations: CO2 = carbon dioxide; CT = computed tomography; ECOG = Eastern Cooperative Oncology Group; GI = gastrointestinal; MDT = multidisciplinary team; MRI = magnetic resonance imaging; PPE = personal protective equipment; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; taTME = transanal total mesorectal excision; WHO = World Health Organization.
Results
A total of 287 surgeons completed the survey. Of the respondents, 90% were colorectal specialists or general surgeons with an interest in colorectal disease, 6% were general surgeons, and 4% were general or colorectal surgical trainees. The largest group of respondents were from the European Union (49%), including 63 (22%) from the United Kingdom (Figure 2 ). Of the 287 respondents, 95% reported a national lockdown of the population with restrictions on social movement and social distancing in their countries. Also, 82% reported that they had been guided by visiting national organizations or their surgical societies’ websites or had received some guidance information from their surgical college or societies regarding how to provide care to their colorectal patients.
Figure 2.
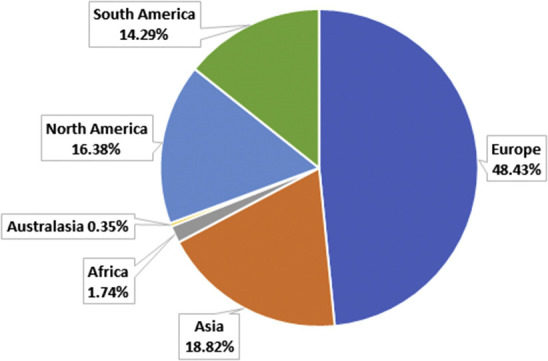
Pie Chart Showing Proportion of Respondents Practicing in Each Country
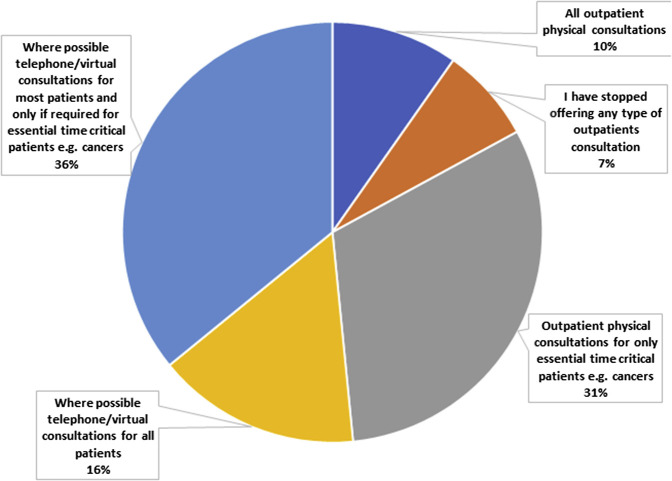
Of the 287 respondents, 96% reported that the pandemic had negatively affected their ability to practice colorectal surgery. Only 10% were offering all patients actual face-to-face physical consultations, and 7% had stopped offering any type of outpatient consultations. Also, 31% were only offering outpatient physical consultations for essential, time-critical patients (eg, those with cancer). In addition, 52% of surgeons reported using telemedicine in their practice, with 16% offering a telephone or virtual consultations to all patients and 36% telephone or virtual consultations to most patients, with in-person, physical consultations only if required for essential time-critical patients (eg, those with cancer; Figure 3 ).
Figure 3.
Pie Chart Showing Outpatient (Office) Services Still Provided by Respondents During the Pandemic
The survey results showed a reduction in the availability of colorectal diagnostic services, with computed tomography (CT)-guided colonoscopy, colonoscopy, and CT and magnetic resonance imaging scans for staging not provided by 82%, 64%, and 13% of the respondents’ practices, respectively.
Of the respondents, 12% did not believe that all major elective colorectal cancer surgical procedures should continue. Moreover, 66% reported that colorectal cancer procedures could proceed but with the caveat of perioperative and intraoperative precautions. However, 22% of the respondents reported that, at present, all major elective colorectal cancer surgical procedures should continue as normal. In addition, 60% of surgeons did not believe that all major, elective, benign colorectal surgical procedures should continue, although 32% thought that these types of procedures could proceed but with personal protective equipment (PPE). A few respondents (8%) reported that benign elective surgery should proceed normally.
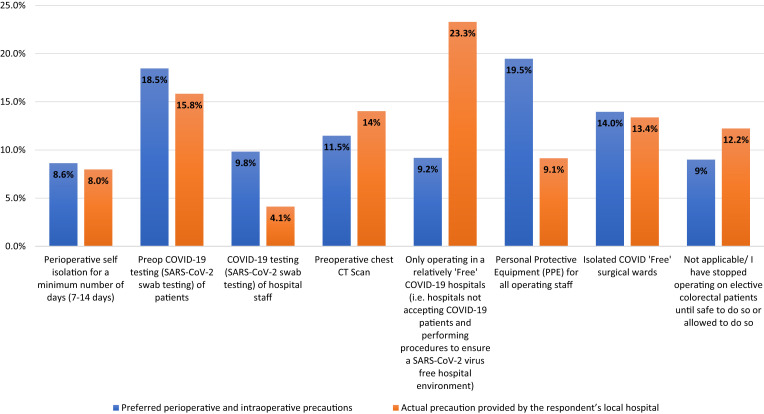
Important perioperative and intraoperative precautions believed prudent to consider and the precautions provided at their local hospitals for elective surgical patients with colorectal cancer are shown in Figure 4 . The most important precaution the respondents recommended was the provision of PPE for all operating staff (19.5%, adjusted for all responses; actual, 81% of respondents). However, the provision of PPE was only achieved by 9.1% of the hospitals. Also, 23.3% of the hospitals were able to provide elective surgical care in relatively “free” COVID-19 hospitals (ie, hospitals not accepting patients with COVID-19 and using strategies to ensure a SARS-CoV-2–free hospital environment). Of the respondents, 18.5% and 9.9% reported that the oronasopharyngeal SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) swab test was important for testing patients and hospital staff. However, testing of patients and hospital staff was only achieved by 15.8% and 4.1% of hospitals, respectively.
Figure 4.
Bar Graph Showing Preferred Perioperative and Intraoperative Precautions and the Actual Precaution Provided by Respondent’s Local Hospitals for Elective Surgical Patients. Because Respondents Could Select ≥ 1 Options, Percentages Were Calculated from the Total Of All Options Chosen for Both Perioperative and Intraoperative Precautions and Actual Precaution Provided by Respondent’s Local Hospital
Abbreviations: COVID-19 = coronavirus disease 2019; CT = computed tomography; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
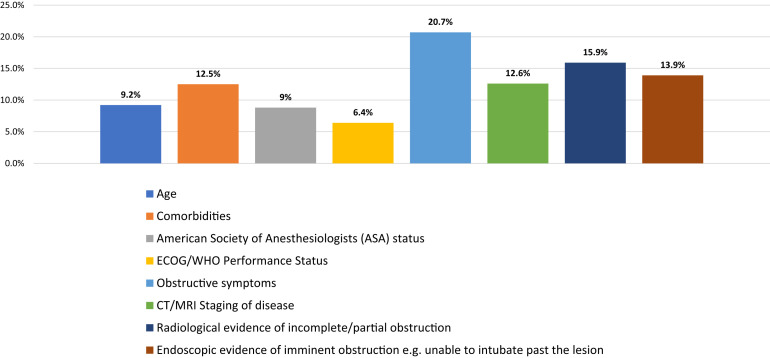
For those surgeons still performing procedures, the factors used to prioritize their patients with cancer who required major elective surgery are shown in Figure 5 . Of the respondents, 20.7% reported that obstructive bowel symptoms were the most important factor for prioritizing their patients for surgery, followed by radiologic evidence of obstruction (reported by 15.9%) and endoscopic evidence of imminent obstruction (reported by 13.9%). Of the surgeons, 26% reported they had stopped performing elective colorectal surgery until safe or allowed to do so. Of the 74% who were still performing surgery, 51% were still performing elective colorectal cancer surgery in their current hospital, which was also admitting patients with COVID-19, 35% were performing surgery in relatively “COVID-19–free” hospitals, and 14% were providing surgery at both types of hospitals. Of those still performing surgery for patients with colorectal cancer, 61% reported they were providing surgery in hospitals able to provide dedicated operating rooms for patients with, or suspected to have, COVID-19 and dedicated operating rooms for COVID-19–negative patients. Finally, 26% reported that their hospitals could not provide this separation, and 14% were not sure whether their hospitals could do so.
Figure 5.
Bar Graph Showing Factors Respondents Considered When Prioritizing Patients for Elective Major Surgery for Colorectal Cancer During Coronavirus Disease 2019 (COVID-19) Pandemic. Because Respondents Could Select ≥ 1 Options, Percentages Were Calculated From the Total of All Selected Options
Abbreviations: CT = computed tomography; ECOG = Eastern Cooperative Oncology Group; MRI = magnetic resonance imaging; WHO = World Health Organization.
Of the surgeons continuing to perform surgery, 46% were providing a combination of open and elective minimally invasive surgery (MIS), 36% were providing open surgery, and 18% MIS. Also, 44% of the surgeons reported that enough information was not available regarding the effects and/or safety of the loss of intra-abdominal insufflation of carbon dioxide gas into the operating room during MIS and that this approach should not be used until further convincing and safe information was available. However, 28% were convinced that sufficient information is available to continue supporting MIS surgery, and 28% were not sure whether it would be safe to perform surgery during the COVID-19 pandemic. Of the surgeons still performing surgery, 72% were doing so with their usual setup of assistance (ie, surgical residents). However, others had been advised or ordered to perform surgery with another specialist or consultant. In addition, 26% of operating surgeons had modified their surgical approach by creating a stoma or proximal diversionary stoma in cases in which they would not have before COVID-19.
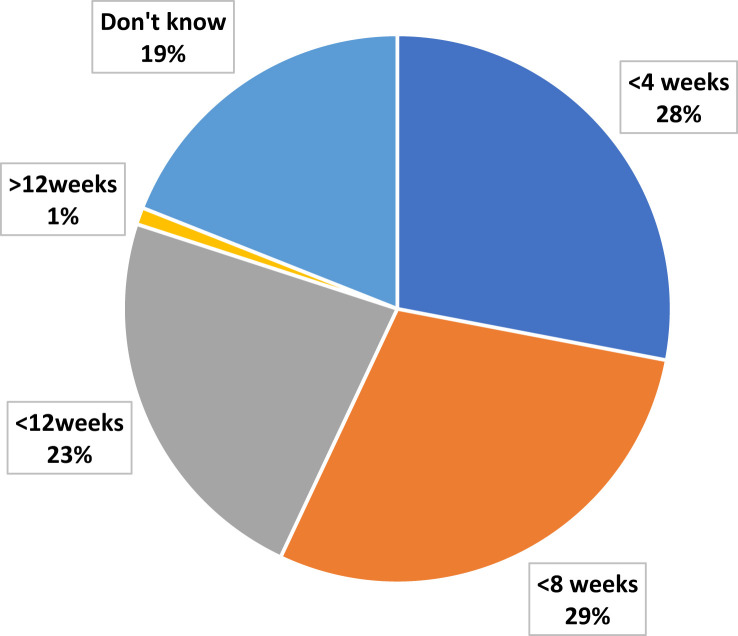
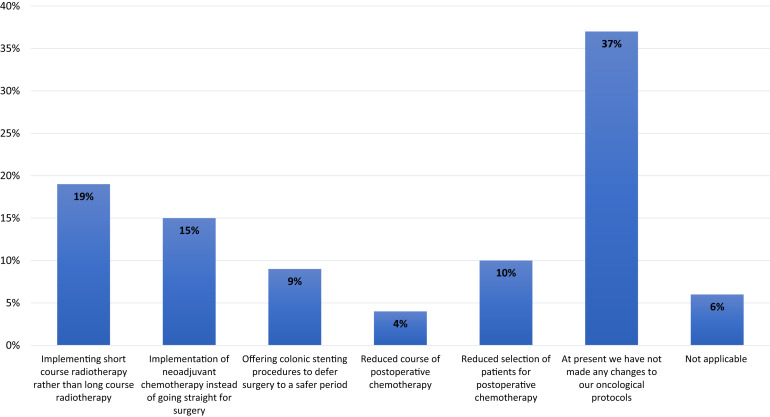
Of the surgeons, 39% were not deferring surgery for asymptomatic, elective patients with colorectal cancer. Most (61%) were prepared to defer surgery for different lengths of time, with the largest group (29%) willing to defer surgery for ≤8 weeks (Figure 6 ). Of the surgeons, 37% reported that their colorectal multidisciplinary teams (MDTs) had also not made any changes to neoadjuvant and adjuvant treatment during the pandemic. However, others reported that the largest change in the MDTs was that 19% had implemented short-course radiotherapy instead of long-course chemoradiotherapy. Other oncologic and practice changes by the MDTs reported by the surgeons are shown in Figure 7 .
Figure 6.
Pie Chart Showing Proportion of Respondents for Each Interval for Deferring Elective Colorectal Cancer Surgery
Figure 7.
Bar Graph Showing Different Changes Implemented by Respondents’ Colorectal Multidisciplinary Team/Practice to Neoadjuvant and Adjuvant Treatment During Coronavirus Disease 2019 pandemic. Because Respondents Could Select ≥ 1 Options, Percentages Were Calculated From the Total of All Selected Options
Discussion
To the best of our knowledge, our survey is the first global survey of colon and rectal surgeons and specialists to assess the effects of COVID-19 on their practice. The response from 287 surgeons, with a wide global distribution, provided a good representative sample to obtain an impression of the present effects of COVID-19 on our specialty of colon and rectal surgery and our patients. In line with national surgical and colorectal society guidance and recommendations, most colorectal surgeons have modified their usual practices to reduce nonessential treatment that could be safely deferred.
However, 36% were still providing outpatient services using traditional in-person consultations, and one of the limitations of our survey was that we did not assess the rationale for this practice. The reasons could include a lack of other methods to conduct outpatient visits (eg, a lack of internet capacity), hospitals reorganizing their infrastructure to allow for a safe flow of patients within the hospital setting to maintain social distancing institutionally, pressure from patients and hospital managers to provide normal services, and financial pressure. In an age of advanced global telecommunications and video conferencing platforms, most outpatient colorectal consultations can be performed through easily accessible and inexpensive platforms and have been shown, surprisingly, to be more efficient than our traditional methods. However, some patients, secondary to socioeconomic status, might not have access to internet-based communication, and some patients might have a generational proclivity against internet-based communication. Platforms specific for medical practice have become available, with virtual waiting rooms and so forth, offering a robust and reliable method for doctors, clinicians, and general practitioners to connect with patients via a secure video link. However, some patients will require education and help to access these platforms.
Despite the great amount of guidance from various colorectal organizations and societies, our survey showed that a vast variation in the opinions of surgeons still exists regarding the preoperative, operative, and oncologic care of patients.
The difficulties in the preoperative identification of patients with COVID-19 or carriers of the SARS-CoV-2 have been compounded by several factors. The testing results have lacked uniformity. The positive rate of RT-PCR for a single oronasopharyngeal SARS-CoV-2 swab test has been reported to be a range from 38% to 71% for patients with COVID-19, and various factors can account for these inconsistencies.2, 3, 4, 5 In a study of 73 hospitalized patients infected with SARS-CoV-2, the stool of 53% of the patients tested positive for SARS-CoV-2 RNA. In addition, 23% of the patients continued to have positive findings in their stool after showing negative results in the respiratory samples.6 The prolonged fecal shedding of viral RNA was also demonstrated with SARS-CoV RNA, which could be detected in the stool of patients for > 10 weeks after symptom onset. Given the biologic similarities between SARS-CoV and SARS-CoV-2, this is concerning owing to the risk of transmission.7 Although the use of a combination of chest CT scans, serial oronasopharyngeal SARS-CoV-2 RT-PCR swabs, and/or other biologic samples can significantly improve the sensitivity for a diagnosis of COVID-19 for 88% to 98% of symptomatic hospitalized patients, such testing might not be practical for the preoperative screening of patients scheduled to undergo elective colorectal surgery.3 , 5 For patients requiring urgent surgery, the results might not be available in time. Thus, some institutions have treated these patients the same as patients confirmed to have COVID-19. For asymptomatic patients, the positivity rate for COVID-19 using chest CT scans remains unknown. Thus, at present, the best preoperative assessment is probably oronasopharyngeal swabs to test for SARS-CoV-2 using RT-PCR. For patients requiring elective surgery who have had COVID-19 and have recovered, if the stage of their colorectal cancer permits a deferral of their definitive procedure, it would probably be best to defer surgery for ~10 weeks from their initial diagnosis to reduce the risk of continued human-to-human cross-contamination and the occurrence of postoperative complications.7 If it is necessary to operate within that 10-week period, it would be sensible to consider these patients as still having positivity for SARS-CoV-2, irrespective of the screening test results. For patients with no history of, and no symptoms consistent with, COVID-19, it will be essential to exclude any history of contact with COVID-19–positive individuals, because of the known asymptomatic incubation period estimated to range from 2 to 10 days, with a mean incubation period of 5.2 days.8, 9, 10 Because the symptomatic period for COVID-19 is ~15 days, preoperative patient self-isolation for a minimum number of 14 days should be included in the preoperative planning schedule to reduce the risk of admitting infected patients who are asymptomatic for surgery.4 Ideally, arrangements should be in place for the appropriate preoperative assessment of patients at not less than 2 to 3 days before surgery to check for symptoms of SARS-CoV-2/COVID-19 infection and patient adherence to self-isolation. Testing with oronasopharyngeal swabs for SARS-CoV-2 using RT-PCR just before surgery can be reassuring. Such testing is prudent and has been required by many institutions before patients can undergo general anesthesia. The timing of the test will depend on the time required for laboratories to provide the results. Ideally, patients should be tested 1 to 2 days before surgery and required to maintain self-isolation thereafter. Postoperative patients should probably be advised to continue to self-isolate for 14 to 28 days after discharge.
To reduce the likelihood of COVID-19 infection after major colorectal cancer surgery, where possible, patients should be admitted to relatively COVID-19–free hospitals that have a strict policy of screening hospital staff and high hospital standards. If that is not possible, these patients should be admitted to COVID- and non–COVID-segregated wards and intensive care units that also have separate personnel. Ideally, patients should undergo surgery by elective colorectal surgeons who have not been participating in emergency on-call rotations. However, this might not be practical. Furthermore, to reduce the risk of hospital-acquired infection of COVID-19, it would be prudent to restrict the number of relatives and friends allowed to visit patients. The risk of asymptomatic hospital staff cross-contaminating patients and colleagues is also a real concern. At a minimum, screening of hospital staff should always include the checking of the temperature of all staff entering the hospital premises and the use of a basic surgical mask within the hospital. Baseline and subsequent COVID screening should also be conducted. Regarding the long scientific and politically debated question in the United Kingdom and United States, Stewart et al11 made a good and convincing argument about the positive merits of the use of face masks in low-risk situations and how droplets 0.1 to 5.0 μm in size that carry SARS-CoV-2 can be trapped by these masks. Apart from aerosolizing procedures, which have been classed as high-risk situations, the use of simple surgical masks should, therefore, be encouraged in the hospital when a social distancing of ≥ 2 meters cannot be maintained.11
Although random oronasopharyngeal swab testing for SARS-CoV-2 using RT-PCR of hospital staff might be ineffective, staff education and vigilance to monitor the compliance of staff to follow the general governmental advice such as that from the United Kingdom and United States should be continued. For individuals with symptoms suggestive of COVID-19, RT-PCR testing should be strongly encouraged. Swab RT-PCR testing of health care workers who are returning to work might be the best use of this test to confirm and prevent asymptomatic health care workers returning to the workplace with COVID-19. The caveat, however, remains the 38% to 71% accuracy of these single swab test results, regardless of how many institutions have begun screening their at-risk health care providers.2, 3, 4, 5
Mounting and convincing evidence has shown that, in addition to the major route of transmission of COVID-19 of droplet transmission and contact with contaminated fomites, aerosols can be another route of transmission.12, 13, 14, 15 The SARS-CoV-2 virion, with a size of 0.07 to 0.09 μm, has the potential to be transmitted as an aerosol and might behave similar to its close relatives of SARS and Middle East respiratory syndrome.16 van Doremalen et al17 recently reported that viable SARS-CoV-2 virion could be detected in aerosols for ≤ 3 hours. Electrosurgery produces surgical smoke, which contains water vapor (95%), inorganic and organic pollutants, and biologic pollutants such as cancer cells, bacteria, and viruses.18 The surgical smoke occurring in laparoscopy during the COVID-19 pandemic is a possible additional source of aerosol airborne pollution generated by the pneumoperitoneum. However, no documented case of the transmission of high-risk coronaviruses (ie, Middle Eastern respiratory syndrome-CoV, SARS-CoV, SARS-CoV-2) to operating room staff during abdominal surgery has been confirmed.19 The risk to operating staff for SARS-CoV-2 is likely related to aerosol-generating ventilatory procedures (ie, tracheal intubation, noninvasive ventilation, mask ventilation, head and neck surgery) and not to abdominal surgical procedures. The latter probably have a negligible risk to operating staff. This negligible risk might be because during such epidemics and pandemics, heightened perioperative procedures could have prevented this occupational hazard.20 A recent review by Mowbray et al21 comprehensively covered the issue of surgical smoke. To date, only a few studies have analyzed the smoke plume during laparoscopic surgery before the COVID-19 pandemic. Those studies isolated hepatitis B virus, human papillomavirus, human immunodeficiency virus in the plume.22, 23, 24 However, very few studies have investigated whether these particles are capable of transmitting disease or even have viral infectivity. Human papillomavirus transmission during anogenital surgery has been the most widely reported and resulted from direct contact with electrocautery in an infected field.21 Because of the unknowns regarding SARS-CoV-2, it would be prudent for all operating staff to adhere to strict perioperative protocols, including full PPE (ie, N95, filtering face piece 2, or filtering face piece 3 respirator masks, eye visors) until it has become safe to not do so.25 , 26 These issues have been extensively covered in a review by Stewart et al.11 The use of routine surgical masks in high-risk situations with a risk of aerosolization of viruses and other dangerous particles will not be enough to contain fine particles.27 The recent Italian single case report of high concentrations of SARS-CoV-2 in peritoneal fluid and the recognition of prolonged fecal shedding of SARS-CoV-2 viral RNA are warnings that should result in an increased level of awareness and the continued protection for surgical staff, even during open surgery and MIS.7 , 28
Because of the potential aerosolization of SARS-CoV-2, surgeons, and others, have had reservations regarding performing colonoscopy and CT colonoscopy, as confirmed by our survey. For patients already awaiting diagnostic tests and those with worrying symptoms, these reservations could be concerning to patients and surgeons. For patients waiting to undergo such diagnostic tests, their priority will need to be readdressed with consideration of either postponing or offering other noninvasive diagnostic tests to exclude colorectal cancer. Noninvasive stool and blood tests to excluded colorectal cancer or inflammatory bowel disease can assist in the prioritization for a confirmatory diagnostic invasive test. Patients with concerning symptoms for colorectal cancer could be offered a fecal immunochemical test (FIT) or multitarget stool DNA test (FIT-DNA), if they do not have rectal bleeding. Both of these tests have proven excellent accuracy for excluding colon cancer.29, 30, 31 Unpublished data from the NICE FIT study which examined the role of FIT on 9822 patients referred with both high- and low-risk symptoms, found that for a threshold of 10 μg hemoglobin/1 g, the negative predictive value was 99.6%. Thus, a negative FIT result can provide reassurance because the chance of the patient not having cancer is 99.6%. Therefore, for patients worried about their symptoms in the era of the pandemic, when health care services are already stretched and physicians cannot provide a timely review, a negative FIT result could be reassuring. For patients with rectal bleeding, the methylated SEPT9 test would be a better option for excluding colorectal cancer.32 The FIT-DNA has increased sensitivity for detecting advanced precancerous lesions compared with FIT alone when screening a population with an average risk of colorectal cancer.30 Mowat et al33 reported that the use of such noninvasive tests could reduce the burden required by invasive tests by > 40%. Because most nations that have been debilitated by the COVID-19 pandemic are now beyond the curve (ie, effective reproduction number < 1) and in the recovery phase, a stepwise resumption of prioritized elective endoscopy services can begin, guided by available hospital endoscopy space, the availability of personnel and equipment supplies, and increased infection prevention training and self-protection for staff. A practice statement from the Asian Pacific Society for Digestive Endoscopy has provided a thorough guide on how to implement endoscopy services.34
The perioperative risk of colorectal surgery in the COVID-19 pandemic must be assessed. In a small retrospective analysis of 34 operative patients who developed COVID-19 pneumonia shortly after surgery, 15 (44.1%) had required admission to the intensive care unit care, and the mortality rate was 20.5%.35 Because the effects of major surgery and infection with COVID-19 in the immediate postoperative phase can lead to significantly increased morbidity and mortality, surgery should continue to be deferred for patients with high-risk factors. These factors include age ≥ 70 years and a history of chronic obstructive pulmonary disease, diabetes, hypertension, cardiovascular disease, and cerebrovascular disease.36 Thus, the perioperative risk of patients should be stratified further using these factors and the recommendations from the American Society of Anesthesiologists.37
Patients with cancer are more susceptible to infection because of the immunosuppressive state caused by the malignancy and anticancer treatment. Thus, one might expect these patients to have an increased risk of COVID-19 and poorer outcomes.38 Colorectal cancer will typically grow for many months and years before clinical presentation; thus, one might expect that delaying surgery would not have negative effects on patient outcomes. A number of studies have shown that the deferral of surgery for 8 to 12 weeks is unlikely to have any effects on the survival outcomes of patients with colon cancer.39, 40, 41 A study of 4685 patients found no association between treatment delays and reduced overall survival in patients with colon cancer.39 Compared with patients who had undergone surgery in the first week after the diagnosis, that study found no increased risk of death with a waiting time of >84 days.39 Their findings were also supported by Hangaard Hansen et al.40 Their systematic review included 5 retrospective and prospective observational studies, with 13,514 patients. The treatment delay intervals ranged from 1 to 56 days, and they concluded that the available data showed no association between treatment delays and reduced overall survival in patients with colon cancer.40
No consensus has been reached regarding the optimal point at which to initiate adjuvant chemotherapy after surgery for stage III colon cancer. Most oncologists, because of the possibility of postoperative complications, have preferred to initiate chemotherapy within 12 weeks after surgery. However, the interval between surgery and beginning adjuvant chemotherapy for stage III colon cancer should be < than 8 weeks because interval of > 8 weeks could be associated with significantly worse overall survival.42 Because the survival benefit of adjuvant therapy given > 3 months after surgery has been questionable, an analysis of the risks and benefits of timely chemotherapy after surgery for patients who might benefit should be performed for each patient.42
For rectal cancer (stage I-II), surgical deferral could be considered. However, for patients with stage T1 lesions of the rectum, a strong argument can be made for transanal endoscopic microsurgery (TEMS) or endoscopic submucosal dissection as an organ preservation procedure, because deferral will likely result in progression and the subsequent requirement for anterior resection. In addition, during the COVID-19 pandemic, one could argue that offering all patients the nonstandard approach of short-course preoperative radiotherapy (SCPRT). A multicenter study of SCPRT, with TEMS performed 8 weeks later, for patients with early-stage rectal cancer (stage T1-T2) showed that 32% of high-risk patients and those refusing total mesorectal excision who had undergone this approach had obtained a complete pathologic response after TEMS of the residual lesion or scar. Because early-stage rectal tumors appear to have a better response to neoadjuvant therapy than advanced lesions, the advantage of this approach is that it reduces the requirement for surgery or endoscopic resection during the peak and aftermath of the pandemic and, therefore, could avoid the need for any interventional procedures after radiotherapy. However, because SCPRT followed by TEMS is a nonstandard approach, discussion and debate will be required in one’s representative colorectal MDT.43
Both SCPRT (5 × 5 Gy) followed by immediate surgery and long-course (25-28 × 2-1.8 Gy) chemoradiotherapy are standard neoadjuvant strategies. However, during the COVID-19 pandemic, providing SCPRT and a delayed of surgery for 8 to 10 weeks might be the better strategy. The latter strategy has been supported by the results from the recently reported Stockholm III trials44 and a review by Bujko et al45 on neoadjuvant radiotherapy (5 × 5 Gy) followed by immediate versus delayed surgery. The approach in the RAPIDO trial for patients with high-risk rectal cancer of SCPRT followed by chemotherapy (6 cycles of CAPOX [capecitabine, oxaliplatin] or 9 cycles of FOLFOX-4 [folinic acid, 5-fluorouracil, oxaliplatin]) and subsequent surgery could be a surgical option during the COVID-19 pandemic.46 However, the primary endpoint and long-term outcomes with this approach have not yet been determined from the trial.46
In patients who have completed chemoradiotherapy and require surgery, one option could be to extend the interval to surgery to 14 to 16 weeks if the restaging magnetic resonance imaging findings after neoadjuvant therapy have demonstrated favorable tumor regression. Favorable tumor regression has generally been associated with good overall and disease-free survival.47, 48, 49, 50 Sloothaak et al47 reviewed the data from 1593 Dutch patients who had undergone preoperative chemoradiotherapy for rectal cancer. They showed that the maximal benefit of neoadjuvant chemoradiotherapy was 16 weeks.47 The role of adjuvant chemotherapy after preoperative chemotherapy is a controversial issue. Other than the recent review by Glimelius51 and the randomized phase II ADORE trial, reported in 2019,52 no new randomized trials using current oncologic drugs and protocols have been performed since 2015. Because the previous studies were small and prematurely terminated owing to poor patient compliance with completing treatment, it is difficult to be certain that adjuvant chemotherapy will significantly reduce the risk of recurrence for patients who have received neoadjuvant chemoradiotherapy. Therefore, it might be very reasonable to be highly selective in recommending adjuvant chemotherapy, thereby reducing unnecessary additional hospital visits and risky immunosuppressive therapy during the COVID-19 pandemic.
For patients presenting with malignant large bowel obstruction and stage IV disease, stenting should be the option of choice. For patients with malignant large bowel obstruction and stage I-III disease, one should follow the European Society of Gastrointestinal Endoscopy 2014 clinical guidelines. These guidelines have stated that self-expandable metal stents (SEMSs) should be used, if possible. Because of the complications and risks of stenting, during COVID-19, stent placement should only be considered as an alternative to emergency surgery for those with an increased risk of postoperative mortality (ie, American Society of Anesthesiologists physical status class ≥ III and/or age > 70 years).53 For healthier patients, the guidelines have not recommended SEMS placement as a bridge to elective surgery to treat malignant colonic obstruction, although during the pandemic, SEMS placement could be considered. However, the stent perforation risk of ~5.88% reported in a recent Cochrane review, concerns regarding the risk of microperforation, which could lead to an increased incidence of perineural invasion, and concerns for poorer oncologic survival and overall systemic recurrence should be considered.54, 55, 56 To reduce the risk of SEMS-related complications, stent procedures will be best performed in units already providing this service. Otherwise, patients should undergo resection or proximal diversion during the pandemic. Stenting of patients without obstruction should not be performed, even if one might not be able to traverse a malignant lesion endoscopically.
For patients already receiving adjuvant or neoadjuvant chemotherapy, whenever possible, the treatment should continue. However, to prevent COVID-19 infection in these immunosuppressed patients, they should be shielded and should self-isolate. For patients who contract COVID-19 during therapy, the treatment can begin again once they have clinically recovered from the disease and have had ≥ 2 negative oronasopharyngeal SARS-CoV-2 RT-PCR swab test results per protocol.
Conclusion
Our global colorectal surgical community has been greatly affected by COVID-19, and we hope that our report has provided some further guidance. Although much of the world is on the downside of the curve with COVID-19, we anticipate the pandemic to continue to severely affect our care of our patients for many more months. Before submitting our report in late May, further communication with the 99 respondents who had provided their e-mail address showed 98% still wished for more guidance from ISUCRS. Therefore, we hope that the present comprehensive report will provide further guidance to our colorectal colleagues who will be attempting to reestablish their practice and reduce risk and unnecessary morbidity and mortality for our patients.
Clinical Practice Points
-
•
The present study used a survey to global colorectal surgeons to assess the effects of COVID-19 on colorectal practice and surgery from April 16 to 28, 2020, which was completed by 287 surgeons.
-
•
Although a number of COVID-19–related studies have been reported, to the best of our knowledge, no survey of colorectal practice and surgery has been as detailed and robust as ours.
-
•
Despite a reasonable number of national surgical and colorectal societies providing guidance and recommendations, further communication with our respondents revealed that 99% still wished for more guidance from the ISUCRS regarding the management of colorectal disease in their practice.
-
•
Although much of the world is on the downside of the curve with COVID-19, we anticipate the pandemic to continue to severely affect our care of our patients for many more months; therefore, we have provided a thorough discussion and extensive review of the reported data to provide a robust and holistic report for colorectal cancer management and all aspects for colorectal surgical practice during the COVID-19 pandemic.
Disclosure
The authors declare that they have no competing interests.
Acknowledgments
We thank Alison Walker for English language proof reading.
Footnotes
A complete list of the participating investigators is provided in Supplemental Appendix 1.
Supplemental Appendix accompanying this article can be found in the online version at https://doi.org/10.1016/j.clcc.2020.05.011.
Contributor Information
ISUCRS COVID-19 Participating Investigator Group:
Abdel Elsayed, Abraham Ayantunde, Ahamaduz Zaman, Ahmed Adam, Aileen McKinley, Alexandre Marsillac, Aliaa Shamardal, Al-Radjid Jamiri, Amjad Khushal, Andrew Allison, Arda Isik, Arman Erkan, Asif Haq, Asif Mehraj, Avdyl Krasniqi, Ayse Unal, Bard Cosman, Ben Griffiths, Bharat Nara, Chang Foo, Christo Lapa, Cristopher Varela, D.K. Dwivedi, Dainius Simcikas, Dragoslav Mladenovikj, Federico Yazyi, Fernanda Elias-Rabelo, Frances McNicol, Georgia Dedemadi, Georgia Dimopoulou, Gian Binda, Giovanni Brandimarte, Giuseppe Brisinda, Glenn Parker, Hamid Khawaja, Harald Geogloman, Hugh Gallagher, Ibrahim Gecim, Igor Pravosudov, Ilario Froehner, Irida Dajti, Islam Abdelmoneim, Jack Lee, James McCormick, Jean-Jacques Tuech, João Rodrigues, Jonathan Robinson, Jorge de-León-Rendón, Jorge Reina, Júlio Leite, Kaluthanthiri De Silva, Katie Cross, Keiichi Takahashi, Lava Kannappa, Lawrence Toquero, Maria Brochado, Rashidul Islam, Mert Tanal, Michele Rubbini, Mihail Slavchev, Mirza Saeed, Mohammad Khalil, Nasir Iqbal, Nirmal Sah, Olu Oluwajobi, Orhan Bulut, Paola De Nardi, Paulo Castro Junior, Puthucode Haray, Rami Makhoul, Raul Fonseca, Robert Talbot, Ruben Martins, Sami Benli, Sebastián Uribe, Sender Liberman, Sergio Martinez, Serkan Tayar, Shingo Tsujinaka, Simon Ng, Stefan Neagu, Sumit Sood, Tahir Saleem, Tahsin Colak, Tamer El Zalabany, Usman Khn, Vladislav Stoyanov, William Cirocco, Wilson Kiraitu, Xavier Delgadillo, Yoshihiko Nakamoto, Yukihiro Hamahata, Vytautas Kvedaras, Zahirul Huq, and Zouari Khadija
Supplemental Appendix 1. Participating Investigators
The participating investigators were as follows: Abdel Elsayed, Abraham Ayantunde, Ahamaduz Zaman, Ahmed Adam, Aileen McKinley, Alexandre Marsillac, Aliaa Shamardal, Al-Radjid Jamiri, Amjad Khushal, Andrew Allison, Arda Isik, Arman Erkan, Asif Haq, Asif Mehraj, Avdyl Krasniqi, Ayse Unal, Bard Cosman, Ben Griffiths, Bharat Nara, Chang Foo, Christo Lapa, Cristopher Varela, D. K. Dwivedi, Dainius Simcikas, Dragoslav Mladenovikj, Federico Yazyi, Fernanda Elias-Rabelo, Frances McNicol, Georgia Dedemadi, Georgia Dimopoulou, Gian Binda, Giovanni Brandimarte, Giuseppe Brisinda, Glenn Parker, Hamid Khawaja, Harald Geogloman, Hugh Gallagher, Ibrahim Gecim, Igor Pravosudov, Ilario Froehner, Irida Dajti, Islam Abdelmoneim, Jack Lee, James McCormick, Jean-Jacques Tuech, João Rodrigues, Jonathan Robinson, Jorge de-León-Rendón, Jorge Reina, Júlio Leite, Kaluthanthiri De Silva, Katie Cross, Keiichi Takahashi, Lava Kannappa, Lawrence Toquero, Maria Brochado, Rashidul Islam, Mert Tanal, Michele Rubbini, Mihail Slavchev, Mirza Saeed, Mohammad Khalil, Nasir Iqbal, Nirmal Sah, Olu Oluwajobi, Orhan Bulut, Paola De Nardi, Paulo Castro Junior, Puthucode Haray, Rami Makhoul, Raul Fonseca, Robert Talbot, Ruben Martins, Sami Benli, Sebastián Uribe, Sender Liberman, Sergio Martinez, Serkan Tayar, Shingo Tsujinaka, Simon Ng, Stefan Neagu, Sumit Sood, Tahir Saleem, Tahsin Colak, Tamer El Zalabany, Usman Khn, Vladislav Stoyanov, William Cirocco, Wilson Kiraitu, Xavier Delgadillo, Yoshihiko Nakamoto, Yukihiro Hamahata, Vytautas Kvedaras, Zahirul Huq, and Zouari Khadija.
References
- 1.CovidSurg Collaborative. Nepogodiev D., Bhangu A. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. https://doi.org/10.1002/bjs.11746 [e-pub ahead of print]. Br J Surg. accessed May 17, 2020. [DOI] [PMC free article] [PubMed]
- 2.Wu J., Liu J., Li S. Detection and analysis of nucleic acid in various biological samples of COVID-19 patients. https://doi.org/10.1016/j.tmaid.2020.101673 [e-pub ahead of print]. Travel Med Infect Dis. accessed May 17, 2020. [DOI] [PMC free article] [PubMed]
- 3.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. https://doi.org/10.1148/radiol.2020200642 [e-pub ahead of print]. Radiology. accessed May 17, 2020. [DOI] [PMC free article] [PubMed]
- 4.Lippi G., Simundic A.-M., Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19) https://doi.org/10.1515/cclm-2020-0285 [e-pub ahead of print]. Clin Chem Lab Med. accessed May 17, 2020. [DOI] [PubMed]
- 5.Fang Y., Zhang H., Xie J. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. https://doi.org/10.1148/radiol.2020200432 [e-pub ahead of print]. Radiology. accessed May 17, 2020. [DOI] [PMC free article] [PubMed]
- 6.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung W.K., To K., Chan P.K.S. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart C.L., Thornblade L.W., Diamond D.J. Personal protective equipment and COVID-19—a review for surgeons. https://doi.org/10.1097/SLA.0000000000003991 [e-pub ahead of print]. Ann Surg. accessed May 17, 2020. [DOI] [PMC free article] [PubMed]
- 12.Kulkarni H., Smith C.M., Lee D.D.H., Hirst R.A., Easton A.J., O’Callaghan C. Evidence of respiratory syncytial virus spread by aerosol: time to revisit infection control strategies? Am J Respir Crit Care Med. 2016;194:308–316. doi: 10.1164/rccm.201509-1833OC. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H., Li X., Ma R. Airborne spread and infection of a novel swine-origin influenza A (H1N1) virus. Virol J. 2013;10:204. doi: 10.1186/1743-422X-10-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adhikari U., Chabrelie A., Weir M. A case study evaluating the risk of infection from Middle Eastern respiratory syndrome coronavirus (MERS-CoV) in a hospital setting through bioaerosols. Risk Anal. 2019;39:2608–2624. doi: 10.1111/risa.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu I.T.S., Li Y., Wong T.W. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 16.Kim J.-M., Chung Y.-S., Jo H.J. Identification of coronavirus isolated from a patient in Korea with COVID-19. Osong Public Health Res Perspect. 2020;11:3–7. doi: 10.24171/j.phrp.2020.11.1.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Song Y., Hu X., Yan L., Zhu X. Awareness of surgical smoke hazards and enhancement of surgical smoke prevention among the gynecologists. J Cancer. 2019;10:2788–2799. doi: 10.7150/jca.31464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veziant J., Bourdel N., Slim K. Risks of viral contamination in healthcare professionals during laparoscopy in the COVID-19 pandemic. https://doi.org/10.1016/j.jviscsurg.2020.04.010 [e-pub ahead of print]. J Visc Surg. accessed May 17, 2020. [DOI] [PMC free article] [PubMed]
- 20.Cook T.M., El-Boghdadly K., McGuire B., McNarry A.F., Patel A., Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: guidelines from the Difficult Airway Society, the Association of Anaesthetists, the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020;75:785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mowbray N.G., Ansell J., Horwood J. Safe management of surgical smoke in the age of COVID-19. https://doi.org/10.1002/bjs.11679 [e-pub ahead of print]. Br J Surg. accessed May 17, 2020. [DOI] [PMC free article] [PubMed]
- 22.Kwak H.D., Kim S.-H., Seo Y.S., Song K.-J. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857–863. doi: 10.1136/oemed-2016-103724. [DOI] [PubMed] [Google Scholar]
- 23.Weyandt G.H., Tollmann F., Kristen P., Weissbrich B. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141–144. doi: 10.1007/s00403-010-1119-3. [DOI] [PubMed] [Google Scholar]
- 24.Ferenczy A., Bergeron C., Richart R.M. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114–118. [PubMed] [Google Scholar]
- 25.Vigneswaran Y., Prachand V.N., Posner M.C., Matthews J.B., Hussain M. What is the appropriate use of laparoscopy over open procedures in the current COVID-19 climate? https://doi.org/10.1007/s11605-020-04592-9 [e-pub ahead of print]. J Gastrointest Surg. accessed May 17, 2020. [DOI] [PMC free article] [PubMed]
- 26.Tan Z., Phoon P.H.Y., Zeng L.A. Response and operating room preparation for the COVID-19 outbreak: a perspective from the National Heart Centre in Singapore. https://doi.org/10.1053/j.jvca.2020.03.050 [e-pub ahead of print]. J Cardiothorac Vasc Anesth. accessed May 17, 2020. [DOI] [PMC free article] [PubMed]
- 27.Weber A., Willeke K., Marchioni R. Aerosol penetration and leakage characteristics of masks used in the health care industry. Am J Infect Control. 1993;21:167–173. doi: 10.1016/0196-6553(93)90027-2. [DOI] [PubMed] [Google Scholar]
- 28.Coccolini F., Tartaglia D., Puglisi A. SARS-CoV-2 is present in peritoneal fluid in COVID-19 patients. Ann Surg. 2020 doi: 10.1097/SLA.0000000000004030. https://journals.lww.com/annalsofsurgery/Documents/SARS-CoV-2%20is%20present%20in%20peritoneal%20fluid%20in%20COVID-19%20patients.pdf Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J.K., Liles E.G., Bent S., Levin T.R., Corley D.A. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160:171. doi: 10.7326/M13-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosch L.J.W., Melotte V., Mongera S. Multitarget stool DNA test performance in an average-risk colorectal cancer screening population. Am J Gastroenterol. 2019;114:1909–1918. doi: 10.14309/ajg.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunin L., Khan A.A., Ibrahim M., Lango A., Klimovskij M., Harshen R. FIT negative cancers: a right-sided problem? Implications for screening and whether iron deficiency anaemia has a role to play. https://doi.org/10.1016/j.surge.2020.02.003 [e-pub ahead of print]. Surgeon. accessed May 17, 2020. [DOI] [PubMed]
- 32.Hariharan R., Jenkins M. Utility of the methylated SEPT9 test for the early detection of colorectal cancer: a systematic review and meta-analysis of diagnostic test accuracy. BMJ Open Gastroenterol. 2020;7:e000355. doi: 10.1136/bmjgast-2019-000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mowat C., Digby J., Strachan J.A. Faecal haemoglobin and faecal calprotectin as indicators of bowel disease in patients presenting to primary care with bowel symptoms. Gut. 2016;65:1463–1469. doi: 10.1136/gutjnl-2015-309579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu P.W.Y., Ng S.C., Inoue H. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements) Gut. 2020;69:991–996. doi: 10.1136/gutjnl-2020-321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lei S., Jiang F., Su W. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;71:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stahel P.F. How to risk-stratify elective surgery during the COVID-19 pandemic? Patient Saf Surg. 2020;14:8. doi: 10.1186/s13037-020-00235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagaria S.P., Heckman M.G., Diehl N.N., Parker A., Wasif N. Delay to colectomy and survival for patients diagnosed with colon cancer. J Invest Surg. 2019;32:350–357. doi: 10.1080/08941939.2017.1421732. [DOI] [PubMed] [Google Scholar]
- 40.Hangaard Hansen C., Gögenur M., Tvilling Madsen M., Gögenur I. The effect of time from diagnosis to surgery on oncological outcomes in patients undergoing surgery for colon cancer: a systematic review. Eur J Surg Oncol. 2018;44:1479–1485. doi: 10.1016/j.ejso.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Turaga K.K., Girotra S. Are we harming cancer patients by delaying their cancer surgery during the COVID-19 pandemic? https://doi.org/10.1097/SLA.0000000000003967 [e-pub ahead of print]. Ann Surg. accessed May 17, 2020. [DOI] [PMC free article] [PubMed]
- 42.Gao P., Huang X.-Z., Song Y.-X. Impact of timing of adjuvant chemotherapy on survival in stage III colon cancer: a population-based study. BMC Cancer. 2018;18:234. doi: 10.1186/s12885-018-4138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smart C.J., Korsgen S., Hill J. Multicentre study of short-course radiotherapy and transanal endoscopic microsurgery for early rectal cancer. Br J Surg. 2016;103:1069–1075. doi: 10.1002/bjs.10171. [DOI] [PubMed] [Google Scholar]
- 44.Erlandsson J., Holm T., Pettersson D. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18:336–346. doi: 10.1016/S1470-2045(17)30086-4. [DOI] [PubMed] [Google Scholar]
- 45.Bujko K., Partycki M., Pietrzak L. Neoadjuvant radiotherapy (5 × 5 Gy): immediate versus delayed surgery. Recent Results Cancer Res. 2014;203:171–187. doi: 10.1007/978-3-319-08060-4_12. [DOI] [PubMed] [Google Scholar]
- 46.van der Valk M.J.M., Marijnen C.A.M., van Etten B. Compliance and tolerability of short-course radiotherapy followed by preoperative chemotherapy and surgery for high-risk rectal cancer—results of the international randomized RAPIDO-trial. Radiother Oncol. 2020;147:75–83. doi: 10.1016/j.radonc.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 47.Sloothaak D.A.M., Geijsen D.E., van Leersum N.J. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg. 2013;100:933–939. doi: 10.1002/bjs.9112. [DOI] [PubMed] [Google Scholar]
- 48.Patel U.B., Taylor F., Blomqvist L. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29:3753–3760. doi: 10.1200/JCO.2011.34.9068. [DOI] [PubMed] [Google Scholar]
- 49.Habr-Gama A., São Julião G.P., Fernandez L.M. Achieving a complete clinical response after neoadjuvant chemoradiation that does not require surgical resection: it may take longer than you think! Dis Colon Rectum. 2019;62:802–808. doi: 10.1097/DCR.0000000000001338. [DOI] [PubMed] [Google Scholar]
- 50.West M.A., Dimitrov B.D., Moyses H.E. Timing of surgery following neoadjuvant chemoradiotherapy in locally advanced rectal cancer—a comparison of magnetic resonance imaging at two time points and histopathological responses. Eur J Surg Oncol. 2016;42:1350–1358. doi: 10.1016/j.ejso.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Glimelius B. Adjuvant chemotherapy in rectal cancer: state of the art and future perspectives. Curr Opin Oncol. 2020;32:377–383. doi: 10.1097/CCO.0000000000000641. [DOI] [PubMed] [Google Scholar]
- 52.Hong Y.S., Kim S.Y., Lee J.S. Oxaliplatin-based adjuvant chemotherapy for rectal cancer after preoperative chemoradiotherapy (ADORE): long-term results of a randomized controlled trial. J Clin Oncol. 2019;37:3111–3123. doi: 10.1200/JCO.19.00016. [DOI] [PubMed] [Google Scholar]
- 53.van Hooft J.E., van Halsema E.E., Vanbiervliet G. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2014;46:990–1053. doi: 10.1055/s-0034-1390700. [DOI] [PubMed] [Google Scholar]
- 54.Sagar J. Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Syst Rev. 2011;11:CD007378. doi: 10.1002/14651858.CD007378.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haraguchi N., Ikeda M., Miyake M. Colonic stenting as a bridge to surgery for obstructive colorectal cancer: advantages and disadvantages. Surg Today. 2016;46:1310–1317. doi: 10.1007/s00595-016-1333-5. [DOI] [PubMed] [Google Scholar]
- 56.Foo C.C., Poon S.H.T., Chiu R.H.Y., Lam W.Y., Cheung L.C., Law W.L. Is bridge to surgery stenting a safe alternative to emergency surgery in malignant colonic obstruction: a meta-analysis of randomized control trials. Surg Endosc. 2019;33:293–302. doi: 10.1007/s00464-018-6487-3. [DOI] [PubMed] [Google Scholar]