Dear Editor,
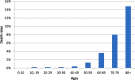
There is a pandemic that suddenly appeared on the agenda of humanity for the past few months. When COVID-19 related deaths are analyzed, higher mortality rates in older people and men are quite evident in all case series [1], [2], [3] (Table1 ). Hypertension is obviously the leading comorbidity in all studies followed by diabetes, obesity and other cardiovascular disorders. Angiotensin-converting enzyme 2(ACE2), which is the receptor for SARS-CoV-2 [4], is a regulator of vascular function by modulating nitric oxide (NO) release and oxidative stress [5]. We realized that the common prominent feature in older vs younger, hypertensive vs healthy, men vs women comparison is decreased endothelial NO production and decreased NO bioavailability which also links cardiovascular and metabolic diseases as their common deficiency.
Table 1.
Case fatality rate in Covid-19 in China according to CDC Weekly / Vol.2 / No.8 data.
 |
Physiological NO signaling is a key determinant of endothelial function, metabolic and vascular health. It is major regulator of vascular tone and has antioxidant, antiinflammatory and antithrombotic activities [6]. Endothelial dysfunction leads to a shift of endothelial cell actions by various chemokines, cytokines and other factors and finally induces proinflammatory, proliferative and prothrombotic status [7], [8].
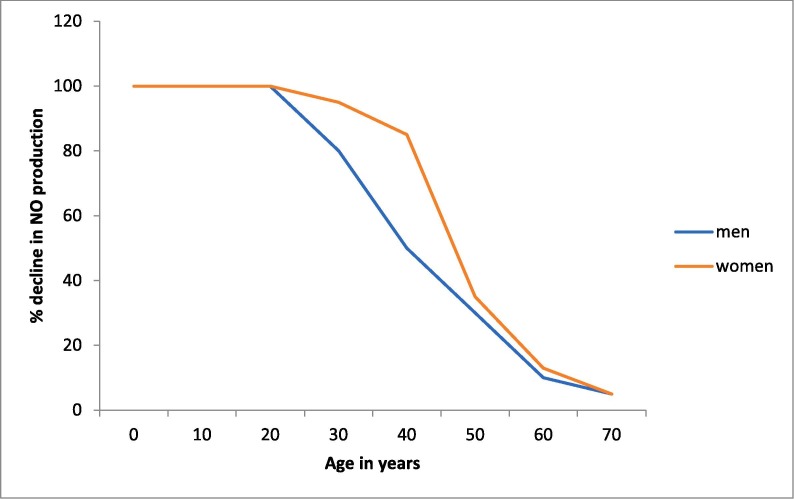
Previous studies have shown that age is the most significant predictor of the endothelium dependent vasodilatation and NO availability progressively declines with aging [9], [10], [11], [12] (Fig. 1 ). The vascular changes associated with essential hypertension, such as endothelial dysfunction, are generally considered to be an accelerated form of changes seen in aging [12].
Fig. 1.
Hypothetical representation of endothelial nitric oxide (NO) production based on sex (adapted from previous published studies [9], [10], [11], [12], [26]).
Estrogen increases expression and activity of endothelial nitric oxide synthase (eNOS) and NO production is higher in the systemic vasculature of females [13]. Females are known also to produce higher innate and adaptive immunity responses than males which can result in faster clearance of viruses [14].
COVID-19 mortality has correlation with the decrease in endothelial NO production and bioavailability in different age groups and/or comorbidities. Whether this is a direct antiviral effect or associated with immune modulation needs to be further investigated. This suggests that increasing endothelial NO availability should be directly effective in antiviral resistance against COVID-19.
Diet rich in polyphenols, caloric restriction and regular exercise are non-pharmacological strategies to increase endothelial NO production and bioavailability [6], [15]. In situations like aging, cardiovascular and metabolic disorders where endothelial NO production is compromised, there are considerable published data suggesting that nitroso compounds can act as storage pools and donors of NO and can compensate for insufficient eNOS activity. Systemic levels of nitroso compounds can be greatly influenced by vegetables rich in nitrates [15], [16], [17], [18], [19], [20].
ACE2 expression was shown in vascular endothelium and arterial smooth muscle of all organs and on alveolar epithelial cells and enterocytes of small intestine [21]. From this aspect it is noteworthy, there are also studies reporting that there is a marked increase in exhaled NO after dietary nitrate consumption [22], [23] and NO produced from nitrite in the upper intestine is up to 10,000 times the concentrations that occur in tissues from enzymatic synthesis [12]. Another interesting point is that despite being an important risk factor in a large spectrum of diseases, cigarette smoke contains huge amounts of nitrogen oxides and smoking habits can increase the blood pool of nitroso compounds [24].
Âkerström et al demonstrated that the inhibitory effect of NO on SARS-CoV is happening inside the cells, where viral material is out of the capsid [25]. We speculate that if we divide the total NO amount in this study to the cell count in the media, the results will be comparable to physiological NO production levels in the vascular endothelium. From this aspect, in the endothelium, especially in early stages of viral invasions, when viral material is in low amounts and out of the capsid, any amount of NO produced inside the cell should have effect on susceptible viruses and a healthy eNOS function may be very valuable as a first line defense.
Conclusions
Due to the urgent nature of the COVID-19 pandemic, simultaneous learning and practice is inevitable. The relation between decreasing NO production and bioavailability with COVID-19 mortality has potential to open alternative preventive and therapeutic ways.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, CDC Weekly 2020; 2(8):1-10. [PMC free article] [PubMed]
- 2.Characteristics of COVID-19 patients dying in Italy report based on available data on March 20th, 2020. Istitvto Svperiore Di Sanita. https://www.epicentro.iss.it.
- 3.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in New York city area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M., Kleine-Weber H., Kruger N. The novel coronavirus 2019(2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRS S2 for entry into target cells. Cell. 2020;181(2):271–280. [Google Scholar]
- 5.Rabelo L.A., Todiras M., Nunes-Souza V. Genetic deletion of ACE2 induces vascular dysfunction in C57BL/6 mice: role of nitric oxide imbalance and oxidative stress. PLoS One. 2016;12(4) doi: 10.1371/journal.pone.0150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine A.B., Punihook D., Levine T.B. The role of nitric oxide and its clinical applications. Cardiology. 2012;122(1):55–68. doi: 10.1159/000338150. [DOI] [PubMed] [Google Scholar]
- 7.Försterman U. Nitric oxide and oxidative stress in vascular disease. Pflügers Arch. 2010;459(6):923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 8.Yang X.Z., Chang Y., Wei W. Endothelial dysfunction and inflammation: immunity in rheumatoid arthritis. Mediators Inflamm. 2016;2016:6813016. doi: 10.1155/2016/6813016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerhard M., Roddy M.A., Creager S.J., Creager M.A. Aging progressively impairs endothelium-dependent vasodilatation in forearm resistance vessels of humans. Hypertension. 1996;27(4):849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- 10.Egashira K., Inou T., Hirooka Y., Kai H., Sugimachi M., Suzuki S. Effects of age on endothelium-dependent vasodilatation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;8(1):77–81. doi: 10.1161/01.cir.88.1.77. [DOI] [PubMed] [Google Scholar]
- 11.Taddei S., Virdis A., Ghisdoni L., Salvetti G., Bernini G., Magagna A. Age related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38(2):274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 12.Torregrossa A.C., Aranke Nathan Bryan. Nitric oxide and geriatrics: implications in diagnostics and treatment of the elderly. J Geriatric Cardiol. 2011;8(4):230–242. doi: 10.3724/SP.J.1263.2011.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nevzati E., Shafighi M., Bakhtian K.D., Treiber H., Fandano J., Fathi A.R. Estrogen induces nitric oxide production via nitric oxide synthase activation in endothelial cells. Acta Neurochir Suppl. 2015;120:141–145. doi: 10.1007/978-3-319-04981-6_24. [DOI] [PubMed] [Google Scholar]
- 14.Klein S.L. Sex influences immune responses to viruses, and efficacy of prophylaxis and therapeutic treatments for viral diseases. BioEssays. 2012;34(12):1050–1059. doi: 10.1002/bies.201200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forte M, Conti V, Damato A, Mariateresa Ambrosio, Annibale A. Puca, Sebastiano Sciarretta, et all. Targeting nitric oxide with naturally derived compounds as a therapeutic strategy in vascular diseases. Oxidative medicine and cellular longevity 2016; 2016: 7364138. [DOI] [PMC free article] [PubMed]
- 16.Lundberg J.O., Weitzberg E. NO generation from nitrite and its role in vascular control. Arterioscler Thromb Vasc Biol. 2005;25(5):915–922. doi: 10.1161/01.ATV.0000161048.72004.c2. [DOI] [PubMed] [Google Scholar]
- 17.Stanaway L., Marwick K.R., Page R., Wong M., Jirangrat W., Teh K.H. Acute supplementation with nitrate rich beetroot juice causes a greater increase in plasma nitrite and reduction in blood pressure of older compared to younger adults. Nutrients. 2019;11(7):1683–1696. doi: 10.3390/nu11071683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raubenheimer K., Hickey D., Leveritt M., Fassett R., de Zevallos Ortiz, Munoz J. Acute effects of nitrate rich beetroot juice on blood pressure, hemostasis and vascular inflammation markers in healthy older adults: a randomized, placebo-controlled crossover study. Nutrients. 2017;9(11):1270–1289. doi: 10.3390/nu9111270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carstrom M., Montenegro M.F. Therapeutic value of stimulating the nitrate-nitrite-nitric oxide pathway to attenuate oxidative stress and restore nitric oxide bioavailability in cardiorenal disease. Intern Med. 2019;285(1):2–18. doi: 10.1111/joim.12818. [DOI] [PubMed] [Google Scholar]
- 20.Mol. Nutr. Food Res. 2016;60:67–78. doi: 10.1002/mnfr.201500153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamming I., Timens W., Bulthuis M.L. Tissue distribution of ACE 2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroll J., Werchan C., Rosenfield D. Acute ingestion of beetroot juice increases exhaled nitric oxide in healthy individuals. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0191030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olin A.-C., Aldenbratt A., Ekman A., Ljungkvist G., Jungersten L., Alving K. Dietary nitrate supplementation increases functional exhaled nitric oxide: implications for the assessment of airway health in athletes. Respir Med. 2001;95:153–158. doi: 10.1053/rmed.2000.1010. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg J.O. Nitric oxide metabolites and cardiovascular disease, markers, mediators, or both. JACC. 2006;47(7):580–581. doi: 10.1016/j.jacc.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Åkerström S., Gunalan V., Keng C.T., Tan Y.J., Mirazimi A. Dual effect of nitric oxide on SARS- CoV: Viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395(1):1–9. doi: 10.1016/j.virol.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celejmayer D.S., Sorensen K.E., Spiegelhalter D.J. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. JACC. 1994;24(2):471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]