Abstract
Context:
Premature canities etiopathogenesis is unclear, and approach to its therapy remains arbitrary. Reactive oxygen species generated during melanin biosynthesis in anagen hair bulb have been implicated in melanocyte apoptosis and hair graying. Extraneous factors, namely environmental pollution, stressful lifestyle, may compound the melanogenesis-induced endogenous oxidative stress.
Aims:
We aimed to investigate the role of systemic oxidative stress in causation of premature canities and its correlation with the severity of hair graying.
Settings and Design:
This was a tertiary care hospital-based cross-sectional study.
Materials and Methods:
Consecutive 50 patients with premature hair graying, aged <25 years, and 30 age and sex-matched healthy controls were recruited. Severity of premature canities was graded based on the total number of gray hair on the scalp. Redox status was evaluated in cases and controls, by malondialdehyde (MDA), reduced glutathione (rGSH), and superoxide dismutase (SOD) measurement in serum, by enzyme-linked immunosorbent assay.
Results:
Serum MDA concentration, an oxidative stress marker, was significantly higher (P < 0.01), while serum rGSH and SOD levels, both indicators of antioxidant potential, were significantly lower (P < 0.0001 and P < 0.01 respectively) in premature canities patients compared to controls. A novel observation was the significant correlation of serum MDA rise and serum rGSH decline with increasing severity of hair graying (P < 0.01 and P = 0.01, respectively).
Conclusion:
Systemic redox imbalance is present in premature canities patients, with the severity of hair graying varying in parallel to the degree of oxidative stress. Antioxidants supplementation is likely to yield therapeutic benefit in premature canities.
Key words: Oxidative stress, premature canities, premature hair graying, redox imbalance
INTRODUCTION
The etiopathogenesis of premature canities, i.e., graying of hair before the usual age, currently remains an enigma.[1] In melanocytes, melanin synthesis from L-tyrosine occurs through a series of oxidative reactions that generate reactive oxygen species (ROS), namely hydrogen peroxide (H2O2) and free radicals in final stages.[2] Oxidative stress with an excessive generation of ROS, due to an imbalance between prooxidant reactions and antioxidant defense mechanisms, leads to macromolecular and cellular damage.[3] Increased melanocyte apoptosis and oxidative stress in the pigmentary unit of graying hair follicles have been documented by few investigators.[4],[5],[6],[7] Melanogenesis-induced oxidative stress in anagen hair bulbs may be compounded by extraneous factors, namely environmental pollution and stressful lifestyle.[8] The role of systemic oxidative stress in the causation of premature hair graying has not been explored. The present study was designed to evaluate oxidative stress parameters in premature canities patients' blood and to correlate the alterations with the severity of hair graying.
MATERIALS AND METHODS
A cross-sectional observational study was undertaken in the dermatology department of a tertiary care hospital. The study was approved by the Institutional Ethics Committee and the procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000.
Study subjects
Fifty clinically diagnosed consecutive patients of premature canities, aged <25 years, and thirty age- and sex-matched healthy controls were recruited. Informed written consent was obtained from the patients as well as controls before inclusion in the study. The natural hair color of patients in the study population was uniformly black/deep dark brown, without much variation. Patients with less than 5 gray hairs on the scalp were excluded from the study. In addition, patients with graying of hair due to other conditions such as vitiligo and cutaneous disease involving the scalp; patients with history of intake of systemic drugs/supplements, namely iron supplementation or those who had used hair dye/gel in the previous 6 months; current, or past smokers; patients with history of fever/systemic disease within 3 months of onset of premature canities and pregnant/lactating females were also excluded.A detailed clinical history and examination was undertaken for each patient. The number of gray hair was counted using a hand lens in five areas of the scalp, i.e., frontal, vertex, occipital, right, and left temporal regions, and summated to determine the total number of scalp gray hair. The severity of premature canities was graded based on the total number of gray hair on the scalp as mild (5–20 gray hair), moderate (21–50 gray hair), and severe (>50 gray hair).
A fasting venous blood sample (5 mL) was drawn after an overnight fast from cases and controls in plain and EDTA Vacutainers. Serum was separated and stored at −70°C until the analysis. Laboratory investigations included complete hemogram and thyroid function tests in both premature canities cases and controls.
Redox status in patients of premature canities/controls was assessed by the measurement of malondialdehyde (MDA), reduced glutathione (rGSH), and total superoxide dismutase (SOD) concentration in the serum, by enzyme-linked immunosorbent assay (ELISA) using commercial kits mentioned below, as per the manufacturer's protocol.
MDA: Sincere Biotech, China, Catalogue no. E13651173 (Type II)
rGSH: Sincere Biotech, China, Catalogue no. E13650807 (Type II)
SOD: Sincere Biotech, China, Catalogue no. E13651677 (Type II) – measures all human SODs, i.e., type I/type II/type III.
Statistical analysis
Data analysis was done using Chi-square/Fisher's exact test in case of categorical variables and by unpaired t-test/Mann–Whitney U-test/ANOVA/Kruskall–Wallis test for continuous variables, as appropriate. P 0.05 was considered as statistically significant. Statistical analysis was done using Statistical Package for the Social Sciences version 21.0 software (SPSS Inc., Chicago, USA).
RESULTS
Demographic features
The mean age at presentation of patients of premature canities was 19.04 ± 4.28 years (range 9–24 years). Among the 50 recruited premature canities patients, 33 (66%) were male and 17 (34%) were female [Table 1].
Table 1.
Demographic and clinical details of premature canities cases and controls
| Cases (n=50) | Controls (n=30) | P | |
|---|---|---|---|
| Age | |||
| Mean age (years) | 19.04±4.28 | 19.70±3.45 | 0.78 |
| Age range (years), n (%) | 9-24 | 12-24 | |
| ≤15 | 12 (24) | 6 (20) | |
| 16-20 | 16 (32) | 10 (33) | |
| >20 | 22 (44) | 14 (47) | |
| Gender, n (%) | |||
| Male | 33 (66) | 18 (60) | 0.37 |
| Female | 17 (34) | 12 (40) | |
| Clinical features | |||
| BMI (kg/m2) | 21.64±2.96 | 21.54±2.32 | 0.87 |
| Anemia, n (%) | 18 (36) | 13 (43) | 0.38 |
| Hypothyroidism, n (%) | 3 (6) | 1 (3.3) | 0.49 |
BMI – Body mass index
Twenty-five (50%) patients suffered from a severe grade of premature hair graying, while 10 (20%) and 15 (30%) patients had mild and moderate grades of premature canities, respectively.
The mean age of onset of premature hair graying was 16.55 ± 3.68 years, while the median age of onset was 16 years. A significantly greater proportion of patients with onset of hair graying at ≤16 years age had a severe grade of premature canities, compared to those with onset at >16 years age (67% vs. 30.5%, respectively; P = 0.03). Besides, a significantly higher percentage of male patients had a severe grade of premature canities, as compared to females (64% vs. 24%, respectively; P = 0.02) in our cohort [Table 2].
Table 2.
Association of severity of premature canities with the age of onset and gender
| Age of onset/gender | Cases, n (%) | Severity grade of premature canities, n (%) |
P | ||
|---|---|---|---|---|---|
| Mild (n=10) | Moderate (n=15) | Severe (n=25) | |||
| ≤16 years | 27 (100) | 3 (11) | 6 (22) | 18 (67) | 0.03 |
| >16 years | 23 (100) | 7 (30.5) | 9 (39) | 7 (30.5) | |
| Male | 33 (100) | 5 (15) | 7 (21) | 21 (64) | 0.02 |
| Female | 17 (100) | 5 (29) | 8 (47) | 4 (24) | |
Family history of premature canities
The number of patients with a positive family history of premature canities in the first-degree relatives was 17 (34%). No significant association of family history of premature canities with an earlier age of onset/disease progression/severity of hair graying was observed.
Scalp area of origin of premature canities
Premature hair graying originated in the frontal, vertex, and temporal region of the scalp in 30 (60%), 15 (30%), and 5 (10%) patients, respectively. There was no correlation of age of onset or gender, with any specific scalp area of origin of premature hair graying.
Obesity, anemia, and thyroid status
The majority of patients, i.e., 28 (56%), were in the normal body mass index (BMI) category, 6 (12%) were underweight, and 10 (20%) and 6 (12%) patients were overweight and obese, respectively. No significant association of severity of premature canities with an increase in BMI was observed.
Anemia was present in 18 (36%) patients and 3 (6%) patients suffered from hypothyroidism.
No significant difference in the mean BMI and presence/absence of anemia or hypothyroidism between premature canities cases and controls was noted [Table 1].
Oxidative stress and antioxidant defense parameters
Serum malondialdehyde
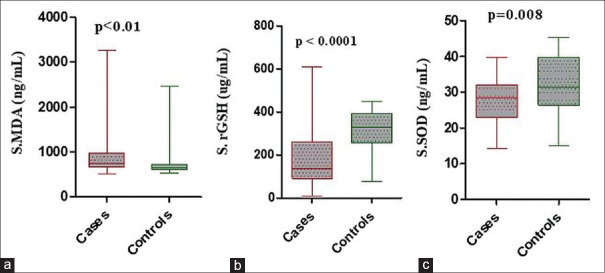
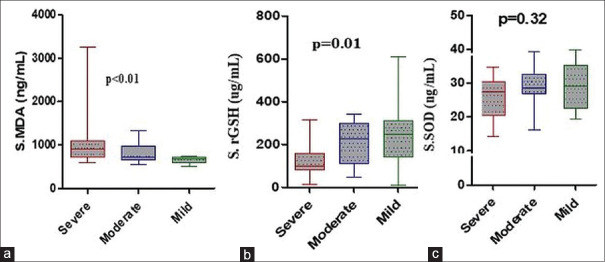
The mean serum MDA levels in premature canities patients (916.0 ± 488.3 ng/mL) were significantly higher than in healthy controls (755.9 ± 363.1 ng/mL) (P < 0.01). Increasing the severity of premature canities correlated with a progressive increase in serum MDA levels, which was statistically significant (P < 0.01) [Tables 3, 4 and Figures 1, 2].
Table 3.
Oxidative stress/antioxidant parameters in sera of premature canities cases and healthy controls
| Parameter (serum concentration) | Mean±SD |
P | |
|---|---|---|---|
| Cases (n=50) | Controls (n=30) | ||
| MDA (ng/mL) | 916.0±488.3 | 755.9±363.1 | <0.01 |
| rGSH (µg/mL) | 177.8±114.5 | 319.0±95.21 | <0.0001 |
| SOD (ng/mL) | 27.45±6.08 | 31.78±8.17 | <0.01 |
SD – Standard deviation; MDA – Malon-di-aldehyde; rGSH – Reduced glutathione; SOD – Superoxide dismutase
Table 4.
Correlation of serum oxidative stress/antioxidant parameters with severity grades in cases of premature canities
| Parameter (serum concentration) | Severity grade of premature canities |
P | ||
|---|---|---|---|---|
| Mild (n=10) | Moderate (n=15) | Severe (n=25) | ||
| MDA (ng/mL) | 660.5±79.18 | 820.6±221.9 | 1075.0±629.6 | <0.01 |
| rGSH (µg/mL) | 248.8±162.9 | 208.7±100.9 | 130.8±76.58 | 0.01 |
| SOD (ng/mL) | 29.03±6.98 | 28.55±5.93 | 26.15±5.75 | 0.32 |
MDA – Malondialdehyde; rGSH – Reduced glutathione; SOD – Superoxide dismutase
Figure 1.
Oxidative stress/antioxidant parameters in sera of premature canities cases and healthy controls. (a) Serum malon-di-aldehyde (b) serum reduced glutathione (c) serum superoxide dismutase
Figure 2.
Association of serum oxidative stress/antioxidant parameters with severity grades in cases of premature canities. (a) Serum malondialdehyde (b) serum reduced glutathione (c) serum superoxide dismutase
Serum reduced glutathione
The mean serum rGSH levels in premature canities cases (177.8 ± 114.5 μg/mL) were significantly lower than in controls (319.0 ± 95.21 μg/mL) (P < 0.0001). A significant parallel decline was observed in serum rGSH levels from mild-to-severe grades of premature canities (P = 0.01) [Tables 3, 4 and Figures 1, 2].
Serum superoxide dismutase
The mean serum SOD levels in cases of premature canities (27.45 ± 6.08 ng/mL) were significantly lower as compared to controls (31.78 ± 8.17 ng/mL) (P < 0.01). A decreasing trend in serum SOD levels was noted with increasing severity of premature canities; however, it did not reach statistical significance [Tables 3, 4 and Figures 1, 2].
DISCUSSION
Premature graying of hair before the usual age (20 years in Caucasians, 25 years in Asians, and 30 years in Africans), though apparently an innocuous disorder, tremendously impacts the self-esteem of young afflicted individuals due to its social perception as a sign of old age.[1] In populations where the natural hair color is deeply pigmented dark brown/black, namely Asians, even few strands of gray hair are noticeable.[9]
The causes of premature canities are not well understood; however, reports in the literature implicate oxidative stress, defective maintenance of melanocyte stem cells, genetic factors, autoimmune disorders, premature aging syndromes, trace element deficiencies, and insufficient neuroendocrine stimulation of melanogenesis, as etiological factors.[1]
Color of human hair depends on melanin biosynthesis, in hair follicular pigmentary unit melanocytes, inside cytosolic organelles termed melanosomes, which are transferred to keratinocytes, mainly in the hair cortex.[10] Melanogenesis in hair follicles is tightly coupled to the hair growth cycle and takes place only during the anagen phase.[11] A marked loss of pigment forming melanocytes from aging hair follicle bulb is central to the pathogenesis of hair graying.[12]
In pigmented hair follicles, melanin synthesis proceeds via tyrosinase-catalyzed initial hydroxylation of L-tyrosine to 3,4-dihydroxy-phenylalanine (DOPA) and subsequent oxidation of DOPA to dopaquinone. Melanins are indole derivatives of dopaquinone, formed through a series of oxidative steps, that in the final stages leading to endogenous production of ROS in bulbar melanocytes.[2]
Excessive generation of ROS leads to macromolecular and cellular damage due to lipid peroxidation, single-strand DNA breaks, mutations, and denaturation of proteins/enzymes.[3] ROS-induced cell membrane damage generates arachidonic acid/other PUFA bicyclic endoperoxides, which subsequently form aldehydes, namely MDA and 4-hydroxynonenal, as secondary end products. MDA crosses cell membranes into the extracellular compartment and its serum concentration represents a sensitive marker of oxidative stress.[13] The in vivo defense against ROS is primarily provided by antioxidant enzymes and Vitamins E and C. The main antioxidant enzymes include superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx).[14]
Harman in 1956 first postulated the “free radical theory of aging,” which implicated a major role of oxidative stress in the aging process.[15] In graying hair follicles, melanocytes are frequently highly vacuolated, a common cellular response to oxidative stress.[16] Redox status in graying hair follicles has been evaluated in mouse models/human subjects in few studies, which suggest oxidative stress as a key underlying mechanism of premature hair graying.[17]
In experimentally created bcl-2-/-mice, Veis et al.[18] observed, in addition to other phenotypes, graying of hair by the second hair growth cycle. They proposed an antioxidant role of the death repressor bcl-2 molecule and suggested that its absence led to derangement of redox-regulated melanin biosynthesis pathway in bcl-2-/-mice.
Nishimura et al.[19] demonstrated a dramatic acceleration of hair graying in a mouse model if the antiapoptotic bcl-2 protein was deficient, which caused a selective apoptosis of melanocyte stem cells in the bulge region of the hair follicle outer root sheath. They concluded that repopulation of early anagen hair bulb with melanocyte precursor cells was likely to fail in advanced age, with resultant graying of hair.
Arck et al.[4] in their study on pigmented, graying, and unpigmented human scalp hair follicles from healthy aging donors demonstrated loss of melanocytes by apoptosis and presence of oxidative damage to DNA in the pigmentary unit of graying hair follicles. Based on these observations, the authors proposed a “free radical theory of graying of hair.”
An in-vivo study by Wood et al.[5] documented accumulation of H2O2 in millimolar concentrations in human scalp gray hair shafts and nearly absent methionine sulfoxide reductases A and B and CAT activity. Limited functionality of tyrosinase, the key enzyme of melanin biosynthesis, observed in gray hair follicles in this study, was consequent to the formation of methionine sulfoxide at the enzyme active site.
It is likely that impairment in antioxidant enzymes with age leads to irreversible damage to melanocytes from their own melanogenesis-related oxidative stress. In an in vitro culture study of human hair follicle melanocytes, from donors of different ages, Kauser et al.[6] observed no difference in the expression of SOD with the advancement of age; however, CAT expression was markedly reduced in melanocytes from older donors. Shi et al.[7] also reported strongly repressed expression of CAT and hydroxyl radical scavenging activities in bulb and bulge region of unpigmented hair follicles, compared to pigmented hair follicles, in patients with premature canities.
In a study conducted in black-haired mouse model, the application of SOD-containing gel on the mice back, was observed to protect against graying of hair, induced by psoralen plus ultraviolet (UV)-A radiation exposure.[20] Transgenic mice expressing Arg213Gly mutation in SOD3 (an extracellular secreted form of SOD) were shown by Kwon et al.[21] to exhibit premature aging, including hair graying.
Hair follicle cells are rapidly proliferating, and therefore, dependent on active DNA synthesis, which requires a sufficient supply of micronutrients, namely Vitamin B12. Sonthalia et al.[22] reported a statistically significant correlation of premature hair graying with a deficiency of Vitamin B12, with 9 out of 71 cases also having anti-parietal cell IgG antibodies, suggestive of pernicious anemia. In this context, an interesting observation is a demonstration of a novel role of Vitamin B12 by Birch et al.,[23] who showed that cobalamins are remarkably effective intracellular antioxidants in vitro.
It has been proposed that the endogenous oxidative stress caused by melanin synthesis in melanocytes in pigmented anagen hair follicles may be aggravated by external factors, namely UV exposure, environmental pollution, psychoemotional stress, smoking, and xenobiotics, which escalate ROS production and deplete the antioxidant capacity. This systemic redox imbalance may lead to permanent damage of hair bulb melanocytes, affecting them selectively and prematurely, thereby hastening the process of hair graying, but its exact role has not been dissected out.[4],[8]
The present study was designed in a clinical setting to assess oxidative stress/antioxidant parameters in the blood of premature canities patients on the premise that it may aid in their prognostic assessment/treatment. No widely accepted or standard scales are available to uniformly assess the severity of premature hair graying, and different scoring systems have been used by various investigators.[24],[25],[26] Systemic biomarkers of redox status measured in blood/serum adequately reflect the redox status of different tissues as reported by Margaritelis et al.,[27] who demonstrated good qualitative and quantitative agreement of MDA, rGSH, and SOD levels in blood and tissues.
In our study, the tilted gender ratio in favor of males could be due to social gender bias and easier tapping of medical services by males and may not reflect true incidence. This male preponderance in premature canities cases has not been observed by other investigators.[26],[28]
We observed significantly higher mean serum MDA levels in cases of premature canities than in controls. Daulatabad et al.[29] also noted significantly higher MDA levels in the sera of premature canities patients in comparison to controls. An interesting observation in our study cohort was the significant progressive elevation in serum MDA levels from mild-to-severe grades of premature canities, which has not been reported earlier.
SOD represents the first line of defense against oxygen-derived free radicals and is rapidly induced in response to oxidative stress.[30] The superoxide free radical is reduced by SOD to less reactive H2O2, which is then degraded to water by the enzymes GPx and/or CAT.[31]
In humans, there are three isoforms of SOD: SOD1 is the major intracellular SOD localized mainly in cytosol (Cu/ZnSOD), SOD2 is located in mitochondrial matrix (MnSOD), and SOD3 is a secretory extracellular Cu/Zn-containing SOD (ecSOD) with primary location in extracellular matrix/cell surfaces and a smaller fraction in plasma/extracellular fluids. In the blood plasma/serum, all the three SOD types may be detectable, as intracellular SOD1 and SOD2 may leak into the circulation due to cellular damage by apoptosis/necrosis.[32]
The measurement of SOD catalytic activity is more biologically relevant than an assay of enzyme mRNA/protein concentration, which may not necessarily reflect an alteration in activity.[33] SOD activity measurement involves the generation of superoxide ion nonenzymically (e.g., by pyrogallol autooxidation) or enzymically (e.g., by xanthine oxidase) in a test medium, which contains an indicator reaction for superoxide (e.g., nitro blue tetrazolium reduction). SOD activity in the test sample is calculated from the degree of SOD-mediated inhibition of indicator reaction.[34],[35] Any entity in reaction mixture which scavenges superoxide/changes rate of superoxide formation/reacts with indicator will lead to erroneous results. These indirect procedures therefore provide only relative estimates of SOD activity in biological samples of comparable composition.[35] There are several reports in the literature of Cu/ZnSOD activity measurement in the blood of normal healthy adults but with variable results.[36],[37]
Direct immunochemical methods, namely ELISA, are highly specific and give an absolute measure of SOD, but the drawback is that antigen concentration is measured and not the catalytic activity.[35] There are no available universally accepted reference ranges in serum for total SOD or specific SOD types. This is apparent from the varying normal ranges quoted by different investigators.[38],[39] Most studies using ELISA for measurement of SOD have compared the levels in cases and matched controls to analyze the alterations, as has been done in the present study.
We observed serum total SOD concentrations measured by ELISA to be significantly lower in premature canities patients compared to controls. A decreasing trend was observed in serum SOD levels from mild-to-severe grades of premature canities; however, the fall was not statistically significant.
We did not come across any report in the literature of measurement of SOD in the blood of premature canities cases. A meta-analysis of different studies to analyze alterations in SOD levels in vitiligo patients reported inconsistent results and suggested that this could be due to variations in activity and duration of disease, different sample sources, or different laboratory analyses.[40] SOD can be rapidly induced on exposure to oxidative stress, which may be followed by a subsequent fall, due to exhaustion of antioxidant potential/enzyme inactivation.[30] This could explain in our study the observed lower SOD levels in premature canities cases and their lack of correlation with disease severity, which may be due to variation in disease activity/duration.
GPx reduces H202 using rGSH, which is simultaneously converted to oxidized glutathione (GSSG). The intracellular redox state is reflected by the levels of rGSH, GSSG as well as the rGSH/GSSG ratio, which is an important indicator of cellular health.[41] The rGSH, GSSG levels, and their ratio in blood mirror the redox status of the whole body since these products leak into the surrounding bloodstream and other body fluids.[42]
We observed significantly lower mean serum rGSH levels in cases of premature canities as compared to controls. Besides, there was a significant parallel decline in serum rGSH levels from mild-to-severe grades of premature canities in our study cohort. Daulatabad et al.[29] reported lower whole blood rGSH levels in cases of premature canities versus controls; however, the difference was not statistically significant in their study.
Alterations in all the three serum parameters in the present study, i.e., MDA, rGSH, and SOD taken together, point to the presence of a high degree of systemic oxidative stress in premature canities patients. Similar studies in autoimmune skin disorders such as vitiligo, alopecia areata, psoriasis, and pemphigus have documented significant differences between patients and healthy controls, in oxidative/antioxidant parameters in the blood/affected skin or both.[43]
It is difficult to ascertain, however, from the present study, whether the systemic oxidative stress in our patients affected the hair follicles secondarily, leading to graying of hair shafts, or the local endogenous oxidative stress in graying hair follicles spilled over into the circulation.
Our study, as well as the aforementioned studies, supports a critical role of oxidative stress in the etiopathogenesis of premature canities. In an attempt to reverse premature hair graying, a number of therapeutic agents, some with an antioxidant role, namely para aminobenzoic acid and l-methionine, have been investigated or are under trial.[1] Randomized double-blind placebo-controlled trials are however lacking, and treatment of premature canities remains arbitrary at present.
CONCLUSION
A systemic redox imbalance was present in premature canities patients in our study cohort due to raised pro-oxidant levels and reduced antioxidant prowess, as evidenced by significantly higher serum MDA and lower serum rGSH and serum SOD levels, in cases as compared to controls. Further, there existed a close correlation of the rise in serum MDA and fall in serum rGSH levels with the severity of premature canities, which to our knowledge is previously unreported.
The measurement of MDA and rGSH in sera of patients with premature canities, as markers of oxidative stress/antioxidant status, may prove useful for monitoring disease progress, and as predictive biomarkers of response to treatment. Further large-scale studies would, however, be necessary to confirm the clinical utility of these parameters. Our study makes a strong case for therapeutic supplementation with antioxidants in patients with premature canities.
Dedication
This paper is dedicated to the second author, Prof. Ram Krishan Gautam, our beloved teacher and mentor, who lost the battle of life during the preparation of this manuscript. He was the guiding spirit behind this paper, and we shall cherish it in his memory forever.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pandhi D, Khanna D. Premature graying of hair. Indian J Dermatol Venereol Leprol. 2013;79:641–53. doi: 10.4103/0378-6323.116733. [DOI] [PubMed] [Google Scholar]
- 2.Nappi AJ, Vass E. Hydrogen peroxide generation associated with the oxidations of the eumelanin precursors 5,6-dihydroxyindole and 5,6-dihydroxyindole-2-carboxylic acid. Melanoma Res. 1996;6:341–9. doi: 10.1097/00008390-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Sies H. Oxidative stress: Introductory remarks. In: Sies H, editor. Oxidative Stress. London: Academic Press; 1985. pp. 1–8. [Google Scholar]
- 4.Arck PC, Overall R, Spatz K, Liezman C, Handjiski B, Klapp BF, et al. Towards a “free radical theory of graying”: Melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006;20:1567–9. doi: 10.1096/fj.05-4039fje. [DOI] [PubMed] [Google Scholar]
- 5.Wood JM, Decker H, Hartmann H, Chavan B, Rokos H, Spencer JD, et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 2009;23:2065–75. doi: 10.1096/fj.08-125435. [DOI] [PubMed] [Google Scholar]
- 6.Kauser S, Westgate GE, Green MR, Tobin DJ. Human hair follicle and epidermal melanocytes exhibit striking differences in their aging profile which involves catalase. J Invest Dermatol. 2011;131:979–82. doi: 10.1038/jid.2010.397. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Luo LF, Liu XM, Zhou Q, Xu SZ, Lei TC. Premature graying as a consequence of compromised antioxidant activity in hair bulb melanocytes and their precursors. PLoS One. 2014;9:e93589. doi: 10.1371/journal.pone.0093589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trüeb RM. Oxidative stress in ageing of hair. Int J Trichology. 2009;1:6–14. doi: 10.4103/0974-7753.51923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daulatabad D, Singal A, Grover C, Chhillar N. Profile of Indian patients with premature canities. Indian J Dermatol Venereol Leprol. 2016;82:169–72. doi: 10.4103/0378-6323.168911. [DOI] [PubMed] [Google Scholar]
- 10.Tobin DJ. Human hair pigmentation biological aspects. Int J Cosmet Sci. 2008;30:233–57. doi: 10.1111/j.1468-2494.2008.00456.x. [DOI] [PubMed] [Google Scholar]
- 11.Slominski A, Paus R, Plonka P, Chakraborty A, Maurer M, Pruski D, et al. Melanogenesis during the anagen-catagen-telogen transformation of the murine hair cycle. J Invest Dermatol. 1994;102:862–9. doi: 10.1111/1523-1747.ep12382606. [DOI] [PubMed] [Google Scholar]
- 12.Tobin DJ, Paus R. Graying: Gerontobiology of the hair follicle pigmentary unit. Exp Gerontol. 2001;36:29–54. doi: 10.1016/s0531-5565(00)00210-2. [DOI] [PubMed] [Google Scholar]
- 13.Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. doi:10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem. 2017;86:715–48. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 15.Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 16.Tobin DJ. The cell biology of human hair follicle pigmentation. Pigment Cell Melanoma Res. 2011;24:75–88. doi: 10.1111/j.1755-148X.2010.00803.x. [DOI] [PubMed] [Google Scholar]
- 17.Seiberg M. Age-induced hair greying - the multiple effects of oxidative stress. Int J Cosmet Sci. 2013;35:532–8. doi: 10.1111/ics.12090. [DOI] [PubMed] [Google Scholar]
- 18.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–40. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: Incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–4. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 20.Emerit I, Filipe P, Freitas J, Vassy J. Protective effect of superoxide dismutase against hair graying in a mouse model. Photochem Photobiol. 2004;80:579–82. doi: 10.1562/0031-8655(2004)080<0579:PEOSDA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Kwon MJ, Lee KY, Lee HW, Kim JH, Kim TY. SOD3 Variant, R213G, altered SOD3 function, leading to ROS-mediated inflammation and damage in multiple organs of premature aging mice. Antioxid Redox Signal. 2015;23:985–99. doi: 10.1089/ars.2014.6035. [DOI] [PubMed] [Google Scholar]
- 22.Sonthalia S, Priya A, Tobin DJ. Demographic characteristics and association of serum Vitamin B12, Ferritin and thyroid function with premature canities in Indian patients from an Urban skin clinic of North India: A retrospective analysis of 71 cases. Indian J Dermatol. 2017;62:304–8. doi: 10.4103/ijd.IJD_221_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birch CS, Brasch NE, McCaddon A, Williams JH. A novel role for vitamin B(12): Cobalamins are intracellular antioxidants in vitro. Free Radic Biol Med. 2009;47:184–8. doi: 10.1016/j.freeradbiomed.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 24.Singal A, Daulatabad D, Grover C. Graying severity score: A useful tool for evaluation of premature canities. Indian Dermatol Online J. 2016;7:164–7. doi: 10.4103/2229-5178.182372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin H, Ryu HH, Yoon J, Jo S, Jang S, Choi M, et al. Association of premature hair graying with family history, smoking, and obesity: A cross-sectional study. J Am Acad Dermatol. 2015;72:321–7. doi: 10.1016/j.jaad.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Bhat RM, Sharma R, Pinto AC, Dandekeri S, Martis J. Epidemiological and investigative study of premature graying of hair in higher secondary and pre-university school children. Int J Trichology. 2013;5:17–21. doi: 10.4103/0974-7753.114706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margaritelis NV, Veskoukis AS, Paschalis V, Vrabas IS, Dipla K, Zafeiridis A, et al. Blood reflects tissue oxidative stress: A systematic review. Biomarkers. 2015;20:97–108. doi: 10.3109/1354750X.2014.1002807. [DOI] [PubMed] [Google Scholar]
- 28.Akin Belli A, Etgu F, Ozbas Gok S, Kara B, Dogan G. Risk factors for premature hair graying in young Turkish adults. Pediatr Dermatol. 2016;33:438–42. doi: 10.1111/pde.12881. [DOI] [PubMed] [Google Scholar]
- 29.Daulatabad D, Singal A, Grover C, Sharma SB, Chhillar N. Assessment of oxidative stress in patients with premature canities. Int J Trichology. 2015;7:91–4. doi: 10.4103/0974-7753.167469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44:532–53. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 31.Latha B, Babu M. The involvement of free radicals in burn injury: A review. Burns. 2017;27:309–17. doi: 10.1016/s0305-4179(00)00127-3. [DOI] [PubMed] [Google Scholar]
- 32.Fukai T, Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010;5:51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katerji M, Filippova M, Duerksen-Hughes P. Approaches and methods to measure oxidative stress in clinical samples: Research applications in the cancer field? Oxid Med Cell Longev. 2019;2019:1279250. doi: 10.1155/2019/1279250. doi:10.1155/2019/1279250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flohé L, Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 37.Minami M, Yoshikawa H. A simplified assay method of superoxide dismutase activity for clinical use. Clin Chim Acta. 1979;92:337–42. doi: 10.1016/0009-8981(79)90211-0. [DOI] [PubMed] [Google Scholar]
- 38.Oka S, Ogino K, Matsuura S, Yoshimura S, Yamamoto K, Okazaki Y, et al. Human serum immuno-reactive copper, zinc-superoxide dismutase assayed with an enzyme monoclonal immunosorbent in patients with digestive cancer. Clin Chim Acta. 1989;182:209–19. doi: 10.1016/0009-8981(89)90079-x. [DOI] [PubMed] [Google Scholar]
- 39.Adachi T, Ohta H, Yamada H, Futenma A, Kato K, Hirano K. Quantitative analysis of extracellular-superoxide dismutase in serum and urine by ELISA with monoclonal antibody. Clin Chim Acta. 1992;212:89–102. doi: 10.1016/0009-8981(92)90176-q. [DOI] [PubMed] [Google Scholar]
- 40.Shi MH, Wu Y, Li L, Cai YF, Liu M, Gao XH, et al. Meta-analysis of the association between vitiligo and the level of superoxide dismutase or malondialdehyde. Clin Exp Dermatol. 2017;42:21–9. doi: 10.1111/ced.12950. [DOI] [PubMed] [Google Scholar]
- 41.Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J, et al. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett. 2012;4:1247–53. doi: 10.3892/ol.2012.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enns GM, Cowan TM. Glutathione as a redox biomarker in mitochondrial disease-implications for therapy. J Clin Med. 2017;6:pii: E50. doi: 10.3390/jcm6050050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah AA, Sinha AA. Oxidative stress and autoimmune skin disease. Eur J Dermatol. 2013;23:5–13. doi: 10.1684/ejd.2012.1884. [DOI] [PubMed] [Google Scholar]