Abstract
Background
Long noncoding RNA X inactivate-specific transcript (lncRNA XIST) has been identified to contribute to the development and progression of non-small cell lung cancer (NSCLC). Thus, it is important to explore more specific functions and molecular mechanisms of XIST in NSCLC tumorigenesis.
Materials and Methods
The expression of XIST, microRNA (miR)-142-5p and paired box 6 (PAX6) was measured using quantitative real-time polymerase chain reaction or Western blot, respectively. Cell proliferation was analyzed using cell counting kit-8 (CCK-8) assay. Flow cytometry was utilized to measure apoptotic cells. Cell migration and invasion were determined by Transwell assay. The interaction between miR-142-5p and XIST or PAX6 was confirmed by the dual-luciferase reporter assay and RNA immunoprecipitation assay. In vivo experiments were performed through the murine xenograft model.
Results
XIST was elevated in NSCLC, and XIST knockdown suppressed cell proliferation, migration, invasion and induced apoptosis in vitro as well as repressed tumor growth in vivo. MiR-142-5p was a target of XIST, and silencing miR-142-5p reversed the anti-tumor functions mediated by XIST knockdown in NSCLC cells. PAX6 was confirmed to be a target of miR-142-5p, and the inhibitory effects caused by miR-142-5p restoration in NSCLC cell malignant phenotypes were attenuated by PAX6 overexpression. Besides that, XIST could indirectly regulate PAX6 expression by sponging miR-142-5p in vivo and in vitro.
Conclusion
XIST suppresses cell tumorigenicity in human NSCLC by regulating miR-142-5p/PAX6 axis, which indicates a novel insight into the pathogenesis of NSCLC and lays a foundation for the molecular therapy of NSCLC.
Keywords: XIST, miR-142-5p, PAX6, NSCLC
Introduction
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for around 80~85% of all lung cancers cases, and has a low five-year survival rate of approximately 15%.1,2 Despite advances in the diagnosis and multimodal treatment, NSCLC patients are still associated with poor prognosis due to the metastasis, recurrence, as well as the limitation on the understanding of NSCLC molecular pathogenesis.3,4 Thus, further investigations to identify novel biomarkers and explore molecular mechanisms of NSCLC pathogenesis are necessary.
Long noncoding RNAs (lncRNAs) are a kind of noncoding RNA molecules without protein coding ability.5 Emerging evidence shows that lncRNAs are key regulators of gene expression and participate in the regulation of various physiological and pathological processes in a wide variety of malignancies, thus controlling the initiation and development of tumors.6,7 To date, the roles and underlying mechanisms of disordered lncRNAs in NSCLC carcinogenesis have been identified, such as SNHG1, CCAT1, HIT and so on.8–10 However, the functions of lncRNAs and the mechanisms by which they act in NSCLC tumorigenesis are still not well understood. X inactivate-specific transcript (XIST) is a lncRNA, which is necessary for transcriptional silencing of X chromosome in female mammals.11 Accumulating evidence has revealed XIST expression is up-regulated in many cancers and involve in the development and progression of tumors, including pancreatic, colorectal, gastric cancer and so on.12–14 In NSCLC, overexpressed XIST has been investigated and has also been verified to contribute to the cancer cell growth, metastasis and drug resistance.15,16 Nevertheless, more specific molecular mechanisms of XIST in NSCLC tumorigenesis still need to be explored.
An increasing number of researches have revealed that lncRNAs can function as competing endogenous RNA (ceRNA), competing for microRNA (miRNA) binding to regulate the targeted mRNA expression.17 MiR-142-5p has been identified to be down-regulated and function as a tumor suppressor to repress cancer cell tumorigenesis in numerous cancers, including NSCLC.18–20 Jia et al found miR-142-5p could serve as a miRNA target of lncRNA TTN-AS1 to participate in TTN-AS1 mediated promotion on cell metastasis in lung adenocarcinoma.21 However, the interaction of lncRNA-miR-142-5p in NSCLC remains unclear yet. Paired box 6 (PAX6) is an important transcription factor, which plays critical roles during embryonic development. Previous studies have shown the abnormal expression of PAX6 in different type of cancers, and deregulation of PAX6 performs oncogenic functions in the development of many cancers.22,23 Thus, deeper investigations on PAX6 in NSCLC are required.
In this study, we explored the function of XIST and miR-142-5p in NSCLC cell carcinogenesis, investigated the relationship between XIST and miR-142-5p, as well as the underlying molecular mechanism by which they affect NSCLC development.
Materials and Methods
Patients and Specimens
30 paired NSCLC tissues and paratumor tissues were obtained from patients who underwent surgical resection at The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital. The samples were stored at −80°C until used. All patients were diagnosed by histopathological examination and did not receive any preoperative treatment. The study was permitted by the Ethics Committee of The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital and all patients had written informed consents.
Cell Culture
Human bronchial epithelial (16HBE) cell lines and NSCLC cell lines (A549, H292, H460, H1299) were purchased from Shanghai Academy of Life Science (Shanghai, China) and grown in the Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA, USA) harboring with 10% fetal bovine serum and 1% penicillin/streptomycin (Gibco) at 37°C with 5% CO2.
Cell Transfection
The miR-142-5p mimic (miR-142-5p), miR-142-5p inhibitor (anti-miR-142-5p), mimic negative control (miR-NC), and inhibitor negative control (anti-miR-NC) were purchased from RIBOBIO (Guangzhou, China). Lentiviral particles stably expressing either short hairpin RNA (shRNA)-targeting XIST (sh-XIST) or a scrambled control sequence (sh-NC), the small interfering RNA (siRNA) sequences targeting XIST (si-XIST), siRNA negative control (si-NC), pcDNA, pcDNA XIST overexpression vector (XIST), pcDNA PAX6 overexpression vector (PAX6) were synthesized by Invitrogen (Carlsbad, CA, USA). All vectors or oligonucleotides were transfected into A549 and H1299 cells using Lipofectamine 2000 (Invitrogen) following the standard procedure.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
TRIzol reagent (Invitrogen) was used to extract RNA from tissues and cells according to the standard procedure. Total RNA was reversely transcribed into complementary DNA (cDNA) using a Reverse Transcription System Kit (Qiagen, Valencia, CA, USA) or One Step PrimeScript® miRNA cDNA Synthesis Kit (Takara, Dalian, China), respectively. Then quantitative PCR was carried out by SYBR™ Green Master Mix (Qiagen). The relative expression was analyzed by 2−ΔΔCt method and normalized by glyceraldehyde 3-phosphate dehydrogenase (GADPH) or U6 small nuclear B noncoding RNA (U6). The same experiment was repeated three times, and the average was taken. The specific primer sequences were listed as follows: XIST: F, 5ʹ-CTAGCTAGCTTTTGTAGTGAGCTTGCTCCT-3ʹ and R, 5ʹ-TGCTCTAGAATGTCTCCATCTCCATTTTGC-3ʹ; PAX6: F, 5ʹ-TCTTTGCTTGGGAAATCCG-3ʹ and R, 5ʹ-CTGGTCTGCAAGGTGTCTGA-3ʹ, R 5ʹ-CTGCCCGTTCAACATCCTTAG-3ʹ; miR-142-5p: F, 5ʹ-GGCCCATAAAGTAGAAAGC-3ʹ and R, 5ʹ-TTTGGCACTAGCACATT-3ʹ; GADPH: F 5ʹ-GATATTGTTGCCATCAATGAC-3ʹ and R 5ʹ-TTGATTTTGGAGGGATCTCG-3ʹ; U6: F 5ʹ-GCTTCGGCAGCACATATACTAAAAT-3ʹ, R 5ʹ-CGCTTCACGAATTTGCGTGTCAT-3ʹ.
Cell Counting Kit-8 (CCK-8) Assay
Transfected cells were seeded into 96-well plates and cultured for 24 h, followed by interaction with 10 μL CCK-8 solution (Beyotime, Beijing, China). Subsequently, the absorbance value at 450 nm was detected in the indicated time to analyze cell proliferation. All experiments were repeated three times independently.
Cell Apoptosis Assay
Apoptotic cells were measured using Annexin V-FITC/PI apoptosis detection kit (BD Biosciences, San Jose, CA, USA) following the standard protocol. Transfected cells were resuspended with binding buffer, followed by staining with 10 μL fluorescein isothiocyanate (FITC) annexin V and propidium iodide (PI). Finally, BD FACSDiva software was used to detect apoptotic cells. Three replicate wells were set in each group, and the experiment was repeated three times.
Cell Migration and Invasion
For the detection of cell migration, cell suspension was added into the top chambers, and then lower chambers were filled with 500 μL DMEM mixed with 10% FBS. After incubation for 24 h at 37°C, migrated cells were fixed with methanol and stained with 0.1% crystal violet. Finally, cells in five randomly selected fields were counted with a microscope. For invasion assay, the matrigel (BD Biosciences) was pre-coated in the chamber membranes and other philosophy of measurement was similar to the steps of cell migration. Experiments were performed three times.
Dual-Luciferase Reporter Assay
The WT or MUT XIST or PAX6 3ʹ-UTR containing with miR-142-5p sequences was amplified and separately cloned into the pmirGLO Vector (Promega, Shanghai, China). A549 and H1299 cells were co-transfected with the wild-type (WT) or mutant-type (MUT) constructed luciferase reporter vectors and miR-142-5p or miR-NC using Lipofectamine 2000 (Invitrogen). Subsequently, a dual-luciferase reporter assay kit (Promega) was utilized to determine the relative luciferase activity.
RNA Immunoprecipitation (RIP) Assay
Transfected cells were lysed, and the lysate was interacted with magnetic beads coated containing anti-Ago2 (Millipore, Billerica, MA, USA) or IgG antibody (Abcam, Cambridge, MA, USA). Finally, purified RNA was examined using qRT-PCR.
Western Blot
Proteins were extracted using RIPA lysis buffer (Beyotime) and quantified by bicinchoninic acid (BCA) method following the standard protocol. An equal amount of extracts was treated with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and shifted onto a nitrocellulose membrane (Millipore). Subsequently, the membranes were incubated with specific primary antibodies PAX6 (1:1000, ab5790, Abcam) and GADPH (1:1000, ab8245, Abcam), and followed by interaction with secondary HRP-conjugated antibody (1:1000, ab9482, Abcam). Finally, protein signals were examined using an ECL method.
Xenograft Experiments in vivo
Five-week-old male BALB/c nude mice were used to establish xenograft models following the guidelines permitted by the Animal Research Committee of The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, and Experiments were approved by the ethics committee of The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital. A549 cells stably infected with lentivirus-sh-NC or lentivirus-sh-XIST were subcutaneously inoculated into the flanks of the nude mice. The tumor size in mice was detected every 4 days. After inoculation for 27 days, the tumor masses were excised and weighed.
Statistical Analysis
Data from three independent experiments were presented as the mean ± standard deviation (SD) and analyzed using GraphPad Prism 7 software (GraphPad Inc., San Diego, CA, USA). Significant differences were analyzed using one-way analysis of variance (ANOVA) or Student’s t-test. P<0.05 suggested statistically significant.
Results
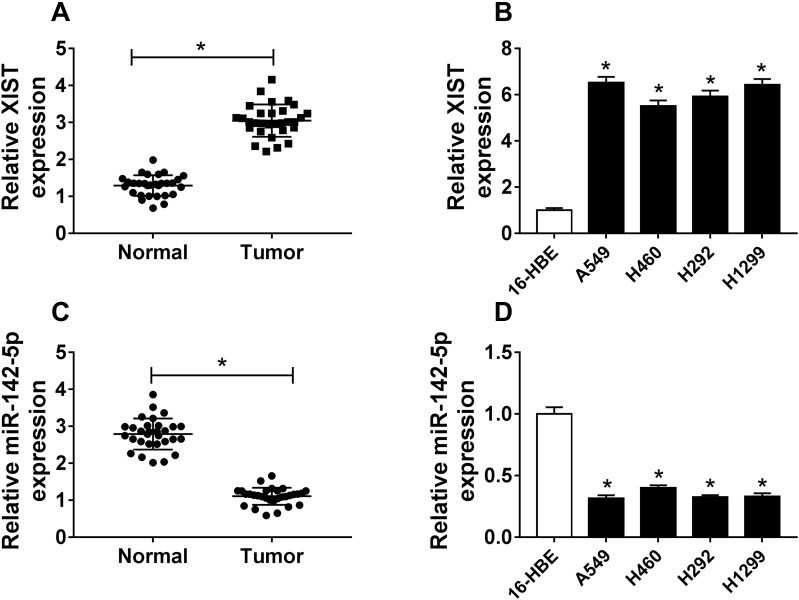
XIST Is Up-Regulated While miR-142-5p Is Down-Regulated in NSCLC Tissues and Cell Lines
The expression of XIST and miR-142-5p was detected. Then qRT-PCR analysis showed that XIST was elevated in NSCLC tissues and cell lines compared with that in controls (Figure 1A and B). Furthermore, compared with the normal controls, miR-142-5p was found to be down-regulated in NSCLC tissues and cell lines (Figure 1C and D). Therefore, XIST and miR-142-5p might be related to cell carcinogenesis in NSCLC.
Figure 1.
XIST is up-regulated while miR-142-5p is down-regulated in NSCLC tissues and cell lines. (A–D) The expression of XIST and miR-142-5p was measured using qRT-PCR in non-tumor tissues and NSCLC tissues, as well as in NSCLC cell lines (A549, H1299, H460, and H292) and human bronchial epithelial (16HBE) cell line. *P<0.05.
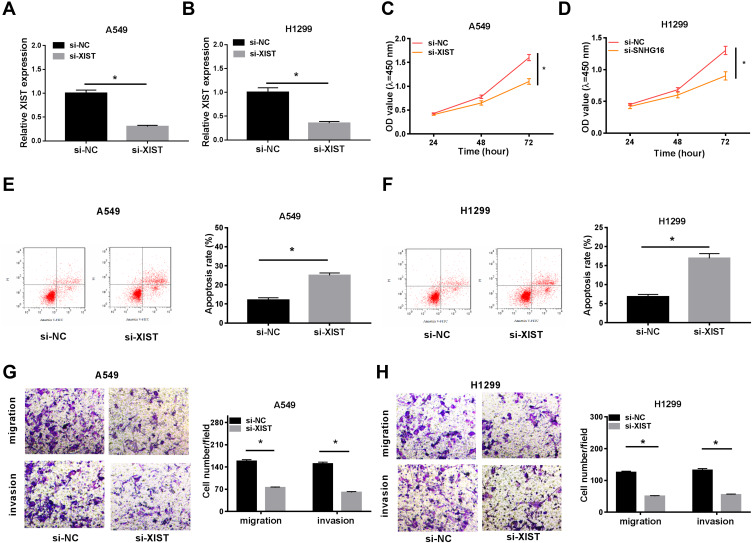
XIST Deletion Inhibits Cell Proliferation, Migration and Invasion but Induces Cell Apoptosis in NSCLC
To explore the potential biological functions of XIST in NSCLC carcinogenesis, we knocked down XIST using siRNA sequences in A549 and H1299 cells, and a significant reduction of XIST expression was observed (Figure 2A and B). Subsequently, CCK-8 assay showed XIST knockdown inhibited NSCLC cell proliferation (Figure 2C and D). Afterwards, we found XIST deletion increased NSCLC cell apoptosis (Figure 2E and F). Besides that, knockdown of XIST also suppressed the migration and invasion abilities of A549 and H1299 cells (Figure 2G and H). These results indicated that XIST could promote cell carcinogenesis of NSCLC.
Figure 2.
XIST deletion inhibits cell proliferation, migration and invasion but induces cell apoptosis in NSCLC. XIST was silenced using siRNA sequences targeting XIST in A549 and H1299 cells. (A and B) The efficiencies of interference were detected using qRT-PCR. (C and D) CCK-8 assay was used to measure cell proliferation. (E and F) Apoptotic cells were examined by flow cytometry. (G and H) Migrated and invaded cells were examined using transwell assay. *P<0.05.
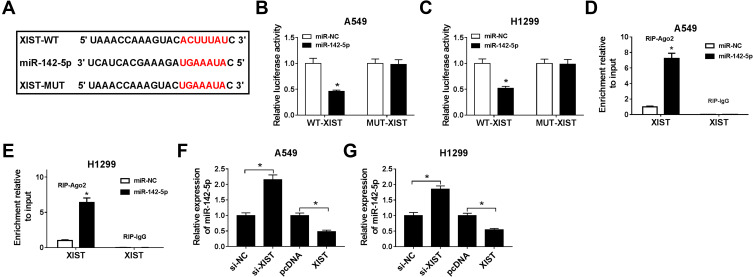
MiR-142-5p Is a Target of XIST in NSCLC Cells
To investigate the biological pathways in which XIST were implicated in NSCLC pathogenesis, the potential miRNA targets of XIST were predicted using StarBase program and we found miR-142-5p had binding sites with XIST (Figure 3A). After that, the dual-luciferase reporter assay was conducted, and we found that luciferase activities of the XIST-WT reporter vector were repressed by miR-142-5p mimics, but there was no obvious change in XIST-MUT reporter vector after miR-142-5p mimic transfection in A549 and H1299 cells (Figure 3B and C). Moreover, this interaction between miR-142-5p and XIST was subsequently confirmed in A549 and H1299 cells by RIP assay (Figure 3D and E). Meanwhile, the level of miR-142-5p was reduced in XIST-overexpressed NSCLC cells, but increased in XIST-down-regulated NSCLC cells (Figure 3F and G). Taken together, XIST directly interacted with miR-142-5p and negatively regulated miR-142-5p expression in NSCLC cells.
Figure 3.
MiR-142-5p is a target of XIST in NSCLC cells. (A) A graphical representation of the miR-142-5p binding sites within XIST was presented. (B–D) The interaction between miR-142-5p and XIST was confirmed using the dual-luciferase reporter assay (B and C) and RIP assay (D and E) in A549 and H1299 cells. (F and G) The expression of miR-142-5p was detected using qRT-PCR in A549 and H1299 cells transfected with si-XIST, si-NC, pc-DNA, or XIST. *P<0.05.
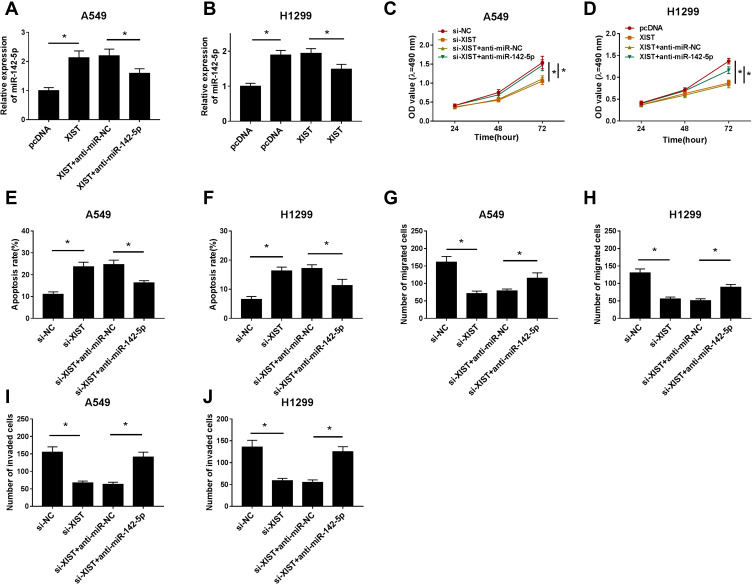
XIST Regulates Cell Carcinogenesis in NSCLC by Sponging miR-142-5p
Based on the relationship between XIST and miR-142-5p, as well as down-regulation of miR-142-5p in NSCLC, we further elucidated whether miR-142-5p involved in si-XIST-mediated inhibition of cell tumorigenesis in NSCLC. Immediately, A549 and H1299 cells were transfected with si-NC, si-XIST, si-XIST + anti-miR-NC, or si-XIST + anti-miR-142-5p and the efficiencies of transfection were confirmed by qRT-PCR (Figure 4A and B). After that, rescue assay suggested that miR-142-5p inhibition could attenuate si-XIST-induced suppression on cell proliferation (Figure 4C and D), migration (Figure 4G and H) and invasion (Figure 4I and J), promotion on cell apoptosis (Figure 4E and F) in NSCLC. These data showed miR-142-5p mediated the anti-tumor effects of si-XIST in NSCLC cells.
Figure 4.
XIST regulates cell carcinogenesis in NSCLC by sponging miR-142-5p. A549 and H1299 cells were transfected with si-NC, si-XIST, si-XIST + anti-miR-NC, or si-XIST + anti-miR-142-5p. (A and B) The efficiencies of transfection were confirmed by qRT-PCR. (C and D) Cell proliferation was analyzed by CCK-8 assay. (E and F) Cell apoptosis was determined by flow cytometry. Cell migration (G and H) and invasion (I and J) abilities were examined using transwell assay. *P<0.05.
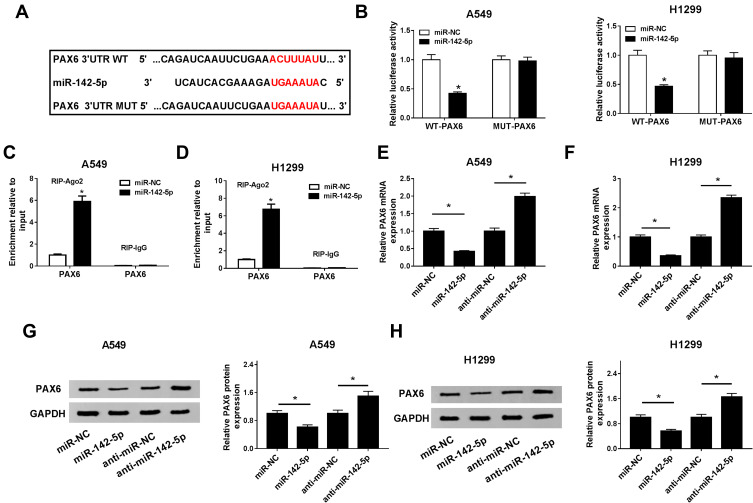
PAX6 Is a Target of miR-142-5p in NSCLC Cells
We further explored the potential molecular mechanisms of XIST/miR-142-5p axis in the regulation of NSCLC development. According to the prediction of TargetScan software, we found miR-142-5p target sites in the 3ʹUTR of PAX6 mRNA (Figure 5A). Afterwards, subsequent dual-luciferase reporter assay showed overexpressed miR-142-5p reduced the luciferase activities of the WT-PAX6 reporter vector but not MUT-PAX6 reporter vector in A549 and H1299 cells (Figure 5B). Furthermore, RIP assay also indicated the expression of PAX6 pull-down by Ago2 was obviously enriched in A549 and H1299 cells transiently overexpressing miR-142-5p (Figure 5C and D). Besides that, qRT-PCR analysis showed the level of PAX6 mRNA was reduced by miR-142-5p overexpression, but increased by the inhibition of miR-142-5p in A549 and H1299 (Figure 5E and F). Meanwhile, the protein changes of PAX6 were also similar to PAX6 mRNA in transfected A549 and H1299 cells (Figure 5G and H). Thus, we confirmed that PAX6 was a target of miR-142-5p in NSCLC cells.
Figure 5.
PAX6 is a target of miR-142-5p in NSCLC cells. (A) The predicted binding sites between miR-142-5p and PAX6 were listed. (B–D) The interaction between miR-142-5p and XIST was confirmed using the dual-luciferase reporter assay (B) and RIP assay (C and D) in A549 and H1299 cells. A549 and H1299 cells were transfected with miR-NC, miR-142-5p, anti-miR-NC, or anti-miR-142-5p. (E–H) The mRNA and protein levels of PAX6 in transfected cells were detected using qRT-PCR (E and F) or Western blot (G and H), respectively. *P<0.05.
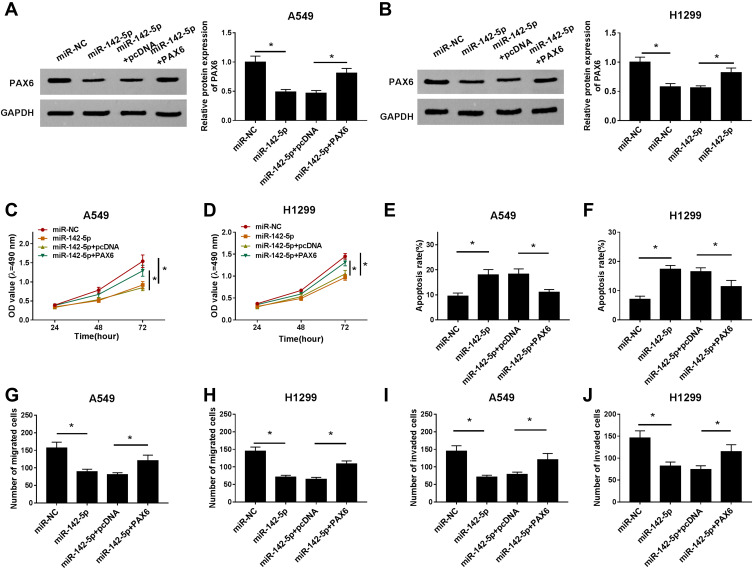
Overexpressed miR-142-5p Inhibits NSCLC Cells Progression by Targeting PAX6
Based on the regulatory network of miR-142-5p/PAX6, we further wanted to know whether miR-142-5p/PAX6 was responsible for cell carcinogenesis in NSCLC. A549 and H1299 cells were transfected with miR-NC, miR-142-5p, miR-142-5p + pcDNA, miR-142-5p + PAX6 and the efficiencies of interference were verified by Western blot (Figure 6A and B). Immediately, CCK-8 assay demonstrated miR-142-5p overexpression inhibited cell proliferation, but this inhibition could be attenuated after PAX6 up-regulation in A549 and H1299 cells (Figure 6C and D). Afterwards, we found apoptotic A549 and H1299 cells were promoted by miR-142-5p overexpression, but were repressed by following PAX6 up-regulation (Figure 6E and F). Moreover, transwell assay also indicated overexpressed PAX6 could partially overturn miR-142-5p-mediated suppression on migration and invasion of A549 and H1299 cells (Figure 6G–J). These data demonstrated miR-142-5p overexpression inhibited NSCLC progression by targeting PAX6.
Figure 6.
Overexpressed miR-142-5p inhibits NSCLC cells progression by targeting PAX6. A549 and H1299 cells were transfected with miR-NC, miR-142-5p, miR-142-5p + pcDNA, miR-142-5p + PAX6. (A and B) The efficiencies of transfection were verified by Western blot. (C and D) CCK-8 assay was used to determine cell proliferation. (E and F) Apoptotic cells were examined by flow cytometry. (G–J) Migrated (G and H) and invaded (I and J) cells were detected using transwell assay. *P<0.05.
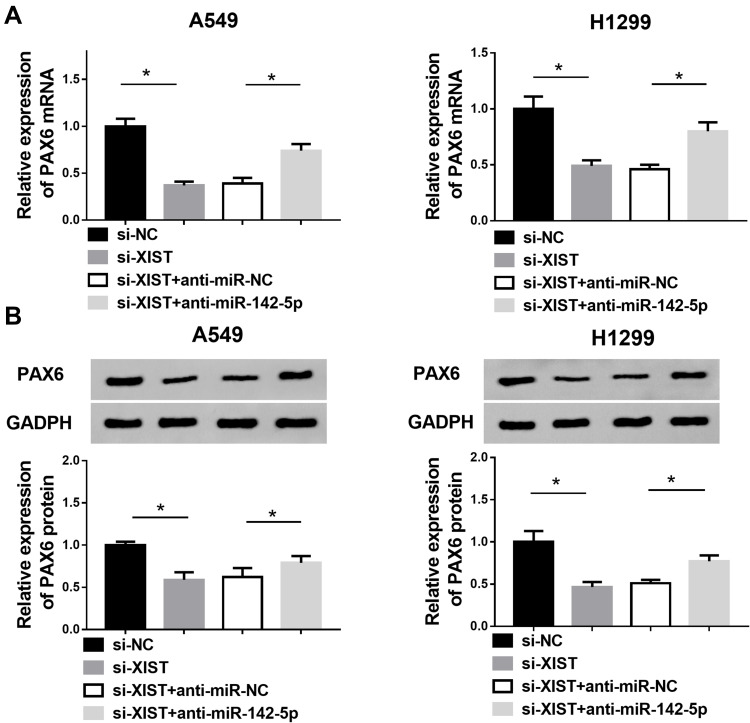
XIST Regulates PAX6 Expression Through Binding to miR-142-5p
To further determine whether XIST regulated PAX6 expression in NSCLC cells by sponging miR-142-5p, the expression of PAX6 in the XIST and miR-142-5p inhibited NSCLC cells were investigated by Western blot. Then results showed a significant reduction PAX6 expression in XIST-deleted A549 and H1299 cells, while miR-142-5p inhibition abolished this effect (Figure 7A and B), indicating PAX6 expression was regulated by XIST/miR-142-5p axis.
Figure 7.
XIST regulates PAX6 expression through binding to miR-142-5p. (A and B) The level of PAX6 in NSCLC cells transfected with si-XIST, si-NC, si-XIST + anti-miR-NC, si-XIST + anti-miR-142-5p was measured by Western blot. *P<0.05.
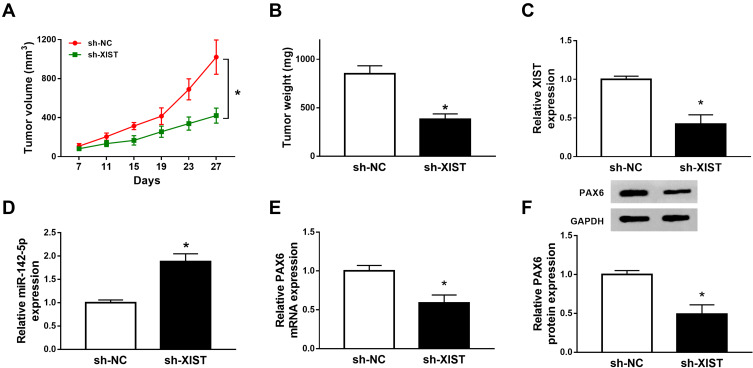
Knockdown of XIST Inhibits NSCLC Tumor Growth in vivo
The carcinogenic roles of XIST in vivo were further investigated, A549 cells stably infected with lentivirus-sh-NC or lentivirus-sh-XIST were used to establish xenograft models. Subsequently, we found knockdown of XIST inhibited NSCLC tumor growth, demonstrated by the reduction of tumor volume and weight in sh-XIST group (Figure 8A and B). In the meanwhile, molecular analysis suggested XIST deletion could regulate miR-142-5p and PAX6 expression in vivo (Figure 8C–F). Collectively, these data demonstrated that knockdown of XIST repressed NSCLC tumor growth in vivo through partially modulating miR-142-5p and PAX6 expression.
Figure 8.
Knockdown of XIST inhibits NSCLC tumor growth in vivo. Xenograft models were established using A549 cells infected with lentivirus-sh-XIST or lentivirus -sh-NC. (A) Tumor volume was calculated every 4 days. (B) Tumor masses were weighed in each group. (C–F) The expression of XIST, miR-142-5p and PAX6 was analyzed in two groups by qRT-PCR or Western blot. *P<0.05.
Discussion
LncRNAs have been reported to act as vital controllers of various aspects of cell biology processes, including the occurrence and development of cancer.6 LncRNA XIST is a well-identified lncRNA; considerable evidence has shown that XIST is associated with the pathogenesis of several type of tumors through regulating cell differentiation, proliferation as well as tumorigenesis.24 Although XIST has been found to be elevated in NSCLC, the functions of XIST in NSCLC cell carcinogenesis are still not fully understood. In the current study, we first detected the expression changes of XIST in 30 paired NSCLC tissues and paratumor tissues, and found XIST was significantly up-regulated in NSCLC tissues. Furthermore, the same expression changes were also observed in NSCLC cell lines. Subsequently, the level of XIST was inhibited in NSCLC cells using siRNA sequences targeting XIST to perform loss-of-function experiments, and we demonstrated XIST deletion inhibited NSCLC cell proliferation, migration, invasion but promoted cell apoptosis. Furthermore, in vivo experiments were performed and knockdown of XIST inhibited NSCLC tumor growth in vivo, further indicating XIST functioned as an oncogene to contribute to NSCLC development.
Based on the ceRNA hypothesis,17 lncRNAs can serve as sponges of miRNAs to regulate target genes expression via absorbing miRNAs. The lncRNA-miRNA-mRNA regulatory network plays an important role in the regulation of many tumors.25,26 In NSCLC, several miRNAs, such as miR-212-3p, miR-16, miR-335 and miR-744,27–30 have been confirmed to serve as targets of XIST to involve in the tumorigenesis of various cancer cells. LncRNAs have been found to contain diverse types and quantities of miRNAs binding sites, thus, lncRNA may have multiple miRNA targets. In this study, we found miR-142-5p was down-regulated in NSCLC tissues and cell lines, subsequent functional analysis indicated re-expression of miR-142-5p exerted anti-tumor effects through inhibiting cell proliferation, metastasis and inducing cell apoptosis in NSCLC. Thus, miR-142-5p is an important regulator in NSCLC development. Besides that, according to the prediction of StarBase program and the confirmation of dual-luciferase reporter assay and RIP assay, we found XIST directly interacted with miR-142-5p in NSCLC cells. Moreover, rescue assay suggested silencing miR-142-5p reversed the anti-tumor functions mediated by XIST knockdown in NSCLC cells. Thus, a XIST/miR-142-5p axis in the progression of NSCLC was identified.
Recent studies have proven that deregulated PAX6 is implicated in cell oncogenesis in NACLC. For instance, methylated PAX6 in the promoter region was associated with poor clinical outcomes and was an available prognostic biomarker for NSCLC.31 Wu et al revealed PAX6 promoted metastasis and cisplatin resistance in NSCLC cells by regulating ZEB2/PI3K/AKT pathway.32 Thus, PAX6 may play roles in the progression of NSCLC. In this study, we found PAX6 was a target of miR-142-5p in NSCLC cells, besides, we also observed that the inhibitory effects caused by miR-142-5p restoration in NSCLC cell malignant phenotypes were attenuated by PAX6 overexpression. In addition, our research indicated XIST regulated PAX6 expression through binding to miR-142-5p in NSCLC. Therefore, a XIST/miR-142-5p/PAX6 regulatory network in NSCLC cells was identified.
In conclusion, our study demonstrated XIST performed oncogenic effects in NSCLC by regulating miR-142-5p/PAX6 axis, indicating a novel insight into the pathogenesis of NSCLC and lays a foundation for the molecular therapy of NSCLC.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet. 2011;378(9804):1727–1740. doi: 10.1016/S0140-6736(10)62101-0 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Bivona TG, Karachaliou N. Genetics and biomarkers in personalisation of lung cancer treatment. Lancet. 2013;382(9893):720–731. doi: 10.1016/S0140-6736(13)61715-8 [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Wang Y, Wang J. MicroRNA-584 inhibits cell proliferation and invasion in non-small cell lung cancer by directly targeting MTDH. Exp Ther Med. 2018;15(2):2203–2211. doi: 10.3892/etm.2017.5624 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Atkinson SR, Marguerat S, Bahler J. Exploring long non-coding RNAs through sequencing. Semin Cell Dev Biol. 2012;23(2):200–205. doi: 10.1016/j.semcdb.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 6.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47–62. doi: 10.1038/nrg.2015.10 [DOI] [PubMed] [Google Scholar]
- 7.Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661–5667. doi: 10.1038/onc.2017.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui Y, Zhang F, Zhu C, et al. Upregulated lncRNA SNHG1 contributes to progression of non-small cell lung cancer through inhibition of miR-101-3p and activation of Wnt/beta-catenin signaling pathway. Oncotarget. 2017;8(11):17785–17794. doi: 10.18632/oncotarget.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu B, Zhang H, Wang Z, et al. LncRNA CCAT1/miR-130a-3p axis increases cisplatin resistance in non-small-cell lung cancer cell line by targeting SOX4. Cancer Biol Ther. 2017;18(12):974–983. doi: 10.1080/15384047.2017.1385679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L, Fang F, Lu S, et al. lncRNA-HIT promotes cell proliferation of non-small cell lung cancer by association with E2F1. Cancer Gene Ther. 2017;24(5):221–226. [DOI] [PubMed] [Google Scholar]
- 11.Brown CJ, Ballabio A, Rupert JL, et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349(6304):38–44. doi: 10.1038/349038a0 [DOI] [PubMed] [Google Scholar]
- 12.Wei W, Liu Y, Lu Y, et al. LncRNA XIST promotes pancreatic cancer proliferation through miR-133a/EGFR. J Cell Biochem. 2017;118(10):3349–3358. doi: 10.1002/jcb.25988 [DOI] [PubMed] [Google Scholar]
- 13.Chen DL, Chen LZ, Lu YX, et al. Long noncoding RNA XIST expedites metastasis and modulates epithelial-mesenchymal transition in colorectal cancer. Cell Death Dis. 2017;8(8):e3011. doi: 10.1038/cddis.2017.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, Chen B, Liu P, et al. XIST promotes gastric cancer (GC) progression through TGF-beta1 via targeting miR-185. J Cell Biochem. 2018;119(3):2787–2796. doi: 10.1002/jcb.26447 [DOI] [PubMed] [Google Scholar]
- 15.Tang Y, He R, An J, et al. lncRNA XIST interacts with miR-140 to modulate lung cancer growth by targeting iASPP. Oncol Rep. 2017;38(2):941–948. doi: 10.3892/or.2017.5751 [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Shen Q, Zhang X, et al. The long non-coding RNA XIST controls non-small cell lung cancer proliferation and invasion by modulating miR-186-5p. Cell Physiol Biochem. 2017;41(6):2221–2229. doi: 10.1159/000475637 [DOI] [PubMed] [Google Scholar]
- 17.Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi: 10.1016/j.cell.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia L, Xi Q, Wang H, et al. miR-142-5p regulates tumor cell PD-L1 expression and enhances anti-tumor immunity. Biochem Biophys Res Commun. 2017;488(2):425–431. doi: 10.1016/j.bbrc.2017.05.074 [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Liu Z, Fang X, et al. MiR-142-5p suppresses tumorigenesis by targeting PIK3CA in non-small cell lung cancer. Cell Physiol Biochem. 2017;43(6):2505–2515. doi: 10.1159/000484459 [DOI] [PubMed] [Google Scholar]
- 20.Islam F, Gopalan V, Vider J, et al. MiR-142-5p act as an oncogenic microRNA in colorectal cancer: clinicopathological and functional insights. Exp Mol Pathol. 2018;104(1):98–107. doi: 10.1016/j.yexmp.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 21.Jia Y, Duan Y, Liu T, et al. LncRNA TTN-AS1 promotes migration, invasion, and epithelial mesenchymal transition of lung adenocarcinoma via sponging miR-142-5p to regulate CDK5. Cell Death Dis. 2019;10(8):573. doi: 10.1038/s41419-019-1811-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou Q, Yi W, Huang J, et al. MicroRNA-375 targets PAX6 and inhibits the viability, migration and invasion of human breast cancer MCF-7 cells. Exp Ther Med. 2017;14(2):1198–1204. doi: 10.3892/etm.2017.4593 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Xia X, Yin W, Zhang X, et al. PAX6 overexpression is associated with the poor prognosis of invasive ductal breast cancer. Oncol Lett. 2015;10(3):1501–1506. doi: 10.3892/ol.2015.3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weakley SM, Wang H, Yao Q, et al. Expression and function of a large non-coding RNA gene XIST in human cancer. World J Surg. 2011;35(8):1751–1756. doi: 10.1007/s00268-010-0951-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He JH, Han ZP, Zou MX, et al. Analyzing the LncRNA, miRNA, and mRNA regulatory network in prostate cancer with bioinformatics software. J Comput Biol. 2018;25(2):146–157. doi: 10.1089/cmb.2016.0093 [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Yin H, Zhang L, et al. The construction and analysis of the aberrant lncRNA-miRNA-mRNA network in non-small cell lung cancer. J Thorac Dis. 2019;11(5):1772–1778. doi: 10.21037/jtd.2019.05.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Yao L, Zhang M, et al. Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging. 2019;11(18):7830–7846. doi: 10.18632/aging.102291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu HB, Yang K, Yu HY, et al. Downregulation of long non-coding RNA XIST inhibits cell proliferation, migration, invasion and EMT by regulating miR-212-3p/CBLL1 axis in non-small cell lung cancer cells. Eur Rev Med Pharmacol Sci. 2019;23(19):8391–8402. doi: 10.26355/eurrev_201910_19150 [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Cai H, Dai Z, et al. Down-regulation of lncRNA XIST inhibits cell proliferation via regulating miR-744/RING1 axis in non-small cell lung cancer. Clin Sci (Lond). 2019;133(14):1567–1579. doi: 10.1042/CS20190519 [DOI] [PubMed] [Google Scholar]
- 30.Zhou X, Xu X, Gao C, et al. XIST promote the proliferation and migration of non-small cell lung cancer cells via sponging miR-16 and regulating CDK8 expression. Am J Transl Res. 2019;11(9):6196–6206. [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Yang X, Wang J, et al. Down-regulation of PAX6 by promoter methylation is associated with poor prognosis in non small cell lung cancer. Int J Clin Exp Pathol. 2015;8(9):11452–11457. [PMC free article] [PubMed] [Google Scholar]
- 32.Wu DM, Zhang T, Liu YB, et al. The PAX6-ZEB2 axis promotes metastasis and cisplatin resistance in non-small cell lung cancer through PI3K/AKT signaling. Cell Death Dis. 2019;10(5):349. doi: 10.1038/s41419-019-1591-4 [DOI] [PMC free article] [PubMed] [Google Scholar]