OVERVIEW
Breast cancer is the most common malignancy among women in the United States, and the primary prevention of this disease is a major public health issue. Because there are relatively few modifiable breast cancer risk factors, pharmacologic interventions with antiestrogens have the potential to significantly affect the primary prevention setting. Breast cancer chemoprevention with selective estrogen receptor modulators (SERMs) tamoxifen and raloxifene, and with aromatase inhibitors (AIs) exemestane and anastrozole, is underutilized despite several randomized controlled trials demonstrating up to a 50% to 65% relative risk reduction in breast cancer incidence among women at high risk. An estimated 10 million women in the United States meet high-risk criteria for breast cancer and are potentially eligible for chemoprevention, but less than 5% of women at high risk who are offered antiestrogens for primary prevention agree to take it. Reasons for low chemoprevention uptake include lack of routine breast cancer risk assessment in primary care, inadequate time for counseling, insufficient knowledge about antiestrogens among patients and providers, and concerns about side effects. Interventions designed to increase chemoprevention uptake, such as decision aids and incorporating breast cancer risk assessment into clinical practice, have met with limited success. Clinicians can help women make informed decisions about chemoprevention by effectively communicating breast cancer risk and enhancing knowledge about the risks and benefits of antiestrogens. Widespread adoption of chemoprevention will require a major paradigm shift in clinical practice for primary care providers (PCPs). However, enhancing uptake and adherence to breast cancer chemoprevention holds promise for reducing the public health burden of this disease.
Unlike cardiovascular disease, limited pharmacologic options exist for the primary prevention of cancer. Antiestrogens, such as SERMs and AIs, have been shown to reduce breast cancer incidence by up to 50% to 65% among women at high risk.1–5 Based on this evidence, the U.S. Preventive Services Task Force (USPSTF) and other professional organizations recommend that clinicians discuss chemoprevention with women at high risk.6–8 An estimated 15% of women age 35 to 79 in the United States may be eligible for chemoprevention,9 but less than 5% of women at high risk who are offered an antiestrogen for primary prevention agree to take it.10 Compounding this underutilization is the large proportion of women who may be unaware of their high-risk status because of an inability to routinely screen for high risk in the primary care setting. Other reasons for low chemoprevention uptake include insuffıcient knowledge about antiestrogens on the part of clinicians and patients, multiple competing demands for PCPs, and concerns about side effects.10,11 Even the term “chemoprevention” has negative connotations, because it sounds like “chemotherapy.” The perception among patients and PCPs is that medications used to treat cancer and prescribed by oncologists may have many toxicities. The risks and benefıts of chemoprevention need to be placed in the context of pharmacologic interventions used to treat or prevent other chronic conditions (e.g., aspirin or statins for cardiovascular disease, bisphosphonates for osteoporosis). Further research is needed to determine how knowledge about breast cancer risk and chemoprevention options are best communicated to women to promote breast cancer prevention strategies.
BREAST CANCER RISK ASSESSMENT
Based on age and breast cancer risk, an estimated 15% of women in the United States meet high-risk criteria and may be eligible for chemoprevention.9 Known breast cancer risk factors include family history, reproductive history, and lifestyle factors, such as alcohol intake and obesity.12 Women with benign breast disease, such as atypical hyperplasia (AH) and lobular carcinoma in situ (LCIS), have up to a 4- to 10-fold increased risk of breast cancer.13 Genetic determinants, such as germ-line mutations in the BRCA1 and BRCA2 genes, confer the greatest effect on breast cancer risk. The Gail model, or Breast Cancer Risk Assessment Tool, which takes into account a woman’s age, race, reproductive history, fırst-degree family history of breast cancer, and benign breast disease including atypia, is the most commonly used model in the United States and has been well validated at the population level.14 It can be administered to women age 35 or older and provides an individual’s absolute 5-year and lifetime risk of invasive breast cancer compared to women of the same age and race in the general population. High-risk criteria used to determine eligibility in chemoprevention trials are at least a 1.67% 5-year risk or 20% or greater lifetime risk of invasive breast cancer. To account for differences in breast cancer risk by race and ethnicity, the Gail model incorporated data from the Women’s Contraceptive and Reproductive Experiences15 and Asian American Breast Cancer Study16 to provide more sensitive estimates for African American and Asian women, respectively. Few studies have used this model in Hispanic populations.17,18 Hispanic women have signifıcantly lower breast cancer risk compared to non-Hispanic white women; however, risk differs among Hispanic subgroups in the United States: according to the Gail model, Cubans have a higher 5-year risk (p < 0.05) and Dominicans have a higher lifetime risk than Mexicans (p < 0.001).19 Interestingly, eligibility for chemoprevention among U.S. women varies dramatically by race and ethnicity, with 18.7% of whites, 5.7% of blacks, and 2.9% of Hispanics meeting high-risk criteria according to the Gail model.9
In women with a strong family history of breast cancer (i.e., two or more affected family members, particularly those with early-age onset), the Tyrer-Cuzick model is useful because it also accounts for second- and third-degree family history of breast and ovarian cancer and age at diagnosis.20 This model may be particularly relevant for estimating risk in women with multiple affected family members, as well as LCIS. Women who had a 10-year risk of breast cancer of 5% of more according to the Tyrer-Cuzick model were included in the International Breast Cancer Intervention Study-I (IBIS-I) of tamoxifen and IBIS-II trial of anastrozole compared with placebo.2,5 A comparison of the breast cancer risk factors included in the Gail and Tyrer-Cuzick models are summarized in Table 1.
TABLE 1.
Comparison of Breast Cancer Risk Factors in the Gail and Tyrer-Cuzick Models
| Gail Model | Tyrer-Cuzick Model |
|---|---|
| Age (35 or older) | Age |
| Race/ethnicity (white, black, Hispanic, Asian American [Chinese, Japanese, Filipino, Hawaiian, other], unknown) | Ashkenazi Jewish descent |
| Age at menarche | Age at menarche |
| Age at first live birth | Age at first live birth |
| Menopausal status | |
| Age at menopause | |
| Use of hormone replacement therapy | |
| Body mass index | |
| Number of benign breast biopsies | |
| Benign breast biopsy with atypical hyperplasia (excludes LCIS, DCIS, or invasive breast cancer) | Benign breast biopsy including hyperplasia with or without atypia and LCIS |
| Number of first-degree relatives with breast cancer | Number of first-, second-, and third-degree relatives with breast or ovarian cancer, bilateral breast cancer, and age at diagnosis |
| BRCA mutation status |
Abbreviations: DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ.
High-risk benign breast disease is an important and under-recognized breast cancer risk factor.21 Over one million benign breast biopsies are performed in the United States each year,22 with approximately 10% showing AH or LCIS—conferring a relative risk of breast cancer of up to 4 to 10.23–27 Long-term studies indicate that absolute breast cancer risk in women with AH is approximately 30% at 25 years of follow-up.25,28 Of note, the Gail model signifıcantly under-predicts breast cancer risk in women with AH (p < 0.001),29 whereas the Tyrer-Cuzick model tends to over-predict risk.30 Because of the high estrogen receptor (ER) expression in AH and the fact that the majority of breast cancers that develop in women with AH are ER+,31 these high-risk women derive a greater benefıt from chemoprevention than the general high-risk population. In the randomized, placebo-controlled chemoprevention trials, relative risk reduction of breast cancer among the subgroup of 2,009 women with AH ranged from 41% to 79%.1,2,4,5,21 In a cohort study of women with atypical breast lesions, 10-year breast cancer risk with chemoprevention was 7.5%, compared to 21.3% without chemoprevention.24 Despite this evidence, chemoprevention uptake remains low among these women at high risk.32
BREAST CANCER CHEMOPREVENTIVE AGENTS
Selective Estrogen Receptor Modulators
Table 2 summarizes results of the major randomized controlled trials of SERMs and AIs for the primary prevention of breast cancer. In 1998, the Breast Cancer Prevention Trial (BCPT) demonstrated that the SERM tamoxifen taken for 5 years reduced breast cancer incidence in women at high risk by 49% (number needed to treat [NNT] to prevent one invasive breast cancer was 95 at 5 years and 56 at 10 years).33,1 The overall results from three additional randomized controlled trials confırmed that tamoxifen decreased breast cancer risk by 30% to 40% compared to placebo.34–37 In particular, long-term follow-up data (median of 16 years) from the IBIS-I trial demonstrated a persistent protective effect of tamoxifen (NNT was 22 at 20 years).2 The magnitude of this risk reduction is comparable to what has been observed with preventive agents for cardiovascular disease.38–40
TABLE 2.
Updated Results from Major Randomized Controlled Trials of Selective Estrogen Receptor Modulators and Aromatase Inhibitors for Breast Cancer Chemoprevention
| Trial | No. of Participants | Eligibility, High-Risk Criteria for Breast Cancer | Intervention | Median Follow-up (Months) | Breast Cancer Incidence | Breast Cancer Risk Reduction RR or HR (95% CI) |
|---|---|---|---|---|---|---|
| BCPT, 20051 | 13,388 | Age ≥ 35, 5-yr Gail risk score > 1.66% if age 35–59 or ≥ 60 or LCIS | Tamoxifen 20 mg/d × 5 yrs versus placebo | 84 | 3.59 versus 6.29a | 0.57 (0.46–0.70) |
| IBIS-I, 20142 | 7,154 | Age 35–70, 10-fold risk if age 35–39, or 4-fold risk if age 40–44, or 2-fold risk if age 45–70 | Tamoxifen 20 mg/d × 5 yrs versus placebo | 192 | 7.0versus 9.8%b | 0.71 (0.60–0.83) |
| STAR, 20103 | 19,747 | Age ≥ 35, postmenopausal, 5-yr Gail risk score > 1.66% | Raloxifene 60 mg/d versus tamoxifen 20 mg/d × 5 yrs | 81 | 5.02 versus 4.04a | 1.24 (1.05–1.47) |
| MAP.3, 20114 | 4,560 | Age ≥ 35, postmenopausal, 5-yr Gail risk score > 1.66% if age 35–59 or age ≥ 60 or AH, LCIS, DCIS with mastectomy | Exemestane 25 mg/d × 5 yrs versus placebo | 35 | 0.19versus 0.55%c | 0.35 (0.18–0.70) |
| IBIS-II, 20135 | 3,864 | Age 40–70, postmenopausal, 4-fold risk if age 40–44, or 2-fold risk if age 45–59, or 1.5-fold risk if age 60–70 | Anastrozole 1 mg/d × 5 yrs versus placebo | 60 | 2% versus 4%b | 0.47 (0.32–0.68) |
Abbreviations: SERM, selective receptor estrogen modulators; AI, aromatase inhibitor; AH, atypical hyperplasia; BCPT, Breast Cancer Prevention Trial; CI, confidence interval; DCIS, ductal carcinoma-in-situ; HR, hazard ratio; IBIS, International Breast cancer Intervention Study; LCIS, lobular carcinoma in situ; MAP, Mammary Prevention Trial; RR, relative risk; STAR, Study of Tamoxifen and Raloxifene.
aInvasive breast cancer incidence rate/1,000 women.
bAll breast cancers, invasive and noninvasive.
cAnnual incidence of invasive breast cancers.
Another SERM, raloxifene, has been shown to reduce the incidence of breast cancer in postmenopausal women for the treatment and prevention of osteoporosis.41,42 Updated analyses from the Study of Tamoxifen and Raloxifene (STAR) trial demonstrated that raloxifene had 76% of the effıcacy of tamoxifen for breast cancer prevention among postmenopausal women at high risk with a more favorable side effect profıle.3 Based on the results of these trials, tamoxifen was approved by the U.S. Food and Drug Administration (FDA) for breast cancer risk reduction among women at high risk in 1998 and raloxifene in 2007.
Aromatase Inhibitors
Data from adjuvant trials have proven to be a useful model for assessing the chemopreventive effects of endocrine therapies, since results of antiestrogens in the primary prevention setting closely mirrored those for adjuvant treatment.37 In 2011, results from the Mammary Prevention Trial-3 (MAP.3) demonstrated that the AI exemestane given to postmenopausal women at high risk reduced invasive breast cancer incidence by 65% compared to placebo (NNT was 26 at 5 years).4 High-risk criteria included age 60 or older (49%), a 5-year Gail risk score 1.66% or greater (40%), AH or LCIS (8%), and ductal carcinoma in situ treated with mastectomy (3%).4 After a median follow-up of 35 months, 11 invasive breast cancers occurred in the exemestane arm compared to 32 in the placebo group (annual incidence of 0.19% vs. 0.55%; p = 0.002).4 In the group comparing exemestane compared to placebo, more grade 2 or higher arthritis (6.5% vs. 4.0%) and hot flashes (18.3% vs. 11.9%) were seen. However, overall quality of life did not differ between the two arms, and no signifıcant differences in new-onset osteoporosis, clinical skeletal fractures, cardiovascular events, or other malignancies were seen.
Another third-generation AI was investigated in the IBIS-II trial, which randomly assigned postmenopausal women at high risk, age 40–70, to receive either anastrozole or placebo for 5 years.5 With a median follow-up of 5 years, 40 breast cancers (invasive and noninvasive) occurred in the anastrozole arm compared to 85 in the placebo group (hazard ratio 0.47; 95% confıdence interval 0.32– 0.68; p < 0.0001). In the anastrozole group compared to placebo, more arthralgia (51% vs. 46%), vasomotor symptoms (57% vs. 49%), vaginal dryness (19% vs. 16%), and hypertension (5% vs. 3%) occurred. In general, there appear to be fewer serious side effects with AIs compared to tamoxifen.
To date, there are no head-to-head trials comparing SERMs to AIs or evaluating extended hormone therapy for up to 10 years in the primary prevention setting. Also, these antiestrogens have no effect on the incidence of ER– tumors, which are associated with a poorer prognosis compared to ER+ breast cancer and are more common in younger women, black women, and BRCA1 mutation carriers. In addition, limited data exist on the effıcacy of antiestrogens for breast cancer risk reduction in women with hereditary breast cancer syndromes, such as BRCA1 and BRCA2 mutation carriers.43,44 Of note, none of these chemoprevention trials were adequately powered to detect a difference in breast cancer–specifıc or overall mortality.
Chemoprevention Guidelines
Based on this evidence, the USPSTF, American Society of Clinical Oncology, and the National Comprehensive Cancer Network published consensus guidelines on breast cancer chemoprevention.6–8 Premenopausal and postmenopausal women at high risk, defıned as a 5-year Gail risk 1.67% or greater or LCIS, may take tamoxifen for 5 years for the primary prevention of breast cancer. Younger women (age 35–50), those without a uterus, and those at higher risk for breast cancer derive the greatest clinical benefıt from tamoxifen. Postmenopausal women at high risk also have the options of raloxifene, exemestane, and anastrozole for breast cancer risk reduction. Because of the increased risk of uterine cancer, follow-up for women on tamoxifen should include annual gynecologic examinations with a timely work-up of abnormal vaginal bleeding, but routine endometrial biopsies in the absence of vaginal symptoms is not recommended. SERMs are contraindicated in women with a history of thromboembolism, such as deep vein thrombosis, pulmonary embolus, stoke, or transient ischemic attack. In addition, the STAR trial excluded women with uncontrolled diabetes or hypertension, those with atrial fıbrillation, and those on hormone replacement therapy.45
Figure 1 depicts a potential algorithm for clinical decision making about antiestrogens for breast cancer chemoprevention based on menopausal status, history of thromboembolism, risk of osteoporosis, and prior hysterectomy. For premenopausal women at high risk, tamoxifen is currently the only FDA-approved drug for the primary prevention of breast cancer. Younger women (age 35–50) at high risk derive the greatest clinical benefıt from tamoxifen because the risk of serious side effects, such as thromboembolism and uterine cancer, is negligible compared to placebo. For postmenopausal women at high risk with an intact uterus, raloxifene may be favored over tamoxifen, whereas tamoxifen may be preferable in women with a prior hysterectomy because of its greater effıcacy in breast cancer risk reduction.3 Both SERMs are contraindicated in women with a prior history of thromboembolism, but AIs may be offered to postmenopausal women. SERMs may be favored over AIs among postmenopausal women at high risk with low bone density, although presence of osteoporosis is not an absolute contraindication to taking an AI. Overall, both SERMs and AIs are effective chemopreventive agents; therefore, the choice will depend on personal preferences and acceptable toxicity profıles.
FIGURE 1. Choice of Selective Estrogen Receptor Modulator or Aromatase Inhibitor for Breast Cancer Chemoprevention.
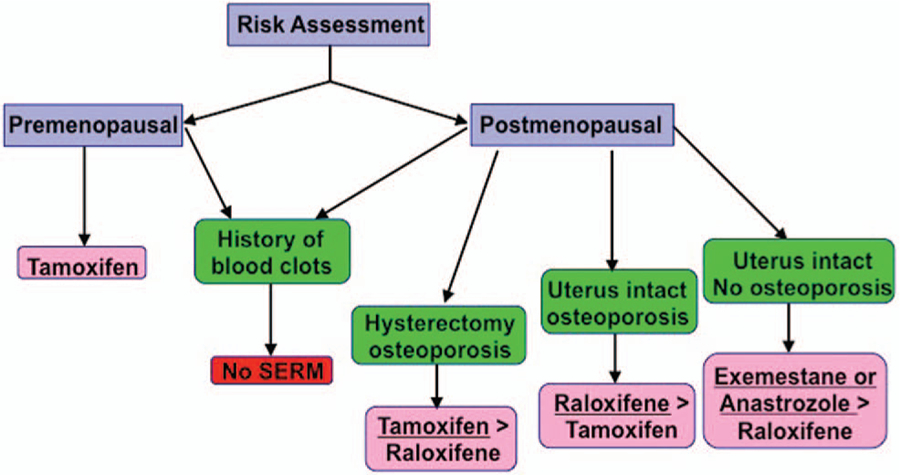
Choice is based on menopausal status, history of thromboembolism, prior hysterectomy, and risk of osteoporosis (based upon the author’s personal algorithm and not a guideline recommendation).
BARRIERS TO UPTAKE OF BREAST CANCER CHEMOPREVENTION
Low Uptake of Breast Cancer Chemoprevention
An estimated 10 million U.S. women age 35 to 79 are eligible for breast cancer chemoprevention.9 Based on a systematic review and meta-analysis of patient decisions about chemoprevention, less than 5% of women at high risk who are offered an antiestrogen for primary prevention agree to take it.10 The main reason for this is the perception of patients and physicians that chemoprevention does not offer a favorable risk– benefıt profıle.46–49 After the 1999 FDA approval of tamoxifen for primary prevention in women at high risk, data from the National Health Interview Survey indicated that the prevalence of tamoxifen use among women without a personal history of breast cancer was 0.2% in 2000 and decreased to 0.08% in 2005.32 Similarly, after raloxifene’s FDA approval in 2007, its use for breast cancer risk reduction decreased.45 It remains to be seen whether there will be greater acceptance of AIs for primary prevention.
Lack of Routine Breast Cancer Risk Assessment in Clinical Practice
Despite the online availability of both the Gail and Tyrer-Cuzick models, only 18% of PCPs report use of software to calculate breast cancer risk.50 In a cross-sectional survey of over 300 PCPs, use of the Gail model for breast cancer risk assessment varied by medical specialty (37% internal medicine, 33% family medicine, 60% gynecology), as well as ever recommending or prescribing breast cancer chemoprevention (9% internal medicine, 8% family medicine, 30% gynecology).51 Barriers to routine breast cancer risk assessment in the primary care setting include time constraints during clinic visits and lack of familiarity with risk assessment tools and chemoprevention.52 There may also be concerns about the accuracy of breast cancer risk prediction models.
Risks and Benefits of Chemoprevention
Concerns about potential side effects, such as uterine cancer, thromboembolic events, and menopausal symptoms, are the main contributors to a woman’s unwillingness to initiate chemopreventive agents for breast cancer and a physician’s reluctance to prescribe them.46–48,53–56 In the BCPT, the net benefıt achieved with tamoxifen varied by age, race, and level of breast cancer risk, such that an estimated 2.5 million women in the United States could derive a net benefıt from the drug.1 In the STAR trial, raloxifene was associated with a lower risk of thromboembolic events, benign uterine complaints, and cataracts than tamoxifen.45,57 Although women on tamoxifen reported more gynecologic and vasomotor symptoms,45 overall quality of life was similar for both SERMs.57 In the MAP.3 and IBIS-II trials, AIs decreased bone mineral density compared to placebo but did not increase the risk of fractures.4 In contrast, SERMs have a favorable effect on bone density with about a 32% reduction in fracture incidence.33,41,42
The general perception among patients and providers is that use of antiestrogens for primary prevention does not confer a favorable risk– benefıt profıle. Based on results from the STAR trial, per 1,000 women at high risk, tamoxifen would prevent 40 breast cancers compared with causing 2.25 uterine cancers and 3.3 thromboembolic events, whereas raloxifene would prevent 31 breast cancers compared with causing 2.47 thromboembolic events.3 Freedman et al developed a model to predict the risks and benefıts of SERMs for women older than 50 based on age, race/ethnicity, breast cancer risk, and presence of a uterus, which may provide a more personalized risk– benefıt profıle.58 Whereas the side effects diminish after stopping chemoprevention, the protective effect on breast cancer risk persists after discontinuation.36 Unlike preventive therapies for other chronic diseases, which often require life-long treatment, breast cancer chemoprevention for 5 years can confer long-term benefıts with side effects limited to during active treatment.
Low chemoprevention uptake occurs because of the lack of effective strategies to inform both PCPs and women at high risk about the risks and benefıts of antiestrogens. Physicians who felt insuffıciently informed about risk-reducing options were less than half as likely to prescribe a SERM for breast cancer prevention than physicians who felt suffıciently trained.59 Physician recommendation and health care provider communication are among the most influential factors to influence chemoprevention uptake.46,48,60
Lack of Intermediate Biomarkers to Predict Response to Chemopreventive Agents
The lack of well-validated intermediate biomarkers for short-term breast cancer risk assessment, analogous to low-density lipoprotein cholesterol for cardiovascular disease or T-score on a bone density scan for osteoporosis, is another barrier to uptake of antiestrogens. Even if a woman at high risk agrees to take chemoprevention, there is no way to assess whether she is deriving a benefıt from the agent except with long-term follow-up to determine whether she remains free of breast cancer. Mammographic density (MD), a strong predictor of breast cancer risk,61,62 may also serve as a predictive biomarker of response to breast cancer chemoprevention. In the IBIS-I trial, tamoxifen given for 18 months caused a signifıcant decrease in MD compared to placebo, particularly among premenopausal women (p < 0.001).63 Cuzick et al demonstrated that at least a 10% reduction in MD with tamoxifen was associated with a 63% reduction in breast cancer risk.64 Compared to other qualitative methods of measuring MD, the Cumulus technique provides quantitative measurements and has been strongly associated with breast cancer risk in epidemiologic studies.65,66 However, more automated methods for measuring MD or volumetric density are needed, which would be applicable in the clinical setting.67,68
Measurement of endogenous hormone levels, such as plasma estrone sulfate, testosterone, prolactin, and sex hormone-binding globulin, have been shown to improve breast cancer risk prediction in postmenopausal women.69 Changes in estradiol and testosterone levels may also serve as good breast cancer risk biomarkers for weight loss interventions.70 However, assay variability with low hormone levels, particularly in postmenopausal women, may hamper their clinical utility.71
Predictors of Poor Adherence to Endocrine Therapy
The effectiveness of chemoprevention depends not only on initiation of therapy but also on long-term adherence. In the chemoprevention trials, adherence at 5 years ranged from 64% to 85%1,4,36,57; however, clinical trial participants are often more compliant than the general population. Veronesi et al reported that women in a chemoprevention trial were less likely to adhere to tamoxifen than patients with breast cancer treated in the adjuvant setting.72 In the Sister Study cohort, 46% of women taking tamoxifen for primary prevention discontinued within 4.5 years.73 In BCPT and MAP.3, ethnic minorities and women with low income had less drug adherence.74,75 Women from racial/ethnic minorities and those who are uninsured are less likely to seek breast cancer preventive care, perhaps contributing to higher rates of late-stage diagnosis.76,77 Understanding predictors of poor uptake and adherence to breast cancer chemoprevention will aid in the development of targeted interventions for certain patient subgroups.
INTERVENTIONS TO INCREASE UPTAKE OF BREAST CANCER CHEMOPREVENTION
Results from recent intervention trials to increase chemoprevention uptake targeting both patients and providers are summarized in Table 3. In a recent randomized controlled trial of a web-based decision aid that informed women about the risks and benefıts of SERMs,78 only 0.5% of eligible participants had started raloxifene and none had started tamoxifen. In a study called the “Ready, Set, GO GAIL!” project, PCPs systematically screened more than 5,700 women age 35–70 with the Gail model; 868 (15.2%) met high-risk criteria and were eligible for chemoprevention, only 128 (14.7%) of these women were referred for specialized risk counseling, 60 (6.4%) completed the consultation, and 17 (2%) started a SERM.79 In the BreastCARE intervention trial, women in the primary care setting were randomly assigned to usual care or a tablet-based patient intake tool that generated individualized breast cancer risk profıles for patients and their physicians.80 Although more women at high risk were referred for specialized risk counseling with the intervention compared to the control arm (18.8% vs. 4.1%), discussions about chemoprevention were still limited (1% vs. 0%).
TABLE 3.
Intervention Trials to Increase Uptake of Breast Cancer Chemoprevention
| Authors, Year | No. of Participants | Eligibility | Intervention | Outcomes |
|---|---|---|---|---|
| Fagerlin et al 201179 | 1,197 | Age 40–74, postmenopausal, 5-yr Gail risk score > 1.66% | Tailored online decision aid “Guide to Decide” | 0% tamoxifen and 0.5% (2 patients) raloxifene uptake with intervention |
| Owens et al 201180 | 868 | Age 35–70, 5-yr Gail risk score ≥ 1.7% or lifetime risk ≥ 20% | “Ready, Set, GO GAIL!” project, clinic-based intervention to implement Gail model in women’s health clinic | Completion of high-risk consultation, 6.4% (60 patients); chemoprevention uptake, 2% (17 patients) |
| Kaplan et al 201481 | 1,235 | Age 40–74, scheduled for clinic visit at two primary care practices, spoke English, Spanish, or Chinese | BreastCARE, tablet-based breast cancer risk assessment that generated individualized reports for patients and their physicians | Referral to high-risk clinic, 18.8% versus 4.1% among women at high risk (307 patients); discussion of chemoprevention, 1% versus 0% |
Interventions designed to increase chemoprevention uptake, involving reading materials or decision aids, met with limited success, ranging from 0.5% to 5.6%.48,49,55,78,81 Few studies have assessed the effect of automated decision support for PCPs.82,83 Two studies used a computer-based tool to improve referrals for genetic testing, but they were not integrated into clinic workflow.84 Given that breast cancer chemoprevention is not widely diffused in the primary care setting, more effective tools are needed to accurately identify women at high risk and educate both patients and providers about the risks and benefıts of chemoprevention options. Studies that involved consultation at a breast clinic reported chemoprevention uptake ranging from 11% to 58%.46,53,54,60,85–87 Therefore, higher chemoprevention uptake may be achieved with health professionals who have suffıcient knowledge and training about breast cancer risk and risk reduction strategies. Given that many community practices may not have access to high-risk clinics, PCPs need to be at the front line of chronic disease prevention, including breast cancer chemoprevention.
Strategies to minimize toxicities to antiestrogens include administering lower or intermittent dosing, developing alternative drug delivery methods such as topical therapy, and identifying novel chemopreventive agents with fewer side effects. For example, clinical trials of oral low-dose tamoxifen of 1, 5, or 10 mg daily or 10–20 mg weekly have demonstrated similar biologic effıcacy to standard-dose tamoxifen (20 mg daily) with fewer side effects.88–95 Since tamoxifen is a prodrug that requires hepatic activation, Mauvais-Jarvis et al developed a topical form of trans-4-hydroxytamoxifen (4-OHT), the active metabolite of tamoxifen, which would maximize local drug levels with fewer systemic side effects.96 Thus far, topical tamoxifen has been tested for the treatment of mastalgia97 and in two presurgical (window of opportunity) trials in women with breast cancer.98,99 Finally, novel chemopreventive agents—including aspirin, NSAIDs, metformin, vitamin D, and vaccines to tumor-associated antigens—which may have a more favorable side effect profıle compared to SERMs and AIs and perhaps activity against ER– breast cancers, are currently under investigation.100
CONCLUSION
Breast cancer chemoprevention with antiestrogens has proven effıcacy in high-risk populations, but uptake remains low. Preventive therapy for cancer is currently less well established compared to other chronic conditions, such as cardiovascular disease, and could benefıt from lessons learned.101 Health care providers can do more in the area of cancer prevention by identifying high-risk populations in the primary care setting. Chemoprevention needs to be integrated into broader strategies of preventive care, which may include nonpharmacologic interventions such as lifestyle modifıcation. Given the high compliance rates for breast cancer screening, incorporating formal risk assessments at the time of screening mammography may represent a “teachable moment” when women are already engaging in a health behavior related to breast cancer. Novel health information technologies such as electronic health records and patient health portals may be a method for integrating information about breast cancer risk and chemoprevention into clinic workflow.
Breast cancer incidence continues to increase in most countries,102 and the economic burden of cancer in the United States is expected to substantially increase103 because of greater intensity of health care usage104,105 and increasing costs of cancer care.106–109 These rising medical costs will disproportionately affect racial/ethnic minorities and low-income and under-insured individuals. U.S. health care providers can do more in the area of cancer prevention by targeting high-risk populations. Promoting chemoprevention uptake among women at high risk will require a major paradigm shift in clinical practice if antiestrogens are to be widely adopted in the primary care setting.
KEY POINTS.
Breast cancer chemoprevention with antiestrogens is underutilized despite several randomized controlled trials demonstrating up to a 50% to 65% relative risk reduction of breast cancer incidence among women at high risk.
Approximately 10 million women in the United States may be eligible for breast cancer chemoprevention, but less than 5% of women at high risk who are offered an antiestrogen for primary prevention agree to take it.
Reasons for low chemoprevention uptake include lack of routine breast cancer risk assessment in the primary care setting, insufficient knowledge about antiestrogens on the part of clinicians and patients, and concerns about side effects.
Interventions designed to increase identification of women at high risk and chemoprevention uptake, including written materials, decision aids, and incorporating breast cancer risk assessment tools into clinical practice, have met with limited success.
Because of the proven efficacy of breast cancer chemopreventive agents, widespread use of antiestrogens for primary prevention among women at high risk has the potential to significantly improve the public health burden of this disease.
Footnotes
Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
References
- 1.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97: 1652–1662. [DOI] [PubMed] [Google Scholar]
- 2.Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goss PE, Ingle JN, Ales-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364: 2381–2391. [DOI] [PubMed] [Google Scholar]
- 5.Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2013;383:1041–1048. [DOI] [PubMed] [Google Scholar]
- 6.Nelson HD, Smith ME, Griffın JC, et al. Use of medications to reduce risk for primary breast cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158:604–614. [DOI] [PubMed] [Google Scholar]
- 7.Visvanathan K, Chlebowski RT, Hurley P, et al. American society of clinical oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27: 3235–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bevers TB. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer Risk Reduction. 2012. http://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf. Accessed February 15, 2015.
- 9.Freedman AN, Graubard BI, Rao SR, et al. Estimates of the number of US women who could benefıt from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526–532. [DOI] [PubMed] [Google Scholar]
- 10.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28:3090–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravdin PM. The lack, need, and opportunities for decision-making and informational tools to educate primary-care physicians and women about breast cancer chemoprevention. Cancer Prev Res (Phila). 2010;3:686–688. [DOI] [PubMed] [Google Scholar]
- 12.Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003; 237:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. [DOI] [PubMed] [Google Scholar]
- 14.Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91:1541–1548. [DOI] [PubMed] [Google Scholar]
- 15.Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99:1782–1792. [DOI] [PubMed] [Google Scholar]
- 16.Matsuno RK, Costantino JP, Ziegler RG, et al. Projecting individualized absolute invasive breast cancer risk in Asian and Pacifıc Islander American women. J Natl Cancer Inst. 2011;103:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu-Rustum NR, Herbolsheimer H. Breast cancer risk assessment in indigent women at a public hospital. Gynecol Oncol. 2001;81:287–290. [DOI] [PubMed] [Google Scholar]
- 18.Grann VR, Jacobson JS, Troxel AB, et al. Barriers to minority participation in breast carcinoma prevention trials. Cancer. 2005;104:374–379. [DOI] [PubMed] [Google Scholar]
- 19.Banegas MP, Leng M, Graubard BI, et al. The risk of developing invasive breast cancer in Hispanic women: a look across Hispanic subgroups. Cancer. 2013;119:1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111–1130. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann LC, Degnim AC, Santen RJ, et al. Atypical hyperplasia of the breast-risk assessment and management options. N Engl J Med. 2015; 372:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutwein LG, Ang DN, Liu H, et al. Utilization of minimally invasive breast biopsy for the evaluation of suspicious breast lesions. Am J Surg. 2011;202:127–132. [DOI] [PubMed] [Google Scholar]
- 23.Simpson JF. Update on atypical epithelial hyperplasia and ductal carcinoma in situ. Pathology. 2009;41:36–39. [DOI] [PubMed] [Google Scholar]
- 24.Coopey SB, Mazzola E, Buckley JM, et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat. 2012;136:627–633. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann LC, Radisky DC, Frost MH, et al. Understanding the premalignant potential of atypical hyperplasia through its natural history: a longitudinal cohort study. Cancer Prev Res (Phila). 2014;7:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.London SJ, Connolly JL, Schnitt SJ, et al. A prospective study of benign breast disease and the risk of breast cancer. JAMA. 1992;267:941–944. [PubMed] [Google Scholar]
- 27.Degnim AC, Visscher DW, Berman HK, et al. Stratifıcation of breast cancer risk in women with atypia: a Mayo cohort study. J Clin Oncol. 2007;25:2671–2677. [DOI] [PubMed] [Google Scholar]
- 28.Page DL, Schuyler PA, Dupont WD, et al. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study. Lancet. 2003;361:125–129. [DOI] [PubMed] [Google Scholar]
- 29.Pankratz VS, Hartmann LC, Degnim AC, et al. Assessment of the accuracy of the Gail model in women with atypical hyperplasia. J Clin Oncol. 2008;26:5374–5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boughey JC, Hartmann LC, Anderson SS, et al. Evaluation of the Tyrer-Cuzick (International Breast Cancer Intervention Study) model for breast cancer risk prediction in women with atypical hyperplasia. J Clin Oncol. 2010;28:3591–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barr FE, Degnim AC, Hartmann LC, et al. Estrogen receptor expression in atypical hyperplasia: lack of association with breast cancer. Cancer Prev Res (Phila). 2011;4:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waters EA, Cronin KA, Graubard BI, et al. Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiol Biomarkers Prev. 2010;19:443–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. [DOI] [PubMed] [Google Scholar]
- 34.Powles TJ, Ashley S, Tidy A, et al. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–290. [DOI] [PubMed] [Google Scholar]
- 35.Veronesi U, Maisonneuve P, Rotmensz N, et al. Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. 2007;99:727–737. [DOI] [PubMed] [Google Scholar]
- 36.Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer-96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–282. [DOI] [PubMed] [Google Scholar]
- 37.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. [DOI] [PubMed] [Google Scholar]
- 38.Dalen JE. Aspirin for the primary prevention of stroke and myocardial infarction: ineffective or wrong dose? Am J Med. 2010;123:101–102. [DOI] [PubMed] [Google Scholar]
- 39.Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396–404. [DOI] [PubMed] [Google Scholar]
- 40.Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011;19: CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–2197. [DOI] [PubMed] [Google Scholar]
- 42.Martino S, Cauley JA, Barrett-Connor E, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761. [DOI] [PubMed] [Google Scholar]
- 43.Narod SA, Brunet JS, Ghadirian P, et al. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Hereditary Breast Cancer Clinical Study Group. Lancet. 2000;356:1876–1881. [DOI] [PubMed] [Google Scholar]
- 44.King MC, Wieand S, Hale K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA. 2001;286:2251–2256. [DOI] [PubMed] [Google Scholar]
- 45.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. [DOI] [PubMed] [Google Scholar]
- 46.Bober SL, Hoke LA, Duda RB, et al. Decision-making about tamoxifen in women at high risk for breast cancer: clinical and psychological factors. J Clin Oncol. 2004;22:4951–4957. [DOI] [PubMed] [Google Scholar]
- 47.Melnikow J, Paterniti D, Azari R, et al. Preferences of Women Evaluating Risks of Tamoxifen (POWER) study of preferences for tamoxifen for breast cancer risk reduction. Cancer. 2005;103:1996–2005. [DOI] [PubMed] [Google Scholar]
- 48.Taylor R, Taguchi K. Tamoxifen for breast cancer chemoprevention: low uptake by high-risk women after evaluation of a breast lump. Ann Fam Med. 2005;3:242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fagerlin A, Zikmund-Fisher BJ, Nair V, et al. Women’s decisions regarding tamoxifen for breast cancer prevention: responses to a tailored decision aid. Breast Cancer Res Treat. 2010;119:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corbelli J, Borrero S, Bonnema R, et al. Use of the Gail model and breast cancer preventive therapy among three primary care specialties. J Womens Health (Larchmt). 2014;23:746–752. [DOI] [PubMed] [Google Scholar]
- 52.Sabatino SA, McCarthy EP, Phillips RS, et al. Breast cancer risk assessment and management in primary care: provider attitudes, practices, and barriers. Cancer Detect Prev. 2007;31:375–383. [DOI] [PubMed] [Google Scholar]
- 53.Metcalfe KA, Snyder C, Seidel J, et al. The use of preventive measures among healthy women who carry a BRCA1 or BRCA2 mutation. Fam Cancer. 2005;4:97–103. [DOI] [PubMed] [Google Scholar]
- 54.Salant T, Ganschow PS, Olopade OI, et al. “Why take it if you don’t have anything?” breast cancer risk perceptions and prevention choices at a public hospital. J Gen Intern Med. 2006;21:779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Port ER, Montgomery LL, Heerdt AS, et al. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann Surg Oncol. 2001;8:580–585. [DOI] [PubMed] [Google Scholar]
- 56.Stacey D, O’Connor AM, DeGrasse C, et al. Development and evaluation of a breast cancer prevention decision aid for higher-risk women. Health Expect. 2003;6:3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2742–2751. [DOI] [PubMed] [Google Scholar]
- 58.Freedman AN, Yu B, Gail MH, et al. Benefıt/Risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaplan CP, Haas JS, Perez-Stable EJ, et al. Factors affecting breast cancer risk reduction practices among California physicians. Prev Med. 2005;41:7–15. [DOI] [PubMed] [Google Scholar]
- 60.Rondanina G, Puntoni M, Severi G, et al. Psychological and clinical factors implicated in decision making about a trial of low-dose tamoxifen in hormone replacement therapy users. J Clin Oncol. 2008;26: 1537–1543. [DOI] [PubMed] [Google Scholar]
- 61.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–1169. [DOI] [PubMed] [Google Scholar]
- 62.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. [DOI] [PubMed] [Google Scholar]
- 63.Cuzick J, Warwick J, Pinney E, et al. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96: 621–628. [DOI] [PubMed] [Google Scholar]
- 64.Cuzick J, Warwick J, Pinney E, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103:744–752. [DOI] [PubMed] [Google Scholar]
- 65.Byng JW, Boyd NF, Fishell E, et al. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39:1629–1638. [DOI] [PubMed] [Google Scholar]
- 66.Pettersson A, Graff RE, Ursin G, et al. Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2014;106. [DOI] [PMC free article] [PubMed]
- 67.Wang J, Azziz A, Fan B, et al. Agreement of mammographic measures of volumetric breast density to MRI. PLoS One. 2013;8:e81653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gubern-Merida A, Kallenberg M, Platel B, et al. Volumetric breast density estimation from full-fıeld digital mammograms: a validation study. PLoS One. 2014;9:e85952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tworoger SS, Zhang X, Eliassen AH, et al. Inclusion of endogenous hormone levels in risk prediction models of postmenopausal breast cancer. J Clin Oncol. 2014;32:3111–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones ME, Schoemaker M, Rae M, et al. Changes in estradiol and testosterone levels in postmenopausal women after changes in body mass index. J Clin Endocrinol Metab. 2013;98:2967–2974. [DOI] [PubMed] [Google Scholar]
- 71.Jones ME, Schoemaker MJ, Rae M, et al. Reproducibility of estradiol and testosterone levels in postmenopausal women over 5 years: results from the breakthrough generations study. Am J Epidemiol. 2014;179: 1128–1133. [DOI] [PubMed] [Google Scholar]
- 72.Veronesi A, Pizzichetta MA, Ferlante MA, et al. Tamoxifen as adjuvant after surgery for breast cancer and tamoxifen or placebo as chemoprevention in healthy women: different compliance with treatment. Tumori. 1998;84:372–375. [DOI] [PubMed] [Google Scholar]
- 73.Nichols HB, DeRoo LA, Scharf DR, et al. Risk-benefıt profıles of women using tamoxifen for chemoprevention. J Natl Cancer Inst. 2015;107:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moy B, Richardson H, Johnston D, et al. NCIC CTG MAP.3: enrollment and study drug adherence of ethnic minority women in a breast cancer prevention trial. Breast Cancer Res Treat. 2007;106:S141–S142. [Google Scholar]
- 75.Land SR, Cronin WM, Wickerham DL, et al. Cigarette smoking, obesity, physical activity, and alcohol use as predictors of chemoprevention adherence in the National Surgical Adjuvant Breast and Bowel Project P-1 Breast Cancer Prevention Trial. Cancer Prev Res (Phila). 2011;4:1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaplan CP, Haas JS, Perez-Stable EJ, et al. Breast cancer risk reduction options: Awareness, discussion, and use among women from four ethnic groups. Cancer Epidemiol Biomarkers Prev. 2006;15:162–166. [DOI] [PubMed] [Google Scholar]
- 77.Jacobson JS, Grann VR, Hershman D, et al. Breast biopsy and race/ ethnicity among women without breast cancer. Cancer Detect Prev. 2006;30:129–133. [DOI] [PubMed] [Google Scholar]
- 78.Fagerlin A, Dillard AJ, Smith DM, et al. Women’s interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid. Breast Cancer Res Treat. 2011;127:681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Owens WL, Gallagher TJ, Kincheloe MJ, et al. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaplan CP, Livaudais-Toman J, Tice JA, et al. A randomized, controlled trial to increase discussion of breast cancer in primary care. Cancer Epidemiol Biomarkers Prev. 2014;23:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loehberg CR, Jud SM, Haeberle L, et al. Breast cancer risk assessment in a mammography screening program and participation in the IBIS-II chemoprevention trial. Breast Cancer Res Treat. 2010;121:101–110. [DOI] [PubMed] [Google Scholar]
- 82.Hilgart JS, Coles B, Iredale R. Cancer genetic risk assessment for individuals at risk of familial breast cancer. Cochrane Database Syst Rev. 2012;2:CD003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akbari A, Mayhew A, Al-Alawi MA, et al. Interventions to improve outpatient referrals from primary care to secondary care. Cochrane Database Syst Rev. 2008:CD005471. [DOI] [PMC free article] [PubMed]
- 84.Wilson BJ, Torrance N, Mollison J, et al. Improving the referral process for familial breast cancer genetic counselling: fındings of three randomised controlled trials of two interventions. Health Technol Assess. 2005;9:iii-iv, 1–126. [DOI] [PubMed] [Google Scholar]
- 85.Sprague BL, Trentham-Dietz A, Nichols HB, et al. Change in lifestyle behaviors and medication use after a diagnosis of ductal carcinoma in situ. Breast Cancer Res Treat. 2010;124:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tchou J, Hou N, Rademaker A, et al. Acceptance of tamoxifen chemoprevention by physicians and women at risk. Cancer. 2004;100:1800–1806. [DOI] [PubMed] [Google Scholar]
- 87.Goldenberg VK, Seewaldt VL, Scott V, et al. Atypia in random periareolar fıne-needle aspiration affects the decision of women at high risk to take tamoxifen for breast cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2007;16:1032–1034. [DOI] [PubMed] [Google Scholar]
- 88.Decensi A, Bonanni B, Guerrieri-Gonzaga A, et al. Biologic activity of tamoxifen at low doses in healthy women. J Natl Cancer Inst. 1998;90: 1461–1467. [DOI] [PubMed] [Google Scholar]
- 89.Decensi A, Robertson C, Viale G, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95:779–790. [DOI] [PubMed] [Google Scholar]
- 90.DeCensi A, Guerrieri-Gonzaga A, Gandini S, et al. Prognostic signifıcance of Ki-67 labeling index after short-term presurgical tamoxifen in women with ER-positive breast cancer. Ann Oncol. 2011;22:582–587. [DOI] [PubMed] [Google Scholar]
- 91.Decensi A, Robertson C, Guerrieri-Gonzaga A, et al. Randomized double-blind 2 × 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol. 2009;27:3749–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bonanni B, Serrano D, Gandini S, et al. Randomized biomarker trial of anastrozole or low-dose tamoxifen or their combination in subjects with breast intraepithelial neoplasia. Clin Cancer Res. 2009;15:7053–7060. [DOI] [PubMed] [Google Scholar]
- 93.Decensi A, Gandini S, Serrano D, et al. Randomized dose-ranging trial of tamoxifen at low doses in hormone replacement therapy users. J Clin Oncol. 2007;25:4201–4209. [DOI] [PubMed] [Google Scholar]
- 94.Guerrieri-Gonzaga A, Botteri E, Lazzeroni M, et al. Low-dose tamoxifen in the treatment of breast ductal intraepithelial neoplasia: results of a large observational study. Ann Oncol. 2010;21:949–954. [DOI] [PubMed] [Google Scholar]
- 95.de Lima GR, Facina G, Shida JY, et al. Effects of low dose tamoxifen on normal breast tissue from premenopausal women. Eur J Cancer. 2003; 39:891–898. [DOI] [PubMed] [Google Scholar]
- 96.Mauvais-Javis P, Baudot N, Castaigne D, et al. trans-4-Hydroxytamoxifen concentration and metabolism after local percutaneous administration to human breast. Cancer Res. 1986;46:1521–1525. [PubMed] [Google Scholar]
- 97.Mansel R, Goyal A, Nestour EL, et al. A phase II trial of Afımoxifene (4-hydroxytamoxifen gel) for cyclical mastalgia in premenopausal women. Breast Cancer Res Treat. 2007;106:389–397. [DOI] [PubMed] [Google Scholar]
- 98.Rouanet P, Linares-Cruz G, Dravet F, et al. Neoadjuvant percutaneous 4-hydroxytamoxifen decreases breast tumoral cell proliferation: a prospective controlled randomized study comparing three doses of 4-hydroxytamoxifen gel to oral tamoxifen. J Clin Oncol. 2005;23:2980–2987. [DOI] [PubMed] [Google Scholar]
- 99.Lee O, Page K, Ivancic D, et al. A randomized phase II presurgical trial of transdermal 4-hydroxytamoxifen gel versus oral tamoxifen in women with ductal carcinoma in situ of the breast. Clin Cancer Res. 2014;20:3672–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Serrano D, Lazzeroni M, Bonanni B. Cancer chemoprevention: Much has been done, but there is still much to do. State of the art and possible new approaches. Mol Oncol. Epub 2014 December 20. [DOI] [PMC free article] [PubMed]
- 101.Meyskens FL Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila). 2011;4:311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 103.Yabroff KR, Lund J, Kepka D, et al. Economic burden of cancer in the United States: Estimates, projections, and future research. Cancer Epidemiol Biomarkers Prev. 2011;20:2006–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Warren JL, Yabroff KR, Meekins A, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dinan MA, Curtis LH, Hammill BG, et al. Changes in the use and costs of diagnostic imaging among Medicare benefıciaries with cancer, 1999–2006. JAMA. 2010;303:1625–1631. [DOI] [PubMed] [Google Scholar]
- 106.Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360:626–633. [DOI] [PubMed] [Google Scholar]
- 107.Tangka FK, Trogdon JG, Richardson LC, et al. Cancer treatment cost in the United States: has the burden shifted over time? Cancer. 2010; 116:3477–3484. [DOI] [PubMed] [Google Scholar]
- 108.Elkin EB, Bach PB. Cancer’s next frontier. Addressing high and increasing costs. JAMA. 2010;303:1086–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]


