Abstract
Background and aims:
Cell-mediated immunity is implicated in atherosclerosis. We evaluated whether innate and adaptive immune cell subsets in peripheral blood are risk factors for coronary heart disease.
Methods:
A nested case-cohort study (n=2,155) was performed within the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS). Cases of incident myocardial infarction (MI) and incident angina (n=880 total cases) were compared with a cohort random sample (n=1,275). Immune cell phenotypes (n=34, including CD14+ monocytes, natural killer cells, γδ T cells, CD4+, CD8+ and CD19+ lymphocyte subsets) were measured from cryopreserved cells by flow cytometry. Cox proportional hazards models with adjustment for cardiovascular disease risk factors were used to evaluate associations of cell phenotypes with incident MI and a composite phenotype of incident MI or incident angina (MI-angina) over a median 9.3 years of follow-up. Th1, Th2, Th17, T regulatory (CD4+CD25+CD127−), naive (CD4+CD45RA+), memory (CD4+CD45RO+), and CD4+CD28− cells were specified as primary hypotheses. In secondary analyses 27 additional cell phenotypes were investigated.
Results:
After correction for multiple testing, there were no statistically significant associations of CD4+ naive, memory, CD28−, or T helper cell subsets with MI or MI-angina in MESA, CHS, or combined-cohort meta analyses. Null associations were also observed for monocyte subsets, natural killer cells, γδ T cells, CD19+ B cell and differentiated CD4+ and CD8+ cell subsets.
Conclusions:
The proportions of peripheral blood monocyte and lymphocyte subsets are not strongly related to the future occurrence of MI or angina in adults free of autoimmune disease.
Keywords: Biomarkers, Cardiovascular Disease, Immunity, Inflammation, Risk Factors, T cells
Graphical abstract
Introduction
Coronary heart disease (CHD) remains a leading cause of death and disability worldwide. The major underlying cause of CHD is atherosclerosis, an inflammatory process initiated by cholesterol retention in the arterial wall. Arterial inflammation is orchestrated by cells of the innate (e.g., monocytes, macrophages) and adaptive (e.g., CD4+ T helper cells and cytotoxic CD8+ T cells) immune systems following their recruitment to the arterial intima [1].
The importance of cell-mediated immunity in atherosclerosis has been established in several experimental mouse models. Accumulation and modification of lipoprotein particles in the arterial intima (e.g., oxidized low-density lipoprotein (LDL)) leads to the recruitment of monocytes which differentiate into lipoprotein-scavenging macrophages [1, 2]. CD4+ T helper cells are recruited to atherosclerotic lesions and play both atherogenic and atheroprotective roles [1]. CD4+ cells include T helper type 1 (Th1), Th2, Th17, and T regulatory (Treg) cells. Interferon-γ (IFN- γ) producing Th1 cells contribute to inflammation and plaque destabilization, whereas IL-10 producing Tregs are anti-inflammatory and atheroprotective [1]. Th2 and Th17 cells have been implicated in atheroprotection and plaque stability, respectively, but their roles in experimental atherosclerosis remain unresolved [1, 3]. CD8+ T cells and humoral immune B cells are suspected as important but their roles are still being clarified [1, 4]. Natural killer cells were shown to have no role in hypercholesterolemia-induced atherosclerosis, but exacerbated atherosclerosis under inflammatory conditions associated with viral infection [5].
Several immune cell populations, including natural killer, CD4+ and CD8+ subsets are present in human atherosclerotic lesions [6–8]. An immune cell atlas of human atherosclerotic plaques was recently generated using single-cell proteomic and transcriptomic analyses [9]. In this study CD4+ and CD8+ T cells isolated from plaques were chronically activated and differentiated, with the majority being effector-memory subsets. Cross-sectional analyses of peripheral blood samples from epidemiologic cohorts have shown associations of higher intermediate and non-classical monocyte subsets, CD19+CD80+ B cells, Th1, CD4+ memory, CD4+ effector-memory, and lower Th2 cells, with the severity of carotid artery intimal medial thickness (IMT) or coronary artery calcification (CAC) [10–15].
Although the involvement of cellular immunity has been established in murine models of atherosclerosis and indicated in cross-sectional human studies, it remains unclear whether these findings translate to risk of clinical cardiovascular disease (CVD) events [16]. Atherosclerotic plaques from patients with recent cerebrovascular events were characterized by an expansion of activated effector-memory CD4+ T cells and gene expression signatures reflecting CD4+ and CD8+ T cell activation, differentiation and exhaustion [9]. Higher levels of intermediate monocytes, Th1, Th17, CD4+CD28−and CD8+CD56+ T cells, and lower Tregs, were reported in the circulating blood of subjects with coronary artery disease compared to those without disease [6, 7, 17]. The prospective Malmö Diet and Cancer Study (MDCS) has evaluated longitudinal relationships of circulating immune cells with risk of incident clinical CVD events [14, 15, 18–21]. In these studies, high levels of CD8+ cells and low levels of Th2 and Tregs, were associated with an increased risk of incident coronary events [14, 18, 20].
To date, no prospective cohort studies have investigated the relationships of a broad panel of innate and adaptive immune cell phenotypes with the risk of incident CHD in a multi-ethnic population. In the present study, we measured 34 immune cell subsets using cryopreserved samples from participants of the population-based Multi-Ethnic Study of Atherosclerosis (MESA) and Cardiovascular Health Study (CHS). We evaluated their prospective relationships with incident myocardial infarction (MI) and incident angina over a follow-up period of up to 15-years. Based on our prior cross-sectional results in MESA [11, 12], and findings from others [10, 14, 20, 22–24], we hypothesized that higher proportions of Th1, CD4+ memory and CD4+CD28− cells, and lower proportions of CD4+ naive, Th2, Th17, and Treg cells, would be associated with increased risk of MI or a composite phenotype of incident MI or incident angina.
Materials and methods
Cohorts
The MESA is a prospective cohort study of subclinical cardiovascular disease [25]. At the baseline examination in 2000–2002, 6,814 men and women aged 45 to 84 years were recruited from six U.S. communities (Baltimore, MD, Chicago, IL, Los Angeles, CA, St. Paul, MN, New York, NY, and Forsyth County, NC). The cohort was 38% white, 28% African-American, 22% Hispanic, and 12% Chinese. Participants were excluded if they had clinical CVD or were undergoing active treatment for cancer. Follow-up examinations occurred in 2002–04, 2004–05, 2005–07, 2010–12, and 2016–18. During the examinations, participants went to the MESA Field Center clinics, underwent assessment for CVD risk factors, provided fasting blood samples, and answered standardized questionnaires [25].
The CHS is a prospective population-based cohort study of risk factors for CHD and stroke in older adults [26]. In 1989–1990, 5,201 men and women aged 65 and older were recruited from 4 U.S. field centers (Forsyth County, NC, Sacramento County, CA, Washington County, MD, and Pittsburgh, PA). An additional cohort of 687 primarily African-American participants were recruited in 1992–93. Participants answered standardized questionnaires assessing medical histories and CVD risk factors, and provided fasting blood samples. Between 1990 and 1999, semi-annual follow-up alternated between clinical examinations and telephone contacts. Since 1999, bi-annual follow-up occurred by telephone. CHS participants with prevalent CVD at the time of the 1998–99 blood draw were excluded from our study.
The current study is a case-cohort study nested within MESA and CHS. The study included 1,195 MESA and 1,197 CHS participants with available samples of cryopreserved cells. The study included all cases of incident MI and incident angina (n=485 MESA, n=492 CHS) and a cohort random sample (n=715 MESA, n=706 CHS). All participants provided written informed consent for participation in the study and all procedures were conducted under institutionally approved protocols for human subjects research.
Covariate data were from the same examination cryopreserved cells were collected: 2000–02 in MESA and 1998–99 in CHS (Year 11). Diabetes mellitus was defined as a fasting blood glucose ≥126 mg/dL or the use of insulin or oral hypoglycemic medications. Smoking was defined as never, former (no cigarettes within the past 30 days), or current. Education status was defined by less than college degree or college degree or above. IgG antibodies to cytomegalovirus (CMV) were measured in serum by enzyme immunoassay (Diamedix Corp., Miami Lakes, FL); the inter-assay coefficients of variation were 5.1%−6.8%.
Cellular phenotyping
Peripheral blood mononuclear cells (PBMCs) were isolated from 8-mL citrate CPT tubes during the MESA baseline examination (2000–02) and the Year 11 CHS examination (1998–99) and cryopreserved at −135°C. Cells were thawed at 37°C for 15 minutes and treated with benzonase (2500 U/mL in RPMI) for 20 minutes. Cells were slowly diluted 10-fold by the addition of RPMI supplemented with fetal bovine serum (10%), L-glutamine (2 mM), penicillin (100 IU/mL) and streptomycin (100 µg/mL) (fsRPMI) with mixing. Cells were pelleted by centrifugation (5 minutes at 200 x g) and treated with 250 U/mL benzonase in fsRPMI for 10 minutes. The pellet was washed and filtered through a 70 µM filter. Cells were placed in 96 well plates, centrifuged and resuspended in phosphate buffered saline for staining with a live/dead stain (Thermo Fisher Scientific, Waltham, MA) for 15 minutes at room temperature.
We performed a pilot study to assess viability of cryopreserved cells in MESA and CHS. We thawed 8 MESA PBMC samples cryopreserved in 2000–02 and 12 CHS samples cryopreserved in 1998–99. Phenotypes from cryopreserved cells were compared to data from the MESA-Inflammation study [11, 12] which evaluated cells isolated from fresh blood samples collected in 2005–07. Results are presented in Supplemental Table I and suggested cell phenotype data from fresh vs. cryopreserved samples were similar. For example, the mean value of CD4+CD45RO+ (memory) cells was 53.9% in fresh samples compared with 56.7% in cryopreserved samples. Mean Th1 (CD4+IFN-γ+) was 16.0% in fresh and 12.3% in cryopreserved samples.
Phenotyping assays for monocyte subsets, innate lymphocytes (i.e., natural killer, γδ T), CD19+ B cell subsets, and naive, memory, differentiated, and CD45RA+ re-expressing effector memory (TEMRA) CD4+ and CD8+ T cell subsets were performed by cell-surface labeling. Cells were incubated with antibodies for 15 minutes in the dark at room temperature according to the manufacturer’s recommendations. Cells were pelleted and fixed in 1% paraformaldehyde (Alfa Asaer, Tewksbury, MA). All antibodies were from Miltenyi Biotec (San Diego, CA) and used dilutions recommended by the manufacturer (Supplemental Material Table II).
In MESA, intracellular cytokine staining was used for Th1, Th2, Th17, CD8+ T cytotoxic type 1 (Tc1), Tc2, and Tc17 phenotyping. These assays used phorbal myristic acetate, ionomycin, and Brefeldin A to activate cells and inhibit cytokine secretion as previously described [11]. Cells were subsequently incubated with APC-conjugated anti-CD4 or VioBrightFITC-conjugated anti-CD8 for 15 minutes at room temperature. Cells were washed with staining buffer and fixed with 2% paraformaldehyde for 10 minutes. Cells were then permeabilized using 0.1% saponin and incubated with PEVio770-anti-IFN-γ, PE-anti-interleukin-4 (IL-4), and APCVio770-anti-IL-17 for 15 minutes at room temperature. Cells were washed and fixed in 2% paraformaldehyde. In CHS, intracellular cytokine assays were not performed due to generally lower cell viability. Th1, Th2, and Th17 phenotypes were evaluated in CHS by cell-surface labeling of the chemokine receptors CXCR3 (VioBright FITC-CXCR3), CCR4 (PE-Vio770-CCR4), and CCR6 (APC-CCR6), as described above for CD4/CD8. Th1 cells were defined as CD4+CXCR3+CCR4−CCR6−. Th2 cells were defined as CD4+CXCR3−CCR4+CCR6− and Th17 cells as CD4+CXCR3−CCR4+CCR6+ [27]. Tc1, Tc2, Tc17, and CD14+ monocyte subsets were not assayed in CHS. All markers used for immunophenotyping are presented in Supplementary Table III.
Cell phenotypes were evaluated using an MQ10 flow cytometry and MACS Quantify software (Miltenyi Biotec). Calibration beads were used for daily calibration. Compensation was set using single color compensation controls. Isotype-matched antibodies were used as controls to set negative gates for each assay. Cell phenotypes were expressed as proportions of larger “parent” populations. For example, T helper cell subsets were expressed as a percentage of CD4+ cells (described in Supplementary Table III). Flow cytometry gating strategies are presented in Supplemental Figures I–VI.
Event ascertainment, definitions, and laboratory measurements
The primary clinical endpoints for this study were incident MI and a composite phenotype comprised of incident MI or incident angina (MI-angina). The MI outcome included non-fatal, fatal, and procedure-related MIs.
MESA participants or their proxies completed telephone interviews every 9–12 months providing information about potential cardiovascular events, including hospitalizations, diagnostic tests, procedures, and deaths [25]. MESA Field Center staff collected information from medical records, death certificates, autopsy reports, and interviews with physicians, participants, or their proxies. Information was reviewed and CVD events were adjudicated by the MESA Events Committee [25]. Follow-up for MESA participants in the present study was through December 31, 2015.
In CHS, events were reported at clinical examinations or during semiannual telephone contacts. Events ascertainment included hospital and out-patient medical records, including copies of ECGs, CT scans, MRI scans, death certificates, and autopsy and coroner reports. These were reviewed and adjudicated by the CHS Cardiac Events Committee [28]. In the current study, follow-up for CHS participants was through June 30, 2015.
Statistical analyses
Our analyses included participants with data for at least one of the immune cell assays (n=2155; MESA: 1113, CHS: 1042). The number of participants with data for each immune cell trait is presented in Supplemental Table IV. Missing cell data were due to poor sample quality and technical assay errors. These types of missing data were determined random, and were not considered to be related to any participant characteristics. We used multiple imputation (100 imputations) with chained equations to impute missing cell phenotype and covariate data from among these 2155 participants. To account for the case-cohort sampling, censoring indicators for MI and angina and time to event data were included in the multiple imputation model [29, 30].
Associations between immune cell traits and MI or composite MI-angina endpoints were evaluated by Cox proportional hazards models that used case-cohort weights and robust standard error (sandwich) estimates. Weights were the inverse sampling probabilities for inclusion in the study [31]. We analyzed results separately in each cohort. For cell assays that were comparable in both studies (e.g., CD4+ naive, memory, and CD28− cells), MESA and CHS results were combined using fixed effects inverse-variance-weighted meta-analysis. Cell phenotypes were analyzed per 1 standard deviation (SD) higher value and modeled singly to avoid collinearity. Demographic models included adjustment for age, sex, race/ethnicity, education, and clinical examination site. CHD risk factor models included these variables plus systolic blood pressure, use of anti-hypertension medications, LDL cholesterol, use of statins, diabetes, and smoking status. A sensitivity model included additional adjustment for log-transformed cytomegalovirus (CMV) antibody concentrations and season of blood draw due to known relationships of these variables with Th1 and Th2 cells in MESA, respectively [11].
Multiple hypothesis testing was controlled for using Bonferroni correction. Primary hypotheses included Th1, Th2, Th17, Treg, CD4+ naive, CD4+ memory, and CD4+CD28− cells and were considered statistically significant if p<0.0071. Secondary analyses evaluated n=27 additional immune cell subsets and used a significance threshold of p<0.0015.
Results
In the combined MESA-CHS case-cohort there were 644 incident MIs and 880 cases of incident MI or incident angina over a median 9.3 years of follow-up (interquartile range (IQR) 4.3–14.0 years). By cohort, there were 296 incident MIs and 451 composite MI-angina events in MESA over a median 11.1 years of follow-up (IQR: 4.9–14.2). In CHS there were 348 incident MIs and 429 incident MI-angina events over a median 7.7 years of follow-up (IQR: 4.0–12.7). Table 1 shows the characteristics of the study population stratified by cohort and case status.
Table 1.
Characteristics of the study population stratified by cohort and case status
| MESA (n=1113) |
CHS (n=1042) |
|||
|---|---|---|---|---|
| No CHD (n=662) |
CHD (n=451) |
No CHD (n=613) |
CHD (n=429) |
|
| Age, years (mean, SD) | 62 (10) | 67 (10) | 80 (5) | 80 (4) |
| Female sex (n, %) | 296 (45%) | 293 (65%) | 408 (66%) | 256 (60%) |
| Race/Ethnicity (n, %) | ||||
| White | 229 (35%) | 192 (43%) | 503 (82%) | 347 (82%) |
| African-American | 209 (32%) | 114 (25%) | 109 (18%) | 77 (18%) |
| Hispanic-American | 132 (20%) | 100 (22%) | - | - |
| Chinese-American | 92 (13%) | 45 (10%) | - | - |
| Bachelor’s degree or above (n, %) | 249 (38%) | 142 (31%) | 142 (23%) | 102 (24%) |
| LDL-cholesterol, mg/dL (mean, SD) | 117 (28) | 119 (34) | 201 (37) | 201 (40) |
| Current smoker (n, %) | 74 (11%) | 74 (16%) | 46 (7%) | 32 (8%) |
| Systolic blood pressure, mm Hg (mean, SD) | 127 (21) | 134 (22) | 135 (20) | 138 (22) |
| Antihypertensive medication use (n, %) | 242 (37%) | 236 (52%) | 318 (52%) | 254 (60%) |
| Statin use (n, %) | 97 (15%) | 99 (22%) | 68 (11%) | 52 (12%) |
| Diabetes (n, %) | 84 (13%) | 103 (23%) | 62 (10%) | 73 (17%) |
| Cytomegalovirus antibodies, EU/mL (mean, SD) | 235 (262) | 242 (262) | 301 (284) | 262 (270) |
Data are from MESA Exam 1 (2000–2002) and the Year 11 CHS examination (1998–1999). Coronary heart disease (CHD) is defined as incident myocardial infarction or incident angina. EU, ELISA units; LDL, low-density lipoprotein.
As primary hypotheses we evaluated associations of Th1 (CD4+IFN-γ+), Th2 (CD4+IL-4+), Th17 (CD4+IL-17A+), Tregs (CD4+CD25+CD127−), naive (CD4+CD45RA+), memory (CD4+CD45RO+), and senescent (CD4+CD28−) CD4+ T cells with incident MI and with incident MI-angina. As shown in Figure 1, there were no statistically significant associations among any of these cell populations with MI in MESA, CHS, or in a combined-cohort meta-analysis (all p>0.05; analyzed per 1-SD higher proportion, adjusted for demographic variables and CHD risk factors). The associations of these cells with incident MI-angina were also not statistically significant (all p>0.05; Figure 2).
Figure 1. Associations of CD4+ T helper cell subsets specified as primary hypotheses with incident myocardial infarction.
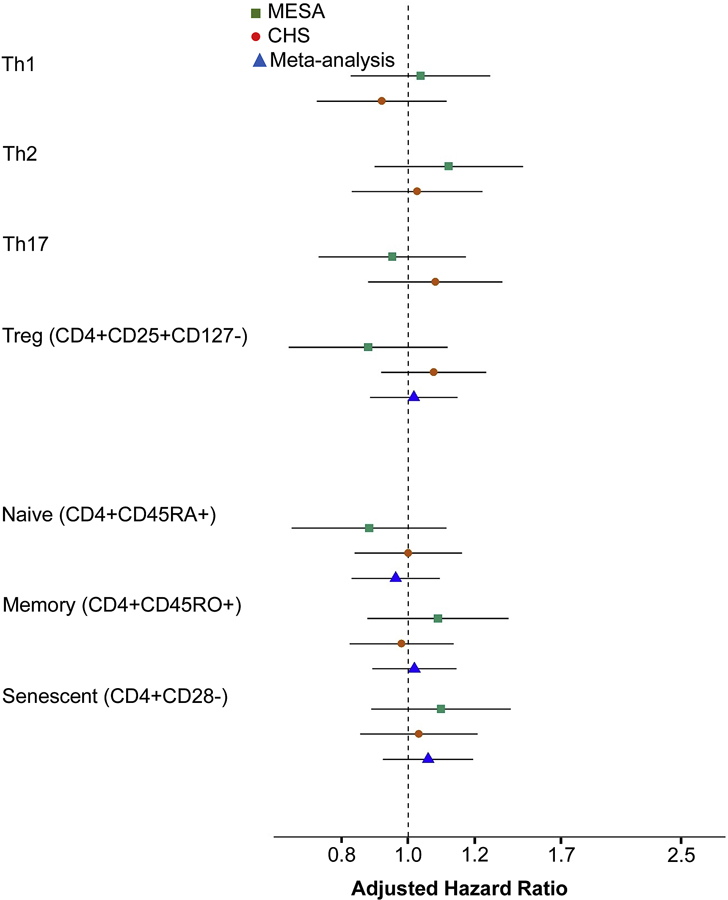
Cell subsets were analyzed per 1-SD higher values using Cox proportional hazards models with sampling weights. Confidence intervals (CIs) used robust (sandwich) standard error estimates and reflect the Bonferroni-adjusted significance level of p<0.0071. Th1, Th2, and Th17 cells were not meta-analyzed due to differences in phenotyping (as described in the Methods). Models were adjusted for age, sex, race/ethnicity, education, clinical site, systolic blood pressure, use of antihypertensive medications, low-density lipoprotein cholesterol, use of statins, smoking, and diabetes.
Figure 2. Associations of CD4+ T helper cell subsets specified as primary hypotheses with incident myocardial infarction or incident angina.
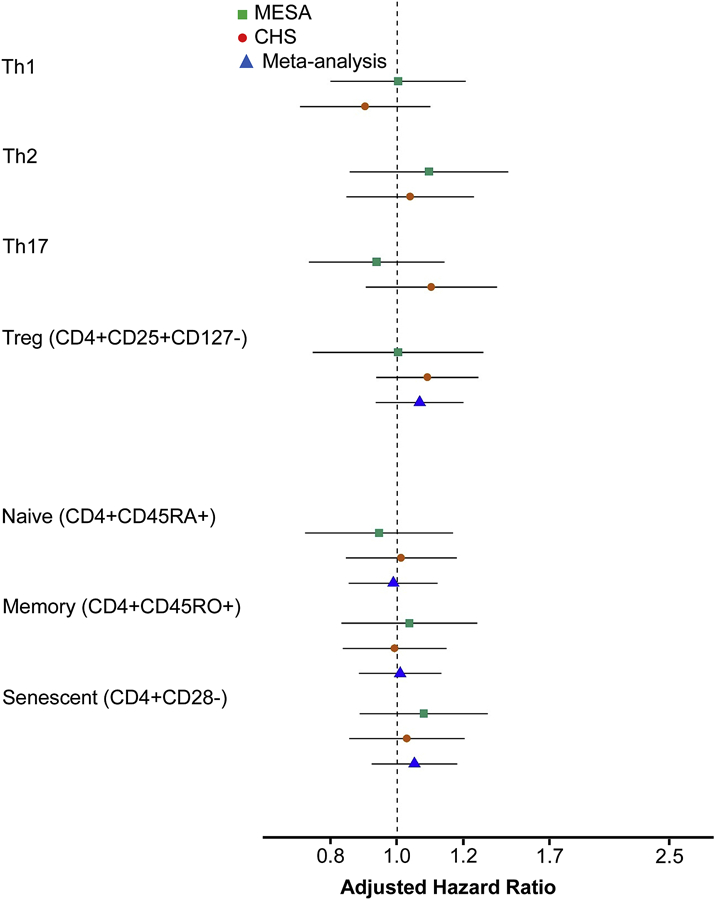
Cell subsets were analyzed per 1-SD higher values using Cox proportional hazards models with sampling weights. Confidence intervals (CIs) used robust (sandwich) standard error estimates and reflect the Bonferroni-adjusted significance level of p<0.0071. Th1, Th2, and Th17 cells were not meta-analyzed due to differences in phenotyping (as described in the Methods). Models were adjusted for age, sex, race/ethnicity, education, clinical site, systolic blood pressure, use of antihypertensive medications, low-density lipoprotein cholesterol, use of statins, smoking, and diabetes.
In secondary analyses, we evaluated associations of 27 additional innate and adaptive immune cell phenotypes with incident MI and incident MI-angina. To correct for multiple hypothesis testing we defined statistical significance as p<0.0015. Cell phenotypes included CD14+ monocyte subsets, natural killer cells, γδ T cells, CD19+ B cell subsets, and differentiated and TEMRA CD4+ and CD8+ cell subsets (as described in Supplemental Table III). In models adjusted for CHD risk factors, none of the cells’ associations with incident MI were statistically significant in either cohort individually or in combined-cohort analyses (all p>0.05 Supplemental Table V). Associations of these cells with the composite endpoint of incident MI-angina were also null (all p>0.20) (Figure 3).
Figure 3. Associations of immune cell subsets included as secondary hypotheses with incident myocardial infarction or incident angina.
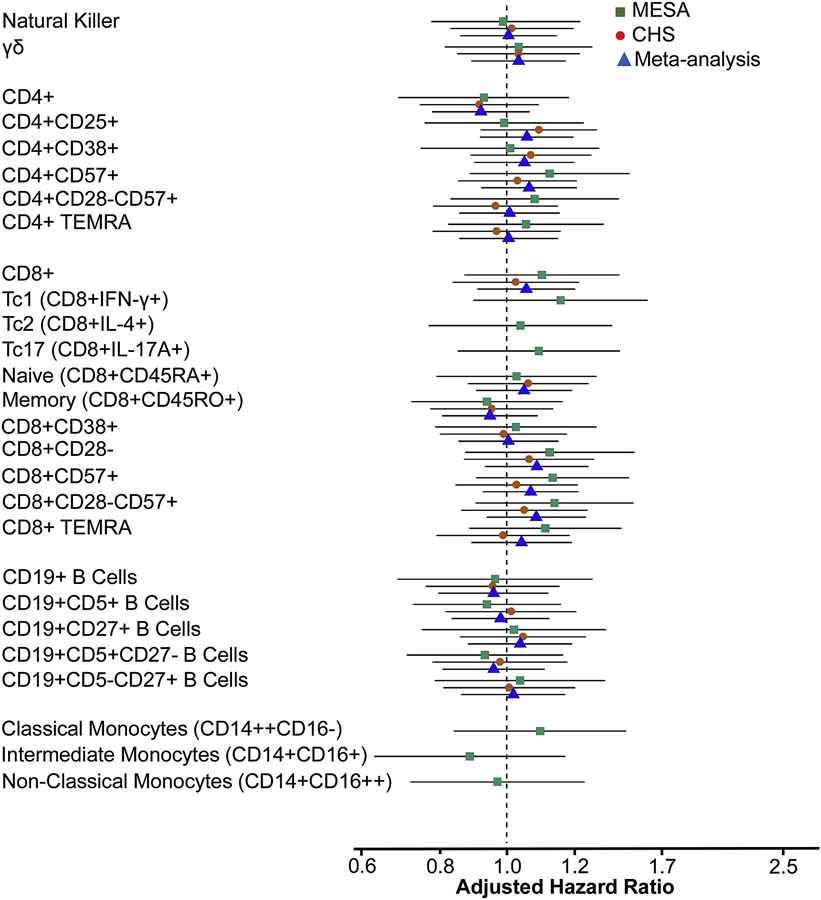
Cell subsets were analyzed per 1-SD higher values using Cox proportional hazards models with sampling weights and robust (sandwich) standard error estimates. Confidence intervals reflect the Bonferroni-adjusted significance level of p<0.0015. Models were adjusted for age, sex, race/ethnicity, education, clinical site, systolic blood pressure, use of statins, low-density lipoprotein cholesterol, use of antihypertensive medications, smoking, and diabetes.
Interpretation of the results was unchanged in models minimally adjusted for demographic variables or in CHD risk factor adjusted models that included season of blood draw and CMV antibody concentrations as additional covariates.
Discussion
In our study of American adults from two longitudinal population-based cohorts, we evaluated the prospective relationships of circulating monocyte and lymphocyte subsets with the risk of incident CHD. We observed no significant associations of CD14+ monocyte, innate lymphocyte (natural killer or γδ), CD19+ B cell, CD4+ or CD8+ T cell subsets with the future occurrence of MI or angina. Associations of each of the 34 immune cell phenotypes investigated were null in MESA and CHS separately, and in a combined-cohort meta-analysis that included 880 composite MI-angina events.
Experimental mouse models have demonstrated distinct roles of several lymphocyte populations in atherosclerosis [1]. Th1 cells promote atherosclerosis, whereas Tregs protect against it [1]. Although less firmly established, Th17, natural killer, γδ T, and B2 B cells have been shown in some mouse models to be atherogenic [1, 7, 32] whereas Th2 and B1a B cells were protective [3, 33]. Overall, the translation of murine results to CVD risk in humans is uncertain.
In human atherosclerotic plaques, there are an abundance of CD4+ and CD8+ effector-memory T cells [9, 34]. Intermediate (CD14++CD16+) and non-classical (CD14+CD16++) monocytes, B cells (CD19+CD80+), Tc1 (CD8+CD56−IFN-γ+), Th1, Th2, memory (CD4+CD45RO+), and effector-memory (CD4+CD45RA−CD45RO+CCR7−) T cells measured in peripheral blood are associated with the severity of atherosclerosis (evaluated by carotid stenosis, IMT, or CAC) [10–15, 18] in cross-sectional analyses, including studies in MESA [11, 12].
Variation in immune cells have also been reported in subjects with clinical CVD [6, 35]. Higher intermediate monocytes (CD14++CD16+), Th1, Th17, effector-memory (CD4+CD45RA−CD45RO+CCR7−), CD4+CD28−, and CD8+CD56+ T cells, and lower Tregs (CD4+CD25+ or CD4+CD25+Foxp3+) were observed in the peripheral blood of patients with coronary artery disease (CAD) and acute coronary syndrome (ACS) [6, 10, 17, 22, 24, 36–42]. Single-cell analyses identified macrophage and T cell dysregulation in atherosclerotic plaques from patients with cerebrovascular events [9]. These collective findings implicate a role of cellular immunity in the development and progression of atherosclerotic CVD. However, as these studies analyzed cells collected from patients with prevalent CVD it is not possible to determine whether variation in immune cells occurred before or after disease onset [43].
To determine the importance of immune cell proportions in peripheral blood as risk factors for the future occurrence of clinical CHD events, we evaluated the prospective relationships of a broad panel of innate and adaptive immune cells with incident MI or incident angina in a population free of prevalent clinical CVD. Our results suggest that higher or lower proportions of CD14+ monocyte, NK, γδ T, CD4+ T helper, cytotoxic CD8+, or CD19+ B cell subsets are not strongly related to the future onset of MI or angina.
To our knowledge, the MDCS and European Prospective Investigation into Cancer and Nutrition (EPIC)-Heidelberg cohorts are the only population-based studies to evaluate longitudinal relationships of immune cell subsets with incident CVD events [14, 15, 18–21, 44]. In these studies, higher peripheral blood CD8+ cells and lower Th2 and Treg (CD4+FoxP3+) cells were associated with increased risk of incident coronary events (defined as fatal or nonfatal MI or death attributed to ischemic heart disease) [14, 18, 20]. Higher classical monocytes (CD14++CD16−) were associated with incident CVD events (defined as MI, CHD death, or stroke) [15]. Apparent differences between these results and those of our study may be explained by the significant association of Th2 cells with MI in women but not men [14], the phenotyping markers used [20], how phenotypes were expressed (i.e., as percentages of parent populations, cell numbers or ratios) [14, 15, 18, 44], or differences in demographic and cultural factors related to CVD risk.
In support of the present null findings, the role of T cell subsets, such as Tregs and CD4+CD28− cells, in CVD prediction has been recently challenged [21, 45]. In the MDCS, lower CD4+FoxP3+ Treg cells were associated with increased risk of coronary events [20], whereas in the EPIC-Heidelberg study a lower ratio of FoxP3+ Treg to CD3+ T cells was not related to incident MI [44]. In a case-control study by Ammirati et al. [46], peripheral blood Tregs (CD3+CD4dimCD25high) were higher in patients with acute ST-elevation MI (STEMI), and lower among those with non-STEMI ACS, compared with CAD-free controls. Among all patients with CAD, however, Treg levels were similar to controls [46].
Tomas et al. recently reported conflicting CVD risk estimates for CD4+CD28− T cells. Higher CD4+CD28− cells were associated with decreased CVD risk in a population without prevalent CVD at baseline, but were associated with increased risk in patients with advanced atherosclerotic disease [21]. Tregs and CD4+CD28− cells were not associated with carotid IMT in these studies [20, 21, 46].
Taken together, these null results and others [14, 15, 18–21, 46] are consistent with the current study and suggest limited use of peripheral blood immune cells as CVD biomarkers.
Findings may suggest that cellular immunity is more strongly related to atherosclerosis than clinically manifest CVD. For example, while Th1, Th2, naive (CD4+CD45RA+) and memory (CD4+CD45RO+) CD4+ cells were associated with subclinical atherosclerosis in prior cross-sectional studies in MESA [11, 12], they were not associated with incident MI or angina in the current prospective study. In the MDCS, intermediate (CD14++CD16+) and non-classical (CD14+CD16++) monocytes, TC1 cells (CD8+CD56−INF-γ+), and CD19+CD86+ B cells were associated with IMT or stenosis in cross-sectional analyses but not with incident coronary events [15, 18, 19]. The magnitude of the associations reported for cell phenotypes and atherosclerosis are typically small. Evidence overall suggests that variation in circulating immune cell levels may explain only a small portion of the risk for atherosclerosis or atherosclerotic CVD. Findings from cross-sectional studies also raise the possibility of reverse causality.
The current study has several limitations. Some cell phenotypes were measured in MESA but not CHS and there was a high degree of missing phenotype data overall. Despite the broad panel of phenotypes investigated, important T cell markers such HLA-DR and CCR7 [10, 47] were not included. The consequences of cryopreservation on PBMC phenotyping results are not well established but some cell surface markers may be affected [48]. A recent study, however, demonstrated several immune cell proportions were not significantly different when measured in cryopreserved samples compared with fresh blood [49]. Evaluation of cell proportions but not their cytokine profiles or antigen specificities is another limitation as changes in cell function may be important in CVD pathogenesis. Similarly, cells measured in peripheral blood may not reflect the populations present in atherosclerotic lesions. For example, immunophenotyping from paired human blood and atherosclerotic plaques showed distinct cell distributions [9]. In particular, macrophage, CD8+, CD4+ & CD8+ effector-memory, and CD4−CD8− T cells were enriched in plaques compared with blood. Finally, it is possible that immune cell frequencies reflect an acute risk of plaque instability [50] and are less strongly related to long-term CVD events [45].
Strengths of our study include the prospective case-cohort design comprised of participants free of prevalent clinical CVD from two well-established independent population-based cohorts. The simultaneous evaluation of a large panel of innate and adaptive immune cell phenotypes is another strength.
In summary, while cell-mediated immunity may play roles in human atherosclerosis, our results suggest peripheral blood monocytes and lymphocytes have limited utility as CHD biomarkers. These findings, however, do not exclude the hypothesis that cellular immunity plays a role in coronary disease. Prospective cohort studies evaluating the relationships of immune cells with the progression of atherosclerosis, as well as a confirmation of our findings in other populations, remain important.
Supplementary Material
Highlights.
Cell-mediated immunity is established in animal models of atherosclerosis
Unclear if immune cell levels in blood are risk factors for future coronary events
CD4+ and CD8+ T cells were not related to incident myocardial infarction or angina
Monocytes, innate lymphocytes, and B cells were not related to coronary risk
Blood lymphocytes and monocytes may have limited utility as CVD biomarkers
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Financial support
The research reported in this article was supported by R00HL129045, R01HL120854, and R01HL135625 from the National Heart, Lung, and Blood Institute (NHLBI).
The MESA was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the NHLBI, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS).
The CHS was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the NHLBI, with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by HHSN268201800001C and U01HL080295 from the NHLBI and R01AG023629 from the National Institute on Aging (NIA).
Abbreviations:
- CHD
coronary heart disease
- CHS
Cardiovascular Health Study
- CMV
cytomegalovirus
- CVD
cardiovascular disease
- MDCS
Malmö Diet and Cancer Study
- MESA
Multi-Ethnic Study of Atherosclerosis
- MI-angina
incident myocardial infarction or incident angina
- Tc
cytotoxic T cell
- Th
cell T helper cell
- Treg
T regulatory cell
- TEMRA
CD45RA+ re-expressing effector memory cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Gistera A and Hansson GK, The immunology of atherosclerosis. Nat Rev Nephrol, 2017. 13(6): p. 368–380. [DOI] [PubMed] [Google Scholar]
- 2.Ley K, Miller YI, and Hedrick CC, Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol, 2011. 31(7): p. 1506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huber SA, et al. , T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation, 2001. 103(21): p. 2610–6. [DOI] [PubMed] [Google Scholar]
- 4.Witztum JL and Lichtman AH, The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol, 2014. 9: p. 73–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nour-Eldine W, et al. , Genetic Depletion or Hyperresponsiveness of Natural Killer Cells Do Not Affect Atherosclerosis Development. Circ Res, 2018. 122(1): p. 47–57. [DOI] [PubMed] [Google Scholar]
- 6.Ammirati E, et al. , The role of T and B cells in human atherosclerosis and atherothrombosis. Clin Exp Immunol, 2015. 179(2): p. 173–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyaw T, et al. , Killer cells in atherosclerosis. Eur J Pharmacol, 2017. 816: p. 67–75. [DOI] [PubMed] [Google Scholar]
- 8.Jonasson L, et al. , Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis, 1986. 6(2): p. 131–138. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez DM, et al. , Single-cell immune landscape of human atherosclerotic plaques. Nat Med, 2019. 25(10): p. 1576–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ammirati E, et al. , Effector Memory T cells Are Associated With Atherosclerosis in Humans and Animal Models. J Am Heart Assoc, 2012. 1(1): p. 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tracy RP, et al. , T-helper type 1 bias in healthy people is associated with cytomegalovirus serology and atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc, 2013. 2(3): p. e000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson NC, et al. , Decreased naive and increased memory CD4(+) T cells are associated with subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. PLoS One, 2013. 8(8): p. e71498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanigawa T, et al. , Relationships of differential leukocyte and lymphocyte subpopulations with carotid atherosclerosis in elderly men. J Clin Immunol, 2003. 23(6): p. 469–76. [DOI] [PubMed] [Google Scholar]
- 14.Engelbertsen D, et al. , T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler Thromb Vasc Biol, 2013. 33(3): p. 637–44. [DOI] [PubMed] [Google Scholar]
- 15.Berg KE, et al. , Elevated CD14++CD16- monocytes predict cardiovascular events. Circ Cardiovasc Genet, 2012. 5(1): p. 122–31. [DOI] [PubMed] [Google Scholar]
- 16.Libby P, Ridker PM, and Hansson GK, Progress and challenges in translating the biology of atherosclerosis. Nature, 2011. 473(7347): p. 317–25. [DOI] [PubMed] [Google Scholar]
- 17.Moroni F, et al. , The Role of Monocytes and Macrophages in Human Atherosclerosis, Plaque Neoangiogenesis, and Atherothrombosis. Mediators Inflamm, 2019. 2019: p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolbus D, et al. , Association between CD8+ T-cell subsets and cardiovascular disease. J Intern Med, 2013. 274(1): p. 41–51. [DOI] [PubMed] [Google Scholar]
- 19.Mantani PT, et al. , Circulating CD40+ and CD86+ B cell subsets demonstrate opposing associations with risk of stroke. Arterioscler Thromb Vasc Biol, 2014. 34(1): p. 211–8. [DOI] [PubMed] [Google Scholar]
- 20.Wigren M, et al. , Low levels of circulating CD4+FoxP3+ T cells are associated with an increased risk for development of myocardial infarction but not for stroke. Arterioscler Thromb Vasc Biol, 2012. 32(8): p. 2000–4. [DOI] [PubMed] [Google Scholar]
- 21.Tomas L, et al. , Low Levels of CD4(+)CD28(null) T Cells at Baseline Are Associated With First-Time Coronary Events in a Prospective Population-Based Case-Control Cohort. Arterioscler Thromb Vasc Biol, 2019: p. Atvbaha119313032. [DOI] [PubMed]
- 22.Cheng X, et al. , The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol, 2008. 127(1): p. 89–97. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, et al. , Correlation of peripheral Th17 cells and Th17-associated cytokines to the severity of carotid artery plaque and its clinical implication. Atherosclerosis, 2012. 221(1): p. 232–41. [DOI] [PubMed] [Google Scholar]
- 24.Liuzzo G, et al. , Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. J Am Coll Cardiol, 2007. 50(15): p. 1450–8. [DOI] [PubMed] [Google Scholar]
- 25.Bild DE, et al. , Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol, 2002. 156(9): p. 871–81. [DOI] [PubMed] [Google Scholar]
- 26.Fried LP, et al. , The Cardiovascular Health Study: design and rationale. Ann Epidemiol, 1991. 1(3): p. 263–76. [DOI] [PubMed] [Google Scholar]
- 27.Pandya JM, et al. , Circulating T helper and T regulatory subsets in untreated early rheumatoid arthritis and healthy control subjects. J Leukoc Biol, 2016. 100(4): p. 823–833. [DOI] [PubMed] [Google Scholar]
- 28.Psaty BM, et al. , Study of Cardiovascular Health Outcomes in the Era of Claims Data: The Cardiovascular Health Study. Circulation, 2016. 133(2): p. 156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Little RJ and Vartivarian S, On weighting the rates in non-response weights. Stat Med, 2003. 22(9): p. 1589–99. [DOI] [PubMed] [Google Scholar]
- 30.Andridge RR and Little RJ, The Use of Sample Weights in Hot Deck Imputation. J Off Stat, 2009. 25(1): p. 21–36. [PMC free article] [PubMed] [Google Scholar]
- 31.Therneau TM and Li H, Computing the Cox model for case cohort designs. Lifetime Data Anal, 1999. 5(2): p. 99–112. [DOI] [PubMed] [Google Scholar]
- 32.Tsiantoulas D, et al. , B cells and humoral immunity in atherosclerosis. Circ Res, 2014. 114(11): p. 1743–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyaw T, et al. , B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res, 2011. 109(8): p. 830–40. [DOI] [PubMed] [Google Scholar]
- 34.Jonasson L, et al. , Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis, 1986. 6(2): p. 131–8. [DOI] [PubMed] [Google Scholar]
- 35.Flego D, et al. , Adaptive Immunity Dysregulation in Acute Coronary Syndromes: From Cellular and Molecular Basis to Clinical Implications. J Am Coll Cardiol, 2016. 68(19): p. 2107–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Y, et al. , Imbalanced frequencies of Th17 and Treg cells in acute coronary syndromes are mediated by IL-6-STAT3 signaling. PLoS One, 2013. 8(8): p. e72804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han SF, et al. , The opposite-direction modulation of CD4+CD25+ Tregs and T helper 1 cells in acute coronary syndromes. Clin Immunol, 2007. 124(1): p. 90–7. [DOI] [PubMed] [Google Scholar]
- 38.Methe H, et al. , Enhanced T-helper-1 lymphocyte activation patterns in acute coronary syndromes. J Am Coll Cardiol, 2005. 45(12): p. 1939–45. [DOI] [PubMed] [Google Scholar]
- 39.Liuzzo G, et al. , Identification of unique adaptive immune system signature in acute coronary syndromes. Int J Cardiol, 2013. 168(1): p. 564–7. [DOI] [PubMed] [Google Scholar]
- 40.Mor A, et al. , Altered status of CD4(+)CD25(+) regulatory T cells in patients with acute coronary syndromes. Eur Heart J, 2006. 27(21): p. 2530–7. [DOI] [PubMed] [Google Scholar]
- 41.Justo-Junior AS, et al. , Monocytes of patients with unstable angina express high levels of chemokine and pattern-recognition receptors. Cytokine, 2019. 113: p. 61–67. [DOI] [PubMed] [Google Scholar]
- 42.Bergstrom I, et al. , Persistent accumulation of interferon-gamma-producing CD8+CD56+ T cells in blood from patients with coronary artery disease. Atherosclerosis, 2012. 224(2): p. 515–20. [DOI] [PubMed] [Google Scholar]
- 43.Libby P and Hansson GK, Adaptive immunity in acute coronary syndromes: chicken or egg? Eur Heart J, 2018. 39(13): p. 1098–1099. [DOI] [PubMed] [Google Scholar]
- 44.Barth SD, et al. , The Ratio of Regulatory (FOXP3+) to Total (CD3+) T Cells Determined by Epigenetic Cell Counting and Cardiovascular Disease Risk: A Prospective Case-cohort Study in Non-diabetics. EBioMedicine, 2016. 11: p. 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjorkbacka H, Can Circulating Regulatory T Cells Predict Cardiovascular Disease? EBioMedicine, 2016. 11: p. 15–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ammirati E, et al. , Circulating CD4+CD25hiCD127lo regulatory T-Cell levels do not reflect the extent or severity of carotid and coronary atherosclerosis. Arterioscler Thromb Vasc Biol, 2010. 30(9): p. 1832–41. [DOI] [PubMed] [Google Scholar]
- 47.Ammirati E, et al. , Reduction of Circulating HLA-DR(+) T Cell Levels Correlates With Increased Carotid Intraplaque Neovascularization and Atherosclerotic Burden. JACC Cardiovasc Imaging, 2016. 9(10): p. 1231–1233. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W, et al. , The effect of cellular isolation and cryopreservation on the expression of markers identifying subsets of regulatory T cells. J Immunol Methods, 2016. 431: p. 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thyagarajan B, et al. , Effect of delayed cell processing and cryopreservation on immunophenotyping in multicenter population studies. J Immunol Methods, 2018. 463: p. 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruggio A, et al. , Correlation between CD4(+)CD28(null) T lymphocytes, regulatory T cells and plaque rupture: An Optical Coherence Tomography study in Acute Coronary Syndromes. Int J Cardiol, 2019. 276: p. 289–292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


