Abstract
Sustained inflammation and matrix metalloproteinase (MMP) activation contribute to vascular occlusive/proliferative disorders. Interleukin-17 (IL-17) is a proinflammatory cytokine that signals mainly via TRAF3 Interacting Protein 2 (TRAF3IP2), an upstream regulator of various critical transcription factors, including AP-1 and NF-κB. Reversion inducing cysteine rich protein with kazal motifs (RECK) is a membrane-anchored MMP inhibitor. Here we investigated whether IL-17A/TRAF3IP2 signaling promotes MMP-13-dependent human aortic smooth muscle cell (SMC) proliferation and migration, and determined whether RECK overexpression blunts these responses. Indeed, IL-17A treatment induced (a) JNK, p38 MAPK, AP-1, NF-κB, and CREB activation, (b) miR-21 induction, (c) miR-27b and miR-320 inhibition, (d) MMP-13 expression and activation, (e) RECK suppression, and (f) SMC migration and proliferation, all in a TRAF3IP2-dependent manner. In fact, gain of TRAG3IP2 function, by itself, induced MMP-13 expression and activation, and RECK suppression. Furthermore, treatment with recombinant MMP-13 stimulated SMC migration in part via ERK activation. Importantly, RECK gain-of-function attenuated MMP-13 activity without affecting its mRNA or protein levels, and inhibited IL-17A- and MMP-13-induced SMC migration. These results indicate that increased MMP-13 and decreased RECK contribute to IL-17A-induced TRAF3IP2-dependent SMC migration and proliferation, and suggest that TRAF3IP2 inhibitors or RECK inducers have the potential to block the progression of neointimal thickening in hyperplastic vascular diseases.
Keywords: inflammation, matrix metalloproteinases, neointima, RECK, restenosis
1 ∣. INTRODUCTION
In-stent restenosis (ISR) is a progressive disease characterized by excessive proliferation of vascular smooth muscle cells (SMCs; neointimal hyperplasia), resulting in narrowing or blockade of vessels (Alfonso, Byrne, Rivero, & Kastrati, 2014). While its overall incidence is decreasing with the advent of second-generation drug-eluting stents, ISR still remains a major problem (Dangas et al., 2010; Jukema, Ahmed, Verschuren, & Quax, 2011; Jukema, Verschuren, Ahmed, & Quax, 2011). Chronic inflammation, foreign body granulation, activation of matrix-degrading metalloproteinases (matrix metalloproteinases [MMPs]), remodeling of extracellular matrix (ECM), and proliferation and migration of vascular SMCs, all contribute to development and progression of ISR (Alfonso et al., 2014; Dangas et al., 2010; Jukema, Ahmed, et al., 2011; Jukema, Verschuren, et al., 2011).
ISR is associated with increased circulating levels of various proinflammatory cytokines, including interleukin-17A (IL-17A). We and others have previously reported that IL-17 exerts both mitogenic and migratory effects in various cell types (Cochaud et al., 2013; Numasaki et al., 2003; Valente, Yoshida, et al., 2012), suggesting its role in ISR. IL-17A signals mainly via the cytoplasmic adapter molecule TRAF3 Interacting Protein 2 (TRAF3IP2; also known as CIKS [Connection To IKK and SAPK/JNK] or Act1 [NFκB-activating protein 1]; Leonardi, Chariot, Claudio, Cunningham, & Siebenlist, 2000; X. Li et al., 2000). It physically associates with IL-17 receptors, and plays a role in the activation of various stress-activated protein kinases, including JNK and p38 MAPK, and the nuclear transcription factors NF-κB and AP-1 (Amatya, Garg, & Gaffen, 2017; Novatchkova, Leibbrandt, Werzowa, Neubuser, & Eisenhaber, 2003; Wu, Zepp, & Li, 2012), suggesting that IL-17A could induce the expression of various promitogenic and migratory factors in vascular SMC, including MMPs.
Nearly 25 MMPs have been identified in humans, and are classified according to their structure and substrate specificity (Mittal, Mishra, Srivastava, Kumar, & Garg, 2014). The vascular SMC express collagenases (MMPs 1, 8, 13, and 18), matrilysins (MMP7), gelatinases (MMPs 2 and 9), membrane-type MMPs (MT1-MMP or MMP14), and macrophage metalloelastase (MMP12). Among the three major collagenases, SMC predominantly expresses MMPs 1 (collagenase-1) and 13 (collagenase-3; Mao, Lee, VanVickle, & Thompson, 1999; Shi, Ji, Berardi, Qazi, & Tarbell, 2010). In addition to functional similarities, these two collagenases show some structural similarities with respect to transcriptional regulation, harboring a TATA box and an AP-1 binding site in their proximal promoter region (Yan & Boyd, 2007). An NF-κB binding site has also been identified in the 5’ cis-regulatory region of both MMPs (Cortez et al., 2007; Liacini, Sylvester, Li, & Zafarullah, 2002), suggesting that the activation of NF-κB and AP-1 can positively regulate their expression. In fact, we have previously demonstrated that IL-17A upregulates MMP1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent NF-κB, AP-1, and C/EBPβ activation (Cortez et al., 2007). However, it is not known whether IL-17A regulates MMP-13 expression. Since IL-17A induces activation of transcription factors known to positively regulate MMP-13 expression, we hypothesized that IL-17A promotes SMC migration and proliferation via induction and activation of MMP-13.
Under physiological conditions, MMP-13 expression is regulated predominantly at the transcriptional level, resulting in low basal expression. However, posttranscriptional regulation also contributes to its expression either positively or negatively. For example, the small endogenous noncoding RNAs, that is, microRNAs, bind the 3’-untranslated region (3’-UTR) of MMP-13 by base pairing, and fine-tune its expression (H. Li, Wang, Yuan, & Min, 2017). Dysregulation in the expression of microRNAs can result in aberrant expression of their target genes. Various microRNAs, including 9, 21, 22, 27b, 145, 156a, 222, and 320, regulate MMP-13 expression either directly or indirectly (H. Li et al., 2017). Interestingly, in addition to directly regulating MMP-13 transcription, activation of stress-activated protein kinases and NF-κB and AP-1 are also shown to regulate the expression of some of these microRNAs (H. Li et al., 2017). Since TRAF3IP2 is an upstream regulator of stress-activated kinases, and NF-κB and AP-1 (Leonardi et al., 2000; X. Li et al., 2000; Somanna et al., 2015; Valente et al., 2013), we hypothesized that IL-17A-mediated differential regulation of microRNAs 21, 27b and 320 plays a role in MMP-13 expression in SMC.
Since MMPs play a critical role in vascular structure and remodeling by degrading matrix as well as nonmatrix substrates, their activation is tightly regulated by various endogenous MMP inhibitors, indicating the existence of a delicate balance in the expression and activation of MMPs and their tissue inhibitors under basal conditions. However, an imbalance in their activation favoring sustained activation of MMPs will result in excessive ECM degradation and adverse remodeling. Several recent studies have described Reversion inducing cysteine rich protein with kazal motifs (RECK) as a novel MMP regulator in various cell types (Oh et al., 2001; Siddesha, Valente, Sakamuri, et al., 2014; Takahashi et al., 1998). RECK is a glycosylphosphatidyl inositol-anchored MMP inhibitor (Takahashi et al., 1998). We have previously reported that ectopic expression of RECK inhibits angiotensin-II (AngII)-induced MMP-9 activation in cultured cardiac fibroblasts (Siddesha et al., 2013). Increased RECK expression also inhibited activation of AngII-induced MMP2 and MMP14 in those cells (Siddesha et al., 2013), thus raising the possibility that RECK could inhibit MMP-13 activation or expression in vascular SMC. Given the opposing activities of MMP-13 and RECK, we tested the hypothesis that IL-17A-mediated SMC migration and proliferation could involve concurrent MMP-13 activation and RECK suppression.
Supporting our hypotheses, the data show that IL-17A differentially regulates MMP-13 and RECK expression in cultured human aortic SMC, induced MMP-13 expression and activation, but suppressed RECK, both in a TRAF3IP2-dependent manner. Moreover, IL-17A-induced miR-21, but suppressed miR-27b and miR-320, resulting in increased MMP-13 expression and SMC migration. Importantly, forced expression of RECK inhibited MMP-13 activity and MMP-13-mediated SMC migration. Together, these data suggest that TRAF3IP2 inhibitors or RECK inducers have the potential to block progression of neointimal thickening in hyperplastic vascular diseases.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Materials
The materials used are detailed in the “Supporting Information Methods” section. Insulin-transferrin-sodium selenite (ITS) liquid media supplement (#I3146) and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Polybrene® (sc-134220) was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). SensoLyte® Plus 520 MMP-13 Assay Kit (Fluorimetric and Enhanced Selectivity; #AS-72019) was from AnaSpec (Fremont, CA). Molecular Probes CyQUANT GR dye (C7026) was purchased from Thermo Fisher Scientific (Waltham, MA). Carrier-free recombinant human IL-17A, endotoxin-free (317-IL-050), and carrier-free recombinant human PDGF-BB (#220-BB) were purchased from R&D Systems (Minneapolis, MN). Recombinant endotoxin-free active human MMP-13 (#444287-5UG) was purchased from MilliporeSigma (Burlington, MA). All inhibitors used in the present study are described in Table S1.
2.2 ∣. Human aortic SMCs
Human aortic SMCs were purchased from Clonetics Corp. (San Diego, CA) and grown as previously described (Valente et al., 2013) in SmGM-2 medium supplied by the manufacturer. The cells were positive for α smooth muscle actin (SMA) and smooth muscle myosin heavy chain (SM-MHC), but not for Von Willebrand factor (quantitative reverse transcription polymerase chain reaction [RT-qPCR]; data not shown). At 70–80% confluency, the culture medium was replaced with basal medium containing 0.5% bovine serum albumin (conditioning medium). After 48 hr incubation, recombinant human IL-17A was added and cultured for the indicated time periods. At the end of the experimental period, culture supernatants were collected into slick tubes, snap-frozen, and stored at −80°C. To investigate the potential signal transduction pathways involved in MMP-13 and RECK regulation, target-specific inhibitors were used at the indicated concentrations and the duration of treatment as described in legends to figures before the addition of IL-17A. Information is also provided in the Supporting Information Tables.
2.3 ∣. Lentiviral and adenoviral transduction
The lentiviral vectors used in the investigation are detailed in Table S2 and have been previously described (Siddesha et al., 2013; Siddesha, Valente, Sakamuri, et al., 2014; Somanna et al., 2015; Valente et al., 2013). For lentiviral infection, SMC at 50–60% confluency was infected with the indicated lentiviral short-hairpin RNA (shRNA) at a multiplicity of infection (moi) of 0.5 for 48 hr in complete media. To increase transfection efficiency, SMC were cotreated with the cationic polymer Polybrene® (5 μg/ml in water). Neither shRNA nor Polybrene® affected cell viability. Recombinant, replication-deficient adenoviral vectors used in the present study are also described in Table S2. For adenoviral transduction, SMC at 70% confluency was infected at ambient temperature in PBS at the indicated moi. After 1 hr, the medium containing adenovirus was replaced with fresh culture medium. Assays were carried out after 24 (expression of target protein) or 48 hr (shRNA). Adenoviral transduction had no off-target effects, and at the indicated moi and duration of treatment, failed to modulate SMC adherence, shape, and viability (trypan blue-dye exclusion; data not shown).
2.3.1 ∣. miRNA expression, mimics, inhibitors, and transfections
For miRNA expression, small RNA-enriched total RNA isolated using the mirVana™ miRNA Isolation Kit (Ambion®, Austin, TX) was used. Expression levels of miRs 21, 27b, and 320 were analyzed by RT-qPCR using miRNA TaqMan® probes (Applied Biosystems, Waltham, MA). Human miR-21 inhibitor, inhibitor control, miR-27b and miR-320 mimics, and mimic control were purchased from Thermo Fisher Scientific (Table S3). SMC were transfected with the mimic, inhibitor or controls (80 nM) using the Neon® transfection system (MPK-5000; Invitrogen, Waltham, MA). SMC were microporated (pulse voltage: 1,300V; pulse width: 20ms; pulse number: 2; the tip type: 10 μl) and then cultured for 24 hr. SMC showed transfection efficiency of 49% with only 7% cell death as determined using the pEGFP-N1 vector. Transfections at the indicated concentration and for the duration of treatment failed to significantly modulate SMC adherence, shape, or viability (trypan blue-dye exclusion; data not shown).
2.3.2 ∣. mRNA expression RT-qPCR
Total RNA was isolated using the TRIzol method. One microgram of DNA-free total RNA was used for the first-strand cDNA synthesis using Quantitect cDNA Synthesis Kit (Qiagen, Germantown, MD). MMP2 and RECK mRNA levels were analyzed by RT-qPCR using TaqMan probes (Table S4) and MasterCycler RealPlex4 from Eppendorf. Glyceraldehyde-3-phosphate dehydrogenase served as an invariant control. Data are shown as fold change (2−ΔΔCt).
2.3.3 ∣. Immunoblotting and ELISA
Preparation of whole cell homogenates, immunoblotting, detection of immunoreactive bands by enhanced chemiluminescence (ECL Plus; GE Healthcare, Bloomington, IL), and their quantification by densitometry were all previously described (Siddesha et al., 2013; Siddesha, Valente, Sakamuri, et al., 2014; Somanna et al., 2015; Valente et al., 2013). In experiments determining MMP-13 activation, trypsinized SMC was resuspended in RPMI 1640 + ITS 1× before IL-17A treatment. The antibodies used are detailed in Table S5. Immunoblotting was performed on at least three separate occasions (biological and not intraassay variables), and a representative immunoblot is shown in the figures. The densitometric data from all three experiments are summarized at the bottom or sides of immunoblots. Secreted cytokine levels in equal amounts of culture supernatants were analyzed using commercially available ELISA kits as detailed in Table S6.
2.3.4 ∣. MMP-13 activity assay
For MMP-13 activity assay, cultured SMC trypsinized and suspended in RPMI 1640 + ITS 1× were stimulated with IL-17A (25 ng/ml for 6 hr). MMP-13 activity was analyzed according to manufacturer’s instructions using the SensoLyte Plus 520 MMP-13 Assay Kit (AnaSpec, Fremont, CA). SMC were incubated with the MMP-13-specific inhibitors CL 82198 or WAY175203 before IL-17A addition.
2.4 ∣. Cell proliferation
SMC were plated into tissue culture treated 96-well clear bottom, black-sided plates (VWR Scientific Products, West Chester, PA) at 1,000 cells/well. Cells were fed with serum-free medium containing 0.5% BSA 48 hr before study. SMC were pretreated for 20min with the p38 MAPK inhibitor SB203580 in dimethyl sulfoxide (DMSO) or MEK inhibitor U0126-EtOH in DMSO or DMSO vehicle (1:1,000). SMC were then exposed to IL-17A (1-50 ng/ml). After 48 hr, the medium was removed and the plates were frozen at −80°C for 2 hr before assay. Plates were then thawed, stained with CyQUANT GR dye according to manufacturer’s protocol (Molecular Probes, Eugene, OR), and read on a FLx800 microplate fluorescence reader (Bio-Tek Instruments, Winooski, VT) using 485/20 excitation and 528/20 emission filters, and analyzed using KC4 software (Bio-Tek Instruments; Das et al., 2018). To determine the role of TRAF3IP2, miRs, inhibitors, or inducers on proliferation, SMC was treated as detailed in figure legends before the addition of IL-17A or recombinant human MMP-13.
2.5 ∣. Cell migration
SMC migration was quantified as previously described using BioCoat™ Matrigel™ migration chambers and 8.0 μm pore polyethylene tersephthalate membranes with a thin layer of Matrigel™ basement membrane matrix (Das et al., 2018; Siddesha et al., 2013; Siddesha, Valente, Sakamuri, et al., 2014; Valente et al., 2013). SMC were made quiescent by incubating cells in medium containing 1× ITS liquid media supplement in place of 0.5% BSA. SMC (2.0 × 105 cells/ml) were layered on the coated insert filters. Cells were stimulated with IL-17A (25 ng/ml). The lower chamber contained 20% fetal bovine serum. Plates were incubated at 37°C for 16 hr. Membranes were washed with phosphate-buffered saline, and noninvading cells on the upper surface were removed using cotton swabs. In addition to taking images of cells on the other side of the membrane, migrated cell numbers were determined at A540 nm using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Numbers of SMC migrating in response to IL-17A were normalized to those of untreated cells and expressed as fold change from untreated. Migration assays were carried out six times. To determine the role of TRAF3IP2, miRs, inhibitors or inducers on IL-17A-induced SMC migration, cells were treated as detailed in Results and figure legends before IL-17A addition of IL-17A or recombinant biologically active human MMP-13.
2.6 I. Cell death detection
To determine whether IL-17A, transduction of viral vectors, and pharmacological inhibitors at the indicated doses and for the duration of treatment affected cell viability, cell death was analyzed by trypan blue-dye exclusion, immunoblotting for total and cleaved caspase-3 levels (Somanna et al., 2015), MTT assay (Alessio et al., 2018), and ELISA that quantifies mono and oligonucleosomal fragmented DNA in cytoplasmic extracts as previously described (Chandrasekar et al., 2006; Das et al., 2018).
2.7 ∣. Statistical analysis
Comparisons between controls and various treatments were performed by analysis of variance with post hoc Dunnett’s t tests. Significance was accepted at p ≤ 0.05. All assays were performed at least three times, and error bars in figures indicate SEs. Further, though a representative immunoblot is shown in main figures, changes in protein/phosphorylation levels from three independent experiments were semiquantified by densitometry and shown as ratios or fold changes from untreated or respective controls at the bottom/side/inset of respective panels. The numbers at the bottom of each panel in figures denote lane numbers.
3 ∣. RESULTS
3.1 ∣. IL-17A induces proliferation and migration of primary human aortic SMCs
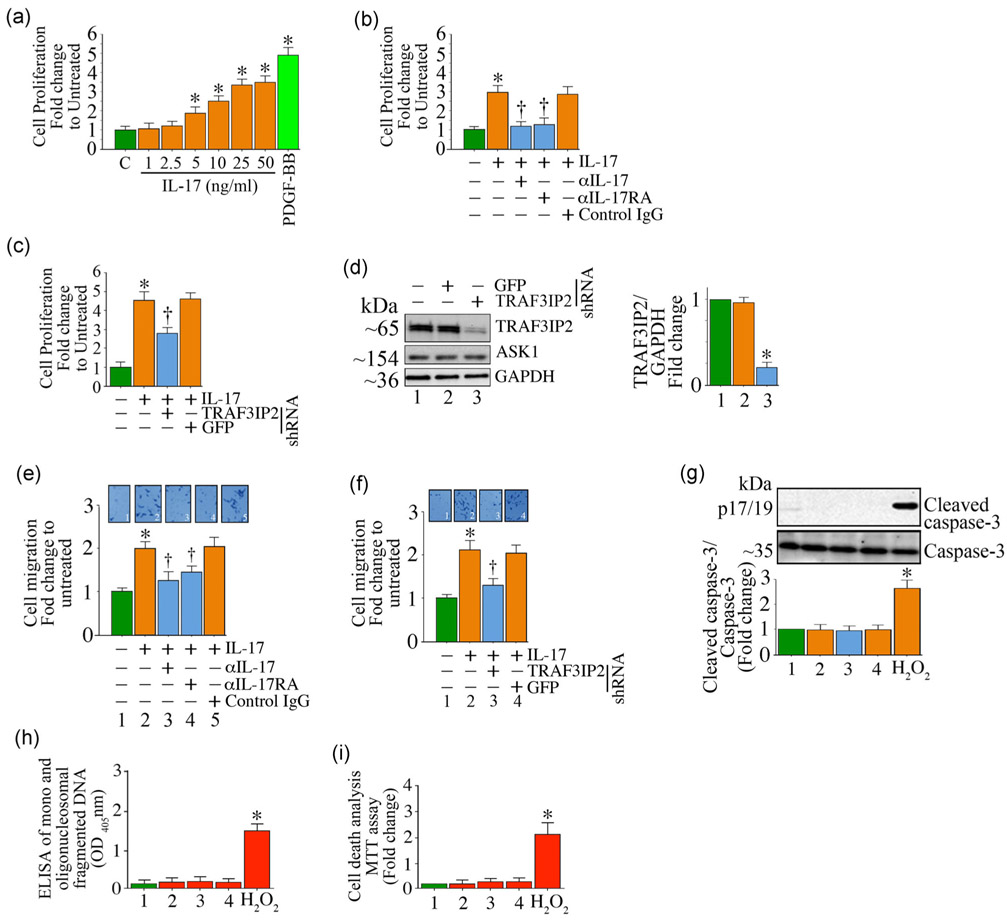
Inflammation plays a critical role in vascular proliferative/occlusive disorders, including atherosclerosis, by promoting adverse vascular remodeling and SMC proliferation and migration. Increased IL-17 expression has been shown to contribute to atherosclerosis development and progression (Nordlohne et al., 2018). We have previously reported that TRAF3IP2, which is critical in IL-17 signal transduction, mediates atherosclerotic plaque development and vulnerability (Sakamuri et al., 2016). Here we investigated whether IL-17A induces SMC proliferation and migration. The serum levels of IL-17A range from undetectable to low levels in normal healthy humans (e.g., 0.0039 ng/ml) and as high as 3.53 ng/ml during inflammation (de Oliveira et al., 2015). However, it is difficult to predict local concentrations of IL-17A that are operative in lesions and diseased tissues. Also, it is known that the affinity of IL-17A to IL-17RA is low and in a nanomolar range (Liu et al., 2013). In addition, several publications reported using IL-17A at 100 ng/ml (e.g., Kudo et al., 2012). Therefore, in pilot experiments we optimized the levels of IL-17A to be used in the present study by testing concentrations between 1 to 50 ng/ml. The data in Figure 1a show that IL-17A induces SMC proliferation in a dose-dependent manner. While the proliferation doubled at 5 ng/ml, it further increased at 10 ng/ml, and peaked at 25 ng/ml (Figure 1a). Increasing IL-17A concentration to 50 ng/ml did not further increase proliferation. Therefore, in all subsequent experiments IL-17A was used at 25 ng/ml. The growth factor PDGF-BB (10 ng/ml) served as a positive control, and increased SMC proliferation by a significant 4.8-fold (Figure 1a). To determine the specificity of IL-17A, we next incubated SMC with neutralizing antibodies against IL-17A or IL-17RA (10 mg/ml for 1hr) before IL-17A addition (25 ng/ml) for 48 hr. The results showed that neutralization of IL-17A significantly attenuated its mitogenic effects (Figure 1b). Since IL-17A predominantly signals via TRAF3IP2, we targeted its expression using adenoviral-mediated shRNA transduction (moi100 for 48 hr) before IL-17A addition (25 ng/ml for 48 hr). The results show that silencing TRAF3IP2, but not the control GFP, significantly inhibited IL-17A-induced SMC proliferation as assessed by the CyQuant assay (Figure 1c), while targeting GFP had no effect. Knockdown of TRAF3IP2 was confirmed by immunoblotting (an average reduction of 72%; ASK1 served as an off-target; quantification data from three independent experiments are shown in Figure 1d right-hand panel). Further, in addition to its mitogenic effects (Figure 1a), IL-17A also induced SMC migration through Matrigel™-coated matrix membranes. In fact, IL-17A treatment doubled SMC migration (Figure 1e), and this effect was blunted by IL-17A or IL-17RA neutralizing antibodies (Figure 1e) or TRAF3IP2 knockdown (Figure 1f). Importantly, the observed inhibition in SMC migration following TRAF3IP2 knockdown was not due to reduced survival, as evidenced by the low levels of active casapse-3 (Figure 1g) and mono and oligonucleosomal fragmented DNA in cytoplasmic extracts (Figure 1h). The MTT cell viability assay confirmed these results (Figure 1i). Together, these results demonstrate that IL-17A/TRAF3IP2 signaling promotes SMC proliferation and migration (Figure 1).
FIGURE 1.
IL-17A induces SMC proliferation and migration via TRAF3IP2. (a,b) Recombinant IL-17A induces SMC proliferation in a dose-dependent manner. Specificity of IL-17A was determined by incubating quiescent SMC with neutralizing IL-17A or IL-17RA antibodies before IL-17A addition (b). (c,d) Adenoviral shRNA-mediated knockdown of TRAF3IP2 leads to reduced IL-17A -induced SMC proliferation. SMC proliferation was not affected by the nonspecific GFP shRNA. (d) shows an immunoblot confirming that the endogenous TRAF3IP2 expression was downregulated by the TRAF3IP2-specific shRNA, but not by the GFP-specific shRNA. Knockdown data from three independent experiments are quantified and summarized in the right-hand side panel. (e,f) IL-17A induces SMC migration via IL-17RA (e) and TRAF3IP2 (f). (g-i) SMC viability following IL-17RA treatment as analyzed by caspase-3 activation (g; data from three independent experiments are summarized in the lower panel), quantification of mono- and oligonucleosomal fragmented DNA in cytoplasmic extracts (h), and MTT cell viability assay (i). In all of the assays, 100 μM hydrogen peroxide (H2O2) served as a positive control, and induced significant cell death. (e) The insets show representative images of cells migrated to the other side of the Matrigel basement membrane. For (d) and (g) the intensity of immunoreactive bands from three independent experiments is semiquantified and summarized in the right-hand panel (d) or bottom panels (g). Data represent mean±SE. GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GFP: green fluorescent protein; IL: interleukin; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; shRNA: short-hairpin RNA; SMC: smooth muscle cell; TRAF3IP2: TRAF3 Interacting Protein 2. *p < at least 0.05 versus untreated; †p < at least 0.05 versus IL-17A
3.2 ∣. IL-17A induces the expression of multiple mediators involved in vascular occlusive disorders
Several cytokines and growth factors exert promitogenic effects in SMC. By inducing MMP activation and ECM degradation, some of these cytokines also promote SMC migration (Davis, Pillai, Lawrence, Sebti, & Chellappan, 2012; Fan, Pech, & Karnovsky, 2000; Tsai et al., 2009). Therefore, we next investigated whether IL-17A induces the expression of cytokines, chemokines, growth factors, and MMPs in SMC. Indeed, treatment with IL-17A (25 ng/ml for 2 hr) significantly upregulated the mRNA and protein expression of proinflammatory cytokines IL-1β, IL-6, IL-17A, IL-18, TNF-α, and the chemokine MCP-1 in SMC (Figure S1). It also stimulated IL-1β secretion. IL-17A also significantly increased the expression of TGFβ (Figure S1), which plays a pivotal role in the pathogenesis of restenosis (McCaffrey, 2009). Both MMPs 2 and 14 play a key role in agonist-induced ECM degradation and SMC migration (Chandrasekar et al., 2006). IL-17A markedly increased their expression as well (Figure S1). Further, using antibodies that specifically detect both pro- and mature forms, we show that IL-17A increased their activation (Figure S1). Importantly, TRAF3IP2 depletion (Ad.TRAF3IP2 shRNA, moi100 for 48 hr) blunted the expression/activation of these inflammatory mediators and MMPs. These in vitro results indicate that sustained increases in IL-17A expression further amplify inflammatory signaling and possibly contribute to adverse vascular remodeling and restenosis in vivo (Figure S1).
3.3 ∣. IL-17A induces MMP-13-dependent SMC migration
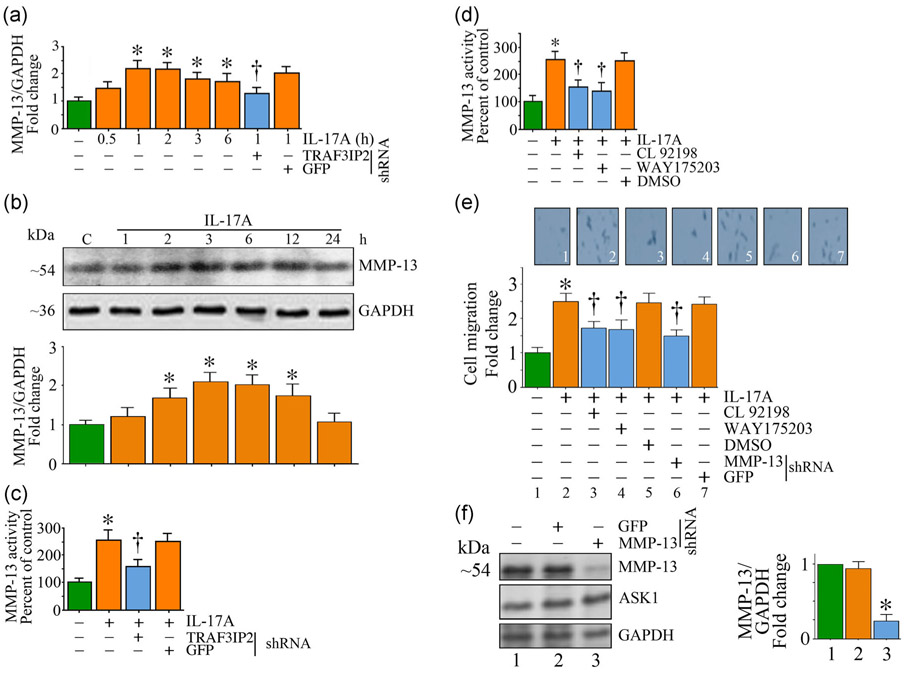
We have demonstrated that IL-17A induces the expression and activation of MMPs 2 and 9 in SMC (Figure S1). However, it is not known whether it regulates MMP-13 expression. Since IL-17A signals mainly via TRAF3IP2-dependent activation of NF-κB and AP-1 that play critical roles in MMP-13 transcription, we postulated that the IL-17A/TRAF3IP2-mediated SMC migration (Figure 1e) is MMP-13-dependent. Indeed, the data in Figure 2a show that IL-17A (25 ng/ml) upregulated MMP-13 mRNA expression in a time-dependent manner. While an increase in MMP-13 mRNA expression was detected as early as 1 hr (~1.29-fold), its levels further increased at 2 hr and then slightly declined, but still remained high relative to the baseline even after 6 hr following initial treatment (Figure 2a). IL-17A treatment also led to increased MMP-13 protein levels in a time-dependent manner with peak levels detected at 2 hr posttreatment (Figure 2b) and paralleled the increase in its mRNA levels. Since MMP-13 is a secreted protein, we next analyzed MMP-13 activity in equal amounts of culture supernatants using the SensoLyte Plus activity kit. The data show that IL-17A induced a 2.6-fold increase in MMP-13 activity at 6 hr (Figure 2c), and this effect was significantly attenuated (~57% reduction) by TRAF3IP2 knockdown. Both CL 82198 and WAY175203 are selective MMP-13 inhibitors, and the data in Figure 2d show that pretreatment for 1 hr with each inhibitor (CL 82198 at 200 μM; WAY175203 at 1 μM) before IL-17A addition reduced MMP-13 activity by at least 50%. Further, both MMP-13 inhibitors as well as shRNA-mediated MMP-13 depletion each inhibited IL-17A-induced SMC migration by at least 60% (Figure 2e). Knockdown of MMP-13 by lentiviral transduction of specific shRNA (moi0.5 for 48 hr) was confirmed by immunoblotting (~74% inhibition; Figure 2f). These results indicate that IL-17A-induced SMC migration is mediated in part via MMP-13 (Figure 2).
FIGURE 2.
IL-17A induces TRAF3IP2-dependent MMP-13 expression and activation, and MMP-13-dependent SMC migration. (a) IL-17A induces time-dependent and TRAF3IP2-mediated MMP-13 mRNA expression. TRAF3IP2 expression was targeted as in Figure 1. (b,c) IL-17A induces MMP-13 protein expression and activity as analyzed by immunoblotting and enhanced fluorometric activity assay. (d) Pharmacological inhibition of MMP-13 by CL92198 and WAY175203 reduced IL-17A-induced MMP-13 activity. DMSO is used as a vehicle control (e). IL-17A mediated SMC migration reduced by either pharmacological inhibition or lentiviral shRNA-mediated knockdown of MMP-13. (f) shRNA-mediated knockdown of MMP-13 expression was confirmed by immunoblotting. (b,f) The intensity of immunoreactive bands from three independent experiments is semiquantified and summarized in the bottom (b) or right-hand side panels (f). Data represent mean ± SE. DMSO: dimethyl sulfoxide; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GFP: green fluorescent protein; IL: interleukin; MMP-13, matrix metalloproteinase-13; SMC: smooth muscle cell; TRAF3IP2: TRAF3 Interacting Protein 2. *p < at least 0.01 versus untreated; †p < at least 0.05 versus IL-17A
3.4 ∣. IL-17A activates stress-activated kinases and cis-regulatory elements involved in MMP-13 induction
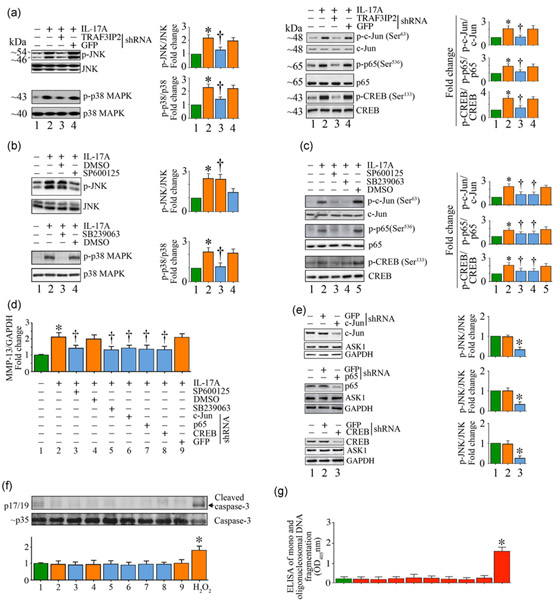
MMP-13 expression is predominantly regulated at the transcriptional level (H. Li et al., 2017). Several stress-activated kinases (e.g., JNK and p38 MAPK) and cis-regulatory elements (e.g., NF-κB, AP-1, and CREB) play a role in its induction (Bui et al., 2012; H. Li et al., 2017). Therefore, we measured activation of JNK, p38 MAPK, NF-κB (p65), AP-1 (c-Jun), and CREB by immunoblotting using cleared whole cell lysates and activation-specific antibodies. The data show that treatment with IL-17A increased JNK and p38 MAPK activation in a time-dependent manner with peak levels of activation detected at 1 hr post-treatment (Figures S2A and S2B). Further, IL-17A induced AP-1 activation in a time-dependent manner, with increased activation detected as early as 30 min as evidenced by the increased levels of phospho-c-Jun (Figure S2C). Its levels peaked at 1 hr and declined thereafter. Similar to its stimulatory effects on AP-1 activation, IL-17A treatment also induced activation of NF-κBp65 (Figures S2D) and CREB (Figures S2E), as evidenced by an increase in their phosphorylated forms. Importantly, silencing TRAF3IP2 (Figure 3a) and pharmacological inhibition of JNK (SP600125; Figure 3b, upper panel) and p38 MAPK (SB239062; Figure 3b, lower panel) each attenuated IL-17A-induced AP-1, NF-κB, and CREB activation (Figure 3c). Importantly, pharmacologic inhibition of JNK and p38 MAPK, and lentiviral mediated AP-1, NF-κB, or CREB knockdown each inhibited IL-17A-induced MMP-13 mRNA expression (35–50%; Figure 3d). AP-1, NF-κB, and CREB knockdown was confirmed by immunoblotting as shown in Figure 3e. However, targeting JNK, p38 MAPK, AP-1 (c-Jun), NF-κB (p65), or CREB did not affect SMC viability as evidenced by no significant increase in the levels of active caspase-3 (Figure 3f) or mono or oligonucleosomal fragmented DNA in cytoplasmic extracts (Figure 3g). Together, these results indicate that IL-17A induced MMP-13 expression in SMC in part via TRAF3IP2-dependent activation of JNK, p38 MAPK, AP-1, NF-κB, and CREB activation (Figure S2 and Figure 3).
FIGURE 3.
IL-17A induces MMP-13 expression via JNK, p38 MAPK, AP-1, NF-κB, and CREB. (a) IL-17A induces JNK, p38 MAPK, AP-1, NF-κB, and CREB activation via TRAF3IP2 as measured by immunoblotting using activation-specific antibodies. (b) IL-17A-induced JNK and p38 MAPK activation was inhibited by SP600125 and SB239063, respectively. (c) Targeting JNK and p38 MAPK attenuates IL-17A-induced AP-1 (c-Jun), NF-κB (p65), and CREB activation. Quiescent SMC treated as in (b) were analyzed for AP-1, NF-κB, and CREB activation by immunoblotting using activation-specific antibodies. (d–f), Targeting JNK, p38 MAPK, AP-1, NF-κB, and CREB suppresses IL-17A-induced MMP-13 induction, without affecting cell viability. JNK and p38 MAPK were targeted by pharmacological inhibitors as in (b), and AP-1, NF-κB, and CREB were targeted by lentiviral shRNA. MMP-13 mRNA expression was measured by RT-qPCR (d), and knockdown of AP-1, NF-κB, and CREB was confirmed by immunoblotting (e). Cell death was analyzed by caspase-3 activation by immunoblotting (f) and quantification of mono- and oligonucleosomal fragmented DNA in cytoplasmic extracts by ELISA (g). (a–c,e,f) The intensity of immunoreactive bands from three independent experiments is semiquantified and summarized in the respective right-hand side or bottom panels. The results are expressed as mean ±SE. DMSO: dimethyl sulfoxide; ELISA: enzyme-linked immunosorbent assay; GFP: green fluorescent protein; IL: interleukin; MMP-13: matrix metalloproteinase-13; OD: optical density; RT-qPCR: quantitative reverse transcription polymerase chain reaction; shRNA: short-hairpin RNA; TRAF3IP2: TRAF3 Interacting Protein 2. *p < at least 0.01 versus untreated; †p < at least 0.01 versus IL-17A
4 ∣. IL-17A/TRAF3IP2 signaling suppresses RECK expression
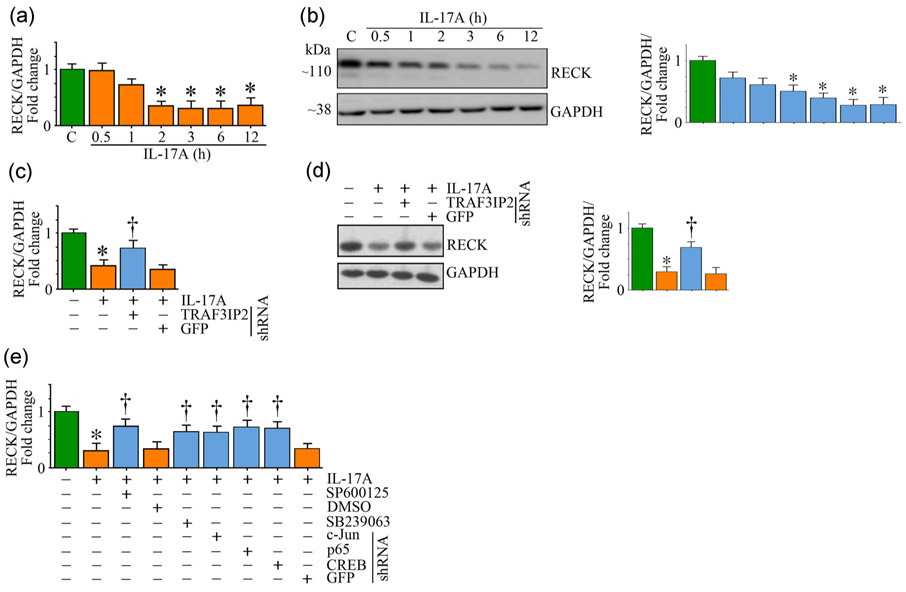
Under physiological conditions, ECM provides structural integrity by promoting cell-cell and cell-matrix interactions as well as sequestration and release of inflammatory mediators and growth factors. However, following injury, increased expression and activation of MMPs, and degradation and excess deposition of ECM, all contribute to adverse vascular remodeling. RECK is an endogenous MMP regulator (Oh et al., 2001). We have previously reported that IL-18, a proinflammatory and proatherogenic cytokine activates multiple MMPs, including MMPs 2, 9, and 14 in cultured cardiac fibroblasts (Siddesha, Valente, Sakamuri, et al., 2014). However, it suppressed RECK, an MMP regulator, in those cells, resulting in increased migration. We also demonstrated that restoring RECK expression by an adenoviral vector reversed these effects (Siddesha, Valente, Sakamuri, et al., 2014). However, it is not known whether IL-17A suppresses RECK expression in SMC. Indeed, IL-17A suppressed RECK mRNA expression in a time-dependent manner (Figure 4a), with significant 65% reduction seen at 2 hr. Its levels remained at these low levels throughout the 6 hr study period. Similar to its inhibitory effects on mRNA (Figure 4a), IL-17A also suppressed RECK protein levels in a time-dependent manner with a significant 40% reduction seen at 3hr (Figure 4b). Its levels were further reduced and were barely detectable at 12 hr post-treatment (Figure 4b). Further, shRNA-mediated TRAF3IP2 depletion reversed the inhibitory effects of IL-17A on RECK mRNA expression (Figure 4c) and protein levels (Figure 4d). Moreover, pharmacological inhibition of p38 MAPK and JNK, and shRNA-mediated AP-1, NF-κB, and CREB depletion each partially reversed its inhibitory effects (Figure 4e). These results indicate that IL-17A differentially regulates MMP-13 and its endogenous inhibitor RECK in SMC; induces MMP-13 (Figure 2), but suppresses RECK, in part via TRAF3IP2 (Figure 4), possibly contributing to increased SMC migration.
FIGURE 4.
IL-17A markedly suppresses RECK expression. (a,b) IL-17A suppresses RECK mRNA (a) and protein (b) expression in a time-dependent manner. Quiescent SMC treated with IL-17A for the indicated time periods were analyzed for RECK mRNA expression by RT-qPCR (a) and its protein expression by immunoblotting (b). (c,d) Adenoviral transduction of TRAF3IP2 shRNA blunts RECK mRNA (c) and protein (d) expression. Adenoviral transduction of GFP shRNA served as a control. (e) Pharmacological inhibitors of JNK and p38 MAPK, and AP-1, NF-κB, and CREB knockdown by lentiviral shRNA each blunted IL-17A-mediated RECK mRNA expression. (b,d) also show the intensity of immunoreactive bands from three independent experiments semiquantified and summarized in the respective right-hand side panels. DMSO, dimethyl sulfoxide; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GFP: green fluorescent protein; IL: interleukin; mRNA, messenger RNA; RECK: reversion-inducing cysteine-rich protein with kazal motifs; RT-qPCR: quantitative reverse transcription polymerase chain reaction; shRNA: short-hairpin RNA; SMC: smooth muscle cell; TRAF3IP2: TRAF3 Interacting Protein 2. (a-e) *p < at least 0.01 versus untreated; †p < at least 0.01 versus IL-17A
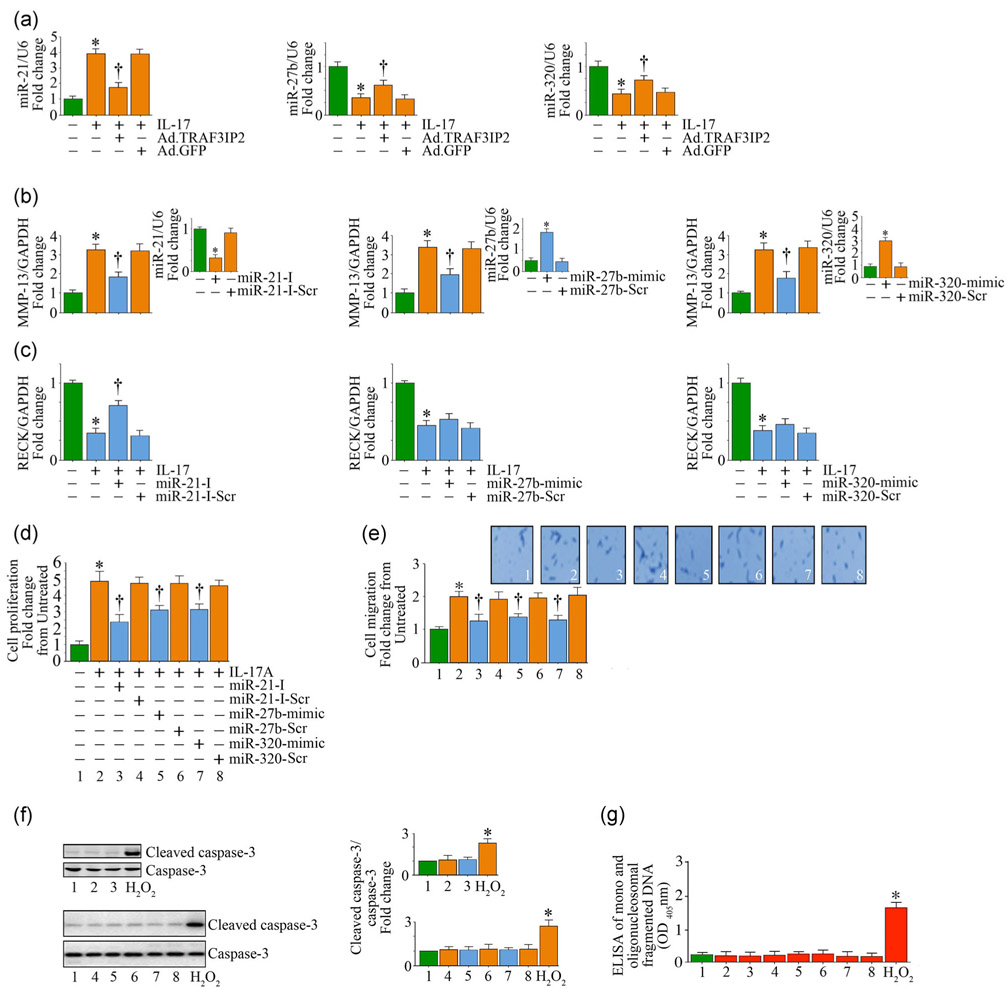
4.1 ∣. IL-17A-induced MMP-13 expression and RECK suppression are differentially regulated by miR-21, miR-27b, and miR-320
In addition to transcription, posttranscriptional regulation plays an important role in MMP-13 and RECK expression (H. Li et al., 2017; Nagini, 2012). In fact, several microRNA (miRs) have been shown to target MMP-13 and RECK, either directly or indirectly (H. Li et al., 2017; Nagini, 2012). For example, miR-21 induces MMP-13 (H. Li et al., 2017), but suppresses RECK (Siddesha, Valente, Yoshida, et al., 2014). Both miRs 27b and 320 have been shown to suppress MMP-13 (Akhtar et al., 2010; Meng et al., 2016), but their role in RECK expression is not known. Of note, the stress-activated kinases JNK and p38 MAPK, and the nuclear transcription factors AP-1 and NF-κB have been shown to regulate the expression of these miRs. For example, we and others have previously shown that activation of AP-1 and NF-κB positively regulates miR-21 expression (Kumarswamy, Volkmann, & Thum, 2011; Siddesha, Valente, Yoshida, et al., 2014), and NF-κB regulates miR-27b expression (Akhtar et al., 2010). Since TRAF3IP2 is an upstream regulator of JNK, p38 MAPK, AP-1 and NF-κB, we next investigated whether these miRs play a role in IL-17A-mediated differential regulation of MMP-13 and RECK in SMC. Indeed, IL-17A induced a significant 3-fold increase in miR-21 expression, but suppressed that of miR-27b and miR-320 by 60–70%, all in a TRAF3IP2-dependent manner (Figure 5a). Further, a miR-21 inhibitor, but not its scrambled control, inhibited IL-17A-induced MMP-13 expression (Figure 5b). However, this treatment partially restored (~50%) RECK expression (Figure 5c). Confirming earlier results, miR-27b and miR-320 mimics each reduced IL-17A-mediated MMP-13 induction (Figure 5b), but had no effect on RECK suppression (Figure 5c). Importantly, the miR-21 inhibitor and miR-27b and miR-320 mimics each blunted IL-17A-mediated SMC proliferation (Figure 5d) and migration (Figure 5e) without affecting cell viability, as evidenced by the low levels of active caspase-3 (Figure 5f) and mono and oligonucleosomal fragmented DNA in cytoplasmic extracts (Figure 5g). These results indicate that IL-17A differentially regulates MMP-13 and RECK expression in SMC in part via miR-21 induction and miR-27b and miR-320 suppression (Figure 5).
FIGURE 5.
IL-17A differentially regulates MMP-13 and RECK in SMC by modulating miRNA expression. (a) IL-17A induces miR-21, but suppresses miR-27b and miR-320 expression in part via TRAF3IP2. Following adenoviral transduction of TRAF3IP2 shRNA, SMC were treated with IL-17A and analyzed for miRNA expression after 2 hr by advanced miRNA assays. (b,c) Forced expression of miR-21 inhibitor or miR-27b and miR-320 mimics blunt IL-17A-induced MMP-13 expression (b), but reverse RECK suppression (c). SMC transduced with the miR-21 inhibitor or miR-27b and miR-320 mimics for 48 hr were treated with IL-17A, and analyzed for MMP-13 (b) or RECK (c) expression by RT-qPCR. Insets show the efficacy of inhibitor or mimics on the expression of respective miRNA. (d-g) microRNA mimic and inhibitors reverse IL-17A-induced SMC proliferation (d) and migration (e) without affecting cell viability. Insets in (e) show images of cells migrated to the other side of the Matrigel™ basement membrane. Cell death was analyzed by immunoblotting using antibodies that detect active and total caspase-3 levels (f) and ELISA that quantifies mono- and oligonucleosomal fragmented DNA in cytoplasmic extracts (g). (f) The intensity of immunoreactive bands from three independent experiments is semiquantified by densitometry and summarized in the right-hand side panels. ELISA: enzyme-linked immunosorbent assay; GFP: green fluorescent protein; IL: interleukin; MMP-13: matrix metalloproteinase-13; RECK: reversion-inducing cysteine-rich protein with kazal motifs; RT-qPCR: quantitative reverse transcription polymerase chain reaction; shRNA: short-hairpin RNA; SMC: smooth muscle cell; TRAF3IP2: TRAF3 Interacting Protein 2. *p < at least 0.01 versus untreated; †p< at least 0.01 versus IL-17A
4.2 ∣. Gain of TRAF3IP2 function induces MMP-13, but suppresses RECK
We have demonstrated that shRNA-mediated TRAF3IP2 depletion blunts IL-17A-induced MMP-13 induction (Figure 2) and RECK suppression (Figure 4). As a proof-of-concept, we next investigated whether gain of TRAF3IP2 function, in the absence of an agonist, mimics IL-17’s effects on MMP-13 induction and RECK suppression. Therefore, we have transduced SMC with an adenoviral vector expressing full-length human TRAF3IP2. Adenoviral vector expressing GFP served as a control. Data show that gain of TRAF3IP2 function (Figure S3A), but not that of control GFP (Figure S3B), at a similar moi increased TRAF3IP2 expression in a dose-dependent manner, with maximal levels detected at a moi of 5 (Figure S3A). Further, while inducing MMP-13 activation (Figure S3C), TRAF3IP2 gain-of-function suppressed RECK expression (Figure S3D). These results confirm the role of TRAF3IP2 in the differential regulation of MMP-13 and RECK in SMC (Figure S3).
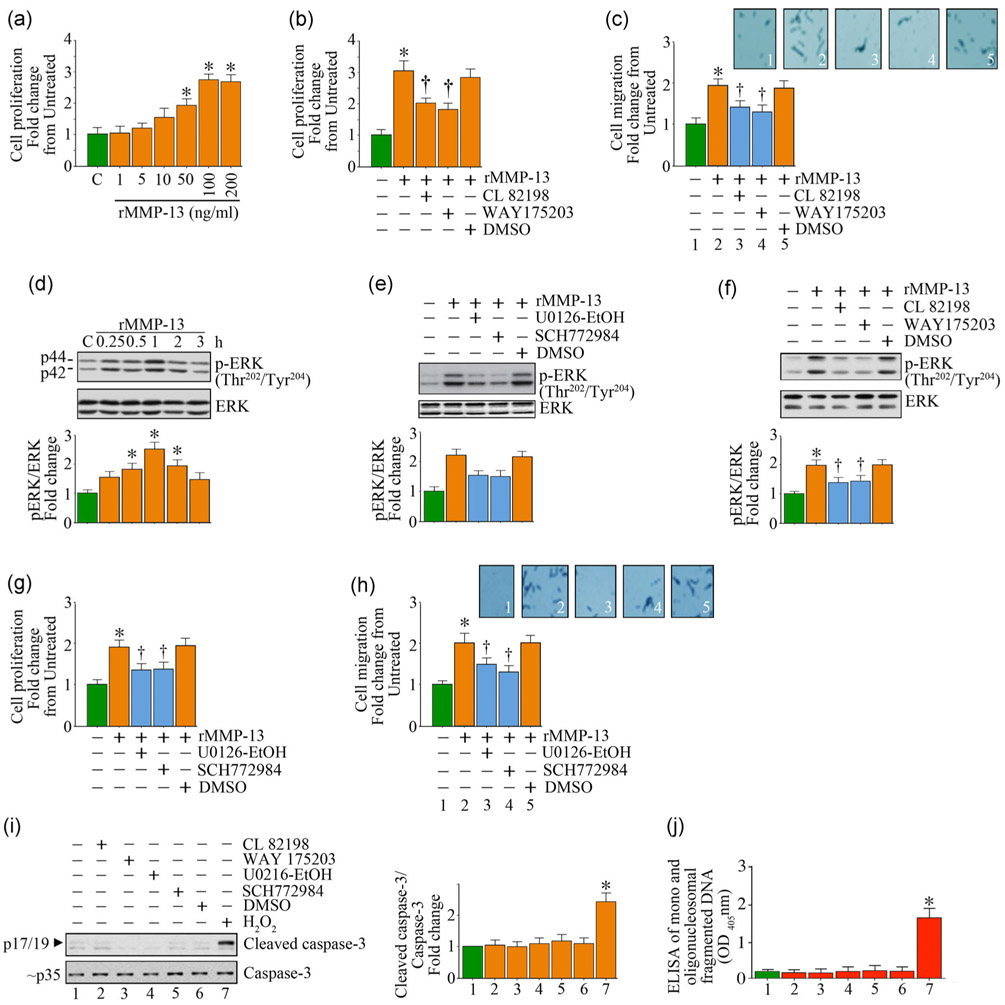
4.3 ∣. Recombinant biologically active MMP-13 induces SMC proliferation and migration in part via ERK activation
We have demonstrated that IL-17A induces MMP-13 expression and activation in SMC (Figure 2). We further demonstrated that silencing MMP-13 attenuates IL-17A-mediated SMC migration (Figure 2D), indicating that MMP-13 mediates IL-17A-induced SMC migration. However, it is not known whether MMP-13, by itself, regulates SMC proliferation and migration. Therefore, we treated SMC with recombinant biologically active human MMP-13 (rMMP-13). In one study involving melanoma patients, most of the serum MMP-13 was found to be the active form, and not the proform, and ranged between 0.079 to 2.850 ng/ml (Nikkola et al., 2005). In a study conducted by Ruan et al., the authors found that the median serum concentration of MMP-13 in Chinese subjects diagnosed with osteoarthritis was 121.48 ng/ml (Ruan et al., 2018). For in vitro studies, recombinant biologically active MMP-13 was used at a concentration of 100–200 ng/ml and pro-MMP-13 at 200 ng/ml (Fu et al., 2016). Therefore, we tested rMMP-13 between 1 and 200 ng/ml. The data show that treatment with rMMP-13 indeed induced SMC proliferation in a dose-dependent manner (Figure 6a), with a significant increase detected at 50 ng/ml. A further increase in cell proliferation was observed when its concentration was increased to 100 ng/ml. CL 82198 and WAY175203 are MMP-13-specific inhibitors, and both inhibitors significantly attenuated rMMP-13-induced SMC proliferation (Figure 6b) as well as migration (Figure 6c). Further investigations revealed that rMMP-13 induces activation of ERK, a member of the MAPK family, in a time-dependent manner, with peak levels of activation observed at 1 hr (Figure 6d), and pretreatment with the MEK inhibitors U0126-EtOH and SCH772984 each attenuated these effects (Figure 6e). Pretreatment with the MMP-13-specific inhibitors also inhibited rMMP-13-induced ERK activation (Figure 6f). Importantly, the MEK inhibitors significantly inhibited rMMP-13-induced SMC proliferation (Figure 6g) and migration (Figure 6h) without inducing cell death as evidenced by no significant changes in the levels of active caspase-3 (Figure 6i) and mono and oligonucleosomal fragmented DNA in cytoplasmic extracts (Figure 6j). Together, these results indicate that, in addition to playing a role in IL-17A-mediated SMC migration, MMP-13 by itself stimulates SMC proliferation and migration in part via ERK activation (Figure 6).
FIGURE 6.
Recombinant biologically active human MMP-13 (rMMP-13) induces SMC proliferation and migration in part via MEK/ERK activation. (a,b) rMMP-13 induces SMC proliferation in a dose-dependent manner as analyzed by the CyQUANT® assay. (b,c) rMMP-13-induced SMC proliferation (b) and migration (c) are attenuated by the MMP-13-specific inhibitors CL 82198 and WAY175203. (d-f) rMMP-13 induces ERK1/2 activation in a time-dependent manner (d), and is inhibited by the MEK (U0126-EtOH) and ERK (SCH772984) inhibitors (e), as well as the MMP-13-specific inhibitors CL 82198 and WAY175203 (f). (g-j), Pharmacological inhibitors of MEK, ERK, and MMP-13 attenuate rMMP-13-induced SMC proliferation (g) and migration (h), without inducing cell death (i,j). Cell death was analyzed by immunoblotting using antibodies that detect active and total caspase-3 levels (i) and ELISA that quantifies mono and oligonucleosomal fragmented DNA in cytoplasmic extracts (j). (c,h) Insets show images of cells migrated to the other side of the Matrigel basement membrane. (d-f,i) The intensity of immunoreactive bands from three independent experiments is semiquantified by densitometry and summarized in the bottom or right hand panels. DMSO: dimethyl sulfoxide; ELISA: enzyme-linked immunosorbent assay; MMP-13: matrix metalloproteinase-13; SMC: smooth muscle cell. *p < at least 0.01 versus untreated; †p < at least 0.01 versus IL-17A
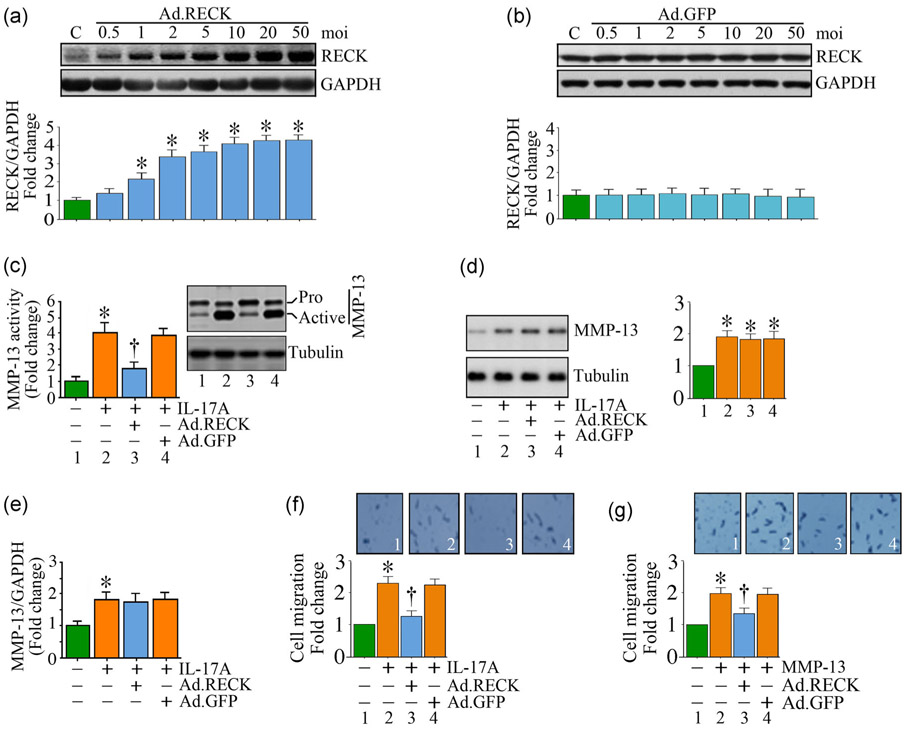
4.4 ∣. Gain of RECK function blunts IL-17A-mediated MMP-13 activation
We have previously demonstrated that RECK gain-of-function by adenoviral transduction inhibits AngII-induced MMP activation and cardiac fibroblast migration (Siddesha et al., 2013). Since IL-17A induced MMP-13, but suppressed RECK expression, we hypothesized that restoring or enhancing RECK expression will suppress IL-17A-mediated MMP-13 activation and SMC migration. The results show that adenoviral-mediated RECK transduction (Figure 7a), compared to control GFP (Figure 7b), increased RECK expression in a dose-dependent manner. Increased RECK expression was detected even at a moi of 1, which further increased at a moi of 2, and peaked at moi 10. Therefore, in all subsequent experiments, Ad. RECK was used at a moi of 10 for 24 hr. Confirming our hypothesis, gain of RECK function significantly reduced MMP-13 activity (Figure 7c), without modulating its total protein levels (Figure 7d) or mRNA expression (Figure 7e). Importantly, RECK gain-of-function inhibited IL-17A-induced (Figure 7f) and MMP-13-induced SMC migration (Figure 7g). These results indicate that restoring RECK blunts the promigratory effects of IL-17A and MMP-13 in SMC (Figure 7).
FIGURE 7.
RECK overexpression blunts IL-17A- and rMMP-13-induced SMC migration. (a,b) Adenoviral transduction of RECK (Ad.RECK; a), but not control GFP (Ad.GFP; b), increases RECK expression in a dose-dependent manner. (c-e) Ectopic expression of RECK by adenoviral transduction (moi 10) inhibits IL-17A-induced MMP-13 activity (c), but not its total protein (immunoblotting; d) or mRNA expression (RT-qPCR; e). (f,g) Ectopic expression of RECK inhibits IL-17A- (f) and rMMP-13 (g)-induced SMC migration. (f,g) Insets show images of cells migrated to the other side of the Matrigel™ basement membrane. (a,b,d) The intensity of immunoreactive bands from three independent experiments is semiquantified by densitometry and summarized in the bottom or right-hand side panels. GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GFP: green fluorescent protein; IL: interleukin; MMP-13: matrix metalloproteinase-13; mRNA: messenger RNA; RECK: reversion-inducing cysteine-rich protein with kazal motifs; RT-qPCR: quantitative reverse transcription polymerase chain reaction; SMC: smooth muscle cell. *p < at least 0.01 versus untreated; †p < at least 0.01 versus IL-17A
5 ∣. DISCUSSION
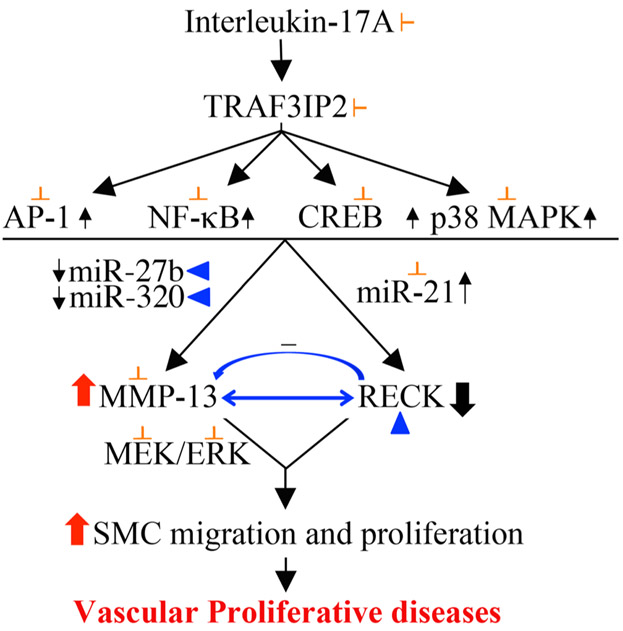
Persistent inflammation plays a critical role in adverse vascular remodeling and vascular proliferative/occlusive disorders, including atherosclerosis, ISR, and vein graft failure, by promoting proliferation and migration of vascular SMC. In the current study, we investigated potential roles of MMP-13 and RECK in IL-17-mediated human aortic SMC proliferation and migration. The major findings are: (a) IL-17A, a proinflammatory and proatherogenic cytokine, induces TRAF3IP2-dependent SMC proliferation and migration. (b) It upregulates the expression of multiple mediators involved in vascular proliferative/occlusive disorders, including TRAF3IP2-dependent IL-1β, IL-6, IL-17A, IL-18, TNF-α, MCP-1, MMPs 2 and 14, and TGFβ. (c) IL-17A induces MMP-13, but suppresses RECK, an MMP inhibitor, in part via TRAF3IP2-dependent activation of JNK, p38 MAPK, NF-κB, AP-1, and CREB. (d) Moreover, IL-17A/TRAF3IP2 signaling induces MMP-13, but suppresses RECK via miR-21 induction, and miR-27b and miR-320 inhibition. (e) Further confirming its causative role in the differential regulation of MMP-13 and RECK, forced expression of TRAF3IP2, by itself, induces MMP-13, but suppresses RECK. (f) Notably, treatment with biologically active recombinant MMP-13, in the absence of IL-17A, induces SMC proliferation and migration in part via ERK activation, and targeting MMP-13 suppresses IL-17A-mediated SMC migration. (g) Finally, ectopic expression of RECK suppresses MMP-13 activity, but not its mRNA or protein levels, and inhibits IL-17A- as well as MMP-13-induced SMC migration. Together, these results demonstrate that IL-17A/TRAF3IP2 signaling induces MMP-13, but suppresses RECK, an MMP inhibitor, resulting ultimately in increased SMC proliferation and migration. These results suggest that TRAF3IP2 inhibitors or RECK inducers have the potential to block the progression of vascular proliferative diseases (Figure 8).
FIGURE 8.
Schema showing possible signal transduction pathways involved in IL-17A-induced differential regulation of MMP-13 and RECK in mediating SMC migration and proliferation. The proinflammatory cytokine IL-17A plays a causal role in atherosclerosis and adverse vascular remodeling, diseased states characterized by increased vascular smooth muscle cell proliferation and migration. Using loss-of-function and gain-of-function studies, we have demonstrated thatTRAF3IP2 plays a critical role in IL-17-mediated MMP-13 induction and RECK suppression. Further, IL-17A induces MMP-13 expression via TRAF3IP2-dependent activation of the stress-activated protein kinases JNK and p38 MAPK, and the nuclear transcription factors AP-1, NF-κB, and CREB. Moreover, IL-17A-induced miR-21, but miR-27b, and miR-320 suppression, results in increased MMP-13 expression. Similar to IL-17A, treatment with biological active recombinant MMP-13 also stimulated SMC proliferation in part via ERK activation. Importantly, RECK gain-of-function promoted MMP-13/RECK physical association, reduced MMP-13 activation and inhibited MMP-13-dependent SMC migration and proliferation. These data suggest that inhibiting TRAF3IP2 or inducing RECK expression has the potential to block progression of neointimal thickening in hyperplastic vascular diseases. IL: interleukin; MMP-13: matrix metalloproteinase-13; RECK: reversion-inducing cysteine-rich protein with kazal motifs; SMC: smooth muscle cell
Balloon angioplasty with and without stenting, surgical removal of plaque as in endarterectomy and bypass surgery all improve patient outcomes by improving patency of the occluded vessel. However, over time, the vessel reoccludes due to inflammation and proliferation and migration of medial SMC (intimal hyperplasia). In fact, injury to a vessel wall has been shown to induce the expression of multiple promitogenic and promigratory inflammatory mediators. Here we show that IL-17A, via TRAF3IP2, induces its own expression and that of IL-1β, IL-6, IL-18, TNF-α, MCP-1, and TGFβ, suggesting that upon induction, IL-17A perpetuates inflammatory signaling in the vessel wall by upregulating multiple inflammatory mediators with a pathological consequence. For example, we have previously demonstrated that IL-18, an IL-17-responsive proatherogenic cytokine, induces SMC migration via IL-18R/Nox1-mediated ROS generation, TRAF3IP2 induction, and TRA-F3IP2-dependent IKK/NF-κB and JNK/AP-1 activation (Valente et al., 2013) . These results suggest that TRAF3IP2 plays a role in the proinflammatory signaling of both IL-17A and IL-18. Since TRAF3IP2 expression is regulated by multiple oxidative stress-responsive nuclear transcription factors, including AP-1, c/EBPβ, and IRF1 (Venkatesan et al., 2013; Zhao et al., 2003), it is highly likely that TRAF3IP2 expression is persistently upregulated in vascular inflammatory diseases. Of note, we have previously reported that TRAF3IP2 gene deletion significantly reduces atherosclerotic burden in ApoE−/− mice fed a high fat diet (Sakamuri et al., 2016).
In addition to inflammatory mediators, IL-17A/TRAF3IP2 signaling also induced the expression and activation of MMPs 2 and 14. IL-17A/TRAF3IP2 signaling also increased the expression and activation of the collagenase MMP-13, suggesting similar cis-elements and trans-activators regulate inflammatory cytokine and MMP expression. For example, activation of AP-1 induces the expression of all three MMPs (Mittal et al., 2014). Activation of NF-κB has also been shown to regulate their expression. In fact, we have previously demonstrated that silencing the NF-κB subunit p65 and the AP-1 subunit c-Jun markedly attenuates IL-18-induced MMP2 and MMP14 expression, and migration of human coronary artery SMC (Valente et al., 2013). Here, we show that targeting p65 and c-Jun inhibits IL-17-mediated MMP-13 expression. Of note, both MMPs 2 and 14 play a role in MMP-13 maturation (Knauper et al., 1996). In addition to silencing NF-κB and AP-1, targeting CREB also attenuated MMP-13 expression. Interestingly, CREB is also shown to play a role in MMP2 and MMP14 expression, indicating that the expression of all three MMPs is regulated by NF-κB, AP-1, and CREB. Since several inflammatory cytokines and growth factors are also responsive to NF-κB, AP-1, or CREB activation, and as TRAF3IP2 is an upstream regulator of these cis-regulatory elements, we hypothesize that targeting TRAF3IP2 will have broader protective effects in vascular proliferative diseases by inhibiting promitogenic and promigratory inflammatory cytokines, chemokines, growth factors, and MMPs.
We have demonstrated that silencing TRAF3IP2 blunts IL-17A-mediated NF-κB and AP-1 activation and the induction of inflammatory mediators. While the mechanisms by which TRAF3IP2 regulates NF-κB and AP-1 are well described, its role in CREB activation is not well understood. For example, the physical association between TRAF3IP2 and members of the IKK signalosome results in activation of NF-κB (Leonardi et al., 2000). We have previously demonstrated that the physical association between TRAF3IP2, IKKγ, and JNK plays a role in AP-1 activation (Valente, Clark, Siddesha, Siebenlist, & Chandrasekar, 2012). Since TRAF3IP2 contains two TRAF-binding sites in its N-terminus (Kanamori, Kai, Hayashizaki, & Suzuki, 2002; Leonardi et al., 2000), and as TRAF6 mediates CREB phosphorylation and activation (Kawaguchi, Kokubu, Fujita, Huang, & Hizawa, 2009), we hypothesize that TRAF3IP2/TRAF6 signaling contributes to IL-17A/TRAF3IP2-mediated CREB activation. It is also possible thatTRAF3IP2/p38 MAPK signaling and the inflammatory mediators induced by IL-17A/TRAF3IP2 signaling might have contributed to CREB phosphorylation. In fact, our published reports and the present study show that p38 MAPK is downstream of TRAF3IP2. Thus, multiple signal transduction pathways downstream of TRAF3IP2 and various inflammatory mediators potentially play a role in IL-17A-mediated MMP-13 induction.
In addition to transcriptional regulation, both MMP-13 and RECK are highly regulated at posttranscriptional levels (H. Li et al., 2017; Nagini, 2012). Though multiple miRs regulate MMP-13 and RECK expression, we focused on miRs 21, 27b, and 320, as they are responsive to stress-activated kinases and NF-κB, AP-1, or CREB. In fact, we previously reported that AngII induces miR-21 expression in cardiac fibroblasts via ERK-dependent AP-1 and STAT3 activation, and while a miR-21 inhibitor reversed AngII-induced RECK suppression, a miR-21 mimic inhibited both RECK expression and AngII-induced fibroblast migration (Siddesha, Valente, Yoshida, et al., 2014). Here we demonstrate that IL-17A differentially regulates the three miRNAs studied; while it induced miR-21, it inhibited miR-27b and miR-320 expression. Moreover, while miR-21 inhibitor suppressed IL-17A-mediated MMP-13 expression, it reversed RECK suppression. Further, while miR-27b and miR-32 mimics inhibited MMP-13 expression, no significant changes were detected in RECK levels, indicating that RECK may not be a target of miR-27b and miR-320. Also, while the roles of miR-27b and miR-320 in MMP-13 suppression are described before (Akhtar et al., 2010; Meng et al., 2016), their role in RECK expression has not been reported. In fact, bioinformatics analysis using TargetScan, mIRDB (MicroRNA Target Prediction and Functional Study Database), miRbase, and PicTar, we found that RECK is not a direct target of miR-27b and miR-320. Thus, our data suggest that both transcriptional and posttranscriptional regulation might have played a role in IL-17A-mediated differential regulation of MMP-13 and RECK in SMC.
As a protease, MMP-13 cleaves multiple components of ECM, including fibrillar collagens, gelatins, and fibronectin under physiological as well as pathological conditions. Here we show that it also stimulates SMC proliferation and migration in part via ERK activation. However, it is not known how MMP-13 elicits signaling in target cells. One possibility is that SMCs express high levels of low-density lipoprotein (LDL) receptor-related protein-1 (LRP1), and both pro- and active forms of MMP-13 have been shown to bind clusters II and III of the receptor, involving the homeopexin domain (Yamamoto et al., 2016). Upon binding, MMP-13 is internalized and activates ERK (Raggatt et al., 2006; Yamamoto et al., 2016). In another study, MMP-13 has been shown to play a role in capillary formation by human vein endothelial cells via activation of FAK and ERK. MMP-13 is also shown to increase the secretion of proangiogenic VEGF by both fibroblasts and endothelial cells, suggesting that MMP-13 exerts biological effects that are independent of its protease function (Kudo et al., 2012).
Under physiological conditions, a delicate balance exists between the expression and activity levels of ECM degrading MMPs and their endogenous inhibitors, contributing to normal ECM assembly and cellular function. However, when the balance shifts in favor of increased MMP activation, ECM degradation ensues, promoting cell proliferation and migration. Recently we have demonstrated that the expression of RECK, an inhibitor of endogenous MMPs and cell migration, is suppressed by various agonists, including the proatherogenic AngII and IL-18 (Siddesha et al., 2013; Siddesha, Valente, Sakamuri, et al., 2014). In those studies, we demonstrated that forced expression of RECK inhibits activity levels of MMPs 2, 9, and 14, and fibroblast migration. Similar to our earlier observations, here we show that while IL-17A suppresses RECK expression, and its forced expression not only inhibited IL-17A, but also MMP-13-induced SMC migration. Importantly, its forced expression inhibited MMP-13 activity, but not its total protein levels or mRNA expression. These results were similar to that reported for MMP-9 (Lee et al., 2018). Since secreted MMP-9 forms a dimer (Dufour, Zucker, Sampson, Kuscu, & Cao, 2010), it was hypothesized that RECK inhibits MMP-9 activity by forming heterodimers involving the Kazal motifs (Lee et al., 2018). Though we have not demonstrated the mechanisms involved in RECK-mediated suppression of MMP-13 activity, we hypothesize that the physically interaction between RECK and MMP-13 might result in its reduced activity. We will investigate this hypothesis in our future studies by GST pull-down assays using various truncated versions of RECK and full-length MMP-13, as we have previously reported for IL-18R and NADPH oxidase interactions (Valente et al., 2013).
Recently, novel alternatively spliced variants of RECK have been reported in cancer with biological implications. In one study, the authors have reported two alternatively spliced variants, RECK-B and RECK-I, of the RECK gene in malignant astrocytomas of different grades (Trombetta-Lima et al., 2015). Those authors observed a higher expression of canonical RECK, and a higher canonical to alternatively spliced RECK variants that positively correlate with higher overall survival rate of GBM patients that underwent chemotherapy, suggesting a positive correlation between high levels of RECK and a better survival (Trombetta-Lima et al., 2015). They further demonstrated that overexpression of RECK-B in malignant glioblastoma cells promotes higher anchorage-independent clonal growth without modulating MMP2 or MMP-9 expression (Trombetta-Lima et al., 2015), suggesting that alternatively spliced RECK variants may have opposing effects compared to canonical RECK, and may serve as prognostic markers in glioblastoma patients. However, those authors did not examine the effects of RECK-B or RECK-I on agonist-induced cell migration that plays a critical role in tumor dissemination. In a second study, a RECK splice variant that is much shorter (25 kDa) than the canonical 110 kDa RECK and different from the variants RECK-B and RECK-I described in the first study, has been shown to arise from alternative splicing and alternative polyadenylation (Lee et al., 2018). Importantly, this shorter form is expressed at higher levels in proliferating and TGF-β-treated cells. Knockdown of this shorter form resulted in reduced fibroblast migration through Matrigel™. Further, this shorter form is shown to compete with MMP-9 binding to the Kazal motifs of canonical RECK, thus liberating MMP-9 from its inactivating interaction (Lee et al., 2018). Whether IL-17A induces the expression of alternatively spliced RECK variants with functional consequences in SMC is not known, and will be investigated in future studies.
As discussed earlier, inflammation is a critical component in vascular proliferative/occlusive disorders. In addition to acting as a MMP inhibitor, RECK also exerts anti-inflammatory effects. For example, ADAM17, also known as TNF-α-converting enzyme (TACE), is a membrane-anchored protein and plays a role in cell–cell and cell–matrix interactions. It mediates neointimal hyperplasia (Takaguri et al., 2011), and its gene deletion blunts pathological neovascularization (Weskamp et al., 2010). It is also a target in various clinical trials involving inflammatory diseases (Moss & Minond, 2017). As a protease, it processes multiple proinflammatory mediators and progrowth factors, including ectodomain shedding of TNF-α, resulting in increased cell proliferation and migration. Importantly, RECK inhibits its activity via physical association (Hong, Wu, Cheng, Chen, & Hung, 2014). Similar to ADAM17, ADAM10 also functions as a sheddase with multiple substrates, including Notch ligands, where Notch signaling plays a critical role SMC proliferation (Sweeney et al., 2004). Importantly, RECK directly inhibits ADAM10 proteolytic activity (Muraguchi et al., 2007). IL-6 signals via IL-6 receptor (IL-6R) and gp130, and IL-6/IL-6R/gp130 signaling plays a role in inflammation and promotes SMC migration. Using immunoprecipitation and immunoblotting, RECK has been shown to bind IL-6R and gp130 (Walsh et al., 2015), possibly inhibiting IL-6 proinflammatory and promigratory effects. RECK has also been shown to inhibit EGFR transactivation, possibly interfering with EGFR-dependent cell growth, proliferation, and migration (Kitajima et al., 2011). These reports suggest that enhancing RECK expression has therapeutic potential in vascular proliferative diseases by targeting multiple mediators involved in vascular inflammation and vascular proliferative diseases.
6 ∣. CONCLUSIONS
In summary, our results demonstrate that IL-17A/TRAF3IP2 signaling differentially regulates MMP-13 and RECK; while inducing MMP-13, it suppresses RECK expression, both in a TRAF3IP2-dependent manner, resulting in increased SMC proliferation and migration. We also demonstrate that ectopic expression of RECK inhibits MMP-13 activity and cell migration. Thus, TRAF3IP2 inhibitors or RECK inducers have the potential to block the progression of neointimal thickening in hyperplastic vascular diseases.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Veterans Affairs Merit (VA-I01-BX004220) and Research Career Scientist (IK6BX004016) to B.C. S.M. is supported by NIH/NIAID R01AI119131, T.Y. by American Heart Association 15SDG25240022, R.I. in part by Elsa U. Pardee Foundation, and U.S. by the Intramural Research Program of the NIH
Funding information
U.S. Department of Veterans Affairs, Grant/Award Numbers: VA-I01-BX002255, I01BX003271, IK6BX004016-01; American Heart Association, Grant/Award Number: 15SDG25240022; National Institute of Allergy and Infectious Diseases, Grant/Award Number: R01AI119131; Veterans Affairs Merit, Grant/Award Number: VA-I01-BX004220; Research Career Scientist, Grant/Award Number: IK6BX004016; NIH/NIAID, Grant/Award Number: R01AI119131; Elsa U. Pardee Foundation; Intramural Research Program of the NIH
Abbreviations:
- Act1
NFκB-activating protein-1
- Ad.GFP
adenoviral vector expressing green fluorescent protein
- ADAM
a disintegrin and metalloproteinase domain-containing protein 10
- Ang
angiotensin
- AP-1
activator protein-1
- ASK1
apoptosis signal regulating kinase 1
- C/EBPβ
CCAAP enhancer binding protein beta
- cAMP
3′,5′-cyclic adenosine monophosphate
- CIKS
connection to connection to IKK and SAPK/JNK
- CREB
cAMP responsive element binding protein
- DES
drug-eluting stents
- DMSO
dimethyl sulfoxide
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- ERK
extracellular regulated kinase
- FAK
focal adhesion kinase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GBM
glioblastoma multiforme
- GPI
glycosylphosphatidylinositol
- IL
interleukin
- ISR
in-stent restenosis
- ITS
insulin-transferrin-sodium selenite
- JNK
c-jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MCP-1
macrophage chemotactic protein-1
- miR
microRNA
- MMP
matrix metalloproteinase
- moi
multiplicity of infection
- MT1-MMP
membrane-type-1 matrix metalloproteinase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PDGF
platelet-derived growth factor
- qPCR
quantitative polymerase chain reaction
- RECK
reversion-inducing cysteine-rich protein with kazal motifs
- rMMP-13
recombinant biologically active human MMP-13
- shRNA
short-hairpin RNA
- SM-MHC
smooth muscle myosin heavy chain
- SMC
smooth muscle cells
- TACE
tumor necrosis factor-α-converting enzyme
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- TRAF
TNF receptor-associated factor
- TRAF3IP2
TRAF3 interacting protein 2
- UTR
untranslated region
- VWF
Von Willebrand factor
- αSMA
α smooth muscle actin
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- Akhtar N, Rasheed Z, Ramamurthy S, Anbazhagan AN, Voss FR, & Haqqi TM (2010). MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthtitis and Rheumatism, 62(5), 1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessio N, Squillaro T, Ozcan S, Di Bernardo G, Venditti M, Melone M, … Galderisi U (2018). Stress and stem cells: Adult Muse cells tolerate extensive genotoxic stimuli better than mesenchymal stromal cells. Oncotarget, 9(27), 19328–19341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso F, Byrne RA, Rivero F, & Kastrati A (2014). Current treatment of in-stent restenosis. Journal of the American College of Cardiology, 63(24), 2659–2673. [DOI] [PubMed] [Google Scholar]
- Amatya N, Garg AV, & Gaffen SL (2017). IL-17 signaling: The Yin and the Yang. Trends in Immunology, 38(5), 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui C, Barter MJ, Scott JL, Xu Y, Galler M, Reynard LN,… Young DA (2012). cAMP response element-binding (CREB) recruitment following a specific CpG demethylation leads to the elevated expression of the matrix metalloproteinase 13 in human articular chondrocytes and osteoarthritis. FASEB Journal, 26(7), 3000–3011. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Mummidi S, Mahimainathan L, Patel DN, Bailey SR, Imam SZ, … Valente AJ (2006). Interleukin-18-induced human coronary artery smooth muscle cell migration is dependent on NF-kappaB- and AP-1-mediated matrix metalloproteinase-9 expression and is inhibited by atorvastatin. Journal of Biological Chemistry, 281(22), 15099–15109. [DOI] [PubMed] [Google Scholar]
- Cochaud S, Giustiniani J, Thomas C, Laprevotte E, Garbar C, Savoye AM, … Bastid J (2013). IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Scientific Reports, 3, 3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, Vincenti M,… Chandrasekar B (2007). IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. American Journal of Physiology: Heart and Circulatory Physiology, 293(6), H3356–H3365. [DOI] [PubMed] [Google Scholar]
- Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, & Mehran R (2010). In-stent restenosis in the drug-eluting stent era. Journal of the American College of Cardiology, 56(23), 1897–1907. [DOI] [PubMed] [Google Scholar]
- Das NA, Carpenter AJ, Yoshida T, Kumar SA, Gautam S, Mostany R,… Chandrasekar B (2018). TRAF3IP2 mediates TWEAK/TWEAKR-induced pro-fibrotic responses in cultured cardiac fibroblasts and the heart. Journal of Molecular and Cellular Cardiology, 121, 107–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R, Pillai S, Lawrence N, Sebti S, & Chellappan SP (2012). TNF-alpha-mediated proliferation of vascular smooth muscle cells involves Raf-1-mediated inactivation of Rb and transcription of E2F1-regulated genes. Cell Cycle, 11(1), 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour A, Zucker S, Sampson NS, Kuscu C, & Cao J (2010). Role of matrix metalloproteinase-9 dimers in cell migration: Design of inhibitory peptides. Journal of Biological Chemistry, 285(46), 35944–35956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan WH, Pech M, & Karnovsky MJ (2000). Connective tissue growth factor (CTGF) stimulates vascular smooth muscle cell growth and migration in vitro. European Journal of Cell Biology, 79(12), 915–923. [DOI] [PubMed] [Google Scholar]
- Fu J, Li S, Feng R, Ma H, Sabeh F, Roodman GD,… Lentzsch S (2016). Multiple myeloma-derived MMP-13 mediates osteoclast fusogenesis and osteolytic disease. Journal of Clinical Investigation, 126(5), 1759–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KJ, Wu DC, Cheng KH, Chen LT, & Hung WC (2014). RECK inhibits stemness gene expression and tumorigenicity of gastric cancer cells by suppressing ADAM-mediated Notch1 activation. Journal of Cellular Physiology, 229(2), 191–201. [DOI] [PubMed] [Google Scholar]
- Jukema JW, Ahmed TA, Verschuren JJ, & Quax PH (2011). Restenosis after PCI. Part 2: Prevention and therapy. Nature Reviews Cardiology, 9(2), 79–90. [DOI] [PubMed] [Google Scholar]
- Jukema JW, Verschuren JJ, Ahmed TA, & Quax PH (2011). Restenosis after PCI. Part 1: Pathophysiology and risk factors. Nature Reviews Cardiology, 9(1), 53–62. [DOI] [PubMed] [Google Scholar]
- Kanamori M, Kai C, Hayashizaki Y, & Suzuki H (2002). NF-kappaB activator Act1 associates with IL-1/Toll pathway adaptor molecule TRAF6. FEBS Letters, 532(1-2), 241–246. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Kokubu F, Fujita J, Huang SK, & Hizawa N (2009). Role of interleukin-17F in asthma. Inflammation and Allergy Drug Targets, 8(5), 383–389. [DOI] [PubMed] [Google Scholar]
- Kitajima S, Miki T, Takegami Y, Kido Y, Noda M, Hara E, … Takahashi C (2011). Reversion-inducing cysteine-rich protein with Kazal motifs interferes with epidermal growth factor receptor signaling. Oncogene, 30(6), 737–750. [DOI] [PubMed] [Google Scholar]
- Knauper V, Will H, Lopez-Otin C, Smith B, Atkinson SJ, Stanton H, … Murphy G (1996). Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. Journal of Biological Chemistry, 271(29), 17124–17131. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Iizuka S, Yoshida M, Tsunematsu T, Kondo T, Subarnbhesaj A, … Takata T (2012). Matrix metalloproteinase-13 (MMP-13) directly and indirectly promotes tumor angiogenesis. Journal of Biological Chemistry, 287(46), 38716–38728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarswamy R, Volkmann I, & Thum T (2011). Regulation and function of miRNA-21 in health and disease. RNA Biology, 8(5), 706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HN, Mitra M, Bosompra O, Corney DC, Johnson EL, Rashed N,… Coller HA (2018). RECK isoforms have opposing effects on cell migration. Molecular Biology of the Cell, 29(15), 1825–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi A, Chariot A, Claudio E, Cunningham K, & Siebenlist U (2000). CIKS, a connection to Ikappa B kinase and stress-activated protein kinase. Proceedings of the National Academy of Sciences of the United States of America, 97(19), 10494–10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang D, Yuan Y, & Min J (2017). New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Research and Therapy, 19(1), 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Commane M, Nie H, Hua X, Chatterjee-Kishore M, Wald D, … Stark GR (2000). Act1, an NF-kappa B-activating protein. Proceedings of the National Academy of Sciences of the United States of America, 97(19), 10489–10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liacini A, Sylvester J, Li WQ, & Zafarullah M (2002). Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biology, 21(3), 251–262. [DOI] [PubMed] [Google Scholar]
- Liu S, Song X, Chrunyk BA, Shanker S, Hoth LR, Marr ES, & Griffor MC (2013). Crystal structures of interleukin 17A and its complex with IL-17 receptor A. Nature Communications, 4, 1888. [DOI] [PubMed] [Google Scholar]
- Mao D, Lee JK, VanVickle SJ, & Thompson RW (1999). Expression of collagenase-3 (MMP-13) in human abdominal aortic aneurysms and vascular smooth muscle cells in culture. Biochemical and Biophysical Research Communications, 261(3), 904–910. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA (2009). TGF-beta signaling in atherosclerosis and restenosis. Frontiers in Bioscience, 1, 236–245. [DOI] [PubMed] [Google Scholar]
- Meng F, Zhang Z, Chen W, Huang G, He A, Hou C,… Liao W (2016). MicroRNA-320 regulates matrix metalloproteinase-13 expression in chondrogenesis and interleukin-1beta-induced chondrocyte responses. Osteoarthritis and Cartilage, 24(5), 932–941. [DOI] [PubMed] [Google Scholar]
- Mittal B, Mishra A, Srivastava A, Kumar S, & Garg N (2014). Matrix metalloproteinases in coronary artery disease. Advances in Clinical Chemistry, 64, 1–72. [DOI] [PubMed] [Google Scholar]
- Moss ML, & Minond D (2017). Recent advances in ADAM17 research: A promising target for cancer and inflammation. Mediators of Inflammation, 2017, 9673537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi T, Takegami Y, Ohtsuka T, Kitajima S, Chandana EP, Omura A, . Takahashi C (2007). RECK modulates Notch signaling during cortical neurogenesis by regulating ADAM10 activity. Nature Neuroscience, 10(7), 838–845. [DOI] [PubMed] [Google Scholar]
- Nagini S (2012). RECKing MMP: Relevance of reversion-inducing cysteine-rich protein with kazal motifs as a prognostic marker and therapeutic target for cancer (a review). Anti-Cancer Agents in Medicinal Chemistry, 12(7), 718–725. [DOI] [PubMed] [Google Scholar]
- Nikkola J, Vihinen P, Vuoristo MS, Kellokumpu-Lehtinen P, Kahari VM, & Pyrhonen S (2005). High serum levels of matrix metalloproteinase-9 and matrix metalloproteinase-1 are associated with rapid progression in patients with metastatic melanoma. Clinical Cancer Research, 11(14), 5158–5166. [DOI] [PubMed] [Google Scholar]
- Nordlohne J, Helmke A, Ge S, Rong S, Chen R, Waisman A, … von Vietinghoff S (2018). Aggravated atherosclerosis and vascular inflammation with reduced kidney function depend on interleukin-17 receptor A and are normalized by inhibition of interleukin-17A. JACC: Basic to Translational Science, 3(1), 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, & Eisenhaber F (2003). The STIR-domain superfamily in signal transduction, development and immunity. Trends in Biochemical Sciences, 28(5), 226–229. [DOI] [PubMed] [Google Scholar]
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T,… Lotze MT (2003). Interleukin-17 promotes angiogenesis and tumor growth. Blood, 101(7), 2620–2627. [DOI] [PubMed] [Google Scholar]
- Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, … Noda M (2001). The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell, 107(6), 789–800. [DOI] [PubMed] [Google Scholar]
- de Oliveira PS, Cardoso PR, Lima EV, Pereira MC, Duarte AL, Pitta Ida R,… Pitta MG (2015). IL-17A, IL-22, IL-6, and IL-21 serum levels in plaque-type psoriasis in Brazilian patients. Mediators of Inflammation, 2015, 819149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggatt LJ, Jefcoat SC Jr., Choudhury I, Williams S, Tiku M, & Partridge NC (2006). Matrix metalloproteinase-13 influences ERK signalling in articular rabbit chondrocytes. Osteoarthritis and Cartilage, 14(7), 680–689. [DOI] [PubMed] [Google Scholar]
- Ruan G, Xu J, Wang K, Wu J, Zhu Q, Ren J, . Ding C (2018). Associations between knee structural measures, circulating inflammatory factors and MMP13 in patients with knee osteoarthritis. Osteoarthritis and Cartilage, 26(8), 1063–1069. [DOI] [PubMed] [Google Scholar]
- Sakamuri S, Higashi Y, Sukhanov S, Siddesha JM, Delafontaine P, Siebenlist U, & Chandrasekar B (2016). TRAF3IP2 mediates atherosclerotic plaque development and vulnerability in ApoE(−/−) mice. Atherosclerosis, 252, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ZD, Ji XY, Berardi DE, Qazi H, & Tarbell JM (2010). Interstitial flow induces MMP-1 expression and vascular SMC migration in collagen I gels via an ERK1/2-dependent and c-Jun-mediated mechanism. American Journal of Physiology: Heart and Circulatory Physiology, 298(1), H127–H135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddesha JM, Valente AJ, Sakamuri SS, Gardner JD, Delafontaine P, Noda M, & Chandrasekar B (2014). Acetylsalicylic acid inhibits IL-18-induced cardiac fibroblast migration through the induction of RECK. Journal of Cellular Physiology, 229(7), 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddesha JM, Valente AJ, Sakamuri SS, Yoshida T, Gardner JD, Somanna N, … Chandrasekar B (2013). Angiotensin II stimulates cardiac fibroblast migration via the differential regulation of matrixins and RECK. Journal of Molecular and Cellular Cardiology, 65, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddesha JM, Valente AJ, Yoshida T, Sakamuri SS, Delafontaine P, Iba H, … Chandrasekar B (2014). Docosahexaenoic acid reverses angiotensin II-induced RECK suppression and cardiac fibroblast migration. Cellular Signalling, 26(5), 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanna NK, Yariswamy M, Garagliano JM, Siebenlist U, Mummidi S, Valente AJ, & Chandrasekar B (2015). Aldosterone-induced cardiomyocyte growth, and fibroblast migration and proliferation are mediated by TRAF3IP2. Cellular Signalling, 27(10), 1928–1938. [DOI] [PubMed] [Google Scholar]
- Sweeney C, Morrow D, Birney YA, Coyle S, Hennessy C, Scheller A, … Cahill PA (2004). Notch 1 and 3 receptor signaling modulates vascular smooth muscle cell growth, apoptosis, and migration via a CBF-1/RBP-Jk dependent pathway. FASEB Journal, 18(12), 1421–1423. [DOI] [PubMed] [Google Scholar]
- Takaguri A, Kimura K, Hinoki A, Bourne AM, Autieri MV, & Eguchi S (2011). A disintegrin and metalloprotease 17 mediates neointimal hyperplasia in vasculature. Hypertension, 57(4), 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi C, Sheng Z, Horan TP, Kitayama H, Maki M, Hitomi K,… Noda M (1998). Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proceedings of the National Academy of Sciences of the United States of America, 95(22), 13221–13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta-Lima M, Winnischofer SM, Demasi MA, Astorino Filho R, Carreira AC, Wei B, . Sogayar MC (2015). Isolation and characterization of novel RECK tumor suppressor gene splice variants. Oncotarget, 6(32), 33120–33133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S, Hollenbeck ST, Ryer EJ, Edlin R, Yamanouchi D, Kundi R,… Kent KC (2009). TGF-beta through Smad3 signaling stimulates vascular smooth muscle cell proliferation and neointimal formation. American Journal of Physiology: Heart and Circulatory Physiology, 297(2), H540–H549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente AJ, Clark RA, Siddesha JM, Siebenlist U, & Chandrasekar B (2012). CIKS (Act1 or TRAF3IP2) mediates angiotensin-II-induced interleukin-18 expression, and Nox2-dependent cardiomyocyte hy-pertrophy. Journal of Molecular and Cellular Cardiology, 53(1), 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente AJ, Yoshida T, Gardner JD, Somanna N, Delafontaine P, & Chandrasekar B (2012). Interleukin-17A stimulates cardiac fibroblast proliferation and migration via negative regulation of the dual-specificity phosphatase MKP-1/DUSP-1. Cellular Signalling, 24(2), 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente AJ, Yoshida T, Izadpanah R, Delafontaine P, Siebenlist U, & Chandrasekar B (2013). Interleukin-18 enhances IL-18R/Nox1 binding, and mediates TRAF3IP2-dependent smooth muscle cell migration. Inhibition by simvastatin. Cellular Signalling, 25(6), 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan B, Valente AJ, Das NA, Carpenter AJ, Yoshida T, Delafontaine JL, … Chandrasekar B (2013). CIKS (Act1 or TRAF3IP2) mediates high glucose-induced endothelial dysfunction. Cellular Signalling, 25(1), 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh LA, Roy DM, Reyngold M, Giri D, Snyder A, Turcan S, . Chan TA (2015). RECK controls breast cancer metastasis by modulating a convergent, STAT3-dependent neoangiogenic switch. Oncogene, 34(17), 2189–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weskamp G, Mendelson K, Swendeman S, Le Gall S, Ma Y, Lyman S, . Blobel CP (2010). Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circulation Research, 106(5), 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zepp J, & Li X (2012). Function of Act1 in IL-17 family signaling and autoimmunity. Advances in Experimental Medicine and Biology, 946, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Okano H, Miyagawa W, Visse R, Shitomi Y, Santamaria S,… Nagase H (2016). MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biology, 56, 57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, & Boyd DD (2007). Regulation of matrix metalloproteinase gene expression. Journal of Cellular Physiology, 211(1), 19–26. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Qian Y, Wald D, Xia YF, Geng JG, & Li X (2003). IFN regulatory factor-1 is required for the up-regulation of the CD40-NF-kappa B activator 1 axis during airway inflammation. Journal of Immunology, 170(11), 5674–5680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.