Abstract
Purpose:
Brain metastases are a common sequelae of breast cancer. Survival varies widely based on diagnosis-specific prognostic factors (PF). We previously published a prognostic index (Graded Prognostic Assessment [GPA]) for patients with breast cancer with brain metastases (BCBM), based on cohort A (1985–2007, n = 642), then updated it, reporting the effect of tumor subtype in cohort B (1993–2010, n = 400). The purpose of this study is to update the Breast GPA with a larger contemporary cohort (C) and compare treatment and survival across the 3 cohorts.
Methods and Materials:
A multi-institutional (19), multinational (3), retrospective database of 2473 patients with breast cancer with newly diagnosed brain metastases (BCBM) diagnosed from January 1, 2006, to December 31, 2017, was created and compared with prior cohorts. Associations of PF and treatment with survival were analyzed. Kaplan-Meier survival estimates were compared with log-rank tests. PF were weighted and the Breast GPA was updated such that a GPA of 0 and 4.0 correlate with the worst and best prognoses, respectively.
Results:
Median survival (MS) for cohorts A, B, and C improved over time (from 11, to 14 to 16 months, respectively; P < .01), despite the subtype distribution becoming less favorable. PF significant for survival were tumor subtype, Karnofsky Performance Status, age, number of BCBMs, and extracranial metastases (all P < .01). MS for GPA 0 to 1.0, 1.5–2.0, 2.5–3.0, and 3.5–4.0 was 6, 13, 24, and 36 months, respectively. Between cohorts B and C, the proportion of human epidermal receptor 2 + subtype decreased from 31% to 18% (P < .01) and MS in this subtype increased from 25 months (P < .01).
Conclusions:
MS has improved modestly but varies widely by diagnosis-specific PF. New PF are identified and incorporated into an updated Breast GPA (free online calculator available at brainmetgpa.com). The Breast GPA facilitates clinical decision-making and will be useful for stratification of future clinical trials. Furthermore, these data suggest human epidermal receptor 2-targeted therapies improve clinical outcomes in some patients with BCBM.
Summary
For patients with breast cancer brain metastases, new prognostic factors are identified and incorporated into an updated Graded Prognostic Assessment (Breast GPA). Over decades, treatment patterns have changed and survival has improved. Median survival for patients in the best prognostic group now exceeds 3 years. Furthermore, these data suggest human epidermal receptor 2–targeted therapies improve outcomes for these patients. The Breast GPA facilitates clinical decision-making, end-of-life care, and appropriate stratification of future clinical trials.
Introduction
In 2019, there will be an estimated 270,000 patients diagnosed with breast cancer in the United States and more than 2 million patients diagnosed with breast cancer worldwide.1 Although the relative mortality rate from breast cancer has dropped by 40% from 1989 to 2016, more than 42,000 deaths are expected this year from the disease in the United States alone.2 Brain metastases (BM) are a serious sequelae of breast cancer, occurring in up to half of patients with metastatic human epidermal receptor 2 (HER2)-positive breast cancer and up to 25% to 46% of patients with metastatic triple negative breast cancer.3–5 The incidence of brain metastases (BCBM) is increasing, likely owing to improvements in systemic therapy, and the resulting decrease in overall mortality puts more patients at risk for the development of this common problem.6,7
Clinical management is complicated by the fact that survival in patients with BM varies widely based on diagnosis-specific prognostic factors (PF). We previously published a series of articles defining and updating the graded prognostic assessment (GPA), a prognostic index for BM patients with cancers of the breast,8 lung,9 kidney,10 gastrointestinal tract,11 and melanoma.12 The original Breast GPA was based on cohort A (1985–2007, n = 642) and found only Karnofsky Performance Status (KPS) to be an independent prognostic factor. It was later updated with the addition of age and tumor subtype in cohort B (1993–2010, n = 400) as additional prognostic factors.13,14
Some of the 21 reports that have independently validated the GPA include those of Villa et al,15 Gilbride et al,16 and Laakman et al.17 Some have suggested modifications of the Breast GPA. Ahn et al (n = 171) proposed that the presence or absence of extracranial metastases (ECM) should be added to the Breast GPA.18 Zhuang et al (n = 282) suggested extracranial disease progression, as opposed to the presence or absence of extracranial metastases, as an independent prognostic factor.19 Ahluwalia et al (n = 371) also suggested the addition of ECM and control of the primary tumor and leptomeningeal involvement.20 More recently, Subbiah et al (n = 1552) suggested the addition of the number of brain metastases based on a study of 1552 patients.21 The purpose of this study is to further refine the Breast GPA based on a larger contemporary cohort (C) and to track changes in patterns of care and survival from 1985 to today.
Methods
Patient population
Our multinational (3), multi-institutional (18) consortium created an institutional review board–approved retrospective database of 2473 evaluable patients with newly diagnosed BCBM treated between January 1, 2006, and December 31, 2017.
All patients had newly diagnosed brain metastases, which we arbitrarily defined as those receiving treatment within 2 months of the diagnosis of BCBM. Patients with recurrent BCBM and those with leptomeningeal metastases were excluded.
Statistical analysis
Associations were analyzed between survival and prognostic factors (PF, Table 1) and treatment. In addition to reported factors, 9 molecular markers (BRCA1, BRCA2, ATM, CDH1, CHEK2, NBN, NF1, PALB2, PTEN, STK11, p53) and body mass index (BMI) were collected, but owing to the extent of incomplete data, only germline BRCA1 status is reported. Median survival (MS) estimates were calculated in months from date of BCBM diagnosis using the Kaplan-Meier method. Survival curves were compared between the current cohort (C) and prior cohorts (A and B) using standard log-rank tests, overall and separately for the 4 tumor subtypes. Time from primary diagnosis to brain metastasis (TPDBM) was described by percentiles (median and interquartile range) and compared using Wilcoxon rank-sum tests.
Table 1.
Patient characteristics by median survival from the TPDBM
| N (%) | Median survival in mo (IQR) | Median mo from primary Dx to BM Dx (TPDBM) (IQR) | |
|---|---|---|---|
| All patients | 2473 (100) | 16 (7–34) | 39 (18–79) |
| Age at BM diagnosis | |||
| <46 | 616 (25) | 20 (9–44) | 28 (15–51) |
| 46–54 | 642 (26) | 18 (7–38) | 40 (18–77) |
| 55–63 | 604 (24) | 17 (7–33) | 44 (18–90) |
| 64–92 | 609 (25) | 12 (5–26) | 51 (22–106) |
| Sex | |||
| Male | 25 (1) | 22 (7–39) | 49 (14–79) |
| Female | 2448 (99) | 16 (7–34) | 39 (18–79) |
| Ethnicity | |||
| Hispanic | 172 (7) | 22 (8–54) | 25 (13–54) |
| Not Hispanic | 2026 (82) | 16 (6–34) | 40 (18–81) |
| Not reported | 275 (11) | 16 (7–32) | 41 (19–74) |
| Race | |||
| White | 1728 (70) | 15 (6–33) | 40 (19–82) |
| Black | 282 (11) | 16 (7–33) | 34 (15–77) |
| Asian | 102 (4.1) | 16 (7–48) | 37 (19–90) |
| Other | 30 (1.2) | 10 (4–22) | 29 (12–46) |
| Not reported | 331 (13) | 23 (12–47) | 37 (17–68) |
| Histology | |||
| Invasive ductal carcinoma | 1967 (80) | 16 (7–35) | 38 (18–75) |
| Invasive lobular carcinoma | 93 (3.8) | 14 (5–47) | 40 (17–96) |
| Mixed | 65 (2.6) | 19 (6–37) | 51 (16–90) |
| Other | 69 (2.8) | 15 (4–38) | 29 (11–67) |
| Not reported | 279 (11) | 17 (6–30) | 46 (20–98) |
| Subtype | |||
| Luminal B (triple positive) | 527 (21) | 27 (13–55) | 46 (21–84) |
| Basal (triple negative) | 595 (24) | 9 (4–19) | 27 (14–49) |
| Her2 (HR-neg, HER2-pos) | 421 (17) | 25 (11–47) | 27 (15–57) |
| Luminal A (HR-pos, HER2-neg) | 772 (31) | 14 (5–30) | 54 (23–103) |
| Unknown | 158 (6.4) | 14 (5–29) | 69 (30–155) |
| No. of BM at initial dx | |||
| 1 | 869 (35) | 20 (9–45) | 39 (19–81) |
| 2–3 | 617 (25) | 15 (7–33) | 42 (19–84) |
| 4–9 | 466 (19) | 13 (6–30) | 39 (17–84) |
| ≥ 10 | 486 (20) | 12 (4–28) | 36 (15–67) |
| Unknown | 35 (1.4) | 23 (16–46) | 42 (16–78) |
| KPS at BM dx | |||
| 100 | 184 (7.4) | 23 (12–42) | 35 (18–69) |
| 90 | 720 (29) | 20 (9–43) | 38 (16–76) |
| 80 | 652 (26) | 15 (6–32) | 42 (20–80) |
| 70 | 371 (15) | 11 (4–22) | 45 (19–92) |
| ≤60 | 195 (7.9) | 5 (2–17) | 35 (14–69) |
| Unknown | 351 (14) | 22 (11–46) | 37 (18–73) |
| Extracranial metastases at BM dx | |||
| Present | 1997 (81) | 15 (6–32) | 45 (20–87) |
| Absent | 454 (18) | 23 (11–51) | 22 (13–40) |
| Unknown | 22 (0.9) | 21 (14–34) | 25 (18–53) |
| BRCA1 mutation | |||
| Present | 57 (2.3) | 16 (7–27) | 37 (21–61) |
| Absent | 426 (17) | 19 (7–47) | 36 (17–71) |
| Unknown | 1990 (80) | 16 (7–33) | 40 (18–81) |
Abbreviations: BM = brain metastasis; dx = diagnosis; HER2 = human epidermal receptor 2; HR = hormone receptor (estrogen or progesterone receptors); IQR = interquartile range; TPDBM = time from primary diagnosis to brain metastases.
Multiple Cox regression was used to initially select and weight variables to be included in the new Breast GPA. The model was stratified by institution. Continuous variables were divided into quartiles to allow for nonlinear effects. Missing observations were included as an unknown category. Factors initially considered were age, sex, ethnicity, race, histology, subtype, number of BCBM, KPS, presence of extracranial metastases, and BMI. Sex was 99% female and not prognostic, and so was dropped from the model. Histology, BMI, and race were not prognostic, so they were dropped from the model. Continuous variables were divided into quartiles to allow for nonlinear effects. The updated Breast GPA was designed such that a GPA of 0 and 4.0 correlate with the worst and best prognosis, respectively, as in all of the prior GPA studies. Factor weights were refined using metrics, such as the concordance index and R-squared. The final GPA was chosen as a balance of performance metrics and simplicity. Analysis was performed using R software, including packages rms and survival. The free online application (brainmetgpa.com) to facilitate calculation of the GPA was updated based on these data.
Results
Patients
Table 1 shows patient characteristics and survival for the entire cohort. Notable findings include the following: median age was 55 years; the frequency of tumor subtype was 31%, 24%, 21%, and 17% in ER-pos/Her2-neg, ER-neg/HER2-neg, ER-pos/HER2-pos, and ER-neg/HER2-pos, respectively; the number of BM was 1 in 35%,≤ 5 in 73% and >10 in 18%; more than 62% had KPS 80 to 100; and 81% had ECM. Only 4.5% (115 of 2473) of patients presented with BCBM synchronous with the diagnosis of primary breast cancer. The tumor subtype distribution for these patients was 41 out of 115 (37%), 28 out of 115 (25%), 19 out of 115 (17%), and 22 out of 115 (22%) for ER-pos/HER2-neg, ER-neg/HER2-neg, ER-pos/HER2-pos, and ER-neg/HER2-pos, subtypes respectively. Male breast cancer represented 1% (25 out of 2473) of the current cohort.
TPDBM
Table 1 shows that TPDBM varied by age, subtype, and ECM. TPDBM was shorter in younger patients (28 months in women <46 years of age compared with 40, 44, and 51 months for patients who were 46–54, 55–63, and 64–92 years of age, respectively). The hormone receptor–negative subtypes had the shortest TPDBM (27 months) compared with ER-pos/HER2-pos (46 months) and ER-pos/HER2-neg (54 months). Table 2 compares the distribution of tumor subtype, survival, and TPDBM between treatment era. The TPDBM was shorter (22 months) for patients without ECM than for patients with ECM (45 months). There was no difference in TPDBM between cohorts B and C.
Table 2.
Distribution of tumor subtype, survival and TPDBM by era
| Tumor subtype | Incidence | Median survival (mo) | TPDBM (mo) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort B | Cohort C | P | Cohort B | Cohort C | P | Cohort B | Cohort C | P | |
| Luminal B (HR/HER2-pos) | 103 of 400 (26%) | 527 of 2315 (23%) | .19 | 21 | 27 | .03 | 47 | 46 | .92 |
| HER2 (HR-neg, HER2-pos) | 122 of 400 (31%) | 421 of 2315 (18%) | <.01 | 18 | 25 | <.01 | 36 | 27 | .35 |
| Luminal A (HR-pos, HER2-neg) | 78 of 400 (20%) | 772 of 2315 (33%) | <.01 | 10 | 14 | .30 | 54 | 54 | .83 |
| Basal (HR/HER2-neg) | 97 of 400 (24%) | 595 of 2315 (26%) | .54 | 6 | 9 | <.01 | 27 | 27 | .94 |
Abbreviations: TPDBM = time from primary diagnosis to brain metastases; HER2 = human epidermal receptor 2; HR = hormone receptor (estrogen or progesterone receptors; MS = median survival.
Incidence data for both cohorts exclude those with unknown subtype.
Survival
Figure 1 shows the overall median survival for cohorts A, B, and C were 11, 14, and 16 months, respectively (P = .01). Table 2 shows that between cohorts B and C, MS has improved significantly for 3 of the 4 subtypes: ER-pos/HER2-pos (21–27 months, P = .03), ER-neg/HER2-pos (18–25 months, P <.01) and ER-neg/HER2-neg (6–9 months, P <.01).
Fig. 1.
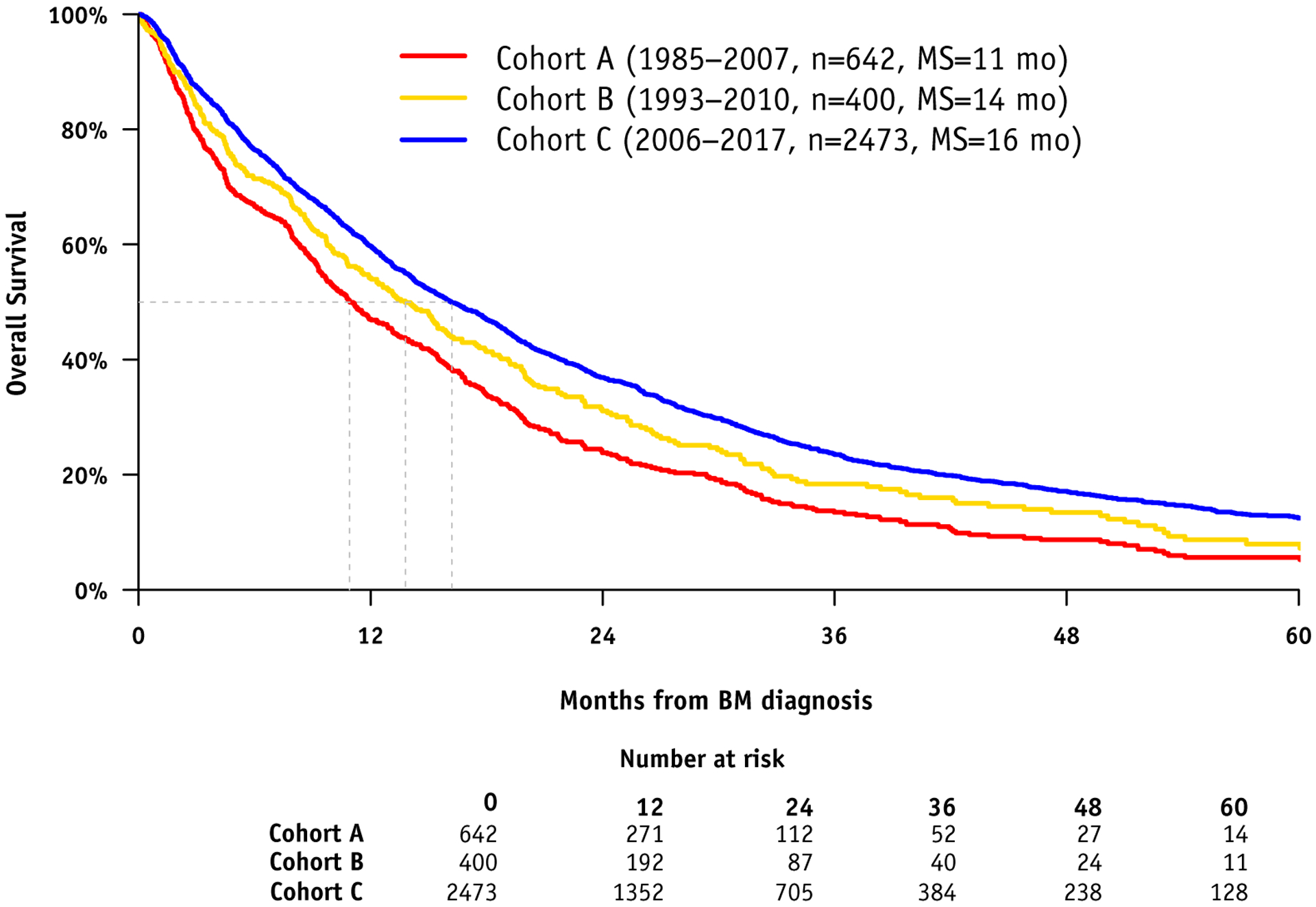
Kaplan Meier Curve for Survival by Era.
Updated Breast GPA
Table 3 shows the definition of the updated Breast GPA in a practical, easy-to-use worksheet format based on the significant prognostic factors and the assigned scoring criteria. It also shows the median survival by GPA. The MS for these 4 groups was 6, 13, 24, and 36 months, for Breast GPA 0 to 1.0, 1.5 to 2.0, 2.5 to 3.0, and 3.5 to 4.0, respectively. This represents significant improvement in MS compare with cohort A (6, 9, 17, 19 months, respectively) and cohort B (3, 8, 15, 25 months, respectively). Figure 2 shows the Kaplan-Meier curves for survival by GPA with clear separation between each subgroup (each P < .001). Figure E1 (available online at https://doi.org/10.1016/j.ijrobp.2020.01.051) shows a comparison of survival by tumor subtype between cohorts B and C.
Table 3.
Definition/worksheet for the updated graded prognostic assessment for breast cancer patients with brain metastases (Breast GPA) and survival by GPA
| Factor | 0 | 0.5 | 1.0 | 1.5 | Patient score |
|---|---|---|---|---|---|
| KPS | ≤60 | 70–80 | 90–100 | NA | |
| Subtype | Basal | Luminal A | NA | HER2, Luminal B | |
| Age | ≤60 | <60 | NA | NA | |
| No. BM | >1 | 1 | NA | NA | |
| ECM | Present | Absent | |||
| Sum total = |
| GPA | N (%) | Median OS | IQR |
|---|---|---|---|
| 0.0–1.0 | 377 (19) | 6.0 | 2.5–12.3 |
| 1.5–2.0 | 780 (39) | 12.9 | 5.6–27.0 |
| 2.5–3.0 | 659 (33) | 23.5 | 11.1–47.0 |
| 3.5–4.0 | 173 (9) | 36.3 | 18.5–78.1 |
Abbreviations: BM = brain metastases; ECM = extracranial metastases; GPA = Graded Prognostic Assessment; HER2 = human epidermal receptor 2; IQR = interquartile range; KPS = Karnofsky Performance Status; OS = overall survival.
Fig. 2.
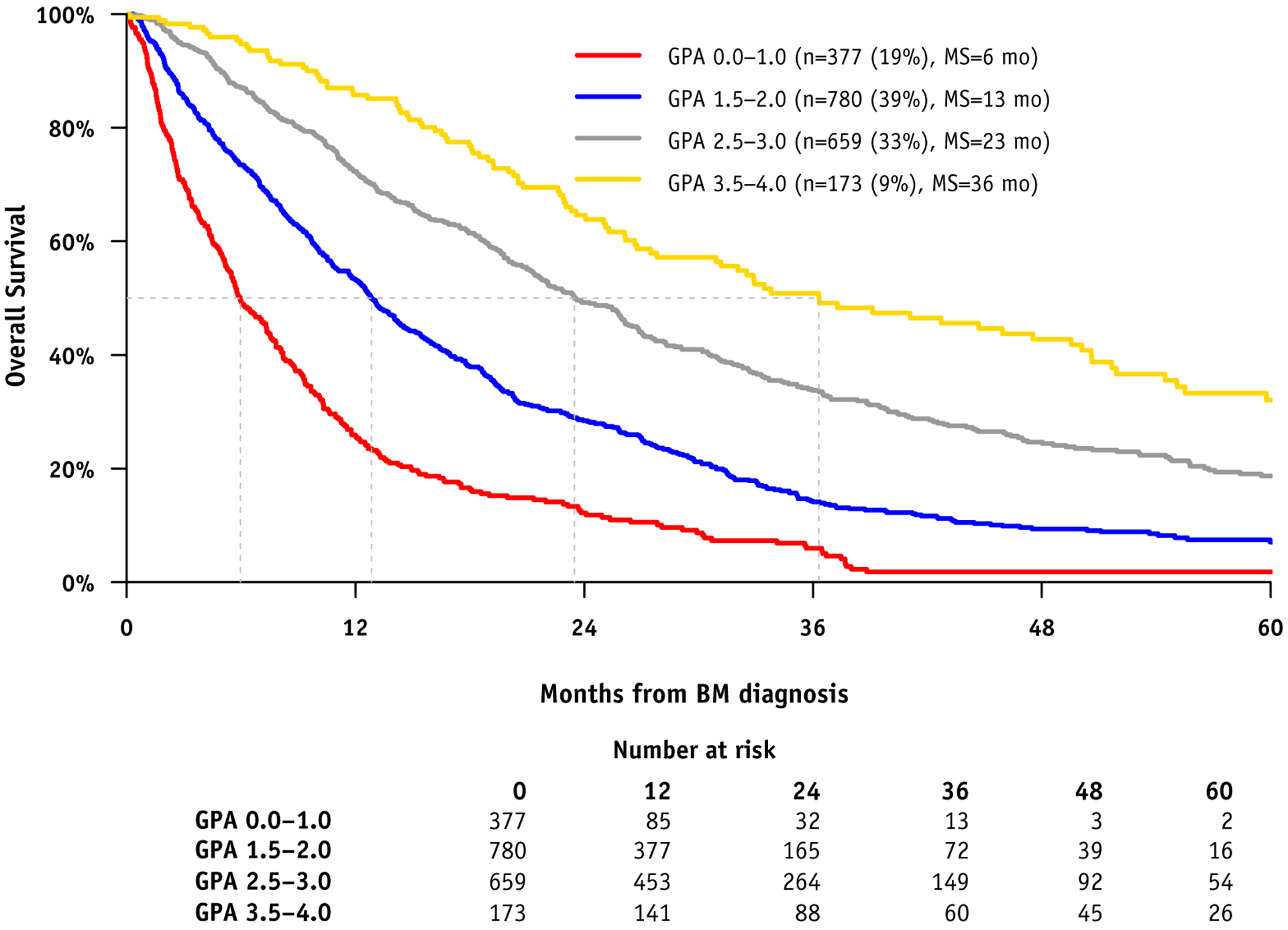
Kaplan Meier Curves for Survival by Breast GPA.
Treatment patterns by era
Table 4 shows survival by era and primary treatment for our 2 prior cohorts (cohort A, n = 642, 1985–2007; cohort B, n = 400, 1993–2010) and the current cohort (cohort C, n = 2473, 2006–2017). The use of whole brain radiation therapy (WBRT) has decreased from 75% to 67% to 47% in cohorts A, B and C, respectively. The use of stereotactic radiosurgery (SRS) alone has increased from 22% to 29% to 34% in cohorts A, B and C, respectively. The combined use of WBRT plus SRS was 19%, 22%, and 4% in cohorts A, B and C, respectively, showing a dramatic decrease in this approach in the current cohort. The use of craniotomy has remained stable (16%−20%) in all 3 cohorts. Table E1 (available online at https://doi.org/10.1016/j.ijrobp.2020.01.051) shows a summary of systemic therapies used before, after and both before and after the diagnosis of BCBM.
Table 4.
Survival by era and primary treatment for brain metastases
| Cohort/era | Overall | WBRT | SRS | WBRT + SRS | S + SRS | S + WBRT | S + WBRT + SRS |
|---|---|---|---|---|---|---|---|
| A (Lin et al5) | |||||||
| 1985–2007 | |||||||
| N (%) | 642 | 277 (43) | 141 (22) | 123 (19) | 19 (3) | 58 (9) | 24 (4) |
| Median survival | 12 | 6 | 14 | 15 | 22 | 18 | 16 |
| Risk of death (HR) | 1.0 | 0.75 | 0.72 | 0.42 | 0.61 | 0.36 | |
| 95% CI | 0.54–1.04 | 0.53–0.98 | 0.21–0.83 | 0.43–0.86 | 0.20–0.63 | ||
| P value vs WBRT | .09 | .04 | .01 | <.01 | <.01 | ||
| B (ref 6–8) | |||||||
| 1993–2010 | |||||||
| N (%) | 400 | 131 (33) | 115 (29) | 86 (22) | 19 (5) | 28 (7) | 20 (5) |
| Median survival | 14 | 7 | 13 | 15 | 24 | 18 | 30 |
| Risk of death (HR)* | 1.0 | 1.07 | 0.74 | 0.59 | 0.72 | 0.47 | |
| 95% CI* | 0.66–1.73 | 0.47–1.16 | 0.28–1.23 | 0.43–1.21 | 0.23–0.96 | ||
| P value vs WBRT* | .80 | .18 | .16 | .72 | .04 | ||
| C | |||||||
| 2006–2017 | |||||||
| N (%) | 2473 | 903 (37) | 840 (34) | 105 (4) | 261 (11) | 136 (5) | 18 (1) |
| Mean GPA | 2.1 | 2.4 | 2.4 | 2.5 | 2.6 | 2.7 | |
| Median survival | 16 | 13 | 16 | 15 | 19 | 25 | 24 |
| Risk of death (HR)* | 1.0 | 0.85 | 1.04 | 0.69 | 0.71 | 0.93 | |
| 95% CI* | 0.75–0.97 | 0.79–1.37 | 0.57–0.84 | 0.55–0.91 | 0.49–1.79 | ||
| P value vs WBRT* | .02 | .77 | <.01 | <.01 | .84 | ||
Abbreviations: CI = confidence interval; SRS = stereotactic radiosurgery.
In cohort C, 106 patients received surgery alone, 47 received fractionated partial brain radiation alone, and 57 received none of the above.
Estimates from multiple Cox regression, adjusted for Graded Prognostic Assessment (GPA) and stratified by institution. Hazard ratios (HR) are relative to whole brain radiation therapy (WBRT) within each cohort/era. Median survival estimates are in months and are not adjusted for any other factors.
Multivariable model
Table E2 (available online at https://doi.org/10.1016/j.ijrobp.2020.01.051) shows the multivariable model for survival used to derive the updated Breast GPA with the hazard ratio for each variable for each prognostic factor. PF significant for survival were tumor subtype, age, KPS, number of BM and ECM (all <0.001). KPS and subtype had the largest effects, with a mortality hazard ratio of 3.4 for KPS ≤ 60 versus KPS = 100 and a hazard ratio of 2.8 for HR-neg/HER2-neg relative to HR-pos/HER2-pos. To compare predictive discrimination of the revised and original Breast GPAs, we calculated the c-index (concordance probability). The c-index for the original GPA was 0.648, which improved to 0.669 using the revised GPA. The latter estimate, however, was made with the sample that was used to derive the model, and a true comparison requires an independent cohort.
BRCA status
BRCA1 status was known in 474 patients and present in 57 of 474 (12%). Among those with BRCA1 present, 33 of 57 (58%) had ER-neg/HER2-neg tumor subtype. There was no significant difference in MS (16 vs 19 months) or TPDBM (37 vs 36 months) in patients with present versus absent BRCA1 mutations (Table 1).
Discussion
Three current evidence-based guidelines22–24 assert the primary role of local therapies (craniotomies, SRS, and WBRT) with or without hippocampal avoidance in the management of patients with BM. These primary therapies are supported by multiple landmark prospective randomized clinical trials, which have changed the standard of care.25–33 Regarding systemic therapy specifically in BCBM, an American Society of Clinical Oncology Clinical Practice Guideline Update recommended that patients with HER2-positive breast cancer and brain metastases receive appropriate local therapies (craniotomy, SRS, WBRT) and HER2-targeted therapies, making it clear that local therapies remain the mainstay of management for most patients with brain metastases, especially on initial diagnosis, although the guideline acknowledges an increasing role of systemic therapy in the options for patients with central nervous system (CNS) progression after initial local therapy.34
Each of these guidelines emphasize the importance of understanding prognosis to optimally individualize management of patients with BCBM. This study, the largest reported series of BCBM, refines our understanding of prognosis and identifies 5 PF. The updated Breast GPA offers a more accurate method to estimate survival. Such information will guide clinical decision-making, patient choice, and end-of-life care. It will be useful in the stratification of future clinical trials to ensure the arms of those trials are comparing truly comparable patients.
We observed a significant decrease between cohorts B and C in the proportion of patients with HR-neg/HER2-pos subtype; although a similar decrease was not observed in the HR-pos/HER2-pos subtype. The reduction in the percentage of HR-neg/HER2-pos patients in Cohort C could be driven by an overall decrease in the risk of distant recurrence among patients presenting with early breast cancer (resulting from improvements in adjuvant therapy) or by a decrease in the risk of brain metastases among patients with established HER2-pos metastatic breast cancer.35,36 Although no adjuvant regimen has been shown to reduce the risk of CNS as the first site of relapse, an overall reduction in the risk of distant metastases reduces the pool of patients at risk for subsequent CNS events.37 Our study cannot directly distinguish between these possibilities, although the lack of change in TPDBM would favor the former explanation. The proportion of tumor subtypes was similar to recent reports by Leone et al38 and Miller et al.39 Leone et al reported a retrospective series of patients (n = 740) with BCBM at the time of the initial breast cancer diagnosis, in which 47% were Luminal A and 14% were HER2 compared with 37% and 22% in our series, respectively. In our series, only 4.5% received a diagnosis of BCBM at the time of primary diagnosis.
MS has improved significantly overall between each era (cohort A to B and B to C) and in 3 (HER2-positive and HR-neg/HER2-neg) of the 4 tumor subtypes subtypes (Tables 2 and 3).
Our data are very similar to the SEER data40 in terms of overall survival by subtype. This is noteworthy because the SEER is population-based, whereas our data are from academic centers. This observation allays the concern that our data may not represent the general population. Of note, the SEER data reflects only patients who had brain metastases at the time of initial diagnosis of breast cancer (de novo brain metastases), whereas our data includes patients with newly diagnosed brain metastases either at the time of primary breast cancer diagnosis or subsequent to the primary diagnosis. The similar survival in our data and the SEER data suggests our data can be applied regardless of whether the brain metastases are diagnosed de novo or subsequent to primary diagnosis.
Our data also provide a unique opportunity to compare survival and treatment patterns across 3 eras (cohorts A, B, C). Survival has improved despite the shift toward a less favorable tumor subtype distribution. The overall MS has improved to 16 months and for patients in the best prognostic group (Breast GPA 3.5–4.0), the MS is now 36 months. Furthermore, in the HER2 subtype, median survival improved from 18 to 25 months between cohorts B and C for this subtype. Although our data set has limitations, in light of the frequent exclusions of patients with brain metastases in prospective clinical trials, and the inclusion only of de novo stage IV patients in the population-based SEER registry, we believe our observations represent perhaps the most persuasive evidence to date of effect of advances in local and systemic therapy upon the outcomes of patients with HER2-positive BCBM.
However, overall survival for patients with breast cancer with brain metastases is still poor, with half of all patients dying by 16 months. The percentage of patients alive at 1, 2, 3, 4, and 5 years is 60%, 37%, 23%, 17%, and 12%, respectively. The percentage of patients alive at 1, 2, 3, 4 and 5 years by subtype were HR-neg/HER2-neg (41%, 20%, 11%, 7%, and 6%); HR-neg/HER2-pos (72%, 51%, 33%, 24%, and 17%); HR-pos/Her2-neg (54%, 31%, 18%, 12%, 9%); and HR-pos/HER2-pos (78%, 54%, 39%, 30%, and 22%), respectively.
Our study showed a TPDBM of 27 months for both HR-neg/HER2-neg and Her2-pos subtypes compared with 46 months for HR-pos/HER2-pos and 54 months for HR-pos/ HER2-neg. This is consistent with other literature, which show BCBM occur later in the disease in patients with HR-pos/HER2-neg patients than other subtypes.41 One observation is that TPDBM was shorter in patients without ECM compared with those with ECM, suggesting that some patients may have occult CNS metastases at presentation of early-stage breast cancer (and not adequately treated with adjuvant systemic therapy) or a more brain-metastatic tumor phenotype.
Table E1 (available online at https://doi.org/10.1016/j.ijrobp.2020.01.051) shows a summary of systemic therapies and timing of those therapies. There are currently no drugs approved by the Food and Drug Administration for use in patients with BCBM, although several regimens have now gained endorsement by the National Comprehensive Cancer Network Neuro-Oncology guidelines committee.42 In particular, multiple HER2-directed regimens have been reported to result in clinically relevant response rates in the CNS.43 For example, Freedman reported a phase II trial (n = 49) with a CNS objective response rate of 49% in HER2-positive BCBM when treated with neratinib plus capcitabine.44
Mounsey et al (n = 123) reported HER2-positive patients who received HER2-targeted therapy (trastuzumab, lapatinib, pertuzumab, or T-DMI) after the diagnosis of BCBM had a MS of 2.1 years compared with 0.65 years in patients who did not receive such therapy.45 Duchnowska et al reviewed the evidence for tyrosine kinase inhibitors (lapatinib, neratinib, afatinib, and tucatinib) in HER2-positive patients, alone and in combination with hormonal therapy and chemotherapy (capcitabine), and concluded their role in BCBM at present is unknown.46
The aforementioned suggestion that HER2-targeted therapy benefits HER2-positive patients with BCBM begs scrutiny of the HR-pos/HER2-neg subtype regarding whether hormonal therapy offers a similar benefit. Bergen et al reported continuing hormonal therapy after the diagnosis of BCBM resulted in improved median survival compared with patients who did not continue hormonal therapy after the diagnosis of BCBM (15 vs 4 months).47 Our data show improvement in MS from 10 to 14 months between cohort B and C but no change in TPDBM (54 months in both cohorts). Although hormonal therapy may have a similar effect in hormone-positive patients, it may be masked by the greater magnitude of effect seen in the HER2-positive patients.
Others have reported there is no difference in outcome for patients with 2 to 4 versus 5 to 15 brain metastases when treated with SRS.48,49 Table E2 (available online at https://doi.org/10.1016/j.ijrobp.2020.01.051) shows the hazard ratios, which confirm there is not much of a survival trend among patients with more than one brain metastasis. Accordingly, the Breast GPA uses the cutoff of 1 versus more than 1. In our study, patients with HR-neg/HER2-neg subtype are most likely to harbor BRCA1 mutations (21%). This is consistent with a meta-analysis of 16 studies with 46,870 patients.50
This study has limitations. The data are retrospective with inherent selection bias, so they cannot be used to prove one treatment is better than another. The type, timing, combination, and sequence of immunotherapy, targeted therapies and chemotherapy, both before and after the diagnosis of brain metastases, varied widely so any conclusions regarding the effect of these interventions warrant cautious interpretation as this may represent bias by reverse causation and thus such conclusions require phase III confirmation. Comparison of survival between eras may suffer from lead-time bias, but we doubt this is a major factor because MRI/CT screening, which could result in earlier detection, is not standard of care. The years of the cohorts overlap somewhat, which could reduce the magnitude and likelihood of detecting a difference between the cohorts; nonetheless the aforementioned differences were found.
Conclusions
Treatment patterns have changed and survival has improved but varies widely by diagnosis-specific prognostic factors. New prognostic factors are identified and the updated Breast GPA is defined. Patients in the best prognostic group (GPA 3.5–4.0) can now expect to live more than 3 years. The GPA facilitates clinical decision-making and stratification of clinical trials. A free app to calculate the GPA is available at brainmetgpa.com. Beyond the updated Breast GPA, these data suggest but do not prove a benefit for systemic therapy. Many questions remain and prospective trials are needed to further improve outcomes for patients with breast cancer with brain metastases.
Supplementary Material
Acknowledgments
Grant support was received from the National Institutes of Health (NIH) grant number UL1TR002494 from the National Center for Advancing Translational Sciences (NCATS). Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Minnesota. In addition, the study was supported by NIH grant number P30 CA77598 using the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and the NCATS.
Disclosures: A.S. reports being an advisor/consultant with Abbvie, Merck, Roche, Varian (Medical Advisory Group), Elekta (Gamma Knife Icon), BrainLAB, and VieCure (Medical Advisory Board); is a board member of the International Stereotactic Radiosurgery Society (ISRS); has presented past educational seminars with Elekta AB, Accuray Inc, Varian (CNS Teaching Faculty), BrainLAB, Medtronic Kyphon; receives research grant from Elekta AB; and received travel accommodations or paid expenses by Elekta, Varian, BrainLAB. A.S. also belongs to the Elekta MR Linac Research Consortium, Elekta Spine, Oligometastases, and Linac Based SRS Consortia. M.P.M. discloses consulting relationships with IBA, Varian, Oncoceutics, Celgene, Abbvie, Astra-Zeneca, Tocagen, and Blue Earth Diagnostics, none of which pertain to the material in the manuscript. In addition, his institution has received research funding from Novocure. He also serves on the Board of Directors of Oncoceutics.
Footnotes
This work was presented at the 2019 Annual Meeting of the American Society of Clinical Oncology in Chicago, Illinois on June 2, 2019.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2020.01.051.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J for Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 3.Pestalozzi BC, Holmes E, de Azambuja E, et al. CNS relapse in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: A retrospective substudy of the HERA trial (BIG 1–01). Lancet Oncol 2013;14:244–248. [DOI] [PubMed] [Google Scholar]
- 4.Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271–3277. [DOI] [PubMed] [Google Scholar]
- 5.Lin N, Vanderplas A, Hughes ME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012;118:5463–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taillibert S, Le Ruhn E. Epidemiology of brain metastases. Cancer Radiother 2015;1:3–9. [DOI] [PubMed] [Google Scholar]
- 7.Loeffler J, Wen PY, Eichler AF. Epidemiology, clinical manifestations, and diagnosis of brain of brain metastases. Waltham, MA: UpToDate; 2019. UpToDate Inc. Available at: https://www.uptodate.com. Accessed March 17, 2019. [Google Scholar]
- 8.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran WJ. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008;70:510–514. [DOI] [PubMed] [Google Scholar]
- 9.Sperduto PW, Chao ST, Sneed P, et al. Diagnosis-specific prognostic factors, indices and treatment outcomes for patients with newly-diagnosed brain metastases: A multi-institutional analysis of 4259 patients. Int J Radiat Oncol Biol Phys 2010;77:655–661. [DOI] [PubMed] [Google Scholar]
- 10.Sperduto PW, Kased N, Roberge D, et al. Summary report on the Graded Prognostic Assessment (GPA): An accurate and facile diagnosis-specific tool to estimate survival, guide treatment and stratify clinical trials for patients with brain metastases. J Clin Onc 2012;30: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the Graded Prognostic Assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys 2012;82:2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sperduto PW, Kased N, Roberge D, et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neuro Oncol 2013;112:467–472. [DOI] [PubMed] [Google Scholar]
- 13.Sperduto PW, Yang TJ, Beal K, et al. Estimating Survival in Patients with Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer using Molecular Markers (Lung-molGPA). JAMA Oncol 2017;3:827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperduto PW, Deegan BJ, Li J, et al. Estimating survival for renal cell carcinoma patients with brain metastases: an update of the Renal Graded Prognostic Assessment tool. Neuro Oncol 2018;20:1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villa S, Moretones C, Weber DC, Manes A. Validation of the new Graded Prognostic Assessment scale for brain metastases: A multicenter prospective study. Radiat Oncol 2011;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbride L, Siker M, Bovi J, Gore E, Schultz C, Hall WA. Current predictive indices and nomograms to enable personalization of radiation therapy for patients with secondary malignant neoplasms of the central nervous system: A review. Neurosurgery 2018;82:595–603. [DOI] [PubMed] [Google Scholar]
- 17.Laakmann E, Riecke K, Goy Y, et al. Comparison of nine prognostic scores in patients with brain metastases of breast cancer receiving radiotherapy of the brain. J Cancer Res Clin Oncol 2016;142:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn HK, Lee S, Park YH, et al. Prediction of outcomes for patients with brain parenchymal metastases from breast cancer: A new breast cancer specific model and nomogram. Neuro-Oncol 2012;14:1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuang Q, Wong RX, Lian WX, et al. Validation of modified breast graded prognostic assessment for breast cancer patients with brain metastases: Extracranial disease progression is an independent risk factor. Ann Palliat Med 2019;8:390–400. [DOI] [PubMed] [Google Scholar]
- 20.Ahluwalia MS, Du L, Venur VA, et al. New graded prognostic index for breast cancer patients with brain metastases. J Clin Oncol 2014;32:637. [Google Scholar]
- 21.Subbiah IM, Lei X, Weinberg JS, et al. Validation and development of a modified breast Graded Prognostic Assessment as a tool for survival in patients with breast cancer and brain metastases. J Clin Oncol 2015; 33:2239–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2012;2:210–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramikrishna N, Temin S, Chandarlapaty, et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor-2-positive breast cancer and brain metastases: ASCO clinical practice guideline update. J Clin Oncol 2018;36:2804–2807. [DOI] [PubMed] [Google Scholar]
- 24.Olson JJ, Kalkanmis SN, Ryken TC. Congress of Neurological Surgeons systematic review and evidence-based guidelines for the treatment of adults with metastatic brain tumors. Neurosurgery 2019;84:550–552. [DOI] [PubMed] [Google Scholar]
- 25.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single brain metastases to the brain. N Engl J Med 1990;322:494–500. [DOI] [PubMed] [Google Scholar]
- 26.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with and without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665–1672. [DOI] [PubMed] [Google Scholar]
- 27.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA 2006;295:2483–2491. [DOI] [PubMed] [Google Scholar]
- 28.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952–26001 study. J Clin Oncol 2010;29:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol 2009;10:1037–1044. [DOI] [PubMed] [Google Scholar]
- 30.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA 2016;316:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown PD, Pugh S, Laack N, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro-Oncol 2013;15:1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J Clin Oncol 2014;32:3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gondi V, Deshmukh S, Brown PD, et al. Preservation of neuro-cognitive function with conformal avoidance of the hippocampus during whole-brain radiotherapy for brain metastases: Preliminary results of phase III trial NRG Oncology CC001. Int J Radiat Oncol Biol Phys 2018;102:1607. [Google Scholar]
- 34.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 2015;372:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019; 380:617–628. [DOI] [PubMed] [Google Scholar]
- 36.von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 2017;377:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson EM, Abdel-Rasoul M, Maly J, et al. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol 2013;24:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leone JP, Leone J, Zwenger AO, et al. Prognostic factors and survival according to tumor subtype in women presenting with breast cancer brain metastases at initial diagnosis. Eur J Cancer 2017;74:17–25. [DOI] [PubMed] [Google Scholar]
- 39.Miller JA, Kotecha R, Ahluwalia MS, et al. Overall survival and the response to radiotherapy among molecular subtypes of breast cancer brain metastases treated with targeted therapies. Cancer 2017;123: 2283–2293. [DOI] [PubMed] [Google Scholar]
- 40.Martin AM, Cagney DN, Catalano PJ, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol 2017; 3:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brosnan EM, Anders CK. Understanding patterns of brain metastases in breast cancer and designing rational therapeutic strategies. Ann Transl Med 2018;6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Comprehensive Cancer Network. About the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Available at: www.nccn.org. Accessed May 13, 2019. [DOI] [PubMed]
- 43.Lin NU, Kieras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res 2009;15:1452–1459. [DOI] [PubMed] [Google Scholar]
- 44.Freedman RA, Gelman RS, Anders C, et al. TBCRC 022: A phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol 2019;37:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mounsey LA, Deal AM, Keith KC, et al. Changing natural history of HER2-positive breast cancer metastatic to the brain in the era of new targeted therapies. Clinical Breast Cancer 2017;18:29–37. [DOI] [PubMed] [Google Scholar]
- 46.Duchnowska R, Loibl S, Jassem J. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer. Cancer Treatment Reviews 2018;61:71–77. [DOI] [PubMed] [Google Scholar]
- 47.Bergen ES, Berghoff AS, Medjedovic M, et al. Continued endocrine therapy is associated with improved survival in patients with breast cancer brain metastases. Clin Cancer Res 2019;18:1968. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol 2014;15: 387–395. [DOI] [PubMed] [Google Scholar]
- 49.Hughes RT, Masters AH, McTyre E, et al. Initial SRS for patients with 5–15 brain metastases: Results of a multi-institutional experience. Int J Radiat Oncol Biol Phys 2018;102:S141–S142. [DOI] [PubMed] [Google Scholar]
- 50.Chen H, Wu J, Zhang Z, et al. Association between BRCA status and triple-negative breast cancer: A meta-analysis. Front Pharmacol 2018;9:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


